Abstract
Establishment and maintenance of pregnancy results from signaling by the conceptus (embryo/fetus and associated extraembryonic membranes) and requires progesterone produced by the corpus luteum (CL). In most mammals, hormones produced by the trophoblast maintain progesterone production by acting directly or indirectly to maintain the CL. In domestic animals (ruminants and pigs), hormones from the trophoblast are antiluteolytic in that they act on the endometrium to prevent uterine release of luteolytic prostaglandin F2 alpha (PGF). In cyclic and pregnant sheep, progesterone negatively autoregulates expression of the progesterone receptor (PR) gene in the endometrial luminal (LE) and superficial glandular epithelium (GE). Available evidence in cyclic sheep indicates that loss of the PR is closely followed by increases in epithelial estrogen receptors (ER) and then oxytocin receptors (OTR), allowing oxytocin to induce uterine release of luteolytic PGF pulses. In pregnant sheep, the conceptus trophoblast produces interferon tau (IFN tau) that acts on the endometrium to inhibit transcription of the ER alpha gene directly and the OTR gene indirectly to abrogate development of the endometrial luteolytic mechanism. Subsequently, sequential, overlapping actions of progesterone, IFN tau, placental lactogen (PL) and growth hormone (GH) comprise a hormonal servomechanism that regulates endometrial gland morphogenesis and terminal differentiated function to maintain pregnancy in sheep. In pigs, the conceptus trophoblast produces estrogen that alters the direction of PGF secretion from an endocrine to exocrine direction, thereby sequestering luteolytic PGF within the uterine lumen. Conceptus estrogen also increases expression of fibroblast growth factor 7 (FGF-7) in the endometrial LE that, in turn, stimulates proliferation and differentiated functions of the trophectoderm, which expresses the FGF-7 receptor. Strategic manipulation of these physiological mechanisms can offer therapeutic schemes to improve uterine capacity, conceptus survival and reproductive health.
Background
The growth and development of the conceptus (embryo/fetus and associated extraembryonic membranes) in mammals unequivocally requires progesterone and placental hormone actions on the uterus that regulate endometrial differentiation and function, pregnancy recognition signaling, uterine receptivity for blastocyst implantation, and conceptus-uterine interactions [1-3]. Hormones from the conceptus act on the uterus in a paracrine manner to establish and maintain pregnancy.
Establishment of pregnancy involves maternal recognition of pregnancy and implantation. Maternal recognition of pregnancy is a phrase coined by Roger Short in 1969 and can be defined as the physiological process whereby the conceptus signals its presence to the maternal system and prolongs lifespan of the corpus luteum (CL). In most mammals, progesterone production by the CL is required for successful pregnancy. Progesterone acts on the uterus to stimulate and maintain uterine functions that are permissive to early embryonic development, implantation, placentation and successful fetal and placental development to term. Prolonged lifespan of the CL is a characteristic feature of mammalian pregnancy in species with a gestation period that exceeds the length of a normal estrous or menstrual cycle, such as domestic animals, laboratory rodents and humans.
Maintenance of pregnancy requires reciprocal interactions between the conceptus and endometrium. Available evidence supports the idea that hormones from the placenta act directly on the uterine endometrium to regulate cell differentiation and function. In domestic animals, the endometrial glands undergo a program of hyperplasia followed by hypertrophy that appears to be dependent on temporal and spatial actions of hormones from the placenta. Endometrial gland morphogenesis during pregnancy allows for the endometrium to increase output of secretory proteins that are transported to the fetus by specialized areas of the placenta termed areolae. Histotrophic nutrition from the endometrium is the first available nutrition for the developing conceptus and appears to be essential for conceptus survival and growth throughout pregnancy in domestic animals. This review summarizes current information on the biology of conceptus signals for establishment and maintenance of pregnancy, with particular emphasis on domestic animals (sheep and pig).
Pregnancy recognition, establishment and maintenance in sheep (Ovis aries)
Luteolytic mechanism
Domestic animals are spontaneous ovulators that undergo uterine-dependent estrous cycles until establishment of pregnancy [4-6]. The estrous cycle is dependent on the uterus, because it is the source of the luteolysin, prostaglandin F2α (PGF). During the estrous cycle, the endometrium releases oxytocin-induced luteolytic pulses of PGF that result in functional and structural regression of the ovarian CL, termed luteolysis. In sheep, the source of luteolytic PGF pulses is the endometrial luminal epithelium (LE) and superficial ductal glandular epithelium (sGE) [7], because they express the oxytocin receptors (OTR) [6] and are the only uterine cell types that express cyclooxygenase 2 (COX-2), a rate limiting enzyme in the synthesis of prostaglandins [8,9]. As illustrated in Figure 1, the luteolytic mechanism that develops in the endometrial LE and sGE requires sequential effects of progesterone, estrogen and oxytocin, acting through their respective receptors [5,10,11]. At estrus (Day 0), estrogens from Graafian follicles stimulate increased uterine estrogen receptor alpha (ERα), progesterone receptor (PR), and OTR expression [12,13]. However, PGF is not secreted, because OT is not present due to the absence of a CL. During early diestrus, progesterone from the newly formed corpus luteum (CL) stimulates accumulation of phospholipids in LE and sGE that can liberate arachidonic acid for synthesis and secretion of PGF [14]. During diestrus, progesterone levels increase and act via PR to "block" expression of ERα and OTR in the endometrial LE and sGE [11]. Therefore, ERα and OTR expression is not detected between Days 5 and 11 of the cycle, i.e., during most of diestrus. The precise molecular mechanism whereby progesterone suppresses ERα gene transcription is unknown. However, the effects of progesterone on OTR gene expression may be indirect through suppression of ERα. The rat OTR gene contains palindromic ER response elements (EREs) that mediate effects of estrogens [15], and the ovine OTR promoter DNA contains several Sp1 elements that appear to mediate responsiveness to ligand activated ERα (Bazer FW, JGW Fleming and TE Spencer, unpublished results). Continuous exposure of the uterus to progesterone for 8 to 10 days down-regulates expression of PR in endometrial LE and sGE after Days 11 to 12 [16], allowing for rapid increases in expression of ERα on Day 13 followed by OTR on Day 14 in LE and sGE [17,18]. Progesterone down-regulation of PR expression may involve PR-mediated decreases in PR gene transcription [19,20]. Oxytocin, secreted from Day 9 of the estrous cycle and pregnancy from the posterior pituitary and/or CL, then induces release of luteolytic PGF pulses from the endometrial LE and sGE on Days 14 to 16 [6]. The CL undergoes regression, allowing the ewe to return to estrus and complete the 17-day estrous cycle. The aforementioned model is based on correlative evidence. Critical experiments in which ERα have been manipulated by pure antiestrogens or knockdown have not been conducted to confirm that induction of ERα in the endometrial LE and sGE is required for OTR gene expression and luteolysis. In ovariectomized ewes, responsiveness to OT develops in the absence of estradiol replacement [21-23]. Given that ERα can be activated by a number of growth factors, such as insulin-like growth factor one and epidermal growth factor, in a ligand-independent manner, estrogen may not be needed during the initial development of the endometrial luteolytic mechanism [24].
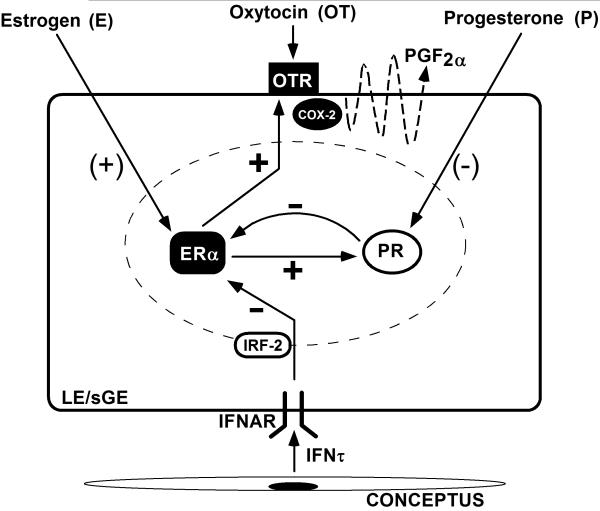
Figure 1.
Schematic illustrating hormonal regulation of the endometrial luteolytic mechanism and antiluteolytic effects of the conceptus on the endometrium in the ovine uterus. During estrus and metestrus, oxytocin receptors (OTR) are present on the uterine lumenal epithelium (LE) and superficial ductal glandular epithelium (sGE), because estrogen (E) levels are high and increase expression of estrogen receptor alpha (ERα) and OTR. The progesterone receptor (PR) is present, but low systemic levels of progesterone result in insufficient numbers of activated PR to suppress ERα and OTR synthesis. During early diestrus, endometrial ERα and estrogen are low, but progesterone levels begin to increase with formation of the CL. Progesterone acts through the PR to suppress ERα and OTR synthesis for 8 to 10 days. Continuous exposure of the endometrium to progesterone eventually down-regulates PR gene expression in the endometrial luminal epithelium (LE) by Days 11 to 12 of the estrous cycle. The loss of PR terminates the progesterone block to ERα and OTR formation. Thus, ERα appears on Days 11 to 12 post-estrus, which is closely followed by OTR on Days 13 and 14. The increase in OTR expression is facilitated by increasing secretion of estrogen by ovarian follicles. In both cyclic and pregnant sheep, oxytocin (OT) is released from the posterior pituitary and corpus luteum beginning on Day 9. In cyclic sheep, OT binds to OTR on the endometrial epithelium and increases release of luteolytic pulses of prostaglandin F2α (PGF2α) to regress the CL through a COX-2 pathway. In pregnant sheep, inteferon tau (IFNτ) is synthesized and secreted by the elongating conceptus beginning on Day 10 of pregnancy. IFNτ binds to Type I IFN receptors (IFNAR) on the endometrial LE and inhibits transcription of the ERα gene through a signaling pathway involving IFN regulatory factor 2 (IRF-2). These actions of IFNτ on the ERα gene prevent OTR formation, thereby maintaining the CL and progesterone production. Legend: E, estrogen; ERα, estrogen receptor alpha; IFNτ, interferon tau; IRF-2, interferon regulatory factor two; OT, oxytocin; OTR, oxytocin receptor; P, progesterone; PGF2α, prostaglandin F2 alpha; PR, progesterone receptor.
Thus, progesterone is paradoxically involved first in suppressing and then inducing development of the endometrial luteolytic mechanism during the estrous cycle. The timing of PR down-regulation by progesterone appears to determine when the luteolytic mechanism develops in the endometrium. This hypothesis is supported by the finding that exogenous progesterone administration during metestrus decreased the interestrus interval in sheep and cattle [25,26]. Further, treatment of cyclic sheep with RU486, a PR antagonist, during the early luteal phase extended the interestrus interval [27]. PR antagonists prevent progesterone down-regulation of PR gene expression, thereby extending the period of PR expression [28].
Pregnancy recognition signaling by interferon tau (IFNτ)
Maternal recognition of pregnancy in ruminants (sheep, cattle, goats) requires that the conceptus elongate from a spherical to a tubular and then filamentous form to produce IFNτ, which is the pregnancy recognition signal that prevents development of the endometrial luteolytic mechanism [5,10,29]. This antiluteolytic effect of IFNτ results in the maintenance of a functional CL and, hence, secretion of progesterone that is essential to maintain a uterine environment that supports events critical to successful development of the conceptus to term.
During maternal recognition of pregnancy, the mononuclear cells of the conceptus trophectoderm synthesize and secrete IFNτ between Days 10 and 21 to 25 with maximal production on Days 14 to 16 [29,30]. In terms of biological activity, a single Day 16 conceptus produces approximately 1 × 108 antiviral units of IFNτ in culture over 24 h [31]. IFNτ appears to be the sole factor produced by the conceptus that prevents development of the endometrial luteolytic mechanism [32]. IFNτ does not act to stabilize PR expression in the endometrial epithelium during pregnancy [13,16,33]. Rather, IFNτ acts in a paracrine fashion on endometrial LE and sGE to suppress transcription of ERα and OTR genes [33,34], thereby abrogating development of the endometrial luteolytic mechanism. Indeed, the increases in ERα and OTR gene expression detected in LE and GE on Days 11 to 17 post-estrus in cyclic sheep do not occur in pregnant sheep [13] or in cyclic sheep infused with IFNτ [35]. By inhibiting increases in OTR expression, IFNτ prevents endometrial production of luteolytic pulses of PGF. However, IFNτ does not inhibit basal production of PGF, which is higher in pregnant than cyclic ewes, and the conceptus and IFNτ do not affect COX-2 expression in the endometrial epithelia of early pregnant sheep [9,36]. Thus, the antiluteolytic actions of IFNτ are to prevent increases in epithelial ERα and OTR gene expression, which are estrogen responsive, by directly inhibiting transcription of the ERα gene and maintaining secretion of progesterone by the CL [34].
IFNτ is a novel member of the Type I IFN family that acts differentially on the endometrial LE, GE and stroma to regulate expression of a number of IFN-stimulated genes (ISGs) that are hypothesized to play roles in endometrial differentiation and conceptus implantation [5,37-39]. The actions of IFNτ to signal pregnancy recognition [40] and induce or increase expression of ISGs, including ISG17 [41] and 2',5'-oligoadenylate synthetase (OAS) [42], is dependent on the effects of progesterone. The Type I IFN receptor subunits, IFNAR1 and IFNAR2, are expressed in all endometrial cell types with highest expression in endometrial LE [43]. However, the majority of ISGs are induced or increased in response to the conceptus or IFNτ only in the endometrial stroma and middle to deep GE of the ovine uterus [37,41,42,44,45]. Interestingly, the induction of many ISGs is also observed in the porcine uterus during early pregnancy, and their expression is limited to the endometrial stroma [46-48]. In the ovine uterus, the lack of ISG induction in the endometrial LE and sGE by IFNτ is apparently due to the expression of IFN regulatory factor two (IRF-2), a potent repressor of gene transcription that is constitutively expressed in the endometrial LE and sGE and increased during early pregnancy [44]. In addition, IRF-2 appears to be involved in IFNτ inhibition of ERα gene transcription in the same endometrial epithelia [34].
The finding that ISGs are induced in the underlying endometrial stroma led to the hypothesis that LE and perhaps GE produce an "interferonomedin" from the basolateral epithelial surface that acts as a paracrine amplifier of IFNτ responses in stroma [10]. A more plausible explanation is that IFNτ produced by the conceptus may be transported across the LE cell layer or move passively into the underlying endometrial stroma. Guillomot and coworkers [49,50] observed that horseradish peroxidase injected into the uterine lumen of pregnant sheep and cattle accumulated in the endometrial stroma beneath the basement membrane of the LE. This transport was mediated via both transepithelial endocytotic activity (vesicles) and passage through intercellular spaces between tight junctions. These phenomena were especially marked when systemic progesterone concentrations were high during late diestrus and when PR is absent from the endometrial LE. The precise nature of the crosstalk between progesterone and IFNτ remains undefined.
Implantation and establishment of pregnancy
Progesterone, the hormone of pregnancy, plays a pivotal and indisputable role in the establishment and maintenance of pregnancy in mammals. In all mammalian uteri, PR is expressed in the endometrial epithelia and stroma during the early luteal phase, allowing direct regulation of a number of genes by progesterone via activation of the PR. However, continuous exposure of the endometrium to progesterone down-regulates PR expression in the endometrial epithelium [16]. Indeed, expression of PR protein is not detectable in endometrial LE and GE in sheep after Days 11 and 13 of pregnancy, respectively [13]. Further, PR expression is only detected in stroma and myometrium throughout most of gestation in the ovine uterus. The paradigm of loss of PR in uterine epithelia immediately prior to implantation is common to sheep [13], cattle [51], pigs [52], western spotted skunks [53], baboons [54], rhesus monkeys [55], humans [55], and mice [56]. Thus, regulation of endometrial epithelial function during the peri-implantation period must be directed by specific factors produced by PR-positive stromal cells in response to progesterone [57]. In sheep, endometrial stromal cells express both fibroblast growth factor 10 (FGF-10) and hepatocyte growth factor (HGF) while endometrial epithelium and trophectoderm express their respective receptors, FGF receptor 2IIIb (FGFR2IIIb) and c-met [58,59]. The tunica intima of uterine blood vessels in sheep also expresses FGF-7, which acts via FGFR2IIIb. Mechanisms regulating these stromal-derived growth factors are not known.
Endogenous Jaagsiekte sheep retroviruses (enJSRVs)
The ovine genome contains 15 to 20 copies of endogenous betaretroviruses that are highly related to two oncogenic exogenous betaretroviruses, JSRV and enzootic nasal tumor virus (ENTV) [60]. Expression of endogenous JSRVs (enJSRVs) in sheep is limited to epithelia of the oviduct, uterus, cervix and vagina [61,62]. The enJSRV RNAs are among the most abundant RNAs in the endometrium, and their expression increases by 15-fold between Days 1 and 13 of the estrous cycle or early pregnancy [62]. Uterine expression of enJSRV RNAs is restricted to the endometrial LE and GE, which suggests physiological roles in regulating conceptus-endometrial interactions, production of IFNτ, and placental differentiation and development [60,63].
The JSRV capsid and envelope proteins are expressed by endometrial LE and GE and detected in binucleate cells of conceptus trophectoderm that forms syncytia with endometrial LE. Indeed, steady-state levels of enJSRV RNAs in LE and GE increase rapidly between Days 1 and 13 in cyclic and pregnant sheep and then decrease to low levels by Day 15 in cyclic sheep and by Day 19 in pregnant sheep. Further, increased expression of enJSRV genes in uterine epithelia is highly correlated with changes in circulating levels of progesterone in peripheral blood and expression of PR in endometrial epithelia. Progesterone, acting via PR, increases transcription of enJSRV genes in vivo and transcriptional activity of several enJSRV long terminal repeats (LTRs) in vitro [62]. Indeed, one or more enJSRV LTR, which contain the retroviral promoter and enhancers, are directly regulated by progesterone [62]. As such, the enJSRVs are among the few known genes in the endometrial epithelium of the ovine uterus directly increased by progesterone via PR.
Extracellular matrix (ECM) and cell adhesion molecules
In both humans and rodents, the expression pattern of the mucin glycoproteins, MUC1 and MUC4, on uterine LE may control accessibility of trophectoderm integrin receptors to their ligands by sterically blocking cell-cell and cell-extracellular matrix (ECM) adhesion and access of conceptus trophectoderm to uterine LE [1,64]. The implantation adhesion cascade in rodents, sheep and pigs is initiated following down-regulation of MUC1, which is coincidental with loss of PR from uterine epithelium [1,65,66]. This pattern of MUC1 expression contrasts with that in rabbits and humans in which there is an overall increase in MUC1 expression during the receptive phase under the influence of progesterone; however, MUC1 is locally reduced at implantation sites, perhaps due to paracrine signals from blastocysts [1,67].
Integrins play a dominant role in interactions with ECM to transduce cellular signals in uterine epithelial cells and conceptus trophectoderm [64]. The endometrium exhibits both constitutive and cycle-dependent expression of integrins and appears to be the only tissue known to exhibit hormone-dependent integrin expression. Three integrins are considered markers of uterine receptivity for implantation in humans, which occurs when the uterus is under the influence of progesterone. The timing of αvβ3 expression correlates with embryo attachment and disappearance of the α4 integrin subunit [68]. The presence of both αvβ3 and α4β1 on the apical surface of uterine LE suggests a role for these integrins in trophectoderm-LE interactions during implantation. In sheep, α(v,4,5) and β(1,3,5) integrin subunit expression occurs in endometrium of both cyclic and pregnant sheep and conceptus trophectoderm [66]. These integrin subunits are detected at the apical surfaces of the LE and GE and on conceptus trophectoderm; expression of these integrins is constitutive and not influenced by pregnancy or presence of the conceptus. In the sheep, receptivity to implantation does not appear to involve changes in temporal or spatial patterns of integrin expression, but may depend on expression of ECM proteins, such as osteopontin (OPN), which are ligands for heterodimers of these integrins [69]. In species such as pigs, mice, sheep and humans, correlative expression patterns between specific integrins and ECM proteins frame the putative window of implantation [1,64,66,70]. In pigs, progesterone increases expression of α4β1 and α5β1 during the peri-implantation period, which may in part define the "implantation window" in this species [64,71].
Uterine gland secretions
In the sheep, continuous exposure of the uterus to progesterone induces expression of proteins in the endometrial glands that are secreted into the uterine lumen. The two best-characterized GE secretory products are the ovine uterine milk proteins (UTMP), also termed ovine uterine serpins, and OPN. UTMPs are members of the serpin family of serine protease inhibitors [72] and serve as excellent markers for endometrial secretory capacity during pregnancy in sheep [73-75]. In pregnant sheep, UTMP mRNA expression is restricted to GE and not in LE or sGE. UTMP mRNA expression is tightly regulated, appearing in GE between Days 15 and 17, and then increasing in abundance during gestation in a manner that parallels fetal growth and development [73,75,76].
OPN is an acidic phophorylated glycoprotein component of the ECM detected in epithelia and in secretions of many tissues, including the uterus [69]. OPN binds to integrin heterodimers (αvβ1, αvβ3, αvβ5, αvβ6, αvβ8, α4β1, α5β1, and α8β1) via its Arg-Gly-Asp (RGD) sequence and to α4β1 and α9β1 by other sequences to promote cell adhesion, spreading and migration [69]. OPN increases in uterine flushings from pregnant sheep during the peri-implantation period (Days 11 to 17) when adherence and attachment of conceptuses to uterine LE occurs [77,78]. Secreted OPN is hypothesized to bind integrin heterodimers expressed by trophectoderm and uterus to: (1) stimulate changes in morphology of conceptus extraembryonic membranes; and (2) induce adhesion between LE and trophectoderm essential for implantation and placentation [69]. Although OPN mRNA increases only in GE of pregnant sheep, OPN protein is localized on the apical aspect of the endometrial LE, GE and conceptus trophectoderm and continues to be present at the uterine-placental interface. Similar to UTMP, the OPN gene is expressed in GE throughout gestation and OPN abundance parallels fetal growth and development [79].
Continuous administration of progesterone to sheep induces UTMP and OPN expression by ovine endometrium [28,73,74]. As revealed by Ing and coworkers [80], treatment of ovariectomized sheep with progesterone for 6 days induced very little UTMP mRNA and protein in the endometrium, whereas treatment with progesterone for 14 or 30 days greatly enhanced UTMP expression. The protracted nature of this progesterone effect is not typical of genes regulated by progesterone through PR in a classic transcriptional manner involving receptor interaction with ligand, homodimerization, and DNA binding and transactivation. Recent studies support the hypothesis that the loss of PR gene expression in GE is required for progesterone induction of UTMP and OPN gene expression [28,74]. Spencer and colleagues [74] found that administration of estrogen with progesterone induced PR expression in endometrial GE and concomitantly ablated effects of progesterone alone to induce UTMP and OPN mRNA expression in GE. Similarly, administration of the PR antagonist ZK136,317 along with progesterone ablated effects of progesterone alone to induce OPN mRNA expression in GE [28]. In that study, the ZK anti-progestin prevented progesterone from down-regulating PR expression. The contention that loss of epithelial PR is required for endometrial GE function during pregnancy is also supported by results from studies of PR gene expression in endometrium from cyclic and pregnant sheep [12,13]. During early pregnancy, PR expression is detectable in LE and GE on Day 11, but PR are undetectable in LE and sGE from Days 13 to 19, and are present only in stromal cells and myometrium after Day 25 of gestation in sheep (Spencer TE and FW Bazer, unpublished results).
Why does progesterone down-regulate expression of the PR gene? Loss of PR by GE appears to be required for GE morphogenesis and differentiated function, as well as to prevent inhibition of these events by progesterone [81,82]. In uterine LE of mice, progesterone inhibits estrogen-induced cyclin D1 and cyclin-dependent kinase 4 (cdk4) nuclear translocation, cyclin E- and cyclin A-cdk2 kinase activation, and cell proliferation [82]. Therefore, liganded PR likely inhibits epithelial morphogenesis due to negative effects on their progression through the cell cycle. It follows that the absence of the PR after Day 15 in GE of sheep uteri is essential for the endometrial glands to undergo a pregnancy-dependent program of hyperplasia from Days 16 to 50 followed by hypertrophy from Days 50 to term [75,83]. Interestingly, the PR gene is also not expressed in the lobuloalveolar epithelium of the mammary gland during lactation [84]. It is reasonable to speculate that the absence of PR is required for secretory epithelia to initiate and maintain expression of genes encoding secretory proteins. Available evidence suggests that disruption of epithelial morphogenesis that involves dysfunction of PR gene expression in the endometrial glands could compromise blastocyst survival and growth during early pregnancy [2].
Maintenance of pregnancy and placental hormone actions on the uterus
In domestic animals, the placenta produces a variety of steroid and protein hormones that act in a paracrine manner on the endometrium to elicit changes in gene expression that support conceptus growth and development. This section of the review describes effects of placental lactogens (PL) and growth hormone (GH) on endometrial support of conceptus growth in sheep.
Uterine gland morphogenesis during pregnancy
In sheep, establishment and maintenance of pregnancy requires integration of endocrine and paracrine signals from the ovary, conceptus, and uterus [5]. Maintenance of pregnancy requires reciprocal communication between the conceptus and endometrium during implantation and synepitheliochorial placentation. In sheep, superficial implantation and placentation begins on Days 15–16, but are not completed until Days 50–60 of pregnancy [83,85]. During this period, the uterus grows and remodels substantially to accommodate rapid conceptus development and growth in the latter two-thirds of pregnancy. In addition to placentomal development in the caruncular areas of the endometrium and changes in uterine vascularity, the intercaruncular endometrial glands grow substantially in length (4-fold) and width (10-fold) and establish additional side-branchings during pregnancy [83]. During gestation, endometrial gland hyperplasia occurs between Days 15 and 50 followed by hypertrophy to increase surface area that allows for maximal production of histotroph after Day 60 [73,75]. These uterine glands synthesize, secrete or transport a variety of enzymes, growth factors, cytokines, lymphokines, hormones, transport proteins and other substances collectively termed histotroph [1,2,86]. Uterine glands undergo a similar morphogenetic pattern of development during pregnancy in other mammals (cow, goat, pig, horse, primates) [2]. Indeed, secretions from the endometrial epithelia influence conceptus survival, growth and development in all mammals [2,87,88].
Hormonal servomechanism regulating uterine gland morphogenesis and differentiated function
In the rabbit and pig, interactions between lactogenic hormones and ovarian steroids have been proposed to constitute a "servomechanism" which regulates endometrial function [89,90]. Interactions between prolactin (PRL) and progesterone increase endometrial proliferation and uteroglobin secretion in long-term ovariectomized rabbits by increasing the concentration of endometrial PR and uterine responsiveness to progesterone [89,91]. This pathway does not appear to be present in the ovine uterus, because neither placental lactogen (PL) nor growth hormone (GH) affect endometrial PR or ERα gene expression [74].
The servomechanism proposed to regulate endometrial gland proliferation and function during pregnancy in sheep is illustrated in Figure 2. The pregnant ovine uterus is exposed sequentially to estrogen, progesterone, IFNτ, PL, and placental GH. These hormones appear to regulate endometrial gland morphogenesis and differentiated secretory function in the ovine uterus [74,92]. The placentae of a number of species, including rodents, humans, nonhuman primates and sheep, secrete hormones structurally related to pituitary GH and PRL that are termed PLs [93,94]. Ovine PL is produced by binucleate cells of the conceptus trophectoderm beginning on Day 16 of pregnancy, which is coincident with the initiation of expression of UTMP and OPN expression by GE [75,77,78]. In maternal serum, PL can be detected as early as Day 50 and peaks between Days 120 to 130 of gestation [94]. A homodimer of the PRL receptor (PRLR) as well as a heterodimer of PRLR and GH receptor (GHR) transduces signals by ovine PL [93]. In the ovine uterus, PL binding sites are specific to GE expressing PRLR [92]. Temporal changes in conceptus production of PL are correlated with endometrial gland morphogenesis and increased production of UTMP and OPN by the GE during pregnancy. The ovine placenta also expresses GH between Days 35 and 70 of gestation [95], which is correlated with onset of GE hypertrophy and maximal increases in UTMP and OPN gene expression by GE. These results suggest that members of the lactogenic and somatogenic hormone family play key roles in stimulating endometrial gland morphogenesis and differentiated function during pregnancy to facilitate conceptus growth and development.
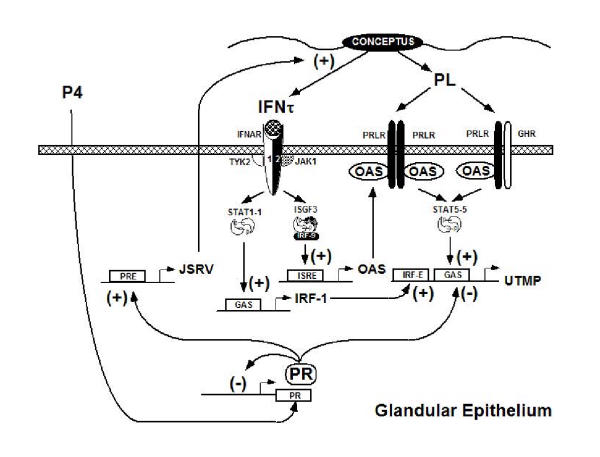
Figure 2.
Schematic illustrating the current working hypothesis on the hormonal servomechanism regulating uterine gland morphogenesis and function in the ovine endometrium during pregnancy. Interferon tau (IFNτ) is produced by the conceptus between Days 11 and 21–25 of pregnancy with maximal production on Days 15–16. High levels of endogenous Jaagsiekte sheep retroviruses (enJSRVs) are expressed in the PR-positive endometrial LE and GE in response to increasing progesterone to stimulate trophoblast proliferation and production of IFNτ. Continuous exposure of the endometrium to progesterone for 8 to 10 days negatively autoregulates PR expression, so that LE and GE are PR-negative by Days 11 and 13, respectively. IFNτ activates the JAK-STAT pathway in the endometrial glands which stimulates formation of STAT1 homodimers (or GAF) as well as the transcription factor IFN stimulated gene factor 3 (ISGF3; heterotrimer of STAT1, STAT2 and IRF-9). STAT1 homodimers or GAF transactivate a GAS element in the IRF-1 gene. IRF-1 then binds to IRF-Es and transactivates the UTMP promoter. ISGF3 transactivates ISREs present in the 2',5' oligoadenylate synthetase (OAS) gene. The 40/46-kDa form of OAS interacts with the intracellular domain of the prolactin receptor (PRLR), which mediates the actions of ovine PL. Specifically, OAS prevents PRLR signaling to STAT1 and promotes signaling through STAT5. Ovine PL is produced by the conceptus beginning on Days 16–17 of pregnancy, which is concomitant with the formation of binucleate cells in the trophectoderm. The actions of PL are mediated by PRLR homodimers or perhaps heterodimers of PRLR and growth hormone receptor (GHR) that stimulate formation of STAT5 homodimers. STAT5 dimers bind and transactivate the GAS element in the UTMP promoter. The induction of UTMP gene expression in the GE by IFNτ-stimulated IRF-1 is maintained by the actions of PL through STAT5.
A number of studies support the idea that sequential exposure of the pregnant ovine endometrium to estrogen, progesterone, IFNτ, PL and placental GH constitutes a "servomechanism", which activates and maintains endometrial remodeling, secretory function and uterine growth during gestation [5]. Intrauterine infusions of recombinant ovine PL or GH increased UTMP and OPN expression by uterine GE of progesterone-treated sheep, but only when the sheep were infused sequentially with IFNτ between Days 11 and 21, and then either PL or GH from Days 16 to 29 after onset of estrus [74]. The mechanism whereby effects of IFNτ permit GE to become responsive to PL and GH is not entirely known, but it may involve IFNτ induction or increases in genes involved in signal transduction including signal transducers and activators of transcription one (STAT1), STAT2, IRF-1, IRF-9, and 40/42-kDa 2',5'-OAS [42,44]. The increase in UTMP expression by endometrial GE was partly attributed to effects of PL and GH to increase the number of endometrial glands, because intrauterine infusion of PL and GH into sheep, treated with progesterone and IFNτ, was found to increase endometrial gland size; an effect not observed in sheep infused with either PL or GH alone [92]. The ability of PL and GH to elicit similar effects on endometrial glands is not surprising since they are members of a unique hormone family based on genetic, structural, binding, receptor signal transduction and function studies [93]. In total, these studies suggest that a developmentally programmed sequence of events, mediated by specific paracrine-acting factors at the conceptus-endometrial interface, stimulate both intercaruncular endometrial remodeling and differentiated function of GE to increase production of histotroph necessary for fetal-placental growth during gestation.
Pregnancy recognition, establishment and maintenance in pigs (Sus scrofa)
Luteolytic mechanism
The pig is a spontaneously ovulating mammal with an estrous cycle of 21 days. Luteolysis is uterine-dependent and occurs during late diestrus following stimulation of the uterine endometrium by progesterone for 10 to 12 days. The uterine luteolysin in pigs is PGF, although endocrine requirements for luteolysis in pigs have not been clearly delineated [96,97]. Hysterectomy extends the estrous cycle and CL function by removing the source of PGF. The CL of pigs are refractory to luteolytic effects of PGF until Days 12–13 of the estrous cycle [98], because of low numbers of luteal receptors for PGF [99]. Luteolysis occurs in response to pulsatile release of PGF into the uterine venous drainage on Days 15 and 16 of the estrous cycle [100].
In ruminants, oxytocin from the CL binds uterine OTR to elicit pulses of PGF, but this mechanism is not well-defined for pigs [97]. The CL of pigs contain very low levels of OT and vasopressin and undetectable levels of OT mRNA, and the role of these neuropeptides of ovarian and/or posterior pituitary origin in luteolysis has not been established [96]. Recent results indicate that the endometrium is a source of OT that may be involved in luteolysis, but the role of endometrial OT remains to be defined. Exogenous OT decreases interestrous interval when administered to cyclic gilts between Days 10 and 16 post-estrus [101]. The endometrium of pigs contains receptors for OT and lysine vasopressin, but only responds to OT with increased secretion of PGF [102].
Concentrations of oxytocin increase in the peripheral circulation during luteolysis in the pig [103]. In addition, oxytocin-induced increases in circulating concentrations of PGF metabolite (PGFM) are reduced in pregnant gilts compared to cyclic gilts or in gilts made pseudopregnant by injection of exogenous estrogen from Days 11–15 post-estrus. Prostaglandins, however, are thought to be critical for establishment of pregnancy in the pig, because inhibition of prostaglandin synthesis results in pregnancy failure and basal peripheral concentrations of PGFM are elevated in pregnant gilts on Day 12 [104].
Endocrine-exocrine theory of pregnancy recognition in pigs
The endocrine-exocrine theory of maternal recognition of pregnancy in pigs was first described by Bazer and Thatcher [105]. The major assumptions are that the uterine endometrium secretes PGF and that the conceptuses secrete estrogens which are antiluteolytic (see Figure 3). In cyclic gilts, PGF is secreted in an endocrine direction, toward the uterine vasculature, and transported to the CL to exert its luteolytic effect. However, in pregnant pigs, the direction of secretion of PGF is exocrine, into the uterine lumen, where it is sequestered away from the CL to exert its biological effects in utero and/or be metabolized to prevent luteolysis. Mean concentrations, peak frequency and peak amplitude of PGF in utero-ovarian vein plasma are lower in pregnant and estrogen-induced pseudopregnant gilts than in cyclic gilts [100,106]. On the other hand, uterine flushings from pseudopregnant and pregnant gilts have significantly higher amounts of PGF than those from cyclic gilts [107]. These results indicate that PGF is released primarily into the uterine venous drainage (endocrine) in cyclic gilts, but into the uterine lumen (exocrine) in pregnant and pseudopregnant pigs and that secretion of PGF is not inhibited during either pregnancy or pseudopregnancy.
Figure 3.
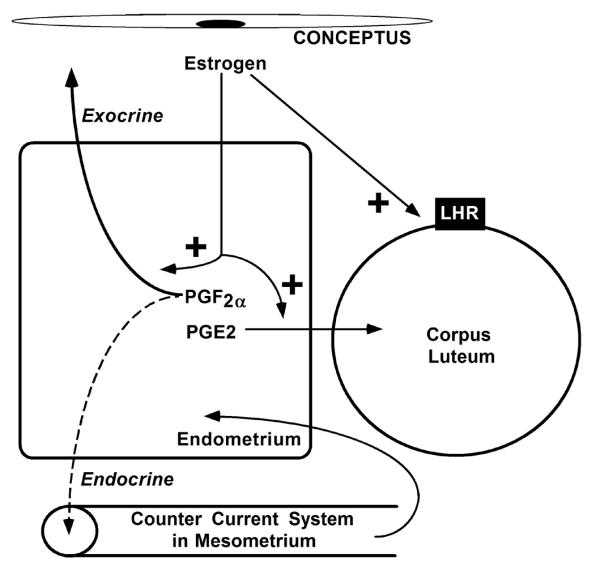
Schematic illustrating the inhibition of luteolysis in pigs. Estrogens produced by the conceptus alters the direction of prostaglandin F2α (PGF2α) secretion from an endocrine to an exocrine direction (uterine lumen). Additionally, PGF2α of uterine origin is taken up by the mesometrium and transferred into the uterus in arterial blood by a counter current system operating in the broad ligament of the uterus. Estrogen also increases prostaglandin E2 (PGE2) secretion by the pig uterus, which is hypothesized to protect the corpus luteum from the luteolytic actions of PGF2α. Estrogen also maintains luteinizing hormone receptors (LHR) in the CL and has a direct luteotropic effect. This figure was modified from Ziecik and coworkers [97].
The transition from endocrine to exocrine secretion occurs between Days 10 and 12 of pregnancy, which is temporally associated with initiation of estrogen secretion by elongating pig conceptuses [108]. Estrogens, either secreted by the conceptus or injected, induce a transient release of calcium into the uterine lumen within 12 h. Re-uptake of that calcium by endometrial and/or conceptus tissues occurs about 12 h after concentrations of calcium in uterine secretions reach maximum values. The switch in direction of endometrial secretion of PGF from an endocrine to an exocrine orientation is closely associated with this period of release and re-uptake of calcium by the endometrium in pregnant and pseudopregnant gilts. When endometrium from Day 14 cyclic gilts was treated with the calcium ionophore A23187 (to induce calcium flux across epithelial membranes), estradiol or prolactin secretion of PGF changed from an endocrine toward an exocrine direction [109]. These results suggest that induction of calcium cycling across endometrial epithelium is associated with redirection of PGF secretion.
Uterine PGF is luteolytic and estrogens produced by conceptuses between Days 11 and 12 of gestation provide the initial signal for maternal recognition of pregnancy in swine [110]. A second period of estrogen production occurs between Days 15 and 25–30 of pregnancy. Injection of exogenous estrogen on Days 11 through 15 of the estrous cycle results in CL maintenance for a period equivalent to or slightly longer than pregnancy (about 120 days later). This condition is referred to as pseudopregnancy [111]. Whereas a single injection of estradiol on either Day 9.5, 11, 12.5, 14, 15.5 or 14–16 results in interestrous intervals of about 30 days, estradiol must be administered to gilts on Day 11 and Days 14–16 or daily from Days 11 to 15 to obtain interestrous intervals of greater than 60 days. This suggests that two phases of estradiol, similar to that produced by conceptuses on Days 11–13 and Days 15 to 25–30, are necessary for prolonged secretion of PGF into the uterine lumen. PGF from the uterus is taken up by the mesometrium and transferred to the ovary in arterial blood by a countercurrent system that exists in the broad ligament of the uterus [112]. Estrogen also changes the ratio of PGE:PGF [113-115] as well as maintains LH receptor levels in both CL and uterus [116,117]. PGE2 may protect the CL from the luteolytic effects of PGF. Indeed, estradiol has been suggested to have a direct luteotropic effect in the pig [118].
Estradiol may induce receptors for maternal hormones, e.g., prolactin, or conceptus secretory proteins, which influence exocrine secretion of PGF [90,119]. The first estrogen signal may induce those receptors and the second estrogen signal may be required to replenish those receptors. Administration of estradiol on Day 9 advances the uterine secretory response in pregnant gilts which leads to conceptus death by Day 16 [120,121]. The "induced" conceptus death results from ansynchrony between development of the conceptus and uterine environment [111]. Available results indicate that estrogens of blastocyst origin are essential for maternal recognition of pregnancy in pigs and that secretory proteins from the conceptus, including interferons, play other roles during early pregnancy in pigs, including cellular changes that lead to increased polarity of endometrial epithelial cells for exocrine secretion of components of histotroph, including PGF [47,96,122].
Implantation and establishment of pregnancy
Placental estrogens and endometrial factors
In the pig, an increase in selected histotroph components occurs in the uterine lumen immediately following the release of estrogens from the conceptus on Day 11 of pregnancy [123]. Placental estrogens also act on the endometrial epithelia in a paracrine manner to increase expression of specific growth factors, including insulin-like growth factor one (IGF-I) and fibroblast growth factor seven (FGF-7; also termed keratinocyte growth factor or KGF), and perhaps OPN, that then act on the trophectoderm to stimulate cell proliferation as well as conceptus attachment and development.
IGF-I is a pleiotropic growth factor required for postnatal uterine growth and conceptus growth and development in the mouse [124]. In the porcine uterus, IGF-I is primarily expressed by endometrial GE of both cyclic and pregnant pigs [125]. Endometrial IGF-I gene expression increases during early pregnancy and peaks on Days 12–13, which is coincidental with production of estrogens by the elongating conceptus [126,127]. Treatment of either ovariectomized or cyclic gilts with estrogen increases IGF-I expression in the uterus [126]. Type I IGF receptors were detected in the endometrium as well as in the conceptus, suggesting paracrine and autocrine modes of action of IGF-I in the uterine microenvironment [124].
FGF-7 is an established paracrine mediator of hormone-regulated epithelial growth and differentiation [128]. In all organs studied before the pig uterus, FGF-7 was uniquely expressed in cells of mesenchymal origin. Intriguingly, expression of FGF-7 in the porcine uterus is particularly abundant between Days 12 and 15 of the estrous cycle and pregnancy. FGF-7 is expressed exclusively in the uterine LE until Day 30 of pregnancy when expression shifts to the GE and is maintained in the GE throughout gestation [129]. Endometrial FGF-7 mRNA levels were highest on Day 12 in pregnant gilts and Day 15 in cyclic gilts, and greater on Day 12 of pregnancy than on Day 12 of the estrous cycle. FGF-7 protein was detected in the uterine flushes of both Day 12 cyclic and pregnant gilts. FGFR2IIIb, the receptor for FGF-7, is expressed in both endometrial epithelia and conceptus trophectoderm. Treatment of endometrial explants from Day 9 cyclic gilts with estradiol-17β increased FGF-7 expression [130]. Further, treatment of porcine trophectoderm cells with recombinant rat FGF-7 increased their proliferation, levels of phosphorylated FGFR2IIIb, activated the mitogen-activated protein kinase (MAPK or ERK1/2) cascade, and increased expression of urokinase-type plasminogen activator, a marker for trophectoderm cell differentiation [130]. Collectively, these results indicate that estrogen, the pregnancy recognition signal from the pig conceptus, increases uterine epithelial FGF-7 expression, and, in turn, FGF-7 stimulates the proliferation and differentiation of conceptus trophectoderm in pigs, which posess a true epitheliochorial placenta [131].
Pigs have a novel expression pattern for OPN in that it is expressed directly in the endometrial LE, supporting the idea that secretion of OPN across the entire diffuse epitheliochorial interface is vital in this species [132]. Expression of OPN mRNA begins in discrete regions of the LE associated with the presence of the conceptus prior to attachment, and expands to the entire LE by Day 20 when firm adhesion of conceptus to uterus has occurred. Glandular expression of OPN mRNA is delayed until between Days 30 and 35 of pregnancy, and GE expression results in 20-fold increases of OPN between Days 25 and 85. Therefore endometrial OPN expression has a bicameral and bimodal nature in pigs. It is hypothesized that factors released from the elongating conceptus exert paracrine effects on the progestational uterus to induce expression of OPN in LE. OPN released from LE then binds integrin receptors on trophectoderm and LE to mediate elongation and attachment. Later in pregnancy, progesterone from the CL induces expression of OPN in GE resulting in secretion and transport of OPN to the embryo as histotrophic support for growth and development throughout gestation.
Trophoblastic interferons
Between Days 12 and 20 of pregnancy in the pig, two unique IFNs were discovered in uterine flushings and supernatants of conceptus-conditioned medium. The predominant species was found to be Type II IFN (IFNγ) [133], while the other species was shown to be a novel Type I IFN (IFNδ), previously known as sp-1 IFN [134,135]. IFNγ is secreted by the pig embryo in substantial amounts (up to 250 μg per uterine horn) with peak of synthesis being observed on Days 15–16 of pregnancy. The secretion of large amounts of IFNγ by non-lymphoid cells makes the pig conceptus unique among mammals.
IFNs from the porcine trophoblast do not appear to affect the trophoblast in an autocrine manner, and they are not detectable in the uterine venous drainage [135,136]. Intrauterine infusion of pCSP on Days 12 to 15 of the estrous cycle had no effect on interestrous interval or temporal changes in concentrations of progesterone in plasma [137]. Similarly, available evidence does not support a role for porcine trophoblast IFNγ to maintain function of the CL [137]. However, recent evidence in the pig uterus supports a role for porcine trophoblast IFNs in the establishment of pregnancy and implantation [46,48].
Blastocyst attachment to the LE, one of the most important functions of the uterine endometrium, can only be achieved by the transitional labilization and remodeling of uterine epithelium polarity, after the synchronous exchange of signals between the conceptus and endometrial cells [138]. Cencic and coworkers [47] hypothesized that trophoblast IFNs in the pig may stimulate the remodeling and/or depolarization of the uterine endometrial epithelium, as a prerequisite for implantation and establishment of a functional placenta. On Day 15 of pregnancy, immunoreactive IFNγ protein was detected in cell clusters unevenly localized along the endometrial LE of the pig uterus, with the underlying stroma, blood vessels and glandular cells mainly negative. Staining in the LE was only visible in areas adjacent to the trophoblast, but was occasionally absent in areas of close apposition between the trophoblast and LE. In the endometrial epithelium of the pregnant Day 15 porcine uterus, de novo appearance of zona occludens one (ZO-1), a marker of epithelial tight junctions, was observed at the basal side of the LE, suggesting significant changes to the endometrial polarity in response to conceptus interferons. IFNγ is known to be a specific and potent inducer of MHC class II molecules [139]. In the stroma of the Day 15 cyclic uterine endometrium, MHC class II molecules were not detected. In contrast, an intense immunoreactivity of MHC class II molecules was observed within the endometrial stroma of pregnant uterus. MHC class II molecules were distributed within the vascular elements beneath the LE of pregnant uterus and within blood vessels further inside the stroma. The LE of the pregnant porcine uterus was completely negative for MHC II molecules, which seems to confirm that these cells are not receptive to IFNγ. However, these observations do not exclude the possibility that they may respond to IFNγ through altered expression of other genes. In addition to this study, other recent studies indicate that a number of IFN stimulated genes, including Mx, ISG15/17, IRF-1, STAT1, and STAT2, are increased specifically in the endometrial stroma during early pregnancy (Days 14–18) in the pig [48] (Johnson GA and MM Joyce, unpublished results). Indeed, induction or increases in ISGs during early pregnancy are observed in the endometrium of many mammals (sheep, cow, pig, mouse, rat, primate, human), suggesting that they are universally involved in pregnancy establishment and perhaps maintenance in mammals. Although little is known about the functions of stromal leukocytes during early pregnancy in the sheep and pig, clear evidence exists for the presence of lymphocytes, granulocytes, and macrophages are present in the endometrial stroma in these species as in rodent species and in human. Indeed, conceptus production of IFNs in both the sheep and pig may regulate endometrial cell function during early pregnancy via stromal immune cells.
Conclusions
Progesterone and PR are critical to changes in uterine biology of the estrous cycle and pregnancy in all mammals. In the sexually mature female, progesterone and PR effects during both the estrous cycle and pregnancy must be understood in the context of temporal and spatial changes in the pattern of PR expression. It is clear that uterine stromal and myometrial cells are always PR-positive and may respond to progesterone by producing paracrine factors that regulate proliferation and/or differentiated functions of endometrial epithelia during pregnancy. This poses a number of interesting questions. Why are PR down-regulated in the endometrial epithelia, but not stromal and myometrial cells? What is the molecular mechanism by which progesterone down-regulates expression of the PR gene in epithelia, but not stromal cells of the endometrium? What are the mechanisms by which stromal cells regulate epithelial cell functions in reproductive tissues? How do cell signaling pathways activated by growth factors, IFNs and somatolactogenic hormones converge to establish the servomechanism that regulates uterine functions critical to maintenance of pregnancy in ruminants? Are there other ERVs, like enJSRV, that are regulated by progesterone and PR in the uterus that affect uterine biology and/or conceptus development? We have learned much about effects of progesterone and expression of PR, but there are many unresolved questions about the extent and magnitude of the effects of this key hormone of the estrous/menstrual cycle and pregnancy and its receptor. There exists a clear need to understand convergence of interactions between cell signaling events mediated by progesterone acting via PR, progestamedin growth factors acting via their respective receptors, lactogenic hormones acting via PRL-R and GH-R, and IFNτ acting through the Type I IFN receptor that regulate proliferation and differentiated functions of uterine endometrial cells throughout gestation. Strategic manipulation of the aformentioned physiological mechanisms may offer insights into therapeutic strategies to improve uterine capacity, conceptus survival and reproductive health in humans and domestic animals.
Acknowledgments
Acknowledgements
Support for this research was provided by NIH Grant HD32534 and US-Israel BARD Grant US-3199-01C.
Contributor Information
Thomas E Spencer, Email: tspencer@tamu.edu.
Fuller W Bazer, Email: fbazer@cvm.tamu.edu.
References
- Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo implantation. Dev Biol. 2000;223:217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. Developmental biology of uterine glands. Biol Reprod. 2001;65:1311–1323. doi: 10.1095/biolreprod65.5.1311. [DOI] [PubMed] [Google Scholar]
- Paria BC, Lim H, Das SK, Reese J, Dey SK. Molecular signaling in uterine receptivity for implantation. Semin Cell Dev Biol. 2000;11:67–76. doi: 10.1006/scdb.2000.0153. [DOI] [PubMed] [Google Scholar]
- McCracken JA, Custer EE, Lamsa JC. Luteolysis: a neuroendocrine-mediated event. Physiological Reviews. 1999;79:263–323. doi: 10.1152/physrev.1999.79.2.263. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW. Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front Biosci. 2002;7:d1879–98. doi: 10.2741/spencer. [DOI] [PubMed] [Google Scholar]
- Wathes DC, Lamming GE. The oxytocin receptor, luteolysis and the maintenance of pregnancy. J Reprod Fertil Suppl. 1995;49:53–67. [PubMed] [Google Scholar]
- Gray C, Bartol FF, Taylor KM, Wiley AA, Ramsey WS, Ott TL, Bazer FW, Spencer TE. Ovine uterine gland knock-out model: effects of gland ablation on the estrous cycle. Biol Reprod. 2000;62:448–456. doi: 10.1095/biolreprod62.2.448. [DOI] [PubMed] [Google Scholar]
- Charpigny G, Reinaud P, Tamby JP, Creminon C, Guillomot M. Cyclooxygenase-2 unlike cyclooxygenase-1 is highly expressed in ovine embryos during the implantation period. Biol Reprod. 1997;57:1032–1040. doi: 10.1095/biolreprod57.5.1032. [DOI] [PubMed] [Google Scholar]
- Kim S, Choi Y, Spencer TE, Bazer FW. Effects of the estrous cycle, pregnancy and interferon tau on expression of cyclooxygenase two (COX-2) in ovine endometrium. Reprod Biol Endocrinol. 2003;1:58. doi: 10.1186/1477-7827-1-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TE, Ott TL, Bazer FW. tau-Interferon: pregnancy recognition signal in ruminants. Proc Soc Exp Biol Med. 1996;213:215–229. doi: 10.3181/00379727-213-44053. [DOI] [PubMed] [Google Scholar]
- McCracken J, Schramm W, Okulicz WC. Hormone Receptor Control of Pulsatile Secretion of PGF-2Alpha from the ovine Uterus During Luteolysis and its Abrogation in Early Pregnancy. Anim Reprod Sci. 1984;7:31–55. [Google Scholar]
- Wathes DC, Hamon M. Localization of oestradiol, progesterone and oxytocin receptors in the uterus during the oestrous cycle and early pregnancy of the ewe. J Endocrinol. 1993;138:479–492. doi: 10.1677/joe.0.1380479. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW. Temporal and spatial alterations in uterine estrogen receptor and progesterone receptor gene expression during the estrous cycle and early pregnancy in the ewe. Biol Reprod. 1995;53:1527–1543. doi: 10.1095/biolreprod53.6.1527. [DOI] [PubMed] [Google Scholar]
- Boshier DP, Holloway H. Effects of ovarian steroid hormones on histochemically demonstrable lipids in the rat uterine epithelium. J Endocrinol. 1973;56:59–67. doi: 10.1677/joe.0.0560059. [DOI] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM. Cloning, novel promoter sequence, and estrogen regulation of a rat oxytocin receptor gene. Endocrinology. 1997;138:1151–1158. doi: 10.1210/endo.138.3.4998. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Becker WC, George P, Mirando MA, Ogle TF, Bazer FW. Ovine interferon-tau regulates expression of endometrial receptors for estrogen and oxytocin but not progesterone. Biol Reprod. 1995;53:732–745. doi: 10.1095/biolreprod53.3.732. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Becker WC, George P, Mirando MA, Ogle TF, Bazer FW. Ovine interferon-tau inhibits estrogen receptor up-regulation and estrogen-induced luteolysis in cyclic ewes. Endocrinology. 1995;136:4932–4944. doi: 10.1210/endo.136.11.7588227. [DOI] [PubMed] [Google Scholar]
- Hixon JE, Flint AP. Effects of a luteolytic dose of oestradiol benzoate on uterine oxytocin receptor concentrations, phosphoinositide turnover and prostaglandin F- 2 alpha secretion in sheep. J Reprod Fertil. 1987;79:457–467. doi: 10.1530/jrf.0.0790457. [DOI] [PubMed] [Google Scholar]
- Read LD, Snider CE, Miller JS, Greene GL, Katzenellenbogen BS. Ligand-modulated regulation of progesterone receptor messenger ribonucleic acid and protein in human breast cancer cell lines. Molecular Endocrinology. 1988;2:263–271. doi: 10.1210/mend-2-3-263. [DOI] [PubMed] [Google Scholar]
- Alexander IE, Clarke CL, Shine J, Sutherland RL. Progestin inhibition of progesterone receptor gene expression in human breast cancer cells. Mol Endocrinol. 1989;3:1377–1386. doi: 10.1210/mend-3-9-1377. [DOI] [PubMed] [Google Scholar]
- Vallet JL, Lamming GE, Batten M. Control of endometrial oxytocin receptor and uterine response to oxytocin by progesterone and oestradiol in the ewe. J Reprod Fertil. 1990;90:625–634. doi: 10.1530/jrf.0.0900625. [DOI] [PubMed] [Google Scholar]
- Lau TM, Gow CB, Fairclough RJ. Increases in the oxytocin-induced prostaglandin F2 alpha response and reduction in the concentrations of endometrial oxytocin receptors in ewes in response to progesterone. J Reprod Fertil. 1992;95:11–18. doi: 10.1530/jrf.0.0950011. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Silvia WJ. Effects of progesterone and estradiol-17 beta on uterine secretion of prostaglandin F2 alpha in response to oxytocin in ovariectomized ewes. Biol Reprod. 1988;38:804–811. doi: 10.1095/biolreprod38.4.804. [DOI] [PubMed] [Google Scholar]
- Coleman KM, Smith CL. Intracellular signaling pathways: nongenomic actions of estrogens and ligand-independent activation of estrogen receptors. Front Biosci. 2001;6:D1379–91. doi: 10.2741/coleman. [DOI] [PubMed] [Google Scholar]
- Woody CO, First NL, Pope AL. Effect of exogenous progesterone on estrous cycle length. J Anim Sci. 1967;26:139–141. doi: 10.2527/jas1967.261139x. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Geisert RD, Zavy MT, Gries LK, Wettemann RP, Buchanan DS. Effect of exogenous progesterone on prostaglandin F2 alpha release and the interestrous interval in the bovine. Prostaglandins. 1988;36:85–96. doi: 10.1016/0090-6980(88)90104-9. [DOI] [PubMed] [Google Scholar]
- Morgan GL, Geisert RD, McCann JP, Bazer FW, Ott TL, Mirando MA, Stewart M. Failure of luteolysis and extension of the interoestrous interval in sheep treated with the progesterone antagonist mifepristone (RU 486) J Reprod Fertil. 1993;98:451–457. doi: 10.1530/jrf.0.0980451. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Spencer TE, Burghardt RC, Taylor KM, Gray CA, Bazer FW. Progesterone modulation of osteopontin gene expression in the ovine uterus. Biol Reprod. 2000;62:1315–1321. doi: 10.1095/biolreprod62.5.1315. [DOI] [PubMed] [Google Scholar]
- Roberts RM, Ealy AD, Alexenko AP, Han CS, Ezashi T. Trophoblast interferons. Placenta. 1999;20:259–264. doi: 10.1053/plac.1998.0381. [DOI] [PubMed] [Google Scholar]
- Bazer FW. Mediators of maternal recognition of pregnancy in mammals. Proc Soc Exp Biol Med. 1992;199:373–384. doi: 10.3181/00379727-199-43371a. [DOI] [PubMed] [Google Scholar]
- Ashworth CJ, Bazer FW. Changes in ovine conceptus and endometrial function following asynchronous embryo transfer or administration of progesterone. Biol Reprod. 1989;40:425–433. doi: 10.1095/biolreprod40.2.425. [DOI] [PubMed] [Google Scholar]
- Vallet JL, Bazer FW, Fliss MF, Thatcher WW. Effect of ovine conceptus secretory proteins and purified ovine trophoblast protein-1 on interoestrous interval and plasma concentrations of prostaglandins F-2 alpha and E and of 13,14-dihydro- 15-keto prostaglandin F-2 alpha in cyclic ewes. J Reprod Fertil. 1988;84:493–504. doi: 10.1530/jrf.0.0840493. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW. Ovine interferon tau suppresses transcription of the estrogen receptor and oxytocin receptor genes in the ovine endometrium. Endocrinology. 1996;137:1144–1147. doi: 10.1210/endo.137.3.8603586. [DOI] [PubMed] [Google Scholar]
- Fleming JA, Choi Y, Johnson GA, Spencer TE, Bazer FW. Cloning of the ovine estrogen receptor-alpha promoter and functional regulation by ovine interferon-tau. Endocrinology. 2001;142:2879–2887. doi: 10.1210/endo.142.7.8245. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Ing NH, Ott TL, Mayes JS, Becker WC, Watson GH, Mirando MA, Brazer FW. Intrauterine injection of ovine interferon-tau alters oestrogen receptor and oxytocin receptor expression in the endometrium of cyclic ewes. J Mol Endocrinol. 1995;15:203–220. doi: 10.1677/jme.0.0150203. [DOI] [PubMed] [Google Scholar]
- Charpigny G, Reinaud P, Tamby JP, Creminon C, Martal J, Maclouf J, Guillomot M. Expression of cyclooxygenase-1 and -2 in ovine endometrium during the estrous cycle and early pregnancy. Endocrinology. 1997;138:2163–2171. doi: 10.1210/endo.138.5.5148. [DOI] [PubMed] [Google Scholar]
- Kim S, Choi Y, Bazer FW, Spencer TE. Identification of genes in the ovine endometrium regulated by interferon tau independent of signal transducer and activator of transcription 1. Endocrinology. 2003;144:5203–5214. doi: 10.1210/en.2003-0665. [DOI] [PubMed] [Google Scholar]
- Kerszberg M, Wolpert L. Mechanisms for positional signalling by morphogen transport: a theoretical study. J Theor Biol. 1998;191:103–114. doi: 10.1006/jtbi.1997.0575. [DOI] [PubMed] [Google Scholar]
- Hansen TR, Austin KJ, Perry DJ, Pru JK, Teixeira MG, Johnson GA. Mechanism of action of interferon-tau in the uterus during early pregnancy. J Reprod Fertil. 1999;54:329–339. [PubMed] [Google Scholar]
- Ott TL, Mirando MA, Davis MA, Bazer FW. Effects of ovine conceptus secretory proteins and progesterone on oxytocin-stimulated endometrial production of prostaglandin and turnover of inositol phosphate in ovariectomized ewes. J Reprod Fertil. 1992;95:19–29. doi: 10.1530/jrf.0.0950019. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Spencer TE, Burghardt RC, Joyce MM, Bazer FW. Interferon-tau and progesterone regulate ubiquitin cross-reactive protein expression in the ovine uterus. Biol Reprod. 2000;62:622–627. doi: 10.1095/biolreprod62.3.622. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Stewart MD, Gray CA, Choi Y, Burghardt RC, Yu-Lee LY, Bazer FW, Spencer TE. Effects of the estrous cycle, pregnancy, and interferon tau on 2',5'- oligoadenylate synthetase expression in the ovine uterus. Biol Reprod. 2001;64:1392–1399. doi: 10.1095/biolreprod64.5.1392. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS, Han CS, Alexenko AP, Spencer TE, Roberts RM. Expression of interferon receptor subunits, IFNAR1 and IFNAR2, in the ovine uterus. Biology of Reproduction. 2002;67:847–853. doi: 10.1095/biolreprod.102.004267. [DOI] [PubMed] [Google Scholar]
- Choi Y, Johnson GA, Burghardt RC, Berghman LR, Joyce MM, Taylor KM, Stewart MD, Bazer FW, Spencer TE. Interferon regulatory factor-two restricts expression of interferon- stimulated genes to the endometrial stroma and glandular epithelium of the ovine uterus. Biol Reprod. 2001;65:1038–1049. doi: 10.1095/biolreprod65.4.1038. [DOI] [PubMed] [Google Scholar]
- Choi Y, Johnson GA, spencer TE, Bazer FW. Pregnancy and interferon tau regulate MHC class I and beta-2-microglobulin expression in the ovine uterus. Biol Reprod. 2003;68:1703–1710. doi: 10.1095/biolreprod.102.012708. [DOI] [PubMed] [Google Scholar]
- Cencic A, La Bonnardiere C. Trophoblastic interferon-gamma: current knowledge and possible role(s) in early pig pregnancy. Veterinary Research. 2002;33:139–157. doi: 10.1051/vetres:2002003. [DOI] [PubMed] [Google Scholar]
- Cencic A, Guillomot M, Koren S, La Bonnardiere C. Trophoblastic interferons: do they modulate uterine cellular markers at the time of conceptus attachment in the pig? Placenta. 2003;24:862–869. doi: 10.1016/s0143-4004(03)00135-8. [DOI] [PubMed] [Google Scholar]
- Hicks BA, Etter SJ, Carnahan KG, Joyce MM, Assiri AA, Carling SJ, Kodali K, Johnson GA, Hansen TR, Mirando MA, Woods GL, Vanderwall DK, Ott TL. Expression of the uterine Mx protein in cyclic and pregnant cows, gilts, and mares. J Anim Sci. 2003;81:1552–1561. doi: 10.2527/2003.8161552x. [DOI] [PubMed] [Google Scholar]
- Guillomot M, Betteridge KJ, Harvey D, Goff AK. Endocytotic activity in the endometrium during conceptus attachment in the cow. J Reprod Fertil. 1986;78:27–36. doi: 10.1530/jrf.0.0780027. [DOI] [PubMed] [Google Scholar]
- Guillomot M, Flechon JE, Wintenberger-Torres S. Conceptus attachment in the ewe: an ultrastructural study. Placenta. 1981;2:169–182. doi: 10.1016/s0143-4004(81)80021-5. [DOI] [PubMed] [Google Scholar]
- Kimmins S, MacLaren LA. Oestrous cycle and pregnancy effects on the distribution of oestrogen and progesterone receptors in bovine endometrium. Placenta. 2001;22:742–748. doi: 10.1053/plac.2001.0708. [DOI] [PubMed] [Google Scholar]
- Geisert RD, Pratt TN, Bazer FW, Mayes JS, Watson GH. Immunocytochemical localization and changes in endometrial progestin receptor protein during the porcine oestrous cycle and early pregnancy. Reprod Fertil Dev. 1994;6:749–760. doi: 10.1071/rd9940749. [DOI] [PubMed] [Google Scholar]
- Mead RA, Eroschenko VP. Changes in uterine estrogen and progesterone receptors during delayed implantation and early implantation in the spotted skunk. Biol Reprod. 1995;53:827–833. doi: 10.1095/biolreprod53.4.827. [DOI] [PubMed] [Google Scholar]
- Hild-Petito S, Verhage HG, Fazleabas AT. Immunocytochemical localization of estrogen and progestin receptors in the baboon (Papio anubis) uterus during implantation and pregnancy. Endocrinology. 1992;130:2343–2353. doi: 10.1210/endo.130.4.1372241. [DOI] [PubMed] [Google Scholar]
- Okulicz WC, Scarrell R. Estrogen receptor alpha and progesterone receptor in the rhesus endometrium during the late secretory phase and menses. Proceedings of the Society for Experimental Biology & Medicine. 1998;218:316–321. doi: 10.3181/00379727-218-44298. [DOI] [PubMed] [Google Scholar]
- Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140:5310–5321. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Bigsby RM, Cooke PS, Sugimura Y. Stromal-epithelial interactions in adult organs. Cell Differ. 1985;17:137–148. doi: 10.1016/0045-6039(85)90481-6. [DOI] [PubMed] [Google Scholar]
- Chen C, Spencer TE, Bazer FW. Fibroblast growth factor-10: a stromal mediator of epithelial function in the ovine uterus. Biol Reprod. 2000;63:959–966. doi: 10.1095/biolreprod63.3.959. [DOI] [PubMed] [Google Scholar]
- Chen C, Spencer TE, Bazer FW. Expression of hepatocyte growth factor and its receptor c-met in the ovine uterus. Biol Reprod. 2000;62:1844–1850. doi: 10.1095/biolreprod62.6.1844. [DOI] [PubMed] [Google Scholar]
- DeMartini JC, Carlson JO, Leroux C, Spencer T, Palmarini M. Endogenous retroviruses related to jaagsiekte sheep retrovirus. Current Topics in Microbiology & Immunology. 2003;275:117–137. doi: 10.1007/978-3-642-55638-8_5. [DOI] [PubMed] [Google Scholar]
- Palmarini M, Hallwirth C, York D, Murgia C, de Oliveira T, Spencer T, Fan H. Molecular cloning and functional analysis of three type D endogenous retroviruses of sheep reveal a different cell tropism from that of the highly related exogenous jaagsiekte sheep retrovirus. J Virol. 2000;74:8065–8076. doi: 10.1128/jvi.74.17.8065-8076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmarini M, Gray CA, Carpenter K, Fan H, Bazer FW, Spencer TE. Expression of endogenous betaretroviruses in the ovine uterus: effects of neonatal age, estrous cycle, pregnancy, and progesterone. J Virol. 2001;75:11319–11327. doi: 10.1128/JVI.75.23.11319-11327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmarini M, Mura M, Spencer TE. Endogenous betaretroviruses of sheep: teaching new lessons in retroviral interference and adaptation. J Gen Virol. 2004;85:1–13. doi: 10.1099/vir.0.19547-0. [DOI] [PubMed] [Google Scholar]
- Burghardt RC, Johnson GA, Jaeger LA, Ka H, Garlow JE, Spencer TE, Bazer FW. Integrins and extracellular matrix proteins at the maternal-fetal interface in domestic animals. Cells Tissues Organs. 2002;171:202–217. doi: 10.1159/000066969. [DOI] [PubMed] [Google Scholar]
- Bowen JA, Bazer FW, Burghardt RC. Spatial and temporal analyses of integrin and Muc-1 expression in porcine uterine epithelium and trophectoderm in vivo. Biol Reprod. 1996;55:1098–1106. doi: 10.1095/biolreprod55.5.1098. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Bazer FW, Jaeger LA, Ka H, Garlow JE, Pfarrer C, Spencer TE, Burghardt RC. Muc-1, integrin, and osteopontin expression during the implantation cascade in sheep. Biol Reprod. 2001;65:820–828. doi: 10.1095/biolreprod65.3.820. [DOI] [PubMed] [Google Scholar]
- Hoffman LH, Olson GE, Carson DD, Chilton BS. Progesterone and implanting blastocysts regulate Muc1 expression in rabbit uterine epithelium. Endocrinology. 1998;139:266–271. doi: 10.1210/endo.139.1.5750. [DOI] [PubMed] [Google Scholar]
- Lessey BA. Endometrial integrins and the establishment of uterine receptivity. Hum Reprod. 1998;13 Suppl 3:247–58; discussion 259-61. doi: 10.1093/humrep/13.suppl_3.247. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Burghardt RC, Bazer FW, Spencer TE. Osteopontin: roles in implantation and placentation. Biol Reprod. 2003;69:1458–1471. doi: 10.1095/biolreprod.103.020651. [DOI] [PubMed] [Google Scholar]
- Lessey BA. Adhesion molecules and implantation. J Reprod Immunol. 2002;55:101–112. doi: 10.1016/s0165-0378(01)00139-5. [DOI] [PubMed] [Google Scholar]
- Bowen JA, Burghardt RC. Cellular mechanisms of implantation in domestic farm animals. Seminars in Cell & Developmental Biology. 2000;11:93–104. doi: 10.1006/scdb.2000.0155. [DOI] [PubMed] [Google Scholar]
- Peltier MR, Hansen PJ. Immunoregulatory activity, biochemistry, and phylogeny of ovine uterine serpin. Am J Reprod Immunol. 2001;45:266–272. doi: 10.1111/j.8755-8920.2001.450502.x. [DOI] [PubMed] [Google Scholar]
- Moffatt RJ, Bazer FW, Roberts RM, Thatcher WW. Secretory function of the ovine uterus: effects of gestation and steroid replacement therapy. J Anim Sci. 1987;65:1400–1410. doi: 10.2527/jas1987.6551400x. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Gray A, Johnson GA, Taylor KM, Gertler A, Gootwine E, Ott TL, Bazer FW. Effects of recombinant ovine interferon tau, placental lactogen, and growth hormone on the ovine uterus. Biol Reprod. 1999;61:1409–1418. doi: 10.1095/biolreprod61.6.1409. [DOI] [PubMed] [Google Scholar]
- Stewart MD, Johnson GA, Gray CA, Burghardt RC, Schuler LA, Joyce MM, Bazer FW, Spencer TE. Prolactin receptor and uterine milk protein expression in the ovine endometrium during the estrous cycle and pregnancy. Biol Reprod. 2000;62:1779–1789. doi: 10.1095/biolreprod62.6.1779. [DOI] [PubMed] [Google Scholar]
- Moffatt J, Bazer FW, Hansen PJ, Chun PW, Roberts RM. Purification, secretion and immunocytochemical localization of the uterine milk proteins, major progesterone-induced proteins in uterine secretions of the sheep. Biol Reprod. 1987;36:419–430. doi: 10.1095/biolreprod36.2.419. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Spencer TE, Burghardt RC, Bazer FW. Ovine osteopontin: I. Cloning and expression of messenger ribonucleic acid in the uterus during the periimplantation period. Biol Reprod. 1999;61:884–891. doi: 10.1095/biolreprod61.4.884. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Burghardt RC, Spencer TE, Newton GR, Ott TL, Bazer FW. Ovine osteopontin: II. Osteopontin and alpha(v)beta(3) integrin expression in the uterus and conceptus during the periimplantation period. Biol Reprod. 1999;61:892–899. doi: 10.1095/biolreprod61.4.892. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Burghardt RC, Joyce MM, Spencer TE, Bazer FW, Pfarrer C, Gray CA. Osteopontin expression in uterine stroma indicates a decidualization-like differentiation during ovine pregnancy. Biol Reprod. 2003;68:1951–1958. doi: 10.1095/biolreprod.102.012948. [DOI] [PubMed] [Google Scholar]
- Ing NH, Francis H, McDonnell JJ, Amann JF, Roberts RM. Progesterone induction of the uterine milk proteins: major secretory proteins of sheep endometrium. Biol Reprod. 1989;41:643–654. doi: 10.1095/biolreprod41.4.643. [DOI] [PubMed] [Google Scholar]
- Musgrove EA, Swarbrick A, Lee CS, Cornish AL, Sutherland RL. Mechanisms of cyclin-dependent kinase inactivation by progestins. Mol Cell Biol. 1998;18:1812–1825. doi: 10.1128/mcb.18.4.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Pollard JW. Progesterone inhibits estrogen-induced cyclin D1 and cdk4 nuclear translocation, cyclin E- and cyclin A-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Mol Cell Biol. 1999;19:2251–2264. doi: 10.1128/mcb.19.3.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimsatt WA. Hew Histological Observations on the Placenta of the Sheep. Am J Anat. 1950;87:391–436. doi: 10.1002/aja.1000870304. [DOI] [PubMed] [Google Scholar]
- Ismail PM, Li J, DeMayo FJ, O'Malley BW, Lydon JP. A novel LacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Molecular Endocrinology. 2002;16:2475–2489. doi: 10.1210/me.2002-0169. [DOI] [PubMed] [Google Scholar]
- Guillomot M. Cellular interactions during implantation in domestic ruminants. J Reprod Fertil Suppl. 1995;49:39–51. [PubMed] [Google Scholar]
- Roberts RM, Bazer FW. The functions of uterine secretions. J Reprod Fertil. 1988;82:875–892. doi: 10.1530/jrf.0.0820875. [DOI] [PubMed] [Google Scholar]
- Bagchi IC, Cheon YP, Li Q, Bagchi MK. Progesterone receptor-regulated gene networks in implantation. Frontiers in Bioscience. 2003;8:s852–61. doi: 10.2741/1148. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. Journal of Clinical Endocrinology & Metabolism. 2002;87:2954–2959. doi: 10.1210/jcem.87.6.8563. [DOI] [PubMed] [Google Scholar]
- Chilton BS, Daniel J. C., Jr. Differences in the rabbit uterine response to progesterone as influenced by growth hormone or prolactin. Journal of Reproduction & Fertility. 1987;79:581–587. doi: 10.1530/jrf.0.0790581. [DOI] [PubMed] [Google Scholar]
- Young KH, Kraeling RR, Bazer FW. Effects of prolactin on conceptus survival and uterine secretory activity in pigs. J Reprod Fertil. 1989;86:713–722. doi: 10.1530/jrf.0.0860713. [DOI] [PubMed] [Google Scholar]
- Randall GW, Daniel J. C., Jr., Chilton BS. Prolactin enhances uteroglobin gene expression by uteri of immature rabbits. Journal of Reproduction & Fertility. 1991;91:249–257. doi: 10.1530/jrf.0.0910249. [DOI] [PubMed] [Google Scholar]
- Noel S, Herman A, Johnson GA, Gray CA, Stewart MD, Bazer FW, Gertler A, Spencer TE. Ovine placental lactogen specifically binds to endometrial glands of the ovine uterus. Biology of Reproduction. 2003;68:772–780. doi: 10.1095/biolreprod.102.009183. [DOI] [PubMed] [Google Scholar]
- Gertler A, Djiane J. Mechanism of ruminant placental lactogen action: molecular and in vivo studies. Mol Genet Metab. 2002;75:189–201. doi: 10.1006/mgme.2002.3303. [DOI] [PubMed] [Google Scholar]
- Anthony RV, Limesand SW, Fanning MD, Liang R. Placental lactogen and growth hormone: regulation and action. In: Bazer F W, editor. The Endocrinology of Pregnancy. Totowa, N. J., Humana Press, Inc.; 1998. pp. 461–490. [Google Scholar]
- Lacroix MC, Devinoy E, Servely JL, Puissant C, Kann G. Expression of the growth hormone gene in ovine placenta: detection and cellular localization of the protein. Endocrinology. 1996;137:4886–4892. doi: 10.1210/endo.137.11.8895361. [DOI] [PubMed] [Google Scholar]
- Bazer FW, Spencer TE, Ott TL. Endocrinology of the transition from recurring estrous cycles to establishment of pregnancy in subprimate mammals. In: Bazer F W, editor. The Endocrinology of Pregnancy. Totowa, N. J., Humana Press, Inc.; 1998. pp. 1–34. [Google Scholar]
- Ziecik AJ. Old, new and the newest concepts of inhibition of luteolysis during early pregnancy in pig. Domest Anim Endocrinol. 2002;23:265–275. doi: 10.1016/s0739-7240(02)00162-5. [DOI] [PubMed] [Google Scholar]
- Moeljono MP, Bazer FW, Thatcher WW. A study of prostaglandin F2alpha as the luteolysin in swine: I. Effect of prostaglandin F2alpha in hysterectomized gilts. Prostaglandins. 1976;11:737–743. doi: 10.1016/0090-6980(76)90073-3. [DOI] [PubMed] [Google Scholar]
- Gadsby JE, Balapure AK, Britt JH, Fitz TA. Prostaglandin F2 alpha receptors on enzyme-dissociated pig luteal cells throughout the estrous cycle. Endocrinology. 1990;126:787–795. doi: 10.1210/endo-126-2-787. [DOI] [PubMed] [Google Scholar]
- Moeljono MP, Thatcher WW, Bazer FW, Frank M, Owens LJ, Wilcox CJ. A study of prostaglandin F2alpha as the luteolysin in swine: II Characterization and comparison of prostaglandin F, estrogens and progestin concentrations in utero-ovarian vein plasma of nonpregnant and pregnant gilts. Prostaglandins. 1977;14:543–555. doi: 10.1016/0090-6980(77)90268-4. [DOI] [PubMed] [Google Scholar]
- Sample GL, Kubotsu SL, Carnahan KG, Mirando MA. Interestrous interval of cyclic gilts is decreased by systemic but not intrauterine administration of exogenous oxytocin. Journal of Animal Science. 2000;78:2393–2398. doi: 10.2527/2000.7892393x. [DOI] [PubMed] [Google Scholar]
- Mirando MA, Prince BC, Tysseling KA, Carnahan KG, Ludwig TE, Hoagland TA, Crain RC. A proposed role for oxytocin in regulation of endometrial prostaglandin F2 alpha secretion during luteolysis in swine. Adv Exp Med Biol. 1995;395:421–433. [PubMed] [Google Scholar]
- Gleeson AR, Thorburn GD. Selected factors that affect the measurement of plasma progesterone concentrations in pregnant ewes. Aust J Biol Sci. 1974;27:659–669. doi: 10.1071/bi9740659. [DOI] [PubMed] [Google Scholar]
- Kraeling RR, Rampacek GB, Fiorello NA. Inhibition of pregnancy with indomethacin in mature gilts and prepuberal gilts induced to ovulate. Biol Reprod. 1985;32:105–110. doi: 10.1095/biolreprod32.1.105. [DOI] [PubMed] [Google Scholar]
- Bazer FW, Thatcher WW. Theory of maternal recognition of pregnancy in swine based on estrogen controlled endocrine versus exocrine secretion of prostaglandin F2alpha by the uterine endometrium. Prostaglandins. 1977;14:397–400. doi: 10.1016/0090-6980(77)90185-x. [DOI] [PubMed] [Google Scholar]
- Frank M, Bazer FW, Thatcher WW, Wilcox CJ. A study of prostaglandin F2alpha as the luteolysin in swine: III effects of estradiol valerate on prostaglandin F, progestins, estrone and estradiol concentrations in the utero-ovarian vein of nonpregnant gilts. Prostaglandins. 1977;14:1183–1196. doi: 10.1016/0090-6980(77)90295-7. [DOI] [PubMed] [Google Scholar]
- Zavy MT, Bazer FW, Thatcher WW, Wilcox CJ. A study of prostaglandin F2 alpha as the luteolysin in swine: V. Comparison of prostaglandin F, progestins, estrone and estradiol in uterine flushings from pregnant and nonpregnant gilts. Prostaglandins. 1980;20:837–851. doi: 10.1016/0090-6980(80)90137-9. [DOI] [PubMed] [Google Scholar]
- Bazer FW. Establishment of pregnancy in sheep and pigs. Reprod Fertil Dev. 1989;1:237–242. doi: 10.1071/rd9890237. [DOI] [PubMed] [Google Scholar]
- Gross TS, Mirando MA, Young KH, Beers S, Bazer FW, Thatcher WW. Reorientation of prostaglandin F secretion by calcium ionophore, estradiol, and prolactin in perifused porcine endometrium. Endocrinology. 1990;127:637–642. doi: 10.1210/endo-127-2-637. [DOI] [PubMed] [Google Scholar]
- Geisert RD, Renegar RH, Thatcher WW, Roberts RM, Bazer FW. Establishment of pregnancy in the pig: I. Interrelationships between preimplantation development of the pig blastocyst and uterine endometrial secretions. Biol Reprod. 1982;27:925–939. doi: 10.1095/biolreprod27.4.925. [DOI] [PubMed] [Google Scholar]
- Geisert RD, Zavy MT, Moffatt RJ, Blair RM, Yellin T. Embryonic steroids and the establishment of pregnancy in pigs. J Reprod Fertil Suppl. 1990;40:293–305. [PubMed] [Google Scholar]
- Krzymowski T, Kotwica J, Stefanczyk-Krzymowska S. Uterine and ovarian countercurrent pathways in the control of ovarian function in the pig. J Reprod Fertil Suppl. 1990;40:179–191. [PubMed] [Google Scholar]
- Akinlosotu BA, Diehl JR, Gimenez T. Prostaglandin E2 counteracts the effects of PGF2 alpha in indomethacin treated cycling gilts. Prostaglandins. 1988;35:81–93. doi: 10.1016/0090-6980(88)90276-6. [DOI] [PubMed] [Google Scholar]
- Christenson LK, Farley DB, Anderson LH, Ford SP. Luteal maintenance during early pregnancy in the pig: role for prostaglandin E2. Prostaglandins. 1994;47:61–75. doi: 10.1016/0090-6980(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Davis DL, Blair RM. Studies of uterine secretions and products of primary cultures of endometrial cells in pigs. J Reprod Fertil Suppl. 1993;48:143–155. [PubMed] [Google Scholar]
- Garverick HA, Polge C, Flint AP. Oestradiol administration raises luteal LH receptor levels in intact and hysterectomized pigs. J Reprod Fertil. 1982;66:371–377. doi: 10.1530/jrf.0.0660371. [DOI] [PubMed] [Google Scholar]
- Ziecik AJ, Jedlinska M, Rzucidlo SJ. Effect of estradiol and progesterone on myometrial LH/hCG receptors in pigs. Acta Endocrinol (Copenh) 1992;127:185–188. doi: 10.1530/acta.0.1270185. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Ford SP. Direct luteotrophic effect of oestradiol-17 beta on pig corpora lutea. J Reprod Fertil. 1989;87:125–131. doi: 10.1530/jrf.0.0870125. [DOI] [PubMed] [Google Scholar]
- Young KH, Kraeling RR, Bazer FW. Effect of pregnancy and exogenous ovarian steroids on endometrial prolactin receptor ontogeny and uterine secretory response in pigs. Biol Reprod. 1990;43:592–599. doi: 10.1095/biolreprod43.4.592. [DOI] [PubMed] [Google Scholar]
- Morgan GL, Geisert RD, Zavy MT, Fazleabas AT. Development and survival of pig blastocysts after oestrogen administration on day 9 or days 9 and 10 of pregnancy. J Reprod Fertil. 1987;80:133–141. doi: 10.1530/jrf.0.0800133. [DOI] [PubMed] [Google Scholar]
- Morgan GL, Geisert RD, Zavy MT, Shawley RV, Fazleabas AT. Development of pig blastocysts in a uterine environment advanced by exogenous oestrogen. J Reprod Fertil. 1987;80:125–131. doi: 10.1530/jrf.0.0800125. [DOI] [PubMed] [Google Scholar]
- Harney JP, Bazer FW. Effect of porcine conceptus secretory proteins on interestrous interval and uterine secretion of prostaglandins. Biol Reprod. 1989;41:277–284. doi: 10.1095/biolreprod41.2.277. [DOI] [PubMed] [Google Scholar]
- Geisert RD, Thatcher WW, Roberts RM, Bazer FW. Establishment of pregnancy in the pig: III. Endometrial secretory response to estradiol valerate administered on day 11 of the estrous cycle. Biol Reprod. 1982;27:957–965. doi: 10.1095/biolreprod27.4.957. [DOI] [PubMed] [Google Scholar]
- Simmen RC, Green ML, Simmen FA. IGF system in periimplantation uterus and embryonic development. In: Dey S K, editor. Molecular and Cellular Aspects of Periimplantation Processes. Springer-Verlag, Inc.; 1995. pp. 185–204. [Google Scholar]
- Persson E, Sahlin L, Masironi B, Dantzer V, Eriksson H, Rodriguez-Martinez H. Insulin-like growth factor-I in the porcine endometrium and placenta: localization and concentration in relation to steroid influence during early pregnancy. Anim Reprod Sci. 1997;46:261–281. doi: 10.1016/s0378-4320(96)01610-7. [DOI] [PubMed] [Google Scholar]
- Simmen RC, Simmen FA, Hofig A, Farmer SJ, Bazer FW. Hormonal regulation of insulin-like growth factor gene expression in pig uterus. Endocrinology. 1990;127:2166–2174. doi: 10.1210/endo-127-5-2166. [DOI] [PubMed] [Google Scholar]
- Simmen FA, Simmen RC, Geisert RD, Martinat-Botte F, Bazer FW, Terqui M. Differential expression, during the estrous cycle and pre- and postimplantation conceptus development, of messenger ribonucleic acids encoding components of the pig uterine insulin-like growth factor system. Endocrinology. 1992;130:1547–1556. doi: 10.1210/endo.130.3.1537304. [DOI] [PubMed] [Google Scholar]
- Rubin JS, Bottaro DP, Chedid M, Miki T, Ron D, Cheon G, Taylor WG, Fortney E, Sakata H, Finch PW, et al. Keratinocyte growth factor. Cell Biol Int. 1995;19:399–411. doi: 10.1006/cbir.1995.1085. [DOI] [PubMed] [Google Scholar]
- Ka H, Spencer TE, Johnson GA, Bazer FW. Keratinocyte growth factor: expression by endometrial epithelia of the porcine uterus. Biol Reprod. 2000;62:1772–1778. doi: 10.1095/biolreprod62.6.1772. [DOI] [PubMed] [Google Scholar]
- Ka H, Jaeger LA, Johnson GA, Spencer TE, Bazer FW. Keratinocyte growth factor is up-regulated by estrogen in the porcine uterine endometrium and functions in trophectoderm cell proliferation and differentiation. Endocrinology. 2001;142:2303–2310. doi: 10.1210/endo.142.6.8194. [DOI] [PubMed] [Google Scholar]
- Jaeger LA, Johnson GA, Ka H, Garlow JG, Burghardt RC, Spencer TE, Bazer FW. Functional analysis of autocrine and paracrine signalling at the uterine-conceptus interface in pigs. Reprod Suppl. 2001;58:191–207. [PubMed] [Google Scholar]
- Garlow JE, Ka H, Johnson GA, Burghardt RC, Jaeger LA, Bazer FW. Analysis of osteopontin at the maternal-placental interface in pigs. Biol Reprod. 2002;66:718–725. doi: 10.1095/biolreprod66.3.718. [DOI] [PubMed] [Google Scholar]
- Lefevre F, Martinat-Botte F, Guillomot M, Zouari K, Charley B, La Bonnardiere C. Interferon-gamma gene and protein are spontaneously expressed by the porcine trophectoderm early in gestation. Eur J Immunol. 1990;20:2485–2490. doi: 10.1002/eji.1830201119. [DOI] [PubMed] [Google Scholar]
- Lefevre F, Boulay V. A novel and atypical type one interferon gene expressed by trophoblast during early pregnancy. J Biol Chem. 1993;268:19760–19768. [PubMed] [Google Scholar]
- Lefevre F, Guillomot M, D'Andrea S, Battegay S, La Bonnardiere C. Interferon-delta: the first member of a novel type I interferon family. Biochimie. 1998;80:779–788. doi: 10.1016/s0300-9084(99)80030-3. [DOI] [PubMed] [Google Scholar]
- D'Andrea S, Chousterman S, Flechon JE, La Bonnardiere C. Paracrine activities of porcine trophoblastic interferons. J Reprod Fertil. 1994;102:185–194. doi: 10.1530/jrf.0.1020185. [DOI] [PubMed] [Google Scholar]
- Lefevre F, Martinat-Botte F, Locatelli A, De Niu P, Terqui M, La Bonnardiere C. Intrauterine infusion of high doses of pig trophoblast interferons has no antiluteolytic effect in cyclic gilts. Biol Reprod. 1998;58:1026–1031. doi: 10.1095/biolreprod58.4.1026. [DOI] [PubMed] [Google Scholar]
- Denker HW. Implantation: a cell biological paradox. J Exp Zool. 1993;266:541–558. doi: 10.1002/jez.1402660606. [DOI] [PubMed] [Google Scholar]
- Young HA, Hardy KJ. Role of interferon-gamma in immune cell regulation. J Leukoc Biol. 1995;58:373–381. [PubMed] [Google Scholar]