Abstract
Background
Viral respiratory infections can cause acute wheezing illnesses in children and exacerbations of asthma.
Objective
We sought to identify variation in genes with known antiviral and pro-inflammatory functions to identify specific associations with more severe viral respiratory illnesses and the risk of virus-induced exacerbations during the peak fall season.
Methods
The associations between genetic variation at 326 SNPs in 63 candidate genes and 10 phenotypes related to viral respiratory infection and asthma control were examined in 226 children enrolled in the RhinoGen study. Replication of asthma control phenotypes was performed in 2,128 children in the Copenhagen Prospective Study on Asthma in Childhood (COPSAC). Significant associations in RhinoGen were further validated using virus-induced wheezing illness and asthma phenotypes in an independent sample of 122 children enrolled in the Childhood Origins of Asthma birth cohort study (COAST).
Results
A significant excess of P values smaller than 0.05 was observed in the analysis of the 10 RhinoGen phenotypes. Polymorphisms in 12 genes were significantly associated with variation in the four phenotypes showing a significant enrichment of small P values. Six of those genes (STAT4, JAK2, MX1, VDR, DDX58, and EIF2AK2) also showed significant associations with asthma exacerbations in the COPSAC study or with asthma or virus-induced wheezing phenotypes in the COAST study.
Conclusions
We identified genetic factors contributing to individual differences in childhood viral respiratory illnesses and virus-induced exacerbations of asthma. Defining mechanisms of these associations may provide insight into the pathogenesis of viral respiratory infections and virus-induced exacerbations of asthma.
Keywords: viral respiratory illness, human rhinovirus, asthma, cold symptoms, wheezing, allergic sensitization, children, genetic association
INTRODUCTION
Infections with respiratory viruses are extremely common during childhood, and all children experience repeated infections during high-prevalence seasons [1]. While most of these infections are relatively short-lived and cause mild respiratory symptoms, some cause severe illnesses that can include episodic wheezing, exacerbations of chronic respiratory diseases, and a need for hospitalization [2]. Furthermore, severe wheezing illnesses may cause airway injury, and thereby promote airway inflammation and remodeling [3, 4]. Indeed, virus-induced wheezing illnesses in early childhood are significantly associated with decreased lung function and increased risk of asthma later in life [5–8]. Rhinovirus (RV) infections are of particular importance in asthma. In early life, wheezing illnesses with RV are associated with a particularly high risk for subsequent asthma [5, 6]. Moreover, once asthma is established, RV infections are the most common cause for virus-induced exacerbations of this chronic airways disease [9].
Because of the significant economic and quality of life costs associated with viral respiratory infections in children with asthma, there is considerable interest in identifying the environmental and genetic factors that underlie individual variation in both susceptibility to and severity of viral infections. Environmental factors, such as exposure to tobacco smoke, day care attendance, and number of siblings, have been identified as risk factors for viral respiratory illnesses [8, 10]. Genetic variation, such as that at the 17q12 locus, has been linked to viral wheezing and asthma risk in children, suggesting a shared genetic architecture for these traits [11]. In addition, cells have multiple systems for recognizing viral infections, as well as effector mechanisms to limit viral replication and/or spread. For example, RV replication produces single and double-stranded RNA that can activate TLR-3, TLR7, PKR and the RNA helicases RIG-I and MDA-5 [12–14]. Which of these molecules is necessary for effective antiviral responses to RV is incompletely understood. In addition, replication of RVs, like most picornaviruses, is sensitive to Type I and Type III interferons [15]. Whether genetic variation in these viral recognition or antiviral effector pathways contributes to illness severity and risk of virus-induced asthma exacerbations is unknown.
The objectives of this study were two-fold: i) to identify antiviral or pro-inflammatory genes that are associated with susceptibility to, and severity of, viral respiratory infections in children, and ii) to identify genetic factors that underlie viral-induced exacerbations of childhood asthma. To this end, we conducted a hypothesis-driven study of 326 SNPs in 63 candidate genes that have known roles in antiviral response, interferon response pathways, or asthma pathogenesis. By integrating genetic data, molecular identification of viruses, and detailed clinical outcomes after naturally acquired respiratory infections in children, we identified host genetic factors that contribute to the initiation and progression of viral respiratory infections and that underlie virus-induced asthma exacerbations during childhood.
METHODS
RhinoGen study subjects
Participants were recruited through the RhinoGen study, a large prospective study of the genetic basis of childhood susceptibility to virus-induced respiratory illness, illness severity and clearance, and virus-induced asthma exacerbations [16]. A total of 383 children, aged 4–12 years, were recruited from the general population in Madison, Wisconsin between 2007 and 2010. This sample included children with and without asthma in approximately equal numbers. After providing informed consent, children underwent a general health screen, provided a blood sample, and completed questionnaires. Any child with or without asthma was eligible for enrollment provided that they did not have one of the following exclusions: history of premature birth, complications at birth, respiratory problems at birth, or any other significant medical illness. Asthma was defined as doctor-diagnosed asthma with either medication use or symptoms in the past 12 months. Written consent was obtained from all families, including assent from children at least 7 years of age. The University of Wisconsin Human Subjects Committee and the University of Chicago Institutional Review Board approved this study.
Phenotype collection
Ten phenotypes (Table E1) were chosen a priori as measures of response to or control of viral respiratory infections. Phenotype data were collected during two 5-week periods, one in the fall (i.e. September) and the other in the spring (i.e. April), that coincided with seasonal peaks of RV infection frequency. During each 5-week period, children or their parents kept daily symptom diaries in which they recorded symptoms related to rhinitis, cough, wheeze, colds, and/or asthma.
Cold and asthma symptom severity was scored based on a four-point scoring system (Table E2), as described previously [17]. Subjects were also trained to collect nasal mucus using a nose-blowing technique as previously described [18, 19]. Nasal samples were collected weekly during each 5-week period and they analyzed for common respiratory viruses using multiplex PCR (MultiCode assay; EraGen Biosciences, Madison, WI) [20]. Specific RV types were identified by partial sequencing [21].
Among the 383 children who participated in RhinoGen, 310 (81%) completed at least 80% of the daily symptom diaries, provided a DNA sample for genetic studies, and submitted at least 8 of 10 scheduled nasal mucus samples (Figure E1). In these 310 children, the mean and median age was 8.4 years and 8.2 years, respectively. For our study, we included the 240 of the 310 children with self-reported European ancestry, which was confirmed by principal component analysis (Figure E2), because there were not enough non-European children to provide statistical power (Figure 1). Six phenotypes were measured in all 240 children: number of illnesses, number of viral illnesses, number of weeks infected, cold symptom burden, viral cold symptom burden, and asthma status (Figure E1). The remaining four asthma-related phenotypes (loss of asthma control, viral loss of asthma control, asthma symptom burden, and viral asthma symptom burden) were studied in the 110 children who were diagnosed with asthma at the completion of the study (Figure E1). See Table E1 for detailed descriptions of all phenotypes.
Figure 1.
Histogram of frequency of P values below defined significant thresholds for 326 SNPs obtained from permutation analysis (n=10,000 runs). The observed number of P values below each threshold is shown in red. (A) Distribution of permutation P values < 0.05. Three hundred and eight of the 10,000 permutations (3.08%) contained more P values <0.05 than the number observed. (B) Distribution of permutation P values < 0.01. Five hundred and fifty of the 10,000 permutations (5.5%) contained more P values <0.01 than the number observed. (C) Distribution of P values <0.001. Two thousand, two hundred and twenty-one of the 10,000 permutations (22.01%) contained more P values <0.001 than that observed.
SNP selection
Sixty-three genes involved in antiviral responses, interferon response pathways, or asthma pathogenesis were selected a priori for genotyping. Sources for gene selection included a study of genes that are highly expressed by RV-infected cells [22] and the available literature. HapMap CEU allele frequencies and linkage disequilibrium (LD) estimates were used to select tag SNPs covering the gene and extending 1500 kb upstream and downstream. Within each LD bin (defined by r2 ≥ 0.80), common SNPs (i.e. minor allele frequency >5%) with known or putative function (i.e. SNPs causing nonsynonymous changes and those located in transcription factor binding sites, microRNA target sites, splice junctions, or conserved noncoding regions) were given priority as tag SNPs. A total of 326 SNPs in 63 genes that were selected passed the Illumina SNP selection process, and were successfully genotyped with high quality using the Illumina GoldenGate custom genotyping assay at the National Heart, Lung, and Blood Institute’s (NHLBI) Resequencing and Genotyping (RS&G) Service at Johns Hopkins University (Table E3).
Genotyping and quality control checks
Peripheral blood samples were collected during the first clinical visit after enrollment. Aliquots of frozen blood were shipped on dry ice to the University of Chicago where DNA was extracted using the Puregene extraction kit, following the manufacturer’s instructions (Gentra Systems, Inc., Minneapolis, MN).
Stringent quality control was used to assure high quality data. Specifically, the 326 SNPs that were included in our analyses all had >95% call rates, Hardy-Weinburg P values >0.001, and minor allele frequencies >5%. After genotype QC, 14 of the 240 children were excluded due to genotyping failure. The final data set was comprised of 226 children of European ancestry genotyped at 326 SNPs (Figure E1).
Genetic association testing
Associations between genetic variation at each of the 326 SNPs and the 10 RhinoGen phenotypes were examined using logistic regression for the 3 qualitative phenotypes (loss of asthma control, viral loss of asthma control, and final asthma diagnosis) and linear regression for the remaining 7 quantitative traits. In both sets of analysis, genetic effects were calculated using an additive model with the age and sex of the subject included as covariates. The results of these association tests are referred to as the observed P values. All analyses were conducted using the ‘glm’ package in the statistical software R (R Project, www.r-project.com).
Because of the non-independence of some SNPs (e.g. due to LD) and phenotypes (e.g. due to biological correlations), permutation tests were applied to assess significance. First, a null distribution of P values was generated from 10,000 permutations, wherein each permutation shuffled the link between phenotype values and the genotype data. Thus, both the observed patterns of linkage disequilibrium among SNPs and correlations among phenotypes were preserved in the generation of the null distribution of P values., Next, we calculated the number of permutation P values that were smaller than the observed P value and divided that number by the total number of permutations performed (n=10,000) to obtain the empirical P value for each test.
An enrichment analysis was performed to test for an enrichment of small (i.e. significant) observed P values for a particular phenotype compared to the null distribution of P values obtained from the permutation analysis (i.e. the null expectation). For each individual RhinoGen phenotype, two statistics were calculated: the number of observed P values across the 326 SNPs that were smaller than a specific significance threshold (either P=0.05, P=0.01, or P=0.001) and the number of permutation P values below that same significance threshold. An empirical P value representing the significance of enrichment for the phenotype was calculated as the number of permutation P values below the threshold divided by the total number of permutations performed (n=10,000).
Replication and validation studies
Significant associations in the RhinoGen analyses were examined in two prospective birth cohorts, the Copenhagen Prospective Study on Asthma in Childhood (COPSAC) and the Childhood Origins of Asthma (COAST) study (Table 1). The COPSAC children served as a replication sample for associations with exacerbations of asthma symptoms, for which RV exposure is the most common cause. The COAST children served as a validation sample because in this cohort we investigated associations with clinical outcomes (wheezing illnesses and asthma).
Table 1.
Baseline characteristics of study subjects.
| RhinoGen children included in genetic analysis | Excluded RhinoGen children* | COAST children (who were not in the RhinoGen study) | COPSAC children | ||
|---|---|---|---|---|---|
| (n=226) | (n=73) | (n=122) | (n=2,128) | ||
| Sex | Male | 57% | 51% | 55% | 60% |
|
| |||||
| Race/Ethnicity | European or European American | 100% | 64% | 100% | 100% |
| African American | 0% | 25% | 0% | 0% | |
| Mixed ancestry | 0% | 11% | 0% | 0% | |
| Hispanic/Latino | 0% | 5% | 0% | 0% | |
|
| |||||
| Asthma | 49% | 63% | 36% | 58% | |
|
| |||||
| Aeroallergen sensitization | 53% | 59% | 50% | N/A | |
Exclusions due to incomplete phenotype data or non-European ancestry.
Cases for the replication analysis were obtained from the COPSACexacerbation study of children with recurrent, severe asthma exacerbations in early childhood [23]. Controls subjects were obtained from the COPSAC2000 and COPSAC2010 birth cohorts [11, 24]. Further descriptions of the COPSAC cohorts and genotyping platforms used in those cohorts are provided in the Supplementary Methods.
The COAST cohort is comprised of 289 newborns enrolled between November 1998 and May 2000 [25]. Genotyping was performed at the same time and on the same platform as that described for the RhinoGen cohort. The diagnostic criteria for asthma and descriptions of the wheezing phenotypes in the first three years of life were as previously described [5, 26]. Further descriptions of the COAST cohort are provided in the Supplementary Methods.
The clinical characteristics of the study populations are described in Table 1.
Prediction of regulatory and functional effects of genetic polymorphisms For SNPs located in non-coding regions, RegulomeDB [27] (http://regulome.stanford.edu/) and HaploReg [28] (http://www.broadinstitute.org/mammals/haploreg/haploreg.php) databases were used to infer potential functional consequences of polymorphisms. To predict the functional and structural effects of SNPs located in the coding region of genes, we used the Poly-Phen2 [29] (http://genetics.bwh.harvard.edu/pph2/) software tool.
RESULTS
Genetic variation and respiratory outcomes in RhinoGen
In the global analysis of associations between the 326 SNPs and the 10 RhinoGen phenotypes, we observed 224 associations with a P value <0.05, which was a significant excess compared to the null expectation (P = 0.031; Figure 1A). There was a nearly significant enrichment of P values <0.01 (P=0.055; Figure 1B), and no evidence of enrichment for P values <0.001 (P=0.22; Figure 1C). These results indicate that this set of SNPs in or near candidate genes is enriched for SNPs associated with viral illness related phenotypes.
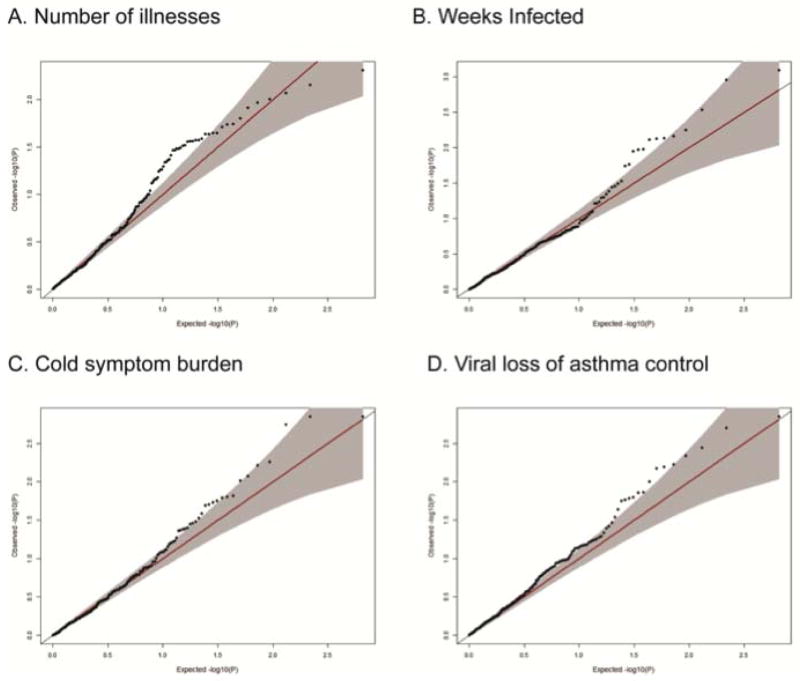
Examining each phenotype individually, we observed a significant enrichment of significant P values for four phenotypes, a nearly-significant enrichment for two additional phenotypes, and no evidence of enrichment for the final two (Table E4). For the four phenotypes showing a significant enrichment, the excess of significant P values is apparent in the Q–Q plots of observed versus expected P values (Figure 2A–D). Among the four phenotypes enriched for small P-values, we identified 12 genes with at least one SNP with an empirical P value <0.01 for those phenotypes (Table 2). Four of those 12 genes contained SNPs with association P values <0.01 for more than one of these four RhinoGen phenotypes (Table 2).
Figure 2.
Q-Q plots of the four RhinoGen phenotypes showing an enrichment of small P values. In each plot (A-D), the observed -log(P value) and expected -log(P value) are plotted.
Table 2.
SNPs with association P-values <0.01 for the four RhinoGen phenotypes showing an enrichment of small p-values. Beta coefficients are shown for three qualitative traits and odds ratio for the one quantitative trait.
| Gene | SNP | Chr | Alleles (min/maj) | Effect allele | RhinoGen Phenotype | P value | Beta coeff | Odds ratio (OR) | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| DDX58 | rs10738889 | 9 | G/A | A | Viral loss of asthma control | 0.0099 | 0.5097 | 0.2879 | 0.8750 | |
| DDX58 | rs10738891 | 9 | T/G | G | Weeks infected | 0.0068 | 0.6158 | 0.2163 | 1.0152 | |
| DDX58 | rs11795343 | 9 | C/T | T | Weeks infected | 0.0076 | −0.5780 | −0.7530 | −0.2015 | |
| DDX58 | rs11795343 | 9 | C/T | T | Viral loss of asthma control | 0.0020 | 0.4243 | 0.2370 | 0.7297 | |
| DDX58 | rs17289402 | 9 | A/G | G | Viral loss of asthma control | 0.0014 | 5.2311 | 1.6722 | 23.2345 | |
| DDX58 | rs3205166 | 9 | G/T | T | Weeks infected | 0.0029 | −0.6637 | −1.0507 | −0.2766 | |
| DDX58 | rs3205166 | 9 | G/T | T | Viral loss of asthma control | 0.0046 | 0.4540 | 0.2585 | 0.7719 | |
| DDX58 | rs6476362 | 9 | C/T | T | Viral loss of asthma control | 0.0067 | 0.3873 | 0.1861 | 0.7669 | |
| DDX58 | rs7034650 | 9 | G/A | A | Weeks infected | 0.0074 | −0.8616 | −1.4358 | −0.2873 | |
| DDX58 | rs944581 | 9 | C/G | G | Weeks infected | 0.0073 | 0.5856 | 0.1928 | 0.9783 | |
|
| ||||||||||
| EIF2AK2 | rs4233920 | 2 | A/G | G | Weeks infected | 0.0008 | 1.4456 | 0.4795 | 2.4116 | |
|
| ||||||||||
| IFNB1 | rs10435777 | 9 | A/T | T | Cold symptom burden | 0.0014 | −10.882 | −16.814 | −4.9498 | |
| IFNB1 | rs7849713 | 9 | C/G | G | Cold symptom burden | 0.0014 | −10.882 | −16.814 | −4.9498 | |
|
| ||||||||||
| IL21 | rs2221903 | 4 | C/T | T | Weeks infected | 0.0056 | −0.6582 | −1.0724 | −0.2441 | |
|
| ||||||||||
| IL28A | rs11671087 | 19 | C/T | T | Cold symptom burden | 0.0084 | −5.9473 | −10.705 | −1.1890 | |
|
| ||||||||||
| IL8 | rs2227306 | 4 | C/T | T | Cold symptom burden | 0.0097 | −5.0177 | −8.9301 | −1.1053 | |
| IL8 | rs2227306 | 4 | C/T | T | Viral loss of asthma control | 0.0036 | 0.4368 | 0.2392 | 0.7650 | |
|
| ||||||||||
| JAK2 | rs6476939 | 9 | A/T | T | Number of illnesses | 0.0099 | −0.4496 | −0.7658 | −0.1334 | |
| JAK2 | rs6476939 | 9 | A/T | T | Cold symptom burden | 0.0061 | −5.7245 | −9.5868 | −1.8622 | |
|
| ||||||||||
| MX1 | rs459498 | 21 | A/G | G | Weeks infected | 0.0011 | −0.6561 | −1.0422 | −0.2699 | |
| MX1 | rs467593 | 21 | G/A | A | Weeks infected | 0.0111 | −0.6896 | −1.1686 | −0.2106 | |
|
| ||||||||||
| SCGB1A1 | rs2509973 | 11 | A/C | C | Cold symptom burden | 0.0018 | 6.3144 | 2.4981 | 10.1307 | |
|
| ||||||||||
| STAT1 | rs11887698 | 2 | G/A | A | Number of illnesses | 0.0070 | −0.5265 | −0.9697 | −0.0833 | |
|
| ||||||||||
| STAT4 | rs16833215 | 2 | G/A | A | Number of illnesses | 0.0085 | −0.4957 | −0.8408 | −0.1506 | |
| STAT4 | rs16833249 | 2 | C/T | T | Number of illnesses | 0.0049 | −0.5064 | −0.8218 | −0.1910 | |
| STAT4 | rs6434435 | 2 | A/G | G | Number of illnesses | 0.0122 | −0.5584 | −0.9715 | −0.1453 | |
|
| ||||||||||
| VDR | rs12721370 | 12 | A/C | C | Weeks infected | 0.0105 | 1.0124 | 0.2930 | 1.7318 | |
| VDR | rs4328262 | 12 | G/T | T | Cold symptom burden | 0.0055 | −6.2878 | −10.376 | −2.1996 | |
| VDR | rs4328262 | 12 | G/T | T | Viral loss of asthma control | 0.0059 | 0.4045 | 0.2039 | 0.7601 | |
| VDR | rs7139166 | 12 | C/G | G | Viral loss of asthma control | 0.0064 | 2.3272 | 1.2885 | 4.4188 | |
Replication in COPSAC and COAST
From the 12 genes showing significant associations in RhinoGen, we selected 57 SNPs with empirical P values <0.05 for replication and validation in the other study populations. For the replication studies in COPSAC, we observed significant genetic associations with asthma exacerbations for four SNPs in two genes (Table 3). In COAST, SNPs in six of the 12 genes were significantly associated with a wheezing or asthma phenotype (Table 3). Overall, SNPs in two genes (MX1 and STAT4) showed significant associations in all three cohorts (Table 3).
Table 3.
Genes and SNPs associated with variation in viral illness response, asthma, and wheezing phenotypes in the RhinoGen discovery population, the COPSAC replication population, and COAST validation population (P ≤0.05 highlighted in bold font).
| RhinoGen P values |
COPSAC P values |
COAST P values |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | SNP | Location | Chr | Alleles (minor/major) |
MAF | Number of illnesses | Number of viral illnesses |
Weeks infected | Cold symptom burden | Viral cold symptom burden |
Asthma symptom burden |
Viral asthma symptom burden |
Asthma | Loss of asthma control | Viral loss of asthma control |
Asthma exacerbations | Asthma (at age 6) | RSV wheezing illness (first 3 years of life) |
RV wheezing illness (first 3 years of life) |
Wheezing illness (first 3 years of life) |
| DDX58 | rs10738889 | intron | 9 | G/A | 0.40 | 0.261 | 0.312 | 0.116 | 0.225 | 0.319 | 0.753 | 0.453 | 0.896 | 0.010 | 0.010 | 0.660 | 0.558 | 0.065 | 0.128 | 0.038 |
|
| ||||||||||||||||||||
| DDX58 | rs3205166 | coding | 9 | G/T | 0.36 | 0.223 | 0.177 | 0.003 | 0.228 | 0.259 | 0.295 | 0.177 | 0.926 | 0.022 | 0.005 | 0.910 | 0.011 | 0.208 | 0.090 | 0.012 |
|
| ||||||||||||||||||||
| DDX58 | rs9695310 | intron | 9 | C/G | 0.47 | 0.109 | 0.948 | 0.744 | 0.324 | 0.108 | 0.770 | 0.589 | 1.000 | 0.015 | 0.073 | 0.367 | 0.015 | 0.126 | 0.810 | 0.482 |
|
| ||||||||||||||||||||
| DDX58 | rs6476362 | intron | 9 | C/T | 0.21 | 0.314 | 0.678 | 0.289 | 0.948 | 0.798 | 0.183 | 0.115 | 0.826 | 0.008 | 0.007 | 0.718 | 0.061 | 0.147 | 0.288 | 0.023 |
|
| ||||||||||||||||||||
| DDX58 | rs11795343 | intron | 9 | C/T | 0.43 | 0.136 | 0.212 | 0.008 | 0.411 | 0.436 | 0.065 | 0.035 | 0.590 | 0.005 | 0.002 | 0.576 | 0.012 | 0.824 | 0.177 | 0.090 |
|
| ||||||||||||||||||||
| DDX58 | rs10813831 | coding | 9 | A/G | 0.27 | 0.388 | 0.942 | 0.296 | 0.642 | 0.509 | 0.034 | 0.022 | 0.919 | 0.005 | 0.014 | 0.292 | 0.035 | 0.396 | 0.762 | 0.207 |
|
| ||||||||||||||||||||
| EIF2AK2 | rs2373114 | intron | 2 | T/C | 0.09 | 0.030 | 0.042 | 0.082 | 0.043 | 0.045 | 0.958 | 0.358 | 0.275 | 0.298 | 0.327 | 0.849 | 0.125 | 0.987 | 0.006 | 0.590 |
|
| ||||||||||||||||||||
| JAK2 | rs913594 | intron | 9 | A/G | 0.28 | 0.018 | 0.185 | 0.429 | 0.065 | 0.203 | 0.420 | 0.353 | 0.393 | 0.962 | 0.788 | 0.415 | 0.027 | 0.035 | 0.018 | 0.036 |
|
| ||||||||||||||||||||
| JAK2 | rs3780375 | intron | 9 | A/G | 0.23 | 0.019 | 0.066 | 0.205 | 0.042 | 0.127 | 0.350 | 0.321 | 0.597 | 0.507 | 0.737 | 0.827 | 0.012 | 0.021 | 0.029 | 0.012 |
|
| ||||||||||||||||||||
| JAK2 | rs3780379 | intron | 9 | A/G | 0.18 | 0.028 | 0.090 | 0.266 | 0.015 | 0.059 | 0.397 | 0.387 | 0.992 | 0.684 | 0.917 | 0.403 | 0.041 | 0.083 | 0.089 | 0.081 |
|
| ||||||||||||||||||||
| MX1 | rs469390 | coding | 21 | G/A | 0.39 | 0.912 | 0.385 | 0.141 | 0.182 | 0.171 | 0.191 | 0.031 | 0.823 | 0.423 | 0.766 | 0.001 | 0.590 | 0.848 | 0.770 | 0.668 |
|
| ||||||||||||||||||||
| MX1 | rs2070229 | coding | 21 | C/T | 0.26 | 0.567 | 0.922 | 0.032 | 0.260 | 0.158 | 0.265 | 0.050 | 0.493 | 0.816 | 0.568 | 0.009 | 0.531 | 0.067 | 0.016 | 0.184 |
|
| ||||||||||||||||||||
| MX1 | rs467593 | intron | 21 | G/A | 0.31 | 0.909 | 0.549 | 0.011 | 0.599 | 0.421 | 0.055 | 0.016 | 0.823 | 0.978 | 0.634 | 0.047 | 0.149 | 0.136 | 0.066 | 0.456 |
|
| ||||||||||||||||||||
| STAT4 | rs16833215 | intron | 2 | G/A | 0.31 | 0.008 | 0.023 | 0.574 | 0.251 | 0.344 | 0.842 | 0.554 | 0.920 | 0.689 | 0.920 | 0.474 | 0.882 | 0.984 | 0.042 | 0.381 |
|
| ||||||||||||||||||||
| STAT4 | rs6434435 | intron | 2 | A/G | 0.17 | 0.012 | 0.319 | 0.221 | 0.584 | 0.356 | 0.991 | 0.807 | 0.905 | 0.576 | 0.719 | 0.754 | 0.020 | 0.815 | 0.220 | 0.970 |
|
| ||||||||||||||||||||
| STAT4 | rs7566274 | intron | 2 | C/A | 0.41 | 0.030 | 0.031 | 0.388 | 0.877 | 0.661 | 0.673 | 0.318 | 0.178 | 0.316 | 0.211 | 0.712 | 0.818 | 0.045 | 0.488 | 0.627 |
|
| ||||||||||||||||||||
| STAT4 | rs11685878 | intron | 2 | T/C | 0.43 | 0.965 | 0.203 | 0.878 | 0.148 | 0.094 | 0.433 | 0.318 | 0.046 | 0.664 | 0.397 | N/A | 0.215 | 0.001 | 0.279 | 0.048 |
|
| ||||||||||||||||||||
| STAT4 | rs4853546 | intron | 2 | A/G | 0.37 | 0.457 | 0.212 | 0.974 | 0.265 | 0.280 | 0.433 | 0.560 | 0.031 | 0.308 | 0.164 | 0.024 | 0.720 | 0.001 | 0.958 | 0.141 |
|
| ||||||||||||||||||||
| STAT4 | rs7574070 | intron | 2 | A/C | 0.39 | 0.946 | 0.188 | 0.566 | 0.089 | 0.045 | 0.212 | 0.143 | 0.385 | 0.340 | 0.228 | 0.147 | 0.034 | 0.001 | 0.312 | 0.023 |
|
| ||||||||||||||||||||
| VDR | rs12721370 | intron | 12 | A/C | 0.09 | 0.392 | 0.318 | 0.010 | 0.433 | 0.655 | 0.762 | 0.993 | 0.838 | 0.831 | 0.679 | 0.206 | 0.010 | 0.825 | 0.553 | 0.454 |
|
| ||||||||||||||||||||
| VDR | rs4328262 | intron | 12 | G/T | 0.40 | 0.018 | 0.046 | 0.445 | 0.005 | 0.009 | 0.016 | 0.047 | 0.269 | 0.002 | 0.006 | 0.973 | 0.021 | 0.892 | 0.338 | 0.964 |
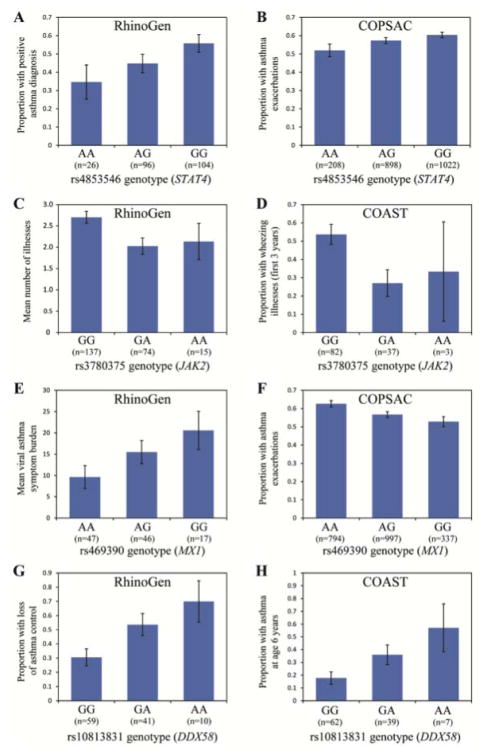
In the STAT4 (signal transducer and activator of transcription 4; OMIM 600558) gene, an intronic SNP, rs16833215, was one of three to be associated with the number of illnesses in RhinoGen and with wheezing or asthma phenotypes in COAST (Table 3). Another intronic SNP in STAT4, rs4853546, was significantly associated with asthma in RhinoGen (Figure 3A), asthma exacerbations in COPSAC (Figure 3B), and RSV wheezing illnesses in COAST (Table 3). STAT4 signals through the Jak-Stat pathway. Notably, an intronic SNP, rs3780375 in the JAK2 (janus kinase 2; OMIM 147796) gene was also associated with number of illnesses and cold symptom burden in RhinoGen (Table 3; Figure 3C) and with asthma at age 6 years and all three wheezing phenotypes in COAST (Figure 3D; Table 3). For all three of these STAT4 and JAK2 SNPs, the direction of effect was consistent across all three cohorts.
Figure 3.
SNP associations with RhinoGen, COPSAC, and COAST phenotypes. STAT4 rs4853546 genotype association with asthma (P=0.031) in RhinoGen (A) and asthma exacerbations (P=0.024) in COPSAC (B). JAK2 rs3780375 genotype association with mean number of illnesses (P=0.019) in RhinoGen (C) and wheezing illnesses (P=0.012) in COAST (D). MX1 rs469390 genotype association with viral asthma symptom burden (P=0.031) in RhinoGen (E) and asthma exacerbations (P=8.8x10−4) in COPSAC (F). DDX58 rs10813831 genotype association with loss of asthma control (P=0.0055) in RhinoGen (G) and asthma at age 6 years (P=0.035) in COAST (H). Error bars show standard error of means or proportions. Total number of individuals is indicated beneath each genotype class.
The MX dynamin-like GTPase 1 gene (MX1; OMIM 147150) SNP, rs469390, which encodes a missense mutation (G/A; Val379Ile) in exon 15 of the MX1 protein, was significantly associated with viral asthma burden in RhinoGen (Figure 3E) and asthma exacerbations in COPSAC (Figure 3F). Surprisingly, the allele (G) associated with increased viral asthma burden in the RhinoGen children was associated with fewer asthma exacerbations in COPSAC children (Figure 3E and 3F). A second independent (r2=0.19) coding SNP, rs2070229, was significantly associated with viral asthma burden and weeks infected in RhinoGen, with RV wheezing in COAST, and with asthma exacerbations in COPSAC (Table 3). This SNP showed an opposite direction of effect in RhinoGen compared to COPSAC and COAST. The third significant MX1 SNP, rs467593, was in linkage disequilibrium (r2=0.78) with rs2070229 and shows the same opposite pattern of association in RhinoGen compared to COPSAC and COAST.
Two additional genes, VDR and DDX58, showed significant associations in the RhinoGen and COAST cohorts, with the same direction of effect. The vitamin D receptor gene (VDR; OMIM 601769) has an intronic SNP (rs4328262) that was significantly associated with variation in eight of the 10 RhinoGen phenotypes and with asthma at age 6 in the COAST validation sample (Table 3). Specifically, the TT genotype at rs4328262 was associated with fewer observed illnesses, decreased cold symptom burden, and less loss of asthma control in children in RhinoGen, and with less asthma at age 6 years in the COAST children.
The DEAD box polypeptide 58 gene (DDX58; OMIM 609631; encoding the RIG-I protein) contained multiple independent SNPs that were associated with RhinoGen and COAST phenotypes. A missense mutation rs10813831 (G/A; Arg7Cys change) was associated with loss of asthma control (Figure 3G), both undefined and of viral origin, and asthma symptom burden in RhinoGen and with asthma at age 6 in COAST (Table 3; Figure 3H). The direction of effect was similar in both cohorts. This mutation results in an Arg7Cys amino acid change in the caspase recruitment domain (CARD) of the protein and is predicted with high confidence to affect protein function or structure according to PolyPhen-2 (Table E5). A second coding mutation in DDX58, rs3205166 (G/T), was associated with increased loss of asthma control, both undefined and of viral origin, and with the number of weeks infected in RhinoGen, and with the occurrence of asthma at age 6 and wheezing in the first 3 years of life in the COAST validation study (Table 3). The rs3205166 SNP represents an independent signal from rs10813831 (LD r2=0.23) and is located in predicted binding motifs for the GATA1, GATA5, and AP-1 proteins (Table E5).
DISCUSSION
In this study, we identified specific genetic variants associated with susceptibility to viral respiratory infections, severity of infection, and virus-induced exacerbations of asthma during childhood that are in or near genes previously implicated in antiviral response, interferon response pathways, or asthma pathogenesis. We observed significant excesses over null expectations of small P values in four of 10 RhinoGen phenotypes. SNPs in 12 genes were significantly associated with variation in at least one of these four RhinoGen phenotypes. Twenty one SNPs in six of those genes were also associated with asthma exacerbations, childhood wheezing, or childhood asthma in children from independent birth cohort, COAST and COPSAC.
Variations in two genes were associated with related phenotypes in all three cohorts and represent the most robust findings from our study. One of those genes, STAT4, has been investigated in previous genetic studies of asthma with inconsistent results [30–32]. STAT4 is essential to IL-12 signal transduction and function, and thus plays an important role in IL-12 mediated IFN-γ production and the differentiation of Th1 cells [33, 34]. We previously showed associations between reduced IFN-γ neonate production and increased frequency and severity of childhood wheezing and respiratory illness [10, 35] and with increased cold symptoms after viral infection [36]. One of the STAT4 SNPs showing associations in all three cohorts, rs4853546, was recently shown to act as a cis-eQTL in CD14+ monocytes stimulated with interferon-γ or LPS [37]. Thus, the associations we observe here between STAT4 genotype and viral illness, asthma exacerbations, and childhood wheezing may be due to defects in IFN-γ response profiles or to a skewing of T-helper cell differentiation towards a Th2 phenotype.
The second gene with associations in all three cohorts, MX1, encodes the MxA protein, an interferon (IFN)-inducible GTPase with antiviral activities against a broad range of RNA viruses [38]. A missense mutation, rs469390 (Val379Ile), was associated with asthma phenotypes in RhinoGen and COPSAC and was also recently reported to be associated with severe RSV disease in childhood [39]. This missense mutation was a cis-eQTL in naïve CD14+ monocytes and monocytes stimulated with interferon-γ [37]. The ancestral G allele, encoding valine, is the minor allele in Europeans although it is the more common allele in African and Asian HapMap populations. The G (valine) allele was associated with viral asthma symptom burden in the RhinoGen children, whereas the A (isoleucine) allele was associated with asthma exacerbations in the COPSAC children in this study, and with RSV-G mRNA expression in 42 HapMap lymphoblastoid cell lines and RSV severity in infants in Buenos Aires [39].
A second coding MX1 SNP (rs2070229) also showed opposite direction of associations, with the allele (C) associated with increased viral asthma burden and weeks infected in RhinoGen being protective against RV wheezing in COAST and asthma exacerbations in COPSAC. These flip-flop associations have been reported for other variants with asthma-related phenotypes and have been attributed to differences in environmental exposures (e.g., CD14 genotype and endotoxin exposure interactions on atopy risk, HLA-G genotype and maternal asthma status interactions on childhood onset asthma risks, or IL13 genotype and cellular environment interactions on IL-13 activity) [40]. The underlying explanation for the opposite effects of alleles at the MX1 locus is less clear but may reflect subtle differences between the cohorts due to sample ascertainment, environmental exposures, or developmental effects, as the COAST and COPSAC wheezing or exacerbations phenotypes were assessed in early life (before age 6), whereas the RhinoGen children were on average older (>8 years of age). Nonetheless, these data combined with the recent studies linking the SNPs to cis-regulation and RSV response suggest a potentially important role for structural variation in the MxA protein modulating individual responses to respiratory viruses, and potentially accounting for differences in downstream effects on asthma symptoms and exacerbations.
Two other genes showed strong associations in the RhinoGen and COAST cohorts. The vitamin D receptor (VDR) is a nuclear protein that serves as the principal receptor for the biologically active form of the vitamin D hormone, and contributes to the development and regulation of the immune system [41]. Genetic variation in the VDR gene has been associated with viral respiratory infection, lung function, atopy, and/or asthma in many [42–49], but not all [50], studies. Most studies of VDR variation focused on a small set of restriction site polymorphisms, including the nonsynonymous Fok-I variant (rs2228570) and a cluster of 3 SNPs in the 3’ end of the gene (Bsm-I, rs1544410; Apa-I, rs7975232; Taq-I, rs731236) that are in LD with each other [51]. Unfortunately, these four SNPs do not capture a large fraction of the known genetic variation at the VDR locus [52]. Our study tagged variation throughout the large VDR gene and flanking regions. As a result, we identified novel associations between VDR variation and viral response and asthma phenotypes. The underlying mechanism for these associations could potentially involve variation in gene expression as both validated SNPs are located in regions predicted to have enhancer properties according to the ENCODE functional annotations [53]: ‘strong enhancer’ for rs12721370 and ‘weak enhancer’ for rs4328262.
Finally, the DDX58 gene, which encodes the antiviral protein, retinoic acid-inducible gene I (RIG-I), contained SNPs with significant associations in both the RhinoGen and COAST populations. Binding of cytoplasmic viral RNA induces a confirmation change in RIG-I and triggers an innate immune signaling cascade that results in the activation of IFN signaling and the production of pro-inflammatory cytokines [54]. A missense mutation, rs10813831, in DDX58 results in an Arg7Cys amino acid change in the CARD domain of the protein that is predicted with high confidence to affect protein function or structure. Indeed, studies have linked the A allele (Cys) to increased DDX58 mRNA expression in Newcastle disease virus-infected human dendritic cells [55] and with decreased type I IFN signaling activity in reporter assays [55, 56]. Thus, our observation that the A allele (Cys) was associated with an increased loss of asthma control in RhinoGen and increased asthma risk in COAST may be partly mediated by its effect on DDX58 gene expression and altered IFN signaling. In contrast, the functional effects of the synonymous mutation rs3205166 are less clear. The mutation has been shown to act as a cis-eQTL in CD14+ monocytes (both unstimulated and LPS-stimulated) [37] and in sputum samples from people with chronic obstructive pulmonary disease [57]. Aside from the potential cis-regulatory implications, the mechanistic link between this SNP or a SNP in LD with rs3205166 and the respiratory and asthma phenotypes in our study remains to be elucidated.
In summary, we identified genetic variation associated with susceptibility to and severity of viral respiratory illnesses in children. Variation in six of these genes was associated with similar or related phenotypes in independent populations, suggesting that these are excellent candidates for further mechanistic studies. We recognize that the SNPs identified in this study may not be the ‘causal’ SNPs but may merely tag the true causal variation. Because we initially prioritized SNPs for selection as tag SNPs based on putative function when possible, it is likely that the variants included in this study were enriched for functional SNPs and therefore may directly influence the phenotypes studied. This may be particularly true for the associated SNPs that result in protein-coding changes. These findings may also provide mechanistic insights into antiviral pathways that are of greatest importance in defenses against infections with RVs and other common respiratory viruses.
Supplementary Material
Acknowledgments
We thank the children and their families that participated in the RhinoGen, COAST, and COPSAC studies and the clinical coordinators who worked so diligently and tirelessly to conduct these studies.
FUNDING DECLARATION: RhinoGen was supported by NIH grant U19 AI070503 and UL1TR000427 (Clinical and Translational Science Award); the COAST study is supported by NIH grant P01 HL070831; DAL was supported by NIH grants F32 HL095268 and T32 HL007605. Genotyping services were provided through the RS&G Service at Johns Hopkins University under U.S. Federal Government contract number HHSN268201100037C from the National Heart, Lung, and Blood Institute.
ABBREVIATIONS
- RV
rhinovirus
- SNP
single nucleotide polymorphism
References
- 1.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 2.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 3.Jackson DJ. The role of rhinovirus infections in the development of early childhood asthma. Current Opinion in Allergy and Clinical Immunology. 2010;10:133–138. doi: 10.1097/ACI.0b013e3283352f7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gavala ML, Bertics PJ, Gern JE. Rhinoviruses, allergic inflammation, and asthma. Immunol Rev. 2011;242:69–90. doi: 10.1111/j.1600-065X.2011.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, DaSilva DF, Tisler CJ, Gern JE, Lemanske RF. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotaniemi-Syrjanen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy-the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilbert TW, Singh AM, Danov Z, Evans MD, Jackson DJ, Burton RM, Roberg KA, Anderson EL, Pappas TE, Gangnon R, Gern JE, Lemanske RF. Decreased lung function after preschool wheezing rhinovirus illnesses in children at risk to develop asthma. J Allergy Clin Immunol. 2011;128:532. doi: 10.1016/j.jaci.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children's Respiratory Study: 1980 to present. J Allergy Clin Immunol. 2003;111:661–675. doi: 10.1067/mai.2003.162. quiz 676. [DOI] [PubMed] [Google Scholar]
- 9.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, Otoole S, Myint SH, Tyrrell DAJ, Holgate ST. Community study of role of viral infections in exacerbations of asthma in 9–11 year-old children. Br Med J. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copenhaver CC, Gern JE, Li ZH, Shult PA, Rosenthal LA, Mikus LD, Kirk CJ, Roberg KA, Anderson EL, Tisler CJ, DaSilva DF, Hiemke HJ, Gentile K, Gangnon RE, Lemanske RF. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med. 2004;170:175–180. doi: 10.1164/rccm.200312-1647OC. [DOI] [PubMed] [Google Scholar]
- 11.Caliskan M, Bochkov YA, Kreiner-Moller E, Bonnelykke K, Stein MM, Du G, Bisgaard H, Jackson DJ, Gern JE, Lemanske RF, Jr, Nicolae DL, Ober C. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gern JE, French DA, Grindle KA, Brockman-Schneider RA, Konno S, Busse WW. Double-stranded RNA induces the synthesis of specific chemokines by bronchial epithelial cells. Am J Respir Cell Mol Biol. 2003;28:731–737. doi: 10.1165/rcmb.2002-0055OC. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, Nagarkar DR, Bowman ER, Schneider D, Gosangi B, Lei J, Zhao Y, McHenry CL, Burgens RV, Miller DJ, Sajjan U, Hershenson MB. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol. 2009;183:6989–6997. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slater L, Bartlett NW, Haas JJ, Zhu J, Message SD, Walton RP, Sykes A, Dahdaleh S, Clarke DL, Belvisi MG, Kon OM, Fujita T, Jeffery PK, Johnston SL, Edwards MR. Coordinated role of TLR3, RIG-I and MDA5 in the innate response to rhinovirus in bronchial epithelium. PLoS Pathog. 2010;6:e1001178. doi: 10.1371/journal.ppat.1001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker TM, Durrani SR, Bochkov YA, Devries MK, Rajamanickam V, Jackson DJ. Effect of exogenous interferons on rhinovirus replication and airway inflammatory responses. Ann Allergy Asthma Immunol. 2013;111:397–401. doi: 10.1016/j.anai.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kloepfer KM, Olenec JP, Lee W, Pappas TE, Liu G, Vrtis RF, Evans MD, Gangnon RE, Gern JE. Increased H1N1 Infection Rate in Asthmatic Children. J Allergy Clin Immunol. 2011;127:1275–1279. [Google Scholar]
- 17.Olenec JP, Kim WK, Lee WM, Vang F, Pappas TE, Salazar LE, Evans MD, Bork J, Roberg K, Lemanske RF, Jr, Gern JE. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125:1001–1006. e1001. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayden FG, Herrington DT, Coats TL, Kim K, Cooper EC, Villano SA, Liu S, Hudson S, Pevear DC, Collett M, McKinlay M Pleconaril Respiratory Infection Study G. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin Infect Dis. 2003;36:1523–1532. doi: 10.1086/375069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell KR, Shorr R, Cherry JD, Hendley JO. Improved method for collection of nasal mucus. J Infect Dis. 1977;136:109–111. doi: 10.1093/infdis/136.1.109. [DOI] [PubMed] [Google Scholar]
- 20.Lee WM, Grindle K, Pappas T, Marshall DJ, Moser MJ, Beaty EL, Shult PA, Prudent JR, Gern JE. High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol. 2007;45:2626–2634. doi: 10.1128/JCM.02501-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee WM, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, Jakiela B, Lemanske RF, Jr, Shult PA, Gern JE. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One. 2007;2:e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bochkov YA, Hanson KM, Keles S, Brockman-Schneider RA, Jarjour NN, Gern JE. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunology. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnelykke K, Sleiman P, Nielsen K, Kreiner-Moller E, Mercader JM, Belgrave D, den Dekker HT, Husby A, Sevelsted A, Faura-Tellez G, Mortensen LJ, Paternoster L, Flaaten R, Molgaard A, Smart DE, Thomsen PF, Rasmussen MA, Bonas-Guarch S, Holst C, Nohr EA, Yadav R, March ME, Blicher T, Lackie PM, Jaddoe VW, Simpson A, Holloway JW, Duijts L, Custovic A, Davies DE, Torrents D, Gupta R, Hollegaard MV, Hougaard DM, Hakonarson H, Bisgaard H. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014;46:51–55. doi: 10.1038/ng.2830. [DOI] [PubMed] [Google Scholar]
- 24.Bisgaard H, Vissing NH, Carson CG, Bischoff AL, Folsgaard NV, Kreiner-Moller E, Chawes BL, Stokholm J, Pedersen L, Bjarnadottir E, Thysen AH, Nilsson E, Mortensen LJ, Olsen SF, Schjorring S, Krogfelt KA, Lauritzen L, Brix S, Bonnelykke K. Deep phenotyping of the unselected COPSAC2010 birth cohort study. Clin Exp Allergy. 2013;43:1384–1394. doi: 10.1111/cea.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemanske RF. The Childhood Origins of Asthma (COAST) study. Pediatr Allergy Immunol. 2002;13:38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 26.Loisel DA, Zheng T, Tisler C, Evans MD, Gangnon R, Jackson DJ, Gern JE, Lemanske RF, Ober C. IFNG genotype and sex interact to influence risk of childhood asthma. J Allergy Clin Immunol. 2011;128:524. doi: 10.1016/j.jaci.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, Karczewski KJ, Park J, Hitz BC, Weng S, Cherry JM, Snyder M. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22:1790–1797. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nature methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Wu B, Xiong H, Zhu C, Zhang L. Polymorphisms of STAT-6, STAT-4 and IFN-gamma genes and the risk of asthma in Chinese population. Respir Med. 2007;101:1977–1981. doi: 10.1016/j.rmed.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Park BL, Cheong HS, Kim LH, Choi YH, Namgoong S, Park HS, Hong SJ, Choi BW, Lee JH, Park CS, Shin HD. Association analysis of signal transducer and activator of transcription 4 (STAT4) polymorphisms with asthma. J Hum Genet. 2005;50:133–138. doi: 10.1007/s10038-005-0232-1. [DOI] [PubMed] [Google Scholar]
- 32.Pykalainen M, Kinos R, Valkonen S, Rydman P, Kilpelainen M, Laitinen LA, Karjalainen J, Nieminen M, Hurme M, Kere J, Laitinen T, Lahesmaa R. Association analysis of common variants of STAT6, GATA3, and STAT4 to asthma and high serum IgE phenotypes. J Allergy Clin Immunol. 2005;115:80–87. doi: 10.1016/j.jaci.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 35.Gern JE, Brooks GD, Meyer P, Chang A, Shen KL, Evans MD, Tisler C, DaSilva D, Roberg KA, Mikus LD, Rosenthal LA, Kirk CJ, Shult PA, Bhattacharya A, Li ZH, Gangnon R, Lemanske RF. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol. 2006;117:72–78. doi: 10.1016/j.jaci.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 37.Fairfax BP, Humburg P, Makino S, Naranbhai V, Wong D, Lau E, Jostins L, Plant K, Andrews R, McGee C, Knight JC. Innate immune activity conditions the effect of regulatory variants upon monocyte gene expression. Science. 2014;343:1246949. doi: 10.1126/science.1246949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verhelst J, Hulpiau P, Saelens X. Mx proteins: antiviral gatekeepers that restrain the uninvited. Microbiol Mol Biol Rev. 2013;77:551–566. doi: 10.1128/MMBR.00024-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciencewicki JM, Wang X, Marzec J, Serra ME, Bell DA, Polack FP, Kleeberger SR. A genetic model of differential susceptibility to human respiratory syncytial virus (RSV) infection. FASEB J. 2014;28:1947–1956. doi: 10.1096/fj.13-239855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ober C, Vercelli D. Gene-environment interactions in human disease: nuisance or opportunity? Trends Genet. 2011;27:107–115. doi: 10.1016/j.tig.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lange NE, Litonjua A, Hawrylowicz CM, Weiss S. Vitamin D, the immune system and asthma. Expert review of clinical immunology. 2009;5:693–702. doi: 10.1586/eci.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daley D, Lemire M, Akhabir L, Chan-Yeung M, He JQ, McDonald T, Sandford A, Stefanowicz D, Tripp B, Zamar D, Bosse Y, Ferretti V, Montpetit A, Tessier MC, Becker A, Kozyrskyj AL, Beilby J, McCaskie PA, Musk B, Warrington N, James A, Laprise C, Palmer LJ, Pare PD, Hudson TJ. Analyses of associations with asthma in four asthma population samples from Canada and Australia. Hum Genet. 2009;125:445–459. doi: 10.1007/s00439-009-0643-8. [DOI] [PubMed] [Google Scholar]
- 43.Maalmi H, Sassi FH, Berraies A, Ammar J, Hamzaoui K, Hamzaoui A. Association of vitamin D receptor gene polymorphisms with susceptibility to asthma in Tunisian children: A case control study. Hum Immunol. 2013;74:234–240. doi: 10.1016/j.humimm.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Raby BA, Lazarus R, Silverman EK, Lake S, Lange C, Wjst M, Weiss ST. Association of vitamin D receptor gene polymorphisms with childhood and adult asthma. Am J Respir Crit Care Med. 2004;170:1057–1065. doi: 10.1164/rccm.200404-447OC. [DOI] [PubMed] [Google Scholar]
- 45.Poon AH, Laprise C, Lemire M, Montpetit A, Sinnett D, Schurr E, Hudson TJ. Association of vitamin D receptor genetic variants with susceptibility to asthma and atopy. Am J Respir Crit Care Med. 2004;170:967–973. doi: 10.1164/rccm.200403-412OC. [DOI] [PubMed] [Google Scholar]
- 46.Saadi A, Gao G, Li H, Wei C, Gong Y, Liu Q. Association study between vitamin D receptor gene polymorphisms and asthma in the Chinese Han population: a case-control study. BMC medical genetics. 2009;10:71. doi: 10.1186/1471-2350-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pillai DK, Iqbal SF, Benton AS, Lerner J, Wiles A, Foerster M, Ozedirne T, Holbrook HP, Payne PW, Jr, Gordish-Dressman H, Teach SJ, Freishtat RJ. Associations between genetic variants in vitamin D metabolism and asthma characteristics in young African Americans: a pilot study. J Investig Med. 2011;59:938–946. doi: 10.231/JIM.0b013e318220df41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roth DE, Jones AB, Prosser C, Robinson JL, Vohra S. Vitamin D receptor polymorphisms and the risk of acute lower respiratory tract infection in early childhood. J Infect Dis. 2008;197:676–680. doi: 10.1086/527488. [DOI] [PubMed] [Google Scholar]
- 49.Kresfelder TL, Janssen R, Bont L, Venter M. Confirmation of an Association Between Single Nucleotide Polymorphisms in the VDR Gene With Respiratory Syncytial Virus Related Disease in South African Children. J Med Virol. 2011;83:1834–1840. doi: 10.1002/jmv.22179. [DOI] [PubMed] [Google Scholar]
- 50.Li F, Jiang L, Willis-Owen SA, Zhang Y, Gao J. Vitamin D binding protein variants associate with asthma susceptibility in the Chinese Han population. BMC medical genetics. 2011;12:103. doi: 10.1186/1471-2350-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 52.Nejentsev S, Godfrey L, Snook H, Rance H, Nutland S, Walker NM, Lam AC, Guja C, Ionescu-Tirgoviste C, Undlien DE, Ronningen KS, Tuomilehto-Wolf E, Tuomilehto J, Newport MJ, Clayton DG, Todd JA. Comparative high-resolution analysis of linkage disequilibrium and tag single nucleotide polymorphisms between populations in the vitamin D receptor gene. Hum Mol Genet. 2004;13:1633–1639. doi: 10.1093/hmg/ddh169. [DOI] [PubMed] [Google Scholar]
- 53.Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, Zhang X, Wang L, Issner R, Coyne M, Ku M, Durham T, Kellis M, Bernstein BE. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473:43–49. doi: 10.1038/nature09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loo YM, Gale M. Immune Signaling by RIG-I-like Receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu JZ, Nistal-Villan E, Voho A, Ganee A, Kumar M, Ding YM, Garcia-Sastre A, Wetmur JG. A Common Polymorphism in the Caspase Recruitment Domain of RIG-I Modifies the Innate Immune Response of Human Dendritic Cells. J Immunol. 2010;185:424–432. doi: 10.4049/jimmunol.0903291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pothlichet J, Burtey A, Kubarenko AV, Caignard G, Solhonne B, Tangy F, Ben-Ali M, Quintana-Murci L, Heinzmann A, Chiche JD, Vidalain PO, Weber ANR, Chignard M, Si-Tahar M. Study of Human RIG-I Polymorphisms Identifies Two Variants with an Opposite Impact on the Antiviral Immune Response. Plos One. 2009;4 doi: 10.1371/journal.pone.0007582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiu W, Cho MH, Riley JH, Anderson WH, Singh D, Bakke P, Gulsvik A, Litonjua AA, Lomas DA, Crapo JD, Beaty TH, Celli BR, Rennard S, Tal-Singer R, Fox SM, Silverman EK, Hersh CP, Investigators E. Genetics of sputum gene expression in chronic obstructive pulmonary disease. PLoS One. 2011;6:e24395. doi: 10.1371/journal.pone.0024395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.