Abstract
Introduction
Although formal spirometry is the gold standard for monitoring respiratory function in patients with myasthenia gravis (MG), such testing is often delayed or unavailable. There is a need for a simple bedside test that can accurately measure respiratory function.
Method
We conducted a prospective, cross-sectional, single-blind study in adults with acetylcholine receptor antibody positive MG. Participants performed the single breath count test (SBCT) and underwent manual muscle strength testing, while a respiratory therapist performed spirometry blinded to SBCT and strength results.
Results
Thirty-one patients, aged 57 ±19 years participated. SBCT showed significant correlations with forced vital capacity (FVC), negative inspiratory force (NIF), and neck flexor strength (P<0.01). FVC showed significant correlation with neck flexor strength (P=0.02) but no correlation with shoulder abductor strength.
Discussion
These data suggest that the SBCT and neck flexor strength testing are valuable tools for bedside assessment of respiratory function in MG patients.
Keywords: Single breath count, myasthenia gravis, spirometry, forced vital capacity, negative inspiratory force, neck flexor strength
Introduction
Myasthenia gravis (MG) is an autoimmune disease characterized by fatigable weakness of skeletal muscles.1, 2 Respiratory muscle weakness leading to hypoventilation and respiratory failure are serious complications that are seen in 20% of patients with generalized MG.3 Respiratory dysfunction may be caused by weakness of bulbar and upper airway muscles as well as diaphragm, intercostal, and accessory breathing muscles.4 Forced vital capacity (FVC) and negative inspiratory force (NIF) are the gold standards for monitoring respiratory muscle strength. 4, 5 Despite their accepted role in identifying respiratory compromise, such testing is often delayed or unavailable. A simple bedside test that accurately measures respiratory function is therefore highly desirable. Anecdotally, surrogate measures such as single breath count test (SBCT) and neck flexor muscle strength (NF) correlate with spirometry and respiratory muscle strength.6 Our objective was to determine the correlation between spirometry and both SBCT and NF.
Methods
This was a prospective, cross-sectional, single blind study performed in subjects ≥ age18 years with acetylcholine receptor antibody positive MG. All participants signed informed consent. The SBCT was performed by asking subjects to take a deep breath and count as far as possible in their normal voice at an approximate rate of 2 counts per second. The better of 2 attempts was recorded. A trained respiratory therapist blinded to SBCT and strength testing then performed spirometry in the seated position. The spirometer was calibrated to 3 liters ± 3%. A nose clip was placed. Subjects were observed while taking several tidal breaths. At the end of expiration the subjects were asked to inhale as deeply as possible through a mouthpiece. At the end of inspiration subjects were asked to exhale as forcefully and as long as possible. The best of 3 attempts was recorded. For NIF measurement, subjects inhaled as forcefully as possible. The best of 3 attempts was recorded. NF and shoulder abductor strength (SA) were tested in the supine and seated positions, respectively, and were graded using the modified medical research council (MRC) grading on a 0-10 point MMT scale.7 Subjects with a history of cervical fusion were excluded. Pearson coefficients were used to determine the association between measures, and P< 0.05 was considered significant.
Results
We enrolled 16 men and 15 women ages 20-85 year (57 ± 19 years, mean ± SD). All patients were able to follow instructions for assessment. Myasthenia Gravis Foundation of America (MGFA) classification at the time of evaluation showed 8 patients were grade 1 (ocular), 20 were grade 2 (mild weakness other than ocular), and 3 were grade 3 (moderate weakness other than ocular). Mean disease duration was 6.3 years, and 90% of patients were on immunosuppressants.
The SBCT (range 16-40; mean 28 ± 7) was lower in patients with more severe disease by MGFA [Pearson coefficient (r): -0.480, P <0.01] (figure 1A). Almost all patients with MGFA-1 counted >30, and none <25. Eleven patients failed to count to 25, including all patients MGFA-3 and 8 of the 20 with MGFA-2. Body mass index and age were similar in MGFA-2 patients counting <25 (33.4±6.3 kg/m2; 56.1±20.3 years; n=8) versus those counting ≥25 (33.8±9.0 kg/m2; 62.4±18.9; n=12) (P =0.91, P= 0.26), respectively. Mean FVC was 3149 ± 880 mL (84% predicted) (median: 3240 mL, 89% predicted; range: 1470 mL, 45% predicted-4470 mL, 119% predicted; n=29). Mean NIF was 56 ±9 cm H2O(median: 60; range: 20-60 cm H2O; n=23). Mean NF was 4.4 ± 0.5(median: 4.3; range: 3.3-5). Mean SA was 4.8 ± 0.5(median: 5; range: 2.7-5).
Figure 1.
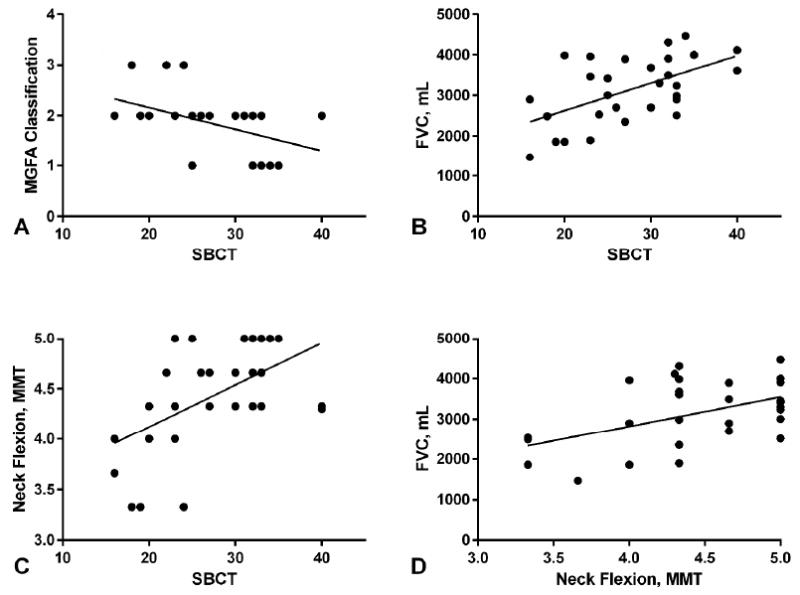
Correlation of single breath count test (SBCT) and neck flexion manual muscle testing. A. SBCT versus Myasthenia Gravis Foundation of America (MGFA) classification (Pearson coefficient -0.480, P<0.01), B. SBCT versus forced vital capacity (FVC) in milliliters (mL) (Pearson coefficient 0.554, P< 0.01), C. SBCT versus neck flexion (Pearson coefficient 0.519, P <0.01), D. Neck flexion versus forced vital capacity (FVC) in milliliters (mL) (Pearson coefficient 0.505, P =0.02)
There was good correlation between SBCT and FVC(r: 0.554, P<0.01) (figure 1B) and NIF(r: 0.530, P<0.01, n=23). Mean FVC for each number counted during the SBCT was 116 ±24 ml (range 76-199). Good correlation was seen between SBCT and NF (r: 0.519, P<0.01) (figure 1C). NF had good correlation with FVC(r: 0.505, P= 0.02) (figure 1D). Two subjects reported mild neck pain that did not limit testing. SBCT did not correlate with SA(r: 0.438, P =0.052).
Discussion
The simplicity of the SBCT and NF strength testing make these tests appealing for rapid assessment of respiratory status. These tests may provide a way to assess patients with significant facial weakness that might otherwise limit the ability to obtain reliable spirometry results.
There is evidence of a role for SBCT in the emergency room setting and in the pediatric pulmonary clinic, and anecdotal evidence suggests SBCT can roughly estimate the FVC (i.e. SBCT# X 100=estimated FVC in milliliters).8,9 Our study showed a mean value remarkably similar to this at SBCT# X 116=actual FVC. A SBCT of 20 has been proposed as an indication of respiratory compromise in MG.10 This study was not designed to define the lower limit of SBCT, but we propose that ≥25 suggests normal respiratory muscle function. Consistent with this, no MGFA-1 patients in this study had SBCT <25. Some individuals were found with low SBCT (<20) but relatively normal FVC. Possible explanations for this finding may include variability in performance of the various assessments, or this could be a direct result of MG such as vocal cord weakness. Additional investigation of severely affected patients is needed to determine a value that indicates impending respiratory failure.
Good correlation was found between SBCT and FVC, which is not unexpected, as FVC measures the maximal volume that can be forcefully expired after full inhalation. Similarly, SBCT measures the maximum number counted after full inhalation. In this study, SBCT and spirometry were performed in the seated position. It is possible that testing in the supine position could reveal additional ventilation deficits. NF strength was more significantly associated with respiratory function compared with shoulder abduction. This likely reflects the role of the neck flexors as accessory muscles of respiration.
Most patients in our cohort had mild disease, which likely resulted from soliciting mostly stable patients in the outpatient setting. Further evaluation of intra- and inter-rater reliability in additional patients with more severe disease is warranted, but this study suggests that SBCT and neck flexion testing are helpful tools for bedside assessment of respiratory function in patients with MG.
Acknowledgments
W.D.A receives funding from a career development award through NIH-NICHD (5K12HD001097-17).
Abbreviations
- MG
Myasthenia gravis
- FVC
Forced vital capacity
- NIF
Negative inspiratory force
- SBCT
single breath count test
- AChR
acetylcholine receptor antibody positive
- MRC
modified medical research council
- MGFA
Myasthenia Gravis Foundation of America
Footnotes
The authors have no relevant financial or conflict of interest disclosures.
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record.
References
- 1.Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8(5):475–902. doi: 10.1016/S1474-4422(09)70063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips LH. The epidemiology of myasthenia gravis. Semin Neurol. 2004;24(1):17–20. doi: 10.1055/s-2004-829593. [DOI] [PubMed] [Google Scholar]
- 3.Thomas CE, Mayer SA, Gungor Y, Swarup R, Webster EA, Chang I, et al. Myasthenic crisis: clinical features, mortality, complications, and risk factors for prolonged intubation. Neurology. 1997;48(5):1253–60. doi: 10.1212/wnl.48.5.1253. [DOI] [PubMed] [Google Scholar]
- 4.Thieben MJ, Blacker DJ, Liu PY, Harper CM, Jr, Wijdicks EF. Pulmonary function tests and blood gases in worsening myasthenia gravis. Muscle Nerve. 2005;32(5):664–7. doi: 10.1002/mus.20403. [DOI] [PubMed] [Google Scholar]
- 5.Rabinstein AA, Wijdicks EF. Warning signs of imminent respiratory failure in neurological patients. Semin Neurol. 2003;23(1):97–104. doi: 10.1055/s-2003-40757. [DOI] [PubMed] [Google Scholar]
- 6.Bartfield JM, Ushkow BS, Rosen JM, Dylon K. Single breath counting in the assessment pulmonary function. Ann Emerg Med. 1994;24(2):256–9. doi: 10.1016/s0196-0644(94)70138-5. [DOI] [PubMed] [Google Scholar]
- 7.Kendall FP, McCreary EK, Provance PG. Muscles: Testing and function. 4. Baltimore: Williams and Wilkins; 1993. [Google Scholar]
- 8.Ali SS, O’Connell C, Kass L, Graff G. Single-breath counting: a pilot study of a novel technique for measuring pulmonary function in children. Am J Emerg Med. 2011;29(1):33–6. doi: 10.1016/j.ajem.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Amato AA, Russel JA. Disorders of neuromuscular transmission in Neuromuscular Disorders. New York: McGraw Hill; 2008. [Google Scholar]
- 10.Juel VC. Myasthenia gravis: management of myasthenic crisis and perioperative care. Seminars in Neurology. 2004;24(1):75–81. doi: 10.1055/s-2004-829595. [DOI] [PubMed] [Google Scholar]


