Abstract
Background and Objectives
We assessed the cumulative incidence, symptoms, and risk factors for upper-extremity lymphedema in breast cancer and melanoma patients undergoing sentinel lymph node biopsy or axillary lymph node dissection.
Methods
Patients were recruited preoperatively (time 0) and assessed at 6, 12, and 18 months postoperatively. Limb volume change (LVC) was measured by perometry. Lymphedema was categorized as none, mild (LVC 5–9.9%), or moderate/severe (LVC≥10%). Symptoms were assessed with a validated lymphedema instrument. Longitudinal logistic regression analyses were conducted to identify risk factors associated with moderate/severe lymphedema.
Results
Among 205 breast cancer and 144 melanoma patients, the cumulative incidence of moderate/severe lymphedema at 18 months was 36.5% and 35.0, respectively. However, in adjusted analyses, factors associated with moderate/severe lymphedema were breast cancer (OR 2.0, p=0.03), body mass index ≥30 kg/m2 (OR 1.6, p=0.04), greater number of lymph nodes removed (OR 1.05, p<0.01), and longer interval since surgery (OR 2.33 at 18 months, p<0.01). Conclusions: Lymphedema incidence increased over time in both cohorts. However, the adjusted risk of moderate/severe lymphedema was two-fold higher in breast cancer patients. These results may be attributed to surgical treatment of the primary tumor in the breast and more frequent use of radiation.
Keywords: lymphedema, melanoma, breast cancer, lymph node excision
INTRODUCTION
Survivorship estimates for both melanoma and breast cancer continue to improve, with 5-year survival rates of 93% and 91%, respectively [1]. While breast-conserving therapy and sentinel lymph node biopsy (SLNB) have reduced patient morbidity, secondary lymphedema remains a vexing complication of treatment for breast cancer and melanoma. The increasing number of cancer survivors demands that clinicians address long-term sequelae of cancer treatment, including lymphedema.
Despite the prevalence of secondary lymphedema, its true incidence in breast cancer and melanoma patients is not precisely known. It affects roughly 13% of patients with upper-extremity melanoma [2], but the reported range varies widely, from 1–39% in individual studies [3–11]. In breast cancer, where more studies have been conducted, a recent meta-analysis of 30 prospective cohort studies estimated that 21% (range 14.9–29.8%) of breast cancer survivors develop lymphedema [12]. However, the definitions and methods used to assess secondary lymphedema have varied, which limits the ability to draw equivalent conclusions across different malignancies. To our knowledge, there are no reports that compare the incidence of upper-extremity lymphedema in breast cancer and melanoma patients using prospective, objective assessment tools. While water displacement is generally considered the standard measurement method, perometry has been adopted in many centers because of its high intra-rater reliability, ease of use, mobility, and reduced hygienic concerns [13, 14].
The objective of this analysis was to compare by perometry18-month cumulative incidence, symptoms, and associated risk factors for secondary lymphedema in patients with upper-extremity melanoma or breast cancer undergoing SLNB or axillary lymph node dissection (ALND). We hypothesized that the incidence of lymphedema would increase over time in all groups and, based on available literature, would be higher in breast cancer patients, those with a higher body mass index (BMI), those treated with radiation therapy, and those undergoing more extensive nodal surgery. We also hypothesized that reported symptoms would be similar across both cancer groups.
MATERIALS AND METHODS
Patient Recruitment, Eligibility, and Enrollment
Patients were recruited prior to surgical intervention. Breast cancer patients were enrolled from 2001–2007 at The University of Missouri and melanoma patients from 2006–2012 at The University of Texas MD Anderson Cancer Center. All patients were required to be fluent in English, at least 18 years old, and capable of providing informed consent. Ineligibility criteria included concurrent treatment for a separate malignancy, implanted hardware, previous surgery to either axillary nodal basin, or prior upper-extremity trauma.
Eligible patients were scheduled to undergo SLNB or ALND per clinical practice guidelines and had pathologically confirmed stage I–III cutaneous melanoma of the upper body or stage 0-IV breast cancer. Surgeons performed primary tumor excisions and axillary lymph node procedures according to their individual surgical preferences but did conform to the National Comprehensive Cancer Network (NCCN) guidelines for axillary surgery in breast cancer and melanoma [15, 16]. Reported stages were determined by final pathologic staging after patients underwent SLNB and/or completion ALND, as appropriate. There were no patients with bilateral breast cancer or melanoma that drained to the bilateral axilla included in the cohort. Radiation therapy was delivered according to NCCN guidelines at the time of treatment. Demographic information was obtained at the time of enrollment. The protocols were approved by the Institutional Review Boards at both institutions.
Measurement of Limb Volume
Patient limb volume was assessed at baseline (time 0) prior to surgery and again at 6 months, 12 months, and 18 months postoperatively using a perometer (Pero-System 1000M or 350S, Juzo, Wuppertal, Germany). Patients were measured by specially trained clinical researchers in rooms where temperature was standardized and held constant across each visit. Measurements were obtained according to protocol by instructing patients to hold a fixed position on the device while the infrared sensors in the frame measured limb volume from the styloid process of the ulna to the axilla. Both the ipsilateral and contralateral upper extremities were assessed at each time point to adjust for weight changes over time. The following mathematical formula was utilized [2, 17]:
where LVC = limb volume change, I = the volume of the ipsilateral extremity, and C = the volume of the contralateral extremity.
Patients with moderate/severe lymphedema (LVC≥10%) were referred to physical therapy for treatment of lymphedema and associated symptoms. Compression garments were prescribed when recommended by lymphedema therapists.
Lymphedema and Breast Cancer Questionnaire
Patient symptoms were assessed at baseline and at 6, 12, and 18 months after surgery with the previously-validated Lymphedema and Breast Cancer Questionnaire (LBCQ) [18], which has also been adapted for melanoma patients [2]. The questionnaire consists of 19 items, which are completed independently by the patient during clinic visits. The survey assesses for symptoms such as swelling, pitting edema, erythema, numbness, and blistering experienced currently or during the past year.
Statistical Analysis
This study is a retrospective analysis of prospectively-collected data from a cohort of breast cancer patients and a cohort of melanoma patients. LVC was calculated for each time point, and patients were grouped into three categories: (1) no lymphedema (LVC<5%), (2) mild lymphedema (LVC 5–9.9%), or (3) moderate/severe lymphedema (LVC≥10%). LVC of 5–9.9% was classified as mild lymphedema because this group has demonstrated significantly worse quality of life and symptom scores than those with LVC of less than 5% [19] and is at risk for progression to more severe lymphedema [20]. The cumulative incidence of lymphedema was calculated at each time point. Generalized estimating equations were used for building the longitudinal logistic regression model to assess which demographic and treatment factors were associated with LVC≥10%. Age, BMI, type of nodal surgery (SLNB vs. ALND), radiation, type of cancer (breast vs. melanoma), number of excised lymph nodes, and time since surgery were included in the model. Factors that were not significant for LVC≥10% were sex, race, disease stage, and surgical site (right vs. left). Affirmative symptom responses from the LBCQ were tallied and stratified by LVC category for breast cancer and melanoma. A Chi-square test was used to perform a stepwise analysis to compare patient-reported symptoms to increasing percentage of limb volume change. All statistical analyses were performed using SAS, version 9.3 (SAS Institute Inc., Cary, North Carolina). P values <0.05 were considered statistically significant.
RESULTS
Patient Characteristics
A total of 205 breast cancer and 144 melanoma patients were enrolled. The demographic, disease, and treatment characteristics are summarized in Table 1. Fifty-two percent of breast cancer patients and 49% of melanoma patients underwent SLNB, and the remaining patients in each group underwent ALND. The median number of nodes removed by SLNB was similar in both groups; however, the median number of lymph nodes removed by ALND in the melanoma cohort was more than double that in the breast cohort (29 vs. 14 nodes). The majority (51%) of breast cancer patients had stage I or in situ disease, whereas the majority of participants with melanoma had stage III disease (52%). Radiation therapy to any anatomic region was much more common in breast cancer patients (62% vs. 14%, p<0.01).
Table 1.
Baseline demographic and clinical characteristics following disease staging
| Variable | Overall n (%) | Breast Cancer n (%) | Melanoma n (%) | P Value |
|---|---|---|---|---|
| (n=349) | (n=205) | (n=144) | ||
| Age, years | 0.03 | |||
| Mean | 57.4 years | 59.1 years | 54.8 years | |
| Under 50 | 97 (28) | 46 (23) | 51 (35) | |
| 50 to Under 65 | 154 (44) | 97 (47) | 57 (40) | |
| 65 or Over | 98 (28) | 62 (30) | 36 (25) | |
| Sex | <0.01 | |||
| Female | 264 (76) | 204 (99.5) | 60 (42) | |
| Male | 85 (24) | 1 (0.5) | 84 (58) | |
| Race | 0.56 | |||
| White | 334 (95) | 194 (94.5) | 140 (97) | |
| African American | 9 (3) | 8 (4) | 1 (1) | |
| Hispanic | 3 (1) | 1 (0.5) | 2 (1) | |
| Native American | 3 (1) | 2 (1) | 1 (1) | |
| Body Mass Index, kg/m2 | 0.55 | |||
| <25 | 87 (24) | 52 (25) | 35 (24) | |
| 25 to <30 | 131 (38) | 72 (35) | 59 (41) | |
| ≥30 | 131 (38) | 81 (40) | 50 (35) | |
| Extremity | 0.66 | |||
| Right | 169 (48) | 101 (49) | 68 (47) | |
| Left | 180 (52) | 104 (51) | 76 (53) | |
| Definitive Nodal Surgery | 0.47 | |||
| SLNB | 177 (51) | 107 (52) | 70 (49) | |
| Number of lymph nodes removed, median (range) | 2 (1–7) | 3 (1–12)* | ||
| ALND | 172 (49) | 98 (48) | 74 (51) | |
| Number of lymph nodes removed, median (range) | 14 (4–39) Missing = 4 |
29 (13–57) | ||
| Number positive, median (range) | 1 (1–25) Missing = 1 |
1 (1–25) | ||
| Disease Stage | <0.01 | |||
| Stage 0 | 17 (5) | 17 (8) | 0 (0) | |
| Stage I | 142 (41) | 88 (43) | 54 (38) | |
| Stage II | 77 (22) | 63 (31) | 14 (10) | |
| Stage III | 100 (28) | 24 (12) | 76 (52) | |
| Stage IV | 6 (2) | 6 (3) | 0 (0) | |
| Unknown | 7 (2) | 7 (3) | 0 (0) | |
| Breast Surgery | ||||
| Excisional Biopsy | 36 (18) | N/A | ||
| Segmental Mastectomy | 97 (47) | N/A | ||
| Mastectomy | 72 (35) | N/A | ||
| Location of Primary Melanoma | ||||
| Head/Neck | N/A | 2 (1) | ||
| Upper Extremity | N/A | 73 (51) | ||
| Trunk | N/A | 64 (44) | ||
| Unknown | N/A | 5 (3) | ||
| Radiation Therapy | <0.01 | |||
| Yes | 147 (42) | 127 (62) | 20 (14) | |
| No | 201 (57) | 77 (37) | 124 (86) | |
| Unknown | 1 (0.3) | 1 (0.5) |
SLNB = Sentinel Lymph Node Biopsy, ALND = Axillary Lymph Node Dissection
SLNB with ≥10 nodes had 4 lymph nodes removed intraoperatively which on formal pathologic review revealed multiple nodes in each tissue specimen
Cumulative Incidence of Lymphedema
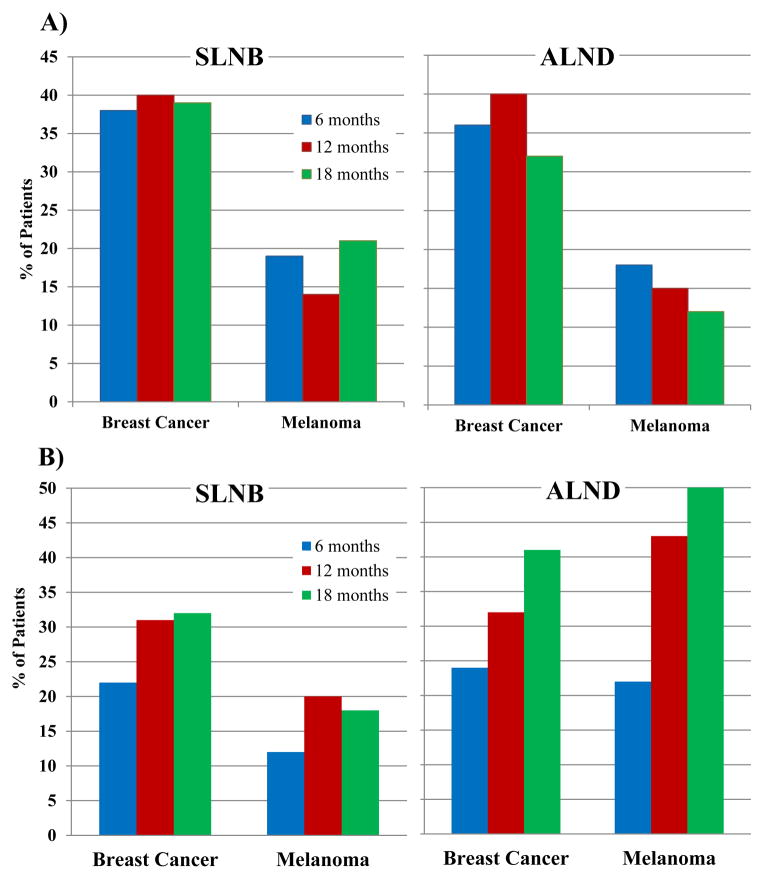
The cumulative incidence of LVC at 6, 12, and 18 months is shown in Table 2 and Figure 1. The cumulative incidence of mild lymphedema appears to decrease at some time points as patients transition from mild to moderate/severe lymphedema (transition from Fig. 1A to 1B). Overall, by 18 months, lymphedema of any severity (LVC≥5%) was seen in 72.5% of breast cancer and 51.3% of melanoma patients. The cumulative incidence of moderate/severe lymphedema increased over time in breast cancer and melanoma patients and was nearly the same at 18 months (35.0% for melanoma, 36.5% for breast cancer). The highest incidence of moderate/severe lymphedema at 18 months was in melanoma patients who had undergone ALND (50%), compared to 41% in breast cancer patients.
Table 2.
Limb volume change over time stratified by lymph node surgery and cancer type
| 6 months, n (%) | 12 months, n (%) | 18 months, n (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <5% LVC | 5–9.9% LVC | ≥10% LVC | <5% LVC | 5–9.9% LVC | ≥10% LVC | <5% LVC | 5–9.9% LVC | ≥10% LVC | ||||
| Breast Cancer | ||||||||||||
| SLNB | 37 (40) | 35 (38) | 21 (22) | 28 (29) | 38 (40) | 30 (31) | 26 (28) | 36 (39) | 30 (32) | |||
| ALND | 32 (40) | 29 (36) | 20 (24) | 24 (28) | 35 (40) | 28 (32) | 20 (27) | 24 (32) | 31 (41) | |||
| Total (Attrition Rate, %) | 69 | 64 | 41 | 174 (15.1) | 52 | 73 | 58 | 183 (10.7) | 46 | 60 | 61 | 167 (18.5) |
|
| ||||||||||||
| Melanoma | ||||||||||||
| SLNB | 41(69) | 11 (19) | 7 (12) | 33 (66) | 7 (14) | 10 (20) | 23 (61) | 8 (21) | 7 (18) | |||
| ALND | 39 (60) | 12 (18) | 14 (22) | 23 (42) | 8 (15) | 24 (43) | 16 (38) | 5 (12) | 21 (50) | |||
| Total (Attrition Rate, %) | 80 | 23 | 21 | 124 (13.9) | 56 | 15 | 34 | 105 (27.1) | 39 | 13 | 28 | 80 (44.4) |
SLNB = Sentinel Lymph Node Biopsy, ALND = Axillary Lymph Node Dissection
Figure 1.
Cumulative incidences of A) mild lymphedema and B) moderate/severe lymphedema at 6, 12, and 18 months stratified by type of surgery for breast cancer and melanoma patients. The cumulative incidence of mild lymphedema is higher in breast cancer patients and relatively stable over time. Moderate/severe lymphedema increases over time in both groups and is highest for melanoma patients after ALND. SLNB = Sentinel Lymph Node Biopsy, ALND = Axillary Lymph Node Dissection
Table 3 shows the cumulative incidence of lymphedema over time by type of primary tumor surgery. Moderate/severe lymphedema at 18 months was more common in patients who had undergone mastectomy for breast cancer or had non-extremity primary melanomas.
Table 3.
Limb volume change over time stratified by cancer type and primary tumor surgery
| 6 months, n (%) | 12 months, n (%) | 18 months, n (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <5% | 5–9% | ≥10% | <5% | 5–9% | ≥10% | <5% | 5–9% | ≥10% | ||||
| Breast Cancer | ||||||||||||
| Mastectomy | 19 (33) | 22 (39) | 16 (28) | 17 (27) | 19 (31) | 26 (42) | 14 (27) | 15 (28) | 24 45) | |||
| Non-Mastectomy | 50 (43) | 42 (36) | 25 (21) | 35 (29) | 54 (45) | 32 (26) | 32 (28) | 45 (40) | 35 (32) | |||
| Total | 69 | 64 | 41 | 174 | 52 | 73 | 58 | 183 | 46 | 60 | 61 | 167 |
|
| ||||||||||||
| Melanoma | ||||||||||||
| Upper Extremity | 39 (69) | 10 (18) | 7 (13) | 28 (60) | 8 (15) | 12 (25) | 18 (47) | 9 (24) | 11 (29) | |||
| Trunk | 27 (55) | 12 (25) | 10 (20) | 18 (44) | 6 (15) | 17 (41) | 15 (47) | 4 (13) | 13 (40) | |||
| Others | 14 (74) | 1 (5) | 4 (21) | 10 (63) | 1 (6) | 5 (31) | 6 (60) | 0 (0) | 4 (40) | |||
| Total | 79 | 23 | 21 | 124 | 56 | 14 | 34 | 105 | 39 | 13 | 28 | 80 |
Risk Factors for Lymphedema
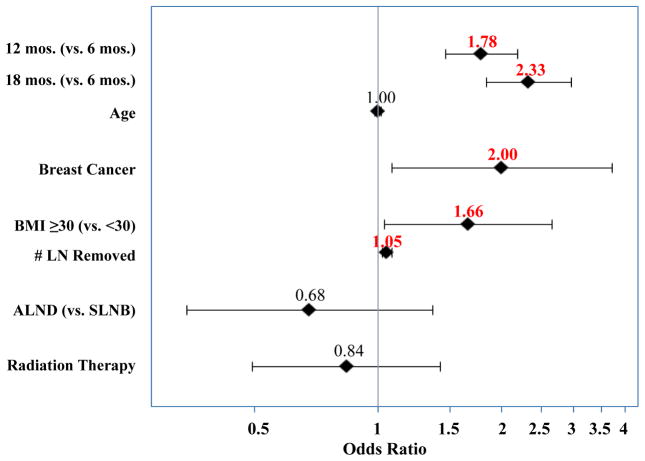
Longitudinal logistic regression identified the following risk factors associated with LVC≥10% (Figure 2): LVC measurement at 12 months (odds ratio [OR] 1.78) and 18 months postoperatively (OR 2.33), breast cancer (OR 2.00), BMI≥30 kg/m2 (OR 1.66), and greater number of lymph nodes removed (OR 1.05). Notably, the type of surgery (ALND vs. SLNB) was not a significant factor when the number of lymph nodes removed was also included in the model.
Figure 2.
Forest plot of longitudinal logistic regression model showing longer interval since surgery, breast cancer, BMI≥30 kg/m2, and greater number of lymph nodes removed to be significant factors for development of moderate/severe lymphedema. LN = Lymph Node, BMI = Body Mass Index, SLNB = Sentinel Lymph Node Biopsy, ALND = Axillary Lymph Node Dissection
Symptom Assessment
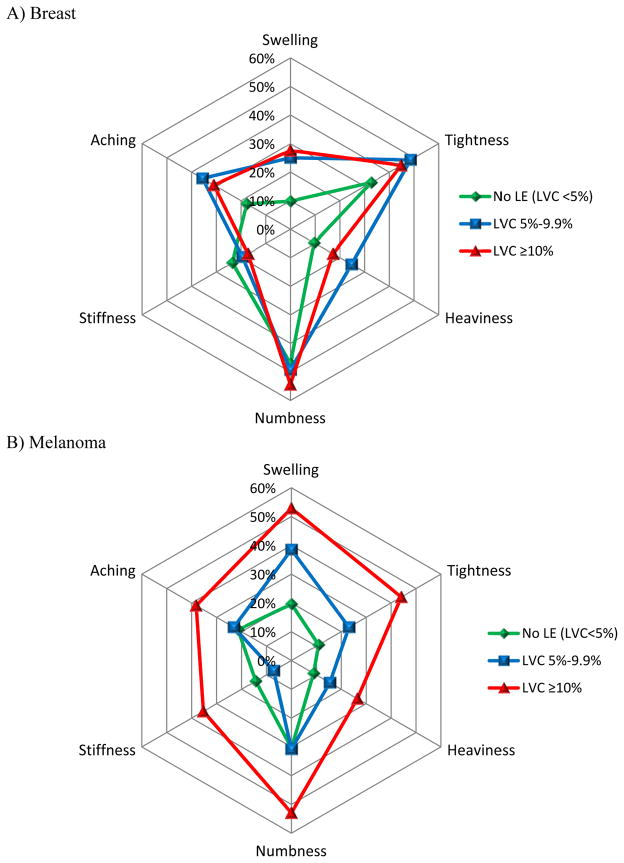
The most frequently reported symptoms at the 12-month interview for all study patients were upper-extremity numbness (45%), tightness (35%), aching (28%), swelling (26%), stiffness (20%), and heaviness (17%). Figure 3 shows the frequency at which these symptoms were experienced in patients with breast cancer (A) or melanoma (B) stratified by LVC. For patients with breast cancer, the frequency of symptoms was similar between the mild and moderate/severe lymphedema groups. In the melanoma group, however, the frequency of reported symptoms differed substantially; melanoma patients with LVC≥10% reported swelling, tightness, numbness, stiffness, heaviness, and aching more frequently than those with LVC 5–9.9%. To further evaluate the observed trends in patient-reported symptoms, a stepwise analysis was performed (not shown). For breast cancer patients, none of the 6 symptoms were significant for LVC≥10%. In contrast, for melanoma patients, all of the 6 most commonly reported symptoms were statistically significantly associated with moderate/severe lymphedema.
Figure 3.
Radar plots of the most commonly reported symptoms at 12 months for A) breast cancer and B) melanoma patients. The frequency of reported symptoms was similar for breast cancer patients with lymphedema, but in melanoma, symptoms increased with worsening lymphedema. LE = lymphedema, LVC = limb volume change
DISCUSSION
Our findings indicate that secondary lymphedema in breast cancer and melanoma patients may be more common than previously reported. The overall 18-month cumulative incidence of moderate/severe lymphedema was nearly identical in the two cancer groups. These results are consistent with those of a prior study from our group in breast cancer patients only, which reported that 31.2% of 269 patients followed at least a year post-operatively had LVC≥10% using similar assessment methods [19]. In the only other published study to assess lymphedema in melanoma patients by perometry, our group reported a 13% incidence of LVC≥10% at 12 months [2]. However, 13% represents the 12-month point incidence of LVC≥10%, whereas our current data reflect the cumulative incidence of lymphedema for all patients up to 18 months. This distinction is important because once diagnosed with moderate/severe lymphedema, patients are usually referred for treatment, which can reduce the limb volume at subsequent visits. Calculating cumulative incidence of lymphedema includes all patients who developed lymphedema over time, even if treatment-related improvements subsequently occurred.
Overall, at the 18-month time point, 72.5% of breast cancer and 51.3% of melanoma patients experienced some form of lymphedema. This is consistent with two prior studies reporting that 61–64% of breast cancer patients experience LVC≥5% [19, 21]. Our group previously found that 22% of upper-extremity melanoma patients were measured to have LVC≥5% at 12 months [2]. The discrepancy between the current findings and our prior results may again be explained partly by the longer, 18-month follow-up and by the cumulative incidence calculation. Traditionally, the LVC 5–9.9% group has been ignored in studies. Our results support the findings of prior studies that have examined this group and support the notion that if LVC is carefully measured and purposefully assessed, lymphedema will be found.
Historically, surgeons consider melanoma patients who undergo ALND to have a lower incidence of postoperative lymphedema than breast cancer patients, despite undergoing a more extensive nodal dissection (levels I, II, and III) [16]. In multivariate analyses with the number of excised lymph nodes evaluated as a continuous variable, the risk of lymphedema increased by about 5% for each additional lymph node removed, with a two-fold lymphedema increase in breast cancer patients. The overall cumulative incidence of LVC≥10% following ALND at 18 months was 50% for melanoma patients compared to 41% for breast cancer patients. The median number of lymph nodes removed differed significantly (29 for melanoma vs. 14 for breast cancer) which may be due to the addition of the level III nodes and possibly overall more thorough nodal dissections in the melanoma population knowing that all nodes must be removed. However, the extended dissection likely only offers a survival benefit to select populations and may confer excessive risk of lymphedema. Another statistical method for direct comparison of outcomes for similar groups would be to examine a stratified subset of homogeneous patients, for example with a BMI between 25 and 35 kg/m2 who underwent ALND and had between 4 and 20 lymph nodes removed with no radiation treatment. For this small stratified subset, the cumulative incidence of LVC≥10% was 50% in breast cancer patients vs. 20% in melanoma patients, which is similar to the adjusted analyses.
Breast cancer, BMI, and the number of lymph nodes removed were significantly associated with development of moderate/severe lymphedema, which is consistent with the literature [12, 17, 22–25]. In adjusted analysis, breast cancer patients were twice as likely as melanoma patients to develop LVC≥10%. This increased risk may be attributable to higher rates of radiation and treatment of the primary tumor in breast cancer patients compared to wide local excisions of primary melanomas. Radiation delivered to the lymphatics is thought to decrease lymph transport, likely secondary to fibrosis of the regional nodal basins [26]. In a systematic review of radiation targets and the development of breast cancer–related lymphedema, the incidence of lymphedema increased with expanded radiation targets [27]. Our current study found no increased risk of lymphedema with radiation to support this hypothesis, potentially due to the low frequency of radiation therapy in the melanoma cohort. Furthermore, detailed radiation therapy data, such as the number of fractions received and the exact anatomic area treated were not collected in this study so residual confounding may remain. We also did not track the type or timing of breast reconstruction so any relationship between reconstruction and lymphedema incidence is not known.
The symptoms of lymphedema can cause daily discomfort and even perceived changes in personal identity [28, 29]. In directly comparing scores from the LBCQ for patients with breast cancer and melanoma, we found that melanoma patients with worse lymphedema reported symptoms more frequently than those with mild or no lymphedema (Figure 3B). However, breast cancer patients with either mild or moderate/severe lymphedema experienced symptoms with similar frequencies, making it difficult to distinguish lymphedema severity based on symptom report alone in the breast cancer cohort (Figure 3A). The stepwise analysis confirmed this observed trend and showed that for melanoma patients, symptom report was a much better indicator of significant limb volume change. Even though experiencing symptoms due to lymphedema is frequent, positive symptom report in breast cancer patients without measurable lymphedema is also common. A recent cross-sectional study of 612 breast cancer patients status post axillary surgery confirmed this finding, showing that 89% of those without lymphedema reported at least one symptom [30]. Perhaps surgery on the breast and/or the increased use of radiation in breast cancer produces more residual symptoms, which are subsequently detected on the LBCQ. Alternatively, it is possible that breast cancer patients are more bothered by breast-related symptoms or altered psychosocial functioning and therefore report less symptoms as compared to melanoma patients with moderate/severe lymphedema.
In a recent European study of 543 cancer patients, the authors estimated that lymphedema management was delayed an average of 3.6 years from the initial onset of symptoms [31]. Lymphedema has been associated with significantly higher medical costs in breast cancer [32], and analysis of a national insurer’s health claims data revealed an estimated annual lymphedema-related cost of $2,243 per patient [33]. Interestingly, annual total healthcare costs were reduced by 18% in the year following prescription of a pneumatic compression device [33], implying that adequate diagnosis and treatment is associated not only with improved quality of life but also with overall cost savings. These data suggest that overall healthcare costs can be curbed by successful treatment of lymphedema, which is encouraging from a public health perspective.
New technologies are emerging for earlier detection of lymphedema. Bioimpedance has been shown to detect early-stage lymphedema, in some cases months before a clinical diagnosis can be made [34, 35]. More importantly, bioimpedance may prevent future lymphedema, as shown in a 2014 study of breast cancer patients by Soran et al [36]. The intervention group in this study was given short-term physical therapy, compression garments, and education upon reaching the threshold for subclinical lymphedema. Of those who received the early intervention therapy, only 4.4% progressed to clinical lymphedema, while 36% of the control group developed lymphedema after an average follow-up of 20 months. Monitoring for mild or subclinical lymphedema as we have done in our study is reasonable, and early intervention may alter future lymphedema formation. As a result, we advocate that all breast cancer and melanoma patients who have undergone surgical treatment be objectively screened for LVC and symptoms throughout their lifetime to assess for secondary lymphedema.
The strengths of our study include longitudinal data collection on several hundred patients, objective assessment with perometry, and linkage of patient-reported symptoms to LVC status. This study is novel in that it used standardized objective methodology to compare the incidence and reported symptoms of lymphedema in breast cancer and melanoma patients. Standardized comparison allows for more accurate conclusions across cancer types.
The current study has several limitations. Most notably, the attrition rate at 18 months was 19% in breast cancer and over 40% in melanoma patients, which introduces the potential for bias from loss to follow-up. It is difficult to determine whether patients who were lost to follow-up experienced lymphedema at an incidence similar to the retained cohort. Fewer complications may make patients less likely to seek follow-up care. The recruitment for each cancer type at two different institutions and variations in treatment for melanoma vs. breast cancer may have also led to variations in outcomes. Furthermore, we did not attempt to control for different surgeons as a number of individual surgeons performed the procedures. While surgical techniques theoretically vary, the procedures were performed by trained specialists whose clinical practices focus on the treatment of melanoma and breast cancer and the analyses were adjusted for the number of lymph nodes removed. It is possible that taken together that these limitations may have resulted in variations in outcomes.
Future work should examine patient outcomes for several additional years. Breast cancer literature reports that lymphedema incidence continues to increase up to 5 years after surgery [37]. However, the data for melanoma are much less robust.
CONCLUSIONS
While the unadjusted incidences of moderate/severe lymphedema were similar at 18 months in both cancers, adjusted analyses showed breast cancer patients to have two-fold the risk of melanoma patients. Longer time since surgery, BMI≥30 kg/m2, and greater number of lymph nodes removed are also risk factors for moderate/severe lymphedema. Symptom reporting on the LBCQ may be a better predictor of lymphedema status in melanoma than in breast cancer patients. Overall, secondary lymphedema is very common in both breast cancer and upper-extremity melanoma and warrants objective, long-term surveillance.
Synopsis.
The cumulative incidence of upper-extremity lymphedema was compared prospectively using perometry over 18 months in breast cancer and melanoma patients. The adjusted risk of moderate/severe lymphedema was two-fold higher in breast cancer patients.
Acknowledgments
This work was supported in part by National Institutes of Health grants R01 CA127328-03, NR05342, NR011093, and T32 CA009599, as well as an MDACC support grant (P30CA016672), University of Missouri PRIME funds, and Ellis Fischel Cancer Center Research Funds. We would like to thank Kathryn Hale for her editorial assistance.
Footnotes
Disclosures: None
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Hyngstrom JR, et al. Prospective assessment of lymphedema incidence and lymphedema-associated symptoms following lymph node surgery for melanoma. Melanoma Res. 2013;23(4):290–7. doi: 10.1097/CMR.0b013e3283632c83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cormier JN, et al. Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer. 2010;116(22):5138–49. doi: 10.1002/cncr.25458. [DOI] [PubMed] [Google Scholar]
- 4.de Vries M, et al. Morbidity after axillary sentinel lymph node biopsy in patients with cutaneous melanoma. Eur J Surg Oncol. 2005;31(7):778–83. doi: 10.1016/j.ejso.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Starritt EC, et al. Lymphedema after complete axillary node dissection for melanoma: assessment using a new, objective definition. Ann Surg. 2004;240(5):866–74. doi: 10.1097/01.sla.0000143271.32568.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serpell JW, Carne PW, Bailey M. Radical lymph node dissection for melanoma. ANZ J Surg. 2003;73(5):294–9. doi: 10.1046/j.1445-2197.2003.t01-1-02622.x. [DOI] [PubMed] [Google Scholar]
- 7.Wrightson WR, et al. Complications associated with sentinel lymph node biopsy for melanoma. Ann Surg Oncol. 2003;10(6):676–80. doi: 10.1245/aso.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Burmeister BH, et al. Radiation therapy following nodal surgery for melanoma: an analysis of late toxicity. ANZ J Surg. 2002;72(5):344–8. doi: 10.1046/j.1445-2197.2002.02405.x. [DOI] [PubMed] [Google Scholar]
- 9.Lawton G, Rasque H, Ariyan S. Preservation of muscle fascia to decrease lymphedema after complete axillary and ilioinguinofemoral lymphadenectomy for melanoma. J Am Coll Surg. 2002;195(3):339–51. doi: 10.1016/s1072-7515(02)01230-9. [DOI] [PubMed] [Google Scholar]
- 10.Bowsher WG, Taylor BA, Hughes LE. Morbidity, mortality and local recurrence following regional node dissection for melanoma. Br J Surg. 1986;73(11):906–8. doi: 10.1002/bjs.1800731119. [DOI] [PubMed] [Google Scholar]
- 11.Urist MM, et al. Patient risk factors and surgical morbidity after regional lymphadenectomy in 204 melanoma patients. Cancer. 1983;51(11):2152–6. doi: 10.1002/1097-0142(19830601)51:11<2152::aid-cncr2820511134>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 12.DiSipio T, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–15. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 13.Deltombe T, et al. Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology. 2007;40(1):26–34. [PubMed] [Google Scholar]
- 14.Adriaenssens N, et al. Comparative study between mobile infrared optoelectronic volumetry with a Perometer and two commonly used methods for the evaluation of arm volume in patients with breast cancer related lymphedema of the arm. Lymphology. 2013;46(3):132–43. [PubMed] [Google Scholar]
- 15.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Breast Cancer. 2015 Available from: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 16.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Melanoma. 2015 [cited 2015 February 23]; Version 2.2015:[Available from: http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf.
- 17.McLaughlin SA, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26(32):5213–9. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armer JM, et al. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52(6):370–9. doi: 10.1097/00006199-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Cormier JN, et al. Minimal limb volume change has a significant impact on breast cancer survivors. Lymphology. 2009;42(4):161–75. [PMC free article] [PubMed] [Google Scholar]
- 20.Specht MC, et al. Defining a threshold for intervention in breast cancer-related lymphedema: what level of arm volume increase predicts progression? Breast Cancer Res Treat. 2013;140(3):485–94. doi: 10.1007/s10549-013-2655-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis WP, et al. Improving surgical outcomes: standardizing the reporting of incidence and severity of acute lymphedema after sentinel lymph node biopsy and axillary lymph node dissection. Am J Surg. 2006;192(5):636–9. doi: 10.1016/j.amjsurg.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Togawa K, et al. Risk factors for self-reported arm lymphedema among female breast cancer survivors: a prospective cohort study. Breast Cancer Res. 2014;16(4):414. doi: 10.1186/s13058-014-0414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilke LG, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13(4):491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Cowher MS, et al. Conservative axillary surgery in breast cancer patients undergoing mastectomy: long-term results. J Am Coll Surg. 2014;218(4):819–24. doi: 10.1016/j.jamcollsurg.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg JI, et al. Morbidity of sentinel node biopsy: relationship between number of excised lymph nodes and patient perceptions of lymphedema. Ann Surg Oncol. 2011;18(10):2866–72. doi: 10.1245/s10434-011-1688-1. [DOI] [PubMed] [Google Scholar]
- 26.Baker A, et al. Lymphatic function is impaired following irradiation of a single lymph node. Lymphat Res Biol. 2014;12(2):76–88. doi: 10.1089/lrb.2013.0036. [DOI] [PubMed] [Google Scholar]
- 27.Shaitelman SF, CK, Chiang YJ, DeSnyder SM, Skoracki RJ, Cormier JN. Radiation targets and the development of breast cancer-related lymphedema: a systematic review of the literature. 11th National Lymphedema Network International Conference; 2014; Washington, DC. [Google Scholar]
- 28.Fu MR, Rosedale M. Breast cancer survivors’ experiences of lymphedema-related symptoms. J Pain Symptom Manage. 2009;38(6):849–59. doi: 10.1016/j.jpainsymman.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cromwell KD, et al. Is surviving enough? Coping and impact on activities of daily living among melanoma patients with lymphedema. Eur J Cancer Care. 2015 doi: 10.1111/ecc.12311. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bundred NJ, et al. Comparison of multi-frequency bioimpedance with perometry for the early detection and intervention of lymphoedema after axillary node clearance for breast cancer. Breast Cancer Res Treat. 2015;151(1):121–9. doi: 10.1007/s10549-015-3357-8. [DOI] [PubMed] [Google Scholar]
- 31.Rucigaj TP, Leskovec NK, Zunter VT. Lymphedema following cancer therapy in Slovenia: a frequently overlooked condition? Radiol Oncol. 2010;44(4):244–8. doi: 10.2478/v10019-010-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih YC, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol. 2009;27(12):2007–14. doi: 10.1200/JCO.2008.18.3517. [DOI] [PubMed] [Google Scholar]
- 33.Brayton KM, et al. Lymphedema prevalence and treatment benefits in cancer: impact of a therapeutic intervention on health outcomes and costs. PLoS One. 2014;9(12):e114597. doi: 10.1371/journal.pone.0114597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cornish BH, et al. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology. 2001;34(1):2–11. [PubMed] [Google Scholar]
- 35.Ward LC. Bioelectrical impedance analysis: proven utility in lymphedema risk assessment and therapeutic monitoring. Lymphat Res Biol. 2006;4(1):51–6. doi: 10.1089/lrb.2006.4.51. [DOI] [PubMed] [Google Scholar]
- 36.Soran A, et al. The importance of detection of subclinical lymphedema for the prevention of breast cancer-related clinical lymphedema after axillary lymph node dissection; a prospective observational study. Lymphat Res Biol. 2014;12(4):289–94. doi: 10.1089/lrb.2014.0035. [DOI] [PubMed] [Google Scholar]
- 37.Armer JM, Stewart BR. Post-breast cancer lymphedema: incidence increases from 12 to 30 to 60 months. Lymphology. 2010;43(3):118–27. [PMC free article] [PubMed] [Google Scholar]