Abstract
Objective
To examine a model addressing the roles of rheumatoid arthritis (RA) disease burden, mood disturbance, and disability as determinants of impairments in role functioning.
Methods
In a cross-sectional design, 103 RA patients recruited from the community to participate in a clinical trial completed assessments of self-assessed disease burden (total joint pain and disease activity), mood disturbance (CES-D depressed mood, somatic symptoms, lack of positive affect, and interpersonal problems), disability (HAQ-DI gross and fine motor), and role functioning (SF-36 physical and social). Structural equation modeling (SEM) was used to examine direct and indirect mechanisms linking disease burden to role functioning.
Results
SEM results indicated that the model had excellent fit, S-Bχ2(30) = 38.59, p = .135, CFI = .977, SRMR = .062, RMSEA = .053. Mediational analyses demonstrated that while disease burden was associated with poor role functioning, its effects were jointly mediated by mood disturbance and disability. After the effects of mood disturbance and disability were taken into account, the effect of disease burden on role functioning was not significant.
Conclusion
The results indicate that mood disturbance and disability may serve as important pathways through which RA disease burden affects role functioning. Future longitudinal research is suggested to replicate these findings and further explore the mediational mechanisms examined in this study.
Rheumatoid arthritis (RA) can interfere significantly with the functional adaptation and emotional wellbeing of patients and their families. While adjustment to RA varies across patients (1,2), research has shown that RA can contribute to widespread disability and impairment in physical functioning for most patients over time (3). Moreover, high levels of disability may persist despite the use of biologic agents that have been successful in reducing inflammation and pain (4,5). Patients may not be able to be gainfully employed due to RA limitations, contributing to an enormous financial burden for patients, families, and society (6,7). Managing the disability and functional impairments due to RA can be enigmatic and confusing for patients and health professionals. It is a challenge for researchers and clinicians to understand the process of disablement that affects the lives of so many RA patients.
In addition to its adverse impact on physical functioning, RA has also been shown to affect patients' psychological wellbeing. As many as 30% to 40% of patients with RA may suffer from dysthymia, adjustment disorder, or major depressive disorder that may be at least partly attributable to the impact of RA (8). Moreover, the existence of depression may exacerbate other comorbid symptoms of RA, such as pain and fatigue (9-11) and contribute to greater disability (12) and poorer quality of life (13,14). Depression may also lead to greater medical utilization and higher health care costs (15). Importantly, biomedical interventions alone may not be sufficient to stop or prevent this downward spiral of functioning (16).
It is critical, therefore, to understand the factors that lead to disability and poor health functioning in RA. While RA pain and disease activity have been shown to contribute to psychological and physical impairments (17), it is unclear how the disease process leads to adverse health outcomes. A biopsychosocial perspective (18), integrating disease activity and psychosocial factors, has been promoted as a heuristic approach to examining individual differences in functional adaptations to RA. There is considerable evidence that psychosocial variables may contribute to functional impairments and deficits in quality of life, along with inflammatory mechanisms and heightened disease activity (19-22). Greater understanding of how such factors contribute to health outcomes could lead to the development of management approaches that may prevent functional declines and adverse psychosocial consequences. Thus, there is a need for research that illustrates the mechanisms through which RA disease activity exerts its effects. A theoretical approach addressing the nature and complexity of such mechanisms, therefore, is warranted.
In this study, we evaluated a comprehensive, integrated model that hypothesized that RA disease burden, assessed by total joint pain and self-reports of disease activity, would have both direct and indirect effects on role functioning. The model makes a distinction between disability, assessed by deficits in fine and gross motor activity, and role functioning, a broader construct assessed by behaviors performed in specific contexts (physical and social). The model analyzed direct and indirect mechanisms accounting for the relationship between disease burden and functional adaptation (see Figure 1). First, the model postulated that RA disease burden would lead to poorer role functioning directly. Secondly, we examined whether higher disease burden would lead to poorer role functioning indirectly, by contributing to higher depression and disability. The model also proposed that depression would affect role functioning indirectly through disability.
Figure 1.
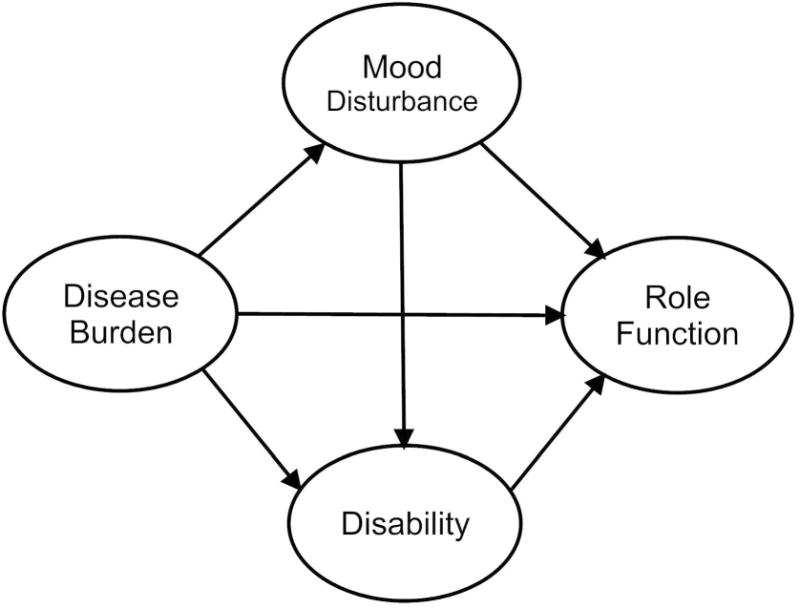
Hypothesized direct and indirect effects of disease burden, mood disturbance and disability on role functioning.
Patients and Methods
Participants and procedure
This study used cross-sectional baseline data from adults with RA from the greater Southern California area who participated in a clinical trial that compared behavioral treatments for RA. The enrollment period was from April 2004 to January 2008. Participants were recruited from clinics in the Divisions of Rheumatology at Cedars Sinai Medical Center (CSMC) and UCLA as well as from the community (via flyers and advertisements in local and regional newspapers) to reduce potential selection bias. After obtaining informed consent, the board-certified study rheumatologist (MW) from CSMC conducted a diagnostic evaluation of all potential participants to verify the RA diagnosis. Reports of medication use were also obtained, including analgesics/nonsteroidal anti-inflammatory drugs, biologic agents, disease-modifying anti-rheumatic drugs (DMARDs), and “other” medication (drugs for other medical conditions, including psychotropic agents). Eligible participants meet the following criteria: (1) 18 years of age or older, (2) fulfill American College of Rheumatology revised criteria for RA, (3) stable disease-modifying drug regimen for three months, (4) stable disease course for three months, (5) free of serious co-morbid medical conditions such as diabetes, renal failure, cancer, or fibromyalgia that would confound interpretations of health status, and (6) not pregnant.
At UCLA, eligible patients gave a second informed consent to participate in an evaluation of psychiatric status, physical functioning, and psychosocial adjustment. The Structured Clinical Interview for DSM Disorders (SCID) (23) was administered, and psychiatric diagnoses were made in a consensus meeting with the principal investigator (PN) and project psychiatrist (MI). Patients who had a serious psychiatric condition such as bipolar disorder or psychosis, or who were at risk for suicide, were ineligible to participate in the study. Research carried out in the current study was in compliance with the Helsinki Declaration and approved by the Institutional Review Boards of UCLA and CSMC.
Measures
The structural model tested in this study (Figure 1) was comprised of the constructs of disease burden, mood disturbance, disability, and role functioning. Multiple reliable and valid measures were used to serve as indicators in the model to increase the reliability of each construct.
The latent variable disease burden included two indicators representing joint pain/tenderness and RA disease activity as measured in The Rapid Assessment of Disease Activity in Rheumatology (RADAR) (24). For joint pain/tenderness, participants were asked to rate pain/tenderness in 10 joints on the right and left sides of the body. Items are rated on a 4-point Likert scale; the aggregate joint pain/tenderness score may range from 0 to 60, with higher scores indicating more severe joint pain/tenderness. For self-perceived RA disease activity, respondents rated “How active has your arthritis been over the past six months?” and “How active is your arthritis today?” on 10-point visual analogue scales, with higher scores indicating greater perceived disease activity. Scores on the two scales were totaled to create a single disease activity variable. Similarities in ratings on self- and clinician-administered RADAR questionnaires suggests it is not unduly affected by self-report bias and may be considered a valid proxy for physician assessments of disease activity and joint pain (25).
Mood disturbance was included as a latent variable with four indicators representing the dimensions of the Center for Epidemiological Studies Depression Scale (CES-D) (26): the 7-item depressed mood subscale, 7-item somatic symptoms subscale, 4-item lack of positive affect subscale, and 2-item interpersonal problems subscale (e.g., feeling disliked and that people are unfriendly). Items are rated on a 4-point Likert scale, and for each subscale a total score was computed, with high scores indicating the presence of more symptomatology. The CES-D has been effectively used to evaluate depression in patients with arthritis (16).
Disability refers to difficulties of an individual in performing tasks and actions and was assessed by the Stanford Health Assessment Questionnaire Disability Index (HAQ-DI) (27). The HAQ-DI reflects difficulties in daily living and contains questions about the ability of patients to perform 20 activities of daily living, classified into 8 categories. Two subscales were derived representing large limb gross movements (i.e., walking, arising, hygiene, and usual activity) and small limb fine movements (i.e., eating, reach, grip, and dressing/grooming) (28). Response options range from 0 (no disability) to 3 (completely disabled), and summary scores were computed based on the average of the category scores for large and small limb movement subscales, respectively. The HAQ-DI is the most widely used self-report measure evaluating disability in RA (27).
Role functioning reflects participation and involvement in life situations and was assessed by the Short Form Health Survey (SF-36) Role Limitations due to Physical Health (Role-Physical) and Social Functioning scales (29). The four-item SF-36 Role-Physical scale measures the impact of physical health on work or other daily activities. The SF-36 Social Functioning scale contains two items and addresses the extent to which health problems interfere with social activities. All SF-36 scales are recalibrated to a 0 to 100 scale with higher scores indicating higher levels of adaptive functioning (rescaled to 1–10 for analysis purposes). The SF-36 has been shown to be a psychometrically sound measure of patient wellbeing in RA with good scale-level reliability (30).
Statistical analyses
Structural equation modeling (SEM) was used to test the proposed model, and the analyses were conducted using EQS 6.1 (31). The associations between medication use (i.e., analgesics/nonsteroidal anti-inflammatory drugs, biologic agents, disease-modifying anti-rheumatic drugs, and other medications) and the model indicator variables were assessed to determine their potential impact on model findings. If statistically significant, the variance from covariates would have been partitioned from relevant indicators prior to analyses. Adequacy of model fit was assessed using multiple criteria: χ2 goodness-of-fit statistic, the Comparative Fit Index (CFI), the Standardized Root Mean Residual (SRMR), and the Root Mean Square Error of Approximation (RMSEA). A statistically nonsignificant χ2 (p > .05) is suggestive of a good match between the data and the hypothesized model. A CFI value greater than .95 is considered evidence of a good fitting model (32). For SRMR and RMSEA, a joint criteria of a SRMR less than .09 and a RMSEA less than .06 is considered optimal to minimize the rates of Type I and Type II error (33). Model modifications were performed based on results from the Wald test and Lagrange multiplier test, along with theoretical considerations.
Mediation analyses examined the extent to which mood disturbance and disability mediated the effect of disease burden on role functioning. First, the preconditions for mediation were assessed to confirm that disease burden was significantly related to role functioning and the mediators (i.e., mood disturbance or disability) (34). Then, a single mediator model was assessed, specifying a direct relationship between disease burden and role functioning and an indirect (mediating) effect through either mood disturbance or disability, thereby testing the mediating effects of mood disturbance and disability separately. Next, a double mediator model was tested in which both mood disturbance and disability were mediators of the relationship between disease burden and role functioning; this examined conditional effects of each mediator after controlling for the effects of the other variable.
Statistical significance of the indirect effect, reflective of a significant decrease in the direct influence of disease burden on role functioning, was taken as evidence of mediation (34). The significance of indirect effect estimates was calculated by EQS, based on the Sobel method (35). Full mediation was indicated if the indirect effect (i.e., disease burden → mood disturbance/disability → role functioning), but not the direct effect (i.e., disease burden → role functioning), was significant; partial mediation was established if both the indirect effect and the direct effect of disease burden on role functioning were significant (34).
Assessment of common method variance
As all study data are self-reported and collected during the same time period, potential common method variance was assessed using Harman's single-factor test and confirmatory factor analysis (CFA) (36,37). Specifically, all 10 model indicator variables were entered into an exploratory factor analysis (EFA), using unrotated principal components analysis and principal component analysis with varimax rotation (36). If a substantial amount of common method variance is present, a single factor will account for the majority of the covariance among the variables. In the confirmatory factor-analytic approach to Harman's single-factor test, all 10 indicator variables were loaded on one latent factor to examine the fit of the CFA model (37). If common method variance is largely responsible for the relationship among the variables, the one-factor CFA model should fit the data well.
Regarding post hoc examination of common method variance, the EFA showed that none of the factors explained the majority of the variance. The first (largest) factor accounted for 43.74% of the variance in the unrotated solution and 32.77% of the variance in the solution after varimax rotation, below the recommended 50% threshold (37). Moreover, the CFA showed that the single latent factor model did not fit the data well, S-Bχ2(35) = 170.46, p < .001, CFI = .638, SRMR = .146, RMSEA = .195. Thus, it seems that common method bias is not a serious problem in the present study.
Results
Sample characteristics
A total of 103 patients were included in the study. The sample size exceeds the minimum of 100 recommended for testing mediation models in SEM (38). The sample consisted of 85 females and 18 males, with an average age of 56.34 years and illness duration of 12.29 years. Participants came from a range of ethnicities (see Table 1). Caucasians were the most prevalent group, but patients from African-American, Hispanic, and Asian ethnicities were also represented. The sample can be characterized as middle to upper middle class, possessing almost 16 years of education on average, and a mean annual income of greater than $50,000.
Table 1. Demographic characteristics of sample (N = 103).
| Mean ± SD or N (%) | Range | |
|---|---|---|
| Age in years | 56.34 ± 12.30 | 22–79 |
| Education in years | 15.99 ± 2.41 | 12–21 |
| Annual income ($) | 50,809 ± 18,911 | 17,644–141,527 |
| Female | 85 (82.52) | |
| Race/Ethnicity | ||
| White | 62 (60.19) | |
| Hispanic | 15 (14.56) | |
| Black | 13 (12.62) | |
| Asian/Pacific Islander | 7 (8.60) | |
| Other race/ethnicity | 6 (5.83) | |
| Marital status | ||
| Never married | 13 (12.62) | |
| Divorced | 29 (28.16) | |
| Widowed | 10 (9.71) | |
| Married | 51 (49.51) | |
| Years since RA diagnosis | 12.29 ± 11.45 | 1–53 |
Table 2 shows the descriptive statistics and intercorrelations among the variables of interest. Preliminary evaluation of the relationships among the latent constructs indicated moderate to strong associations between disease burden and role functioning (r = -.68, p < .001), and between disease burden and the two posited mediators (for mood disturbance: r = .38, p < .001; for disability: r = .73, p < .001), confirming that the preconditions for mediation were present.
Table 2. Intercorrelations, means, and standard deviations for study variables (N = 103).
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Joint pain/tenderness | -- | |||||||||
| 2. | RA disease activity | .631*** | -- | ||||||||
| 3. | Depressed mood | .247* | .266** | -- | |||||||
| 4. | Somatic symptoms | .237* | .254** | .684*** | -- | ||||||
| 5. | Lack of positive affect | .207* | .222* | .598*** | .572*** | -- | |||||
| 6. | Interpersonal problems | .194* | .208* | .559*** | .535*** | .468*** | -- | ||||
| 7. | Gross motor disability | .510*** | .548*** | .215* | .206* | .180 | .168 | -- | |||
| 8. | Fine motor disability | .419*** | .450*** | .176 | .169 | .148 | .138 | .686*** | -- | ||
| 9. | Physical-role function | -.438*** | -.470*** | -.398*** | -.382*** | -.333*** | -.312** | -.524*** | -.431*** | -- | |
| 10. | Social functioning | -.387*** | -.416*** | -.352*** | -.337*** | -.295** | -.276** | -.464*** | -.381*** | .585*** | -- |
|
| |||||||||||
| M | 11.22 | 6.17 | 1.71 | 3.92 | 1.88 | 0.27 | 3.06 | 3.68 | 5.49 | 7.90 | |
|
| |||||||||||
| SD | 9.30 | 4.77 | 2.41 | 3.56 | 2.49 | 0.48 | 2.59 | 2.57 | 4.14 | 1.97 | |
p < .05;
p < .01;
p < .001.
SEM results
Preliminary data screening revealed a violation of multivariate normality. Therefore, the ML robust test statistics, which correct for non-normal data, are reported. In the assessment of covariates, none of the associations between medication use and the indicators variables were found to be statistically significant. The hypothesized model provided a good fit of the data, S-Bχ2(29) = 38.08, p = .121, CFI = .976, SRMR = .063, RMSEA = .055. However, the Wald test indicated that the impact of deleting the path from mood disturbance to disability on the χ2 of the model would be minimal. As such, in an effort to attain parsimony and based on theoretical plausibility, this path was removed. The fit of this revised model was also good, S-Bχ2(30) = 38.59, p = .135, CFI = .977, SRMR = .062, RMSEA = .053. As shown in Figure 2, disease burden contributed to mood disturbance and higher levels of disability, whereas mood disturbance and disability related to lower levels of role functioning. Overall, the specified predictors explained 15% of the variance in mood disturbance, 53% of the variance in disability, and 69% of the variance in role functioning.
Figure 2.
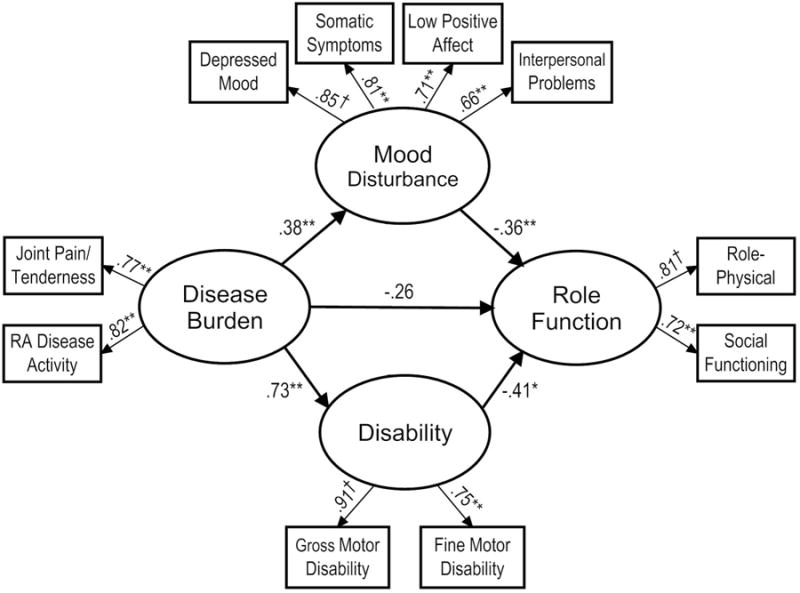
Final model with estimated path coefficients and factor loadings.
Note. †pathway fixed to 1.0.
*p < .01; **p < .001.
Single mediator models
In the single mediator models, a direct relationship was specified between disease burden and role functioning, and an indirect (mediating) effect through either mood disturbance or disability. The two single mediator models fit the data well [S-Bχ2(17) = 21.26, p = .215, CFI = .982, SRMR = .055, RMSEA = .050 for mood disturbance; S-Bχ2(6) = 3.36, p = .762, CFI = 1.00, SRMR = .018, RMSEA < .001 for disability]. For both models, disease burden was predictive of the mediator (mood disturbance: β = .40, p < .001; disability: β = .74, p < .001) which, in turn, were each predictive of role functioning (mood disturbance: β = -.38, p = .003; disability: β = -.35, p = .030). Stated otherwise, mood and disability each separately mediated the relationship between the predictor and outcome, as indicated by a significant indirect path between disease burden and role functioning in the mood disturbance model (βindirect = -.15, p = .048) as well as the disability model (βindirect = -.26, p = .043). These findings, in combination with the attenuated but still significant direct effect of disease burden in each model (mood disturbance: β = -.55, p < .001; disability: β = -.42, p = .007), suggest that the association between disease burden and role functioning was partially mediated by mood disturbance and disability, respectively (also see Table 3).
Table 3.
Direct, indirect and total effects from structural equation models.
| Direct | Indirect | Total | |
|---|---|---|---|
| Single mediator model (Mood disturbance) | |||
| Disease burden → Mood disturbance | .40*** | ― | .40*** |
| Mood disturbance → Role function | -.38** | ― | -.38** |
| Disease burden → Role function | -.55*** | -.15* | -.70*** |
| Single mediator model (Disability) | |||
| Disease burden → Disability | .74*** | ― | .74*** |
| Disability → Role function | -.35* | ― | -.35* |
| Disease burden → Role function | -.42** | -.26* | -.68*** |
| Multiple mediator model (Mood disturbance & Disability) | |||
| Disease burden → Mood disturbance | .38*** | ― | .38*** |
| Disease burden → Disability | .73*** | ― | .73*** |
| Mood disturbance → Role function | -.36*** | ― | -.36*** |
| Disability → Role function | -.41** | ― | -.41** |
| Disease burden → Role function | -.26 | -.44** | -.70*** |
p < .05;
p < .01;
p < .001.
Multiple mediator model
Results for the multiple mediator model indicated that the collective indirect effect of disease burden on role functioning via mood disturbance and disability was significant (βindirect = -.44, p = .002). Moreover, the initially significant direct path from disease burden to role functioning (β = -.68, p < .001) was no longer significant in the full model (β = -.26, p = .111; see Table 3 and Figure 2). As such, mediation was formally tested in accordance with a method recommended for use with SEM (39). Specifically, the fit of the full model was compared with the fit of a model that constrained the path (i.e., the path was set to 0) between disease burden and role functioning. If there is a mediational effect, the inclusion of the disease burden to role functioning path should not improve model fit. Because the S-Bχ2 statistic using maximum likelihood robust estimation is not appropriate for comparing models, a log likelihood-based difference test incorporating scaling correction factor was used. When compared with the combined effects model, the constrained model did not show worse fit [ΔS-Bχ2(1) = 2.50, p = .114], indicating that inclusion of the disease burden–role functioning path did not significantly affect model fit and that mood disturbance and disability jointly fully mediated the association between disease burden and role functioning.
Discussion
Our main objective in this research was to shed light on the factors associated with role functioning in RA, a chronic illness associated with multiple psychological and physical co-morbidities (40,41). Using SEM, we examined a model in which disease burden would contribute to poor role functioning directly, and indirectly, though mood disturbance and disability. The model had excellent fit, with the predictors explaining 69% of the variance in role functioning. The results showed that disease burden was associated with greater mood disturbance and disability which, in turn, contributed to poorer role functioning. Single mediation models found that both mood disturbance and disability partly mediated the relationship between disease burden and role functioning, with disease burden also showing a direct effect. However, when mood disturbance and disability were examined jointly as mediators, the relationship between disease burden and role functioning was no longer significant.
The results indicate that the adjustment to RA is individualized in nature and may not be adequately predicted by RA disease burden alone (12,16). Multiple psychological, social, and biomedical factors affect health outcomes in RA. Previous research has shown that psychological variables predict role functioning in RA independently of pain and disease activity (42,43). The findings of this study showed that while disease burden possessed a significant zero-order correlation with role functioning, this effect was jointly mediated by mood disturbance and disability. The modeling approach that was adopted helped to illustrate this pattern, demonstrating the value of an integrated, theoretical framework that enables examination of potential underlying mechanisms, in addition to direct effects.
This research has shown that mood disturbance and disability may serve as important pathways through which disease burden contributes to poor health functioning. It is understandable that when patients are limited in their fine and gross motor skills, their role functioning is likely to be impaired. Moreover, depression has been shown to contribute to greater pain, functional impairment, inflammation, and health care use in patients with arthritis (44,45). Depression can also lead to disengagement, causing limitations in social and physical activities. When RA disease activity is high, depression is more likely to occur, which may further contribute to this downward spiral of functioning. Confirmatory longitudinal research is suggested to further explore the proposed model and replicate study findings.
While the findings have illustrated the value of a systemic theoretical framework for analyzing role functioning in RA, some limitations should be noted. First, the cross-sectional nature of the study precludes inferences regarding causality and the directionality of relationships among variables. Although cross-sectional data have been used to examine mediational relationships (34), alternate causal relations are possible. Diminished role functioning, for example, may lead to exacerbations in depression and disability that, in turn, may increase disease activity. Longitudinal studies are necessary to clarify the direction of associations and mediational mechanisms that we examined. A second limitation is the reliance on self-report measures, raising the possibility that shared method variance may have contributed to the magnitude of the relationships observed among model constructs. Although post hoc statistical tests provided evidence that common method bias was not a major source of the variation underlying the observed effects, these results do not preclude the possibility of common method effect. As such, future studies might consider obtaining measures of the examined variables from additional sources (e.g., behavioral data and observer ratings).
Finally, since the study included only patients who volunteered to be enrolled in a larger clinical trial and who were concurrently being treated by a rheumatologist, the data may not be generalizable to those not receiving care for their RA. Larger, epidemiological studies would add clarity regarding the robustness of these results. Nevertheless, the excellent fit of the model argues against the spurious nature of the findings. Future longitudinal research examining the mechanisms that contribute to deficits in role functioning in RA is needed to corroborate these findings and suggest new avenues for clinical management.
Significance and Innovations.
This study examined an integrated biomedical and psychosocial model of disease burden, mood disturbance and disability as determinants of role functioning in patients with rheumatoid arthritis (RA).
Results suggest the effect of RA disease burden on role functioning is not simply a direct linear relationship and that mood disturbance and disability may each play important mediational roles.
Biomedical interventions alone may be insufficient to address functional impairments in RA patients who experience mood disturbance and/or are confronted with significant disability.
The findings support the value of an integrated biopsychosocial model for understanding determinants of role functioning in RA, and suggest that interventions that target mood disturbance and/or disability may facilitate functional recovery in cases where reduction in RA disease activity is, or is not, possible.
Acknowledgments
The authors wish to acknowledge Mara Custodio, B.A., Kate Jackson, B.A., and Sarosh J. Motivala, Ph.D., who contributed to the diagnostic evaluation and assessment of participants in the project, and reviewers for helpful comments on the manuscript.
Supported by AR R01-049840 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institute of Health to Dr. Nicassio. Also supported in part by HL R0-079955, R01-AG034588, R01-AG026364, R01-CA119159, R01 HL095799, P30-AG028748, UL RR 033176 to Dr. Irwin, and the Cousins Center for Psychoneuroimmunology.
References
- 1.Kojima M, Kojima T, Ishiguro N, Oguchi T, Oba M, Tsuchiya H, et al. Psychosocial factors, disease status, and quality of life in patients with rheumatoid arthritis. J Psychosom Res. 2009;67:425–31. doi: 10.1016/j.jpsychores.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Escalante A, Del Rincon I. The disablement process in rheumatoid arthritis. Arthritis Rheum. 2002;47:333–42. doi: 10.1002/art.10418. [DOI] [PubMed] [Google Scholar]
- 3.Verbrugge LM, Juarez L. Profile of arthritis disability: II. Arthritis Rheum. 2006;55:102–13. doi: 10.1002/art.21694. [DOI] [PubMed] [Google Scholar]
- 4.Lovinger SP. Use of biologics for rheumatoid arthritis tempered by concerns over safety, cost. JAMA. 2003;289:3229–30. doi: 10.1001/jama.289.24.3229. [DOI] [PubMed] [Google Scholar]
- 5.DeWitt EM, Lin L, Glick HA, Anstrom KJ, Schulman KA, Reed SD. Pattern and predictors of the initiation of biologic agents for the treatment of rheumatoid arthritis in the United States: an analysis using a large observational data bank. Clin Ther. 2009;31:1871–80. doi: 10.1016/j.clinthera.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics. 2004;22:1–12. doi: 10.2165/00019053-200422001-00002. [DOI] [PubMed] [Google Scholar]
- 7.Yelin E, Murphy L, Cisternas MG, Foreman AJ, Pasta DJ, Helmick CG. Medical care expenditures and earnings losses among persons with arthritis and other rheumatic conditions in 2003, and comparisons with 1997. Arthritis Rheum. 2007;56:1397–407. doi: 10.1002/art.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Covic T, Tyson G, Spencer D, Howe G. Depression in rheumatoid arthritis patients: demographic, clinical, and psychological predictors. J Psychosom Res. 2006;60:469–76. doi: 10.1016/j.jpsychores.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 9.van Hoogmoed D, Fransen J, Bleijenberg G, van Riel P. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology (Oxford) 2010;49:1294–302. doi: 10.1093/rheumatology/keq043. [DOI] [PubMed] [Google Scholar]
- 10.Treharne GJ, Lyons AC, Hale ED, Goodchild CE, Booth DA, Kitas GD. Predictors of fatigue over 1 year among people with rheumatoid arthritis. Psychol Health Med. 2008;13:494–504. doi: 10.1080/13548500701796931. [DOI] [PubMed] [Google Scholar]
- 11.Waltz M, Kriegel W, van't Pad Bosch P. The social environment and health in rheumatoid arthritis: marital quality predicts individual variability in pain severity. Arthritis Care Res. 1998;11:356–74. doi: 10.1002/art.1790110507. [DOI] [PubMed] [Google Scholar]
- 12.Rupp I, Boshuizen HC, Dinant HJ, Jacobi CE, van den Bos GA. Disability and health-related quality of life among patients with rheumatoid arthritis: association with radiographic joint damage, disease activity, pain, and depressive symptoms. Scand J Rheumatol. 2006;35:175–81. doi: 10.1080/03009740500343260. [DOI] [PubMed] [Google Scholar]
- 13.Whalley D, McKenna SP, de Jong Z, van der Heijde D. Quality of life in rheumatoid arthritis. Br J Rheumatol. 1997;36:884–8. doi: 10.1093/rheumatology/36.8.884. [DOI] [PubMed] [Google Scholar]
- 14.Pollard L, Choy EH, Scott DL. The consequences of rheumatoid arthritis: quality of life measures in the individual patient. Clin Exp Rheumatol. 2005;23:S43–52. [PubMed] [Google Scholar]
- 15.Dickens C, Creed F. The burden of depression in patients with rheumatoid arthritis. Rheumatology (Oxford) 2001;40:1327–30. doi: 10.1093/rheumatology/40.12.1327. [DOI] [PubMed] [Google Scholar]
- 16.Nicassio PM, Kay MA, Custodio MK, Irwin MR, Olmstead R, Weisman MH. An evaluation of a biopsychosocial framework for health-related quality of life and disability in rheumatoid arthritis. J Psychosom Res. 2011;71:79–85. doi: 10.1016/j.jpsychores.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakobsson U, Hallberg IR. Pain and quality of life among older people with rheumatoid arthritis and/or osteoarthritis: a literature review. J Clin Nurs. 2002;11:430–43. doi: 10.1046/j.1365-2702.2002.00624.x. [DOI] [PubMed] [Google Scholar]
- 18.Engel GL. The clinical application of the biopsychosocial model. Am J Psychiatry. 1980;137:535–44. doi: 10.1176/ajp.137.5.535. [DOI] [PubMed] [Google Scholar]
- 19.Bowling A, Browne PD. Social networks, health, and emotional well-being among the oldest old in London. J Gerontol. 1991;46:S20–32. doi: 10.1093/geronj/46.1.s20. [DOI] [PubMed] [Google Scholar]
- 20.Kendig H, Browning CJ, Young AE. Impacts of illness and disability on the well-being of older people. Disabil Rehabil. 2000;22:15–22. doi: 10.1080/096382800297088. [DOI] [PubMed] [Google Scholar]
- 21.Lambert VA. Study of factors associated with psychological well-being in rheumatoid arthritic women. Image J Nurs Sch. 1985;17:50–3. doi: 10.1111/j.1547-5069.1985.tb01417.x. [DOI] [PubMed] [Google Scholar]
- 22.van Lankveld W, Naring G, van der Staak C, van't Pad Bosch P, van de Putte L. Stress caused by rheumatoid arthritis: relation among subjective stressors of the disease, disease status, and well-being. J Behav Med. 1993;16:309–21. doi: 10.1007/BF00844762. [DOI] [PubMed] [Google Scholar]
- 23.Spitzer R, Williams JB, Gibbon M. Instruction manual for the Structured Clinical Interview for DSM-III-R (SCID) New York: Biometrics Research Department, New York State Psychiatric Institute; 1987. [Google Scholar]
- 24.Wong AL, Wong WK, Harker J, Sterz M, Bulpitt K, Park G, et al. Patient self-report tender and swollen joint counts in early rheumatoid arthritis. Western Consortium of Practicing Rheumatologists. J Rheumatol. 1999;26:2551–61. [PubMed] [Google Scholar]
- 25.Calvo FA, Calvo A, Berrocal A, Pevez C, Romero F, Vega E, et al. Self-administered joint counts in rheumatoid arthritis: comparison with standard joint counts. J Rheumatol. 1999;26:536–9. [PubMed] [Google Scholar]
- 26.Radloff LS. The CES-D Scale. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 27.Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: a review of its history, issues, progress, and documentation. J Rheumatol. 2003;30:167–78. [PubMed] [Google Scholar]
- 28.Milligan SE, Hom DL, Ballou SP, Persse LJ, Svilar GM, Coulton CJ. An assessment of the Health Assessment Questionnaire functional ability index among women with systemic lupus erythematosus. J Rheumatol. 1993;20:972–6. [PubMed] [Google Scholar]
- 29.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 30.Kanecki K, Tyszko P, Wisłowska M, Łyczkowska-Piotrowska J. Preliminary report on a study of health-related quality of life in patients with rheumatoid arthritis. Rheumatol Int. 2013;33:429–34. doi: 10.1007/s00296-012-2421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bentler PM. EQS 6 structural equations program. Encino, CA: Multivariate Software; 2005. [Google Scholar]
- 32.Kaplan D. Structural equation modeling: foundations and extensions. Los Angeles: Sage; 2009. [Google Scholar]
- 33.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 34.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 35.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Podsakoff PM, Organ DW. Self-report in organizational research: problems and prospects. J Manage. 1986;12:531–44. [Google Scholar]
- 37.Podsakoff PM, MacKenzie SB, Lee JY, Podsakoff NP. Common method biases in behavioral research: a critical review of the literature and recommended remedies. J Appl Psychol. 2003;88:879–903. doi: 10.1037/0021-9010.88.5.879. [DOI] [PubMed] [Google Scholar]
- 38.Kline RB. Principles and practice of structural equation modeling. New York: Guilford Publications; 2005. [Google Scholar]
- 39.Holmbeck GN. Toward terminological, conceptual, and statistical clarity in the study of mediators and moderators: examples from the child-clinical and pediatric psychology literatures. J Consult Clin Psychol. 1997;65:599–610. doi: 10.1037//0022-006x.65.4.599. [DOI] [PubMed] [Google Scholar]
- 40.Gettings L. Psychological well-being in rheumatoid arthritis: a review of the literature. Musculoskeletal Care. 2010;8:99–106. doi: 10.1002/msc.171. [DOI] [PubMed] [Google Scholar]
- 41.Michaud K, Wolfe F. Comorbidities in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:885–906. doi: 10.1016/j.berh.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Escalante A, Del Rincon I. How much disability in rheumatoid arthritis is explained by rheumatoid arthritis? Arthritis Rheum. 1999;42:1712–21. doi: 10.1002/1529-0131(199908)42:8<1712::AID-ANR21>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 43.Scharloo M, Kaptein AA, Weinman JA, Hazes JM, Breedveld FC, Rooijmans HG. Predicting functional status in patients with rheumatoid arthritis. J Rheumatol. 1999;26:1686–93. [PubMed] [Google Scholar]
- 44.Holzberg AD, Robinson ME, Geisser ME, Gremillion HA. The effects of depression and chronic pain on psychosocial and physical functioning. Clin J Pain. 1996;12:118–25. doi: 10.1097/00002508-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]


