Abstract
Although considerable advances in our understanding of mammalian and avian embryonic coronary development have occurred during the last decade, our current knowledge of this topic in humans is limited. Accordingly, the aim of this study was to determine if the development of the human coronary vasculature in humans is like that of other mammals and avians. The data document a progression of events involving mesenchymal cell-containing villi from the proepicardium, establishment of blood islands and a capillary network. The major finding of the study is direct evidence that the capillary plexus associated with spindle cells and erythroblasts invades the base of the aorta to form coronary ostia. A role for the dorsal mesocardium is also indicated by the finding that cells from this region are continuous with the aorta and pulmonary artery. The development of the tunica media of the coronary arteries follows the same base-apex progression as in other species, with the development of branches occurring late in the embryonic period. The fetal period is characterized by 1) growth and a numerical increase in the smallest arterial branches, veins and venules, 2) innervation of arteries, 3) inclusion of elastic fibers in the tunica media of the coronary arteries and development of the tunica adventitia. In conclusion, the data demonstrate that the development of the coronary system in humans is similar to that of other mammalian and avian species, and for the first time documents that the formation of the ostia and coronary stems in humans occurs by ingrowth of a vascular plexus and associated cells from the epicardium.
Key terms: proepicardium, coronary ostia, mesenchymal cells, angiogenesis, vascular smooth muscle, heart development
INTRODUCTION
During the last two decades our knowledge concerning the development of coronary vessels in experimental animals has greatly increased. For example, numerous growth factor signaling mechanisms and the roles of progenitor cells have been identified (reviewed in Tomanek, 2013; Dyer, et al., 2014). It is now well-known that the epicardium is derived from the proepicardium in both avian and mammalian species. In mice, proepicardial cells are derived from the septum transversum, adjacent to the sinoatrial junction and form a continuous mesothelial sheet, the epicardium (Viragh and Challice, 1981). That the cells forming the coronary vessels derive from independent precursors that migrate to the heart with the developing epicardium was documented in embryonic chicks (Mikawa and Fischman, 1992; Mikawa and Gourdie, 1996). Moreover, epicardial cells are involved in numerous signaling pathways that influence formation of coronary vessels (reviewed in Pérez-Pomares and de la Pompa, 2011).
Recently Wu, et al., (2013) established that Tbx18, a T-box family member, is expressed in the proepicardium and controls key steps in the coronary development in mice. Moreover, the formation of the coronary ostia and stems by an ingrowth of capillaries and progenitor cells from extracardiac sources into the aorta has been documented in quail (Bogers et al., 1989), chick (Waldo, et al., 1990), rat (Tomanek, et al., 1996; Ratajska and Fieka, 1999) and mouse (Tian, et. al., 2013a, 2013b). The findings from these experimental animal models indicate that the coronary vasculature forms by an elaborate process involving vasculogenesis, angiogenesis and arteriogenesis.
In contrast, our understanding of this topic in humans remains very limited. During the 1970s, blood islands, capillary growth and the pattern of vascularization on the surface of the human heart were described (Obrucnik, et al., 1972; Rychter, et al., 1975; Lichnovsky, et al., 1978). A decade later Hirakow (1983) studied 52 embryos from the Kyoto University collection and reported that the middle cardiac vein is present at stage (S) 16, i.e., about 37 days, and that the coronary ostia and stems develop between S18 and S19 (during the 6th week). A description of cell groups at the atrioventricular junction and subepicardium along with “vascular buds” were reported by Conte and Pellegrini (1984). Both Hutchins et. al. (1988), who studied the Carnegie collection, and Mandarin-de-Lacerda (1990), who examined the Paris Embryonic Collection, confirmed the earlier findings of Hirakow. Subsequently, Hirakow (1992) delineated the formation of the epicardium and its associated villous protrusions of mesothelial cells.
Formation of coronary arteries was believed to occur by the formation of sprouts at the sites of the coronary ostia, i.e., an outgrowth from the aorta (Hirakow, 1983; Boucek, et al., 1984; Conte and Pellegini, 1984). In retrospect, one can understand this assumption, because these investigators did not find a communication between the artery and preformed vascular channels in the myocardium at the time of coronary artery stem formation. This assumption was later questioned by Bogers and colleagues (1988). They examined human and rat embryos, and noted that “a coronary orifice was never seen in the absence of a proximal coronary artery.” Based on their observations, they concluded that the existing theories regarding proximal coronary artery development, which mainly assumed an outgrowth of the arteries, were inadequate to explain their observations. Subsequently, using serial sections, these investigators were able to show that the coronary ostia were formed by ingrowth of a capillary plexus in quail embryos (Bogers, et al, 1989).
The use of digitized microscopic images during the last two decades has facilitated our study of serially-sectioned organs, enabling better detail of various components. Accordingly, the current study utilized microscopic slides from the Carnegie Collection in order to 1) elucidate the sequence of events that constitute the development of the human coronary vasculature and 2) compare these events to those noted in experimental animal models. A major objective of this study was to determine if mesenchymal cell-containing villi from the proepicardium are the primary source of cells that form the coronary vasculature and that the coronary ostia form by an ingrowth of a capillary plexus. A second objective of this study was to elucidate the growth of the coronary vasculature in the fetus based on the slides from the Patten collection.
MATERIALS AND METHODS
The data in this study are based on digitized images from embryonic (Carnegie Collection) and fetal (Patten Collection) hearts archived at the National Museum of Health and Medicine, Silver Springs, MD. Histological serial sections from a total of 79 hearts were examined and selected images were digitized. The number of embryonic hearts studied from various stages (S) was as follows: S15 – 5, S16 – 8, S17 –7, S18 – 10, S19 – 9, S20 – 8, S21 – 7, S22 – 6, S23 – 6. The number of fetal hearts studied at 12–13, 15–16, 18–19 and 26–30 weeks was 5, 4, 2 and 2 respectively. Embryonic “stages” in the Carnegie Collection are based on the morphological state of development and therefore not on chronological age. Similarly, fetal age of specimens in the Patten Collection is also an estimate based on the morphological state of development.
The specimens used for figures that were digitized from the from the Carnegie Collection are: Fig. 1 A & B – 3512, C – 6931, D – 5515. Fig. 2 A, B & C – 6520, D – 6521. Fig. 3 A - 613, B – 6529. Fig. 4 A – 6528, B – 4430, C – 4480, D – 4430. Fig. 5 A – 432, B – 9113, C & D –8965. Fig. 6 A & B – 6426, C – 7272, D & E – 9614, F – 4960. Fig. 7A – 782, B – 4289. Those from the Patten Collection are: Fig. 8 A–D – 853. Fig. 9 – 318. Fig. 10A – 318, B – 433. Fig. 11 – 838. Fig. 12A & B – 831. Most of the sections were stained with hematoxylin and eosin. Usage of other stains is indicated in the Figure legends.
Figure 1.
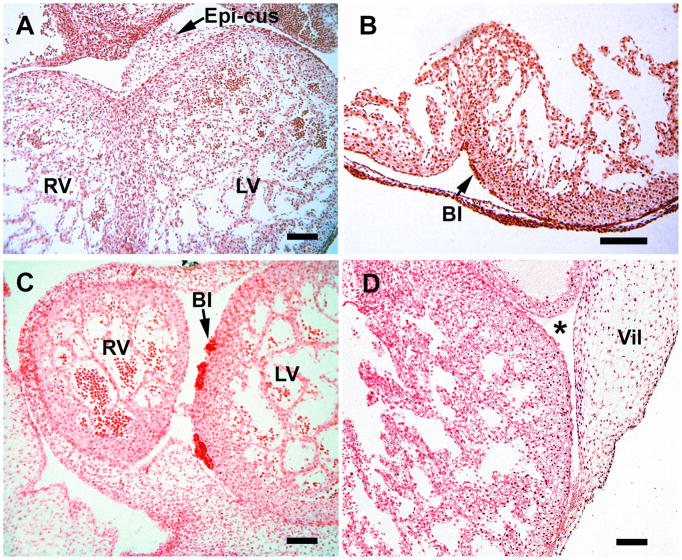
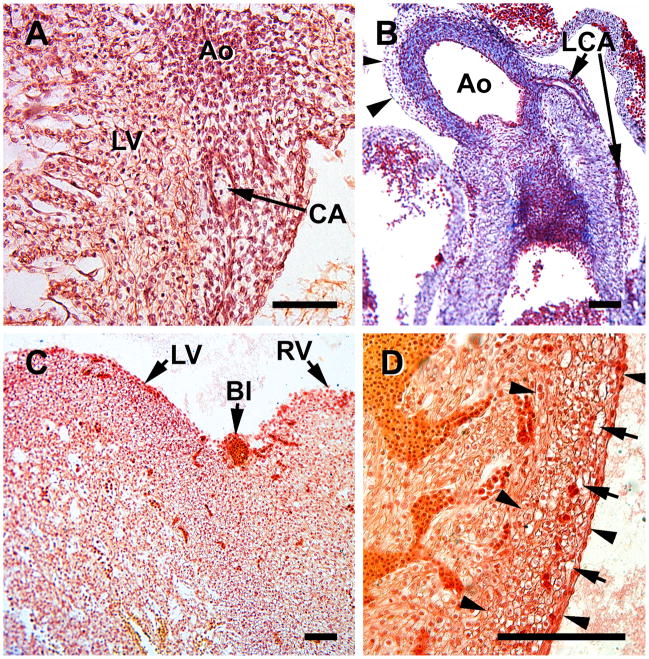
Early cardiac vascularization during S15 (A &B) and S16 (C & D). A, mesenchymal-containing epicardial atrioventricular cushions (E-cus) at the interventricular groove (RV, right ventricle; LV, left ventricle). B, blood islands (BI) are present in the subepicardium, the site of early capillary formation. C, blood islands increase in size. D, a proepicardial villus is attached to the left ventricle near the A-V junction (*). Note that a large portion of the mesothelial-lined villus, which contains mesenchymal cells, is still unattached. Magnification bar = 100 μm.
Figure 2.
Hearts from S17 embryos illustrating the importance of the mesenchymal-containing epicardial atrioventricular cushions (Epi-cus), at the atrioventricular groove. These structures represent the expanded subepicardium. A, an overview of the heart indicates that they are present at both right (RV) and left (LV) ventricles near the endocardial cushions (EC); the sinus venosus (SV) is also seen in this image. B, a blood island (BI) appears within the epicardial cushion. C, the endocardial cushion and the mesenchyme-filled epicardial cushion are seen at a higher power than in A. D, subepicardial blood islands (BI) are seen in close association with epicardial-derived subepicardial cells (*). Magnification bar = 100 μm.
Figure 3.
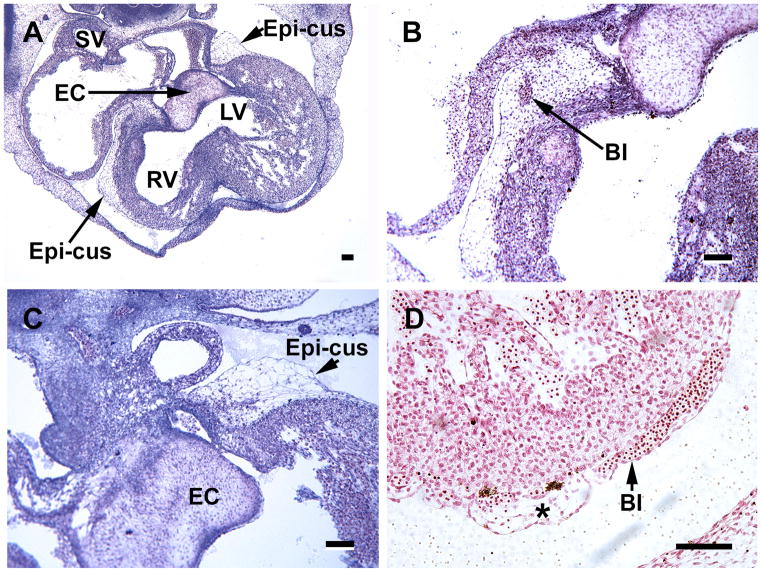
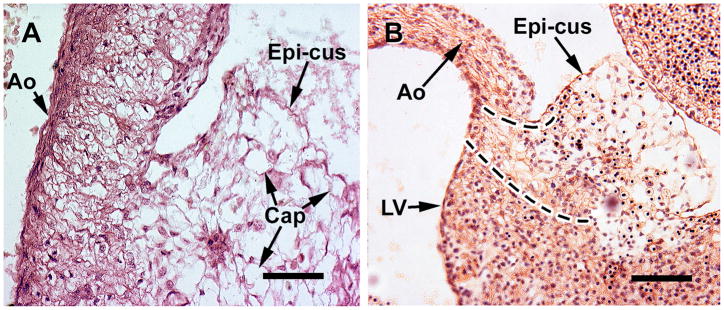
A. this coronal section includes a capillary plexus (Cap) that is established within a epicardial cushion (Epi-cus) at the root of the aorta (Ao) in a S17 embryonioc heart. B, the capillary plexus (in this oblique section) penetrates the aorta (S18 embryo), which results in the formation of a coronary ostium. Note the presence of erythroblasts that accompany the vascular plexus at the site of the coronary ostium. The region between the broken lines contains the capillaries and cells that are penetrating the aortic root. Magnification bar = 100 μm.
Figure 4.
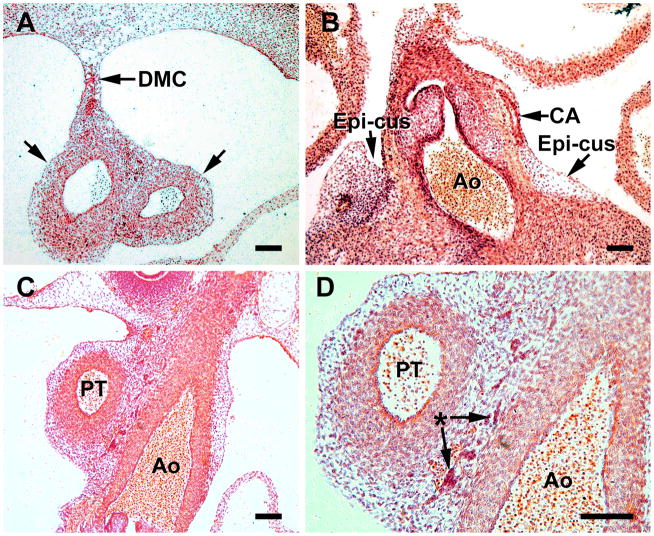
S18 heart sections comprise this figure, which represents the time of coronary ostia and stem formation. A, In this transverse section at the level of the arterial trunk, the dorsal mesocardium (DMC) is seen attaching to the aorta and pulmonary trunk (between arrows) and serves as a source of cells for the arterial trunk. B, formation of a coronary artery (CA) passing through the persisting epicardial cushion (epi-cus) at the aortic root (Ao). C, a network of cells (mostly mesenchymal) surrounds the pulmonary trunk (PA) and aorta (Ao). D, a higher power of C. The asterisk indicates blood islands and isolated erythrocytes that are characteristic of this region. Magnification bar = 100 μm. B,C and D are from coronal sections.
Figure 5.
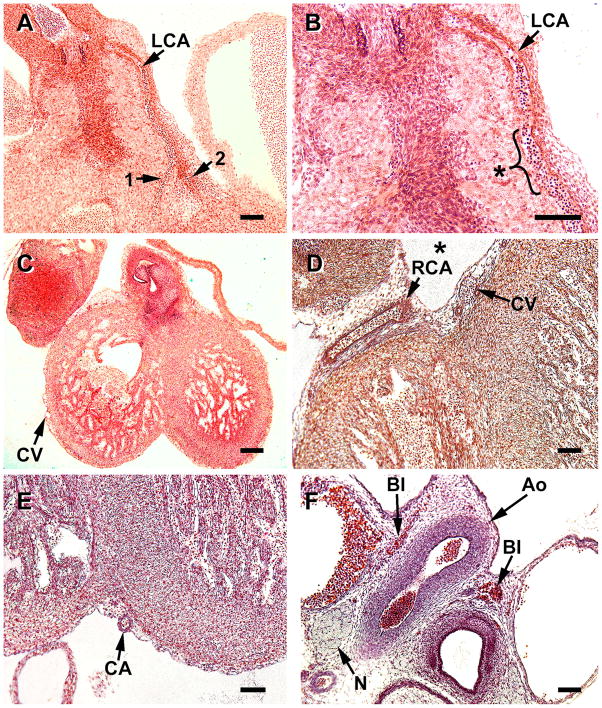
S19 hearts, a stage that is characterized by the presence of both ostia and coronary stems. A, this coronal section shows a coronary artery (CA) with a single layer of VSMCs at the point of emergence from the aorta (Ao) into the left ventricle (LV). B. a coronary artery with a complete layer of smooth is seen at the aortic root, but lacks VSMCs more distally, (azure-Mallory stain). C, downstream development of the tunica media of the left anterior descending coronary artery at the apical atrioventricular groove is preceded by a blood island (BI). D, with the establishment of coronary flow by S19, perfusion of the preformed capillary bed occurs as capillaries (arrows) appear dilated in the compact region (between arrowheads) of the ventricle. Magnification bar = 100 μm.
Figure 6.
S20 (A–C) and S21 (D–F) heart specimens. A, during these stages the formation of primary branches (1, 2) of the main coronary arteries occurs, but the development of the tunica media is still limited to the proximal portion of the left, main coronary artery (LCA). B, a higher magnification of A, the limitation of VSMC recruitment to more distal region of the artery (asterisk) can be seen. Note that distally the VSMCs are only on the artery’s epicardial side. C and D, at this stage epicardial veins and venules (CV) are more common; in the atrioventricular groove (*) they are adjacent to coronary arteries (CA). Development of the tunica media is limited to the largest veins at this stage. E, the left anterior descending coronary artery (CA) is undergoing development of its tunica media in the apical atrioventricular groove during the S21 stage. This is the site where the blood island was noted in Figure 4C. F, a nerve (N) and blood islands (BI) (aluminum cochineal stain), are seen near the aortic root (Ao). Magnification bar = 100 μm.
Figure 7.
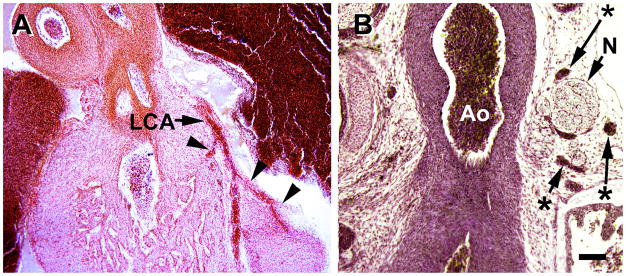
Coronal sections of S23 hearts (the last stage of the embryonic period). A, smooth muscle development of the left coronary artery (LCA) has progressed to its branches (arrowheads). B, chromaffin cell clusters (asterisks) along the aorta (Ao) are closely associated with numerous nerve bundles (N). Magnification bar = 100 μm.
Figure 8.
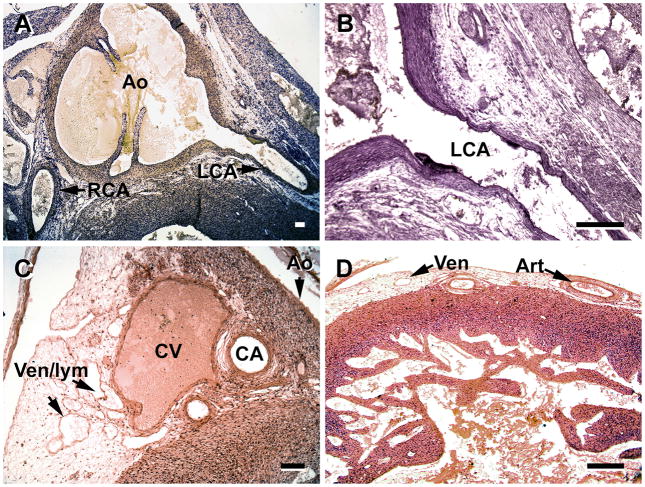
A and B, by the 12th week the coronary arteries (CA) of fetal hearts have a well-developed tunica media. C and D, the venous drainage has expanded as seen by numerous venules, although some of the structures may be lymphatic vessels (Ven/lym ). C, great cardiac vein (CV) at the base of the aorta (Ao) has a complete tunica media, and the coronary artery branches (CA) have well-developed meda. D, the number of veins and arteries (in the left ventricle) have increased substantially in the subepicardium. Ven/lym, veins/lymphatic vessels; RCA, right coronary artery, LCA, left coronary artery. Slides in A and B were stained with Masson trichrome. Magnification bar = 100 μm.
Figure 9.
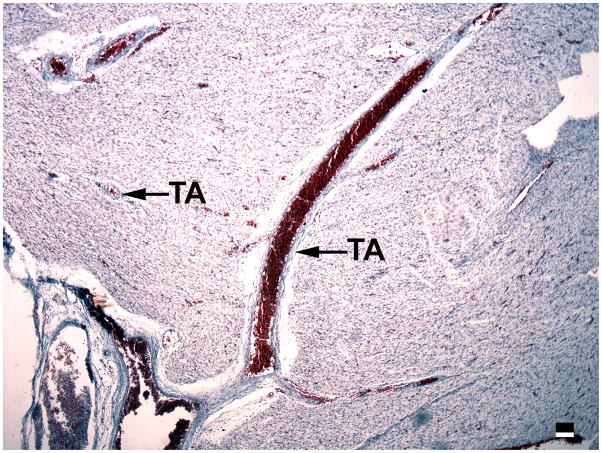
Development of transmural arteries (TA) in the left ventricle is evident in 16 week fetuses, as seen here in a transverse section (Masson trichrome stain). Magnification bar = 100 μm.
Figure 10.
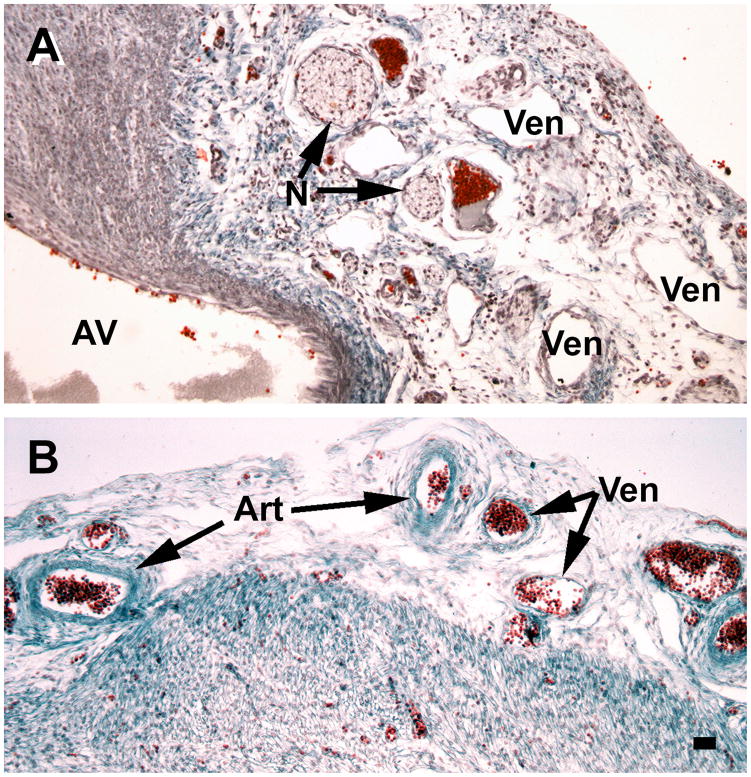
A major characteristic of 16 week fetuses is the substantial growth of subepicardial vessels and nerve fibers. A, the atrioventricular junction (AV) is especially rich in nerves (N) and veins (Ven). B, development of arteries (Art) and veins has progressed to the apical region of the ventricles. Slides were stained with Masson trichrome. Magnification bar = 100 μm.
Figure 11.
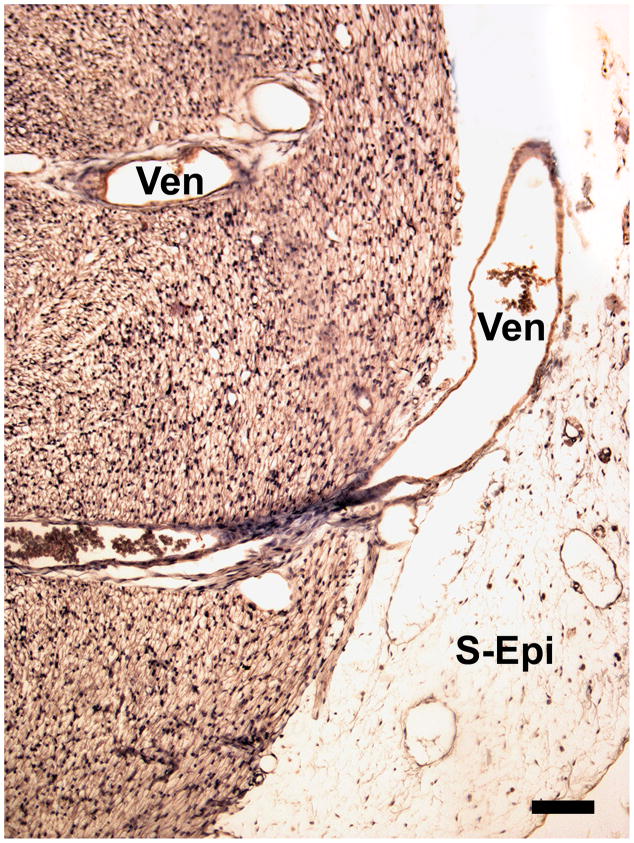
The transmural venous (Ven) drainage is further developed by the 18th and 19th weeks. Note the well-developed subepicardium (S-Epi) at the site of venous drainage in this transverse section. Magnification bar = 100 μm.
Figure 12.
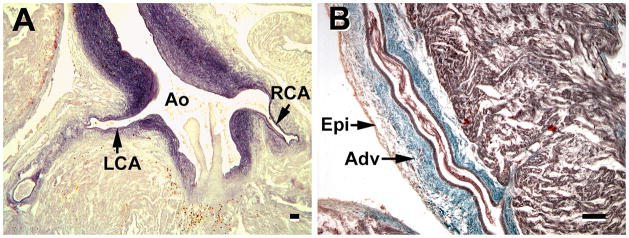
Late coronary development, as seen in 26–30 week fetuses. A, a major development is the deposition of elastic fibers in the major coronary arteries (CA). B, a second characteristic is the accumulation of collagen to the adventitia (Adv) of the major coronary arteries; thus the tunica adventitial becomes thicker than the media. Ao, aorta; RCA & LCA, right & left coronary arteries; Epi, epicardium; Adv, adventitia. A stained for elastin, B stained with Masson trichrome. Magnification bar = 100 μm.
RESULTS
Table 1 provides some characteristics of the embryonic heart at Carnegie Stages (S) 13–23. Carnegie Stages are based on Streeter’s definitive classification of human embryos with regard to their characteristics rather than age (Streeter, 1942). S23 was considered the end of the embryonic period as defined by the onset of marrow formation in the humerus (Streeter, 1951). The formation of the coronary vasculature is initiated at the time of septation of the truncus arteriosus, which begins at S14 – S15 and is completed at S16 (Orts-Llorca, et al., 1982).
Table 1.
Some developmental events of the embryonic heart at various stages (S); post-ovulatory days (D)*
| S13 (28D) Heart with primitive ventricle and common atrium |
| S14 (43D) Pulmonary vein present: onset of aorticopulmonary septation |
| S15 (33D) Outflow tract cushions form in looping heart |
| S16 (37D) Semilunar valves begin forming in looped heart |
| S17 (41D) Ostium secundum and semilunar valves are formed; completion of aorticopulmonary septation |
| S18 (44D) Interventricular septum complete; septation of 4 chambers complete; appearance of septum secundum and foramen ovale |
| S19 (47D) Fusion of aortic and mitral endocardial cushion material; outflow tract septum formed and aortic and pulmonary streams divided |
| S20 (50D) Nerve processes and collagen appear in sinus node region |
| S21 (52D) Subepicardium becomes thicker |
| S22 (54D) Chordae tendinae begin forming |
| S23 (56D) SA and AV nodes evident; ganglion cells and immature neurons appear between aorta and pulmonary trunk; embryo’s length is double that of S18 Data from: McBride, et al., 1981; Orts-Llorca, et al, 1982; Leatherbury and Waldo, 1995; Conte and Pellegrini, 1984; Boucek, et al., 1984; Lomonico, et al., 1986; Wessels, et al., 2000; Magovern et al., 1986. |
The approximate age for each stage is according to Olivier and Pineau, 1962.
Events Preceding Formation of Coronary Arteries
At S15 and S16 (34 and 37 days) two key structures are evident in all specimens: blood islands at the atrioventricular and interventricular grooves, and epicardial atrioventricular cushions, a term used in this study to denote the thickened portion of the subepicardium that is characteristic of this region (Figure 1). The latter contains a network of loose cells, including mesothelial cells and erythroblasts, which are associated with the formation of the coronary ostia, and the two coronary artery stems, processes that occur between S17 and S19 (days 41 and 48). Epicardial atrioventricular cushions, as well as the epicardium, are derived from proepicardial villi; a portion of one is seen in Figure 1D). Villi are projections of the proepicardium that attach to the dorsal surface of the heart and form the epicardium. The epicardial cushions at the atrioventricular groove are thicker persist throughout the embryonic period. The ventricles contain both spongy and compact myocardium and the subepicardium displays expanded regions, which contain various cell types and some capillaries. The subepicardium is the main site of blood islands (Figure 1B,C). Endothelial cell precursors migrate to the subepicardium, as shown in animal models (Mikawa and Fischman, 1992; Mikawa and Gourdie, 1996), and are the source endothelial cells that form a vascular network in the compact myocardium (reviewed in Tomanek, 2013).
By S17 (41 days) the compact regions of the ventricles are thicker, as seen in Figure 2, compared to S15 (Figure 1A, B). The epicardial cushions at the atrioventricular groove contain not only spindle-shaped mesothelial cells, erythroblasts, but also blood islands (Figure 2B). Blood islands, as seen in Figure 2D, are located throughout the subepicardium, and consist of erythroblasts and spindle-shaped mesothelial cells. They extend to the aortic-ventricular junction, where the coronary ostia and coronary stems form, a process initiated at S17. The endocardial cushions are aligned with the atrioventricular grooves (Figure 2A, B, C). Separation of the atrial and ventricular myocardium in the human heart begins during the seventh week of development and provides insulation between the chambers (Wessels, et al., 1996). Therefore, the formation of the coronary ostia, as documented here, occurs concurrently with atrial-ventricular septation. The epicardial cushions, adjacent to the endothelial cushions, are also in proximity of the sinus venosus Figure 2A). They persist throughout the period of arterial stem formation, which is completed by S19 in all hearts observed in this study, and throughout embryonic development (up to S23). They contain the precursors of a capillary system that originates in this region.
Formation of the ostia and coronary arteries
The formation of coronary ostia and coronary artery stems has been shown in various mammalian and avian species to occur via ingrowth of a capillary network, and has been suggested to occur in humans as well. Here I show, for the first time, that in the human heart, cells and capillaries from the villus are seen penetrating the aortic root, thus forming a coronary ostium similar to that seen in experimental animals. The presence of capillaries in the epicardial cushions becomes clear at S17 (Figure 3A), and by S18 the capillary and cell network invades the aortic wall (Figure 3B). Erythroblasts are also included in the cell network that invades the aorta. Note, as seen in Figure 3B, that the endothelium of the aorta is still intact at this point in time.
A portion of the dorsal mesocardium remains attached to the outflow tract in S18 (44 days) hearts, where various cell types are continuous with the external tunic of the truncus arteriosus (Figure 4A). A loose cell network and blood islands between the aorta and pulmonary artery is seen, and persists during later time periods (Figure 4C, D). The presence of a single (left) coronary artery was seen in 4 of 6 hearts, that were sectioned at the aortic root, at S18 (Figure 4B) and both left and right arteries were noted in all S19 specimens. As in other mammalian and avian species, cells exterior to the aorta are required for the formation of the two ostia and coronary arteries in the human heart. The sites where the ostia form (above the left and right aortic cusps) the subepicardium remains wide and is rich in erythroblasts and spindle-shaped cells (Figures 4B and 5B). The forming coronary artery stem passes through this enlarged subepicardium (subepicardial cushion) at the atrioventricular groove (Figures 4B and 5B).
Newly formed coronary ostia and stems (Figure 5A, B) consist of a single layer of vascular smooth muscle cells (VSMC). With the formation of coronary ostia and the onset of coronary flow, the capillary bed is dilated and more readily observed (5D). Recruitment and assembly of VSMC occurs first at the ostium and progresses distally as seen in Figure 6A, B. These micrographs illustrate that, at S19, VSMCs have yet to be recruited to the branches of the main coronary arteries. However, far removed from the atrioventricular groove, another site of angiogenesis occurs at the apex of the heart, in the interventricular groove, as first noted by the presence of a blood island (Figure 5C).
By S20, subepicardial venules and veins are more characteristic (Figure 6C, D). However, the veins have not yet developed a tunica media. They are joined to the vascular plexus, that originates in the subepicardium, as well as to the sinus venosus. Formation of the left anterior descending artery in the interventricular groove at the heart’s apex is documented from an S21 specimen in figure 6E. This is the site where a blood island is found at earlier stages (Figure 5C). Thus, VSMC incorporation into this major coronary artery extends distally to the apex by S21. This stage is also characterized by the appearance of nerve bundles at the aortic root (6F). At this stage, and later, dark cells, shown to be chromaffin cells with nerve fibers that innervate the coronary arteries (Gardner and O’Rahilly, 1976; Pauza, et al., 2000), are often present (not shown here but seen in Figure 7B).
Final stages of the embryonic period (S22 and S23)
Following the establishment of coronary ostia and coronary circulation, several morphological changes occur in a short period of time, i.e., about 3 days. First the compact portions of the two ventricles expand. Second, coronary artery branches begin developing a tunica media (Figure 7A), and the main coronary arteries develop a partial second layer of VSMC in their proximal portions. Some small branches begin to take a transmural course, but remain limited close to the epicardium. Third, nerves with associated chromaffin (ganglionic) cells course along the aorta (Figures 7B).
Coronary development during the fetal period
Vessel maturation is a key characteristic of the fetal period. Between the end of the embryonic period (8 weeks) and 12 weeks, or one month into the fetal period, the major coronary arteries increase both their luminal diameters and the thickness of the tunica media (Figure 8A–D). Medial development of smaller arteries has occurred and larger veins have a distinct tunica media (Figure 8C, D). The number of veins, venules, arteries and arterioles has greatly increased. By 16 weeks the transmural arteries and arterioles are well-developed (Figure 9), in response to the thickening of the compact region of the ventricle. Other characteristics include increases in the density of epicardial vessels, especially in the apical region of the ventricles, and in the number of nerve branches associated with innervation of the coronary arteries (Figure 10).
In hearts of 18–19 week fetuses transmural veins are more numerous and larger than those observed in their 16 week counterparts (Figure 11). A later fetal period (26–30 weeks) is characterized by the inclusion of elastic fibers and expansion of the tunica adventitia in the main coronary arteries. As seen in Figure 12, the thickness of the tunica adventitia, by this stage, exceeds that of the media (Figure 12). Thus, between the 16th and 18–19th weeks the coronary arteries and veins increase in number and develop a thicker tunica media. Then the arteries undergo maturation, involving the addition of elastic fibers and expansion of the tunica adventitia, as noted in third trimester (26–30 week) fetuses.
DISCUSSION
This study is the first to document the time course of coronary vascular development in the human embryo and fetus and supports three important conclusions. First, the coronary ostia form by an ingrowth of capillaries and spindle cells from epicardial cushions that are derived from the proepicardium. Proepicardial villi preferentially target the atrioventricular groove and the aortic root. This evidence indicates that human coronary ostia formation is virtually identical to that of other mammalian and avian species. Second, a role for the mesocardium in coronary development is suggested by the continuity of cells of the dorsal thoracic wall and those surrounding the aorta and pulmonary artery. Third, erythroblasts appear to play a key role in the formation of coronary vessels, as evidenced not only in their presence in blood islands, but also as free cells associated with the formation of the coronary ostia and arterial stems. A recent study on mice (Jankowska-Steifer et al., 2015), based on immunohistochemical and ultrastructural methods, found that the blood islands in the subepicardium of interventricular sulci consist of both vasculogenic and hematopoietic progenitors and macrophages. The latter would appear to be necessary for the penetration of endothelial and other cell types that form the coronary ostia. Thus, blood islands contribute to both the formation of the early cardiac vascular tubes and to the establishment of coronary ostia and stems.
Summary of events comprising coronary vessel formation
A review of prenatal coronary morphogenesis (Tomanek, 2013, pp.25–46) provides some details that pertain to the events described in this study. The proepicardium and the sinus venosus region provide the precursor cells required for the formation of coronary tree. Villi from the proepicardium attach to the dorsal surface of the heart and form the epicardium. Mesothelial cells under the epicardium contribute to the formation of the early vascular plexus by the process of vasculogenesis, i.e., the assembly of cells to form vascular channels, which grow by establishing branches and extending the vessels (angiogenesis). One of the regions of the vascular plexus surrounds the aortic root and provides cells that penetrate the great vessel to establish the two coronary ostia. The recruitment of vascular smooth muscle cells enables the establishment of the main coronary arteries (arteriogenesis), a process that occurs in a base to apex direction. An important concurrent event is the remodeling of the vascular bed to enable blood flow from the coronary arteries through the capillaries and into the venous system.
Pericardial villi and the dorsal mesocardium
There is ample evidence that proepicardial cells in both avian and mammalian models reach the dorsal surface of the heart, via multicellular villous projections, where they proliferate and spread over the surface of the heart (reviewed in Tomanek, 2013; Dyer et al., 2014). More than two decades ago Männer (1992) found that the chick coelomic epithelium of the ventral wall of the sinus venosus forms villous protrusions that contact the dorsal wall of the heart to form a secondary dorsal mesocardium (a connection between the ventral wall of the sinus venosus and the dorsal surface of the heart). He then reported that the attachments of the secondary dorsal mesocardium form the epicardium (Männer, 1993). Subsequently it was found that mesothelial cells of the septum transversum are components of the epicardium and subepicardium in the mice (Komiyama et al., 1987) and rats (Van den Eijnde et al., 1995). Colonization of proepicardial cells in the developing avian heart were shown to occur via a bridge from the posterior thoracic wall (Nahirney et al., 2003). Nesbitt and colleagues (2006) then found that a direct attachment of these cells to the heart in rats occurs in a manner similar to that documented in avians. A direct contact of the multicellular proepicardial villi with the myocardium to form the epicardium has also been established in mice (Rodgers et al., 2008).
As seen in Figure 1D, the villus at the atrioventricular groove has a mesothelial outer layer and an inner core of mesenchymal cells, as has been described in various species (reviewed in Tomanek, 2013). Some epicardial cells undergo epithelial-mesenchymal transition and differentiate into various lineages. These cells, along with those of the villus core invade the myocardium and contribute to the formation of the coronary vasculature. A subpopulation of these cells selectively migrates to the base of the aorta where they contribute to the formation of the coronary ostia. As seen in Figure 3, a capillary plexus and erythroblasts penetrate the aorta, a phenomenon that is similar to that which occurs in other mammals and in avians. In humans, the mesothelial cells of the villous protrusions migrate to form the epicardium between stages S12 – S16 (Hirakow, 1992).
Importance of atrioventricular groove
Villous protrusions have been found to contact the dorsal walls of the ventricles in human ventricles as early as S12 (Hirakow, 1992). In that study the epicardium was present in all S13 specimens studied. The current study reveals that these villous projections target the atrioventricular groove. Moreover, it illustrates that the dorsal mesocardium surrounds the aorta and pulmonary trunk and contains cells similar to those on the dorsal wall. Thus, the cells from the dorsal wall are likely derivatives of the so-called “second heart field”. A review of the literature supports the conclusion that the dorsal mesenchymal protrusion provides an extracardiac population of mesenchyme for the common atrium and contributes to the atrioventricular complex (Briggs, et al., 2012). It has also been found to be important for the development of pulmonary veins in humans (Bliss and Hutchins, 1994). This is consistent with the finding of the current study that the villi consistently appear at the atrioventricular groove. Indeed, the inner curvature of the heart is selectively permissive for the penetration of endothelial precursor cells as noted in chicks (Lie-Venema et al., 2005); these cells then spread radially from the atrioventricular groove (Ratajska, et al., 2008). Using specific antibodies (QH1) for endothelial cells in the embryonic quail heart, Kattan et al. (2004) demonstrated that the atrioventricular groove is the earliest site of endothelial cell appearance. Moreover, the work revealed that CD45+ hematopoietic precursors are closely related to the vasculogenic/angiogenic process.
These findings support the observations in the current human heart study that the atrioventricular groove is a focal point for the development of the coronary ostia and arteries as well as endothelial tube formation. As found in the current human study, the expanded subepicardium at the atrioventricular junctions persists during the embryonic period and is closely associated with the coronary ostia. As shown in mice, this site of epithelial – mesenchymal transition provides cells for the atrioventricular cushions, as well as coronary vessels (Wessels et al., 2012).
Ostial and coronary artery stem formation
The assumption that the coronary artery stems arise from the aorta was well engrained in the literature until Bogers, et al., (1989) questioned this notion, since their observations in quail embryos indicated that the coronary ostia were always connected to a proximal coronary artery. Waldo et al., (1990) then demonstrated that a capillary ring surrounding the aortic and pulmonary outflow tracts, in chicks, penetrates the aorta to form channels giving rise to the two coronary ostia. As noted earlier, a similar phenomenon has been found to occur in mammals. This process, as demonstrated in the chick, requires apoptosis of aortic cells enabling the influx of the capillary ring plexus (Velkey and Bernanke, 2001). Subsequently, Ando and colleagues (2004) documented multiple endothelial strands entering the aorta at several sites in quail hearts, but only two of the sites persisted with fusion of the endothelial strands. This event is followed by the development of a tunica media and the formation of coronary artery stems. Details of VSMC recruitment and assembly in experimental animals have been recently reviewed (Tomanek, 2013; Wu, et al., 2013).
Earlier studies in humans assumed that the “endothelial buds” observed on the outside of the aorta were the consequence of endothelial outgrowths (Hirakow, 1983; Conte and Pellegrini, 1984). However, Hutchins, et al. (1988) were uncertain how the ostia connected with the coronary artery stems and Bogers and colleagues (1988), as previously noted, doubted the endothelial outgrowth explanation of coronary stem formation. The current study certifies a series of events underlying the formation of the coronary ostia and stems in the human heart. First the villus structure, firmly attached in the atrioventricular groove contains erythroblasts and a capillary plexus. Second, these are the structures that are shown to invade the aorta. Third, the aortic wall is modified, i.e. a loss of aortic VSMCs at the site of capillary and cell invasion. Finally, these events occur only during the time frame of ostial formation, which has been shown to occur between S17 and S20 (Hirakow, 1983; Conte and Pellegrini, 1984; Hutchins et al., 1988; Mandarin-de-Lacerda, 1990). The finding that the left coronary artery forms before the right, also reported earlier (Bogers, et al., 1988; Mandarin-de-Lacerda, 1990), relates to the observation that the left ventricle is vascularized before the right ventricle (Rychter et al., 1975). That left coronary artery formation precedes that of the right is consistent with data from chickens (Rychter and Ostadal, 1971) and rats (Bogers, et al., 1988; Ratajska and Fiejka, 1999).
Coronary vessel growth and maturation
The earliest signs of venous connections in human hearts occur in S16 – S17 embryos (Hirakow, 1983; Hutchins et al., 1988). The images in the current study indicate that at these stages the structures are mainly venules, whereas veins are more obvious at the time of arterial formation and later. A study in quails found that arteriovenous anastomoses, present when ostia form, disappear as veins mature (Vrancken Peeters, et al., 1997). Prior to that time the coronary vascular plexus consists only of endothelial-lined tubes lacking a tunica media. These are, at least in part, derivatives of the early vascular plexus that originated from the subepicardium. Thus, the development of these venous channels requires the fusion between the capillary plexus, a derivative of the cells of the subepicardium, and the endothelial sprouts of the sinus venosus. Hutchins, et al. (1988) noted that this “establishment of venous connection of epicardial plexus to the coronary sinus” was not established in most embryos until S17. The muscularization of coronary veins, like that of arteries, is dependent on the establishment of coronary flow, which occurs at S19 (about 47 days). For this reason, muscular veins are not seen until later. However, the development of the tunica media in larger veins occurs rapidly as seen in S21 (Figure 6D) and 12 week hearts (Figure 8C).
Some of the vessels seen in the fetal hearts that have characteristics of venules (small diameters and no smooth muscle) may be lymphatic vessels. These channels are preceded by the development of the coronary arteries, “both in time and place” (Wilting and Männer, 2013). Cardiac lymphatic vessels arise from the jugular lymph sac and grow out through the subepicardium toward the apex (reviewed in Wilting and Männer, 2013). These thin-walled channels are positioned around the coronary arteries.
The development of a distinct pulmonary vein was observed in the left atrium in close proximity to the atrioventricular groove in a S17 (about 41 days) heart, and already at S14 in previous studies (Bliss and Hutchins, 1995; Wessels et al., 2000). The pulmonary vein “contributes to the posterior part of the atrial septum and is continuous with the dorsal sinuatrial fold,” which is the future left venous valve (Blom, 2001). It arises from the dorsal mesocardium and is a prominent structure in S16 hearts (Wessels, et al., 2000).
Branches of the two main coronary arteries develop during the late embryonic period. Licata (1954) noted branches to the sinuatrial node and main AV bundle in a 9 week human heart (about S22). However, the process of VSMC recruitment is gradual and continues into the fetal period. Although, myocardial perfusion commences with the formation of the coronary ostia, much of the pre-capillary vasculature is not yet muscularized. With the thickening of the ventricular walls, transmural vessels begin attaining a tunica media; however, this occurs primarily during the fetal period.
Since vascular growth and maturation are dependent on signaling mechanisms, the presence of receptors on coronary vessels is of importance. A differential expression of endothelial growth factor receptors was found in human embryos and fetuses (Partanen et al., 1999). VEGFR-1, VEGFR-2, Neuropilin-1, Tie-1 and Tie-2 were expressed in the endothelia of myocardial arteries, veins and capillaries, whereas epicardial coronaries (these are the larger arteries) lacked VEGFR-2. VEGFR-3 expression was more intense in 5 week embryos, but was downregulated after that. These findings are consistent with the well-established evidence that the developing coronary vasculature requires a variety of growth factors associated with angiogenesis and vessel growth (Tomanek, et al., 2010; Tomanek, 2013).
Nerves, ganglia and neural crest cells
Gardner and O’Rahilly (1976), who studied the nerve supply and conduction system in a S23 human embryonic heart, described ganglia consisting of immature neurons with large pale nuclei that they considered to be sympathetic, parasympathetic or sensory. They also noted dark-staining chromaffin cells, satellite cells and fibroblasts. Preganglionic coronary nerves, in humans have been shown to enter the heart between the aorta and pulmonary trunk ganglia and are considered to be mainly post-ganglionic fibers innervating the coronary arteries (Pauza, et al., 2000). These coronary subplexuses have a dense distribution in this region and account for about 10% of all ganglia to the heart. Navaratnum (1965), who studied 91 human embryonic and fetal hearts, found that the nerves first pass through the venous mesocardium at S17 and then through the arterial mesocardium at S18. By 2 months the nerves coalesce to form the deep cardiac plexus. Subsequently, the plexuses associated with the coronary arteries increase in size and arterial innervation is initiated by S23 (Smith, 1970). As seen in Figure 10, numerous nerve branches are found in the supepicardium of 16 week fetuses. A review of neural crest cells indicates that they may contribute to the development of the autonomic nerves to the heart (Hildreth, et al., 2008).
Neural crest cells have also long been considered to play, at least, a regulatory role in coronary ostial formation. In chick embryos the development of coronary artery vascular smooth muscle was dependent upon the presence of neural crest cells, since their ablation resulted in disrupted development of the tunica media of the coronary arteries (Hood and Rosenquist, 1992). Using quail-to-chick chimeras, a subsequent study verified that neural crest cell-derived parasympathetic ganglia are associated with the survival of the persistent coronary arteries (Waldo, et al., 1994). The study revealed that branches from the parasympathetic nerves entered the stems of the coronary arteries near the sites of neural crest cells. Although these studies did not find that neural crest cells contributed VSMCs to the coronary arteries, a recent study has suggested that neural crest cells from the preotic hindbrain region (mice and chicks) contribute mesenchymal-like cells and VSMCs to the heart (Arima, et al., 2012). Ablation of these cells resulted in altered sites of coronary artery origins and asymmetry in VSMC deployment.
The fetal period
The findings of the current study indicate that a maturation process of the coronary vasculature is the major characteristic of the fetal period. Development of the tunica media occurs by the addition of VSMC layers in the coronary arteries and the recruitment of VSMCs to the smallest arterioles. Development of the latter increases their numerical density. During the first month of the fetal period (9 – 12 weeks) the major arteries increase in diameter and the number of veins increases. Formation of transmural arteries and arterioles becomes evident at 16 weeks. In agreement with previous work (Matonoha and Zechmeister, 1992), the elastic components of arteries are not yet well developed at this time. Elastic development occurs during the late fetal period, along with a marked expansion of the tunica adventitia, a finding also reported by Matonoha and Zechmeister (1992). A distinct and continuous internal elastic membrane in the main coronary arteries is evident at 5 months (Moon, 1957). Laurie and Woods (1958) noted coronary anastomoses in the hearts of neonates. Four decades later, Cortis and Serrato (1998) utilized barium sulfate angiograms to document coronary collateral vessels (ranging 3–50 μm in diameter) in 19–32 week fetuses. Previous work had reported coronary anastomoses in neonates, but noted that they tended to involute during childhood (Reiner, 1961). Coronary collateral vessels may be remnants of the extensive vascular connections that precede the establishment of a functional coronary circulation. The involution of collateral channels is likely the consequence of limited flow in some of the channels.
In the absence of immuno-staining, it is difficult to distinguish lymphatic vessels from venules and veins. However, the development of the former occurs later than that of the latter in most species, including humans (reviewed in Wilting and Männer, 2013). Lymphatic channels are derived from the jugular lymph sacs. In humans the lymph sacs were found in 6–7 week embryos (Van der Putte, 1975), or after 8 weeks (Kampmeier, 1928). The latter study found that during the third month a net of lymphatic vessels grows from the lymphatic sacs and spreads over the heart. Lymphatic vessels are usually in close association with the coronary arteries and their development proceeds toward the apex of the heart (Wilting and Männer, 2013).
During the fetal period the coronary nerves passing between the aorta and pulmonary trunk give rise to a dense subplexus of nerve fibers that are more numerous at the left than at the right coronary artery (Pauza, et al., 2000). The plexus of nerves increases in size and extent at about the 9th week (Navaratnam, 1965; Smith, 1970), and as documented in the current study, numerous nerve branches extend into the subepicardium by the 16th week. Human coronary epicardial arteries and veins have sympathetic, parasympathetic and sensory nerves at their adventitial-medial borders (reviewed in Saetrum Opgaard et al, 1997). Smaller vessels have only a sparse plexus of nerve fibers; veins have fewer fibers than arterioles (Smith, 1970).
Concluding remarks
This study documents the events that constitute the development of the human coronary vasculature during the embryonic and fetal periods. These events are similar to those that have been detailed in avian and mammalian models. One of the most important findings in this study is the formation of the coronary ostia by ingrowth of capillaries, which occurs in conjunction with a blood island positioned in the epicardial cushion, situated in the atrioventricular groove. Compared to the capillary ingrowth noted in experimental animal models, which emanates from a broad capillary plexus, the ingrowth that occurs in humans appears to involve a less extensive capillary plexus and therefor is more focused. It appears that the cells contributing to the formation of the coronary ostia and arterial stems are derived from both the mesenchyme of the villus core and from epithelial-mesenchymal transition. Moreover, mesenchymal stem cells may influence the signaling of growth factors, as documented in cultured embryonic mouse hearts (Tomanek, et al., 2010).
Although the current study reveals morphological events in humans that are similar to those in other mammals and avians, it remains to be determined if the signals regulating these events are the same as those in other species. The identity of coronary vessel progenitors, their pathways, and the signals that activate these cells are topics of current investigations in animal models. One area of investigation concerns the origins of the various cells that form the coronary hierarchy. Our current understanding of this topic is that they may arise from proepicardial cells contributing to the epicardium, the sinus venosus or the endocardium (Perez-Pomeres and de la Pompa, 2011; Chen, et al., 2014). Of the many growth factors implicated in vascularization of the heart, VEGF family members to play key roles, as established in avian (Tomanek, et al., 2006) and mouse (Chen, et al., 2014) models. As shown by Chen, et al. (2014) VEGF-C from the epicardium plays a specific role in regulating dorsal and lateral coronary growth. Based on our current state of knowledge, it would not be surprising to find that the progenitor cells and their signaling events are conserved through evolution.
Table 2.
Milestones in coronary vessel formation and development. S, Carnegie Stage.
| S15 Blood islands, capillaries and epicardium are present |
| S17–18 Subepicardial expansion at AV groove contains capillaries, mesenchymal cells and erythroblasts, which invade the aortic wall to form coronary ostia; primitive coronary plexus connects with sinus venosus sprouts |
| S19 Both coronary ostia and stems are present; smooth muscle recruitment occurs in the proximal portions of coronary artery stems |
| S22–23 Tunica media formation in coronary artery branches; nerves with associated chromaffin (ganglionic) cells invade region around the aortic base: onset of lymphatic development |
| 9–12 weeks Formation of tunica media in large veins and small arteries |
| 16 weeks Transmural arteries and arterioles are well-developed; epicardial vessel density is increased |
| 26–30 weeks Elastic fibers added to main coronary arteries; the thickness of the adventitia is greatly increased |
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: HL075446
The author gratefully acknowledges Elizabeth Lockett of the National Museum of Health and Medicine, Silver Springs, MD for her assistance regarding the slides used in this study.
LITERATURE CITED
- Ando K, Nakajima Y, Yamagishi T, Yamamoto S, Nakamura H. Development of proximal coronary arteries in quail embryonic heart: multiple capillaries penetrating the aortic sinus fuse to form main coronary trunk. Circ Res. 2004;94:346–352. doi: 10.1161/01.RES.0000112963.79064.09. [DOI] [PubMed] [Google Scholar]
- Arima Y, Miyagawa-Tomita S, Maeda K, Asai R, Seya D, Minoux M, Rijli FM, Nishiyama K, Kim KS, Uchijima Y, Ogawa H, Kurihara Y, Kurihara H. Preotic neural crest cells contribute to coronary artery smooth muscle involving endothelin signalling. Nat Commun. 2012;3:1267. doi: 10.1038/ncomms2258. [DOI] [PubMed] [Google Scholar]
- Bliss DF, Hutchins GM. The dorsal mesocardium and development of the pulmonary veins in human embryos. Am J Cardiovasc Pathol. 1995;5:55–67. [PubMed] [Google Scholar]
- Blom NA, Gittenberger-de Groot AC, Jongeneel TH, DeRuiter MC, Poelmann RE, Ottenkamp J. Normal development of the pulmonary veins in human embryos and formulation of a morphogenetic concept for sinus venosus defects. Am J Cardiol. 2001;87:305–309. doi: 10.1016/s0002-9149(00)01363-1. [DOI] [PubMed] [Google Scholar]
- Bogers AJ, Gittenberger-de Groot AC, Dubbeldam JA, Huysmans HA. The inadequacy of existing theories on development of the proximal coronary arteries and their connexions with the arterial trunks. Int J Cardiol. 1988;20:117–123. doi: 10.1016/0167-5273(88)90321-x. [DOI] [PubMed] [Google Scholar]
- Bogers AJ, Gittenberger-de Groot AC, Poelmann RE, Péault BM, Huysmans HA. Development of the origin of the coronary arteries, a matter of ingrowth or outgrowth? Anat Embryol (Berl) 1989;180:437–441. doi: 10.1007/BF00305118. [DOI] [PubMed] [Google Scholar]
- Boucek RJ, Moralwes AR, Romanelli R, Judkins MP. Coronary Artery Disease. Pathological and Clinical Assessment. Balitmore: Williams and Wilkins; 1984. Embryology and Congenital Anomalies of the Coronary Arteries; pp. 38–65. [Google Scholar]
- Briggs LE, Kakarla J, Wessels A. The pathogenesis of atrial and atrioventricular septal defects with special emphasis on the role of the dorsal mesenchymal protrusion. Differentiation. 2012;84:117–130. doi: 10.1016/j.diff.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HI, Sharma B, Akerberg BN, Numi HJ, Kivelä R, Saharinen P, Aghajanian H, McKay AS, Bogard PE, Chang AH, Jacobs AH, Epstein JA, Stankunas K, Alitalo K, Red-Horse K. The sinus venosus contributes to coronary vasculature through VEGFC-stimulated angiogenesis. Development. 2014;141:4500–4512. doi: 10.1242/dev.113639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte G, Pellegrini A. On the development of the coronary arteries in human embryos, stages 14–19. Anat Embryol (Berl) 1984;169:209–218. doi: 10.1007/BF00303151. [DOI] [PubMed] [Google Scholar]
- Cortis BS, Serratto M. The collateral coronary circulation in the human fetus: angiographic findings. Cardiologia. 1998;43:77–81. [PubMed] [Google Scholar]
- Dyer L, Pi X, Patterson C. Connecting the coronaries: how the coronary plexus develops and is functionalized. Dev Biol. 2014;395:111–119. doi: 10.1016/j.ydbio.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner E, O’Rahilly R. The nerve supply and conducting system of the human heart at the end of the embryonic period proper. J Anat. 1976;121:571–587. [PMC free article] [PubMed] [Google Scholar]
- Hildreth V, Webb S, Bradshaw L, Brown NA, Anderson RH, Henderson DJ. Cells migrating from the neural crest contribute to the innervation of the venous pole of the heart. J Anat. 2008;212:1–11. doi: 10.1111/j.1469-7580.2007.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakow R. Development of the cardiac blood vessels in staged human embryos. Acta Anat (Basel) 1983;115:220–230. doi: 10.1159/000145693. [DOI] [PubMed] [Google Scholar]
- Hirakow R. Epicardial formation in staged human embryos. Kaibogaku Zasshi. 1992;67:616–622. [PubMed] [Google Scholar]
- Hood LC, Rosenquist TH. Coronary artery development in the chick: origin and deployment of smooth muscle cells, and the effects of neural crest ablation. Anat Rec. 1992;234:291–300. doi: 10.1002/ar.1092340215. [DOI] [PubMed] [Google Scholar]
- Hutchins GM, Kessler-Hanna A, Moore GW. Development of the coronary arteries in the embryonic human heart. Circulation. 1988;77:1250–1257. doi: 10.1161/01.cir.77.6.1250. [DOI] [PubMed] [Google Scholar]
- Jankowska-Steifer E, Madej M, Niderla-Bielińska J, Ruminski S, Flaht-Zabost A, Czarnowska E, Gula G, Radomska-Leśniewska DM, Ratajska A. Vasculogenic and hematopoietic cellular progenitors are scattered within the prenatal mouse heart. Histochem Cell Biol. 2015;143:153–169. doi: 10.1007/s00418-014-1269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampmeier O. On the lymph flow of the human heart, with reference to the development of the channels and the first appearance, distribution, and physiology of their valves. Am Heart J. 1928;4:210–222. [Google Scholar]
- Kattan J, Dettman RW, Bristow J. Formation and remodeling of the coronary vascular bed in the embryonic avian heart. Dev Dyn. 2004;230:34–43. doi: 10.1002/dvdy.20022. [DOI] [PubMed] [Google Scholar]
- Komiyama M, Ito K, Shimada Y. Origin and development of the epicardium in the mouse embryo. Anat Embryol (Berl) 1987;176:183–189. doi: 10.1007/BF00310051. [DOI] [PubMed] [Google Scholar]
- Laurie W, Woods JD. Anastomosis in the coronary circulation. Lancet. 1958;2:812–816. doi: 10.1016/s0140-6736(58)90374-x. [DOI] [PubMed] [Google Scholar]
- Leatherbury L, Waldo K. Visual understanding of cardiac development: the neural crest’s contribution. Cell Mol Biol Res. 1995;41:279–291. [PubMed] [Google Scholar]
- Licata RH. The human embryonic heart in the ninth week. Am J Anat. 1954;94:73–125. doi: 10.1002/aja.1000940104. [DOI] [PubMed] [Google Scholar]
- Lichnovský V, Obrucník M, Kraus J. A quantitative morphometric study of capillary length and ventricular volume and surface area in the human embryonic and foetal heart [proceedings] Folia Morphol (Praha) 1978;26:187–193. [PubMed] [Google Scholar]
- Lie-Venema H, Eralp I, Maas S, Gittenberger-De Groot AC, Poelmann RE, DeRuiter MC. Myocardial heterogeneity in permissiveness for epicardium-derived cells and endothelial precursor cells along the developing heart tube at the onset of coronary vascularization. Anat Rec A Discov Mol Cell Evol Biol. 2005;282:120–129. doi: 10.1002/ar.a.20154. [DOI] [PubMed] [Google Scholar]
- Lomonico MP, Moore GW, Hutchins GM. Rotation of the junction of the outflow tract and great arteries in the embryonic human heart. Anat Rec. 1986;216:544–549. doi: 10.1002/ar.1092160412. [DOI] [PubMed] [Google Scholar]
- Magovern JH, Moore GW, Hutchins GM. Development of the atrioventricular valve region in the human embryo. Anat Rec. 1986;215:167–181. doi: 10.1002/ar.1092150210. [DOI] [PubMed] [Google Scholar]
- Mandarim-de-Lacerda CA. Development of the coronary arteries in staged human embryos (the Paris Embryological Collection revisited) An Acad Bras Cienc. 1990;62:79–84. [PubMed] [Google Scholar]
- Matonoha P, Zechmeister A. Structure of the coronary arteries during the prenatal period in man. Funct Dev Morphol. 1992;2:209–212. [PubMed] [Google Scholar]
- Mcbride RE, Moore GW, Hutchins GM. Development of the outflow tract and closure of the interventricular septum in the normal human heart. Am J Anat. 1981;160:309–331. doi: 10.1002/aja.1001600308. [DOI] [PubMed] [Google Scholar]
- Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci U S A. 1992;89:9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- Moon HD. Coronary arteries in fetuses, infants, and juveniles. Circulation. 1957;16:263–267. doi: 10.1161/01.cir.16.2.263. [DOI] [PubMed] [Google Scholar]
- Männer J. The development of pericardial villi in the chick embryo. Anat Embryol (Berl) 1992;186:379–385. doi: 10.1007/BF00185988. [DOI] [PubMed] [Google Scholar]
- Männer J. Experimental study on the formation of the epicardium in chick embryos. Anat Embryol (Berl) 1993;187:281–289. doi: 10.1007/BF00195766. [DOI] [PubMed] [Google Scholar]
- Nahirney PC, Mikawa T, Fischman DA. Evidence for an extracellular matrix bridge guiding proepicardial cell migration to the myocardium of chick embryos. Dev Dyn. 2003;227:511–523. doi: 10.1002/dvdy.10335. [DOI] [PubMed] [Google Scholar]
- Navaratnam V. Development of the nerve supply to the human heart. Br Heart J. 1965;27:640–650. doi: 10.1136/hrt.27.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt T, Lemley A, Davis J, Yost MJ, Goodwin RL, Potts JD. Epicardial development in the rat: a new perspective. Microsc Microanal. 2006;12:390–398. doi: 10.1017/S1431927606060533. [DOI] [PubMed] [Google Scholar]
- Obrucník M, Malínský J, Lichnovský V. The early stages of differentiation of the vascular bed in the ventricular wall of the human embryonic heart as seen in the electron microscope. Folia Morphol (Praha) 1972;20:49–51. [PubMed] [Google Scholar]
- Oliver G, Pineau H. Horizons de Streeter et age embryonnaire. Bull Ass Anat. 1962;47:573–576. [Google Scholar]
- Orts-Llorca F, Puerta Fonolla J, Sobrado J. The formation, septation and fate of the truncus arteriosus in man. J Anat. 1982;134:41–56. [PMC free article] [PubMed] [Google Scholar]
- Partanen TA, Makinen T, Arola J, Suda T, Weich HA, Alitalo K. Endothelial growth factor receptors in human fetal heart. Circulation. 1999;100:583–586. doi: 10.1161/01.cir.100.6.583. [DOI] [PubMed] [Google Scholar]
- Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec. 2000;259:353–382. doi: 10.1002/1097-0185(20000801)259:4<353::AID-AR10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Pérez-Pomares JM, de la Pompa JL. Signaling during epicardium and coronary vessel development. Circ Res. 2011;109:1429–1442. doi: 10.1161/CIRCRESAHA.111.245589. [DOI] [PubMed] [Google Scholar]
- Ratajska A, Czarnowska E, Ciszek B. Embryonic development of the proepicardium and coronary vessels. Int J Dev Biol. 2008;52:229–236. doi: 10.1387/ijdb.072340ar. [DOI] [PubMed] [Google Scholar]
- Ratajska A, Fiejka E. Prenatal development of coronary arteries in the rat: morphologic patterns. Anat Embryol (Berl) 1999;200:533–540. doi: 10.1007/s004290050301. [DOI] [PubMed] [Google Scholar]
- Reiner L, Molnar J, Jimenez FA, Freudenthal RR. Interarterial coronary anastomoses in neonates. Arch Pathol. 1961;71:103–112. [PubMed] [Google Scholar]
- Rodgers LS, Lalani S, Runyan RB, Camenisch TD. Differential growth and multicellular villi direct proepicardial translocation to the developing mouse heart. Dev Dyn. 2008;237:145–152. doi: 10.1002/dvdy.21378. [DOI] [PubMed] [Google Scholar]
- Rychter Z, Jirásek JE, Rychterová V, Uher J. Vascularization of heart in human embryo: location and shape of non-vascularized part of cardiac wall. Folia Morphol (Praha) 1975;23:88–96. [PubMed] [Google Scholar]
- Rychter Z, Ostádal B. Mechanism of the development of coronary arteries in chick embryo. Folia Morphol (Praha) 1971;19:113–124. [PubMed] [Google Scholar]
- Saetrum Opgaard O, Gulbenkian S, Edvinsson L. Innervation and effects of vasoactive substances in the coronary circulation. Eur Heart J. 1997;18:1556–1568. doi: 10.1093/oxfordjournals.eurheartj.a015135. [DOI] [PubMed] [Google Scholar]
- Smith RB. Development of innervation of coronary arteries in human foetus up until 230 mm. stage (mid-term) Br Heart J. 1970;32:108–113. doi: 10.1136/hrt.32.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter GL. Contribution to Embryology. Washington DC: Carnegie Institute of Washington; 1942. Developmental horizons in human embryos. Description of age group XI, 13 to 20 somites and age group XII, 21 to 29 somites; pp. 211–245. Publication 541. [Google Scholar]
- Stretter GL. Developmental horizens in human embryos. Description of age groups XIX, XX, XXI, XXII and XXIII. In: Heuser CH, Corner GW, editors. Being the fifth issue of a survey of the Carnegie Collection. Washington, D.C: Carnegie Institution of Washington; 1951. pp. 165–196. [Google Scholar]
- Tian X, Hu T, He L, Zhang H, Huang X, Poelmann RE, Liu W, Yang Z, Yan Y, Pu WT, Zhou B. Peritruncal coronary endothelial cells contribute to proximal coronary artery stems and their aortic orifices in the mouse heart. PLoS One. 2013a;8:e80857. doi: 10.1371/journal.pone.0080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Hu T, Zhang H, He L, Huang X, Liu Q, Yu W, Yang Z, Zhang Z, Zhong TP, Yang X, Yan Y, Baldini A, Sun Y, Lu J, Schwartz RJ, Evans SM, Gittenberger-de Groot AC, Red-Horse K, Zhou B. Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell Res. 2013b;23:1075–1090. doi: 10.1038/cr.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomanek RJ. Coronary Vasculature: Development, Structure-Function and Adaptations. New York: Springer; 2013. [Google Scholar]
- Tomanek RJ, Christensen LP, Simons M, Murakami M, Zheng W, Schatteman GC. Embryonic coronary vasculogenesis and angiogenesis are regulated by interactions between multiple FGFs and VEGF and are influenced by mesenchymal stem cells. Dev Dyn. 2010;239:3182–3191. doi: 10.1002/dvdy.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomanek RJ, Haung L, Suvarna PR, O’Brien LC, Ratajska A, Sandra A. Coronary vascularization during development in the rat and its relationship to basic fibroblast growth factor. Cardiovasc Res. 1996;31(Spec No):E116–126. [PubMed] [Google Scholar]
- Tomanek RJ, Ishii Y, Holifield JS, Sjogren CL, Hansen HK, Mikawa T. VEGF family members regulate myocardial tubulogenesis and coronary artery formation in the embryo. Circ Res. 2006;98:947–953. doi: 10.1161/01.RES.0000216974.75994.da. [DOI] [PubMed] [Google Scholar]
- Van den Eijnde SM, Wenink AC, Vermeij-Keers C. Origin of subepicardial cells in rat embryos. Anat Rec. 1995;242:96–102. doi: 10.1002/ar.1092420113. [DOI] [PubMed] [Google Scholar]
- van der Putte SC. The development of the lymphatic system in man. Adv Anat Embryol Cell Biol. 1975;51:3–60. [PubMed] [Google Scholar]
- Velkey JM, Bernanke DH. Apoptosis during coronary artery orifice development in the chick embryo. Anat Rec. 2001;262:310–317. doi: 10.1002/1097-0185(20010301)262:3<310::AID-AR1040>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Virágh S, Challice CE. The origin of the epicardium and the embryonic myocardial circulation in the mouse. Anat Rec. 1981;201:157–168. doi: 10.1002/ar.1092010117. [DOI] [PubMed] [Google Scholar]
- Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, Hungerford JE, Little CD, Poelmann RE. The development of the coronary vessels and their differentiation into arteries and veins in the embryonic quail heart. Dev Dyn. 1997;208:338–348. doi: 10.1002/(SICI)1097-0177(199703)208:3<338::AID-AJA5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Kirby ML. Association of the cardiac neural crest with development of the coronary arteries in the chick embryo. Anat Rec. 1994;239:315–331. doi: 10.1002/ar.1092390310. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Willner W, Kirby ML. Origin of the proximal coronary artery stems and a review of ventricular vascularization in the chick embryo. Am J Anat. 1990;188:109–120. doi: 10.1002/aja.1001880202. [DOI] [PubMed] [Google Scholar]
- Wessels A, Anderson RH, Markwald RR, Webb S, Brown NA, Viragh S, Moorman AF, Lamers WH. Atrial development in the human heart: an immunohistochemical study with emphasis on the role of mesenchymal tissues. Anat Rec. 2000;259:288–300. doi: 10.1002/1097-0185(20000701)259:3<288::AID-AR60>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Wessels A, Markman MW, Vermeulen JL, Anderson RH, Moorman AF, Lamers WH. The development of the atrioventricular junction in the human heart. Circ Res. 1996;78:110–117. doi: 10.1161/01.res.78.1.110. [DOI] [PubMed] [Google Scholar]
- Wessels A, van den Hoff MJ, Adamo RF, Phelps AL, Lockhart MM, Sauls K, Briggs LE, Norris RA, van Wijk B, Perez-Pomares JM, Dettman RW, Burch JB. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev Biol. 2012;366:111–124. doi: 10.1016/j.ydbio.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilting J, Manner J. Development and patterning of the cardiac lymphatic network. In: GK, editor. The Cardiac Lymphatic System: An Overview. New York: Springer; 2013. pp. 17–31. [Google Scholar]
- Wu SP, Dong XR, Regan JN, Su C, Majesky MW. Tbx18 regulates development of the epicardium and coronary vessels. Dev Biol. 2013;383:307–320. doi: 10.1016/j.ydbio.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]