Abstract
Background
Storage-dependent damage to RBCs varies significantly. Identifying RBC units that will undergo higher levels of hemolysis during storage may allow for more efficient inventory management decision-making. Oxidative-stress mediates storage-dependent damage to RBCs and will depend on the oxidant : antioxidant balance. We reasoned that this balance or redox tone will serve as a determinant of how a given RBC unit stores and that its assessment in “young” RBC will predict storage-dependent hemolysis.
Study Design and Methods
RBCs were sampled from bags and segments stored for 7d – 42d. Redox tone was assessed by nitrite oxidation kinetics and peroxiredoxin-2 (Prx-2) oxidation. In parallel, hemolysis was assessed by measuring cell-free hemoglobin and free-heme (hemin). Correlation analyses were performed to determine if d7 measurements predicted either the level of hemolysis at d35 or the increase in hemolysis during storage.
Results
Higher d7 Prx-2 oxidation was associated with higher d35 Prx-2 oxidation suggesting that early assessment of this parameter may identify RBCs that will incur the most oxidative damage during storage. RBCs that oxidized nitrite faster at d7 were associated with greatest levels of storage-dependent hemolysis and increases in Prx-2 oxidation. An inverse relationship between storage-dependent changes in oxyhemoglobin and free heme was observed underscoring an unappreciated reciprocity between these molecular species. Moreover, free heme was higher in the bag compared to paired segments, with opposite trends observed for free hemoglobin.
Conclusion
Measurement of Prx-2 oxidation, nitrite oxidation kinetics early during RBC storage may predict storage-dependent damage to RBC including hemolysis-dependent formation of free hemoglobin and heme.
Keywords: nitric oxide, hemolysis, hemin, oxidative stress, storage lesion
Introduction
Transfusion with RBCs is used for multiple clinical indications. Current guidelines allow RBCs to remain in ‘storage’ solutions at 4°C, for up to 42d. However, recent studies highlight the potential for older stored RBCs, compared to younger RBCs, to elicit transfusion-related toxicity1. Several retrospective studies, together with experimental studies in different animal models, have shown that older RBC provide a second hit that augments microcirculatory dysfunction, inflammatory tissue injury and infection. These consequences underscore the associations between the age and number of RBCs transfused and morbidity and mortality in transfusion recipients2-16. Specific proposed mechanisms of injury include increased oxidative stress and inflammation, inhibition of nitric oxide (NO) signaling and perturbation of iron homeostasis. Mediating this gain of toxicity, are several storage-dependent changes to the RBCs that include formation of smaller and more dense cells (that display increased rates of NO and nitrite-oxidation), release of microparticles, hemolysis and release of free hemoglobin (Hb) and free heme (hemin), loss of ATP and increased levels of products from oxidative stress4,7,14,17-19.
It is also evident that storage lesion toxicity is variable20. The variance stems from both the diverse patient populations that receive RBCs, but also the heterogeneity intrinsic to the RBCs that reflect donor variations in how cells respond to mechanical and biochemical stresses during collection, processing and cold storage. This variation leads to the question: are there biomarkers that could be evaluated early during storage to predict how well or poorly RBCs from a given donor would subsequently store? To date there are no consensus markers that can be used to evaluate the quality of stored RBCs, although recent studies using metabolomic signatures are beginning to suggest that this may be possible21-23. Oxidative stress is a common feature of storage-related damage to the RBCs reflected by increases in lipid and protein oxidation products, and a concomitant decrease in the amount / activity of antioxidants19,23. RBCs are endowed with multiple antioxidant and oxidant sources, however, limiting this candidate approach to identify biomarkers. Our recent work has shown that during storage, basal oxidation of peroxiredoxin-2 (Prx-2) is elevated24. Prx-2 has emerged as the key antioxidant protein that protects RBCs against biologically relevant concentrations of H2O2 produced endogenously (via hemoglobin autoxidation) or exogenously by inflammatory cells25,26.
We have also shown that older RBCs oxidize nitrite with faster rates compared to younger RBCs, which may account for decreased circulating nitrite levels in trauma patients receiving older RBCs14. Nitrite oxidation by oxyhemoglobin is a complex, but well-characterized reaction that proceeds via a lag phase followed by an autocatalytic propagation phase. As elucidated by Keszler et al27, during the lag phase, the rate limiting steps are comprised of nitrite oxidation by oxyferrous heme (Hb2+O2) to form nitrate (NO3-), ferric (met) hemoglobin (Hb3+) and H2O2, with the latter two species then reacting to form ferryl hemoglobin radical (Hb4+-P•)(Equations 1-2). During the propagation phase, nitrite reduces ferryl hemoglobin radical to ferryl hemoglobin (reaction 3), and ferryl hemoglobin to ferric hemoglobin (reaction 4) with concomitant formation of the nitrogen dioxide radical (NO2•), that is then oxidized to nitrate by oxyHb (reaction 5).
| Equation 1 |
| Equation 2 |
| Equation 3 |
| Equation 4 |
| Equation 5 |
Importantly, the length of the lag phase and rate of metHb formation in either lag or propagation phase will reflect the balance between reactive species (H2O2 and NO2•) formation and endogenous antioxidant systems thereby integrating information about oxidative / antioxidant balance. In the current study, we evaluated the inter-relationships between nitrite oxidation, Prx-2 oxidation and storage-dependent changes in hemolysis with the goal of identifying potential parameters that could be measured early to predict how a given unit of RBCs will store.
Materials and Methods
Materials
Antibodies against Peroxiredoxin-2 were purchased from Abcam (Cambridge, MA). Goat anti-rabbit IgG antibody conjugated to alkaline phosphatase was purchased from Millipore (Billerica, MA). All other materials were purchased from Sigma–Aldrich (St Louis, MO).
Human RBC collection and processing
Leukoreduced RBCs (RBCs), in Adsol stored for 7 days or 35 days were collected from segments (∼1ml) attached to units from the UAB Hospital blood bank. All protocols were approved by the UAB Institutional Review Board. Two study designs were employed. The first used a cross-sectional study to develop a protocol to assess nitrite oxidation. In the second, a paired study design was employed whereby for each RBC unit, 2 segments were collected. One segment was processed immediately for storage day 7 (d7) measurements of biochemical indices described below. The remaining segment was stored at 4°C, in the dark until reaching 35 days and then processed as matched d7 samples. At each time point segments were emptied into a 1.7ml Eppendorf tube and red cells pelleted by centrifugation (1500g, 10min, 4°C). The supernatant was collected to measure cell-free hemoglobin and free heme. In a separate study, leukoreduced RBC units with attached segments were purchased from the American Red Cross to assess potential differences between RBCs present in segments and bags. Units and segments were sampled using aseptic approaches at d35 and d42 and selected assays performed.
Measurement of hemoglobin concentration
For intracellular hemoglobin, packed RBCs were hemolysed in water (1:10) and hemoglobin concentration determined by visible spectroscopy (450-700nm) followed by deconvolution using standard spectra for oxyhemoglobin, deoxyhemoglobin, methemoglobin and heme (hemin) as previously described28. A similar approach was used for extracellular hemoglobin and heme measurement from the supernatant. Standard spectra measured at pH 7.4 and 6.8 were used for d7 and d35 samples respectively reflecting measured pH of these samples. Ten of 42 samples were excluded due to extensive turbidity that precluded obtaining an accurate absorbance spectrum. Centrifugation of these samples (20,800×g for 10min, 4°C) did not improve their clarity. For subsequent experiments, all samples were diluted 10-fold into Adsol resulting in turbidity levels that did not interfere with spectra
Nitrite oxidation kinetics
RBCs were pelleted, washed once in ice-cold isotonic phosphate buffer [80 mM NaPi, 40 mM NaCl, 10 mM KCl, 100μM DTPA] by centrifugation (1500g, 10min, 4°C) and then lysed 1:8 (vol: vol (50μl RBC + 350μl ultrapure deionized water). The lysates were diluted to 100μM hemoglobin concentration in PBS containing 100 μM DTPA, pH 7.4. Sodium nitrite (0-500μM final) was added (total assay volume of 200μl) and time dependent disappearance of oxyhemoglobin was assessed at 570nm measured in a Perkin Elmer Victor2 plate reader at 25°C for 3h. The length of the lag phase, rate in the lag phase and rate during propagation phase was determined as shown in Figure 1 and previously described27,29.
Figure 1. Effects of storage on hemolysate mediated nitrite oxidation kinetics.
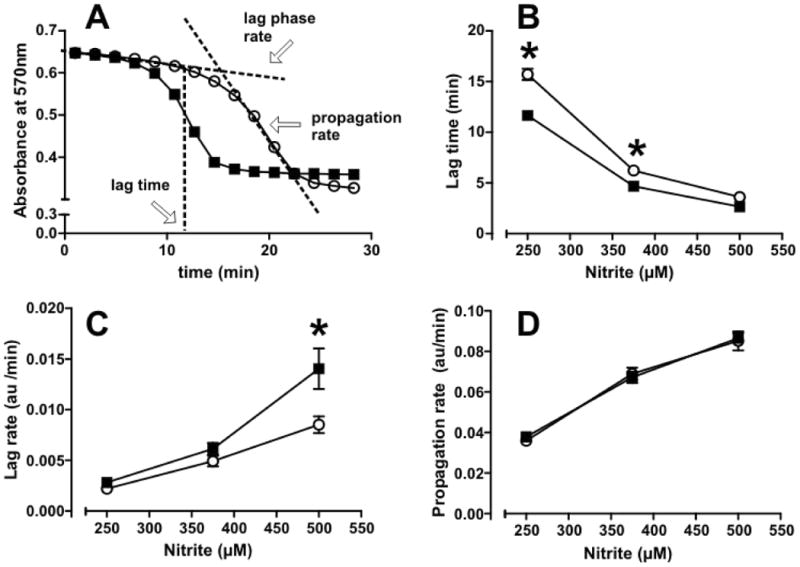
Panel A shows a representative tracing for nitrite (250μM)-mediated oxidation by d7 and d35 RBC hemolysates (25μM hemoglobin, (concentrations reflect heme) assessed by absorbance change at 570nm, at 25°C, in PBS + 100μM DTPA. Panel B-D show respectively, lag times, lag rates and propagation rates plotted as a function of nitrite added to d7 (○) and d35 (■) hemolysates. Data are mean ± SEM, n=10. *p<0.05 by 2-way ANOVA with Bonferroni post tests.
Peroxiredoxin-2 oxidation
Pelleted RBCs were washed 3 times with isotonic saline buffer [80 mM NaPi, 40 mM NaCl, 10 mM KCl, 100μM DTPA] at 4°C by centrifugation (1500g, 10min per wash). The dimeric (disulfide) and monomeric (reduced) forms of peroxiredoxin-2 (Prx-2) was determined by Western blotting under non-reducing conditions as previously described30,31. Briefly, immediately upon collection of cells from segments, RBCs were diluted to 50μM heme in isotonic saline and treated with 100mM NEM for 15 minutes at 37°C to alkylate thiols and prevent Prx-2 oxidation. RBC were then pelleted (2000g, 30 seconds) at room temperature and lysed in 100mM NEM containing SDS-PAGE sample buffer without beta-mercaptoethanol. Proteins were resolved by SDS-PAGE and transferred onto nitrocellulose. Membranes were blocked with 5% non-fat dried skimmed milk powder (w/v) in TBS-T (137mM NaCl, 2.6mM KCl, 0.05% Tween-20 (v/v) in 25mM Tris-HCl pH 7.4) for 1h at room temperature and then washed with TBS-T for 30 minutes. Membranes were incubated with rabbit poly or monoclonal antibody against Prx-2 followed by secondary alkaline phosphatase-linked goat anti-rabbit IgG antibody (AP132A, Millipore). Blots were developed with Duo-Lux Chemiluminescent / Fluorescent substrate (Vector Laboratories, Inc.) and exposed to film. Prx-2 oxidation was assessed by the amount of dimeric Prx-2 (MWt ∼38KDa) divided by the total Prx-2 (dimeric + monomeric (19KDa) forms).
Statistical Analyses
Paired t-test was performed to determine significance between paired groups. For testing of correlations, data normality was first determined by the D'Agostino and Pearson omnibus normality test and then Pearson or Spearman correlations performed accordingly. Statistical analyses were performed using GraphPad Prism.
Results
Effects of storage on nitrite oxidation
The goal of this study was to assess storage-dependent damage to RBCs using selected end-points related to structural stability / hemolysis, oxidative stress and nitrite metabolism, and to determine if evaluation of these parameters early during storage, could predict ensuing storage-dependent damage. We initially established a plate-reader assay that allows assessment of nitrite oxidation by RBC hemolysates in a high throughput setting (as described in Methods). Figure 1 shows representative absorbance vs time traces after addition of nitrite to a d7 and d35 RBC hemolysate. A typical time course for nitrite-dependent hemoglobin oxidation is observed comprising an initial lag phase followed by a propagation phase. The length of the lag phase, and rates of oxidation in the lag and propagation phases were determined as shown in Figure 1A (as previously described27,29). Figures 1B-1C show that lag times were lower and lag rates faster in d35 RBC hemolysates compared to d7. No differences in propagation rates were observed (Figure 1D).
Effects of storage on hemolysis and Prx-2 oxidation
Next, using a paired study design, we tested if there were any inter-relationships between nitrite oxidation kinetics, Prx-2 oxidation and hemolysis-derived free hemoglobin and free-heme. Figures 2A-F show changes in oxyhemoglobin, methemoglobin and free heme in both the intraerythrocytic and supernatant fractions in both d7 and d35 RBC. Significant storage-dependent increases for all species in the cell-free fraction were observed, with no storage-dependent differences observed in the erythrocyte. Figure 2G shows storage also resulted in a significant increase in Prx-2 oxidation.
Figure 2. Effects of storage on hemolysis and Prx-2 oxidation.
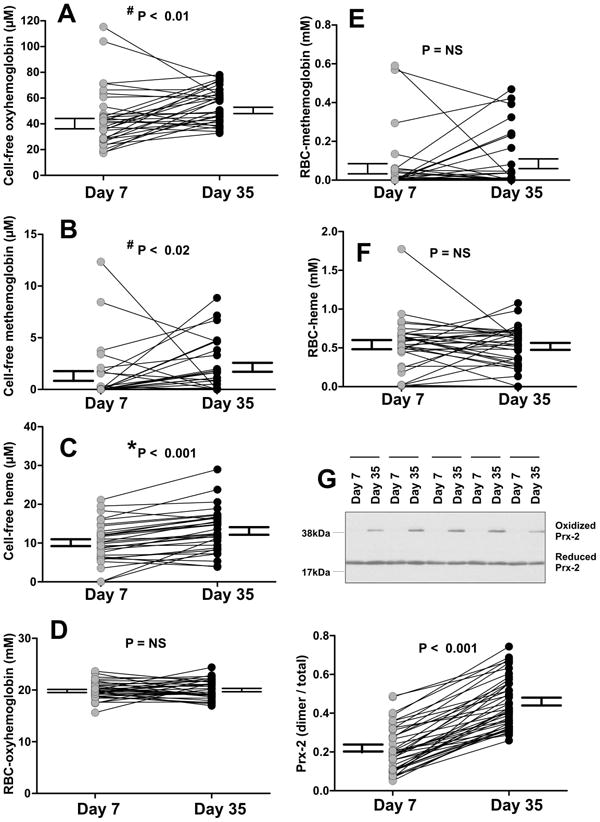
Shown are paired assessments in d7 and d35 supernatant fraction for cell-free oxyhemoglobin (panel A), cell-free methemoglobin (Panel B), cell-free heme (Panel C), RBC oxyhemoglobin (Panel D), RBC-methemoglobin (Panel E) and RBC-heme (Panel F) Each point (n=32) denotes sampling from an individual donated RBC unit. Panel G shows oxidized Prx-2 : total Prx-2 ratio determined by western blotting (n= 42). A representative western blot with 5 paired d7 and d35 samples are shown. Indicated P-values by paired t-test using *normal or #non-normal (Wilcoxon signed rank test) distribution.
Figure 3 shows the storage-dependent change (d35 – d7) in cell-free oxyhemoglobin methemoglobin, free heme and Prx-2 oxidation. For all these parameters, the effects of storage varied ∼5-10 fold (around the mean) underscoring the heterogeneity between RBCs from different donors to storage-dependent changes. In some cases opposite trends were observed; for example cell-free oxy or methemoglobin and free heme was lower at 35 days in 10-15% of RBC segments.
Figure 3. Heterogeneity of storage dependent changes in hemolysis and Prx-2 oxidation.
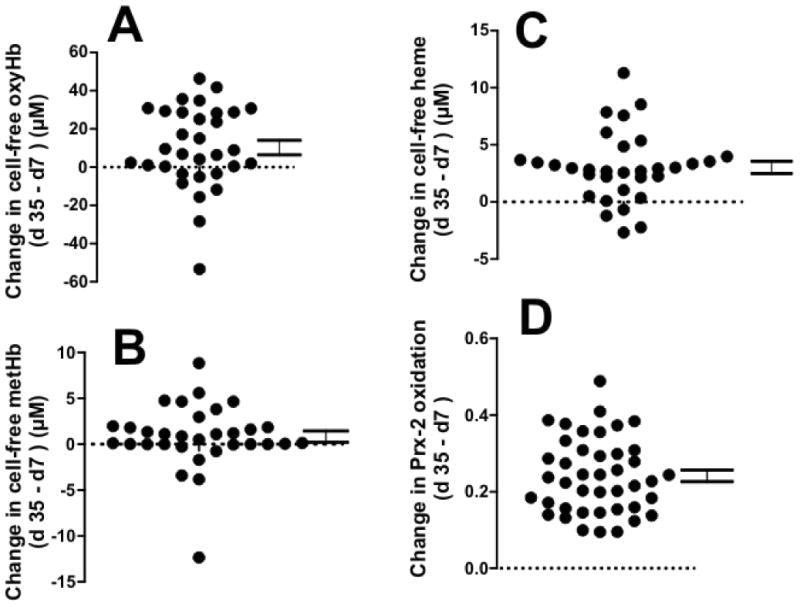
Storage dependent changes (d35 – d7) in cell-free oxyhemoglobin (Panel A), methemoglobin (Panel B), free heme (Panel C) and Prx-2 oxidation in RBC (Panel D) were calculated. Each symbol represents a unique RBC unit.
We next performed correlation analysis to determine if there were any associations between the selected oxidative stress, hemolysis and nitrite oxidation indices, and to assess if d7 measurements could predict changes observed in d35 samples.
Prx-2 oxidation
Figure 4A shows d7 Prx-2 oxidation was a strong predictor of Prx-2 oxidation at d35. Figures 4B-D shows that d7 Prx-2 oxidation correlates positively with nitrite oxidation kinetics in the lag phase (i.e. inversely with lag time, positively with lag rates, Figures 4B-C respectively). However, no correlation with propagation phase kinetics was observed (Figure 4D), Moreover, no correlations between d7 Prx-2 oxidation and d35 nitrite oxidation kinetics were observed.
Figure 4. Basal Prx-2 oxidation dependent correlations.
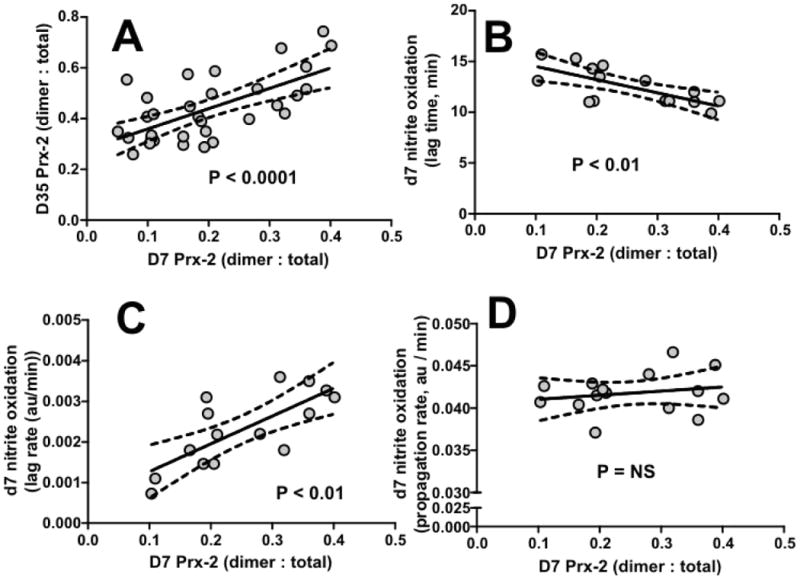
Panel A shows correlations between Prx- oxidation at d7 and d35. Panels B-D show respectively, d7 Prx-2 oxidation correlations with d7 lag times, d7 lag rates and d7 propagation rates for nitrite oxidation. P-values indicate significance by Pearson correlations; line show linear regression with 95% confidence intervals of fit.
Nitrite oxidation
Interestingly, the lag phase and propagation phase of nitrite oxidation showed correlations selectively with changes in extracellular free-heme or hemoglobin respectively. Figures 5A-5B, show that at d7, faster rates for nitrite oxidation in the lag phase were positively associated with higher concentrations of extracellular free heme at d7 and d35. Figures 5C-5D show that d7 propagation rates for nitrite oxidation correlate with both d7 and d35 cell-free oxyhemoglobin. Figure 5E shows that d7 nitrite oxidation propagation rates also positively correlate with the extent of Prx-2 oxidation that occurred during storage (i.e. d35 – d7 Prx-2 oxidation in RBC).
Figure 5. Nitrite oxidation kinetics dependent correlations.
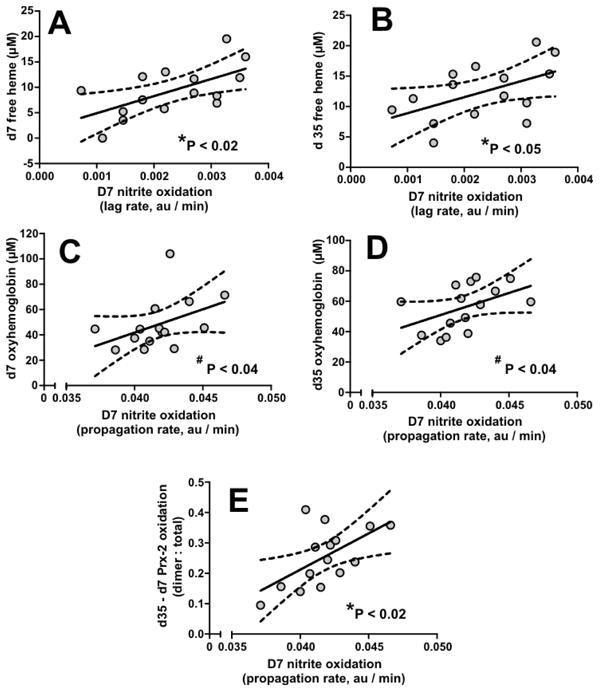
Panel A-B show correlations between rate of nitrite oxidation in the lag phase from d7 RBC with d7 and d35 free heme levels, respectively. Panel C-D show correlations between rate of nitrite oxidation in the propagation phase from d7 RBCs with d7 and d35 extracellular free hemoglobin levels respectively. Panel E shows the correlation between rate of nitrite oxidation in the propagation phase from d7 RBCs with storage dependent increases in Prx-2 oxidation (d35 – d7). P-values indicate significance by *Pearson or #Spearman correlations; line show linear regression with 95% confidence intervals of fit.
Heme and hemoglobin
Free heme levels at d7 positively correlated with d35 free heme (Figure 6A). Day 35 free heme and d35 oxyhemoglobin levels were inversely correlated (Figure 6B). Interestingly, d7 oxyhemoglobin strongly and inversely correlated with the storage-dependent changes in cell-free oxyhemoglobin (Figure 6C), with the opposite association observed for storage-dependent changes in cell-free heme (Figure 6D). Consistent with this observation, an inverse correlation between storage-dependent changes in cell-free oxyhemoglobin and free heme was observed (Figure 6E).
Figure 6. Heme and hemoglobin dependent correlations.
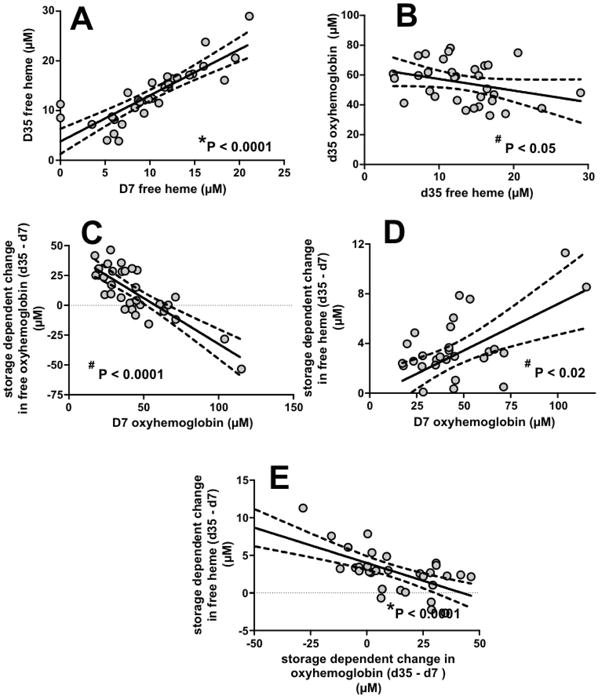
Panel A-B show correlations between d7 free heme levels and d35 free heme and d35 oxyhemoglobin, respectively. Panel C-D show correlations between d7 oxyhemoglobin and storage-dependent increases in free oxyhemoglobin and heme, respectively. Panel E shows the correlation between storage dependent changes in free oxyhemoglobin and free heme. P-values indicate significance by *Pearson or #Spearman correlations; line show linear regression with 95% confidence intervals of fit.
Bag vs segment sampling
The data presented above were collected using RBCs obtained from segments attached to RBC units. Recent studies have shown that segments can differ from the bag; for example hemolysis may be higher, but complement levels lower in the segment compared to the paired bag32,33. We therefore compared nitrite oxidation kinetics, Prx-2 oxidation, hemoglobin and heme levels in samples collected from paired bag and segments stored for 35 and 42 days. Figures 7A-C show that nitrite oxidation kinetics were faster with d42 compared to d35 RBC. However, no difference between bag and segments was observed except for ∼25% slower lag rates in d35 segments vs d35 bags (Figure 7B). Figures 7D-E shows that there was a modest increase in hemolysis-derived hemoglobin from d35 to d42 in segments, but no changes in free heme. Interestingly, while a trend towards increase hemoglobin in the segments vs bags was observed, consistent with previous studies33, the opposite was observed for free heme (Figure 7E). Figure 7F plots the total hemolysis-derived heme containing products (hemoglobin + heme) and shows that hemolysis per se between segments and bags was not different. No differences in basal Prx-2 oxidation between bags and segment were observed (not shown).
Figure 7. Effects of bag vs segment sampling.
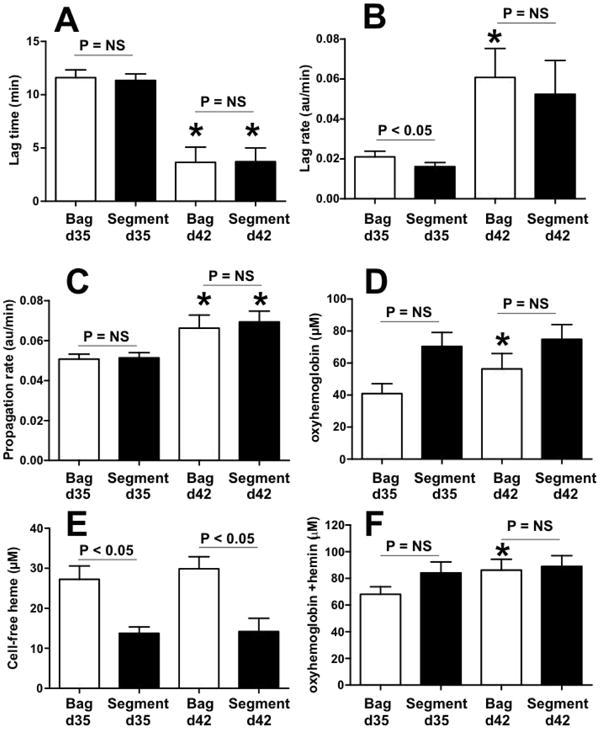
RBC collected from bags and paired segments after storage for 35 days or 42 days and nitrite oxidation kinetics measured by lag times (Panel A using 250μM nitrite), lag rates (Panel B using 500μM nitrite), propagation rates (Panel C using 250μM nitrite). Panels D-F show respectively levels of oxyhemoglobin, free heme and the total (oxyhemoglobin + free heme) in paired bags and segments. *P < 0.05 by paired t-test relative to respective bag or segment at d35. NS = not significant by paired t-test. Data show mean ± SEM, n=4-5.
Discussion
Our goals in this study were to evaluate potential relationships / associations between biochemical indices of RBC storage and specifically whether measurement of some of these parameters early during storage could predict the effects of storage on RBC. Storage is known to result in increased hemolysis which in turn results in loss of NO-signaling, oxidative stress and inflammation post-transfusion. More recently, we have also shown that free heme is also released during storage and may mediate further inflammation28. Thus, identifying RBC units that are likely to undergo higher levels of hemolysis / oxidation during storage may allow inventory management decision-making to transfuse these units earlier rather than later. A common element of storage-dependent changes in RBCs is oxidative stress. We therefore focused on Prx-2 oxidation and oxidation of exogenously added nitrite. Prx-2 oxidation - reduction cycling has emerged as the predominant pathway for detoxifying H2O2 in RBC and is subject to loss of activity during RBC storage24. Moreover, stored RBCs display faster rates of nitrite oxidation compared to younger RBCs and this reaction is sensitive to metabolism of both reactive oxygen (H2O2) and nitrogen (NO2•) species. Assessing nitrite oxidation may provide a sensitive and functional index of RBC damage by integrating information about the general oxidative / antioxidant balance in RBCs. We also reasoned that evaluating these associations may generate new hypotheses regarding potential biochemical mechanisms underlying storage-dependent damage to the RBC. Next we discuss salient observations from these analyses:
The higher the basal (d7) level of Prx-2 oxidation, the higher the level of Prx-2 oxidation in the same RBC after 35 days of storage. These data suggest that early assessment of Prx-2 oxidation may identify RBCs that will have the greatest degree of oxidative damage at d35. In addition, nitrite oxidation kinetics correlated with Prx-2 oxidation. Specifically, shorter lag times and faster rates of oxidation in the lag phase (both indicative of higher oxidative stress) correlated with Prx-2 oxidation consistent with lag phase kinetics and Prx-2 both being sensitive to H2O2 reactivity. Furthermore, propagation rates, which are sensitive to NO2• reactivity, did not correlate with Prx-2 oxidation supporting the conclusion that lag phase kinetics and Prx-2 report on H2O2 metabolism in RBCs. These data also suggest that evaluation of Prx-2 oxidation may provide insights into the potential of stored RBCs to deplete nitrite in the recipient.
-
Nitrite oxidation parameters on d7 RBC also predicted changes in hemolysis. Specifically, lag phase kinetics positively correlated with both d7 and d35 free heme levels, whereas d7 propagation phase kinetics correlated with d7 and d35 free oxyhemoglobin levels. These data raise the hypothesis that nitrite oxidation kinetics analyses of RBCs may identify those units that will be associated with the greatest amount of storage-dependent hemolysis. Furthermore, d7 propagation kinetics also positively correlated with the magnitude of storage-dependent Prx-2 oxidation. In other words, the faster rate of d7 nitrite oxidation predicted greater extents of oxidative damage incurred by the RBCs during storage. Taken together these data suggest the potential for assessing nitrite oxidation in “young” or “fresh” RBCs to identify those units that will incur the greatest oxidative and structural damage.
Moreover we note that while both nitrite oxidation and Prx-2 oxidation are sensitive to H2O2 metabolism, only nitrite oxidation correlated with hemolysis. Although why remains to be determined,we speculate that nitrite oxidation is a more sensitive readout for oxidant : antioxidant status as it integrates both H2O2 and •NO2 formation / metabolism, whereas Prx-2 only informs on H2O2-dependent processes.
-
Higher levels of extracellular free heme at d7 predicted higher levels at d35 also, although it should be noted that the magnitude by which heme increased during storage was relatively low (∼30%). Therefore, the majority of extracellular free heme present is formed during collection and processing. More intriguingly are the relationships between extracellular free heme and hemoglobin. A modest but significant negative correlation was observed between free heme and hemoglobin levels at d35 consistent with the hypothesis that free heme emanates from hemoglobin breakdown, i.e., as the latter decreases, the former increases. This process is not quantitative however, and perhaps reflects an incomplete understanding of free heme metabolism and compartmentalization. One limitation of our assay is that it does not detect free heme in hydrophobic compartments. We also note that significant levels of free heme were measured in the RBC itself, and thus, free heme measured outside the RBC may arise from extracellular hemoglobin denaturation and / or from RBC lysis.
We observed an intriguing relationship between d7 cell-free oxyhemoglobin predicting the storage-dependent changes in hemolysis. The higher d7 cell-free oxyhemoglobin level the greater the degree of free heme formation during storage. However, the opposite was observed with respect to storage-dependent changes in cell-free oxyhemoglobin and an inverse relationship between the storage-dependent changes in oxyhemoglobin and free heme was also observed. These data underscore the reciprocity between oxyhemoglobin and free heme levels discussed in iii) above, and support the hypothesis that metabolism of oxyhemoglobin and free heme are linked. Indeed, in some cases, cell-free hemoglobin in fact decreased with storage which may reflect hemoglobin degradation with the concomitant rise in free heme, although variation between segments cannot be excluded. More surprising is the observation that higher levels of oxyhemoglobin at d7 are associated with lower storage-dependent hemolysis. Cell-free hemoglobin is a pro-oxidant and we speculate that its presence early during storage catalyzes further oxidative damage and hemolysis, with the inverse relationship between d7 oxyhemoglobin and extent of hemolysis reflecting the fact that hemoglobin can be a more potent stimulator of lipid peroxidation at lower total concentrations, where there is a greater concentration of hemoglobin dimers; hemoglobin dimers are more potent pro-oxidant than hemoglobin tetramers.
Differences between red cells in bags compared to their partnered segments have been noted. Assessing RBC storage parameters only from the bag would be ideal and provide direct insights into what transfusion recipients receive; however, this is not practical for experimental testing especially for temporal-based (longitudinal) studies or when higher numbers of replicates are required. We tested if bag vs segment differences exist in nitrite oxidation kinetics, however no differences were observed with the exception of slightly slower rates in the lag phase in segments. Interestingly, segment-bag differences in hemolysis was observed with a trend towards increased hemoglobin in the segment, consistent with published reports 33. In contrast, free heme was higher in the bags compared to segments. However, when total heme-containing products were assessed, no difference in hemolysis between bag and segments were observed. In other words, hemolysis per se is the same, while the composition is different between bags and segments. We do not have an explanation for this result, but note that these data suggest that measuring only hemoglobin is not sufficient to assess for hemolysis in RBCs. Furthermore, our data show that free heme comprises a significant portion of extracellular heme containing species (∼30-40% for d35-42 RBCs). Combined with our recent studies showing a role for free heme in mediating stored RBC toxicity in a model of trauma hemorrhage34, we hypothesize that free heme is another key player with hemoglobin, in the storage lesion toxicity mechanisms.
Finally, we acknowledge limitations of this study including lack of insight to whether hemin is free or bound to a protein. Also, we have not determined the mechanisms underlying increasing nitrite oxidation rates in older stored RBC. To determine this systematic depletion / repletion of multiple antioxidants from RBC (e.g. catalase, Prx-2, Gpx, GSH, ascorbate) coupled with storage dependent assessment of nitrite oxidation kinetics is required to test the hypothesis that increased nitrite oxidation rates in older stored RBC is due to decreasing antioxidant capacity. It is also important to point out that, approximately 85% of free heme (hemin) is released during processing i.e., by d7. For Hb this is approximately 75% of the total, indicating that for both hemin and Hb, a large fraction is released early. These calculations are based on measurements in segments however, and as shown in Figure 7, differences between segments and bags exist for free heme and Hb. We note that when blood was collected, leukoreduced and stored for experimental purposes as reported in our previous work14, which does not model commercial processing, d7 levels of Hb was significantly lower than those reported here, representing approximately 30% of d35 levels. Clearly, additional studies investigating when hemoglobin and free heme (hemin) are released, and the sensitivities of this release to the different protocols for RBC processing, sampling and storage are required.
In summary, we show inter-relationships between nitrite oxidation kinetics, Prx-2 oxidation and hemolysis-derived hemoglobin and free heme, and that measurements of these parameters early during storage of RBCs may predict how they will store during their shelf-life. Future studies evaluating whether these parameters can be used to determine if donor-specific differences are genetically determined and can predict different 24h recoveries post-transfusion are warranted. Even more clinically relevant, will be to determine if there is a relationship between biochemical changes in stored RBCs and their ability to provide a second hit resulting in patient harm.
Acknowledgments
Source of support: This study was supported by grants from the National Institutes of Health (HL095468 to RPP. JW) and RS was supported by a NIH T32 HL007918 research training grant.
Footnotes
Conflict of Interest: RPP is a co-inventor on a patent for use of nitrite salts for the treatment of cardiovascular conditions. All other authors (JO, RS, VH, MM) declare no conflicts.
References
- 1.Glynn SA. The red blood cell storage lesion: a method to the madness. Transfusion. 2010;50:1164–9. doi: 10.1111/j.1537-2995.2010.02674.x. [DOI] [PubMed] [Google Scholar]
- 2.Vandromme MJ, McGwin G, Jr, Weinberg JA. Blood transfusion in the critically ill: does storage age matter? Scandinavian journal of trauma, resuscitation and emergency medicine. 2009;17:35. doi: 10.1186/1757-7241-17-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, Hendrickson JE, Zimring JC, Brittenham GM, Spitalnik SL. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–92. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spinella PC, Sparrow RL, Hess JR, Norris PJ. Properties of stored red blood cells: understanding immune and vascular reactivity. Transfusion. 2011;51:894–900. doi: 10.1111/j.1537-2995.2011.03103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinberg JA, Barnum SR, Patel RP. Red blood cell age and potentiation of transfusion-related pathology in trauma patients. Transfusion. 2011;51:867–73. doi: 10.1111/j.1537-2995.2011.03098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu H, Zennadi R, Xu BX, Eu JP, Torok JA, Telen MJ, McMahon TJ. Impaired adenosine-5′-triphosphate release from red blood cells promotes their adhesion to endothelial cells: a mechanism of hypoxemia after transfusion. Critical care medicine. 2011;39:2478–86. doi: 10.1097/CCM.0b013e318225754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baek JH, D'Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. The Journal of clinical investigation. 2012;122:1444–58. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belizaire RM, Makley AT, Campion EM, Sonnier DI, Goodman MD, Dorlac WC, Friend LA, Lentsch AB, Pritts TA. Resuscitation with washed aged packed red blood cell units decreases the proinflammatory response in mice after hemorrhage. The journal of trauma and acute care surgery. 2012;73:S128–33. doi: 10.1097/TA.0b013e3182606301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberg JA, Maclennan PA, Vandromme-Cusick MJ, Angotti JM, Magnotti LJ, Kerby JD, Rue LW, 3rd, Barnum SR, Patel RP. Microvascular response to red blood cell transfusion in trauma patients. Shock. 2012;37:276–81. doi: 10.1097/SHK.0b013e318241b739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangalmurti NS, Xiong Z, Hulver M, Ranganathan M, Liu XH, Oriss T, Fitzpatrick M, Rubin M, Triulzi D, Choi A, Lee JS. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood. 2009;113:1158–66. doi: 10.1182/blood-2008-07-166264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds JD, Hess DT, Stamler JS. The transfusion problem: role of aberrant S-nitrosylation. Transfusion. 2011;51:852–8. doi: 10.1111/j.1537-2995.2011.03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander JT, El-Ali AM, Newman JL, Karatela S, Predmore BL, Lefer DJ, Sutliff RL, Roback JD. Red blood cells stored for increasing periods produce progressive impairments in nitric oxide-mediated vasodilation. Transfusion. 2013 doi: 10.1111/trf.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stapley R, Owusu BY, Brandon A, Cusick M, Rodriguez C, Marques MB, Kerby JD, Barnum SR, Weinberg JA, Lancaster JR, Jr, Patel RP. Erythrocyte storage increases rates of NO and nitrite scavenging: implications for transfusion-related toxicity. The Biochemical journal. 2012;446:499–508. doi: 10.1042/BJ20120675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuman R, Hayek S, Rahman A, Poole JC, Menon V, Sher S, Newman JL, Karatela S, Polhemus D, Lefer DJ, De Staercke C, Hooper C, Quyyumi AA, Roback JD. Effects of storage-aged red blood cell transfusions on endothelial function in hospitalized patients. Transfusion. 2014 doi: 10.1111/trf.12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirby BS, Hanna G, Hendargo HC, McMahon TJ. Restoration of intracellular ATP production in banked red blood cells improves inducible ATP export and suppresses RBC-endothelial adhesion. Am J Physiol Heart Circ Physiol. 2014;307:H1737–44. doi: 10.1152/ajpheart.00542.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis RO, Jhang JS, Pham HP, Hod EA, Zimring JC, Spitalnik SL. Glucose-6-phosphate dehydrogenase deficiency in transfusion medicine: the unknown risks. Vox sanguinis. 2013 doi: 10.1111/vox.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumont LJ, Yoshida T, AuBuchon JP. Anaerobic storage of red blood cells in a novel additive solution improves in vivo recovery. Transfusion. 2009;49:458–64. doi: 10.1111/j.1537-2995.2008.02038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rinalducci S, D'Amici GM, Blasi B, Zolla L. Oxidative stress-dependent oligomeric status of erythrocyte peroxiredoxin II (PrxII) during storage under standard blood banking conditions. Biochimie. 2011;93:845–53. doi: 10.1016/j.biochi.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–60. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 21.Zimring JC, Smith N, Stowell SR, Johnsen JM, Bell LN, Francis RO, Hod EA, Hendrickson JE, Roback JD, Spitalnik SL. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion. 2014;54:137–48. doi: 10.1111/trf.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roback JD, Josephson CD, Waller EK, Newman JL, Karatela S, Uppal K, Jones DP, Zimring JC, Dumont LJ. Metabolomics of ADSOL (AS-1) red blood cell storage. Transfus Med Rev. 2014;28:41–55. doi: 10.1016/j.tmrv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Alessandro A, D'Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97:107–15. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harper VM, Oh JY, Stapley R, Marques MB, Wilson L, Barnes S, Sun CW, Townes T, Patel RP. Peroxiredoxin-2 recycling is inhibited during erythrocyte storage. Antioxid Redox Signal. 2015;22:294–307. doi: 10.1089/ars.2014.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayer SB, Maghzal G, Stocker R, Hampton MB, Winterbourn CC. Neutrophil-mediated oxidation of erythrocyte peroxiredoxin 2 as a potential marker of oxidative stress in inflammation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013 doi: 10.1096/fj.13-227298. [DOI] [PubMed] [Google Scholar]
- 26.Cho CS, Yoon HJ, Kim JY, Woo HA, Rhee SG. Circadian rhythm of hyperoxidized peroxiredoxin II is determined by hemoglobin autoxidation and the 20S proteasome in red blood cells. Proc Natl Acad Sci U S A. 2014;111:12043–8. doi: 10.1073/pnas.1401100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keszler A, Piknova B, Schechter AN, Hogg N. The reaction between nitrite and oxyhemoglobin: a mechanistic study. The Journal of biological chemistry. 2008;283:9615–22. doi: 10.1074/jbc.M705630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stapley R, Rodriguez C, Oh JY, Honavar J, Brandon A, Wagener BM, Marques MB, Weinberg JA, Kerby JD, Pittet JF, Patel RP. Red blood cell washing, nitrite therapy, and antiheme therapies prevent stored red blood cell toxicity after trauma-hemorrhage. Free Radic Biol Med. 2015 doi: 10.1016/j.freeradbiomed.2015.04.025. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piknova B, Keszler A, Hogg N, Schechter AN. The reaction of cell-free oxyhemoglobin with nitrite under physiologically relevant conditions: Implications for nitrite-based therapies. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2009;20:88–94. doi: 10.1016/j.niox.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Low FM, Hampton MB, Peskin AV, Winterbourn CC. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood. 2007;109:2611–7. doi: 10.1182/blood-2006-09-048728. [DOI] [PubMed] [Google Scholar]
- 31.Vitturi DA, Sun CW, Harper VM, Thrash-Williams B, Cantu-Medellin N, Chacko BK, Peng N, Dai Y, Wyss JM, Townes T, Patel RP. Antioxidant functions for the hemoglobin beta93 cysteine residue in erythrocytes and in the vascular compartment in vivo. Free radical biology & medicine. 2013;55:119–29. doi: 10.1016/j.freeradbiomed.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu X, Marques MB, Weinberg JA, Patel RP, Barnum SR. The level of complement activation fragments is higher in red blood cell units than segments. Transfus Apher Sci. 2013 doi: 10.1016/j.transci.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Kurach JD, Hansen AL, Turner TR, Jenkins C, Acker JP. Segments from red blood cell units should not be used for quality testing. Transfusion. 2013 doi: 10.1111/trf.12303. [DOI] [PubMed] [Google Scholar]
- 34.Stapley R, Rodriguez C, Oh JY, Honavar J, Brandon A, Wagener B, Marques MB, Weinberg JA, Kerby JD, Pittet J, Patel RP. RBC washing, nitrite therapy, and anti-heme therapies prevent RBC storage toxicity after trauma-hemorrhage. Free Radic Biol Med. 2015 doi: 10.1016/j.freeradbiomed.2015.04.025. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]


