Abstract
The complement system has been implicated in the removal of dysfunctional synapses and neurites during development and in disease processes in the mouse, but it is unclear how far the mouse data can be transferred to humans.
Here, we co-cultured macrophages derived from human THP1 monocytes and neurons derived from human induced pluripotent stem cells, to study the role of the complement system in a human model. Components of the complement system were expressed by the human macrophages and human neuronal culture, while receptors of the complement cascade were expressed by human macrophages as shown via gene transcript analysis and flow cytometry. We mimicked pathological conditions leading to an altered glycocalyx by treatment of human neurons with sialidases. Desialylated human neurites were opsonized by the complement component C1q. Furthermore, human neurites with an intact sialic acid cap remained untouched, while desialylated human neurites were removed and ingested by human macrophages. While blockage of the complement receptor 1 (CD35) had no effect, blockage of CD11b as part of the complement receptor 3 (CR3) reversed the effect on macrophage phagocytosis of desialylated human neurites.
Data demonstrate that in the human system sialylation of the neuronal glycocalyx serves as an inhibitory flag for complement binding and CR3 mediated phagocytosis by macrophages.
Keywords: Human, sialic acid, neurons, macrophage, phagocytosis, complement
Introduction
Microglia form the resident immune cells of the central nervous system (CNS). They populate the CNS during early neuroectodermal development (Ginhoux et al. 2010; Kierdorf et al. 2013) and participate in tissue homeostasis, innate and adaptive immunity (Hanisch and Kettenmann 2007; Ransohoff and Perry 2009). In the mouse, microglia remove unwanted neuronal structures that have been marked by complement C1q during development via their complement receptor 3 (CR3; CD11b/CD18) (Schafer et al. 2012; Stevens et al. 2007). They also actively phagocytose neurons in neurodegenerative diseases, thus potentially contributing to disease progression (Brown and Neher 2014). However, little is known about the target structures opsonized by complement and the molecules preventing complement opsonization of intact cellular structures. Since the glycosylation pattern reflects the cellular phenotypic state (Varki 2011), sialylated carbohydrate structures may serve as a flag for preventing complement opsonization and microglial phagocytosis (Linnartz and Neumann 2013). Recently, we showed on cultured mouse neurons that desialylation of neurites triggered complement binding and microglial phagocytosis via complement receptors (Linnartz et al. 2012). In vitro, desialylated neurites of the mouse were opsonized by C1q and removed by microglia in a CR3-dependent manner (Linnartz et al. 2012). Thus, in the murine system C1q opsonized neuronal structures are recognized and ingested by phagocytic receptors on microglia such as the CR3. However, emerging data demonstrate that the murine system cannot be directly compared to the human system due to species specific differences, particularly for microglia (Smith and Dragunow 2014). While the complement receptor 1 (CR1) and complement receptor 2 (CR2) are alternatively spliced from one gene in the mouse, the two receptors are encoded by two different genes in humans (Jacobson and Weis 2008). The human complement C3b/C4b specific CR1 has phagocytic capacity for complement-bound immune complexes (Holers et al. 1992). Interestingly, CR3 is involved in the engulfment and CR1 mediates the adhesion of the particles (Fallman et al. 1993); although in an “activated” state such as in macrophages or by stimulants such as phorbol 12,13-dibutyrate and other synergistic surface receptors, it can also mediate phagocytosis of C3b/C4b opsonized targets (Bobak et al. 1988). Thus, both receptors seem to initiate the transmembrane signaling leading to phagocytosis (Fallman et al. 1993). We now investigated whether results that have been obtained in the murine system on CR3-mediated phagocytosis of desialylated neurites (Linnartz et al. 2012) can be transferred into the human system and can be further clarified.
Our results show that in a human neuron-macrophage co-culture system the enzymatic elimination of the sialic acid cap of neurons resulted in a C1q opsonization and phagocytosis by human macrophages. Inhibition of CR3, but not CR1, prevented the removal and uptake of desialylated neurites by the human macrophages.
Materials and Methods
Cell culture
Human monocytes (THP1 cells, kindly provided by Prof. Hornung, Bonn, Germany) were cultured in RPMI medium supplemented with 1% chicken serum (Gibco, Germany), 1% penicillin/streptomycin (Gibco, Germany), 1% L-glutamine (Gibco, Germany), 1% sodium pyruvate (Gibco, Germany) and 1% N2 supplement (Gibco, Germany). For differentiation, floating THP1 were plated and 0.5 µM Phorbol-12-Myristate-13-Acetate (PMA, Sigma) was added to the cells for three hours to allow adherence to the bottom of the plate. Cells were washed three times with pre-warmed medium and the cells were left for 18 hours at 37°C and 5% CO2. After 18 hours, cells show a macrophage-like morphology and they can be used for further experiments (further described as macrophages).
Induced pluripotent stem (iPS) cell-derived neural stem cells derived neurons (further described as neurons) were used in all the experiments. They were differentiated according to the protocol of Li and colleagues (Li et al. 2011). Primitive neural stem cells (pNSCs) were kept in neural stem cell medium containing 10 ng/ml human leukemia inhibiting factor (Millipore, Germany), 4 µM CHIR99021 (inhibitor of glycogen synthase kinase (GSK) 3 β and GSK-3α; Axon Medchem, Netherlands), and 3 µM SB431542 (inhibitor of transforming growth factor β1 and activin receptors; Axon Medchem, Netherlands) on poly-L-ornithine (PLO; Sigma, Germany) plus fibronectin (Sigma, Germany) coated dishes in an incubator with 5% CO2 at 37°C. Confluent cell dishes were split 1:10. For differentiation into human neurons, pNSCs were split onto PLO plus laminin (Sigma, Germany) coated chamber slides (100,000 cells/chamber) in neural stem cell medium. When cells attached and started to form small colonies medium was changed to differentiation medium containing 10 ng/ml brain derived neurotrophic factor (BDNF; Prospect, Israel), 10 ng/ml glial cell line-derived neurotrophic factor (GDNF; Prospect, Israel), 0.2 mM ascorbic acid (Tocris, United Kingdom) and 300 ng/ml cyclic adenosine monophosphate (Sigma, Germany) for two weeks by changing medium every second day. The differentiation status was additionally checked with a microscope (Axiovert 40 CFL; Zeiss).
Immunocytochemistry of human neurons for different markers
Differentiated human neurons were fixed and stained with antibodies directed against neurofilament (1:1000; Sigma, Germany), βIII tubulin (1:500; Sigma, Germany), microtubule-associated protein 2 (MAP2; 1:200; Millipore, Germany), vesicular glutamate transporter 1 (vGLUT1; 1:300; Synaptic Systems, Germany), gamma aminobutyric acid (GABA; 1:500; Sigma, Germany), tyrosine hydroxylase (TH; 1:1000; Sigma, Germany), choline acetyltransferase (ChAT; 1:100; Millipore, Germany), glial fibrillary acidic protein (GFAP; 1:500; DAKO, Denmark) or the ionized calcium binding adapter molecule 1 (Iba1; 1:500; Wako, Japan) followed by the corresponding Alexa488-conjugated antibodies (1:500; Invitrogen, Germany). Neurons were counterstained with an antibody directed against human neuronal nuclei (NeuN; 1:50; Millipore, Germany) or βIII tubulin (1:500) followed by the corresponding Cy3-conjugated secondary antibody (1:500; Dianova, Germany). Nuclei of cells were subsequently labeled with 4´,6-diamidino-2-phenylindole (DAPI; 1:10,000; Sigma, Germany). Images were taken by confocal laser scanning microscopy (Fluoview 1000, Olympus) or fluorescence microscope (AxioImager.Z1).
Sialidase-treatment of human neurons
Differentiated human neurons were either untreated (medium only, control cells) or treated with 12.5 U/ml exo-α-sialidase (neuraminidase, number: 3.2.1.18, New England BioLabs, Germany) plus 0.5 U/ml endo-α-sialidase (endoneuramidase N, number 3.2.1.129, Linaris, Germany) for 5 hours at 37°C to remove α2.3-, α2.6- and α2.8-linked sialic acid residues. To check successful removal of sialic acids from the cell surface, cells were fixed and stained with an antibody directed against polysialylated neural cell adhesion molecule (PSA-NCAM; 1:500; Millipore, Germany) followed by the corresponding Alexa488-conjugated secondary antibody (1:500). Neurons were counterstained with an antibody directed against neurofilament (1:1000) followed by the corresponding Cy3-conjugated secondary antibody (1:500). Nuclei of cells were subsequently labeled with DAPI (1:20,000). For quantification of PSA-NCAM staining intensity of neurites, in total 15 images per condition out of three independent experiments were collected with a confocal laser scanning microscope (Fluoview 1000, Olympus) maintaining the same settings. Images were analyzed by a blinded investigator with ImageJ software (MBF). Intensity of PSA-NCAM staining was defined as PSA-NCAM intensity minus background intensity, divided by area of neurofilament-positive cells.
Immunocytochemistry of complement C1q
Cells, either untreated or sialidase-treated, were incubated for 1 hour at 37°C in human purified C1q (Benoit and Tenner 2011). Cells were fixed and stained with an antibody directed against human C1q (1:200; Quidel, USA) followed by the corresponding Cy3-conjugated secondary antibody (1:200). Neurons were double labeled with an antibody directed against neurofilament (1:1000) followed by an Alexa488-conjugated secondary antibody (1:500). Nuclei of cells were subsequently labeled with DAPI (1:20,000). Images were taken by confocal laser scanning microscopy (Fluoview 1000, Olympus). For quantification of C1q binding to neurites, staining intensity of C1q was determined selectively on processes not on cell bodies identified by double-labeling with an antibody directed against neurofilament. In total 15 pictures per condition out of three independent experiments were collected maintaining the same settings and analyzed by ImageJ software (NIH) by a blinded investigator. The measured mean values of the staining intensities were compared.
RT-PCR analysis of complement genes
Total RNA of macrophages and neurons was isolated by adding 1 ml QIAzol (Qiagen, Germany) lysis buffer to the cells. The lysed cells were transferred to a 1.5 ml tube and incubated for 5 minutes at room temperature. 200 µl of chloroform were added and the cells were shaken for 20 seconds. After another incubation step of 3 minutes at room temperature, the cells were centrifuged at 12,000 rpm for 15 minutes at 4°C. The colorless aqueous upper phase was transferred into a new tube; isopropanol (Carl Roth GmbH and Co KG, Germany) was added 1:1 and incubated at −20°C overnight. After a centrifugation step at 12,000 rpm for 20 minutes at 4°C, the supernatant was removed and three washing steps with 300 µl 70% ethanol (Carl Roth GmbH and Co KG, Germany) and a centrifugation step at 12,000 rpm for 5 minutes at 4°C followed. The supernatant was removed and the pellet was dried for 2–3 minutes at room temperature. The RNA was resuspended in 11 µl RNase free water, reverse transcription (RT) was performed and the resulting cDNA was amplified by polymerase chain reaction (PCR). The following oligonucleotides were used for RT-PCR: C1q, forward (for) GCCCCGCAGTGGCAAGTTCA, C1q reverse (rev) ACCTTCTGTGCACGCTCCCG; C1r for ATGACCACCAGCAAGTACAC, rev TGCCATCATCCTGGCAGACA; C1s, for AAGAGCGTTTTACGGGGTTT, rev CACTTGGAACCCTTTCTCCA; C2, for TATGACTGAGGTGATCAGCA, rev GGACCCTAGCTCATTCAGTT; C3, for TCAGCATGTCGGACAAGAAAGGGA, rev TGCAGAAGGCTGGATTGTGGAGTA; C4, for CGGGTCTTTGCACTGCATCA, rev CTTCACCTCAAAGTTGGGAA and GAPDH for CTGCACCACCAACTGCTTAG, rev TTCAGCTCAGGGATGACCTT.
Flow cytometry of CD11b, CD18 and CD35
Macrophages were mechanically detached and stained with a biotinylated anti-CD11b, anti-CD18 (both 1:200; BD Bioscience, Germany) or anti-CD35 antibody (CR1; 1:50; antibodies-online, Germany) followed by PE-conjugated streptavidin or corresponding PE-conjugated secondary antibody (1:200; Dianova, Germany). Isotype-matched control antibodies (BD Bioscience, Germany) were used as negative controls. Analysis was done with a FACSCalibur flow cytometer (BD Bioscience, Germany) and analysis was performed with FlowJo Software.
Neurite length analysis after co-culture with macrophages and phagocytic uptake of neurites into macrophages
Human neurons were either untreated or treated with both sialidases (see above). Human macrophages (20,000 cells/chamber) and a blocking antibody directed against CD11b (2 µg/ml; BD Bioscience 553307, Germany), that has been shown to block human CR3 (Beller et al. 1982), or the corresponding rat IgG2b,κ isotype control (2 µg/ml; BD Bioscience, Germany), or CD35 (CR1; 2 µg/ml; J3D3; antibodies-online GmbH, Germany), that has been shown to block the human CR1 (Crehan et al. 2013), or the corresponding mouse IgG1ĸ isotype control (2 µg/ml; BD Bioscience, Germany) were added. After 48 hours cells were fixed and immunostained with an anti-Iba1 (1:250) antibody followed by Alexa488-conjugated secondary antibody (1:500). Cells were double labeled with anti-βIII tubulin (1:500) followed by Cy3-conjugated secondary antibody (1:500). For phagocytosis, human neurons were incubated with the red fluorescent membrane dye PKH26 (10 nM final concentration; Sigma, Germany) for 5 minutes at 37°C before the addition of human macrophages (20,000 cells/chamber) and blocking antibodies or the corresponding isotype controls (see above) for 18 hours. In all conditions, co-cultures were incubated in differentiation medium plus growth factors (ascorbic acid, cyclic adenosine monophosphate, BDNF and GDNF) before cells were fixed. The nuclei were labeled with DAPI (1:20,000). Images (normal or z-stack) were collected with a laser scanning confocal microscope (Fluoview 1000, Olympus) and analyzed by a blinded observer. Images were equally processed and the mean length of βIII tubulin positive neurites, the number of cell bodies or the uptake of red fluorescent-labeled material was quantified by ImageJ/NeuronJ software (NIH, MBF). Using the NeuronJ plugin the mean length of the neurites per picture can be calculated. The mean length of the control cells without sialidase treatment was always set as 100% in all experiments. At least 5 pictures out of three independent experiments per condition (15 pictures in total) were taken. By co-localizing DAPI-positive cells with either Iba1- or βIII tubulin-positive cells, the number of cell bodies of different cell types were counted and distinguished. For the quantification of the cell body density 5 images per condition of at least three independent experiments were taken and the relative cell number of neurons per image was calculated. For the uptake of red fluorescent-labeled material, all macrophages per picture were counted and the macrophages having ingested red material. The percentage of phagocytic cells per picture was calculated and the control without sialidase treatment was always set as 100%. In total, 25 pictures out of five independent experiments were taken into account.
Statistical Analysis
Data are presented as mean±SEM of at least three independent experiments. Data were analyzed by independent student´s t-test (2 groups only) or One-Way ANOVA (more than 2 groups) followed by Bonferroni using SPSS computer software.
Results
Human neuronal cultures derived from induced pluripotent stem cells
Neural stem cells (NSCs) were obtained from human induced pluripotent stem (iPS) cells that were derived from a healthy donor. Differentiation of NSCs into neurons was induced by neuronal differentiation medium in the presence of brain-derived neurotrophic factor (BDNF) and glial cell-derived neurotrophic factor (GDNF) for two weeks. After one week the NSCs formed small colonies and established first neuritic processes. The NSCs further differentiated within the next week and build distinct colonies with neurites (data not shown). To confirm the successful differentiation into neurons and to investigate the obtained neuronal subtypes the neuronal cultures were stained with different markers. The neurons were positively immunostained for the neuronal markers NeuN, neurofilament, βIII-tubulin and microtubule-associated protein 2 (MAP2) (Fig. 1A). Furthermore, the neuronal subtypes were analyzed. While many neurons expressed the excitatory glutamate transporter vGLUT1 or the inhibitory transmitter GABA, only few neurons were positive for the dopaminergic marker TH (Fig. 1B). Positive staining for the choline acetyltransferase ChAT was mainly found around the neuronal cell bodies (Fig. 1B). Since NSCs are multipotent (Li et al. 2011), the neuronal cultures were also stained for non-neuronal cell types. While the cultures were free of macrophages and microglia, dispersedly distributed clusters of astrocytes were found (Fig. 1C).
Figure 1. Differentiation and characterization of human neuronal culture.
A. The human neurons were stained for neuron-specific markers. The neurons were positive for the neuronal markers neuronal nuclei (NeuN, red), neurofilament, βIII tubulin and MAP2 (green). Scale bar: 50 µm. Representative images out of at least three independent experiments are shown.
B. The human neurons were also stained for subtypes of neurons (green) co-stained with the neuronal marker βIII tubulin (red). Many neurons were positive for vesicular glutamate transporter 1 (vGLUT1) and gamma aminobutyric acid (GABA), while fewer neurons were positive for tyrosine hydroxylase (TH). Positive choline acetyltransferase (ChAT) staining was mainly found at the cell bodies. Scale bar: 50 µm, higher magnification: 20 µm. Representative images out of at least three independent experiments are shown.
C. Differentiated human neurons were stained for the non-neuronal markers ionized calcium binding adaptor molecule 1 (Iba1; microglia/macrophages, green) and glial fibrillary acidic protein (GFAP; astrocytes, green) and co-stained with the neuronal marker βIII tubulin (red). Nuclei were stained with DAPI (blue). While the neuronal culture was free of microglia/macrophages, some clusters of GFAP-positive cells were found. Scale bar: 20 µm. Representative images out of at least three independent experiments are shown.
Thus, the differentiated human neurons are a mixed neuronal culture with few astrocytes.
Binding of complement C1q to the desialylated neuronal glycocalyx
Differentiated human neurons were treated with a combination of an exo- and an endo-α-sialidase to remove the sialic acid residues from the cell surface. The absence of sialic acids from the neuronal glycocalyx was confirmed via staining of neurons with an antibody directed against polysialic acid neural cell adhesion molecule (PSA-NCAM). In the control situation without sialidase treatment a strong polysialic acid staining was observed on the neuronal glycocalyx while the staining was strongly reduced after enzymatic removal of sialic acids from the cell surface (Fig 2A). In detail, quantification of the relative fluorescent intensity revealed a significant reduction (p<0.001) from 100 ± 3.8% in the untreated to 14.9 ± 2.2% in the sialidase-treated situation (Fig. 2B). In neural cultures of the mouse, desialylation of the neuronal glycocalyx has initiated complement C1q binding (Linnartz et al. 2012). To investigate whether in the human system the complement cascade led to an opsonization of sialidase-treated neurons as well, human neurons were treated with sialidases and then incubated with human purified C1q. Afterwards human neurons were stained with an antibody directed against human C1q. After incubation with human purified C1q several degenerated neuronal cell bodies demonstrated binding of C1q in the untreated as well as in the sialidases-treated condition. However, only desialylated neurites showed opsonization by C1q, while untreated neurites were unaffected (Fig. 2C). For the quantification of C1q binding, the relative fluorescence intensity of the C1q staining on neurites was quantified. Specifically, untreated and sialidase-treated neurites without incubation of C1q showed nearly no relative fluorescent intensities (0.33 ± 0.12% and 0.65 ± 0.3%, respectively; Fig. 2D). Untreated neurites displayed a relative C1q fluorescent intensity of 100 ± 21.86%, while the sialidase-treatment increased the C1q binding to neurites (388.84 ± 80.45%, p<0.001; Fig. 2D).
Figure 2. Binding of complement C1q to the desialylated neuronal glycocalyx.
A. Human neurons were either untreated or sialidase-treated, fixed and stained with an antibody directed against polysialic acid (PSA-NCAM; green) and co-stained with the neuron-specific marker neurofilament (red). While untreated human neurons are highly positive for PSA-NCAM, the staining intensity was highly reduced after sialidase-treatment. Scale bar: 50 µm. Representative images out of at least three independent experiments are shown.
B. Quantification of the relative fluorescent intensity revealed a significant reduction (*** p<0.001) of the PSA-NCAM staining after sialidase treatment. n=3.
C. Human neurons were incubated with human purified C1q and then immunostained with an anti-C1q antibody (red) and double-immunolabeled with anti-neurofilament antibody (green). Confocal images of untreated or sialidase-treated neurons were taken. Incubation of human neurons with human purified C1q led to a binding to degenerated cell bodies. Removal of sialic acids from the glycocalyx led to additional C1q staining of morphologically intact human neurites. Scale bar: 50 µm, higher magnification 10 µm. Representative images out of at least three independent experiments are shown.
D. Quantification of C1q binding to human neurites. Binding of human purified C1q to desialylated neurites was increased compared to untreated neurites (*** p<0.001). n=3.
Thus, removal of sialic acid from the neuronal glycocalyx led to a binding of C1q to desialylated neurites.
Components of the classical complement cascade are produced by neuronal cultures and macrophages
To analyze the source of complement components, the human induced pluripotent stem (iPS) cell-derived neuronal cultures that contained neurons and few astrocytes and macrophages were investigated via RT-PCR. Gene transcripts of the following components of the classical complement cascade were constitutively detected in the neuronal cultures: C1r, C1s, C2 and C3 (Fig. 3A). The macrophages transcribed C1q, C1s, C2, C3 and C4 at high levels while C1r was only detected at very low level (Fig. 3A). Moreover, expression levels of the CR3, which consists of the two integrin subunits, CD11b and CD18, and CR1 (CD35) were analyzed via flow cytometry. The human macrophages expressed CR3 (CD11b and CD18) and CR1 (CD35; Fig. 3B).
Figure 3. Complement expression by human neuronal cultures and macrophages.
A. RT-PCR of neuronal cultures and macrophages. Transcripts for C1r, C1s, C2 and C3 were detected in unstimulated neuronal cultures. Macrophages transcribed C1q, C1s, C2, C3 and C4 at high levels and C1r at very low level. GAPDH served as internal loading control. H2O control: PCR without cDNA. Representative images out of at least three independent experiments are shown.
B. Flow cytometry analysis of human macrophages. Human macrophages express CD11b and CD18, the two integrin subunits of the complement receptor 3, and CD35 (complement receptor 1). The isotype controls are shown in red and the markers in blue. Representative images out of at least three independent experiments are shown.
Thus, neural cells produce complement components and macrophages express complement components as well as the complement receptors CR1 and CR3.
Desialylated human neurites are removed and ingested by human macrophages
Human neurons were either untreated or treated with sialidases and then co-cultured with human macrophages. Neurons and macrophages were immunostained with antibodies directed against βIII tubulin and ionized calcium-binding adapter molecule 1 (Iba1), respectively. In co-culture with normal untreated neurons human macrophages did not affect the neurons and their neurites. However, human macrophages in co-culture with neurons displaying less sialic acid on their cell surface resulted in interrupted neurites, while the neuronal cell body density remained unaltered (Fig. 4A). In detail, the relative neurite length was not significantly changed by the sialidase-treatment itself (92.3 ± 5.4%) or by adding macrophages to the culture (88.3 ± 4.9%) compared to untreated neurons without macrophages (100 ± 7.1%). However, after sialidase-treatment the relative neurite length was reduced in co-culture with macrophages (50.9 ± 3.5%, p<0.001 vs untreated, +sialidase and +macrophages; Fig. 4B). Neither the sialidase-treatment nor the addition of macrophages altered the relative neuronal cell body density (Fig. 4C).
Figure 4. Macrophage-dependent removal of desialylated neurites.
Human neurons were untreated or sialidase-treated and then co-cultured for 48 hours with human macrophages. Cells were immunostained with antibodies directed against βIII tubulin (red) and Iba1 (green) and nuclei were stained with DAPI (blue).
A. After co-culture of neurons with macrophages, sialidase-treated neurons displayed shorter and discontinuous neurites (as indicated by arrows) compared to untreated neurons. Scale bar: 25 µm, higher magnification: 10 µm. Representative images out of at least three independent experiments are shown.
B. Normal or desialylated human neurons (+sialidase) were either left alone or co-cultured for 48 hours with human macrophages (+macrophages) and the relative neurite length was determined. Addition of human macrophages reduced the length of desialylated neurites (*** p<0.001 vs untreated, vs treated, and vs +macrophages). n=3.
C. The relative cell body number of human neurons in the different culture conditions was unchanged. n=3.
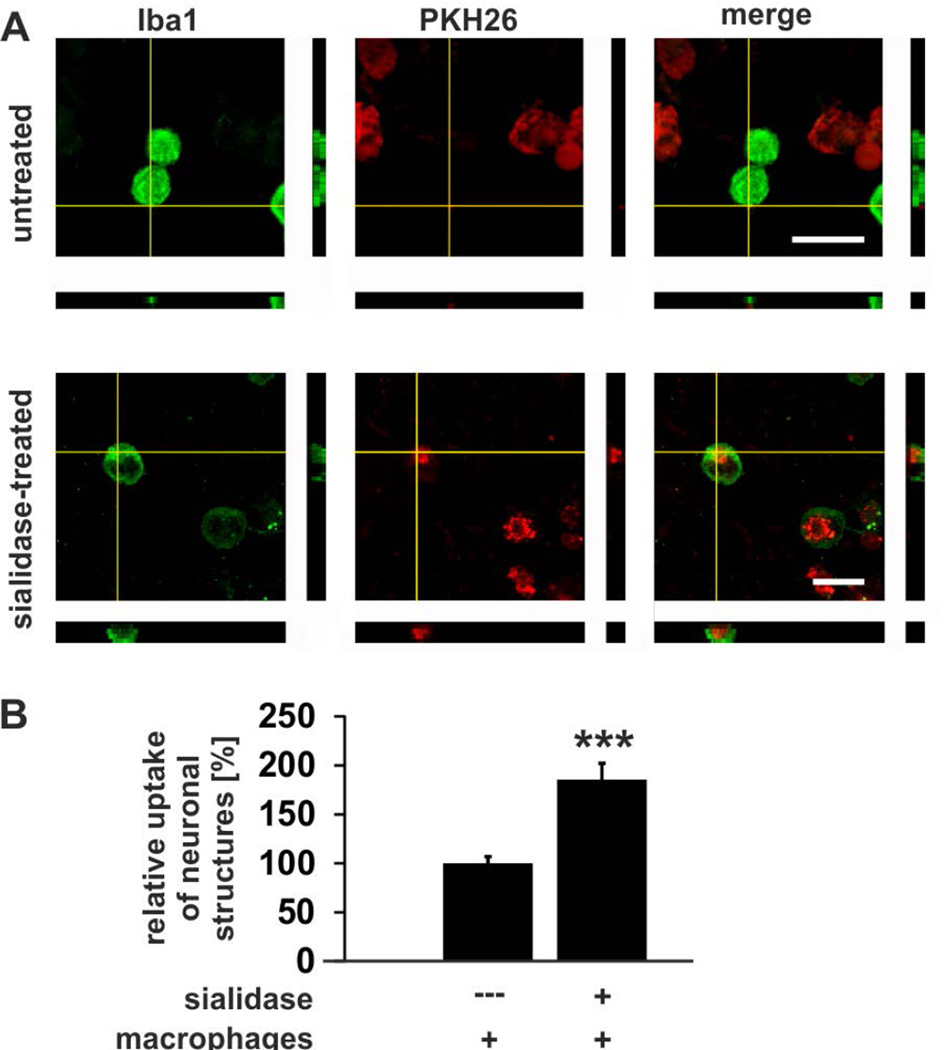
To investigate whether desialylated neuronal material was taken up by human macrophages, the neurons were stained with the membrane dye PKH26. After 18 hours of co-culture the cells were fixed and the macrophages were stained with Iba1. The uptake of neuronal membranes into the macrophages was visualized by confocal microscopy and 3D–reconstruction. Red fluorescent-marked neuronal membranes were detected in the macrophages after co-culture with desialylated neurons (Fig. 5A). In detail, the uptake of red fluorescent labeled neuronal membranes was increased (p<0.001) from 100 ± 6.9% in the untreated situation to 185.5 ± 16.5% after removal of the sialic acid cap from the neuronal glycocalyx (Fig. 5B).
Figure 5. Uptake of desialylated neurons by macrophages.
Human macrophages (green) were co-cultured for 18 hours with untreated or sialidase-treated human neurons, which were labeled with a red fluorescent membrane dye (PKH26). Uptake of human neuronal membranes by human macrophages was visualized by confocal microscopy and 3D–reconstruction.
A. After co-culture of human macrophages with desialylated human neurons fluorescent-marked neuronal membranes were detected inside the macrophages. Scale bars: 20 µm. Representative images out of at least three independent experiments are shown.
B. Quantification of uptake. Few neuronal membranes were detected in human macrophages co-cultured with untreated human neurons. Uptake of red fluorescent neuronal membranes by human macrophages was increased after sialidase treatment (*** p<0.001). n=5.
Thus, loss of sialic acid on neurites is a trigger for clearance and ingestion by human macrophages.
Involvement of complement receptor 3, but not complement receptor 1 in macrophage removal of desialylated neurites
In the mouse system, CR3 was involved in the microglial removal and uptake of desialylated neuronal material (Linnartz et al. 2012). Thus, we investigated whether this complement receptor as well plays a role in the human macrophage removal and uptake of human neuronal material displaying less sialic acid on the cell surface. In the human system, CR1 like CR3 is capable of binding C3 fragments, although CR1 had a greater affinity for C3b while CR3 bound iC3b with higher affinity (Ross et al. 1983). Thus, in the human system both receptors principally could be involved in the microglial/macrophage removal of opsonized material. Therefore, we investigated the role of both complement receptors by the addition of blocking antibodies for either CD11b (one integrin subunit of CR3) or CR1 (CD35) to the co-culture system. The relative neurite length was reduced in the co-culture of treated neurons and macrophages (50.9 ± 3.5%) compared to untreated neurons with macrophages (88.3 ± 4.9%, p<0.001). After the addition of a corresponding isotype control antibody for the CD11b blockade the relative neurite length was still reduced (58.0 ± 2.7%) in co-cultures of human macrophages and sialidase-treated neurons compared to the untreated condition (p<0.001). However, the addition of the CD11b blocking antibody reversed the effect to 87.7 ± 4.1% (p<0.001 vs treated neurons+macrophages and vs treated neurons+isotype blocked macrophages; Fig. 6A). This effect was not detected after blocking CR1 (CD35). After sialidase-treatment, the relative neurite length was reduced independently of the addition of the corresponding isotype control or the CR1 (CD35) specific blocking antibody (treated+macrophages 50.9 ± 3.5%, p<0.001; treated+macrophages+isotype antibody 56.7 ± 3.8%, p<0.001; treated+macrophages+CR1 (CD35) antibody 61.9 ± 4.4%, p<0.001) compared to the untreated neurons co-cultured with macrophages (88.3 ± 4.9%; Fig. 6A). The quantification of the neuronal cell bodies also showed no differences in-between the various blocking conditions (data not shown). The uptake of red fluorescent labeled neuronal membrane particles by human macrophages was also reversed to 128.0 ± 9.7% after the blockade of CD11b (one subunit of CR3) compared to the treated neurons (185.5 ± 16.5%, p<0.01) or the blockade with the corresponding isotype control (193.3 ± 13.6%, p<0.01; Fig. 6B). Again, this effect was not visible after blocking the CR1. After addition of the CD35 blocking antibody the uptake of red fluorescent labeled neuronal membranes (180.3 ± 13.9%) was comparable to treated neurons (185.5 ± 16.5%, p=1) or after the addition of the corresponding isotype control antibody (186.0 ± 14.1%, p=1; Fig. 6B).
Figure 6. Removal and uptake of desialylated neurons by macrophages is mediated via CD11b.
A. Normal or desialylated human neurons (+sialidase) were either left alone or co-cultured for 48 hours with human macrophages (+macrophages) blocked with the corresponding isotype control or antibodies directed against CD11b (left graph) or CD35 (right graph) and the relative neurite length was determined after the immunostaining with an antibody directed against βIII tubulin. The relative neurite length was reduced in a co-culture of treated neurons and macrophages compared to untreated neurons with macrophages (*** p<0.001). While the addition of an isotype control antibody (+isotype CD11b) showed the same effect (*** p<0.001 vs untreated+macrophages), the addition of a CD11b blocking antibody reversed the reduction of the neurite length (+CD11b; *** p<0.001 vs isotype; left graph; n=3). This antagonizing effect was not observed after the addition of an antibody against CD35 (+CD35; right graph; *** p<0.001 vs untreated+macrophages; n=3).
B. Human macrophages were co-cultured for 18 hours with untreated or sialidase-treated human neurons, which were labeled with a red fluorescent membrane dye (PKH26). Uptake of human neuronal membranes by human macrophages was quantified by confocal microscopy. The uptake of red fluorescent neuronal membranes by human macrophages was increased after sialidase treatment (*** p<0.001). After the addition of an isotype control antibody (+isotype; left graph) the uptake of human neuronal material was still increased (*** p<0.001 vs untreated+macrophages). However, the addition of a CD11b blocking antibody reversed the effect (+CD11b; ** p<0.01 vs +isotype; left graph; n=5). This effect has not been observed after the addition of a CD35 blocking antibody (+CD35; right graph; p = 1 vs treated and p = 1 vs +isotype; n=5).
In summary, desialylated neurites are mainly removed by human macrophages via CR3-mediated phagocytosis.
Discussion
In the mouse system, CR3 of microglia has been shown to contribute to neurodevelopment and neurodegenerative processes (Schafer et al. 2012; Stevens et al. 2007). However, it is still unclear how far the mouse system data can be transferred to the human system due to several differences between mouse and human microglia/macrophages (Smith and Dragunow 2014). Shay et al. found cell surface markers, regulators and 169 one-to-one orthologs with highly distinct expression patterns between human and mouse immune cell types (Shay et al. 2013). Interestingly, phagocytosis and its associated molecules are quite diverse between mouse and human; for example while Toll-like receptor 4 is expressed on mouse microglia, this receptor is difficult to detect on human microglia (reviewed in (Smith and Dragunow 2014)). Fc-gamma receptors show differences in their expression patterns and binding abilities between human and mouse systems (Bruhns 2012). Other phagocytosis-associated receptors with differences in their expression and function between mouse and human are the microglial/macrophage immunoreceptor tyrosine based inhibition motif (ITIM)-signaling Siglecs (sialic acid binding immunoglobulin like lectins) (Linnartz-Gerlach et al. 2014). These inhibitory Siglec receptors also counter-act receptors leading to a phagocytosis signal via an immunoreceptor tyrosine based activation motif (ITAM) such as the CR3 that signals via the ITAM-containing adaptor molecule DAP12/TYROBP (Linnartz and Neumann 2013). For some human SIGLECS (e.g. human SIGLEC-11 gene) no homologue can be found in the mouse (Hayakawa et al. 2005; Varki 2010). Furthermore, there are often structural differences like for the human SIGLEC-3 (CD33), which signals via an ITIM in its cytoplasmic tail, while this motif is absent in mouse Siglec-3 that contain an ITIM-like motif and a charged residue in principal capable to interact with DAP12/TYROBP (Brinkman-Van der Linden et al. 2003; Crocker et al. 2007; Tchilian et al. 1994). Furthermore, humans in contrast to mice have two different genes for encoding CR1 and CR2 (Jacobson and Weis 2008).
In the mouse system desialylated neuronal material is removed by complement and the microglial CR3 (Linnartz et al. 2012). We now used a co-culture system of THP1-derived human macrophages and iPS-derived human neurons. We found that the complement components C1r, C1s, C2 and C3 are constitutively transcribed by the human neuronal culture that also contains some astrocytes and C1q, C1r, C1s, C2, C3 and C4 by human macrophages. Moreover, the complement receptors CR1 and CR3 are expressed on human macrophages. Astrocytes have already been described before as the major cell type in the CNS that are capable of synthesizing complement components, while complement receptors CR1 and CR3 have been shown to be expressed by microglia (Barnum 1995). Moreover, astrocytes have been shown to be involved in the elimination of synapses (Chung et al. 2013). However, complement alone from astrocytes in our neuronal culture failed to reduce the neurite length after a desialylation stimulus, since the relative neurite length of sialidase-treated neuronal culture was comparable to the untreated situation. Only after the addition of macrophages to the neuronal cultures, neuronal material was ingested via CR3 by the macrophages as demonstrated by the uptake of PKH26 positive neuronal material. Thus, astrocytes can be a source of complement components, but were not involved in the complement-mediated removal of desialylated neuronal material. In addition, the removal of sialic acid residues from the glycan cell surface led to the binding of human C1q to neurites. Therefore, the classical complement cascade might be activated leading to the production and the cleavage of the complement component C3 to C3a and C3b/iC3b. CR3 and CR1 are both capable of binding to C3 fragments (Ross et al. 1983; Sutterwala et al. 1996). Genome-wide association studies identified the human-specific CR1 gene to be implicated in late onset Alzheimer´s disease (AD) (Brouwers et al. 2011; Lambertsen et al. 2009). The involvement of CR1 in AD could be explained by one of its isoforms having an increased number of C3b/C4b binding sites (Brouwers et al. 2011). Therefore, we studied the involvement of both complement receptors, CR3 and CR1, in our human macrophages-neuron co-culture system. While the reduction of the relative neurite length after addition of macrophages to desialylated neurons was reversed by blocking CD11b as one part of the CR3, blocking CR1 with a CD35 blocking antibody had no neutralizing effect. Another study demonstrated the phagocytic capacity of CR1 for complement-bound immune complexes on the surface of neutrophils (Holers et al. 1992), but this function was redundant compared to the CR3 (Jacobson and Weis 2008). However, we cannot fully rule out that the CR1 might play a role in the recognition and modulation of the complement system during phagocytosis. Furthermore, we do not know the exact mechanism that triggers microglial/macrophage phagocytosis after removal of sialic acid. In principal, loss of sialic acid could either unmask target structures (e.g. pentraxin PTX3, (Inforzato et al. 2006)) that induce complement binding or lead to loss of inhibitory complement components (e.g. complement factor H, (Ferreira et al. 2010)) that enable complement opsonization on the desialylated neurites.
However, data clearly show that an altered glycocalyx might be a flag for complement binding and phagocytic clearance. Microglia/macrophages express a whole set of diverse recognition receptors that monitor the neuronal physical condition and respond accordingly (Linnartz-Gerlach et al. 2014; Linnartz and Neumann 2013). An intact glycocalyx displays a sialic acid cap on the periphery of glycoconjugates. It is becoming clear that the sialic acid glycosylation pattern reliably reflects an intact cellular phenotypic state (Varki 2011). Interestingly, sialic acid incorporation is also a common strategy acquired by bacteria to prevent complement opsonization and complement-mediated lysis (Ferreira et al. 2010). Changes in the cellular glycosylation pattern can be a sign for a phenotypic alteration that in response alarms the immune system and initiates the removal of the altered structure by phagocytes (Rachmilewitz 2010). During pathological conditions the glycosylation composition might be changed. Under certain conditions or stimuli, cellular sialidases/neuraminidases can be mobilized to the cell surface (Cross et al. 2003; Liang et al. 2006; Lukong et al. 2001) leading to the desialylation of glycoconjugates. Neuraminidase-1 (Neu1) for instance plays a role in the regulation of phagocytosis by altering the sialylation status (Seyrantepe et al. 2009). Moreover, during ischemia a process called acidosis occurs that could lead to the hydrolysis of sialic acids (Barrier et al. 1997; Inoue and Inoue 2001; Rodriguez et al. 1996). However, desialylation itself had no influence on the viability of the neurons as the sialidase treatment per se did not change the morphology of the neurons, nor the neuronal cell body density. These results are in line with former publications on sialidase-treated cells (Linnartz et al. 2012; Meesmann et al. 2010).
In summary, viable human neuronal structures, which display less sialic acid on the cell surface, are susceptible to be taken up by phagocytes. Therefore, the glycosylation pattern of human neurons might be a hallmark of cellular well-being and a recognition signal for microglia/macrophages.
Main points.
-
-
Desialylation on neurites resulted in complement C1q opsonization and removal in a human neuron-macrophage co-culture system.
-
-
Desialylated human neurites were removed and ingested by human macrophages via complement receptor 3-mediated phagocytosis.
Acknowledgments
This project was supported by the BONFOR-program of the University Hospital Bonn, by the Deutsche Forschungsgemeinschaft (KFO177, SFB704, FOR1336) and the Hertie-foundation and the UC, Irvine contribution by NIH AG 00538. BLG and HN are members of the DFG funded Excellence Cluster ImmunoSensation (EXC 1023). We thank Ajit Varki for helpful comments. We thank Jessica Reinartz and Rita Jietou for excellent technical support of cultures and molecular biology.
Footnotes
Competing financial interests.
The authors have no conflicting financial interests.
References
- Barnum SR. Complement biosynthesis in the central nervous system. Crit Rev Oral Biol Med. 1995;6:132–146. doi: 10.1177/10454411950060020301. [DOI] [PubMed] [Google Scholar]
- Barrier L, Barrier J, Arnaud M, Piriou A, Tallineau C. Alterations in the ganglioside composition of rat cortical brain slices during experimental lactic acidosis: implications of an enzymatic process independent of the oxidative stress. Biochim Biophys Acta. 1997;1336:15–22. doi: 10.1016/s0304-4165(97)00004-4. [DOI] [PubMed] [Google Scholar]
- Beller DI, Springer TA, Schreiber RD. Anti-Mac-1 Selectively Inhibits the Mouse and Human Type 3 Complement Receptor. Journal of Experimental Medicine. 1982;156:1000. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit ME, Tenner AJ. Complement Protein C1q–Mediated Neuroprotection Is Correlated with Regulation of Neuronal Gene and MicroRNA Expression. J Neurosci. 2011;31:3459–3469. doi: 10.1523/JNEUROSCI.3932-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobak DA, Frank MM, Tenner AJ. C1q acts synergistically with phorbol dibutyrate to activate CR1-mediated phagocytosis by human mononuclear phagocytes. Eur J Immunol. 1988;18:2001–2007. doi: 10.1002/eji.1830181220. [DOI] [PubMed] [Google Scholar]
- Brinkman-Van der Linden EC, Angata T, Reynolds SA, Powell LD, Hedrick SM, Varki A. CD33/Siglec-3 binding specificity, expression pattern, and consequences of gene deletion in mice. Mol Cell Biol. 2003;23:4199–4206. doi: 10.1128/MCB.23.12.4199-4206.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers N, Cauwenberghe CV, Engelborghs S, Lambert JC, Bettens K, Bastard NL, Pasquier F, Montoya AG, Peeters K, Mattheijssens M, and others Alzheimer risk associated with a copy number variation in the complement receptor 1 increasing C3b/C4b binding sites. Mol Psychiatry. 2011;17:223–233. doi: 10.1038/mp.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15:209–216. doi: 10.1038/nrn3710. [DOI] [PubMed] [Google Scholar]
- Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–5649. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, and others Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crehan H, Hardy J, Pocock J. Blockage of CR1 prevents activation of rodent microglia. Neurobiol Dis. 2013;54:139–149. doi: 10.1016/j.nbd.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- Cross AS, Sakarya S, Rifat S, Held TK, Drysdale BE, Grange PA, Cassels FJ, Wang LX, Stamatos N, Farese A, and others Recruitment of murine neutrophils in vivo through endogenous sialidase activity. J Biol Chem. 2003;278:4112–4120. doi: 10.1074/jbc.M207591200. [DOI] [PubMed] [Google Scholar]
- Fallman M, Andersson R, Andersson T. Signaling properties of CR3 (CD11b/CD18) and CR1 (CD35) in relation to phagocytosis of complement-opsonized particles. J Immunol. 1993;151:330–338. [PubMed] [Google Scholar]
- Ferreira VP, Pangburn MK, Cortes C. Complement control protein factor H: the good, the bad, and the inadequate. Mol Immunol. 2010;47:2187–2197. doi: 10.1016/j.molimm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, and others Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Angata T, Lewis AL, Mikkelsen TS, Varki NM, Varki A. A human-specific gene in microglia. Science. 2005;309:1693. doi: 10.1126/science.1114321. [DOI] [PubMed] [Google Scholar]
- Holers VM, Kinoshita T, Molina H. The evolution of mouse and human complement C3-binding proteins: divergence of form but conservation of function. Immunol Today. 1992;13:231–236. doi: 10.1016/0167-5699(92)90160-9. [DOI] [PubMed] [Google Scholar]
- Inforzato A, Peri G, Doni A, Garlanda C, Mantovani A, Bastone A, Carpentieri A, Amoresano A, Pucci P, Roos A, and others Structure and function of the long pentraxin PTX3 glycosidic moiety: Fine-tuning of the interaction with C1q and complement activation. Biochemistry. 2006;45:11540–11551. doi: 10.1021/bi0607453. [DOI] [PubMed] [Google Scholar]
- Inoue S, Inoue Y. Developmental profile of neural cell adhesion molecule glycoforms with a varying degree of polymerization of polysialic acid chains. J Biol Chem. 2001;276:31863–31870. doi: 10.1074/jbc.M103336200. [DOI] [PubMed] [Google Scholar]
- Jacobson AC, Weis JH. Comparative functional evolution of human and mouse CR1 and CR2. J Immunol. 2008;181:2953–2959. doi: 10.4049/jimmunol.181.5.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Holscher C, and others Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, Nielsen HH, Haugaard LS, Wirenfeldt M, Nielsen M, Dagnaes-Hansen F, and others Microglia Protect Neurons against Ischemia by Synthesis of Tumor Necrosis Factor. Journal of Neuroscience. 2009;29:1319. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Sun W, Zhang Y, Wei W, Ambasudhan R, Xia P, Talantova M, Lin T, Kim J, Wang X, and others Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc Natl Acad Sci U S A. 2011;108:8299–8304. doi: 10.1073/pnas.1014041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Seyrantepe V, Landry K, Ahmad R, Ahmad A, Stamatos NM, Pshezhetsky AV. Monocyte differentiation up-regulates the expression of the lysosomal sialidase, Neu1, and triggers its targeting to the plasma membrane via major histocompatibility complex class II-positive compartments. J Biol Chem. 2006;281:27526–27538. doi: 10.1074/jbc.M605633200. [DOI] [PubMed] [Google Scholar]
- Linnartz-Gerlach B, Mathews M, Neumann H. Sensing the neuronal glycocalyx by glial sialic acid binding immunoglobulin-like lectins. Neuroscience. 2014;275C:113–124. doi: 10.1016/j.neuroscience.2014.05.061. [DOI] [PubMed] [Google Scholar]
- Linnartz B, Kopatz J, Tenner AJ, Neumann H. Sialic acid on the neuronal glycocalyx prevents complement C1 binding and complement receptor-3-mediated removal by microglia. J Neurosci. 2012;32:946–952. doi: 10.1523/JNEUROSCI.3830-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnartz B, Neumann H. Microglial activatory (immunoreceptor tyrosine-based activation motif)- and inhibitory (immunoreceptor tyrosine-based inhibition motif)-signaling receptors for recognition of the neuronal glycocalyx. Glia. 2013;61:37–46. doi: 10.1002/glia.22359. [DOI] [PubMed] [Google Scholar]
- Lukong KE, Seyrantepe V, Landry K, Trudel S, Ahmad A, Gahl WA, Lefrancois S, Morales CR, Pshezhetsky AV. Intracellular distribution of lysosomal sialidase is controlled by the internalization signal in its cytoplasmic tail. J Biol Chem. 2001;276:46172–46181. doi: 10.1074/jbc.M104547200. [DOI] [PubMed] [Google Scholar]
- Meesmann HM, Fehr EM, Kierschke S, Herrmann M, Bilyy R, Heyder P, Blank N, Krienke S, Lorenz HM, Schiller M. Decrease of sialic acid residues as an eat-me signal on the surface of apoptotic lymphocytes. J Cell Sci. 2010;123:3347–3356. doi: 10.1242/jcs.066696. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz J. Glycosylation: An intrinsic sign of “danger”. Self Nonself. 2010;1:250–254. doi: 10.4161/self.1.3.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Rodriguez PE, Maggio B, Cumar FA. Acid and enzymatic hydrolysis of the internal sialic acid residue in native and chemically modified ganglioside GM1. J Lipid Res. 1996;37:382–390. [PubMed] [Google Scholar]
- Ross GD, Newman SL, Lambris JD, Devery-Pocius JE, Cain JA, Lachmann PJ. Generation of three different fragments of bound C3 with purified factor I or serum. II. Location of binding sites in the C3 fragments for factors B and H, complement receptors, and bovine conglutinin. J Exp Med. 1983;158:334–352. doi: 10.1084/jem.158.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyrantepe V, Iannello A, Liang F, Kanshin E, Jayanth P, Samarani S, Szewczuk MR, Ahmad A, Pshezhetsky AV. Regulation of phagocytosis in macrophages by neuraminidase 1. J Biol Chem. 2009;285:206–215. doi: 10.1074/jbc.M109.055475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay T, Jojic V, Zuk O, Rothamel K, Puyraimond-Zemmour D, Feng T, Wakamatsu E, Benoist C, Koller D, Regev A, and others Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc Natl Acad Sci U S A. 2013;110:2946–2951. doi: 10.1073/pnas.1222738110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Dragunow M. The human side of microglia. Trends Neurosci. 2014;37:125–135. doi: 10.1016/j.tins.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, and others The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Sutterwala FS, Rosenthal LA, Mosser DM. Cooperation between CR1 (CD35) and CR3 (CD 11b/CD18) in the binding of complement-opsonized particles. J Leukoc Biol. 1996;59:883–890. doi: 10.1002/jlb.59.6.883. [DOI] [PubMed] [Google Scholar]
- Tchilian EZ, Beverley PC, Young BD, Watt SM. Molecular cloning of two isoforms of the murine homolog of the myeloid CD33 antigen. Blood. 1994;83:3188–3198. [PubMed] [Google Scholar]
- Varki A. Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proc Natl Acad Sci U S A 107 Suppl. 2010;2:8939–8946. doi: 10.1073/pnas.0914634107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Since there are PAMPs and DAMPs, there must be SAMPs? Glycan "self-associated molecular patterns" dampen innate immunity, but pathogens can mimic them. Glycobiology. 2011;21:1121–1124. doi: 10.1093/glycob/cwr087. [DOI] [PMC free article] [PubMed] [Google Scholar]