Summary
Obesity is recognized as a chronic disease and one of the major healthcare challenges facing us today. Weight loss can be achieved via lifestyle, pharmacological and surgical interventions, but weight maintenance remains a lifetime challenge for individuals with obesity. Guidelines for the management of obesity have highlighted the role of primary care providers (PCPs). This review examines the long‐term outcomes of clinical trials to identify effective weight maintenance strategies that can be utilized by PCPs. Because of the broad nature of the topic, a structured PubMed search was conducted to identify relevant research articles, peer‐reviewed reviews, guidelines and articles published by regulatory bodies. Trials have demonstrated the benefit of sustained weight loss in managing obesity and its comorbidities. Maintaining 5–10% weight loss for ≥1 year is known to ameliorate many comorbidities. Weight maintenance with lifestyle modification – although challenging – is possible but requires long‐term support to reinforce diet, physical activity and behavioural changes. The addition of pharmacotherapy to lifestyle interventions promotes greater and more sustained weight loss. Clinical evidence and recently approved pharmacotherapy has given PCPs improved strategies to support their patients with maintenance of weight loss. Further studies are needed to assess the translation of these strategies into clinical practice.
Keywords: Maintenance, obesity, primary care, weight loss
Introduction
Obesity is now widely recognized as a disease 1, 2, 3 and its increasing prevalence makes it one of the major global health challenges of our time 4, 5, 6. In 2008, more than 200 million men and nearly 300 million women were estimated to be obese, which represents more than 10% of the world's adult population 4. An additional 1.4 billion adults are overweight 4, and both overweight and obesity are increasing in children 7. Obesity is associated with high morbidity and mortality; complications include cancer, cardiovascular disease, depression, dyslipidaemia, hypertension, obstructive sleep apnoea, osteoarthritis and type 2 diabetes 8, 9, 10, 11. The World Health Organization estimates that overweight and obesity are the fifth leading cause of death globally 4. This disease, therefore, represents a critical health challenge.
Consequently, there is an emphasis on public health campaigns aimed at the primary prevention of obesity, but these must be complemented by strategies to manage the disease in individuals who are already obese. Patients with overweight and obesity require secondary prevention and treatment, or tertiary interventions if there are associated weight‐related complications 3, as is the case with other diseases such as type 2 diabetes and hypertension.
Currently, patients with overweight or obesity can be treated with lifestyle interventions alone, or with such interventions in combination with weight‐loss medications or with bariatric surgery. However, even when therapy involves medications or surgery, lifestyle intervention continues to be critically important for achieving treatment goals 1, 12, 13, 14, 15. Following weight loss, weight maintenance is a challenge, regardless of the initial modality used for weight loss. Behavioural interventions are successful at delivering 5–10% weight loss, but maintaining this weight loss is challenging, over the longer term 16, 17, 18.
New tools and the role of primary care providers
Primary care providers (PCPs) play a critical role in supporting individuals attempting to lose weight and in maintaining their weight loss for long‐term 19. This important role is acknowledged in the American Heart Association (AHA)/American College of Cardiology (ACC)/The Obesity Society (TOS) obesity guidelines, which are aimed at PCPs and designed to aid treatment decisions to support weight loss and maintenance 1, 12, 13. Patients often seek and trust the advice of PCPs on weight management 20. PCPs, therefore, have the opportunity and credibility to educate their patients on the negative health outcomes associated with obesity, the treatment options available to them, the challenges of weight maintenance and the various approaches to successful weight‐loss maintenance. As with other chronic diseases, such as diabetes and hypertension, it is important that PCPs discuss obesity with their patients and how this disease can negatively impact health. However, it must also be acknowledged that it is challenging for PCPs to balance time constraints and competing demands in order to treat obesity effectively 21. Questions still remain on the effectiveness of obesity treatment in primary care and the optimal role of PCPs in the maintenance of weight loss 22. A team‐based approach that includes dietitians, exercise trainers and behaviourists could assist in the time‐efficient and comprehensive management of patients with overweight or obesity.
Several effective and well‐tolerated pharmacotherapies are now available that complement both the weight‐loss and weight‐maintenance efforts of an individual, and other pharmacotherapies are under development 23. These new treatment options have enabled the development of more robust approaches to medical care that not only optimize the benefit–risk balance and improve outcomes but also are cost‐effective 1, 12, 13, 14, 15.
This article overviews the various strategies for weight maintenance, based upon a review of what we know from clinical trials, and examines how these strategies can be applied in real‐world settings.
Methods
A PubMed search was conducted for articles related to the review topic and published in the English language prior to 28 February 2015. The search method employed was a structured rather than a systematic approach; this strategy was judged to be more appropriate because of the broad nature of the review topic. The search terms included ‘(weight) AND (loss OR reduc* OR decreas*) AND (maintenance OR maintain* OR sustain* OR control*) AND (manag* OR treat* OR therap*) AND (obesity) AND (benefit)’. This search provided 825 articles. The search was further refined by adding more specific search terms to the string. Additional terms included: ‘metabol*’, ‘disease’, ‘cardiovasc*’, ‘diabet*’, ‘risk’, ‘chronic’, ‘vascul*’, ‘complications’, ‘primary care provider/specialist/multidisciplin*’, ‘educat*’, ‘pharmacother*’, ‘lifestyle’, ‘diet’, ‘exercise/physical activity’, ‘behavio*’, ‘gene/genetics’, ‘mechanism’, ‘regain’, ‘guideline’ and ‘surg*’. Articles were scanned for relevance to the review topic and included if they were considered to be related to weight‐loss maintenance. Selected articles included peer‐reviewed reviews and original research articles, guidelines and articles published by regulatory bodies. The bibliographies of selected articles were also searched for any additional relevant literature. Article selection was based upon the author's own judgement, clinical experience and knowledge of the literature. The following types of articles were excluded: those considered not relevant to the topics covered by the review, single case studies, short commentaries, letters and interviews.
Weight maintenance: definition, benefits and potential drawbacks
The initial weight‐loss goal is 5–10% – a reduction that is sufficient to improve health and prevent or ameliorate many weight‐related complications 18, 24, 25. Currently, there is no consensus on a definition of ‘effective’ or ‘sufficient’ weight‐loss maintenance (Box 1) 25. Although such a definition should be based upon the degree of sustained weight loss needed to optimize health outcomes, there is a lack of evidence on the minimum weight loss needed to achieve these outcomes over the long term 25. Nevertheless, the data suggest that maintaining weight loss for at least 1 year can be sufficient to ameliorate many of the complications of obesity 18, 24, 25.
Box 1. Descriptions that have been used to define weight‐loss maintenance 25 .
Maintaining ‘new weight’ for 2 years after weight loss 34
Gaining no more than 5 lb (2.27 kg) or 5% of weight after weight loss (4 years) 35
Maintaining weight loss of 5–10% 36
Remaining within ±5 lb (2.27 kg) of goal weight 37
A weight change of less than ±3% of a designated body weight under standardized conditions
The evidence base for the benefits of weight loss and maintenance is substantial. Such benefits include improvements in cardiometabolic disease (cardiovascular disease risk, diabetes, dyslipidaemia, hypertension, metabolic syndrome, non‐alcoholic fatty liver disease and prediabetes) 12, 26, depression 27, gastroesophageal reflux disease 28, osteoarthritis 9, polycystic ovary syndrome 29, sleep apnoea 30, urinary incontinence 31 and others. Sustained weight loss (3 kg lost over 2–3 years) leads to reductions in blood pressure 32, 33. Long‐term weight loss is also associated with a reduced risk of developing type 2 diabetes and improved glucose control in patients with type 2 diabetes 38. In men with obesity with a waist circumference >100 cm, weight maintenance (–4.8 kg after 23 months) is associated with decreased glucose and insulin concentrations 39. Some studies have suggested that intentional long‐term (>2 years) weight loss may reduce the risk of all‐cause mortality for women and people with diabetes 40. In a retrospective cohort study, 2,500 patients (74% men) undergoing bariatric surgery had significantly lower mortality than matched controls after 1–5 years (hazard ratio [HR]: 0.45; 95% confidence interval [CI]: 0.36–0.56) and after 5–14 years (HR: 0.47; 95% CI: 0.39–0.58) 41. Furthermore, a 23‐year follow‐up study showed that a 6‐year lifestyle intervention programme (diet or exercise, or both) for Chinese subjects with impaired glucose tolerance had reduced the incidence of all‐cause mortality (HR: 0.71; 95% CI: 0.51–0.99) and diabetes (HR: 0.55; 95% CI: 0.40–0.76), in comparison with controls 42.
Many people with obesity, however, struggle to maintain their weight, following weight loss 43. A variety of effects following weight regain have been reported. There is evidence that some benefits are sustained following transient weight loss, despite weight regain. For example, if patients with diabetes, hypertension or sleep apnoea are able to sustain lower glycosylated haemoglobin A1c (HbA1c) 44, lower blood pressure 33, and alleviate apnoea 10 over the period of weight loss, this can lower the health risks these diseases exert on patients over their lifetime. With better glycaemic control, fewer microvascular complications 45, 46, and reduced cardiovascular disease 47 have been observed. In the Diabetes Prevention Program (DPP), subjects randomized to intensive lifestyle therapy experienced a 4–7% weight loss over the 4 years of the study, but continued to exhibit decreased rates of progression to type 2 diabetes after 10 years, despite regaining weight to a level equal to that in the control (placebo) group 44. Thus, there was a residual effect of preventing future type 2 diabetes, even though subjects had long regained the weight they had lost during the earlier intervention.
However, evidence also indicates that some benefits may be lost. Minimal weight regain (i.e. 2–6%) has been reported to cause metabolic risk factors (e.g. plasma lipids, blood pressure, fasting glucose and insulin concentrations) to revert to baseline levels 48. For postmenopausal women, partial weight regain following intentional weight loss is associated with an increased cardiometabolic risk 49. Serum triglycerides and low‐density lipoprotein‐cholesterol levels typically decrease with weight loss, but return to former levels after weight regain 38, 50, 51. Therefore, PCPs are in a unique position to educate their patients about the challenges of weight maintenance and the positive residual effect of weight loss, and to emphasize the need to maintain weight loss over the long term.
Lifestyle modification and weight maintenance
Lifestyle modification is effective in achieving weight loss. Landmark studies on the maintenance of weight loss with lifestyle modification are reviewed in this section. When reviewing these data, it is important to consider factors such as duration and intensity of treatment, as these can influence outcome. Overall, these studies demonstrate that weight maintenance with lifestyle modification – although challenging – is possible, and this message should be clearly communicated to patients.
The National Weight Control Registry (NWCR), a US database founded in 1993, has provided evidence in relation to specific strategies for achieving and maintaining weight loss. This registry has identified the lifestyle modifications practiced by those individuals who were able to successfully maintain weight loss. It is important that PCPs are aware of this information so that they can better educate and support their patients in their weight maintenance journey (Box 2). For inclusion of patients in this database as weight maintainers, their weight loss had to be ≥13.6 kg and their new weight sustained for ≥1 year. A 10‐year observational study of 2,886 participants found that mean weight loss was 23.1 ± 0.4 kg at 10 years and that ≥87% of participants maintained a weight loss of ≥10% 52. A decrease in leisure‐time physical activity, dietary restraint and frequency of self‐weighing and an increase in the proportion of energy intake derived from fat were associated with a greater weight regain 52. Participants with ≥2 years of weight loss maintenance at enrolment continued to maintain larger weight losses at 5 and 10 years 52. Longer duration of weight maintenance was associated with better long‐term weight loss outcomes. The study concluded that most weight loss can be maintained over 10 years, but that it requires a sustained behaviour change 52. The NWCR found that characteristics common to successful weight‐loss maintainers included eating breakfast 53, high levels of volitional physical activity 54, reduced fat intake 54, self‐monitoring of dietary intake and physical activity, consumption of low or no calorie‐sweetened beverages to limit total energy intake 57, greater dietary restraint 54, regular self‐monitoring of weight, and limited television viewing time (≤10 h week−1; Box 2) 58.
Box 2. NWCR key patient data 55 .
Registry members lost an average of 30 kg (66 lb) and maintained their reduced weight for 5.5 years
45% lost weight by themselves; 55% lost weight with the help of some type of programme
98% modified their food intake to lose weight
94% increased their physical activity – walking being the most frequent activity
Most kept their weight off by continuing to maintain a low‐calorie (1,360 kcal d−1), low‐fat diet (24% of calories from fat) and by high levels of physical activity (∼2,786 kcal week−1) 56
Most self‐monitored their dietary intake and physical activity 56
78% ate breakfast every day
75% weighed themselves once‐weekly or more frequently
62% watched ≤10 h of television per week
90% exercised, on average, about 1 h per day of physical activity
The Look AHEAD (Action for Health in Diabetes) study assessed the effects of intensive lifestyle intervention (ILI) on clinically important health outcomes in people with overweight or obesity with type 2 diabetes 59, 60, 61. Participants were randomly assigned to one‐on‐one ILI or conventional diabetes support and education (DSE) 59. ILI included diet modification and physical activity to induce and maintain ≥7% weight loss at 1 year and beyond 59, 60. ILI participants had goals of 1,200–1,800 kcal d−1, based upon initial weight, and physical activity of >175 min week−1 60. At 1 year, the ILI group, compared with the DSE group, had lost a greater percentage of their initial weight (−8.6% vs. −0.7%); had improved fitness (assessed by treadmill test); had improvements in HbA1c, systolic and diastolic blood pressure, triglyceride, high‐density lipoprotein (HDL)‐cholesterol and urine albumin/creatinine; and had a reduced need for type 2 diabetes, hypertension and lipid‐lowering medications 59. Although the greatest benefits were often seen at 1 year, the ILI group still had greater improvements than the DSE group in terms of weight reduction (−6.15% vs. −0.88%), fitness, HbA1c levels, systolic blood pressure and HDL‐cholesterol levels at 4 years 61. Factors indicative of the long‐term success of ILI included use of meal replacements, high levels of exercise, self‐monitoring and individualized diets using ‘healthy meal plans’ that worked with personal and cultural food preferences 61. The study also found that participants who maintained their weight loss after 4 years reported more favourable physical activity and food intake, and attended more treatment sessions than those who had not maintained their weight loss – indicating the importance of a sustained lifestyle change in successful weight‐loss maintainers 62.
The DPP evaluated if modest weight loss through dietary changes and increased physical activity or treatment with metformin could prevent or delay the onset of type 2 diabetes. Participants were randomized into three groups as follows: (i) the lifestyle intervention group received intensive training in diet, physical activity and behaviour modification (24‐week curriculum), with the aim of losing ≥7% of their body weight and maintaining that weight loss; (ii) a second group received 850 mg of metformin twice daily, and (iii) a third group received placebo 44, 63. The metformin and placebo groups both received information on diet and exercise but received no intensive or individualized counselling 63. Half of the lifestyle intervention group achieved the 7% weight reduction goal after 24 weeks 63. After 2.8 years, participants assigned to the lifestyle intervention group had shown a greater increase in physical activity and greater weight loss (−5.6 kg) than those on metformin (−2.1 kg) or placebo (−0.1 kg) (P < 0.001 for both) 63. Compared with placebo, lifestyle intervention and treatment with metformin had reduced the incidence of type 2 diabetes by, respectively, 58% and 31% 63, and had reduced the incidence of metabolic syndrome by 41% and 17% 64. These findings demonstrate that intense lifestyle intervention can be effective in long‐term weight loss, weight maintenance and in risk associated with comorbidities. In the 10‐year follow‐up 44, the lifestyle group had lost, on average, 7 kg by year 1 and then had partly regained weight (although still 2 kg less than at randomization), while the metformin group had maintained a modest weight loss of 2.5 kg. An additional analysis of the lifestyle intervention arm found that the overall 2‐year weight loss (from baseline) was the strongest predictor of a reduced incidence of type 2 diabetes and improved cardiometabolic risk 65. Furthermore, the early rapid weight loss (and its subsequent maintenance for 2 years) had also provided the additional benefit of a reduction in the risk of developing type 2 diabetes 65.
A 3.5‐year observational study of 110 women with obesity who had received an initial 6‐month lifestyle intervention, followed by a 1‐year extended‐care phase (comprising face‐to‐face counselling, telephone counselling or mail‐only contact) with no further treatment until follow‐up, found that 41.8% of ‘successful’ participants had maintained weight reductions of ≥5% from baseline to follow‐up 66. These subjects reported that they had planned meals in advance and selected lower calorie foods, and had self‐monitored their food intake, calories and weight. The intensive weekly group sessions emphasizing behaviour skills – particularly self‐monitoring – were a key component of successful long‐term weight management 66.
In the 2‐year ‘Keep It Off’ trial, in participants who lost ≥10% of their body weight, sustained, supportive phone‐ and mail‐based intervention was shown to have improved weight maintenance (defined as a regain of <2.5% baseline body weight), compared with brief intervention alone 67, 68. The probability of successfully maintaining a reduced body weight at 2 years was 1.37 times higher in the group receiving intervention than in the group not receiving it, showing the benefit of sustained, supportive intervention over self‐directed support 67.
In the weight‐loss maintenance trial, adults with overweight or obesity (body mass index [BMI] 25–45 kg m−2) who lost ≥4 kg in a 6‐month behaviour weight‐loss intervention (phase I) were randomized to one of three 30‐month maintenance interventions (phase II) 69, 70, 71. In phase II, participants received behaviour intervention via interactive internet‐based technology (IT), monthly personal counselling (PC) or no further intervention (self‐directed control, SD) 71. Mean weight changed range from −2.3% (African–American women) to −4.5% (Caucasian men) after 36 months. Although participants regained some weight during phase II, mean weight at the end of the study was significantly lower than entry weight in phase I (P = 0.0002) 71. Participants in both the IT‐ and the PC‐based groups regained significantly less weight than the SD group over a period of up to 24 months (P < 0.05) 69. The PC group maintained significantly higher weight loss than the SD‐ (P = 0.003) and IT‐based groups (P = 0.03) 69.
Some clinical trials and commercial weight‐loss programmes have shown that meal replacements are highly effective in producing significant sustainable weight loss 62, 72, 73, 74. Other studies have found that behaviour changes involving diet (e.g. increased consumption of fruits, vegetables and whole grains), self‐monitoring of caloric intake, self‐weighing, planning meals in advance and moderate‐intensity physical activity (150–250 min week−1) are important factors in maintaining a reduced body weight over the long term 66, 75, 76, 77, 78. A staged approach to weight management – including monitoring weight fluctuations and having a clear signal for weight regain that triggers immediate action – is also a common characteristic of successful weight maintainers 79.
The clinical trials described previously provide ample evidence that lifestyle interventions can successfully produce clinically meaningful weight loss and weight maintenance, and also identify those components or practices within lifestyle modification programmes that are the most effective (Box 3). The US Preventive Services Task Force Recommendation Statement calls for patients with BMI ≥30 kg m−2 to receive intensive, multicomponent behaviour interventions, e.g. involving weight‐loss goals, improving diet or nutrition and increasing physical activity, addressing barriers to change, self‐monitoring and strategizing how to maintain lifestyle changes 80. Over the long term, however, people with obesity will need additional ongoing support to reinforce diet and physical activity changes.
Box 3. Components of a weight maintenance lifestyle intervention programme of proven efficacy in clinical trials.
Diet
Individualized calorie goals, based upon an individual's desire to maintain or lose more weight 61, 62
Use of meal replacements for one or more meals or snacks per day 61
Planning meals in advance 66
Individualized healthy meal plans that accommodate personal and cultural food preferences 61, 62
Self‐monitoring of food intake and calories 66
Exercise
Behaviour
Pathophysiology of obesity and the physiological responses to weight loss that promote weight regain
Obesity susceptibility genes constitute an important determinant of body weight and act to influence the homeostatic processes that regulate food intake and energy balance 81. These genetic factors interact with the environment and behaviour to determine the degree of obesity and whether or not the excess adiposity is associated with complications 81. Evidence indicates that, following weight loss, there are compensatory changes in the homeostatic processes that result in increased hunger and energy storage, favouring weight regain 43, 83, 84, 85, 86. These changes are part of the pathophysiology that characterizes obesity as a disease – namely, the exaggerated compensatory mechanisms that work to return the patient back to the original (high) body weight. They include changes in the levels of circulating appetite‐related hormones – such as increases in orexigenic hormones (e.g. ghrelin), which make you feel hungry, and decreases in anorexigenic hormones (e.g. leptin, cholecystokinin, glucagon‐like peptide‐1 [GLP‐1], amylin and peptide YY), which make you feel satiated, with the net result that appetite is increased 83, 86, 87. Clearly, increased hunger in an environment that promotes the availability of energy‐dense foods makes it challenging for people to maintain a healthy weight. If individuals do not self‐monitor and revert to disinhibited or unhealthy eating habits, they will not be able to overcome these regulatory changes, and weight regain occurs 52. Furthermore, in response to weight loss, resting energy expenditure rates are decreased, and the energy that muscles use for any given amount of work is also decreased (i.e. increased muscle energy efficiency). These energetic changes also promote weight regain (Fig. 1) 86, 88. To maintain weight loss, individuals must adhere to behaviours that oppose these physiological adaptations and the other factors favouring weight regain. However, it is difficult for people with obesity to overcome physiology with behaviour over the long term. Common reasons for weight regain include decreased caloric expenditure, decreased self‐weighing frequency, increased caloric intake, increased fat intake and eating disinhibition over time 52. As a sustained change in behaviour can be challenging, patients need help, which can be provided by prescribing medications as an adjunct to lifestyle modification.
Figure 1.
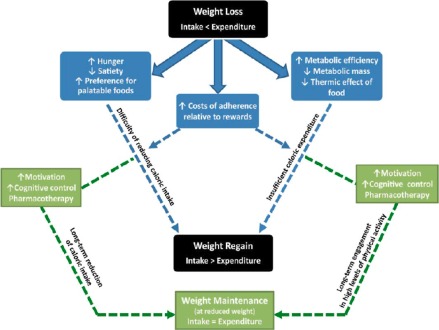
The balance between weight loss and weight regain and the associated physiological and psychological changes involved. Physiological and psychological changes that occur as a result of weight loss are shown in blue, and the pathway to overcome the propensity for weight regain in green. This pathway, which may involve pharmaceutical and behavioural interventions that improve adherence, counteracts the physiological and behavioural adaptations and re‐establish the intake–expenditure balance. (Adapted, with permission, from MacLean et al.) 82.
Pharmacotherapy
Since 2012, there have been four new weight‐loss medications approved for the chronic treatment of obesity by the US Food and Drugs Administration (FDA). These medications are in addition to orlistat (120 mg), approved in 1999 89. The newer medications include lorcaserin, phentermine/topiramate extended release (ER), naltrexone ER/bupropion ER, and high‐dose liraglutide (3.0 mg), which is the most recently approved weight‐loss medication (US FDA, December 2014; European Medicines Agency, March 2015) 90, 91. All these medications are approved in the US as adjuncts to lifestyle modification in patients with overweight with BMI 27–29.9 kg m−2 having ≥1 weight‐related comorbidity – generally taken to be type 2 diabetes, hypertension or dyslipidaemia – or in patients with obesity (BMI ≥30 kg m−2) whether or not comorbidities are present 90, 92, 93, 94. Orlistat is the only approved long‐term drug for adolescents ≥12 years of age with obesity 95, while all others have been approved only for adults. With the exception of orlistat, these medications act on the mechanisms regulating appetite and satiety, and help combat the pathophysiological adaptations that drive weight regain 13. These effects help patients sustain weight loss and help them comply with calorie‐reduced diets. Clinical trials have shown that the addition of pharmacotherapy to lifestyle interventions promotes greater weight loss and sustains weight loss for a longer period of time than lifestyle interventions alone 13, 96, 97, 99, 100, 116. The available data, while only encompassing ≤2 years of follow‐up, demonstrate that these pharmacotherapies sustain weight loss while patients randomized to lifestyle interventions plus placebo are regaining weight (Table 1) 13, 96, 97, 99, 100, 116.
Table 1.
Anti‐obesity medications currently approved for use by the US FDA
| Drug (dose) | Mechanism of action | Weight loss | AEs |
|---|---|---|---|
| Phentermine/topiramate extended release (ER) 96, 97 (3.75 mg/23 mg [phentermine 3.75 mg/ topiramate 23 mg ER] daily for 14 d; then increase to 7.5 mg/46 mg daily 92) | Appetite suppressant; other central nervous system actions or metabolic effects also involved 91 | Up to −10.9% vs. placebo (1.6%) after 56 weeks 98 | Paraesthesia, dizziness, dysgeusia, dry mouth, constipation 92 |
| Lorcaserin 99, 100 (10 mg twice daily 93) | Selective serotonin 2C receptor agonist; increases feelings of satiety 93 | −4.5% to −5.8% vs. placebo (−1.5% to −2.5%) in obese patients with/without diabetes after 1 year 99, 100 | Headache, dizziness, fatigue, nausea, dry mouth and constipation, and in diabetic patients hypoglycaemia, headache, back pain, cough and fatigue 93 |
| Naltrexone/bupropion ER (8 mg naltrexone HCl/90 mg bupropion HCl; escalation dose up to week 4 94) | Opioid receptor agonist/noradrenaline and dopamine uptake inhibitor; dual action: reduces appetite/ enhances control of eating behaviour 94 | −5.0% to −9.3% vs. placebo (–1.2 to 5.1%) after 56 weeks 101, 102, 103 | Nausea, constipation, headache, vomiting, dizziness, insomnia, dry mouth, diarrhoea 94 |
| Orlistat 104 (120 mg three times daily 105) | Gastrointestinal lipase inhibitor; induces dietary fat malabsorption 106 | −5.8 kg vs. −3.0 kg with placebo after 4 years; P < 0.001 104 | Higher frequency of gastrointestinal AEs vs. placebo: oily spotting, flatus with discharge, faecal urgency, fatty/oily stool, oily evacuation, increased defaecation and faecal incontinence 105 |
| Meta‐analysis (8 trials, n = 1,738): −1.80 kg (−2.54 to −1.06) vs. placebo after 1 year 107 | |||
| Liraglutide (3.0 mg once daily) 108 | GLP‐1 analogue that stimulates insulin secretion/inhibits glucagon output in a glucose‐dependent manner, slows gastric emptying and promotes satiety/decreases appetite 110, 111, 112 | −6.2 to −8.0% vs. placebo (−0.2 to −2.6%) after 56 weeks 113, 114 | Side effects are consistent with the known effects of GLP‐1 receptor agonists 115. Nausea, hypoglycaemia, diarrhoea, constipation, vomiting, headache, decreased appetite, dyspepsia, fatigue, dizziness, abdominal pain and increased lipase 108, 113, 114 |
| Lower doses of liraglutide (1.2–1.8 mg once daily) produce less weight loss and are approved to treat type 2 diabetes 109 |
AEs, adverse events.
ER, extended release.
GLP, glucagon like peptide.
Of the new anti‐obesity medications in the pipeline 23, many target the endogenous endocrine circuits regulating energy homeostasis, including central neuropeptide signalling (melanocortin receptor, neuropeptide Y), intestinal peptide hormone signalling (GLP‐1, oxyntomodulin), pancreatic hormone signalling (pancreatic polypeptide, amylin), adipose tissue hormone signalling (leptin) and inhibition of pancreatic lipase 23. These future drugs offer the potential for replacing currently used drugs or combining them, with possible synergistic effects, and of improved safety and efficacy.
It is important to consider that obesity is a chronic disease and requires long‐term management, which calls for long‐term efficacy and safety data on the use of the newly approved medications. Indeed, longer‐term data (over 2 years) are available for several approved agents 97, 104, 117 and further studies are ongoing 118, 119. Given the need to maintain weight loss, clinical experience needs to be developed for the use of medications to sustain weight loss over the patient's lifetime. The options include long‐term use of medication and combination therapy, but there are only sparse data to support or guide their use in therapy intended for prolonged time periods. Furthermore, although these new anti‐obesity medications have been approved, several cardiovascular outcome trials are pending or ongoing and may take several years to complete 120.
Surgery
Bariatric surgery is the most effective method for the treatment of severe obesity 112, 121, 122. Referral is considered for patients with a BMI ≥35 kg m−2 and associated comorbidities, or a BMI of 40 kg m−2, after failure of lifestyle modification and pharmacotherapies 124. Bariatric surgery can result in substantial weight loss of >28 kg 121, 122, 123, but losses vary depending upon the procedure used (e.g. laparoscopic Roux‐en‐Y gastric bypass [RYGB], adjustable gastric banding, sleeve gastrectomy, biliopancreatic diversion with or without duodenal switch) 125, 126. Many, although not all, patients achieve long‐term weight loss following surgery 127. However, sustained weight loss also depends upon the re‐education of patients in terms of active lifestyle changes, and long‐term medical follow‐up 122, 126.
Adams and colleagues examined the association of RYGB surgery with weight loss, type 2 diabetes and other health risks 6 years after surgery 127. Weight‐loss maintenance was superior in patients with severe obesity (BMI ≥35 kg m−2) receiving RYGB surgery compared with non‐surgical controls, with 76% of patients who had received RYGB surgery maintaining ≥20% weight loss over 6 years 127. RYGB was also associated with higher rates of diabetes remission, and with lower cardiovascular disease risk and other health outcomes over 6 years 127. The Swedish Obese Subjects (SOS) study reported a 10‐year follow‐up that compared patients with obesity who had undergone bariatric surgery with matched conventionally treated obese controls (non‐standardized care; lifestyle intervention, behaviour modification or no treatment) 121. In total, 73.5% of the gastric bypass subgroup maintained ≥20% weight loss over 10 years compared with only 3.8% of the control group 121. The authors also reviewed the key SOS study results published between 2004 and 2012, with follow‐up periods of 10–20 years. The mean reported body weight changes after 2, 10, 15 and 20 years were, respectively, −23%, −17%, −16% and −18% in the surgery group, and 0%, 1%, −1% and −1% in the conventionally treated care group 123. Compared with standard care, bariatric surgery reduced overall mortality and decreased the incidence of diabetes, cardiovascular disease events and cancer. Study results have shown that, provided a 10–30% weight loss is maintained, the effects on risk factors remain for over 10 years 123. However, Cooper and colleagues demonstrated that weight regain is a common complication following RYGB surgery. In their study, the mean weight regain following surgery (6.9 ± 4.9 years [mean ± SD]) for all patients was 23.4% 128. Furthermore, over one‐third of patients experienced excessive weight regain (≥25% of lost weight) 128. To counteract this weight regain, weight‐loss medication could be considered as an adjunct to lifestyle intervention in this population 1, 12, 129, 130.
Medical models and guidelines
Individuals seeking medical care usually do so in the primary care setting, so PCPs are the first line of defense to support patients with their weight loss and its maintenance. Once lifestyle intervention is initiated, patients typically achieve maximum weight loss at 6 months followed by plateau and gradual weight regain. PCPs should acknowledge and educate their patient on the challenges of weight maintenance. It is difficult to prevent weight regain in an environment where palatable and energy‐dense foods are readily available and sedentary behaviour is prevalent. PCPs should encourage long‐term follow‐up, ≥1 year of monthly or more frequent visits, in person or by phone to improve weight maintenance success. The long‐term follow‐up will give the PCP an opportunity to address small weight gains by reinstituting the lifestyle intervention and to assess the need for weight‐loss medications or bariatric surgery 12. Several models have been developed to help PCPs manage obesity and provide behaviour support to promote sustained weight loss (Box 4) 1, 14, 15.
Box 4. Models to guide primary care providers in managing obesity.
Relatively BMI‐centric: AHA/ACC/TOS 12, 13
Evaluate and stage patients, decide who needs treatment based upon BMI, and explain the possible health benefits
Begin with comprehensive lifestyle intervention to achieve 5–10% weight loss
Consider medications if 5–10% weight loss is not achieved by lifestyle intervention within 6 months
Pharmacotherapy: BMI 27–29.9 kg m−2 with comorbidity, BMI ≥30 kg m−2
Counsel patients on weight‐loss effects on cardiovascular risk factors
Continue to provide dietary strategies, lifestyle intervention, and counselling
Consider bariatric surgery in patients with BMI ≥35 kg m−2 with comorbidity or ≥40 kg m−2 who are motivated to lose weight but do not respond to nonsurgical treatments
Relatively complications‐centric: AACE 1, 13
Evaluate and stage patients according to cardiometabolic or mechanical or functional obesity‐related complications plus BMI
Initiate weight‐loss programme with sufficient intensity to achieve weight‐loss goals (primary treatment)
Prevent further weight gain and appearance of complications (secondary treatment or prevention)
Treat existing weight‐related complications (tertiary treatment)
Employ lifestyle intervention, with or without medications as needed, to achieve degree of weight loss required to attain therapeutic goals. Refer for bariatric surgery in select patients
Assess degree of improvement in complications after equilibrium weight loss is achieved and intensify weight‐loss therapy and/or use complications‐specific medication if goals of weight loss therapy are not achieved
These models advocate the involvement of effective multidisciplinary healthcare teams, although such teams are not a standard component of many healthcare systems. The team approach should include PCPs, registered dietitians, psychologists, or other counselling professionals and exercise specialists 131. If the supporting professionals (dietitians, exercise trainers, etc.) are not available to the PCP, patients should be referred to a wellness centre, obesity medicine specialist or an online programme. In a recent review of the best role for primary care in the management of obesity in the US, Ard suggested that there should be a stepped approach to interventions, such that therapy is intensified in those patients who respond partially to initial treatment. However, in those patients with serious weight‐related complications, it may be beneficial to initiate therapy with aggressive weight‐loss management 22. Evidently, however, it appears that patients are not usually offered these therapeutic options, which increases the likelihood for weight regain. Ko and colleagues reported that only 39% of 1,873 obese adults surveyed had been advised to lose weight. The authors concluded that patients should receive more weight‐loss counselling from allied health professionals as follow‐up advice to lose weight 132.
Guidelines for the management of obesity have been proposed by several professional organizations, including the American Association of Clinical Endocrinologists (AACE) 1, 13, AHA/ACC/TOS, and the Endocrine Society 12. The AHA/ACC/TOS guidelines were specifically developed for PCPs. Multiple guidelines can be confusing, but it is important to consider their points of consensus that are relevant to weight loss and prevention of weight regain. All guidelines agree on the following:
Obesity is a chronic disease that requires long‐term management. It is important to approach patients with information regarding the health implications.
The goal of obesity treatment is to improve the health of the patient, and it is not intended for cosmetic purposes.
The cornerstone of therapy is comprehensive lifestyle intervention from informed PCPs or other healthcare professionals.
The initial goal of therapy is a weight loss of 5–10% in most patients, as this is sufficient to ameliorate many weight‐related complications. However, weight loss of ≥10% may be needed to improve certain weight‐related complications, such as obstructive sleep apnoea.
Consideration should be given to the use of a weight‐loss medication or possible bariatric surgery, as the addition of these treatment modalities to lifestyle therapy can promote greater weight loss and maintain the weight loss for a longer period of time.
It is important for clinicians to evaluate the patient for weight‐related complications, that can be improved by weight loss, and to consider such patients for more aggressive treatment. The AACE guidelines represent a more ‘complications‐centric’ approach 1, where the risk or presence of weight‐related complications is a primary indication for weight‐loss therapy. Similarly, the impact of weight loss and the need to prevent weight regain are considered in relationship to the prevention or improvements in weight‐related complications.
What can primary care providers do?
Weight loss and maintenance are critical for managing obesity and its associated weight‐related complications 133. PCPs need to address the problem of obesity in their patients, just as they would with any other chronic condition such as hypertension or type 2 diabetes, and to ensure that their patients are aware of the health risks of obesity. While no consensus exists for defining weight maintenance 25, the most commonly used definition is an initial weight loss of 5–10% and maintaining that loss for at least 1 year 18, 24, 25. Clinical trials have shown that many of the positive benefits of weight loss are sustained only if weight loss is maintained over the long term. However, it is clear that additional research is needed to better define how PCPs can be more effective in treating overweight and obesity 22. In the real‐world setting, PCPs should evaluate their patients for the risk or presence of weight‐related complications, both initially and during longer‐term therapy, as these patients may derive the greatest benefits from weight loss and the prevention of weight regain.
The cornerstone of obesity treatment is lifestyle modification, and this is best achieved by structured intervention programmes that involve a multidisciplinary team of healthcare professionals. PCPs should aim to develop programmes or refer patients to programmes featuring lifestyle intervention practices that are known to sustain weight loss for longer periods of time as a strong component. However, through our improved knowledge of the mechanisms regulating energy balance, we know that pathophysiological processes promote weight regain, and that other therapeutic modalities will often be necessary to help patients sustain weight loss. New medications are now available to help combat these processes, and they may be used as adjuncts to lifestyle therapy. However, longer‐term efficacy and safety data beyond 2 years are required for these medications.
Obesity is one of the major healthcare challenges facing us today. Clinical trials have demonstrated the benefit of sustained weight loss in managing the disease and its various comorbidities. Advances in our knowledge of the pathophysiology of obesity, and the new therapy options now available, mean that we are better equipped than ever before to manage the disease effectively over the long term. It now remains for us to translate such advances into standard clinical practice, and this is where PCPs have a critical role.
Conflict of interest statement
W. Timothy Garvey, MD, FACE; Advisory Boards: Astra Zeneca, Boehringer‐Ingelheim, Daiichi‐Sankyo, Eisai, Janssen, Liposcience, Novo Nordisk, Takeda, Vivus; Research: Astra Zeneca, Eisai, Lexicon, Merck, Sanofi, Pfizer, Weight Watchers; Stocks/shares: Affymetrix, Bristol‐Myers Squibb, Isis, Lilly, Merck, Novartis, Pfizer. Sunil Daniel, MD; Speakers bureau and Advisory Boards: Novo Nordisk. Taraneh Soleymani, MD; no disclosures to declare.
Authors' contributions
TS, SD and WTG wrote and revised the manuscript. All authors gave final approval of the manuscript.
Acknowledgements
WTG acts as guarantor and takes responsibility for the content of the article. Editorial assistance was provided by Dr Ian Seymour from AXON Communications, and funded by Novo Nordisk A/S. The authors acknowledge the support of the Diabetes Research Center at the University of Alabama at Birmingham, funded by an award from the National Institutes of Health (DK‐079626).
Soleymani, T. , Daniel, S. , and Garvey, W. T. (2016) Weight maintenance: challenges, tools and strategies for primary care physicians. Obesity Reviews, 17: 81–93. doi: 10.1111/obr.12322.
[Corrections added on 7 December 2015, after first online publication: The author requested some additional changes in the paper which do not alter the meaning of the content.]
The copyright line for this article was changed on 28 October 2016 after original online publication
References
- 1. Mechanick JI, Garber AJ, Handelsman Y, Garvey WT. American Association of Clinical Endocrinologists' position statement on obesity and obesity medicine. Endocr Pract 2012; 18: 642–648. [DOI] [PubMed] [Google Scholar]
- 2. Tsigos C, Hainer V, Basdevant A et al Management of obesity in adults: European clinical practice guidelines. Obes Facts 2008; 1: 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garvey WT, Garber AJ, Mechanick JI et al American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the 2014 advanced framework for a new diagnosis of obesity as a chronic disease. Endocr Pract 2014; 20: 977–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Obesity and overweight. Fact Sheet No. 311. 2014. [WWW document]. URL http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed April 2015).
- 5. World Health Organization . Obesity. [WWW document]. URL http://www.euro.who.int/en/health‐topics/noncommunicable‐diseases/obesity (accessed April 2015).
- 6. Fruhbeck G, Toplak H, Woodward E, Yumuk V, Maislos M, Oppert JM. Obesity: the gateway to ill health – an EASO position statement on a rising public health, clinical and scientific challenge in Europe. Obes Facts 2013; 6: 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 2010; 92: 1257–1264. [DOI] [PubMed] [Google Scholar]
- 8. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health 2009; 9: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vincent HK, Heywood K, Connelly J, Hurley RW. Obesity and weight loss in the treatment and prevention of osteoarthritis. PM R 2012; 4: S59–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuna ST, Reboussin DM, Borradaile KE et al Long‐term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep 2013; 36: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garber AJ. Obesity and type 2 diabetes: which patients are at risk? Diabetes Obes Metab 2012; 14: 399–408. [DOI] [PubMed] [Google Scholar]
- 12. Jensen MD, Ryan DH, Apovian CM et al 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014; 63: 2985–3023. [DOI] [PubMed] [Google Scholar]
- 13. Garvey WT. New tools for weight‐loss therapy enable a more robust medical model for obesity treatment: rationale for a complications‐centric approach. Endocr Pract 2013; 19: 864–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med 2009; 24: 1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jensen MD, Ryan DH, Apovian CM et al 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129: S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stalonas PM, Perri MG, Kerzner AB. Do behavioral treatments of obesity last? A five‐year follow‐up investigation. Addict Behav 1984; 9: 175–183. [DOI] [PubMed] [Google Scholar]
- 17. Wadden TA, Sternberg JA, Letizia KA, Stunkard AJ, Foster GD. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five‐year perspective. Int J Obes 1989; 13(Suppl. 2): 39–46. [PubMed] [Google Scholar]
- 18. Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 2001; 21: 323–341. [DOI] [PubMed] [Google Scholar]
- 19. Bray G, Look M, Ryan D. Treatment of the obese patient in primary care: targeting and meeting goals and expectations. Postgrad Med 2013; 125: 67–77. [DOI] [PubMed] [Google Scholar]
- 20. Jarlenski MP, Gudzune KA, Bennett WL, Cooper LA, Bleich SN. Insurance coverage for weight loss: overweight adults' views. Am J Prev Med 2013; 44: 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Waring ME, Roberts MB, Parker DR, Eaton CB. Documentation and management of overweight and obesity in primary care. J Am Board Fam Med 2009; 22: 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ard J. Obesity in the US: what is the best role for primary care? BMJ 2015; 350: g7846. [DOI] [PubMed] [Google Scholar]
- 23. Kim GW, Lin JE, Blomain ES, Waldman SA. Antiobesity pharmacotherapy: new drugs and emerging targets. Clin Pharmacol Ther 2014; 95: 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wing RR, Phelan S. Long‐term weight loss maintenance. Am J Clin Nutr 2005; 82: 222S–225. [DOI] [PubMed] [Google Scholar]
- 25. Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J Obes (Lond) 2006; 30: 391–399. [DOI] [PubMed] [Google Scholar]
- 26. Eckel RH, Jakicic JM, Ard JD et al 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63: 2960–2984. [DOI] [PubMed] [Google Scholar]
- 27. Strain GW, Kolotkin RL, Dakin GF et al The effects of weight loss after bariatric surgery on health‐related quality of life and depression. Nutr Diabetes 2014; 4: e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Emerenziani S, Rescio MP, Guarino MP, Cicala M. Gastro‐esophageal reflux disease and obesity, where is the link? World J Gastroenterol 2013; 19: 6536–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Legro RS. Obesity and PCOS: implications for diagnosis and treatment. Semin Reprod Med 2012; 30: 496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Foster GD, Borradaile KE, Sanders MH et al A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med 2009; 169: 1619–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whitcomb EL, Subak LL. Effect of weight loss on urinary incontinence in women. Open Access J Urol 2011; 3: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aucott L, Poobalan A, Smith WCS, Avenell A, Jung R, Broom J. Effects of weight loss in overweight/obese individuals and long‐term hypertension outcomes: a systematic review. Hypertension 2005; 45: 1035–1041. [DOI] [PubMed] [Google Scholar]
- 33. Aucott L, Rothnie H, McIntyre L, Thapa M, Waweru C, Gray D. Long‐term weight loss from lifestyle intervention benefits blood pressure? A systematic review. Hypertension 2009; 54: 756–762. [DOI] [PubMed] [Google Scholar]
- 34. Crawford D, Jeffery RW, French SA. Can anyone successfully control their weight? Findings of a three year community‐based study of men and women. Int J Obes Relat Metab Disord 2000; 24: 1107–1110. [DOI] [PubMed] [Google Scholar]
- 35. Field AE, Wing RR, Manson JE, Spiegelman DL, Willett WC. Relationship of a large weight loss to long‐term weight change among young and middle‐aged US women. Int J Obes Relat Metab Disord 2001; 25: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 36. Klein S. Outcome success in obesity. Obes Res 2001; 9(Suppl. 4): 354S–358. [DOI] [PubMed] [Google Scholar]
- 37. Lowe MR, Miller‐Kovach K, Phelan S. Weight‐loss maintenance in overweight individuals one to five years following successful completion of a commercial weight loss program. Int J Obes Relat Metab Disord 2001; 25: 325–331. [DOI] [PubMed] [Google Scholar]
- 38. Avenell A, Broom J, Brown TJ et al Systematic review of the long‐term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004; 8: iii–iv, 1–182. [DOI] [PubMed] [Google Scholar]
- 39. Kukkonen‐Harjula KT, Borg PT, Nenonen AM, Fogelholm MG. Effects of a weight maintenance program with or without exercise on the metabolic syndrome: a randomized trial in obese men. Prev Med 2005; 41: 784–790. [DOI] [PubMed] [Google Scholar]
- 40. Poobalan AS, Aucott LS, Smith WC, Avenell A, Jung R, Broom J. Long‐term weight loss effects on all cause mortality in overweight/obese populations. Obes Rev 2007; 8: 503–513. [DOI] [PubMed] [Google Scholar]
- 41. Arterburn DE, Olsen MK, Smith VA et al Association between bariatric surgery and long‐term survival. JAMA 2015; 313: 62–70. [DOI] [PubMed] [Google Scholar]
- 42. Li G, Zhang P, Wang J et al Cardiovascular mortality, all‐cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23‐year follow‐up study. Lancet Diabetes Endocrinol 2014; 2: 474–480. [DOI] [PubMed] [Google Scholar]
- 43. Maclean PS, Bergouignan A, Cornier MA, Jackman MR. Biology's response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol 2011; 301: R581–R600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knowler WC, Fowler SE, Hamman RF et al 10‐year follow‐up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009; 374: 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Purnell JQ, Zinman B, Brunzell JD, Group DER. The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC). Circulation 2013; 127: 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol 1999; 48: 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mannucci E, Dicembrini I, Lauria A, Pozzilli P. Is glucose control important for prevention of cardiovascular disease in diabetes? Diabetes Care 2013; 36(Suppl. 2): S259–S263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kroeger CM, Hoddy KK, Varady KA. Impact of weight regain on metabolic disease risk: a review of human trials. J Obes 2014; 2014: 614519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beavers DP, Beavers KM, Lyles MF, Nicklas BJ. Cardiometabolic risk after weight loss and subsequent weight regain in overweight and obese postmenopausal women. J Gerontol A Biol Sci Med Sci 2013; 68: 691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Klein S, Burke LE, Bray GA et al Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation 2004; 110: 2952–2967. [DOI] [PubMed] [Google Scholar]
- 51. Wadden TA, Anderson DA, Foster GD. Two‐year changes in lipids and lipoproteins associated with the maintenance of a 5% to 10% reduction in initial weight: some findings and some questions. Obes Res 1999; 7: 170–178. [DOI] [PubMed] [Google Scholar]
- 52. Thomas JG, Bond DS, Phelan S, Hill JO, Wing RR. Weight‐loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med 2014; 46: 17–23. [DOI] [PubMed] [Google Scholar]
- 53. Wyatt HR, Grunwald GK, Mosca CL, Klem ML, Wing RR, Hill JO. Long‐term weight loss and breakfast in subjects in the National Weight Control Registry. Obes Res 2002; 10: 78–82. [DOI] [PubMed] [Google Scholar]
- 54. Catenacci VA, Odgen L, Phelan S et al Dietary habits and weight maintenance success in high versus low exercisers in the national weight Control Registry. J Phys Act Health 2014; 11: 1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. National Weight Control Registry . Facts. [WWW document]. URL http://www.nwcr.ws/Research/default.htm (accessed April 2015).
- 56. Phelan S, Wyatt HR, Hill JO, Wing RR. Are the eating and exercise habits of successful weight losers changing? Obesity (Silver Spring) 2006; 14: 710–716. [DOI] [PubMed] [Google Scholar]
- 57. Catenacci VA, Pan Z, Thomas JG et al Low/no calorie sweetened beverage consumption in the National Weight Control Registry. Obesity (Silver Spring) 2014; 22: 2244–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Raynor DA, Phelan S, Hill JO, Wing RR. Television viewing and long‐term weight maintenance: results from the National Weight Control Registry. Obesity (Silver Spring) 2006; 14: 1816–1824. [DOI] [PubMed] [Google Scholar]
- 59. Pi‐Sunyer X, Blackburn G, Brancati FL et al Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one‐year results of the look AHEAD trial. Diabetes Care 2007; 30: 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wing RR. Long‐term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four‐year results of the Look AHEAD trial. Arch Intern Med 2010; 170: 1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Look Ahead Research Group . Eight‐year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring) 2014; 22: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wadden TA, Neiberg RH, Wing RR et al Four‐year weight losses in the Look AHEAD study: factors associated with long‐term success. Obesity (Silver Spring) 2011; 19: 1987–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Knowler WC, Barrett‐Connor E, Fowler SE et al Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Orchard TJ, Temprosa M, Goldberg R et al The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 2005; 142: 611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Delahanty LM, Pan Q, Jablonski KA et al Effects of weight loss, weight cycling, and weight loss maintenance on diabetes incidence and change in cardiometabolic traits in the diabetes prevention program. Diabetes Care 2014; 37: 2738–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Milsom VA, Middleton KM, Perri MG. Successful long‐term weight loss maintenance in a rural population. Clin Interv Aging 2011; 6: 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sherwood NE, Crain AL, Martinson BC et al Enhancing long‐term weight loss maintenance: 2 year results from the Keep It Off randomized controlled trial. Prev Med 2013; 56: 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sherwood NE, Crain AL, Martinson BC et al Keep it off: a phone‐based intervention for long‐term weight‐loss maintenance. Contemp Clin Trials 2011; 32: 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Svetkey LP, Stevens VJ, Brantley PJ et al Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA 2008; 299: 1139–1148. [DOI] [PubMed] [Google Scholar]
- 70. Tyson CC, Appel LJ, Vollmer WM et al Impact of 5‐year weight change on blood pressure: results from the Weight Loss Maintenance trial. J Clin Hypertens (Greenwich) 2013; 15: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Svetkey LP, Ard JD, Stevens VJ et al Predictors of long‐term weight loss in adults with modest initial weight loss, by sex and race. Obesity (Silver Spring) 2012; 20: 1820–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Heymsfield SB, van Mierlo CA, van der Knaap HC, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord 2003; 27: 537–549. [DOI] [PubMed] [Google Scholar]
- 73. Finley CE, Barlow CE, Greenway FL, Rock CL, Rolls BJ, Blair SN. Retention rates and weight loss in a commercial weight loss program. Int J Obes (Lond) 2007; 31: 292–298. [DOI] [PubMed] [Google Scholar]
- 74. Foster GD, Borradaile KE, Vander Veur SS et al The effects of a commercially available weight loss program among obese patients with type 2 diabetes: a randomized study. Postgrad Med 2009; 121: 113–118. [DOI] [PubMed] [Google Scholar]
- 75. Wing RR, Hamman RF, Bray GA et al Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res 2004; 12: 1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Coughlin JW, Gullion CM, Brantley PJ et al Behavioral mediators of treatment effects in the weight loss maintenance trial. Ann Behav Med 2013; 46: 369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Raynor HA, Van Walleghen EL, Bachman JL, Looney SM, Phelan S, Wing RR. Dietary energy density and successful weight loss maintenance. Eat Behav 2011; 12: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Donnelly JE, Blair SN, Jakicic JM et al American College of Sports Medicine position stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2009; 41: 459–471. [DOI] [PubMed] [Google Scholar]
- 79. Chambers JA, Swanson V. Stories of weight management: factors associated with successful and unsuccessful weight maintenance. Br J Health Psychol 2012; 17: 223–243. [DOI] [PubMed] [Google Scholar]
- 80. Moyer VA. Screening for and management of obesity in adults: U.S. preventive services task force recommendation statement. Ann Intern Med 2012; 157: 373–378. [DOI] [PubMed] [Google Scholar]
- 81. Levian C, Ruiz E, Yang X. The pathogenesis of obesity from a genomic and systems biology perspective. Yale J Biol Med 2014; 87: 113–126. [PMC free article] [PubMed] [Google Scholar]
- 82. MacLean PS, Wing RR, Davidson T et al NIH working group report: Innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 2015; 23: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995; 332: 621–628. [DOI] [PubMed] [Google Scholar]
- 84. Ebbeling CB, Swain JF, Feldman HA et al Effects of dietary composition on energy expenditure during weight‐loss maintenance. JAMA 2012; 307: 2627–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sumithran P, Prendergast LA, Delbridge E et al Long‐term persistence of hormonal adaptations to weight loss. N Engl J Med 2011; 365: 1597–1604. [DOI] [PubMed] [Google Scholar]
- 86. Sumithran P, Proietto J. The defence of body weight: a physiological basis for weight regain after weight loss. Clin Sci (Lond) 2013; 124: 231–241. [DOI] [PubMed] [Google Scholar]
- 87. Doucet E, Imbeault P, St‐Pierre S et al Appetite after weight loss by energy restriction and a low‐fat diet‐exercise follow‐up. Int J Obes Relat Metab Disord 2000; 24: 906–914. [DOI] [PubMed] [Google Scholar]
- 88. Ochner CN, Barrios DM, Lee CD, Pi‐Sunyer FX. Biological mechanisms that promote weight regain following weight loss in obese humans. Physiol Behav 2013; 120: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. US Food and Drug Administration . Orlistat (marketed as Alli and Xenical) Information. 2013. [WWW document]. URL http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm180076.htm (accessed April 2015).
- 90. Food and Drug Administration . FDA news release. 2014. FDA approves weight‐management drug Saxenda. [WWW document]. URL http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm (accessed July 2015).
- 91. Novo Nordisk . Saxenda® approved in Europe for the treatment of obesity. 2015. [WWW document]. URL http://www.novonordisk.com/bin/getPDF.1905678.pdf (accessed July 2015).
- 92. US Food and Drug Administration . Qysmia (phentermine/topiramate ER). [WWW document]. URL http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022580s000lbl.pdf (accessed July 2015).
- 93. Arena Pharmaceuticals GmbH . Belviq (lorcaserin) prescribing information. 2012. [WWW document]. URL https://www.belviq.com/pdf/Belviq_Prescribing_information.pdf (accessed July 2015).
- 94. Takeda Pharmaceuticals USA Inc . Contrave (naltrexone/bupropion ER) prescribing information. 2014. [WWW document]. URL http://general.takedapharm.com/content/file.aspx?filetypecode=CONTRAVEPI&cacheRandomizer=305dd67e‐2a45‐4f66‐a1ca‐6a0f3eb17d31 (accessed July 2015).
- 95. US Food and Drug Administration . Patient Information XENICAL (orlistat) capsules. 2013. [WWW document]. URL http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020766s033lbl.pdf. (accessed July 2015).
- 96. Garvey WT. Phentermine and topiramate extended‐release: a new treatment for obesity and its role in a complications‐centric approach to obesity medical management. Expert Opin Drug Saf 2013; 12: 741–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Garvey WT, Ryan DH, Look M et al Two‐year sustained weight loss and metabolic benefits with controlled‐release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo‐controlled, phase 3 extension study. Am J Clin Nutr 2012; 95: 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Allison DB, Gadde KM, Garvey WT et al Controlled‐release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring) 2012; 20: 330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fidler MC, Sanchez M, Raether B et al A one‐year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab 2011; 96: 3067–3077. [DOI] [PubMed] [Google Scholar]
- 100. O'Neil PM, Smith SR, Weissman NJ et al Randomized placebo‐controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM‐DM study. Obesity (Silver Spring) 2012; 20: 1426–1436. [DOI] [PubMed] [Google Scholar]
- 101. Makowski CT, Gwinn KM, Hurren KM. Naltrexone/bupropion: an investigational combination for weight loss and maintenance. Obes Facts 2011; 4: 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Greenway FL, Fujioka K, Plodkowski RA et al Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR‐I): a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2010; 376: 595–605. [DOI] [PubMed] [Google Scholar]
- 103. Wadden TA, Foreyt JP, Foster GD et al Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR‐BMOD trial. Obesity (Silver Spring) 2011; 19: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004; 27: 155–161. [DOI] [PubMed] [Google Scholar]
- 105. Genentech . Xenical (orlistat) prescribing information. 2013. [WWW document]. URL http://www.gene.com/download/pdf/xenical_prescribing.pdf (accessed July 2015).
- 106. Hill JO, Hauptman J, Anderson JW et al Orlistat, a lipase inhibitor, for weight maintenance after conventional dieting: a 1‐y study. Am J Clin Nutr 1999; 69: 1108–1116. [DOI] [PubMed] [Google Scholar]
- 107. Dombrowski SU, Knittle K, Avenell A, Araujo‐Soares V, Sniehotta FF. Long term maintenance of weight loss with non‐surgical interventions in obese adults: systematic review and meta‐analyses of randomised controlled trials. BMJ 2014; 348: g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Novo Nordisk . Saxenda (liraglutide [rDNA origin] injection). Highlights of prescribing information. 2014. [WWW document]. URL http://novo‐pi.nnittest.com/saxenda.pdf (accessed July 2015).
- 109. Novo Nordisk . Victoza (liraglutide [rDNA origin] injection). Highlights of prescribing information. 2013. [WWW document]. URL http://www.novo‐pi.com/victoza.pdf (accessed July 2015).
- 110. Madsbad S. Exenatide and liraglutide: different approaches to develop GLP‐1 receptor agonists (incretin mimetics) – preclinical and clinical results. Best Pract Res Clin Endocrinol Metab 2009; 23: 463–477. [DOI] [PubMed] [Google Scholar]
- 111. Astrup A, Rossner S, Van Gaal L et al Effects of liraglutide in the treatment of obesity: a randomised, double‐blind, placebo‐controlled study. Lancet 2009; 374: 1606–1616. [DOI] [PubMed] [Google Scholar]
- 112. Flint A, Raben A, Astrup A, Holst JJ. Glucagon‐like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998; 101: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wadden TA, Hollander P, Klein S et al Weight maintenance and additional weight loss with liraglutide after low‐calorie‐diet‐induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 2013; 37: 1514. [DOI] [PubMed] [Google Scholar]
- 114. Pi‐Sunyer X, Astrup A, Fujioka K et al A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015; 373: 11–22. [DOI] [PubMed] [Google Scholar]
- 115. Nauck M, Frid A, Hermansen K et al Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)‐2 study. Diabetes Care 2009; 32: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. European Medicines Agency . Questions and answers on the review of orlistat‐containing medicines. 2012. [WWW document]. URL http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Orlistat_31/WC500122883.pdf (accessed April 2015).
- 117. Smith SR, Weissman NJ, Anderson CM et al Multicenter, placebo‐controlled trial of lorcaserin for weight management. N Engl J Med 2010; 363: 245–256. [DOI] [PubMed] [Google Scholar]
- 118. Novo Nordisk . Treatment with Saxenda® for three years reduced the risk of developing type 2 diabetes compared with placebo. 2015. [WWW document]. URL http://www.novonordisk.com/bin/getPDF.1923542.pdf (accessed July 2015).
- 119. ClinicalTrials.gov . Effect of liraglutide on body weight in non‐diabetic obese subjects or overweight subjects with co‐morbidities: SCALE™ – obesity and pre‐diabetes. [WWW document]. URL https://clinicaltrials.gov/ct2/show/NCT01272219?term=scale+and+prediabetes&rank=1 (accessed July 2015).
- 120. ClinicalTrials.gov . Cardiovascular outcomes study of naltrexone SR/bupropion SR in overweight and obese subjects with cardiovascular risk factors (the Light Study). [WWW document]. URL https://clinicaltrials.gov/ct2/show/NCT01601704?term=Naltrexone%2Fbupropion&rank=14 (accessed December 2014).
- 121. Sjostrom L, Lindroos AK, Peltonen M et al Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351: 2683–2693. [DOI] [PubMed] [Google Scholar]
- 122. Madura JA, 2nd , Dibaise JK. Quick fix or long‐term cure? Pros and cons of bariatric surgery. F1000 Med Rep 2012; 4: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med 2013; 273: 219–234. [DOI] [PubMed] [Google Scholar]
- 124. Garber AJ, Abrahamson MJ, Barzilay JI et al American Association of Clinical Endocrinologists' comprehensive diabetes management algorithm 2013 consensus statement – executive summary. Endocr Pract 2013; 19: 536–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. U. S. Preventive Services Task Force . Screening for obesity in adults: recommendations and rationale. Ann Intern Med 2003; 139: 930–932. [DOI] [PubMed] [Google Scholar]
- 126. Kissler HJ, Settmacher U. Bariatric surgery to treat obesity. Semin Nephrol 2013; 33: 75–89. [DOI] [PubMed] [Google Scholar]
- 127. Adams TD, Davidson LE, Litwin SE et al Health benefits of gastric bypass surgery after 6 years. JAMA 2012; 308: 1122–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Cooper TC, Simmons EB, Webb K, Burns JL, Kushner RF. Trends in weight regain following roux‐en‐Y gastric bypass (RYGB) bariatric surgery. Obes Surg 2015; 25: 1474–1481. [DOI] [PubMed] [Google Scholar]
- 129. Johnson Stoklossa C, Atwal S. Nutrition care for patients with weight regain after bariatric surgery. Gastroenterol Res Pract 2013; 2013: 256145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kalarchian MA, Marcus MD, Courcoulas AP, Cheng Y, Levine MD, Josbeno D. Optimizing long‐term weight control after bariatric surgery: a pilot study. Surg Obes Relat Dis 2012; 8: 710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Carvajal R, Wadden TA, Tsai AG, Peck K, Moran CH. Managing obesity in primary care practice: a narrative review. Ann N Y Acad Sci 2013; 1281: 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ko JY, Brown DR, Galuska DA, Zhang J, Blanck HM, Ainsworth BE. Weight loss advice U.S. obese adults receive from health care professionals. Prev Med 2008; 47: 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Madigan CD, Daley AJ, Lewis AL, Jolly K, Aveyard P. Which weight‐loss programmes are as effective as Weight Watchers®? Non‐inferiority analysis. Br J Gen Pract 2014; 64: e128–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]


