Abstract
Dopamine neurons in the ventral tegmental area (VTA) have important functions related to rewards but are also activated in aversive contexts. Electrophysiology studies suggest that the degree to which VTA dopamine neurons respond to noxious stimuli is topographically organized across the dorsal-ventral extent. We used c-fos immunohistochemistry to examine the responses of VTA dopamine neurons in contexts of social defeat and social approach. Studying monogamous California mice (Peromyscus californicus) allowed us to observe the effects of social defeat on both males and females. Females exposed to three episodes of defeat, but not a single episode, had more tyrosine hydroxylase (TH)/c-fos positive cells in ventral (but not dorsal) VTA compared to controls. This observation suggests that repeated exposure to aversive contexts is necessary to trigger activation of VTA dopamine neurons. Defeat did not affect TH/c-fos colocalizations in males. We also examined long term effects of defeat on c-fos expression in a social interaction test. As previously reported, defeat reduced social interaction in females but not males. Surprisingly, there were no effects of defeat stress on TH/c-fos colocalizations in any subregion of the VTA. However, females had more TH/c-fos positive cells than males across the entire VTA, and also had greater c-fos positive cell counts in posterior subregions of the nucleus accumbens (NAc) shell. Our results show that dopamine neurons in the VTA are more responsive to social contexts in females and that the ventral VTA in particular is sensitive to aversive contexts.
Keywords: stress, dopamine, sex differences, behavioral neuroscience, behavior
Graphical Abstract
Introduction
Dopamine neurons in the ventral tegmental area (VTA) are highly responsive to pleasurable contexts, including natural rewards and drugs of abuse (Reviewed in (Wise 2008)). Furthermore, the activity of VTA dopamine neurons is strongly influenced by cues for rewards. The difference between an expected reward and the actual reward has important effects on the direction and magnitude of activity in dopamine neurons. For example, VTA dopamine neurons will respond with burst firing following an unexpected reward or become inhibited when an expected reward is absent (Hollerman and Schultz 1998). This has been hypothesized to play an important role in Pavlovian reward learning (i.e., prediction error; Reviewed in (Schultz 2013)) or to trigger internal motivational states (i.e. incentive salience; reviewed in (Berridge 2012)). Although the exact mechanism of action is debated, dopaminergic signaling clearly is an important modulator of goal-directed behavior (Reviewed in (Pignatelli and Bonci 2015). The function of VTA dopamine neurons has been studied primarily in the context of appetitive rewards. However, these neurons are also highly responsive to aversive contexts.
Dopamine release in the mesolimbic system is induced by aversive stimuli such as electric shocks and social stressors (Reviewed in (Miczek et al. 2008; Trainor 2011; Polter and Kauer 2014). An important question is whether the same cells activated during appetitive contexts are also activated by aversive contexts or their cues. Electrophysiological recordings of VTA neurons in Rhesus monkeys showed that most VTA neurons were excited by cues for appetitive and aversive stimuli (Matsumoto and Hikosaka 2009). However, electrophysiological criteria were used to identify dopamine neurons, which can sometimes lead to misclassification of cells (Margolis et al. 2010). A transgenic mouse study tagged either dopaminergic or GABAergic neurons with channelrhodopsin-2 so that they could be identified during optical stimulation (Cohen et al. 2012). Most dopamine neurons were sensitive to cues predicting rewards (water to water-deprived mice) and less than 20% of dopamine neurons responded to cues predicting an aversive stimulus (an air puff to the face). However air puffs are not particularly robust stimuli. About 25% of putative VTA dopamine neurons (as identified using electrophysiological criteria) responded to cues predicting a 30 cm free fall (Wang and Tsien 2011). An alternative line of evidence shows that aversive contexts can induce long lasting changes in the activity of VTA dopamine neurons. Social defeat stress induces hyperactivity in VTA dopamine neurons in multiple species of rodent, and has been observed by multiple research groups (Krishnan et al. 2007; Anstrom et al. 2009; Cao et al. 2010). Indeed, optogenetic inhibition of VTA dopamine neurons projecting to nucleus accumbens (NAc) in mice exposed to social defeat reversed stress-induced social withdrawal, but inhibition of VTA neurons projecting to prefrontal cortex (PFC) facilitated social withdrawal (Chaudhury et al. 2012). One question that is unclear is whether the effect of social defeat stress on VTA dopamine neural activity is topographically organized.
Anterior-posterior and medial-lateral divisions of VTA have been studied extensively in reward- and drug-related contexts (Sanchez-Catalan et al. 2014). For example, overexpression of the glutamate receptor Glur1 in the anterior VTA increased morphine reward (as measured by place preference), while Glur1 overexpression in posterior VTA produced morphine-induced aversion (Carlezon Jr et al. 2000). Similarly, the effects of CREB overexpression were anatomically specific, enhancing morphine place preferences in anterior VTA but inducing aversion in posterior VTA (Olson et al. 2005). These functional differences may be related to differences in axonal projections (See (Ikemoto 2007) for review), as more posterior VTA dopamine neurons project to NAc than anterior neurons (Swanson 1982). Interestingly, the CREB gene transfer experiments indicated that posterior VTA can be altered to induce aversive states. Recent evidence indicates that there is topographical organization in the dorsal-ventral extent of the VTA. Anesthetized rats exposed to footshocks showed selective activation of dopamine neurons in ventral but not dorsal VTA (Brischoux et al. 2009). In contrast, dopamine neurons in dorsal VTA were inhibited by footshocks. So far, no study has tested whether other types of aversive contexts, such as social defeat, selectively activate VTA dopamine neurons in a topographically specific orientation.
We used c-fos immunohistochemistry to examine the responses of VTA dopamine neurons under both positive and negative social contexts. We studied the California mouse, a monogamous species in which both males and females defend territories (Ribble and Salvioni 1990; Ribble and Millar 1996). This social organization is unique and allows for the study of males and females exposed to an equivalent intensity of defeat stress in a consistent ethological context (Trainor et al. 2013). The long term effects of defeat stress are sex-specific and generally consistent with reactive and proactive coping strategies described by Koolhaas and colleagues (Koolhaas et al. 1999). In females, the long term effects of social defeat are more consistent with reactive coping strategies such as social withdrawal (Greenberg et al. 2014), reduced aggression (Steinman et al. 2015) and behavioral flexibility (Laredo et al. 2015). We examined TH/c-fos cells in mice exposed to either one or three episodes of defeat stress, and recorded behavioral observations immediately before episodes of defeat to estimate anticipation of repeated episodes of social stress. Finally, we examined the long term effect of social defeat on both social interaction behavior and TH/c-fos immunostaining. The social interaction test generates strong approach responses in mice naïve to defeat (Trainor et al. 2011). In this study we also examined c-fos in the NAc, PFC, and bed nucleus of the stria terminalis (BNST). The NAc and PFC receive strong dopaminergic projections from the VTA while the BNST is an important node connecting the VTA with circuits controlling social behaviors (O’Connell and Hofmann 2011). We previously found that social withdrawal in stressed female mice during the social interaction test is mediated by neurotrophin signaling and dopamine signaling in the BNST (Greenberg et al. 2014) and NAc (Campi et al. 2014), respectively. Together these studies form a unique examination of the effects of different social contexts on VTA dopamine neurons in both males and females.
Materials and Methods
Animals
Adult (3–6 months old) male and female California mice (Peromyscus californicus) were bred in our laboratory colony and housed in same sex groups of 2–3 per cage on Sani-Chips bedding with cotton nestlets in clear polypropylene cages. Mice were kept on a 16h light/8h dark cycle (lights on 2300 h) with Harlan Teklad 2016 food (Hayward, CA) and water provided ad libitum. All procedures were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of California, Davis. Estrous cycles were not monitored in experiments. Although estrous cycle can impact behavior and neural function, a recent metanalysis of 293 studies showed that randomly cycling female mice were no more variable than males across a range of behavioral and neurobiological processes (Prendergast et al. 2014). We have also demonstrated defeat-induced social aversion is present across all stages of the estrous cycle (Trainor et al. 2011) and that ovariectomy has no effect on defeat-induced social aversion, (Trainor et al. 2013). Sample sizes for each experiment are listed in Table 1.
Table 1.
Sample sizes for all experiments
| Study | Design | Male Control | Male Stress | Female Control | Female Stress |
|---|---|---|---|---|---|
| Exp 1 | Single Defeat | 4 | 6 | 4 | 6 |
| Exp 2 | 3x Defeat | 8 | 9 | 7 | 15 |
| Exp 3 | 3x Defeat + Social Interaction | 8 | 7 | 6 | 8 |
Behavioral Testing and Tissue Collection
All behavioral testing was performed during lights out under dim red light (3 lux). For all experiments, mice were anesthetized with isoflurane gas and euthanized via rapid decapitation 1 h following the final behavioral test. Brains were removed and fixed for 24h in 5% acrolein (Sigma, St. Louis, MO) in 0.1 M phosphate buffered saline (PBS) at 4 °C. Brains were then transferred to 20% sucrose in PBS for an additional 24h before being frozen at −40° C.
Behavioral Testing
In experiment 1, mice were randomly assigned to a single episode of social stress or control conditions (Trainor et al. 2013). Mice assigned to defeat were placed into the cage of an aggressive, same-sex resident for 7 min or until they received 10 bites from the resident, whichever occurred first. Control mice were placed in an empty cage for 7 min. At the end of this period each mouse was returned to its home cage. Mice were euthanized one hour later, as described above.
In experiment 2, mice were randomly assigned to undergo one episode of social defeat or control conditions per day for three consecutive days. After each episode of defeat or control conditions, each mouse was returned to its home cage and left undisturbed until the following day. On the third day, mice were euthanized 1 hr after the third episode as in experiment 1.
In experiment 3, mice were randomly assigned to social defeat or control conditions for three consecutive days. In this study we made additional behavioral observations immediately before each episode of defeat or control. Each cage of mice was transferred from the colony room to an adjacent behavior testing room. Within 1 min we started a 3 min video recording. This recording was then scored for indices of activity and anxiety-like behavior using behavioral scoring software (Behavior Tracker 1.5, www.behaviortracker.com) by an observer without knowledge of treatment assignments. The frequencies of backflips, autogrooming, digging and rearing were recorded. Durations of autogrooming and digging were also scored. Immediately after this 3 min recording, focal mice were transferred to defeat or control conditions. After defeat or control conditions focal mice were returned to their home cage and left undisturbed (besides routine animal husbandry) until social interaction testing.
Two weeks following the third day of social defeat or control conditions all mice underwent social interaction testing as described previously (Trainor et al. 2013; Greenberg et al. 2014). Mice were first habituated to a large Plexiglas box (89 × 63 × 60 cm) after being placed in a neutral zone (the center of the arena) from which they were free to move about. Each mouse spent 3 min in the arena during this open field phase. A video tracking system (Any-Maze, Stoelting, Wood Dale, IL) recorded the total distance travelled and the durations mice spent within 8 cm of the sides and within a center zone located 14 cm from the sides. An empty wire cage was then placed against a wall of the arena for a second 3-minute stage (acclimation phase). Distance travelled, time spent within 8 cm from the wire cage, and time spent in a corners zone opposite of the cage zone were recorded. Finally, a same-sex novel “target” mouse was placed into the wire cage, and the same variables were once again recorded. Mice were euthanized 1 hr after testing as in experiment 1.
Immunohistochemistry
A cryostat was used to collect 40 μm thick coronal sections through the hypothalamus. Previous studies of the VTA have used both coronal and horizontal sections. Coronal sections have been used to examine rostral versus caudal topography in anatomical (Carlezon et al. 2000) and functional analyses (Mahler and Aston-Jones 2012). Horizontal sections provide an alternate approach for examining the VTA in a dorsal-ventral axis. Cell bodies in the VTA that express TH have a horizontal orientation, and horizontal sections allow for better visualization of these cells (Phillipson 1979). Horizontal sectioning also facilitates discrimination of the VTA from the substantia nigra (SN) by having both the interpenduncular nucleus (IPN) and medial terminal nucleus of the accessory optic tract (MT) in the same section (Margolis et al. 2006b; Margolis et al. 2012). We used horizontal sections because we predicted that dorsal and ventral subregions of the VTA would have different responses to defeat stress.
The two primary antibodies used in our studies have been previously evaluated for specificity by preabsorption with an immunizing peptide (Schiltz and Sawchenko 2007) or immunoblotting (Haycock and Waymire 1982). In addition, these antibodies produced immunostaining congruent with previously published reports for TH (Margolis et al. 2010) and c-fos (Justice et al. 2008). In a control experiment, omission of TH or c-fos primary antibody abolished positive staining for TH and c-fos, respectively. In a second pilot experiment, we ran both primary antibodies with respective secondary antibodies in striatal brain slices in which TH is selectively expressed in fibers. We only detected signal for TH (green staining) in fibers and signal for c-fos (red staining) in nuclei. This experiment indicates that the antibodies used for our experiments do not cross-react.
For all experiments, every third section (every 120 μm) containing the ventral tegmental area (VTA) and substantia nigra (SN) (40 μm thick) was processed for immunostaining, resulting in one “ventral,” one “middle,” and one “dorsal” section (Figure 1, (Margolis et al. 2006a,b). Immunofluorescent procedures were used to facilitate the determination of colocalizations. Chromagenic immunostaining procedures have a greater ability to amplify signals, but are less optimal for determining colocalizations because separate images of proteins cannot be obtained. Sections were first washed three times for 5 min in PBS. Sections were then incubated for 10 min in 0.1M sodium borohydride in PBS and then blocked in 10% normal donkey serum (NDS) in PBS for 2 h. Following a 5 min wash in PBS, they were then incubated overnight at 4° C in rabbit anti-c-fos (1:2500, EMD Biosciences Cat# PC38T) in PBS with 0.5% Triton X (Tx) and 2% NDS. The next day sections underwent 3 washes (5 min each) in PBS followed by incubation in Alexa Fluor 555 conjugated donkey anti-rabbit lgG (1:250, Life Technologies Cat# A31572) for 2 h at room temperature. Following three 5 min washes in PBS, sections were blocked in 10% normal rabbit serum (NRS) in PBS for 2 h before being incubated for 48 h at 4° C in sheep anti-TH (1:600, Millipore Cat# AB1542) diluted in PBS with 0.5% Tx and 2% NRS. After this incubation, sections were washed three times for 5 min each in PBS. Sections were then incubated 2 h at room temperature with biotin-conjugated rabbit anti-sheep (1:500, Vector Laboratories Cat# BA-6000) in PBS with 0.5% Tx and 2% NRS for 2 h. After three 5 min washes in PBS, sections were incubated for 30 min in Alexa Fluor 488 conjugated streptavidin (1:250, S-11223, Life Technologies) diluted in PBS with 0.5% Tx. Sections underwent three last washes: first in PBS alone for 5 min, a second wash for 10 min in PBS, followed by a 30 min wash in PBS with 0.1% Tx and a final wash in PBS alone. Stained sections were then dipped in Nanopure water before being mounted onto Superfrost plus slides (Fisher, Pittsburgh, PA) and coverslipped using Vectashield Mounting Medium (Vector Laboratories).
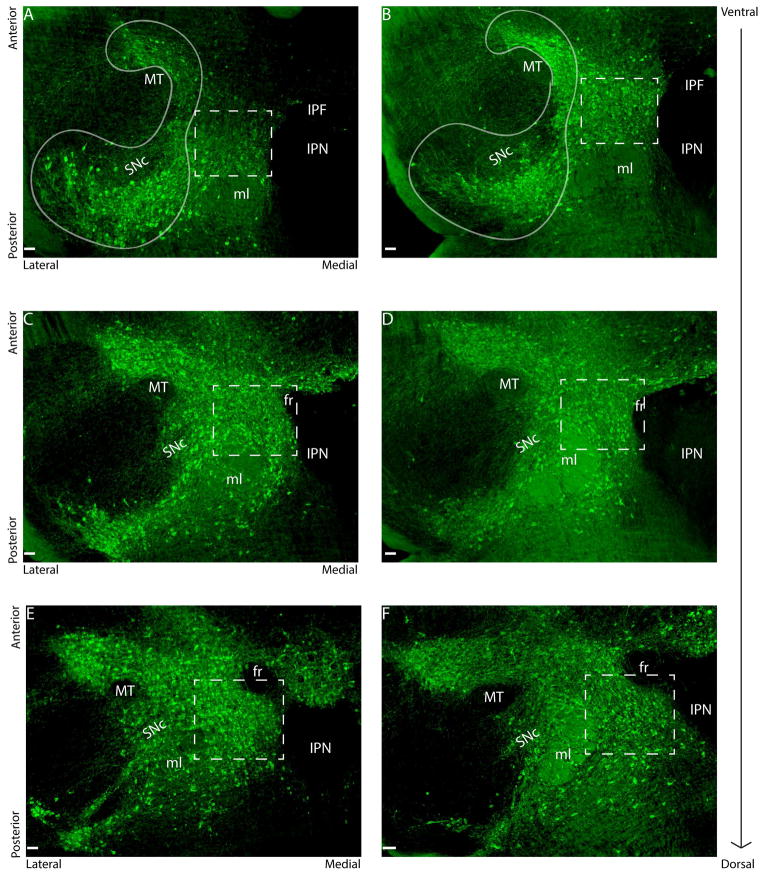
Fig. 1.
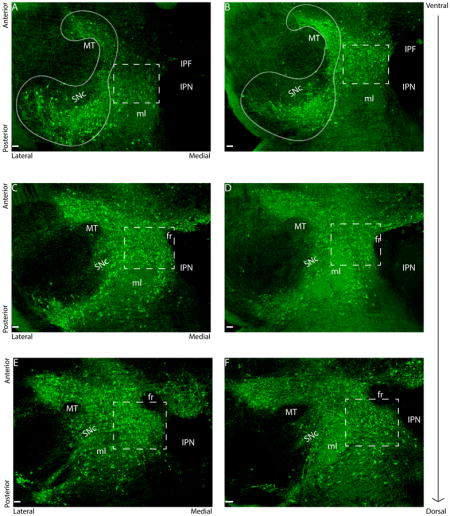
Representative photomicrographs depicting regions of ventral tegmental area (VTA, dotted lines) and substantia nigra compacta (SNc, solid lines). Fluorescent staining is for tyrosine hydroxylase (TH) in ventral (A), middle (C) and dorsal (E) subdivisions of a representative control female. Stressed females had more TH-ir neurons in ventral (B) and dorsal (F) VTA compared to control females. Stressed females did not differ from control females for number of TH positive cells in middle VTA (D). Scale bars = 100μm. Abbreviations: fasciculus retroflexus (fr), interpeduncular fossa (IPF), interpeduncular nucleus (IPN), medial lemniscus (ml), and medial terminal nucleus of the accessory optical tract (MT) are labeled.
For experiment 3, coronal sections containing the prefrontal cortex (PFC), nucleus accumbens (NAc) and bed nucleus of the stria terminalis (BNST) (40 μm thick) were collected on the cryostat prior to collection of horizontal sections for VTA and SN. Every third section (every 120 μm) was processed for immunostaining. These sections were stained for c-fos alone using the methods previously described for experiments 1 and 2. For all immunostaining, both sides of the section were quantified to increase accuracy of the counts.
Immunohistochemistry Micrographs and Quantification
Images were captured with a Zeiss Axioimager microscope (Carl Zeiss Meditec, Inc., Dublin, CA) with a monochromatic Zeiss Axiocam (MRm). Axioimage software was used to take images with an exposure time of 4–10 ms. Quantification was performed using ImageJ software (NIH, Bethesda, MD). Although it is possible that some cells may have been over- or under-counted, we took steps to ensure that inaccuracies in counts were not confounded with group differences. First, mice were randomly assigned to control or defeat conditions. Second, quantifications were performed by observers that had no knowledge of experimental conditions. Our data should be considered estimates of relative differences in TH and c-fos immunoreactivity between groups rather than estimates of actual cell numbers.
The subdivisions of VTA have been increasingly refined for their functional specificity, cytoarchitecture, and heterogeneity of axonal projections (see (Ikemoto 2007; Lammel et al. 2014) for review). In horizontal sections, the number of TH-ir neurons, c-fos-ir cells and TH/c-fos colocalizations were counted for the substantia nigra pars compacta (SNc) and VTA. The growing consensus is that the use of TH immunohistochemistry on horizontal sections is the best approach for identifying dopaminergic cells within the VTA (Ikemoto 2007; Margolis et al. 2010). We delineated boundaries between the SNc and VTA by combining methods using horizontal sectioning in rats (Margolis et al. 2006a,b) and by use of tracer injections in the dorsal striatum that we have previously described (Campi et al. 2013). Due to the presence of dopamine neurons in midline nuclei, such as the IPN and interfascicular nucleus, earlier studies incorporated these regions as parts of the VTA, but they have been more-recently excluded from analyses due to anatomical differences (Reviewed in (Ikemoto 2007). We did not include the midline nuclei in our VTA analyses, and we used the IPN as a medial boundary for the VTA, as previously described (Campi et al. 2013). Cell counts were made from three sections spanning the ventral-dorsal extent of the VTA, and these three sections were termed “ventral,” “middle,” and “dorsal” (Fig. 1). This quantification area consisted of box sizes of 0.52 × 0.47mm for ventral VTA, 0.63 × 0.50mm for middle VTA, and 0.67 × 0.51mm for dorsal VTA. Boxes were placed between the lateral edge of the IPN and the dorsal edge of the medial lemniscus (ml) for ventral and middle sections. Boxes were placed between the lateral edge of the IPN and the medial edge of the ml for dorsal sections. Boxes were aligned with the anterior edge of the VTA, which previous electrophysiological studies showed is more responsive to aversive contexts (Brischoux et al. 2009). The box sizes and their placement across all three sections allowed for quantification of VTA neurons while avoiding counts of “wrapping” TH-positive neurons around the MT, as described in (Campi et al. 2013) and illustrated in Figure 1. These neurons were previously determined to be part of SNc by their configuration and from cell-labeling in this area by retrograde tracers infused into dorsal striatum.
In experiment 3, every third coronal section was used to quantify c-fos cell counts in the prefrontal cortex, NAc and BNST. In the prefrontal cortex a box (0.5 × 0.67 mm) was aligned just lateral to the corpus callosum beginning approximately at Bregma 1.70 mm for prelimbic cortex (PRLCx). Images of infralimbic cortex were taken on the same sections just dorsal to PRLCx with a box of the same size. Subregions of NAc and BNST were quantified using previous descriptions of California mouse NAc and BNST (Trainor et al. 2011; Campi et al. 2013; Greenberg et al. 2014). For the NAc core, a box (0.5 × 0.67 mm) was placed medial to the anterior commissure (AC) and ventral to the lateral ventricle. A box of identical size was placed directly medial and ventral to this NAc core box to quantify NAc shell. Anterior (Bregma 1.23 mm) and posterior (Bregma 0.51 mm) subregions of the NAc were quantified separately based on previous reports that glutamate signaling in these subregions had opposing effects on motivation valence (Richard and Berridge 2011). For the anterolateral BNST (BNSTal), a box (0.5 × 0.67 mm) was placed adjacent to the dorsal edge of the AC and ventral to the lateral ventricle beginning at approximately Bregma 0.45 mm. For the anteromedial BNST (BNSTam), a box was placed dorsal to the AC and directly medial to the box for BNSTal. For ventrolateral BNST (BNSTvl) and ventromedial BNST (BNSTvm), quantification areas were placed on the ventral edge of the AC and aligned medial-laterally on the same section with BNSTal and BNSTam, respectively. Identical quantification areas from middle BNST sections were positioned similarly but posterior to anterior BNST regions. For the posterior dorsal division of the BNST (BNSTpr) a box (0.45 × 0.37mm) was placed just lateral to the fornix and dorsal to the AC at approximately Bregma 0.10 mm. For the posterior lateral division of the BNST (BNSTlp), a box (0.59 × 0.29 mm) was placed just lateral to the AC at approximately Bregma 0.10 mm.
Statistical Analysis
Normality for each variable was assessed using Q-Q plots and histograms. Levene’s test was used to confirm homogeneity of variance. Two-way ANOVA was used to test for effects of sex and social defeat stress on behavior and cell counts. All significant sex*treatment interactions were followed up with planned comparisons to test for effects of treatment within sex. When criteria for normality and homogeneity were not met, data were corrected with log transformations. Autogrooming data and c-fos cell count data in Experiment 3 were positively skewed, and log transformations were used to normalize the distributions of these variables for ANOVA. Colocalization data (TH/c-fos) from all three experiments had substantial positive skew that could not be normalized by transformations, so the raw data were analyzed with nonparametric Mann-Whitney U tests. For Experiment 3, repeated measures three-way ANOVA, using day of testing as a within-subjects factor, was used to test for effects of sex and stress on behavior immediately prior to episodes of social defeat or control conditions. Follow-up paired t-tests were used to compare individual time points. In order to test connectivity between regions, nonparametric Spearman correlations were used correlate colocalizations in VTA with c-fos counts in other forebrain areas. False discovery rate (FDR) adjustments were applied to p-values obtained from forebrain ANOVA analyses and correlational analyses. Untransformed means and s.e. are presented in all figures and tables.
Results
Experiments 1&2: Effects of social defeat on dopamine circuitry immediately following acute and chronic social defeat stress
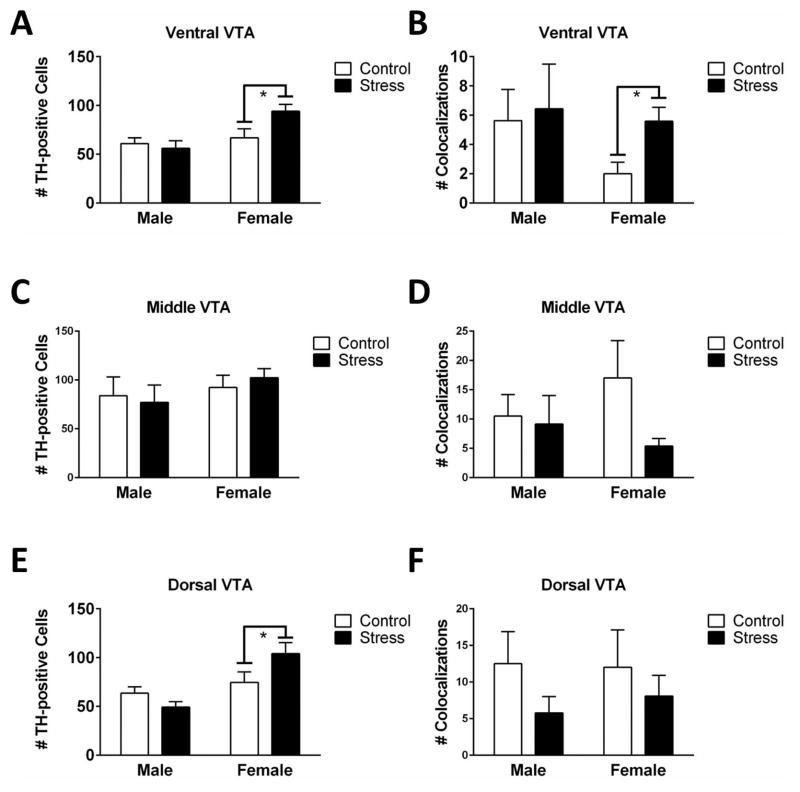
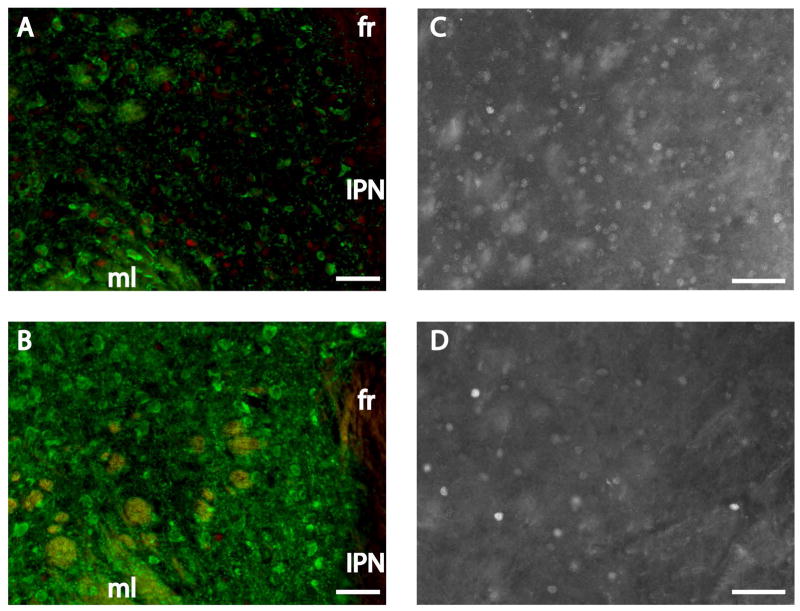
There were no differences in VTA TH cell number or TH/c-fos colocalizations in experiment 1 in which mice were exposed to one episode of defeat or control conditions (Table 2). In contrast, in experiment 2, in which three episodes of social defeat or control conditions were used, there was a sex*stress interaction on the total number of TH-ir cells (F1,35 = 4.06, p = 0.05) in ventral VTA sections. Stressed females (Fig. 1B) had more TH-ir cells than control females (Fig. 1A; Fig. 3A; F1,35 = 6.37, p = 0.02) and there was no difference in males. In ventral VTA sections, stressed females had significantly more TH/c-fos colocalizations than control females (Fig. 2; Fig. 3B; Mann-Whitney U = 21.00, p = 0.03). There were no differences in total c-fos-ir cells in the ventral VTA (Table 2). In middle sections of the VTA, there were no significant effects of sex or stress on total TH neurons or TH/c-fos colocalizations (Fig. 1C, 1D; Fig. 3C, 3D). There was a sex*stress interaction for total c-fos-ir cells (F1,34=5.86, p = 0.02). In middle VTA planned comparisons showed that stressed females had fewer c-fos-ir cells than control females (F1,34=11.87, p = 0.002) and that there was no effect of stress in males (Table 2). In the dorsal VTA, there was a sex*stress interaction (Fig. 3E, F1,34= 3.98, p=0.05) on TH-ir neurons. Planned comparisons indicated a trend for stressed females (Fig. 1F) to have significantly more TH-ir cells than control females (Fig. 1E, F1,34=3.96, p = 0.06) and that there was no difference between control and stressed males. There were no significant effects of sex or stress on TH/c-fos colocalizations (Fig. 3F) or total c-fos counts in dorsal VTA sections.
Table 2.
Mean ± SE cell counts from mice undergoing a single episode of defeat or control conditions. Data are shown through the ventral-dorsal extent of VTA and SNc.
| Region and Count | Male Control | Male Stress | Female Control | Female Stress |
|---|---|---|---|---|
| Ventral SN TH | 112.0 ± 10.0 | 139.7 ± 17.2 | 173.0 ± 2.0 | 134.5 ± 12.2 |
| Middle SN TH | 123.0 ± 51.0 | 174.0 ± 31.5 | 230.5 ± 7.5 | 155.3 ± 11.4 |
| Dorsal SN TH | 157.5 ± 26.5 | 172.0 ± 10.1 | 170.5 ± 14.5 | 154.8 ± 7.2 |
| Ventral SN c-fos | 12.5 ± 2.5 | 13.3 ± 2.2 | 18.0 ± 6.0 | 17.3 ± 2.3 |
| Middle SN c-fos | 13.0 ± 7.0 | 8.0 ± 2.5 | 13.5 ± 4.5 | 10.3 ± 1.8 |
| Dorsal SN c-fos | 13.5 ± 6.5 | 7.0 ± 3.1 | 11.5 ± 2.5 | 10.2 ± 2.0 |
| Ventral SN TH/cfos | 0.0 ± 0.0 | 1.0 ± 1.0 | 0.0 ± 0.0 | 1.3 ± 0.6 |
| Middle SN TH/cfos | 0.5 ± 0.5 | 1.0 ± 0.6 | 0.0 ± 0.0 | 0.3 ± 0.2 |
| Dorsal SN TH/cfos | 1.0 ± 1.0 | 0.3 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Ventral VTA TH | 74.0 ± 13.1 | 79.2± 5.3 | 69.3 ± 6.9 | 54.5 ± 7.5 |
| Middle VTA TH | 101.0 ± 20.9 | 116.4 ± 9.7 | 125.0 ± 19.5 | 97.2 ± 15.8 |
| Dorsal VTA TH | 77.0 ± 13.0 | 70.2 ± 6.0 | 73.2 ± 9.2 | 48.0 ± 8.2 |
| Ventral VTA c-fos | 17.0 ± 6.2 | 29.2 ± 7.5 | 24.3 ± 15.8 | 17.3 ± 6.0 |
| Middle VTA c-fos | 23.3 ± 4.4 | 18.0 ± 5.4 | 17.3 ± 10.7 | 31.6 ± 12.2 |
| Dorsal VTA c-fos | 22.3 ± 9.4 | 22.8 ± 6.0 | 23.5 ± 15.0 | 21.0 ± 10.5 |
| Ventral VTA TH/cfos | 8.0 ± 3.8 | 6.5 ± 2.3 | 2.8 ± 1.0 | 5.2 ± 1.9 |
| Middle VTA TH/cfos | 7.5 ± 3.4 | 8.2 ± 2.2 | 4.0 ± 2.5 | 6.8 ± 2.5 |
| Dorsal VTA TH/cfos | 4.0 ± 3.4 | 3.6 ± 2.2 | 9.7 ± 3.3 | 9.6 ± 2.9 |
Fig. 3.
Cell count data following 3 episodes of social defeat or control conditions (n = 7–15 per group). Females, but not males, had more TH-ir neurons (A) and more TH/c-fos colocalizations (B) in the ventral VTA after stress. There were no effects of stress on TH-positive neurons (C) or TH/c-fos colocalizations (D) in the middle sections of VTA. Females, but not males, had more TH-ir neurons in the dorsal VTA after stress (E). There were no effects of stress on TH/c-fos colocalizations (F) in dorsal sections of VTA. Graphs display mean ± SE. *p<0.05 effect of stress.
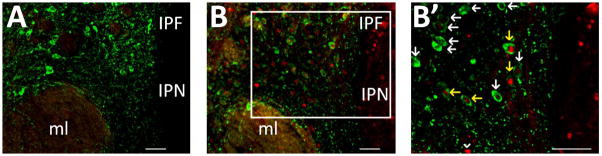
Fig. 2.
Representative images of c-fos (fluorescent red staining) and TH (fluorescent green staining) expression in a control female (A) and stressed female mouse (B) following 3 consecutive days of treatment; scale bars = 100 μm. Markers interpenduncular nucleus (IPN), interpeduncular fossa (IPF), and medial lemniscus (ml) are labeled. (B′) magnified view of area enclosed in the white box in the corresponding panel; scale bar = 100 μm. Yellow arrows designate c-fos immunoreactive nuclei within TH immunoreactive cell bodies, white arrows designate cells immunoreactive for TH alone, and white arrow heads designate cells immunoreactive for c-fos only.
In the SNc, there were no effects of sex or stress on total dopamine neurons, TH/c-fos colocalizations or total c-fos cells within ventral, middle or dorsal sections following 3 episodes of social defeat stress or control conditions (Table 3). Following a single episode of social defeat stress, there were no effects of sex or stress on SN TH neurons, TH/c-fos colocalizations, or total c-fos cell counts in the ventral, middle, or dorsal sections (Table 3).
Table 3.
Table showing mean ± SE cell counts from mice undergoing three episodes of defeat or control conditions. Data are shown through the ventral-dorsal extent of VTA and SNc.
| Region and Count | Male Control | Male Stress | Female Control | Female Stress |
|---|---|---|---|---|
| Ventral SN TH | 142.6 ± 12.6 | 130.1 ± 15.4 | 155.3 ± 35.0 | 174.7 ± 23.8 |
| Middle SN TH | 165.7 ± 25.3 | 163.4 ± 27.1 | 172.5 ± 28.8 | 177.1 ± 23.0 |
| Dorsal SN TH | 133.0 ± 27.9 | 151.4 ± 29.6 | 139.0 ± 22.7 | 134.0 ± 16.4 |
| Ventral SN c-fos | 10.3 ± 2.8 | 13.0 ± 4.5 | 7.0 ± 2.0 | 9.0 ± 1.7 |
| Middle SN c-fos | 13.0 ± 2.2 | 9.1 ± 2.8 | 13.3 ± 4.6 | 8.6± 1.9 |
| Dorsal SN c-fos | 6.6 ± 2.4 | 6.6 ± 2.1 | 7.5 ± 1.9 | 6.3 ± 1.9 |
| Ventral SN TH/c-fos | 1.00± 0.5 | 1.8 ± 0.6 | 2.8 ± 1.4 | 2.1 ± 0.9 |
| Middle SN TH/c-fos | 0.8 ± 0.5 | 0.7 ± 0.2 | 2.5 ± 1.0 | 1.0 ± 0.5 |
| Dorsal SN TH/c-fos | 1.4 ± 1.3 | 2.0 ± 0.9 | 2.0 ± 1.1 | 0.9± 0.7 |
| Ventral VTA TH | 60.9 ± 6.0 | 56.2 ± 7.7 | 66.9 ± 9.3 | 94.2 ± 6.9* |
| Middle VTA TH | 83.9 ± 19.2 | 77.1 ± 17.9 | 92.4 ± 12.4 | 102.5 ± 9.2 |
| Dorsal VTA TH | 63.5 ± 6.5 | 49.3 ± 5.7 | 74.7 ± 10.7 | 104.1 ± 11.2+ |
| Ventral VTA c-fos | 9.6 ± 2.9 | 11.3 ± 2.9 | 8.0 ± 4.6 | 8.7 ± 1.4 |
| Middle VTA c-fos | 21.1 ± 4.2 | 21.9 ± 5.6 | 27.0 ± 6.1 | 8.3 ± 1.5* |
| Dorsal VTA c-fos | 19.6 ± 5.7 | 31.0 ± 8.3 | 23.9 ± 9.3 | 11.1 ± 4.2 |
| Ventral VTA TH/c-fos | 5.6 ± 2.1 | 6.4 ± 3.1 | 2.0 ± 0.8 | 5.6 ± 1.0* |
| Middle VTA TH/c-fos | 10.5 ± 3.7 | 9.1 ± 4.9 | 17.0 ± 6.4 | 5.4 ± 1.3 |
| Dorsal VTA TH/c-fos | 12.5 ± 4.4 | 5.8 ± 2.3 | 12.0 ± 5.1 | 8.1 ± 2.9 |
p < 0.05 vs female control,
p = 0.06 vs female control
Experiment 3: Long term effects of social defeat on mesolimbic circuits
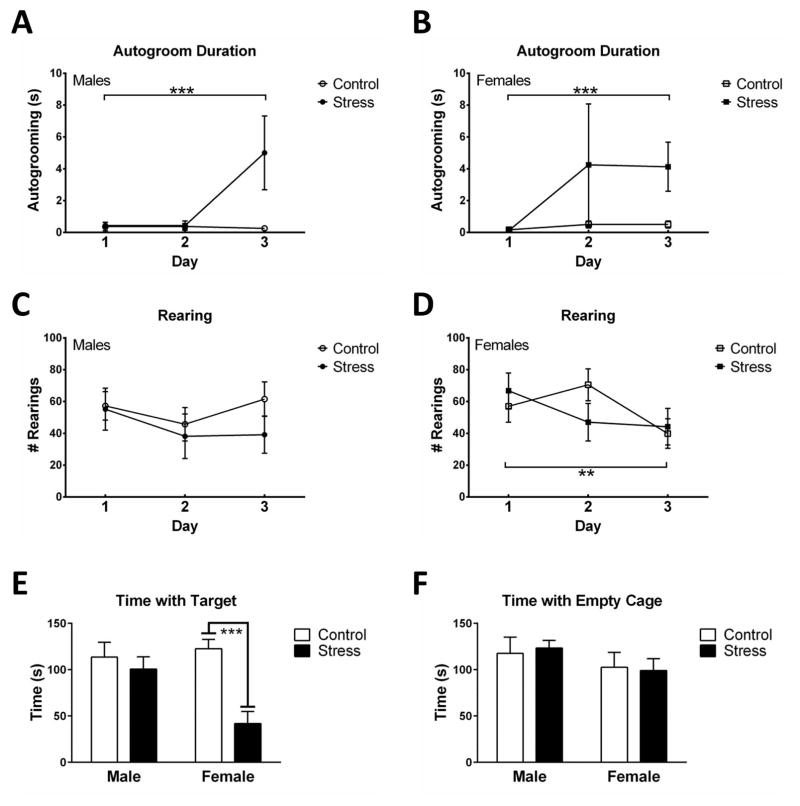
Immediately prior to social defeat episodes or control conditions, the duration of autogrooming increased over three days in stressed mice but not controls (Repeated measures ANOVA, F2,50=6.66, p<0.01), and there were no sex differences (p’s > 0.5). The duration of autogrooming increased on day 3 compared to day 1 in stressed mice (Fig. 4A; paired t14=4.812, p<0.001) but not controls. The effect of stress on rearing was different in males and females across the three day testing period (Repeated measures ANOVA, F2,50 = 3.39, p = 0.04). Rearing decreased on day 3 compared to day 1 in females (paired t13=2.83, p=0.01) but not males. There were no differences in number of backflips, number of digs, or digging duration (Table 4).
Fig. 4.
Autogrooming increased in male and female mice from day 1 to day 3 during three consecutive days prior to social defeat (A,B, n = 7–8 per group). Rearing decreased from day 1 to day 3 in females (D) but not males (C). Female mice that had been exposed to defeat stress 2 weeks prior to social interaction tests spent significantly less time in the cage zone when a target mouse was present compared to control females (E). There were no differences in time spent in the cage zone when the cage was empty (F). Graphs display mean ± SE. ** p<0.01, *** p<0.001 effect of stress.
Table 4.
Behavioral data from Experiment 3
| Behavior | Male Control | Male Stress | Female Control | Female Stress |
|---|---|---|---|---|
| Before Social Defeat | ||||
| Backflips Day 1 | 18.9 ± 8.8 | 28.3 ± 14.1 | 2.8 ± 1.3 | 5.5 ± 4.4 |
| Backflips Day 2 | 32.8 ± 19.6 | 51.0 ± 21.8 | 19.3 ± 11.1 | 19.9 ± 13.3 |
| Backflips Day 3 | 17.9 ± 11.8 | 34.9 ± 14.9 | 15.7 ± 8.3 | 22.4 ± 12.6 |
| Digs Day 1 | 1.3 ± 0.7 | 2.1 ± 1.4 | 1.8 ± 0.8 | 1.0 ± 0.7 |
| Digs Day 2 | 1.4 ± 0.7 | 1.0 ± 0.7 | 0.8 ± 0.5 | 1.3 ± 0.9 |
| Digs Day 3 | 1.0 ± 0.8 | 0.7 ±0.3 | 1.2 ± 0.6 | 1.5 ± 0.8 |
| Digging Duration Day 1 (s) | 2.5 ± 1.7 | 1.6 ± 0.9 | 1.8 ± 1.1 | 0.9 ± 0.5 |
| Digging Duration Day 2 (s) | 1.3 ± 0.7 | 0.4 ± 0.3 | 0.0 ± 0.2 | 0.8 ± 0.4 |
| Digging Duration Day 3 (s) | 0.9 ± 0.7 | 0.7 ± 0.3 | 0.5 ± 0.2 | 7.6 ± 7.3 |
| Social Interaction Test | ||||
| Open Field Center Time (s) | 41.9 ± 11.8 | 34.1 ± 3.1 | 29.8 ± 4.6 | 34.8 ± 4.3 |
| Open Field Distance (m) | 28.5 ± 3.0 | 23.5 ±3.6 | 25.1 ± 3.1 | 24.6 ± 2.9 |
| Time in cage zone (no target, s) | 117.6 ± 17.5 | 123.4 ± 8.3 | 102.6 ± 16.0 | 99.2 ± 12.8 |
| Time in cage zone (target mouse, s) | 113.6 ± 16.1 | 100.6 ± 13.2 | 122.6 ± 10.0 | 41.8 ± 13.1*** |
p < 0.01 vs corresponding control,
p < 0.001 vs corresponding control
Two weeks later, during the social interaction test there was a significant sex*stress interaction for time spent in the cage zone when a target mouse was present (Fig. 4C; F1,26=6.31, p=0.02). Planned comparisons showed that stressed female mice spent significantly less time in the interaction zone than control females (F1,26=17.87, p<0.001), but this difference was not present in male mice. There were no significant effects of sex or stress on time in the cage (Fig. 4D) or corners zones during the acclimation (no target) phase. There were also no significant effects of sex or stress on distance travelled during the test, time in the center during the open field phase or time in the corners zone during the interaction phase (Table 4).
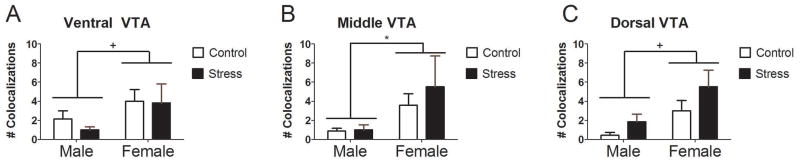
Following the social interaction test, there were no effects of sex or stress on SN or VTA total TH-ir neurons, or total c-fos-ir cell counts in the ventral, middle, or dorsal sections (Table 5. However, there were significantly more TH/c-fos colocalizations in the middle VTA of female (Fig. 5B) mice than male mice (Fig. 5A; Fig. 6B; Mann-Whitney U = 149.0, p=0.017) and similar nonsignificant trends in ventral VTA (Fig. 6A; Mann-Whitney U = 138.0, p=0.07) and dorsal VTA (Fig. 6C; Mann-Whitney U = 138.0, p=0.07). If all three subregions are combined, females had significantly more TH/c-fos colocalizations than males (Mann-Whitney U = 160.5, p < 0.01). There were also no effects of sex or stress on total c-fos cell counts in BNSTpr, BNSTlp, infralimbic cortex (ILCx), or prelimbic cortex (PRLCx), NAc core, or anterior NAc shell (Table 5). In anterior BNST subregions, average c-fos cell counts were higher in females than males but this difference was not significant (main effects of sex p’s 0.14–0.17). Females mice showed more c-fos counts in posterior NAc shell (Fig. 5C, 5D; F1,24=10.15, p<0.01, FDR corrected p = 0.06) than males. In correlational analyses of cell count data a near-significant, positive correlation was detected between ventral VTA colocalizaitons and posterior NAc shell (ρ=0.702, p<0.001, FDR corrected p = 0.06), and this correlation was stronger in females (ρ=0.707, p=0.01) than males (ρ=0.513, p=0.06), with the strongest correlation in stressed females when broken down by sex and treatment (ρ=0. 880, p=0.020).
Table 5.
Mean ± SE cell counts from mice in experiment 3
| Region and Count | Male Control | Male Stress | Female Control | Female Stress |
|---|---|---|---|---|
| Ventral SN TH | 100.1 ± 17.1 | 116.6 ± 19.6 | 123.4 ± 10.8 | 138.3 ± 16.0 |
| Middle SN TH | 134.3 ± 8.5 | 114.0 ± 19.6 | 128.6 ± 18.4 | 144.3 ± 11.6 |
| Dorsal SN TH | 143.9 ± 8.9 | 130.9 ± 16.8 | 135.9 ± 13.8 | 155.5 ± 14.6 |
| Ventral SN c-fos | 5.6 ± 1.4 | 3.7 ± 1.1 | 8.9 ± 2.4 | 7.2 ± 1.3 |
| Middle SN c-fos | 4.9 ± 1.0 | 5.1 ± 2.1 | 8.7 ± 3.0 | 7.2 ± 1.9 |
| Dorsal SN c-fos | 3.0 ± 1.0 | 5.9 ± 2.1 | 7.1 ± 2.3 | 7.7 ± 1.5 |
| Ventral SN TH/c-fos | 0.4 ± 0.2 | 0.1 ± 0.1 | 0.6 ± 0.4 | 0.8 ± 0.3 |
| Middle SN TH/c-fos | 0.8 ± 0.3 | 0.1 ± 0.1 | 0.7 ± 0.4 | 0.7 ± 0.3 |
| Dorsal SN TH/c-fos | 0.3 ± 0.2 | 0.7 ± 0.4 | 0.3 ± 0.2 | 0.5 ± 0.3 |
| Ventral VTA TH | 103.6 ± 6.4 | 100.4 ± 11.7 | 95.9 ± 8.1 | 120.2 ± 15.1 |
| Middle VTA TH | 120.0 ± 11.4 | 105.7 ± 8.1 | 107.6 ± 16.8 | 126.7± 10.70 |
| Dorsal VTA TH | 106.6 ± 5.1 | 98.9 ± 8.1 | 108.6 ± 9.3 | 129.2 ± 12.8 |
| Ventral VTA c-fos | 11.4 ± 2.2 | 13.6 ± 4.7 | 34.9± 11.1 | 19.3 ± 5.2 |
| Middle VTA c-fos | 10.5 ± 3.6 | 9.6 ± 4.4 | 31.9 ± 8.6 | 23.2 ± 4.9 |
| Dorsal VTA c-fos | 12.8 ± 5.3 | 14.3 ± 5.9 | 26.3 ± 5.3 | 24.3 ± 5.0 |
| Ventral VTA TH/c-fos | 2.1 ± 0.9 | 1.0 ± 0.3 | 4.00 ± 1.2† | 3.83 ± 2.0† |
| Middle VTA TH/c-fos | 0.9 ± 0.3 | 1.0 ± 0.5 | 3.6 ± 1.2* | 5.5 ± 3.3* |
| Dorsal VTA TH/c-fos | 2.4 ± 2.0 | 1.9 ± .8 | 3.0 ± 1.1† | 5.5 ± 1.8† |
| ILCx c-fos | 108.7 ± 27.2 | 104.3 ± 29.4 | 88.4 ± 14.8 | 93.4 ± 15.0 |
| PRLCx c-fos | 81.7 ± 28.3 | 46.6± 8.7 | 52.9 ± 11.1 | 59.8 ± 36.4 |
| Anterior NAcC c-fos | 259.6 ± 101.2 | 263.4 ± 100.6 | 210.7 ± 54.6 | 233.6 ± 47.4 |
| Anterior NAcSh c-fos | 234.7 ± 98.9 | 123.9 ± 30.7 | 139.3 ± 30.4 | 144.8± 38.1 |
| Posterior NAcC c-fos | 104.7 ± 23.5 | 85.0 ± 16.9 | 123.0 ± 22.2 | 134.5 ± 24.4 |
| Posterior NAcSh c-fos | 60.4 ± 8.4 | 60.7 ± 8.7 | 112.3 ± 18.0† | 112.0 ± 22.3† |
| BNSTal c-fos | 216.6 ± 65.0 | 226.3 ± 49.9 | 327.8 ± 95.9 | 333.5 ± 72.1 |
| BNSTam c-fos | 236.4± 67.5 | 227.4 ± 50.7 | 339.8 ± 100.0 | 339.4 ± 71.4 |
| BNSTvl c-fos | 161.3 ± 62.0 | 186.1 ± 49.1 | 273.7 ± 79.1 | 244.8 ± 54.7 |
| BNSTvm c-fos | 222.1 ± 68.0 | 196.9 ± 40.4 | 308.2 ± 87.8 | 309.2 ± 62.9 |
| BNSTpr c-fos | 3.7 ± 1.1 | 6.8 ± 2.0 | 5.0 ± 1.5 | 5.9 ± 1.3 |
| BNSTlp c-fos | 5.0 ± 1.3 | 11.7 ± 1.9 | 12.1 ± 3.4 | 13.9 ± 2.3 |
p < 0.05 vs corresponding male group
p = 0.06 vs corresponding male group
Fig. 5.
Representative photomicrographs of TH (green)/c-fos (red) immunostaining in the middle sections of VTA in control female (A) and male (B) mice following the social interaction test. Markers fasciculus retroflexus (fr), interpenduncular nucleus (IPN) and medial lemniscus (ml) are labeled. Representative photomicrographs of fos staining in the same order by sex are shown for posterior NAc shell (C,D). There was a significant sex difference, with females having higher counts than males, in all of these regions. Scale bars = 100 μm for all images.
Fig. 6.
Cell count data following social interaction with 3 episodes of social defeat or control conditions 2 weeks prior (n=6–8 per group). There were no effects of stress or sex on TH/c-fos colocalizations in ventral VTA sections (A). Females had significantly more TH/c-fos colocalizations in middle VTA sections than males (B). There were no effects of stress or sex on TH/c-fos colocalizations in dorsal VTA sections (C). Graphs display mean ± SE. *p<0.05 sex difference, +nonsignificant trend for sex difference (p = 0.07).
Discussion
Here, we tested for dorsal-ventral topographical specificity of VTA dopamine neuron responses to aversive contexts by examining c-fos and TH expression immediately following a single episode or three consecutive days of social defeat stress. We also examined whether effects of social defeat were long lasting and extended to a non-agonistic social context two weeks after the final day of chronic social defeat stress. We demonstrated that a third episode of defeat stress selectively increased c-fos expression in dopamine neurons in the ventral VTA of females. This suggests that there is dorsal-ventral organization in responsiveness of VTA dopamine neurons to aversive contexts. This was previously observed in anesthetized rats exposed to electric shocks (Brischoux et al. 2009), and we now extend this finding to social defeat in awake female California mice. Furthermore, no increases in TH/c-fos colocalizations were observed in any subregion of the adjacent SNc, which indicates that this neural response is highly localized to the ventral VTA. Intriguingly, patterns of TH/c-fos expression were much different following social interaction tests. Although no effect of defeat stress on TH/c-fos staining was observed in the VTA, females had more than twice as many TH/c-fos stained neurons across the entire VTA compared to males. In experiments 1 and 2 there were no sex differences in TH/c-fos cell counts in control animals, which were not exposed to social stimuli. This indicates that the sex difference in TH/c-fos colocalizations observed in the social context of experiment 3 is not simply a baseline sex difference in TH/c-fos staining. This difference most likely reflects increased responsiveness of VTA dopamine neurons to social contexts in females versus males.
Short term effects of defeat stress in ventral VTA
To our knowledge, this is the first report of dorsal-ventral organization in the responsiveness of VTA dopamine neurons to a stressor in a non-anesthetized rodent. Although the overall number of TH/c-fos neurons in stressed females were low (11 or less), the location of this increase is consistent with a previous study on anesthetized animals. Brischoux and colleagues (2009) demonstrated that the majority of recorded dopamine neurons were inhibited or showed no response following repeated footshocks administered to an anesthetized male rat. However, a small number of dopaminergic neurons (5/14 neurons) had increased firing rates, and these neurons were confined to anterior medioventral parts of the VTA. In non-anesthetized rabbits, neurons activated by an acoustic stimulus paired with an electric shock were mostly confined to the ventral VTA (i.e., ventral to the apex of the fossa), and neurons that showed decreased activity to the conditioned stimulus were more dorsal (Guarraci and Kapp 1999). In this study, however, it was not clear whether these cells responding to conditioned stimuli were dopamine neurons.
In addition to the heterogeneity of VTA subpopulations that have been found to respond to aversive contexts, several studies have also reported the number of dopaminergic neurons responding to aversive contexts to be highly variable. Previous studies examining the impact of aversive contexts on dopamine neurons have examined many types of aversive stimuli and have observed variable results (Marinelli and McCutcheon 2014). In a TH/c-fos double-label study similar to our study, 30 minutes of restraint stress increased the number of TH-positive neurons that displayed fos-like immunoreactivity in VTA by 7-fold compared to controls when brains were collected 4.5 hr after restraint (Deutch et al. 1991). However, when brains were collected 2.25 hrs after restraint, there was no difference from controls. Similarly, a single electric shock inhibited the activity of putative dopamine neurons in anesthetized rats but repeated foot shocks generated increased activity in a population of putative dopamine neurons in medial VTA (Valenti et al. 2011). A recent study used in vivo calicium imaging to visualize the activity of VTA dopamine neurons during fear conditioning in freely moving mice. Calcium transients were present following the conditioned stimulus on day 2 of conditioning in a small percentage of dopaminergic neurons that were not present following the conditioned stimulus during the first day of conditioning, suggesting fear learning induces plasticity with experience (Gore et al. 2014). A common thread is that when VTA dopamine neurons respond to noxious contexts with increased activity, it is usually after repeated exposure. Similarly, previous studies reporting defeat-induced increases in dopamine content (Mos and Van Valkenburg 1979; Puglisi-Allegra and Cabib 1990) or dopamine release (Tidey and Miczek 1996) in the NAc observed these effects after multiple episodes of social defeat. In rats, about 50% of dopamine neurons in the ventral VTA project to the NAc shell (Melis et al. 2007). Overall, it appears that the duration of the stressor, and also its predictability, are important factors determining the responsiveness of VTA dopamine neurons.
In male rodents defeat-induced social withdrawal is modulated by excitability of VTA dopamine neurons (Chaudhury et al. 2013), and these changes in neurophysiology are present 4 weeks post-stress (Krishnan et al. 2008; Razzoli et al. 2011). Defeat stress also increased dopamine and HVA content in the nucleus accumbens of both male and female California mice two weeks following defeat (Campi et al. 2014). However, it appears that c-fos immunohistochemistry may not be an optimal technique for detecting these forms of long-lasting changes. Optical inhibition of VTA dopamine neurons projecting to the NAc attenuated defeat-induced social withdrawal in male mice (Chaudhury et al. 2013), indicating that hyperactivity of VTA dopamine neurons plays an important role in mediating social withdrawal. Optical activation of VTA dopamine neurons has been reported to reduce behavioral despair and anhedonia in male mice exposed to chronic mild stress (Tye et al. 2013). At first glance, the two optogenetic studies appear to produce contradictory results. However, social defeat stress induces chronic hyperactivity in VTA dopamine neurons while chronic mild stress reduces the activity of VTA dopamine neurons (Chang and Grace 2014). In both studies, manipulations that minimized the impact of psychosocial stress on VTA dopamine neurons had antidepressant effects. Thus, while different forms of stress may have different effects on VTA dopamine neurons, interventions that restore normal activity appear to have therapeutic effects.
In addition to dorsal-ventral topographic organization, tracing studies have found medial-lateral organization of VTA dopamine neuron populations in relation to their projections to medial and lateral subdivisions of NAc shell, NAc core, and PFC (Lammel et al. 2008). An aversive hindpaw formalin injection increased AMPA/NMDAR ratios in PFC-projecting and NAc-projecting medial and lateral VTA dopamine neurons, respectively (Lammel et al. 2011). The increased AMPA/NMDAR ratio in medial VTA mesocortical dopamine neurons paralleled the increase in excited dopamine neurons identified in the ventromedial VTA by Brischoux and colleagues (2009) following noxious footshock. Interestingly, NAc shell-projecting neurons in lateral VTA, but not medial VTA, were found to have importance for aversive signaling. Thus, these experiments suggest that increased responses of VTA dopamine neurons following aversive contexts occur across the medial-lateral extent of the VTA, and that track tracing can further refine a medial/lateral gradient of aversive signaling within VTA subdivisions. Although our approach did not allow for the refined analysis of VTA subdivisions along a medial-lateral axis, future studies that combine track tracing with dorsal/ventral divisions could further distinguish the topographical specificity of dopamine neurons that differentially respond to appetitive and aversive contexts.
Effects of stress on TH cell number
Intriguingly, a third episode of defeat stress increased the total number of TH-positive cells in the ventral VTA of females. A similar trend was observed in the dorsal VTA. This effect could be mediated by sex differences in glucocorticoid levels. Dexamethasone (a synthetic glucocorticoid) rapidly increases TH protein expression but not TH mRNA (Chen et al. 2008; Tank et al. 2008). Interestingly, female California mice have higher corticosterone levels immediately following social conflict than males. This effect is observed in female residents (Trainor et al. 2010) and intruders (Trainor et al. 2013). This sex difference is dependent on gonadal hormones (Trainor et al. 2013), similar to other rodent species (Viau 2002; Handa and Weiser 2014). Chronic mild stress increases corticosterone levels (Grippo et al. 2005) and was found to increase TH protein in the VTA as measured with western blot (Ortiz et al. 1996). It is possible that high corticosterone levels need to be maintained in order to promote increased TH expression in the VTA (Ortiz et al. 1995). An important aspect of experiment 3 is that animals were not exposed to any stressors outside of routine husbandry after the last episode of defeat. In previous studies using this design, corticosterone levels in stressed female California mice are no different from control females two weeks following control or stress manipulations are performed (Trainor et al. 2011). This could explain why a change in TH cell number was observed immediately after but not two weeks after a third episode of defeat.
Glucocorticoid signaling in the mesolimbic dopamine system has been previously identified as important for defeat-induced social withdrawal in male rodents. In male mice, selective deletion of glucocorticoid receptors (GR) on D1 receptor expressing neurons in the NAc (but not VTA dopamine neurons) blocked defeat-induced social withdrawal (Barik et al. 2013). In California mice, ovariectomy blocks post-defeat increases in corticosterone levels in females but does not block defeat-induced social withdrawal (Trainor et al. 2013). Although this would appear to contradict the GR deletion study, the GR deletion was accomplished using a dopamine transporter regulatory element, which results in GR deletion occurring early in life. Intriguingly, developmental exposure to dexamethasone induced stronger increases in TH immunoreactivity in the VTA of female rats versus males (McArthur et al. 2006). Plasticity in TH immunostaining has also been observed in adults. In prairie voles, androgens increase TH positive cell counts in the BNST and medial amygdala, and these differences were hypothesized to reflect different levels of TH synthesis that facilitated detection via immunohistochemistry (Northcutt et al. 2007). These findings suggest that sex-specific changes in hormone responses have important effects on dopamine cells. Similarly, we speculate that repeated stress in female California mice increases TH production and therefore facilitates the detection of TH neurons via immunohistochemistry. Increased TH immunoreactivity in VTA was observed in rats exposed to chronic unpredictable stress for 10 days (Ortiz et al. 1996), and this increase was hypothesized to enhance the magnitude of dopamine synthesis. Since we only observed increased TH immunoreactivity immediately after a third episode of defeat, this suggests that TH expression is affected by ongoing stressors.
Sex differences in TH/c-fos following a social interaction test
Although we did not observe effects of social defeat on TH/c-fos colocalizations in the social interaction test, females had more TH/c-fos colocalizations across the entire extent of the VTA compared to males. There were no sex differences in TH/c-fos colocalizations in control animals in experiments 1 and 2, which were placed into a novel empty cage. This indicates that the sex differences in TH/c-fos in experiment 3 cannot solely be attributed to exposure to a novel environment and is most likely mediated by the social context. Several reports suggest that the mesolimbic dopamine system responds more strongly to appetitive contexts in females than males. Following i.p. injection of amphetamine greater increases of dopamine release in the NAc was observed in female rats compared to males (Virdee et al. 2014). Consistent with this effect, females are more likely to develop conditioned place preference (CPP) to psychostimulants (Lynch et al. 2002; Russo et al. 2003; Carroll and Anker 2010; Trainor 2011). Natural rewards also generate stronger responses in VTA dopamine neurons in females. In juvenile rats, social play induces significant increases in TH/c-fos cells in the VTA of females but not males (Northcutt and Nhuyen 2014). Interestingly, a recent study used in vivo calcium imaging to demonstrate increased activity of VTA dopamine neurons in female mice during social interactions with an unfamiliar females (Gunaydin et al. 2014). Together, these data support the hypothesis that across the entire VTA, dopamine neurons respond to social interactions more strongly in females compared to males.
It is possible that gonadal hormones might have an effect on TH/c-fos expression in male and female California mice. In the VTA, androgen receptors are expressed on VTA dopamine neurons and nondopamine neurons express estrogen receptor α (Kritzer and Creutz 2008). We did not monitor estrous cycle, so it’s possible that the estrous stage may have affected cell counts. However, gonadectomy has no effect on behavior in the social interaction test and does not alter sex differences in social withdrawal (Trainor et al. 2013). In addition, in both control and stressed female California mice, there were no differences in social interaction behavior across different stages of the estrous cycle (Trainor et al. 2011). Furthermore a recent metanalysis showed that variability in studies of females that do not control for estrous cycle stage was no different than studies of males (Prendergast et al. 2014). Thus although estrous cycle could have had an impact on cell counts, it does not alter the fact that important sex differences were observed in both experiment 2 and 3.
Anxiety-like behavior prior to a third episode of defeat stress
Behavioral data in experiment 3 showed that anxiety-like behaviors were induced immediately before a third episode of defeat while mice assigned to control conditions showed no changes in behavior. Over the course of the three day testing period, only mice exposed to defeat increased autogrooming behavior immediately prior to each testing session. Autogrooming is an anxiety-like behavior that is reduced by anxiolytic drugs such as diazepam (Dunn et al. 1981; Moody et al. 1988) and is induced by chronic mild stress in California mice (Harris et al. 2013) and restraint stress in prairie voles (Smith and Wang 2014). The increased autogrooming observed in mice assigned to defeat suggests that transportation to the testing room on day 3 triggers a conditioned response of anxiety-like behavior. Although we did not directly compare mice exposed to one versus three episodes of stress, it was only after a third episode of defeat that increases in TH/c-fos colocalizations were observed. At this point it is not clear why three episodes of defeat stress, which induced increased autogrooming behavior, did not increase TH/c-fos colocalizations in males. Experiments 1 and 2 did not include an experimental group with a sedated resident, which raises the possibility that changes in TH/c-fos cell counts may have been caused by exposure to olfactory cues from the resident. Whether simply placing a socially stressed animal into the same room it experienced previous episodes of defat could increase TH/c-fos colocalizations would also be an intriguing future experiment.
In experiment 3 females had more c-fos positive cells in the posterior NAc shell. Ventral VTA colocalizations were also positively correlated with c-fos in the posterior NAc shell in females but not males, and this correlation was strongest in stressed females. Posterior subregions of the NAc have been found to have stronger effects on aversive behavioral responses while more anterior subregions of NAc have important effects on appetitive behavioral responses (Richard and Berridge 2011). Posterior regions of NAc are activated by BLA stimulation (Gill and Grace 2011) and also receive stronger norepinephrine input (Berridge et al. 1997). A previous study showed that females exposed to defeat had more pCREB positive cells in the NAc compared to control females immediately following a social interaction test (Trainor et al. 2011). In the 2011 study pCREB cell counts were taken primarily in posterior regions of NAc, but anterior-posterior subregions were not directly compared. Together these results suggest that anterior and posterior NAc neurons have different effects on the valence generated by social stimuli, and suggest that there are sex differences in how these neurons are affected by social defeat.
Conclusions
We demonstrated important sex differences in how VTA dopamine neurons respond to social contexts generating approach and aversion. Although it is well established that social stressors have important effects on the function of VTA dopamine neurons, few studies have considered females. Our results indicate that in the aversive context of repeated social defeat, neurons in the ventral VTA are preferentially activated in females. In contrast, in the context of a social interaction test (which normally induces strong approach responses), dopamine neurons across the entire VTA are more responsive in females. Together these results suggest that VTA dopamine neurons are more responsive to both positive and negative social contexts in females, and that the short term effect of negative contexts on VTA dopamine neurons is localized primarily to the ventral VTA. This suggests that further study of sex differences in VTA function is warranted, given that depression is often linked to altered function in the mesolimbic dopamine system (Russo and Nestler 2013). Future studies looking at effects of stressors on the mesolimbic dopamine system should consider males and females as well as topographical organization in both the NAc and VTA.
Acknowledgments
We thank Elyssa Margolis and Katharine Campi for advice on neuroanatomy. We thank Dr. Cindy Clayton for veterinary assistance; David Gibson, Kyle Scroggins, Claire Manning, Elizabeth Takahashi, and Shannon Yeh for technical assistance. This work was supported by NIH T32 MH 82174-3 (GDG), F31 MH095253 (MQS), and R01 MH097714 01A1 (BCT).
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest
Contributors
This study was designed by GDG, BCT and MQS. All authors acquired data and contributed to its interpretation. Statistical analyses were performed by GDG and BCT. GDG and BCT wrote the manuscript.
References
- Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G, Tassin JP, Mombereau C, Faure P, Tronche F. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. 2013;339:332–335. doi: 10.1126/science.1226767. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Stratford TL, Foote SL, Kelley AE. Distribution of dopamine beta-hydroxylase-like immunoreactive fibers within the shell subregion of the nucleus accumbens. Synapse. 1997;27:230–241. doi: 10.1002/(SICI)1098-2396(199711)27:3<230::AID-SYN8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci. 2012;35:1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proceedings of the National Academy of Sciences. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi KL, Greenberg GD, Kapoor A, Ziegler TE, Trainor BC. Sex differences in effects of dopamine D1 receptors on social withdrawal. Neuropharmacology. 2014;77:208–216. doi: 10.1016/j.neuropharm.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi KL, Jameson C, Trainor BC. Sexual dimorphism in the brain of the monogamous California mouse (Peromyscus californicus) Brain Behav Evol. 2013;81:236–249. doi: 10.1159/000353260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi KL, Jameson CE, Trainor BC. Sexual dimorphism in the brain of the monogamous California mouse (Peromyscus californicus) Brain, behavior and evolution. 2013;81:236–249. doi: 10.1159/000353260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JL, Covington HE, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, Nestler EJ, Han MH. Mesolimbic Dopamine Neurons in the Brain Reward Circuit Mediate Susceptibility to Social Defeat and Antidepressant Action. The Journal of Neuroscience. 2010;30:16453–16458. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Haile CN, Coppersmith R, Hayashi Y, Malinow R, Neve RL, Nestler EJ. Distinct sites of opiate reward and aversion within the midbrain identified using a herpes simplex virus vector expressing GluR1. J Neurosci. 2000;20:RC62. doi: 10.1523/JNEUROSCI.20-05-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WAJ, Haile CN, Coppersmith R, Hayashi Y, Malinow R, Neve RL, Nestler EJ. Distinct sites of opiate reward and aversion within the midbrain identified using a herpes simplex virus vector expressing GluR1. J Neurosci. 2000;20(5):RC62. doi: 10.1523/JNEUROSCI.20-05-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Hormones and Behavior. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Chang CH, Grace AA. Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry. 2014;76:223–230. doi: 10.1016/j.biopsych.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Xu L, Radcliffe P, Sun B, Tank AW. Activation of tyrosine hydroxylase mRNA translation by cAMP in midbrain dopaminergic neurons. Molecular Pharmacology. 2008;73:1816–1828. doi: 10.1124/mol.107.043968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch AY, Lee MC, Gillham MH, Cameron DA, Goldstein M, Iadarola MJ. Stress selectively increases fos protein in dopamine neurons innervating the prefrontal cortex. Cerebral Cortex. 1991;1:273–292. doi: 10.1093/cercor/1.4.273. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Guild AL, Kramarcy NR, Ware MD. Benzodiazepines decrease grooming in response to novelty but not ACTH or b-endorphin. Pharm Biochem Behav. 1981;15:605–608. doi: 10.1016/0091-3057(81)90217-3. [DOI] [PubMed] [Google Scholar]
- Gill KM, Grace AA. Heterogeneous processing of amygdala and hippocampal inputs in the rostral and caudal subregions of the nucleus accumbens. Int J Neuropsychopharmacol. 2011;14:1–14. doi: 10.1017/S1461145710001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore BB, Soden ME, Zweifel LS. Visualization of plasticity in fear-evoked calcium signals in midbrain dopamine neurons. Learning & Memory. 2014;21:575–579. doi: 10.1101/lm.036079.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, Trainor BC. Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front Behav Neurosci. 2014;7:223. doi: 10.3389/fnbeh.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Kapp BS. An electrophysiological characterization of ventral tegmental area dopaminergic neurons during differential pavlovian fear conditioning in the awake rabbit. Behavioural Brain Research. 1999;99:169–179. doi: 10.1016/s0166-4328(98)00102-8. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BN, de Jong TR, Yang V, Saltzman W. Chronic variable stress in fathers alters paternal and social behavior but not pup development in the biparental California mouse (Peromyscus californicus) Hormones and Behavior. 2013;64:799–811. doi: 10.1016/j.yhbeh.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock JW, Waymire JC. Activating antibodies to tyrosine hydroxylase. J Biol Chem. 1982;257:9416–9423. [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward learning. Nat Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Research Reviews. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice NJ, Yuan ZF, Sawchenko PE, Vale W. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin releasing factor system. J Comp Neurol. 2008;511:479–496. doi: 10.1002/cne.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas J, Korte S, De Boer S, Van Der Vegt B, Van Reenen C, Hopster H, De Jong I, Ruis M, Blokhuis H. Coping styles in animals: current status in behavior and stress-physiology. Neuroscience & Biobehavioral Reviews. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, LaPlant Q, Graham A, Lutter M, Lagace DC. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han M-H, Mazei-Robison M, Iñiguez SD, Ables JL, Vialou V, Berton O, Ghose S, Covington HE, III, Wiley MD. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biological psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Creutz LM. Region and sex differences in consituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci. 2008;28:9525–9535. doi: 10.1523/JNEUROSCI.2637-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique Properties of Mesoprefrontal Neurons within a Dual Mesocorticolimbic Dopamine System. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76:351–359. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laredo SA, Steinman MQ, Robles CF, Ferrer E, Ragen BJ, Trainor BC. Effects of defeat stress on behavioral exibility in males and females: modulation by the mu-opioid receptor. Eur J Neurosci. 2015;41:434–441. doi: 10.1111/ejn.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch W, Roth M, Carroll M. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2012;32:13309–13325. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Coker AR, Driscoll JR, Lemaitre A-I, Fields HL. Reliability in the Identification of Midbrain Dopamine Neurons. PLoS ONE. 2010;5:e15222. doi: 10.1371/journal.pone.0015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Himmels P, Morales M, Fields HL. Identification of rat ventral tegmental area GABAergic neurons. Plos One. 2012;7:e42365. doi: 10.1371/journal.pone.0042365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. κ opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2006a;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? The Journal of Physiology. 2006b;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, McCutcheon JE. Heterogeneity of dopamine neuron activity across traits and states. Neuroscience. 2014;282:176–197. doi: 10.1016/j.neuroscience.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur S, McHale E, Gillies GE. The size and distribution of midbrain dopaminergic populations are permanently altered by perinatal glucocorticoid exposure in a sex-region-and time-specific manner. Neuropsychopharmacology. 2006;32:1462–1476. doi: 10.1038/sj.npp.1301277. [DOI] [PubMed] [Google Scholar]
- Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, Boi A, Ferri GL, Argiolas A. Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur J Neurosci. 2007;26:1026–1035. doi: 10.1111/j.1460-9568.2007.05721.x. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE. Social stress, therapeutics and drug abuse: Preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody TW, Merali Z, Crawley JN. The effects of anxiolytics and other agents on rat grooming behavior. Ann N Y Acad Sci. 1988;525:281–290. doi: 10.1111/j.1749-6632.1988.tb38613.x. [DOI] [PubMed] [Google Scholar]
- Mos J, Van Valkenburg CFM. Specific effect on social stress and aggression on regional dopamine metabolism in rat brain. Neurosci Lett. 1979;15:325–327. doi: 10.1016/0304-3940(79)96134-2. [DOI] [PubMed] [Google Scholar]
- Northcutt KV, Nhuyen JM. Female juvenile play elicits Fos expression in dopaminergic neurons of the VTA. Behav Neurosci. 2014;128:178–186. doi: 10.1037/a0035964. [DOI] [PubMed] [Google Scholar]
- Northcutt KV, Wang Z, Lonstein JS. Sex and species differences in tyrosine hydroxylase-synthesizing cells of the rodent olfactory extended amygdala. J Comp Neurol. 2007;500:103–115. doi: 10.1002/cne.21148. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. The Vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. The Journal of Comparative Neurology. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, Hughes T, Self DW, Neve RL, Nestler EJ. Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. The Journal of Neuroscience. 2005;25:5553–5562. doi: 10.1523/JNEUROSCI.0345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, DeCarpio JL, Kosten TA, Nestler EJ. Strain-selective effects of corticosterone on locomotor sensitization to cocaine and on levels of tyrosine hydroxylase and glucocorticoid receptor in the ventral tegmental area. Neuroscience. 1995;67:383–397. doi: 10.1016/0306-4522(95)00018-e. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Lane S, Terwilliger R, Nestler EJ. Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology. 1996;14:443–452. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- Phillipson O. A Golgi study of the ventral tegmental area of Tsai and interfascicular nucleus in the rat. Journal of Comparative Neurology. 1979;187:99–115. doi: 10.1002/cne.901870107. [DOI] [PubMed] [Google Scholar]
- Pignatelli M, Bonci A. Role of Dopamine Neurons in Reward and Aversion: A Synaptic Plasticity Perspective. Neuron. 2015;86:1145–1157. doi: 10.1016/j.neuron.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Polter AM, Kauer JA. Stress and VTA synapses: implications for addiction and depression. European Journal of Neuroscience. 2014;39:1179–1188. doi: 10.1111/ejn.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Cabib S. Effects of defeat experiences on dopamine metabolism in different brain areas of the mouse. Aggressive Behavior. 1990;16:271–284. [Google Scholar]
- Razzoli M, Andreoli M, Michielin F, Quarta D, Sokal D. Increased phasic activity of VTA dopamine neurons in mice 3 weeks after repeated social defeat. Behavioural BrainResearch. 2011;218:253–257. doi: 10.1016/j.bbr.2010.11.050. [DOI] [PubMed] [Google Scholar]
- Ribble DO, Millar JS. The mating system of northern populations of Peromyscus maniculatus as revealed by radiotelemetry and DNA fingerprinting. Ecoscience. 1996;3:423–428. [Google Scholar]
- Ribble DO, Salvioni M. Social organization and nest coocupancy in Peromyscus californicus, a monogamous rodent. Behav Ecol Sociobiol. 1990;26:9–15. [Google Scholar]
- Richard JM, Berridge KC. Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D1 alone for appetitive eating but D1 and D2 together for fear. J Neurosci. 2011;31:12866–12879. doi: 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian MR, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Catalan MJ, Kaufling J, Georges F, Veinante P, Barrot M. The antero-posterior heterogeneity of the ventral tegmental area. Neuroscience. 2014;282:198–216. doi: 10.1016/j.neuroscience.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Schiltz JC, Sawchenko PE. Specificity and generality of the involvement of cetecholaminergic afferents in hypothalamic responses to immune insults. J Comp Neurol. 2007;502:455–467. doi: 10.1002/cne.21329. [DOI] [PubMed] [Google Scholar]
- Schultz W. Updating dopamine reward signals. Current Opinion in Neurobiology. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Wang Z. Hypothalamic Oxytocin Mediates Social Buffering of the Stress Response. Biological psychiatry. 2014;76:281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Laredo SA, Lopez EM, Manning CE, Hao RC, Doig IE, Campi KL, Flowers AE, Knight JK, Trainor BC. Hypothalamic vasopressin systems are more sensitive to social defeat in males versus females. Psychoneuroendocrinology. 2015;51:122–134. doi: 10.1016/j.psyneuen.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Research Bulletin. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tank AW, Xu LC, Chen X, Radcliffe P, Sterling CR. Post-transcriptional regulation of tyrosine hydroxylase expression in adrenal medulla and brain. Ann N Y Acad Sci. 2008;1148:238–248. doi: 10.1196/annals.1410.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimibic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Trainor BC. Stress responses and the mesolimbic dopamine system: social contexts and sex differences. Horm Behav. 2011;60:457–469. doi: 10.1016/j.yhbeh.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, Crean KK. Sex Differences in Social Interaction Behavior Following Social Defeat Stress in the Monogamous California Mouse (Peromyscus californicus) PLoS One. 2011;6:e17405. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, Crean KK. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus) PLOS One. 2011;6:e17405. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, Laredo SA, Orr VN, Silva AL, Steinman MQ. Sex differences in stress-induced social withdrawal: independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Horm Behav. 2013;63:543–550. doi: 10.1016/j.yhbeh.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Takahashi EY, Silva AL, Crean KK, Hostetler C. Sex differences in hormonal responses to social conflict in the monogamous California mouse. Horm Behav. 2010;58:506–512. doi: 10.1016/j.yhbeh.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai H-C, Finkelstein J, Kim S-Y, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti O, Lodge DJ, Grace AA. Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. The Journal of Neuroscience. 2011;31:4280–4289. doi: 10.1523/JNEUROSCI.5310-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Virdee K, McArthur S, Brischoux F, Caprioli D, Ungless MA, Robbins TW, Dalley JW, Gillies GE. Antenatal glucocorticoid treatment induces adaptations in adult midbrain dopamine neurons, which underpin sexually dimorphic behavioral resilience. Neuropsychopharmacology. 2014;39:339–350. doi: 10.1038/npp.2013.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DV, Tsien JZ. Convergent processing of both positive and negative motivational signals by the VTA dopamine neuronal populations. PloS one. 2011;6:e17047–e17047. doi: 10.1371/journal.pone.0017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotoxicity research. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]