Abstract
The assembly of the upper jaw is a pivotal moment in the embryonic development of amniotes. The upper jaw forms from the fusion of the maxillary, medial nasal, and lateral nasal prominences, resulting in an intact upper lip/beak and nasal cavities; together called the primary palate. This process of fusion requires a balance of proper facial prominence shape and positioning to avoid craniofacial clefting, whilst still accommodating the vast phenotypic diversity of adult amniotes. As such, variation in craniofacial ontogeny is not tolerated beyond certain bounds. For clarity, we discuss primary palatogenesis of amniotes into in two categories, according to whether the nasal and oral cavities remain connected throughout ontogeny or not. The transient separation of these cavities occurs in mammals and crocodilians, while remaining connected in birds, turtles and squamates. In the latter group, the craniofacial prominences fuse around a persistent choanal groove that connects the nasal and oral cavities. Subsequently, all lineages except for turtles, develop a secondary palate that ultimately completely or partially separates oral and nasal cavities. Here, we review the shared, early developmental events and highlight the points at which development diverges in both primary and secondary palate formation.
Introduction
The jaws represent a pivotal evolutionary innovation in vertebrates (Ohno, 1970; Cohn, 2002; Kuratani, 2003; Shigetani et al., 2005; Cerny et al., 2010). Gnathostome (jawed vertebrate) jaws consist of two elements, the lower jaw (mandible) and the upper jaw (maxilla), connected by the temporomandibular joint in mammals or the quadrato-articular joint in nonavian reptiles and birds (Richman et al., 2006; Cerny et al., 2010; Anthwal et al., 2013). The evolution of the jaw allowed gnathostomes to expand into a variety of new ecological niches within their environment by attaining an active, predatory lifestyle which ushered in vast diversity not only in general body shape and size, but also in jaw structure and use. After diversification, adaptations such as teeth and beaks evolved in order to facilitate more efficient and specialized feeding mechanisms (Davit-Beal et al., 2009). The diversity in adult morphology initiates during embryonic development, where subtle, interspecific differences in molecular signaling lead to tissue restructuring in a lineage-specific manner (Abzhanov et al., 2004; Tokita et al., 2013; Abramyan et al., 2014; Bhullar et al., 2015; Lainoff et al., 2015).
Morphological diversity is particularly enhanced in the shape and structure of the amniote jaw (Manzanares and Nieto, 2003; Richman et al., 2006; Young et al., 2014; Hu et al., 2015b). Amniote craniofacial development begins with dorsoventral migration of neural crest cells, from the border of the neural plate and surface ectoderm, to populate the pharyngeal arches (Creuzet et al., 2005; Graham et al., 2005). The first pharyngeal arch is the only arch that contributes to the face. The lower jaw forms from the mandibular prominences, which derive from the first pharyngeal arch (Chai et al., 1997; Bush and Jiang, 2012). The upper jaw, on the other hand, is more complex. It assembles from the medial nasal, lateral nasal and maxillary prominences (Fig. 1). The maxillary prominence originates partially from the first pharyngeal arch and also from the post-optic mesenchyme (Lee et al., 2004). The medial and lateral nasal prominences are derived from fore and midbrain neural crest cells that migrate medial to the optic placodes (Couly et al., 1993).
Figure 1.
Digitally reconstructed optical projection tomography (OPT) scans of embryos depicting the medial nasal prominences, mnp (purple); lateral nasal prominence, lnp (red); maxillary prominence, mxp (green), and reptile frontonasal mass, fnm (purple). A) E11.5 mouse (Mus musculus); B) 10-day crocodile (Crocodilus niloticus(Peterka et al., 2010); C) stage 28 chicken (Gallus gallus - (Hamburger and Hamilton, 1951); D) stage 4 turtle (Emydura subglobosa - (Werneburg et al., 2009); E) stage 34 chameleon (Chamaeleo calyptratus(Blanc, 1974). A’) High magnification of mouse fusion zone depicting the lambdoid (λ) junction as white dashed lines at the boundary where all three prominences meet. Figure modified from (Abramyan et al., 2015).
Despite having slightly different embryonic origins, the morphogenesis of the upper and lower jaws is coordinated so that adult teeth or beaks contact each other during function. In this manuscript, we will predominantly focus on the upper jaw, which consists of two distinct regions with different embryonic origins: the primary palate, which is common to all amniotes and the secondary palate, which is present in all major amniote lineages with the exception of most turtles. The primary palate begins to form in the second half of the embryonic period and encompasses the nasal cavities and upper lip (including the four upper incisor teeth in humans). The secondary palate forms below the roof of the stomodeum; either partially or completely separating the oral and nasal cavities. Secondary palate formation begins at the end of the embryonic period and extends into the early fetal period.
Primary palate formation establishes the intact upper jaw as a single structure. The medial nasal prominences lie medial to the nasal pits, the lateral nasal prominences lie lateral to the nasal pits, and the maxillary prominences flank the oral cavity, directly inferior to the lateral nasal prominences (Fig. 1). In birds and turtles, there is a single midline structure medial to the nasal pits called the frontonasal mass (Fig. 1C,D). In mammals and crocodilians, this midline structure has a deep furrow through the middle called the midsagittal groove, which separates it into the paired structures called medial nasal prominences (Fig. 1A,A’, B) (Tamarin and Boyde, 1977). The frontonasal mass and the medial nasal prominences represent homologous structures with identical neural crest cell origins (Santagati and Rijli, 2003). Failure of the medial nasal, lateral nasal, and maxillary prominences to unite causes the most common craniofacial malformation, cleft lip with or without cleft palate (CL/P) (Dixon et al., 2011; Leslie and Marazita, 2013). CL/P has recently been shown to be a developmental risk factor shared amongst all amniotes (Young et al., 2014).
Posterior to the primary palate, and entirely inside the oral cavity, is the secondary palate. In birds and lizards, the secondary palate consists of paired, soft-tissue outgrowths from the maxillary prominences commonly referred to as palatine processes since they are retained into adulthood (Richman et al., 2006; Bush and Jiang, 2012; Jankowski, 2013; Abramyan et al., 2014). The palatine processes in these groups do not connect, instead leaving a midline cleft, resulting in a persistent connection between the nasal and oral cavities. Mammals and crocodilians (crocodiles and alligators), on the other hand, possess a complete secondary palate which develops from the midline fusion of the embryonic palatal shelves, early in development. The union of the palatal shelves functionally separates the oral and nasal cavities in these groups. The reptilian palatine processes and embryonic palatal shelves are likely evolutionarily homologous structures since secondary palates occur in most amniote lineages and form in a similar manner. Thus, we will utilize the term “palatal shelves” for these structures in all lineages in order to avid confusion with the skeletal palatine processes which we introduce later in this manuscript.
The posterior-most portion of the secondary palate in both mammals and crocodilians is comprised of a muscular structure called the soft palate in mammals (Bush and Jiang, 2012) or the palatal valve (alternatively called the gular valve or basihyal valve) (Putterill and Soley, 2006; Jankowski, 2013; Abramyan et al., 2014) in crocodilians. These structures are utilized for separating the oral or nasal cavities from the nasopharynx during processes such as breathing, swallowing or vocalization (Putterill and Soley, 2006; Lane and Kaartinen, 2014; Kummer et al., 2015).
Many recent reviews have performed an exemplary job of covering the underlying molecular signaling in primary and secondary palate development (Cox, 2004; Hilliard et al., 2005; Szabo-Rogers et al., 2010; Dixon et al., 2011; Bush and Jiang, 2012; He and Chen, 2012; Brinkley et al., 2013; Hall, 2014; Lane and Kaartinen, 2014). Therefore, this commentary will instead compare the mechanisms of facial morphogenesis during amniote ontogeny. We anticipate that closer examination of lineage-specific morphological variation during ontogeny will allow the research community to better understand how embryonic morphology is linked to adult phenotype. Furthermore, our commentary will highlight similarities and differences between the two main model amniotes in the field of developmental biology, the mouse and chicken, relative to the larger constellation of amniotes.
Developmental Mechanisms Shared Across Amniotes
Craniofacial prominence outgrowth
The prominences in the upper face (medial nasal, lateral nasal and maxillary) become demarcated by enlarging rapidly around nasal placodes, which will later develop into the nasal pits. The gradual deepening of the nasal pit relative to the facial prominences has been investigated in chicken embryos and found to be the result of differential proliferation. Proliferation drops at the base of the nasal pit while relatively higher proliferation is maintained in the surrounding facial prominences (Minkoff and Kuntz, 1977; Abramyan et al., 2015). The maxillary prominences also become enlarged during this period through differential proliferation relative to the head mesenchyme, as shown in chicken (Minkoff and Kuntz, 1978; Bailey et al., 1988; Abramyan et al., 2014) and turtle embryos (Abramyan et al., 2014). We have previously also shown this pattern of cellular proliferation in the bearded dragon lizard (Pogona vitticeps) (Abramyan et al., 2015), indicating that these processes are likely similar across all amniotes.
Soon after these three prominences have become sufficiently enlarged, they demarcate a junction point between them termed the lambdoid or lambdoidal junction (λ-junction), due to the “λ” shape found at the confluence of the prominences (Fig. 1A’) (Tamarin and Boyde, 1977; Depew and Simpson, 2006; Depew and Compagnucci, 2008; Compagnucci et al., 2011). All amniotes develop paired lambdoidal junctions at the craniolateral corners of the stomodeum at early embryonic stages (Depew and Compagnucci, 2008; Compagnucci et al., 2011).
Fusion and merging
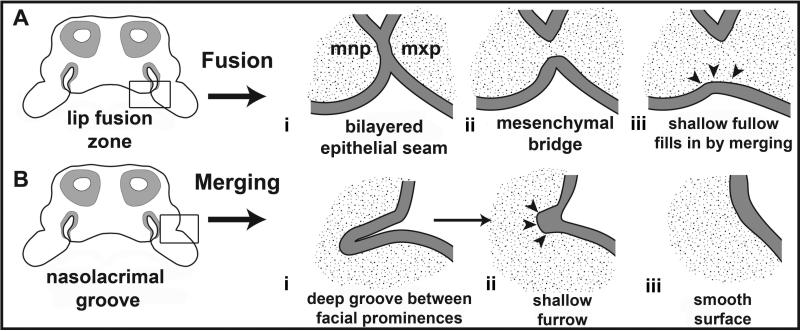
Once individual prominences develop, they begin to connect and unify through two main mechanisms, in order to form an intact face. The first mechanism is commonly utilized in the connection of freely projecting prominences and is termed “fusion” (Pruzansky, 1961; Cox, 2004) (Fig. 2A). There are several locations where fusion occurs in the face, including the tips of the maxillary, medial nasal and lateral nasal prominences during primary palate formation (shared across amniotes) (Jiang et al., 2006), as well as in the palatal shelves during secondary palate formation in mammals and crocodilians (Bush and Jiang, 2012). During fusion, a bilayered epithelial seam forms at the fusion zone and subsequently degrades through a combination of mechanisms such as apoptosis (Martinez-Alvarez et al., 2000; Cuervo and Covarrubias, 2004; Jiang et al., 2006; Nawshad, 2008), cell migration (Carette and Ferguson, 1992; Cuervo and Covarrubias, 2004; Jin and Ding, 2006), and epithelial-mesenchymal transformation (Fitchett and Hay, 1989; Griffith and Hay, 1992; Shuler et al., 1992; Shuler, 1995; Martinez-Alvarez et al., 2000; Nawshad, 2008). Once the intervening epithelium has degraded, the mesenchyme becomes continuous across the respective facial prominences, forming a “mesenchymal bridge”.
Figure 2.
Illustration depicting the two mechanisms of craniofacial prominences unification. A) Fusion is depicted in posterior frontal section of E11.5 mouse embryo, between the maxillary and the medial nasal prominences. Fusion is illustrated as a three stage process: stage i, prominences attach and a bilayered epithelial seam forms between the tissues; stage ii, the bilayered epithelial seam begins to break down through apoptosis, cell migration, or epithelialmesenchymal transformation; stage iii, the mesenchyme between the two prominences unifies into a single structure with shared mesenchyme and tissues fill in. B) Merging is depicted in the same embryo as occurring in the nasolacrimal groove, which demarcates the boundary between the lateral nasal and maxillary prominences. During merging, the superficial groove fills in and becomes a smooth surface on the embryo. Figure modified from our previous work (Abramyan et al., 2015).
The second mechanism of prominence connection is termed “merging” and utilizes cellular proliferation and migration to unify structures which are either recently fused, or have a deep groove between them with a shared mesenchyme (Pruzansky, 1961; Cox, 2004; Jiang et al., 2006; Szabo-Rogers et al., 2010) (Fig. 2B). The process of merging is used to form the smooth external surfaces of the embryo. Merging is observed in the nasolacrimal groove between the maxillary and lateral nasal prominences (Fig. 1A,A’ – red arrowhead) and the midline of the mandibular prominences. In the human embryo, the furrows or grooves in these two regions are present at 44 days post fertilization but are mostly filled out by 47 days (Hinrichsen, 1985). Similarly, merging in the aforementioned regions occurs in the mouse embryo between embryonic day 11.5 (E11.5) and embryonic day 12 (E12) (https://www.facebase.org/mouseanatomy). In the chicken embryo, the midline of the mandibular prominence is grooved at stage 24 and almost entirely merged by stage 28-29 (1.5-2 days later). In general, fusion is a more complex process than merging, due to the added requirement for the facial prominences to grow out enough to meet each other, in addition to being in the correct relative position. It is important to note that fusion and merging are not mutually exclusive processes and that fusion is usually followed by a period of merging where the tissues is filled in to make a smooth surface (Fig. 2A)
Cellular Migration
The formation of the continuous mesenchymal bridge not only serves to permanently connect freely projecting tissues, but also facilitates the passage of cells as documented using dye (McGonnell et al., 1998) or tritiated thymidine (Patterson et al., 1984) labeling. Labeled cells from the anterior tip of the maxillary prominence were identified in the frontonasal mass after fusion (Patterson et al., 1984; McGonnell et al., 1998). Thus, failure of prominence fusion not only results in an overt cleft in the intact structure, but may also reduce overall cell numbers in the contributing facial prominences; especially in the primary palate where extensive cell mixing occurs McGonnell et al., 1998.
Curiously, there are several places in the developing face where cell migration unexpectedly fails to take place. The first location is the boundary between the lateral nasal and maxillary prominences. The nasolacrimal groove (Fig. 1A – red arrowhead; Fig. 2B) serves as a barrier for cell migration, despite shared mesenchymal tissue between the structures. There is little to no mesenchymal cell migration or mixing across this landmark (Patterson et al., 1984; McGonnell et al., 1998). The nasolacrimal groove demarcates the future nasolacrimal duct and the presence of cells fated to become the duct may act as a physical barrier to block cell migration. One study does report an epithelial barrier between the lateral nasal and maxillary prominences in the human embryo (Rudé et al., 1994). Another location where little mesenchymal migration takes place is in the midline of the recently fused mammalian secondary palate. Mouse organ culture experiments demonstrating fusion between cultured palatal shelves revealed no mesenchymal cell migration between the two tissues, despite epithelial cells migrating across the fusion zone (Cuervo and Covarrubias, 2004; Jin and Ding, 2006).
Incidentally, cell mixing and cellular migration are not necessarily linked to the developmental processes of fusion or merging. There are regions in the embryonic face that do not undergo fusion or merging and still have directional cell movement. For example, unidirectional cell migration proceeds from the frontonasal mass into the lateral nasal prominence, but not vice versa (McGonnell et al., 1998). This process is hypothesized to give rise to a more prominent lateral nasal prominence, as well as deepen the nasal pits.
Divergent mechanisms used during primary palate development
Initiation of facial prominence fusion varies across amniotes
While the process of primary palate morphogenesis is superficially similar amongst all amniotes, the details of fusion initiation, as well as the relative sizes, shapes, and positions of the facial prominences, vary. Indeed there has been a general lack of consensus on which prominences initiate fusion in amniotes. A series of studies in chicken (Sun et al., 2000; Ashique et al., 2002; Cox, 2004) and human (Pruzansky, 1961; Diewert and Wang, 1992; Rudé et al., 1994; Sperber, 2002; Kim et al., 2004) concluded that the maxillary and medial nasal prominences are the first to contact each other. On the other hand, most studies carried out on mouse embryos found that the medial nasal and lateral nasal prominences are the first to fuse at E10.5-E11.0 (Trasler, 1968; Gaare and Langman, 1980; Wang et al., 1995; Gong and Guo, 2003).
To address the question of which facial prominences are utilized during primary palate formation, we recently carried out a comparative 3D study on mouse, crocodile, chicken, turtle and lizard. We paid especially close attention to the specific prominences which fuse first and initiate primary palate formation using the nasolacrimal groove as a landmark (Abramyan et al., 2015). Our analyses showed that the medial nasal prominence/frontonasal mass is always involved in primary palate fusion. However, contact may be made with both the maxillary and lateral nasal prominences as in mouse, only with the lateral nasal prominence as in turtle (Emydura subglobosa) or only with the maxillary prominence as in chicken (Abramyan et al., 2015).
There is even variation within the mouse literature as to which facial prominences initiate primary palate fusion. Most studies report that the lateral nasal and medial nasal prominences contact each other first in the mouse embryo (Trasler, 1968; Gaare and Langman, 1980; Wang et al., 1995; Gong and Guo, 2003). However, others have found that the medial nasal prominence joins with both the lateral nasal and the maxillary prominences at the nasolacrimal groove (Song et al., 2009). Thus, even within the arguably best-studied model organism in development, there is disagreement over which prominences are involved in primary palatogenesis (Jiang et al., 2006).
The confusion over which prominences meet first may have resulted from the previously unacknowledged anteroposterior difference in the prominences that are involved in murine primary palate formation. Specifically, anterior histological sections in the frontal plane show that fusion takes place between the medial nasal and lateral nasal prominences (illustrated in Fig. 3A), also demonstrated in the following studies: (Gaare and Langman, 1980 - figures 3,4); (Song et al., 2009 - figure 1I); (Kosaka et al., 1985 - figures 6,7); (Thomason et al., 2008 - figure 2G); (Iamaroon et al., 1996 - figures 2,6). However, more posterior sections of the same animals distinctly show that the medial nasal prominence joins with the maxillary prominence and not the lateral nasal prominence (Fig. 3B), (Gaare and Langman, 1980 - figure 5); (Weingaertner et al., 2006 - figure 4A in rat); (Song et al., 2009 - figure 1 K,M); (Thomason et al., 2008 - figure 2I); (Iamaroon et al., 1996 - figure 4,7). This anteroposterior difference in the fusion of prominences has likely added to the discrepancies within the current scientific literature since most studies of mouse embryo will readily observe the externally visible lateral nasal and medial nasal fusion.
Figure 3.
Illustrations of histological sections of E11.5 mouse embryo in the frontal plane. A) Anterior-most section illustrates fusion between the medial nasal and lateral nasal prominences. B) Posterior sections, on the other hand, show fusion as occurring between the medial nasal and maxillary prominences. The exact position of the prominences is demarcated by the nasolacrimal groove (black arrowhead). The site of bucconasal membrane formation at the site of fusion is also between the maxillary and medial nasal prominences (red arrowhead). Epithelial tissue in the nasal cavity and brain are depicted in grey. br, brain; lnp, lateral nasal prominence; mnp, medial nasal prominences; mxp, maxillary prominence; md, mandible; nc, nasal cavity; nf, nasal fin.
In human embryos, the involvement of the three prominences in the formation of the primary palate is reversed compared to the mouse. The medial nasal prominence is fused with the maxillary prominence anteriorly and the lateral nasal prominence posteriorly, as seen with histological sections (Pruzansky, 1961; Tamarin and Boyde, 1977; Diewert and Wang, 1992; Rudé et al., 1994; Kim et al., 2004) and in scanning electron micrographs (Sperber, 2002; Jiang et al., 2006).
In the chicken, primary palate fusion is initiated only between the medial nasal prominence and the maxillary prominence (Sun et al., 2000; Ashique et al., 2002; Cox, 2004; Abramyan et al., 2015), similar to the human embryo. However, the phenotypic similarity between human and chicken is likely an example of convergent evolution (where two distantly related lineages independently evolve similar features) (Darwin, 1859; Losos, 2011). This is supported by the fact that both prominence outgrowth and subsequent fusion differ significantly in these two, distantly related lineages. In contrast to the chicken, in the red-bellied short-necked turtle (Emydura subglobosa), primary palate formation initiates between the frontonasal mass and lateral nasal prominences and not the maxillary prominence (Abramyan et al., 2015). We also observed the same mechanism in three, distantly related species of lizards (Abramyan et al., 2015). Interestingly, in the red-eared slider turtle (Trachemys scripta) and the painted turtle (Chrysemys picta), fusion is described between the frontonasal mass and maxillary prominence, similar to the chicken (Cordero and Janzen, 2014; Lainoff et al., 2015). This may be linked to the caudally extended frontonasal mass, which approximates the maxillary prominence just prior to fusion (Lainoff et al., 2015 - figure 1B,C).
In addition to differences in which prominences initiate fusion, we also observed two main modes of primary palate assembly which we will categorize here as: Fusion Mode 1, where the lateral and medial components of the primary palate fuse together as they grow out, completely (but temporarily) closing off the nasal cavity from the stomodeum and Fusion Mode 2, where the prominences fuse around a patent choanal groove.
Fusion Mode 1
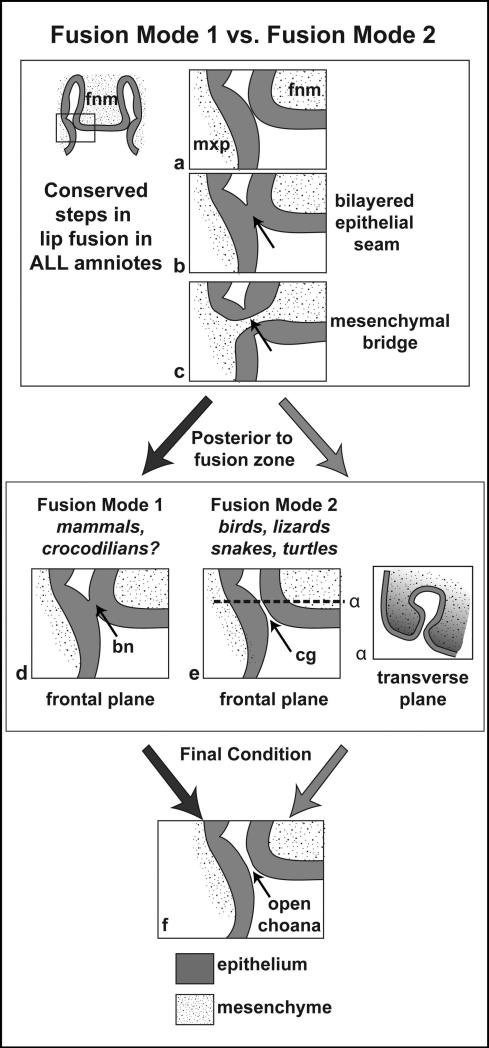
Primary palate Fusion Mode 1 has convergently evolved in mammals and crocodilians. In mode 1, fusion between the facial prominences occurs at the lambdoidal junction and is in line with the nasolacrimal groove (Abramyan et al., 2015) (Fig. 1A’, 3). As the medial nasal, lateral nasal and maxillary prominences enlarge, they begin fusing with each other though a “posterior-anterior zipping-up” process (Kosaka et al., 1985) (Movie S1), cutting the deepening nasal pit off from the stomodeum (Depew and Compagnucci, 2008; Compagnucci et al., 2011; Griffin et al., 2013) (Fig. 3). The mouse is an example of an animal that uses Fusion Mode 1.
The second feature that defines Mode 1 is the transient epithelial seam, called the nasal fin, which forms between the lateral nasal-medial nasal and maxillary-medial nasal fusion zones, completely separating the oral and nasal cavities (Fig. 3A, 4b; Movie S1) (Gaare and Langman, 1980; Jirásek, 1983; Diewert and Wang, 1992; Rudé et al., 1994). The murine nasal fin, for example, degrades as ontogeny progresses, first between the medial nasal and the maxillary prominences, inferior to the nasolacrimal groove (Gaare and Langman, 1980 - figure 5); (Weingaertner et al., 2006 - figure 4a in rat); (Song et al., 2009 - figure 1 K,M); (Thomason et al., 2008 - figure 2I); (Iamaroon et al., 1996 - figures 4,7) and later between the medial nasal and lateral nasal prominences (Gaare and Langman, 1980 - figures 3,4); (Weingaertner et al., 2006 in rat); (Song et al., 2009 - figure 1I); (Kosaka et al., 1985 - figures 6,7); (Thomason et al., 2008 - figure 2G); (Iamaroon et al., 1996 - figures 2,6).
Figure 4.
Schematic summarizing the two modes of primary palate fusion. Steps a - c depict processes in primary palate fusion that are conserved in all amniotes, although the actual prominences which initiate the fusion varies according to taxon. Steps d and e depict Fusion Modes 1 and 2 respectively. In step d, just posterior to the mesenchymal bridge unifying the primary palate, the epithelial seam (nasal fin) persists, forming a transient bucconasal membrane. In e, there is no epithelial seam posterior to the primary palate; instead entering the choanal groove. In the transverse plane (α), the decline in proliferation at the base of the choana (lighter grey) allows for the groove to remain as the prominences grow around it. The final state (f) in all amniotes is in an open choana connecting the oral and nasal cavities. bn, bucconasal membrane; cg, choanal groove. Figure modified from (Abramyan et al., 2015).
The posterior-most portion of the nasal fin (between the medial nasal and maxillary prominences in the mouse) is eventually thought to thin and form the bucconasal (or oronasal) membrane (Fig. 4b; Movie S1). In mammals, the bucconasal membranes demarcate the locations of the paired early ‘primitive’ choanae (internal nares) (Gaare and Langman, 1980; Diewert and Wang, 1992; Kim et al., 2004; Oisi et al., 2013; Som and Naidich, 2013). After lip fusion, the degradation of the nasal fin and bucconasal membrane is necessary for reestablishing contact between the neonatal oral and nasal cavities through an open choana (Fig. 4f). This vital connection will allow for nasal breathing in neonate animals. If the bucconasal membrane fails to break, the choana will be obstructed, resulting in a potentially life-threatening condition called choanal atresia (Kim et al., 2004). Choanal opening also coincides with completion of lip fusion between the distal tips of the medial nasal and lateral nasal prominences.
Despite the aforementioned differences in primary palate fusion between humans and mice, humans undergo the same epithelial seam changes as in rodents. The sequence of nasal fin formation, transient separation of the oral and nasal cavities, and subsequent rupture of the bucconasal membrane, also places humans into Fusion Mode 1.
Recently, using a combination of 3D imaging and histology, we showed that, rather surprisingly, crocodilians also utilize Fusion Mode 1 (Abramyan et al., 2015). In contrast, the majority of reptiles use Mode 2. Further analyses, at more stages of development, are required in order to delineate the exact order of prominence fusion and choanal opening mechanism in crocodilians. It is interesting that crocodilians, of all reptiles, resemble mammals in terms of primary palate ontogeny. Crocodilians also share another craniofacial feature with mammals: the presence of a complete, secondary hard palate (Ferguson, 1981). The correlation between primary and secondary palate morphogenesis in mammals and crocodilians is intriguing and may suggest a previously unrecognized developmental connection between the two structures.
Fusion Mode 2
Fusion Mode 2 is utilized by birds and the majority of nonavian reptiles (with the exception of crocodilians), and is characterized by a persistent connection, via open choanae, between the oral and nasal cavities throughout ontogeny (Fig. 3e; Movie S2). This is accomplished through the formation of a choanal groove (Bertmar, 1969; Abramyan et al., 2015). The choanal groove develops at the lambdoidal junction separating the frontonasal mass from the lateral components of the primary palate (the lateral nasal and maxillary prominences), and lies inferior to the nasal cavity. Relative proliferation differences allow for the craniofacial prominences to grow out and around the choanal groove without filling it in (Minkoff and Kuntz, 1977; Minkoff and Kuntz, 1978; Abramyan et al., 2015).
Taken together, the identification of Mode 1 fusion in the two distinct branches of amniotes (mammals and reptiles) demonstrates that the fusion mode that is utilized is likely dictated by morphological features which the embryonic face has to accommodate in space and/or time, rather than being a lineage-specific, selectively advantageous trait. The driver of this diversity may either be morphological limitations (such as the case of the primary palate and the threat of clefting), or simply, shifts in morphospace to accommodate other developmental processes which may take precedent (such as the precocious eye development observed in embryonic reptiles or the development of a secondary palate). The surprising degree of variation in lip closure within the Mode 2 animals may make some animals more resistant to clefting, while still allowing for evolutionary plasticity. For example, in our previous study, we proposed that the involvement of only two of the three prominences in primary palate fusion may allow organisms like the chicken or Emydura to tolerate more morphological variation in the shape and size of the free prominence (the lateral nasal prominences or the maxillary prominences respectively), without increasing the risk of craniofacial clefting (Abramyan et al., 2015).
Recently, Young et al., (2014) applied geometric morphometrics to 3D scans of amniote faces to measure shape changes during craniofacial ontogeny. In this study, mammals, birds, snakes, lizards and alligators were utilized to assess geometric morphospace. While each species has a unique growth trajectory, overall diversity in morphospace narrowed between lineages during primary palate fusion. Once the primary palate is fused, the morphospace is expanded, allowing for the variety of adult shapes and sizes to reach full potential (Young et al., 2014). In other words, there is selective pressure to limit size and shape variation at this critical time in order to ensure proper fusion of the primary palate in all amniotes. As demonstrated in chicken embryos, a relatively minor spatiotemporal deviation in the prominences results in cleft lip (Ashique et al., 2002; Szabo-Rogers et al., 2008; Higashihori et al., 2010; Young et al., 2014; Hu et al., 2015a), which can be detrimental, if not fatal, for the organism. The one caveat of the analyses performed by Young et al., (2014) was that the lateral nasal and maxillary prominences were considered together as a single region. Thus the bottleneck in morphospace occurring during lip fusion may not be as narrow as previously thought if individual facial prominences are taken into account. Finer delineation of prominences could be beneficial in future studies.
Divergence in Form and Function in the Secondary Palate
Palatal shelf outgrowth
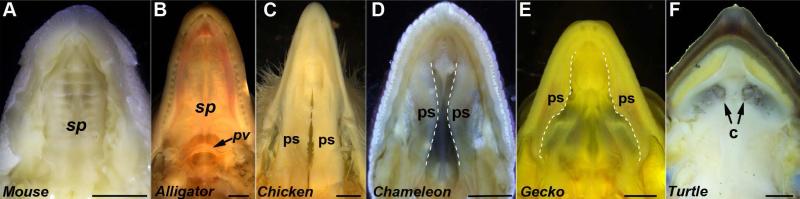
The secondary palate, which occurs in only select lineages, is a uniquely amniote structure which partakes in a variety of functions ranging from feeding to vocalization. Basal tetrapods such as amphibians do not possess any type of secondary palate architecture, instead having their choanae open directly into the oral cavity (Jankowski, 2013). Amniotes, on the other hand, develop paired outgrowths called palatal shelves, from the maxillary prominences towards the midlines of the oral cavity, during embryonic development (Tamarin, 1982; Jiang et al., 2006; Bush and Jiang, 2012; Abramyan et al., 2014). In crocodilians and mammals, the shelves grow towards the midline and fuse to form a continuous secondary palate (Fig. 5A,B). While they ultimately form similar structures, the palatal shelves develop differently in the aforementioned groups. The mammalian palatal shelves begin development in a vertical plane; eventually shifting into a horizontal position before connecting with one another (Ferguson, 1987; Bush and Jiang, 2012). In crocodilians however, the palatal shelves have a more reptilian pattern of development in forming horizontally towards each other from the outset and connecting in the midline (Ferguson, 1981; Ferguson, 1987). In birds and lizards, the palatal shelves grow towards each other horizontally but never fuse, leaving a natural cleft in the midline (Fig. 5C,D,E) (Richman et al., 2006; Kimmel et al., 2009; Jankowski, 2013; Abramyan et al., 2014).
Figure 5.
External views of amniote palates at late fetal stages. The secondary palate in the mouse and alligator is completely closed (A,B). In the chicken, there is a cleft between the palatal shelves, but the shelves still cover most of the palate (C). The chameleon also exhibits relatively substantial palatal shelves (D). In the leopard gecko, the palatal shelves are highly reduced (demarcated by white, dashed lines) (E). This gives access to the vomeronasal organ, located in the roof of the mouth. In the turtle, there are no palatal shelves, and the choanae (internal nares) open directly into the oral cavity (F). c, choana; ps, palatal shelf; pv, palatal valve; sp, secondary palate. Scale bars: 2mm (A,B,C,E,F); 1mm (D)
The process of outgrowth, or budding, of the embryonic palatal shelves from the medial surfaces of the maxillary prominences is similar to that originally described in the limb bud. The budding structure maintains the original, high proliferation index characteristic of younger stage embryos whereas proliferation in the basal mesenchyme drops (Saunders, 1948). Previous studies by our group on chicken (Abramyan et al., 2014), and others on mouse (Iwabe et al., 2005), have shown that palatal shelf outgrowth occurs through this classic mechanism. Thus, this mechanism is conserved in all species in which palatal shelves develop, and furthermore, is likely utilized in animals to form tissue outgrowths in general.
Turtles are a unique group amongst amniotes in that they do not develop embryonic palatal shelves, allowing direct opening of the choanae into the oral cavity (Fig. 5F). In our study of the red-bellied short-necked turtle (Emydura subglobosa) (Abramyan et al., 2014), we identified a complete lack of medial outgrowths from the maxillary prominence during embryogenesis, a trait also observed in the red-eared slider turtle (Trachemys scripta) (Tulenko and Sheil, 2007). Since embryonic palatal shelves in amniotes are likely synapomorphic structures (inherited structures which are shared across related taxa and inferred to have arisen in their common ancestor), we hypothesized that shelves were secondarily lost in the ancestral lineage leading to all extant turtles. We draw this conclusion from the fact that the two aforementioned species are each representative of the two distinct suborders of extant turtles, Pleurodira and Cryptodira (Crawford et al., 2015).
Through comparison of cellular and molecular mechanisms underlying chicken palatal shelf outgrowth with that of E. subglobosa, we identified an absence of differential proliferation in the medial maxillary prominence compared to the head mesenchyme in turtle embryos (Abramyan et al., 2014). The loss of the proliferation gradient likely results in failure of palatal shelf budding in the turtle; a phenotype previously described in chicken embryos in which maxillary proliferation was reduced through molecular manipulation (Ashique et al., 2002). Some lineages of turtles do develop a secondary palate, although there is a lack of embryological evidence as to the exact developmental processes involved in the assembly of this structure.
Skeletal patterning in the secondary palate
The amniote palate is comprised of several intramembranous bones. The bones that are conserved in all amniotes, regardless of palate morphology, include the premaxillae (derived from the medial nasal prominences), the palatine processes of the maxillary bones and the palatine bones (derived from the maxillary prominences) (Richman et al., 2006). However there are major differences in the posterior bones between reptiles and mammals. These differences are especially obvious when we compare the lineages that have independently evolved a bony, secondary palate.
The pterygoid bone in particular plays a much larger role in the reptilian palate than it does in mammals (Richman et al., 2006). Mammalian pterygoids are reduced and allow for lateral movement of the jaw during mastication (Crompton, 1995). This modification in turn allowed for the upper and lower jaws to function as scissors, bringing with it morphological diversification in teeth (Crompton and Parker, 1978). In mammals, the pterygoid bones are reduced to the pterygoid plates of the sphenoid bone and articulate with the vertical plate of the palatine bones anteriorly and the basisphenoid posteriorly. The pterygoid-basisphenoid articulation is at the base of the mammalian skull and is in a superior/cranial plane relative to the secondary palate. This difference in plane of articulation is particularly magnified in the human where the head is positioned at a right angle to the spine (due to our bipedal gate), as opposed to being comparatively parallel in other tetrapods (Russo and Kirk, 2013). Therefore, the pterygoid plates do not take part in the structure of the secondary palate in mammals. It is important to note that there has historically been some controversy whether the reptilian pterygoid bone, which is much more structurally substantial, is homologous to the mammalian pterygoid. However, comparative studies of adult anatomy as well as embryology have concluded that they are indeed homologous structures (Presley and Steel, 1978).
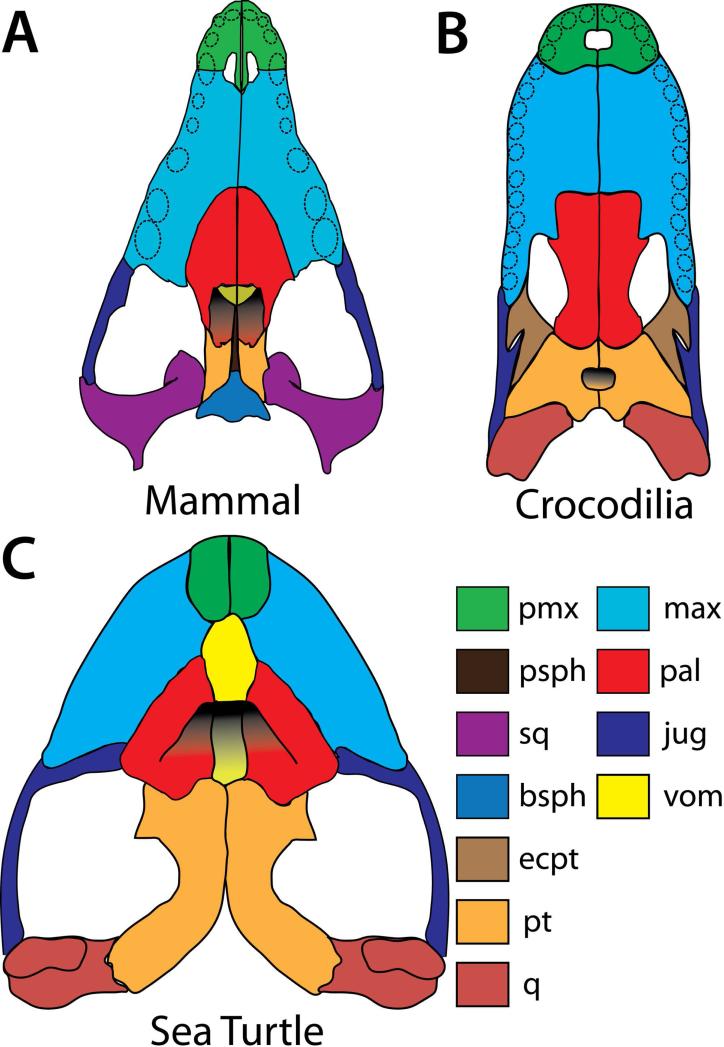
Since all amniotes exhibit a generally similar pattern of ossification in the palate, one might predict that convergent evolution of a secondary palate would correlate with similar underlying skeletal morphology. Lineages with open secondary palates, such as birds and lizards exhibit a generally similar skeletal morphology, however those with closed secondary palates differ. The two lineages of reptiles that have evolved secondary, bony palates (crocodilians and sea turtles) exhibit some ossification patterns which are unique to each group when compared to each other and to mammals. In the crocodilian secondary palate, there is substantial structural input from the pterygoid bones posteriorly and the choanal opening is within the pterygoids, instead of being anterior to the pterygoids as in mammals (Fig. 6B). This pattern allows the choana to open further back in the snout than in mammals. There is also an extra bone in the crocodilian palate called the ectopterygoid, which lies between the maxillary, jugal and pterygoid (Fig. 6B).
Figure 6.
Illustrations depicting palatal views of the three lineages with secondary, bony palates which have independently evolved in amniotes. A) The canine skull represents the mammalian lineage. The mammalian secondary palate involves only three bones, the premaxilla, maxilla and the palatine. B) The crocodilian phenotype is represented by the American alligator (Alligator mississippiensis). In the crocodilian secondary palate, the secondary palate involves substantial input from the pterygoids, which also envelop the choanae entirely. C) The sea turtle is represented by the olive ridley turtle (Lepidochelys olivacea) (illustrated from (Wyneken and Witherington, 2001). In the sea turtle, the secondary palate has substantial input from the vomer, which is not observed in either mammals or crocodilians. pmx, premaxilla; psph, presphenoid; sq, squamosal; bsph, basisphenoid; ecpt, ectopterygoid; pt, pterygoid; q, quadrate; max, maxilla; pal, palatine; j, jugal; vom, vomer.
There are several extant (as well as extinct) species of turtles that do possess a secondary bony palate (Fig. 6C). Examples of this palate phenotype can be seen in the superfamily Chelonioidea (Sea Turtles) (Hirayama, 1994; Meylan et al., 2000; Wyneken and Witherington, 2001). The adaptation of palatal ossifications to form a secondary palate is a derived trait in turtles and has likely occurred at least six times (Hirayama, 1994; Meylan et al., 2000). The difference in secondary palate ossification of sea turtles compared to mammals or crocodilians is the inclusion of the vomer in the midline of the structure (Fig. 6C) (Gaffney, 1979; Meylan et al., 2000; Wyneken and Witherington, 2001). In some lineages such as the Hawksbill turtle (Eretmochelys imbricata) and the extinct species Sandownia harrisi, there is also input from the jugal bones, which articulate with the palatine and maxillary bones posteriorly (Meylan et al., 2000; Wyneken and Witherington, 2001). The pterygoids, while extensive in the turtle, do not take part in secondary palate. Instead, they articulate with the palatine bones in a plane superior to the secondary palate and in line with the skull base. The putative loss of palatal shelves early in turtle evolution, combined with the unique pattern of secondary palate ossification, gives credibility to the aforementioned hypotheses of secondary palates re-evolving in sea turtles, likely through an as-yet unknown mechanism of development.
Evolution of biomechanics and function in the open and closed secondary palate
Amniotes exhibit great morphological diversity in secondary palates which often correlate with life-history traits and function (Fig. 5). In mammals, the closed secondary palate is required for suckling (Maier et al., 1996), mastication (Prinz and Lucas, 1997), buttressing the skull (in order to undertake greater masticatory loads) (Menegaz et al., 2009), breathing and vocalization (Kummer et al., 2015). The crocodilian palate serves similar functions, with the exception of suckling and mastication. Crocodilians are semi-aquatic reptiles and use their palates to exclude water from the nasal cavity when submerged (since the oral cavity fills with water during submersion). The secondary palate, along with the palatal valve and modifications of the external nares, enable the oral cavity to be closed off from the nasopharynx. Crocodilians can then breathe while partially submerged with their external nares extending above the water level (a method of stalking prey commonly used by this group) (Ferguson, 1981; Putterill and Soley, 2006; Jankowski, 2013). The ability to physically separate the nasal cavity from the pharynx is also utilized when crocodilians submerge with their jaws open, as when dragging prey underwater (Jankowski, 2013). Studies also suggest that the crocodilian secondary palate may also partake in strengthening the rostrum of these powerful predators, in a similar manner to the mammalian palate (Jankowski, 2013).
Birds also develop a substantial secondary palate which almost entirely covers the roof of the oral cavity, however, their palatal shelves never fuse, facilitating permanent contact between the oral and nasal cavities (Fig. 5C). The avian secondary palate houses the majority of taste receptors, as opposed to the localization of taste buds in the mammalian tongue. The avian tongue is keratinized rather than covered with mucosa and primarily serves as an organ for food intake (Berkhoudt, 1992; Mason and Clark, 2000; Crole and Soley, 2009; Scanes, 2014). The lack of teeth in birds has also makes it compulsory for them to swallow food without substantial processing inside the mouth. During swallowing, the tongue is elevated to fit into the recess within the palate, obliterating the cleft and keeping food from passing into the nasal cavity as it is consumed (Jankowski, 2013).
Squamates (lizards and snakes) generally exhibit highly reduced secondary palates (Fig. 5E), allowing greater exposure of the vomeronasal (or Jacobson's) organ, which is located in the anterior roof of the oral cavity. The reason for this particular phenotype may be due to their heavy reliance on chemosensory information for basic functions ranging from mating to foraging for food (Halpern, 1992; Mason, 1992; Schwenk, 1995; Filoramo and Schwenk, 2009). The tongue is used to deliver chemosensory information from their environment to the vomeronasal organ; hence the characteristic tongue flick often associated with snakes and lizards. The vomeronasal organ is connected to the oral cavity though vomeronasal fenestrae which open directly into the mouth and associate with the tongue during this process (Filoramo and Schwenk, 2009). In addition to the abovementioned adaptations, snakes are also unique in possessing dentate palatine and pterygoid bones which provide a second, antero-posterior row of teeth in the palate (Richman et al., 2006; Buchtova et al., 2008).
An exception to the traditional squamate palate can be found in the veiled chameleon (Chamaeleo calyptratus) (Fig. 5D). In this group, the palatal shelves are quite substantial compared to other lizards, and almost cover the entire roof of the oral cavity (Richman et al., 2006) – figure 3). We hypothesize that this morphology is likely due to the fact that chameleons primarily hunt by eyesight, using especially evolved and unique binocular vision (Ott et al., 1998), thus minimizing the need for chemosensory input during foraging. Additionally, the chameleon tongue is highly specialized and used as an instrument for prey capture (Wainwright and Bennett, 1992a; Wainwright and Bennett, 1992b). Due to these specializations, the chameleon tongue is likely not utilized as a chemosensory device and the roof of the oral cavity does not require substantial access to a vomeronasal organ. Indeed the chameleon has been cited as having a degenerated vomeronasal organ (Haas, 1937; Schwenk, 1985; Døving and Trotier, 1998). Thus, we can reasonably expect that based on the foraging mechanism, we may be able to predict the secondary palate phenotype in distinct lizard species or lineages.
In turtles that do not possess a secondary palate, the maxillary and palatine bones form a large, flat surface which may be used as a triturating surface for food processing or handling underwater (Gaffney, 1979; Natchev et al., 2009; Heiss et al., 2010) (Fig. 5F). In fact, extinct turtle species commonly possessed palatine teeth to aid in these processes (Gaffney et al., 1987; Gaffney, 1990; Davit-Beal et al., 2009). In extant sea turtles, the secondary hard palate is likely used for structural reinforcement, and may also function in excluding water from the nasopharynx during submersion.
Conclusion
In this commentary, we have compiled data from earlier studies, and our own work, to clarify the key differences in facial development in both model and non-model amniotes. We highlight the fact that primary and secondary palatogenesis passes through conserved early stages and subsequent, lineage-specific and divergent later stages. These deviations have likely contributed to the difficulty in identifying a common developmental paradigm for palatogenesis in amniotes as a group.
In order to delineate a more general developmental pattern, we assign the primary palate assembly mechanisms in amniotes into two broad categories: one utilized by crocodilians and mammals, which completely separates the nasal cavities from the stomodeum after fusion and a second utilized by birds and nonavian reptiles, which retain a permanent connection between their oral and nasal cavities throughout development. While birds and nonavian reptiles likely inherited their mechanism from basal tetrapods, the mammalian and crocodilian primary palates are independently derived. We propose that the convergent evolution of both primary and secondary palates in mammals and crocodilians suggests an intricate developmental link between the two structures, perhaps mediated by signaling from the primitive choana. Specifically, the unique position of the choana between the primary and secondary palate is shared by both groups and may allow for proper spatial positioning of the palatal shelves which will develop later in ontogeny.
Secondary palatogenesis is also initiated similarly in all lineages, but later developmental patterns diverge in a lineage-specific manner. This process can also be divided into three broad categories. Mammals, crocodilians and some turtles exhibit a bony secondary palate which completely separates the oral and nasal cavities. Since the bony palate has evolved independently in each of the aforementioned lineages, specific osseous articulations are unique to each group. Birds and squamates exhibit cleft secondary palates comprised of paired palatal shelves with differing degrees of palatal closure. Lastly, the majority of turtles and tortoises do not form a secondary palate, instead exhibiting choanal openings directly into the oral cavity, in a similar manner to basal vertebrates.
Our commentary resolves some of the gaps in understanding which have beset the research community regarding the morphogenesis of early craniofacial development in amniotes. We furthermore highlight the notion that the specific developmental mechanism utilized by a species or lineage involves a combination of an inherited developmental plan in conjunction with the need to accommodate lineage-specific morphological traits in space and/or time. This concept is best illustrated by the similar mechanisms of primary and secondary palate development in crocodilians and mammals, or the differences in primary palate development even within the same lineage, such as turtles.
Supplementary Material
Acknowledgements
This work was funded by an NSERC discovery grant #326908 to J.M.R. J.A. is a recipient of an NIH Ruth L. Kirschstein NRSA Postdoctoral Fellowship (award no.: 1F32 DE022999-03).
Footnotes
The authors declare no conflict of interest.
REFERENCES
- Abramyan J, Leung KJ, Richman JM. Divergent palate morphology in turtles and birds correlates with differences in proliferation and BMP2 expression during embryonic development. J Exp Zool B Mol Dev Evol. 2014;322:73–85. doi: 10.1002/jez.b.22547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramyan J, Thivichon-Prince B, Richman JM. Diversity in primary palate ontogeny of amniotes revealed with 3D imaging. Journal of Anatomy. 2015 doi: 10.1111/joa.12291. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. Bmp4 and morphological variation of beaks in Darwin's finches. Science. 2004;305:1462–5. doi: 10.1126/science.1098095. [DOI] [PubMed] [Google Scholar]
- Anthwal N, Joshi L, Tucker AS. Evolution of the mammalian middle ear and jaw: adaptations and novel structures. Journal of anatomy. 2013;222:147–160. doi: 10.1111/j.1469-7580.2012.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashique AM, Fu K, Richman JM. Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development. 2002;129:4647–60. doi: 10.1242/dev.129.19.4647. [DOI] [PubMed] [Google Scholar]
- Bailey LJ, Minkoff R, Koch WE. Relative growth rates of maxillary mesenchyme in the chick embryo. J Craniofac Genet Dev Biol. 1988;8:167–77. [PubMed] [Google Scholar]
- Berkhoudt H. Avian taste buds: Topography, structure and function, Chemical Signals in Vertebrates 6. Springer; 1992. pp. 15–20. [Google Scholar]
- Bertmar G. The vertebrate nose, remarks on its structural and functional adaptation and evolution. Evolution. 1969:131–152. doi: 10.1111/j.1558-5646.1969.tb03500.x. [DOI] [PubMed] [Google Scholar]
- Bhullar BA, Morris ZS, Sefton EM, Tok A, Tokita M, Namkoong B, Camacho J, Burnham DA, Abzhanov A. A molecular mechanism for the origin of a key evolutionary innovation, the bird beak and palate, revealed by an integrative approach to major transitions in vertebrate history. Evolution. 2015 doi: 10.1111/evo.12684. [DOI] [PubMed] [Google Scholar]
- Blanc F. Table de développement de Chamaeleo lateralis Gray, 1831. Ann Embryol Morphol. 1974;7:99–115. [Google Scholar]
- Brinkley JF, Borromeo C, Clarkson M, Cox TC, Cunningham MJ, Detwiler LT, Heike CL, Hochheiser H, Mejino JL, Travillian RS, Shapiro LG. The ontology of craniofacial development and malformation for translational craniofacial research. Am J Med Genet C Semin Med Genet. 2013;163C:232–45. doi: 10.1002/ajmg.c.31377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchtova M, Handrigan GR, Tucker AS, Lozanoff S, Town L, Fu K, Diewert VM, Wicking C, Richman JM. Initiation and patterning of the snake dentition are dependent on Sonic hedgehog signaling. Dev Biol. 2008;319:132–45. doi: 10.1016/j.ydbio.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Bush JO, Jiang R. Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Development. 2012;139:231–243. doi: 10.1242/dev.067082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette MJ, Ferguson MW. The fate of medial edge epithelial cells during palatal fusion in vitro: an analysis by DiI labelling and confocal microscopy. Development. 1992;114:379–88. doi: 10.1242/dev.114.2.379. [DOI] [PubMed] [Google Scholar]
- Cerny R, Cattell M, Sauka-Spengler T, Bronner-Fraser M, Yu F, Medeiros DM. Evidence for the prepattern/cooption model of vertebrate jaw evolution. Proc Natl Acad Sci U S A. 2010;107:17262–7. doi: 10.1073/pnas.1009304107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Sasano Y, Bringas P, Mayo M, Kaartinen V, Heisterkamp N, Groffen J, Slavkin H, Shuler C. Characterization of the fate of midline epithelial cells during the fusion of mandibular prominences in vivo. Developmental dynamics. 1997;208:526–535. doi: 10.1002/(SICI)1097-0177(199704)208:4<526::AID-AJA8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Cohn MJ. Evolutionary biology: lamprey Hox genes and the origin of jaws. Nature. 2002;416:386–7. doi: 10.1038/416386a. [DOI] [PubMed] [Google Scholar]
- Compagnucci C, Fish JL, Schwark M, Tarabykin V, Depew MJ. Pax6 regulates craniofacial form through its control of an essential cephalic ectodermal patterning center. Genesis. 2011;49:307–25. doi: 10.1002/dvg.20724. [DOI] [PubMed] [Google Scholar]
- Cordero GA, Janzen FJ. An enhanced developmental staging table for the painted turtle, Chrysemys picta (Testudines: Emydidae). Journal of morphology. 2014;275:442–455. doi: 10.1002/jmor.20226. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993;117:409–29. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- Cox TC. Taking it to the max: the genetic and developmental mechanisms coordinating midfacial morphogenesis and dysmorphology. Clin Genet. 2004;65:163–76. doi: 10.1111/j.0009-9163.2004.00225.x. [DOI] [PubMed] [Google Scholar]
- Crawford NG, Parham JF, Sellas AB, Faircloth BC, Glenn TC, Papenfuss TJ, Henderson JB, Hansen MH, Simison WB. A phylogenomic analysis of turtles. Molecular phylogenetics and evolution. 2015;83:250–257. doi: 10.1016/j.ympev.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Creuzet S, Couly G, Le Douarin NM. Patterning the neural crest derivatives during development of the vertebrate head: insights from avian studies. J Anat. 2005;207:447–59. doi: 10.1111/j.1469-7580.2005.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crole MR, Soley JT. Morphology of the tongue of the emu (Dromaius novaehollandiae). II. Histological features. Onderstepoort J Vet Res. 2009;76:347–61. doi: 10.4102/ojvr.v76i4.18. [DOI] [PubMed] [Google Scholar]
- Crompton AW. Masticatory function in nonmammalian cynodonts and early mammals. In: Thomason JJ, editor. Functional Morphology in Vertebrate paleontology. Cambridge University Press; New York: 1995. pp. 55–75. [Google Scholar]
- Crompton AW, Parker P. Evolution of the Mammalian Masticatory Apparatus: The fossil record shows how mammals evolved both complex chewing mechanisms and an effective middle ear, two structures that distinguish them from reptiles. American Scientist. 1978:192–201. [PubMed] [Google Scholar]
- Cuervo R, Covarrubias L. Death is the major fate of medial edge epithelial cells and the cause of basal lamina degradation during palatogenesis. Development. 2004;131:15–24. doi: 10.1242/dev.00907. [DOI] [PubMed] [Google Scholar]
- Darwin C. On the origin of species. Murray; London: 1859. p. 434. [Google Scholar]
- Davit-Beal T, Tucker AS, Sire JY. Loss of teeth and enamel in tetrapods: fossil record, genetic data and morphological adaptations. J Anat. 2009;214:477–501. doi: 10.1111/j.1469-7580.2009.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew MJ, Compagnucci C. Tweaking the hinge and caps: testing a model of the organization of jaws. J Exp Zool B Mol Dev Evol. 2008;310:315–35. doi: 10.1002/jez.b.21205. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Simpson CA. 21st century neontology and the comparative development of the vertebrate skull. Developmental dynamics. 2006;235:1256–1291. doi: 10.1002/dvdy.20796. [DOI] [PubMed] [Google Scholar]
- Diewert VM, Wang KY. Recent advances in primary palate and midface morphogenesis research. Crit Rev Oral Biol Med. 1992;4:111–30. doi: 10.1177/10454411920040010201. [DOI] [PubMed] [Google Scholar]
- Dixon MJ, Marazita ML, Beaty TH, Murray JC. Cleft lip and palate: understanding genetic and environmental influences. Nat Rev Genet. 2011;12:167–78. doi: 10.1038/nrg2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Døving KB, Trotier D. Structure and function of the vomeronasal organ. The Journal of experimental biology. 1998;201:2913–2925. doi: 10.1242/jeb.201.21.2913. [DOI] [PubMed] [Google Scholar]
- Ferguson MW. The structure and development of the palate in Alligator mississippiensis. Arch Oral Biol. 1981;26:427–43. doi: 10.1016/0003-9969(81)90041-8. [DOI] [PubMed] [Google Scholar]
- Ferguson MW. Palate development: mechanisms and malformations. Irish journal of medical science. 1987;156:309–315. doi: 10.1007/BF02951261. [DOI] [PubMed] [Google Scholar]
- Filoramo NI, Schwenk K. The mechanism of chemical delivery to the vomeronasal organs in squamate reptiles: a comparative morphological approach. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology. 2009;311:20–34. doi: 10.1002/jez.492. [DOI] [PubMed] [Google Scholar]
- Fitchett JE, Hay ED. Medial edge epithelium transforms to mesenchyme after embryonic palatal shelves fuse. Dev Biol. 1989;131:455–74. doi: 10.1016/s0012-1606(89)80017-x. [DOI] [PubMed] [Google Scholar]
- Gaare JD, Langman J. Fusion of nasal swellings in the mouse embryo. DNA synthesis and histological features. Anat Embryol (Berl) 1980;159:85–99. doi: 10.1007/BF00299258. [DOI] [PubMed] [Google Scholar]
- Gaffney ES. Comparative cranial morphology of Recent and fossil turtles. Bulletin of the AMNH. 1979;164 article 2. [Google Scholar]
- Gaffney ES. The comparative osteology of the Triassic turtle Proganochelys. Bulletin of the AMNH. 1990;(194) [Google Scholar]
- Gaffney ES, Hutchison JH, Jenkins FA, Meeker LJ. Modern turtle origins: the oldest known cryptodire. Science. 1987;237:289–291. doi: 10.1126/science.237.4812.289. [DOI] [PubMed] [Google Scholar]
- Gong SG, Guo C. Bmp4 gene is expressed at the putative site of fusion in the midfacial region. Differentiation. 2003;71:228–36. doi: 10.1046/j.1432-0436.2003.710304.x. [DOI] [PubMed] [Google Scholar]
- Graham A, Okabe M, Quinlan R. The role of the endoderm in the development and evolution of the pharyngeal arches. J Anat. 2005;207:479–87. doi: 10.1111/j.1469-7580.2005.00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JN, Compagnucci C, Hu D, Fish J, Klein O, Marcucio R, Depew MJ. Fgf8 dosage determines midfacial integration and polarity within the nasal and optic capsules. Dev Biol. 2013;374:185–97. doi: 10.1016/j.ydbio.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith CM, Hay ED. Epithelial-mesenchymal transformation during palatal fusion: carboxyfluorescein traces cells at light and electron microscopic levels. Development. 1992;116:1087–99. doi: 10.1242/dev.116.4.1087. [DOI] [PubMed] [Google Scholar]
- Haas G. The structure of the nasal cavity in Chamaeleo chameleon (Linnaeus). Journal of Morphology. 1937;61:433–451. [Google Scholar]
- Hall BK. Summarizing craniofacial genetics and developmental biology (SCGDB). Am J Med Genet A. 2014;164A:884–91. doi: 10.1002/ajmg.a.35288. [DOI] [PubMed] [Google Scholar]
- Halpern M. Nasal chemical senses in reptiles: structure and function. University of Chicago Press; Chicago, London: 1992. [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- He F, Chen Y. Wnt signaling in lip and palate development. Front Oral Biol. 2012;16:81–90. doi: 10.1159/000337619. [DOI] [PubMed] [Google Scholar]
- Heiss E, Natchev N, Beisser C, Lemell P, Weisgram J. The fish in the turtle: on the functionality of the oropharynx in the common musk turtle Sternotherus odoratus (Chelonia, Kinosternidae) concerning feeding and underwater respiration. Anat Rec (Hoboken) 2010;293:1416–24. doi: 10.1002/ar.21185. [DOI] [PubMed] [Google Scholar]
- Higashihori N, Buchtova M, Richman JM. The function and regulation of TBX22 in avian frontonasal morphogenesis. Dev Dyn. 2010;239:458–73. doi: 10.1002/dvdy.22182. [DOI] [PubMed] [Google Scholar]
- Hilliard SA, Yu L, Gu S, Zhang Z, Chen YP. Regional regulation of palatal growth and patterning along the anterior-posterior axis in mice. J Anat. 2005;207:655–67. doi: 10.1111/j.1469-7580.2005.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen K. The early development of morphology and patterns of the face in the human embryo. Adv Anat Embryol Cell Biol. 1985;98:1–79. doi: 10.1007/978-3-642-70754-4. [DOI] [PubMed] [Google Scholar]
- Hirayama R. Phylogenetic systematics of chelonioid sea turtles. Island Arc. 1994;3:270–284. [Google Scholar]
- Hu D, Young NM, Li X, Xu Y, Hallgrimsson B, Marcucio RS. A dynamic Shh expression pattern, regulated by SHH and BMP signaling, coordinates fusion of primordia in the amniote face. Development. 2015a;142:567–74. doi: 10.1242/dev.114835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Young NM, Xu Q, Jamniczky H, Green RM, Mio W, Marcucio RS, Hallgrimsson B. Signals from the brain induce variation in avian facial shape. Developmental Dynamics. 2015b doi: 10.1002/dvdy.24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iamaroon A, Tait B, Diewert VM. Cell proliferation and expression of EGF, TGF-α, and EGF receptor in the developing primary palate. Journal of dental research. 1996;75:1534–1539. doi: 10.1177/00220345960750080301. [DOI] [PubMed] [Google Scholar]
- Iwabe N, Hara Y, Kumazawa Y, Shibamoto K, Saito Y, Miyata T, Katoh K. Sister group relationship of turtles to the bird-crocodilian clade revealed by nuclear DNA-coded proteins. Mol Biol Evol. 2005;22:810–3. doi: 10.1093/molbev/msi075. [DOI] [PubMed] [Google Scholar]
- Jankowski R. The Complex Formation of the Secondary Palate and Nose in Evolution, The Evo-Devo Origin of the Nose, Anterior Skull Base and Midface. Springer; 2013. pp. 41–61. [Google Scholar]
- Jiang R, Bush JO, Lidral AC. Development of the upper lip: morphogenetic and molecular mechanisms. Dev Dyn. 2006;235:1152–66. doi: 10.1002/dvdy.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J-Z, Ding J. Analysis of cell migration, transdifferentiation and apoptosis during mouse secondary palate fusion. Development. 2006;133:3341–3347. doi: 10.1242/dev.02520. [DOI] [PubMed] [Google Scholar]
- Jirásek JE. Atlas of human prenatal morphogenesis. Martinus Nijhoff Publishers; Boston: 1983. [Google Scholar]
- Kim CH, Park HW, Kim K, Yoon JH. Early development of the nose in human embryos: a stereomicroscopic and histologic analysis. Laryngoscope. 2004;114:1791–800. doi: 10.1097/00005537-200410000-00022. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Sidlauskas B, Clack JA. Linked morphological changes during palate evolution in early tetrapods. Journal of anatomy. 2009;215:91–109. doi: 10.1111/j.1469-7580.2009.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K, Hama K, Eto K. Light and electron microscopy study of fusion of facial prominences. A distinctive type of superficial cells at the contact sites. Anat Embryol (Berl) 1985;173:187–201. doi: 10.1007/BF00316300. [DOI] [PubMed] [Google Scholar]
- Kummer AW, Marshall JL, Wilson MM. NON-CLEFT CAUSES OF VELOPHARYNGEAL DYSFUNCTION: IMPLICATIONS FOR TREATMENT. International journal of pediatric otorhinolaryngology. 2015 doi: 10.1016/j.ijporl.2014.12.036. [DOI] [PubMed] [Google Scholar]
- Kuratani S. Evolution of the vertebrate jaw: homology and developmental Constraint Paleonotol Res. 2003;7:89–102. [Google Scholar]
- Lainoff AJ, Moustakas-Verho JE, Hu D, Kallonen A, Marcucio RS, Hlusko LJ. A comparative examination of odontogenic gene expression in both toothed and toothless amniotes. J Exp Zool B Mol Dev Evol. 2015;324:255–69. doi: 10.1002/jez.b.22594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J, Kaartinen V. Signaling networks in palate development. Wiley Interdisciplinary Reviews: Systems Biology and Medicine. 2014;6:271–278. doi: 10.1002/wsbm.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Bedard O, Buchtova M, Fu K, Richman JM. A new origin for the maxillary jaw. Dev Biol. 2004;276:207–24. doi: 10.1016/j.ydbio.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Leslie EJ, Marazita ML. Genetics of cleft lip and cleft palate. Am J Med Genet C Semin Med Genet. 2013;163C:246–58. doi: 10.1002/ajmg.c.31381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losos JB. Convergence, adaptation, and constraint. Evolution. 2011;65:1827–1840. doi: 10.1111/j.1558-5646.2011.01289.x. [DOI] [PubMed] [Google Scholar]
- Maier W, Heever J.d., Durand F. New therapsid specimens and the origin of the secondary hard and soft palate of mammals. Journal of Zoological Systematics and Evolutionary Research. 1996;34:9–19. [Google Scholar]
- Manzanares M, Nieto MA. A celebration of the new head and an evaluation of the new mouth. Neuron. 2003;37:895–8. doi: 10.1016/s0896-6273(03)00161-2. [DOI] [PubMed] [Google Scholar]
- Martinez-Alvarez C, Tudela C, Perez-Miguelsanz J, O'Kane S, Puerta J, Ferguson MW. Medial edge epithelial cell fate during palatal fusion. Dev Biol. 2000;220:343–57. doi: 10.1006/dbio.2000.9644. [DOI] [PubMed] [Google Scholar]
- Mason JR, Clark L. The chemical senses in birds. Sturkie's avian physiology. 2000:39–56. [Google Scholar]
- Mason RT. Reptilian pheromones. In: Gans C, Crews D, editors. Biology of the Reptilia. University of Chicago Press; 1992. [Google Scholar]
- McGonnell IM, Clarke JD, Tickle C. Fate map of the developing chick face: analysis of expansion of facial primordia and establishment of the primary palate. Dev Dyn. 1998;212:102–18. doi: 10.1002/(SICI)1097-0177(199805)212:1<102::AID-AJA10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Menegaz RA, Sublett SV, Figueroa SD, Hoffman TJ, Ravosa MJ. Phenotypic plasticity and function of the hard palate in growing rabbits. Anat Rec (Hoboken) 2009;292:277–84. doi: 10.1002/ar.20840. [DOI] [PubMed] [Google Scholar]
- Meylan PA, Moody RTJ, Walker CA, Chapman SD. Sandownia harrisi, a highly derived trionychoid turtle (Testudines: Cryptodira) from the Early Cretaceous of the Isle of Wight, England. Journal of Vertebrate Paleontology. 2000;20:522–532. [Google Scholar]
- Minkoff R, Kuntz AJ. Cell proliferation during morphogenetic change; analysis of frontonasal morphogenesis in the chick embryo employing DNA labeling indices. J Embryol Exp Morphol. 1977;40:101–13. [PubMed] [Google Scholar]
- Minkoff R, Kuntz AJ. Cell proliferation and cell density of mesenchyme in the maxillary process and adjacent regions during facial development in the chick embryo. J Embryol Exp Morphol. 1978;46:65–74. [PubMed] [Google Scholar]
- Natchev N, Heiss E, Lemell P, Stratev D, Weisgram J. Analysis of prey capture and food transport kinematics in two Asian box turtles, Cuora amboinensis and Cuora flavomarginata (Chelonia, Geoemydidae), with emphasis on terrestrial feeding patterns. Zoology (Jena) 2009;112:113–27. doi: 10.1016/j.zool.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Nawshad A. Palatal seam disintegration: to die or not to die? that is no longer the question. Dev Dyn. 2008;237:2643–56. doi: 10.1002/dvdy.21599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. Evolution by gene duplication. Springer-Verlag; London, Berlin, Heidelberg, New York: 1970. [Google Scholar]
- Oisi Y, Ota KG, Kuraku S, Fujimoto S, Kuratani S. Craniofacial development of hagfishes and the evolution of vertebrates. Nature. 2013;493:175–80. doi: 10.1038/nature11794. [DOI] [PubMed] [Google Scholar]
- Ott M, Schaeffel F, Kirmse W. Binocular vision and accommodation in prey41 catching chameleons. Journal of Comparative Physiology A. 1998;182:319–330. [Google Scholar]
- Patterson SB, Johnston MC, Minkoff R. An implant labeling technique employing sable hair probes as carriers for 3H-thymidine: applications to the study of facial morphogenesis. Anat Rec. 1984;210:525–36. doi: 10.1002/ar.1092100313. [DOI] [PubMed] [Google Scholar]
- Peterka M, Sire JY, Hovorakova M, Prochazka J, Fougeirol L, Peterkova R, Viriot L. Prenatal development of Crocodylus niloticus niloticus Laurenti, 1768. J Exp Zool B Mol Dev Evol. 2010;314:353–68. doi: 10.1002/jez.b.21335. [DOI] [PubMed] [Google Scholar]
- Presley R, Steel FLD. The pterygoid and ectopterygoid in mammals. Anatomy and Embryology. 1978;154:95–110. doi: 10.1007/BF00317957. [DOI] [PubMed] [Google Scholar]
- Prinz JF, Lucas PW. An optimization model for mastication and swallowing in mammals. Proc Biol Sci. 1997;264:1715–21. doi: 10.1098/rspb.1997.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzansky S. Congenital Anomalies of the Face and Associated Structures. In: Pruzansky Samuel., editor. Proceedings of an international symposium. Charles C. Thomas; Springfield, Ill.: 1961. [Google Scholar]
- Putterill JF, Soley JT. Morphology of the gular valve of the Nile crocodile, Crocodylus niloticus (Laurenti, 1768). Journal of morphology. 2006;267:924–939. doi: 10.1002/jmor.10448. [DOI] [PubMed] [Google Scholar]
- Richman JM, Buchtova M, Boughner JC. Comparative ontogeny and phylogeny of the upper jaw skeleton in amniotes. Dev Dyn. 2006;235:1230–43. doi: 10.1002/dvdy.20716. [DOI] [PubMed] [Google Scholar]
- Rudé FP, Anderson L, Conley D, Gasser RF. Three-dimensional reconstructions of the primary palate region in normal human embryos. The Anatomical Record. 1994;238:108–113. doi: 10.1002/ar.1092380112. [DOI] [PubMed] [Google Scholar]
- Russo GA, Kirk EC. Foramen magnum position in bipedal mammals. Journal of human evolution. 2013;65:656–670. doi: 10.1016/j.jhevol.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Santagati F, Rijli FM. Cranial neural crest and the building of the vertebrate head. Nature Reviews Neuroscience. 2003;4:806–818. doi: 10.1038/nrn1221. [DOI] [PubMed] [Google Scholar]
- Saunders JW., Jr. The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. J Exp Zool. 1948;108:363–403. doi: 10.1002/jez.1401080304. [DOI] [PubMed] [Google Scholar]
- Scanes CG. Sturkie's avian physiology. Elsevier; 2014. [Google Scholar]
- Schwenk K. Occurrence, distribution and functional significance of taste buds in lizards. Copeia; 1985. pp. 91–101. [Google Scholar]
- Schwenk K. Of tongues and noses: chemoreception in lizards and snakes. Trends in ecology & evolution. 1995;10:7–12. doi: 10.1016/s0169-5347(00)88953-3. [DOI] [PubMed] [Google Scholar]
- Shigetani Y, Sugahara F, Kuratani S. A new evolutionary scenario for the vertebrate jaw. Bioessays. 2005;27:331–8. doi: 10.1002/bies.20182. [DOI] [PubMed] [Google Scholar]
- Shuler CF. Programmed cell death and cell transformation in craniofacial development. Crit Rev Oral Biol Med. 1995;6:202–17. doi: 10.1177/10454411950060030301. [DOI] [PubMed] [Google Scholar]
- Shuler CF, Halpern DE, Guo Y, Sank AC. Medial edge epithelium fate traced by cell lineage analysis during epithelial-mesenchymal transformation in vivo. Dev Biol. 1992;154:318–30. doi: 10.1016/0012-1606(92)90071-n. [DOI] [PubMed] [Google Scholar]
- Som PM, Naidich TP. Illustrated review of the embryology and development of the facial region, part 1: Early face and lateral nasal cavities. AJNR Am J Neuroradiol. 2013;34:2233–40. doi: 10.3174/ajnr.A3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Li Y, Wang K, Wang YZ, Molotkov A, Gao L, Zhao T, Yamagami T, Wang Y, Gan Q, Pleasure DE, Zhou CJ. Lrp6-mediated canonical Wnt signaling is required for lip formation and fusion. Development. 2009;136:3161–71. doi: 10.1242/dev.037440. [DOI] [PubMed] [Google Scholar]
- Sperber GH. Formation of the primary palate. In: F., W.D., editors. Cleft lip and palate: from origin to treatment. Oxford University; New York: 2002. pp. 5–13. [Google Scholar]
- Sun D, Baur S, Hay ED. Epithelial-mesenchymal transformation is the mechanism for fusion of the craniofacial primordia involved in morphogenesis of the chicken lip. Dev Biol. 2000;228:337–49. doi: 10.1006/dbio.2000.9946. [DOI] [PubMed] [Google Scholar]
- Szabo-Rogers HL, Geetha-Loganathan P, Nimmagadda S, Fu KK, Richman JM. FGF signals from the nasal pit are necessary for normal facial morphogenesis. Dev Biol. 2008;318:289–302. doi: 10.1016/j.ydbio.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Szabo-Rogers HL, Smithers LE, Yakob W, Liu KJ. New directions in craniofacial morphogenesis. Dev Biol. 2010;341:84–94. doi: 10.1016/j.ydbio.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Tamarin A. The formation of the primitive choanae and the junction of the primary and secondary palates in the mouse. Am J Anat. 1982;165:319–37. doi: 10.1002/aja.1001650308. [DOI] [PubMed] [Google Scholar]
- Tamarin A, Boyde A. Facial and visceral arch development in the mouse embryo: a study by scanning electron microscopy. Journal of anatomy. 1977;124:563. [PMC free article] [PubMed] [Google Scholar]
- Thomason HA, Dixon MJ, Dixon J. Facial clefting in Tp63 deficient mice results from altered Bmp4, Fgf8 and Shh signaling. Dev Biol. 2008;321:273–82. doi: 10.1016/j.ydbio.2008.06.030. [DOI] [PubMed] [Google Scholar]
- Tokita M, Chaeychomsri W, Siruntawineti J. Developmental basis of toothlessness in turtles: insight into convergent evolution of vertebrate morphology. Evolution. 2013;67:260–273. doi: 10.1111/j.1558-5646.2012.01752.x. [DOI] [PubMed] [Google Scholar]
- Trasler DG. Pathogenesis of cleft lip and its relation to embryonic face shape in A-J and C57BL mice. Teratology. 1968;1:33–49. doi: 10.1002/tera.1420010106. [DOI] [PubMed] [Google Scholar]
- Tulenko FJ, Sheil CA. Formation of the chondrocranium of Trachemys scripta (Reptilia: Testudines: Emydidae) and a comparison with other described turtle taxa. Journal of morphology. 2007;268:127–151. doi: 10.1002/jmor.10487. [DOI] [PubMed] [Google Scholar]
- Wainwright PC, Bennett AF. The mechanism of tongue projection in chameleons: I. Electromyographic tests of functional hypotheses. Journal of Experimental Biology. 1992a;168:1–21. [Google Scholar]
- Wainwright PC, Bennett AF. The mechanism of tongue projection in chameleons: II. Role of shape change in a muscular hydrostat. Journal of experimental biology. 1992b;168:23–40. [Google Scholar]
- Wang KY, Juriloff DM, Diewert VM. Deficient and delayed primary palatal fusion and mesenchymal bridge formation in cleft lip-liable strains of mice. J Craniofac Genet Dev Biol. 1995;15:99–116. [PubMed] [Google Scholar]
- Weingaertner J, Proff P, Bienengraeber V, Gedrange T, Fanghaenel J, Kristina L. In vivo study of apoptosis as a creative agent of embryonic development of the primary nasal duct in rats. Journal of Cranio-Maxillofacial Surgery. 2006;34:3–7. doi: 10.1016/S1010-5182(06)60002-4. [DOI] [PubMed] [Google Scholar]
- Werneburg I, Hugi J, Muller J, Sanchez-Villagra MR. Embryogenesis and ossification of Emydura subglobosa (Testudines, Pleurodira, Chelidae) and patterns of turtle development. Dev Dyn. 2009;238:2770–86. doi: 10.1002/dvdy.22104. [DOI] [PubMed] [Google Scholar]
- Wyneken J, Witherington D. The anatomy of sea turtles. Southeast Fisheries Science Center, National Marine Fisheries Service, National Oceanic and Atmospheric Administration, US Department of Commerce; 2001. [Google Scholar]
- Young NM, Hu D, Lainoff AJ, Smith FJ, Diaz R, Tucker AS, Trainor PA, Schneider RA, Hallgrimsson B, Marcucio RS. Embryonic bauplans and the developmental origins of facial diversity and constraint. Development. 2014;141:1059–63. doi: 10.1242/dev.099994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.