Abstract
Background
Erwinia asparaginase is antigenically distinct from E.coli‐derived asparaginase and may be used after E.coli‐derived asparaginase hypersensitivity. In a single‐arm, multicenter study, we evaluated nadir serum asparaginase activity (NSAA) and toxicity with intravenously administered asparaginase Erwinia chrysanthemi (IV‐Erwinia) in children and adolescents with acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma with hypersensitivity to E.coli‐derived asparaginase.
Patients and Methods
Between 2012 and 2013, 30 patients (age 1–17 years) enrolled from 10 centers. Patients received IV‐Erwinia, 25,000 IU/m2/dose on Monday/Wednesday/Friday, for 2 consecutive‐weeks (6 doses = 1 cycle) for each dose of pegaspargase remaining in the original treatment plan. The primary objective was to determine the proportion of patients achieving NSAA ≥0.1 IU/ml 48 hr after dose 5 in Cycle 1. Secondary objectives included determining the proportion achieving NSAA ≥0.1 IU/ml 72 hr after Cycle 1 dose 6, and the frequency of asparaginase‐related toxicities.
Results
Twenty‐six patients completed Cycle 1; 24 were evaluable for NSAA assessment. In Cycle 1, NSAA ≥0.10 IU/ml was detected in 83% of patients (95% confidence interval [CI], 63–95%) 48 hr post‐dose 5 (mean ± SD; 0.32 IU/ml ± 0.23), and in 43% (95% CI, 22–66%) 72 hr post‐dose 6 (mean ± SD; 0.089 IU/ml ± 0.072). For all 30 patients over all cycles, hypersensitivity/infusional reactions with IV‐Erwinia occurred in 37%, pancreatitis 7%, and thrombosis 3%.
Conclusions
IV‐Erwinia administration in children/adolescents appeared feasible and tolerable. A therapeutically‐effective NSAA (≥0.10 IU/ml) was achieved in most patients at 48 hr, but in fewer than half 72 hr post‐dosing, suggesting that monitoring NSAA levels and/or every 48 hr dosing may be indicated. Pediatr Blood Cancer 2015. © 2015 Wiley Periodicals, Inc.
Keywords: acute lymphoblastic leukemia, childhood ALL, clinical trials, Erwinia asparaginase, pharmacokinetics
INTRODUCTION
L‐asparaginase is an important component of therapy for acute lymphoblastic leukemia (ALL) and acute lymphoblastic lymphoma (LBL). However, up to 30% of patients experience a hypersensitivity reaction to Escherichia coli (E. coli)‐derived asparaginase.1, 2, 3 The development of hypersensitivity reaction to E. coli‐derived asparaginase generally precludes further administration of the same agent. Patients developing a clinical allergy as well as some patients without any clinical signs of hypersensitivity reactions can have reduced asparaginase activity levels due to the presence of neutralizing antibody.4, 5, 6 However, patients who do not receive their full asparaginase treatment‐course due to asparaginase‐related toxicity may have inferior outcomes compared with those who receive all intended asparaginase doses.1 In patients with allergy to E. coli‐derived asparaginase, Erwinia asparaginase, an antigenically distinct preparation derived from Erwinia chrysanthemi with a shorter biological half‐life, can be substituted.4, 7, 8, 9, 10, 11
Erwinia asparaginase has typically been administered intramuscularly in North America, but intravenous (IV) administration would be less painful and more convenient. In an open‐label, single‐arm, multi‐center study, we evaluated nadir serum asparaginase activity (NSAA) and toxicity associated with intravenously administered Erwinia asparaginase in children and adolescents with ALL or LBL who had developed hypersensitivity to pegylated E. coli‐asparaginase (pegaspargase).
PATIENTS AND METHODS
Patients
Patients (aged ≥1 to ≤30 years) undergoing therapy for newly diagnosed ALL or LBL who developed hypersensitivity (≥ grade 2) to pegaspargase, with further asparaginase therapy intended in their treatment plan, were eligible for enrollment. Patients with a history of any of the following were ineligible: ≥ grade 3 pancreatitis, thrombosis requiring anticoagulation, asparaginase‐associated hemorrhage, or prior Erwinia asparaginase treatment. The Institutional Review Board at each participating institution approved the protocol before patient enrollment. Informed consent was obtained from parents or guardians, and consent/assent from patients as appropriate by age, before starting therapy.
Therapy
Patients received Erwinia asparaginase, 25,000 IU/m2/dose, administered intravenously over 1 hr, on a Monday/Wednesday/Friday schedule for two consecutive weeks (6 doses = 1 cycle) as a substitution for each dose of pegaspargase remaining in each patient's original treatment‐plan. All other chemotherapy was continued according to the original treatment plan.
Nadir Serum Asparaginase Activity Assessment
The primary study objective was to determine the proportion of patients who achieved NSAA ≥0.10 IU/ml at 48 hr after dose 5 in Cycle 1. Secondary objectives included determining the proportion of patients who achieved NSAA ≥0.10 IU/ml at 72 hr post‐dose 6 in Cycle 1 (72 hr after a Friday IV‐Erwinia administration). NSAA levels ≥0.10 IU/ml have been associated with serum asparagine depletion; enzymatic activity levels at or above this threshold have been considered therapeutic by many investigators and used in clinical trials.9, 10, 12, 13, 14, 15, 16 Asparaginase activity was determined with a coupled enzymatic assay as previously described,8 conducted at AIBio Tech (Richmond, VA), with lower limit of detection 0.0128 IU/ml. Blood samples were collected during the first treatment cycle, 48 hr post‐dose 5 (primary end‐point) and 72 hr post‐dose 6 (secondary end‐point), as well as prior to dose 1, 5 min after the end of the 1 hr infusion of dose 1, 48 hr post‐dose 1, 48 hr post‐dose 2, 72 hr post‐dose 3, 5 min post‐dose 4, and 48 hr post‐dose 4. Only patients who completed Cycle 1 with samples obtained for the 48 hr post‐dose 5 assessment within the pre‐specified time‐frame (±3hr) were considered evaluable for NSAA assessment.
Asparaginase‐Related Toxicity
Secondary objectives also included determining the frequency of asparaginase‐related toxicities. Toxicity data were collected prospectively. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 4.03. Reactions that occurred during the infusion of Erwinia asparaginase were classified as hypersensitivity and acted upon as such.
Anti‐Asparaginase Antibody Testing
Antibodies against Erwinia asparaginase were measured by enzyme‐linked immunosorbent assay with confirmatory competitive‐inhibition assay. Blood samples for assessment for antibodies to Erwinia were collected pre‐dose 1 and 48 hr post‐dose 5 in Cycle 1 and in all subsequent cycles. Only IgG antibodies were assessed with no isotyping.
Statistical Analysis
The NSAA analysis included all evaluable patients who completed Cycle 1 (6 doses of Erwinia asparaginase) and had evaluable NSAA samples. The proportion of patients with NSAA ≥0.10 IU/ml 48 hr post‐dose 5 and 72 hr post‐dose levels 6 in Cycle 1 were calculated with 95% confidence interval (CI). All enrolled patients who received at least one dose of intravenously administered Erwinia asparaginase were included in the toxicity analysis. Toxicity data were summarized using descriptive statistics. Data analysis was performed using SAS® Version 9.1.3. In order to estimate the half‐life of IV Erwinia asparaginase, a population PK model was used. The population PK of IV Erwinia was characterized by nonlinear mixed‐effects modeling using NONMEM, Version VI, Level 1.0 (ICON Development Solutions, Ellicott City, MD).
RESULTS
Between November 2012 and June 2013, 30 patients enrolled at 10 centers in the United States. Age at enrollment ranged from 1 to 17 years (median 6.5 years), 19 (63%) were male. The majority, 23 patients (77%), had B ALL, 6 patients (20%) had T ALL, and 1 (3%) had LBL (Table I).
Table I.
Characteristics of 30 Enrolled Patients
| Age (years) | |
|---|---|
| Mean ± SD | 7.90 ± 5.08 |
| Median (range) | 6.5 (1–17) |
| Sex, n (%) | |
| Male | 19 (63%) |
| Female | 11 (37%) |
| Race, n (%) | |
| Caucasian | 25 (83%) |
| Black | 1 (3%) |
| Asian | 2 (7%) |
| Other | 2 (7%) |
| Diagnosis, n (%) | |
| B ALL | 23 (77%) |
| T ALL | 6 (20%) |
| LBL | 1 (3%) |
| Body surface area, m2 | |
| Mean ± SD | 1.09 ± 0.52 |
| Median (range) | 0.85 (0.42–2.39) |
SD, standard deviation; ALL, acute lymphoblastic leukemia; LBL, acute lymphoblastic lymphoma.
Twenty‐six patients (87%) completed Cycle 1; in Cycle 1, 3 patients discontinued Erwinia asparaginase due to hypersensitivity and one discontinued due to pancreatitis (Fig. 1). Twenty‐four patients were evaluable for the primary NSAA endpoint (two patients had samples obtained outside the protocol‐specified time‐frame). Of the 26 patients who completed Cycle 1, one patient declined further therapy after four cycles and one was withdrawn after two cycles (an updated informed consent form was not offered after a protocol amendment). Eight additional patients withdrew for adverse events: seven with reactions designated as hypersensitivity reactions (including one designated as urticaria), and one with pancreatitis. Therefore, 16 patients (53%) completed their full course of planned asparaginase, receiving from 6 to 30 total doses (1–5 cycles) of intravenously administered Erwinia asparaginase. Overall, there were a total of 71 6‐dose cycles completed. Based on a population PK model, the mean (%CV) half‐life of intravenous Erwinia asparaginase was 7.51 (23.9%) hours.
Figure 1.
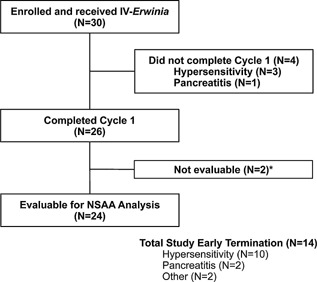
Patients evaluable for primary nadir serum asparaginase activity (NSAA) analysis. *Sample obtained outside the protocol‐specified time‐frame.
Nadir Serum Asparaginase Activity (NSAA) with Intravenous Erwinia Asparaginase
With intravenously administered Erwinia asparaginase, 20 of 24 (83%, 95% CI: 63–95%) evaluable patients achieved NSAA levels ≥0.10 IU/ml 48 hr post‐dose 5. Mean NSAA was 0.32 ± 0.23 IU/ml at this 48 hr time‐point. Similar findings were noted 48 hr post‐dosing at other time‐points throughout Cycle 1 (Table II). Seventy‐two hours post‐dose 6, 9 of 21 patients (43%, 95% CI: 22–66%) achieved NSAA levels ≥0.10 IU/ml. Mean NSAA was 0.09 ± 0.072 IU/ml at this 72 hr time‐point, with similar findings post‐dose 3 in Cycle 1.
Table II.
Serum Asparaginase Activity in Cycle 1 in patients receiving IV‐Erwinia, 25,000 IU/m2/dose, on a Monday/Wednesday/Friday Schedule for 2 Consecutive‐Weeks (6 doses = 1 Cycle)
| Dose 1 | Dose 2 | Dose 3 | Dose 4 | Dose 5 | Dose 6 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre (N = 24) | 5 min post (N = 22) | 48 hr post (N = 19) | 48 hr post (N = 23) | 72 hr post (N = 21) | 5 min post (N = 21) | 48 hr post (N = 22) | 48 hr post (N = 24) | 72 hr post (N = 21) | |
| Mean (IU/ml) ±SD | 12.65 ± 3.16 | 0.37 ± 0.28 | 0.42 ± 0.36 | 0.088 ± 0.095 | 12.10 ± 3.11 | 0.32 ± 0.24 | 0.32 ± 0.23 | 0.089 ± 0.072 | |
| % Patients ≥0.05 IU/ml | 0 | 100 | 100 | 100 | 52 | 100 | 95 | 92 | 52 |
| % Patients ≥0.10 IU/ml | 0 | 100 | 89 | 91 | 38 | 100 | 82 | 83 a | 43 b |
| % Patients ≥0.4 IU/ml | 0 | 100 | 32 | 43 | 0 | 100 | 36 | 29 | 0 |
Primary end‐point.
Secondary end‐point.
Based on the 72 hr findings, the protocol was amended for patients continuing on the trial beyond Cycle 1 to receive intravenous Erwinia asparaginase with dosing every 48 hr for seven doses, instead of on a Monday/Wednesday/Friday schedule. After switching to every 48 hr dosing, asparaginase activity was assessed prior to the first dose of the cycle and 48 hr after each delivered dose. Two patients continued on the trial with every 48 hr dosing. In these two patients, all NSAA levels measured 48 hr after dosing remained above 0.10 IU/ml, with levels ranging from 0.282 to 1.485 IU/ml (median 0.472 IU/ml). One patient developed pancreatitis after six doses and received no further Erwinia asparaginase and the other completed three Cycles (completing planned asparaginase with this dosing schedule).
Toxicities
Observed toxicities are summarized in Tables III and IV. Over all cycles, hypersensitivity/infusion reactions (either reported as hypersensitivity or urticaria) occurred in 37% of patients, pancreatitis in 7%, and thrombosis in 3% of patients. The one urticarial reaction was grade 3; all other hypersensitivity/infusion reactions (n = 10) were ≤ grade 2. No episodes of anaphylaxis were reported. No deaths were reported. Of the 11 patients with hypersensitivity/infusion reactions, 10 discontinued intravenous Erwinia asparaginase (three patients in Cycle 1 and seven patients in subsequent cycles), and one patient continued intravenous Erwinia (Table V).
Table III.
Frequency of Selected Toxicities by Cycle: Targeted Asparaginase‐Associated Toxicities and Most Commonly‐Reported Adverse Events
| Adverse event, N (%) | Cycle 1 (N = 30) | Cycle 2 (N = 21) | Cycle 3 (N = 15) | Cycle 4 (N = 10) | Cycle 5 (N = 6) | Cycle 6 (N = 1) | Overall a (N = 30) |
|---|---|---|---|---|---|---|---|
| ≥1 Adverse event, any grade | 15 (50) | 11 (52) | 6 (40) | 2 (20) | 1 (17) | 1 (100) | 23 (77) |
| ≥1 Grade 3–4 adverse event b | 5 (17) | 4 (19) | 4 (27) | 1 (10) | 1 (17) | 0 | 10 (33) |
| Hypersensitivity/infusion reaction c | 5 (17) | 2 (10) | 2 (13) | 0 | 1 (17) | 1 (100) | 11 (37) |
| Pancreatitis | 1 (3) | 0 | 0 | 1 d (10) | 0 | 0 | 2 (7) |
| Thrombosis | 1 (3) | 0 | 0 | 0 | 0 | 0 | 1 (3) |
| Hyperglycemia | 2 (7) | 4 (19) | 0 | 0 | 0 | 0 | 5 (17) |
| Nausea and/or vomiting | 7 (23) | 2 (10) | 0 | 1 (10) | 0 | 0 | 7 (23) |
| Transaminase increase | 0 | 1 (5) | 0 | 0 | 0 | 0 | 1 (3) |
| Fever ± neutropenia | 1 (3) | 1 (5) | 2 (13) | 0 | 0 | 0 | 4 (13) |
| Infection/sepsis | 1 b (3) | 1 (5) | 0 | 0 | 0 | 0 | 2 (7) |
Each patient is counted only once for a given toxicity.
The only grade 4 adverse event was an occurrence of sepsis; all other adverse events were grade 1–3.
Included is one event reported as urticaria (grade 3); all other reactions were ≤ grade 2.
Pancreatitis occurred after six doses administered with every 48 hr dosing.
Table IV.
Frequency of Grade 3 and 4 Toxicities During All Cycles
| N (%) | |
|---|---|
| ≥1 Grade 3–4 adverse event | 10 (33) |
| Hypersensitivity (urticaria) | 1 (3) |
| Pancreatitis | 2 (7) |
| Hyperglycemia | 1 (3) |
| Nausea and/or vomiting | 2 (7) |
| Fever/neutropenia | 4 (13) |
| Stomatitis | 2 (7) |
| Fatigue | 1 (3) |
| Bacteremia | 1 (3) |
| Sepsis | 1 (3) |
| Decreased appetite | 1 (3) |
| Peripheral motor neuropathy | 1 (3) |
| Posterior reversible encephalopathy syndrome | 1 (3) |
| Pain in extremity | 1 (3) |
| Abdominal pain | 1 (3) |
Table V.
Timing, Grade, and Outcome of Each Reported Hypersensitivity Reaction
| Cycle number | Dose number | Grade | Disposition |
|---|---|---|---|
| 1 | 5 | 1 | Infusion interrupted and then continued with symptom improvement |
| 1 | 1 | 2 | Withdrawn |
| 1 | 2 | 2 | Withdrawn |
| 1 | 4 | 2 | Withdrawn |
| 1 | 6 | 2 | Withdrawn |
| 2 | 1 | 2 | Withdrawn |
| 2 | 5 | 2 | Withdrawn |
| 3 | 2 | 2 | Withdrawn |
| 3 | 4 | 2 | Withdrawn |
| 5 | 1 | 3 | Withdrawn |
| 6 | 1 | 2 | Withdrawn |
Anti‐Asparaginase Antibodies
From the 30 enrolled patients, 155 samples were tested for antibodies against Erwinia asparaginase. Five samples in four patients (13% of patients) were positive for anti‐asparaginase antibodies (Supplemental Table I). Three of four patients with positive anti‐asparaginase antibody samples experienced a hypersensitivity/infusion reaction. Eight of the 11 patients who developed hypersensitivity/infusion reactions were found negative for anti‐asparaginase antibodies.
DISCUSSION
In children and adolescents with ALL or LBL who develop hypersensitivity to E. coli‐derived asparaginase, Erwinia asparaginase can be substituted. Erwinia asparaginase has typically been administered intramuscularly in North America and was previously FDA approved only for intramuscular administration. We aimed to better define the pharmacokinetics of intravenously administered Erwinia asparaginase in children and adolescents with ALL or LBL who had developed allergy to pegaspargase. Based on the data from this study, Erwinia asparaginase is now FDA‐approved for intravenous administration.
In this study, utilizing a NSAA threshold of 0.10 IU/ml, a therapeutically effective NSAA (≥0.10 IU/ml) was achieved in most patients at 48 hr after IV administration, but in fewer than half of patients 72 hr post‐dosing. NSAA levels ≥0.10 IU/ml have been associated with serum asparagine depletion and enzymatic activity levels at or above this threshold have been considered therapeutic by many investigators.[9, 10, 12, 13, 14, 15, 16] The target trough of 0.10 IU/ml was selected for this study in accordance with the existing literature because of the association with desired therapeutic effect and in order to be comparable, for descriptive purposes, with prior reports. The precise threshold for optimal therapeutic effect of Erwinia asparaginase is unclear, and whether lower NSAA levels would be adequate is an area worthy of future investigation. In a previous study from Salzer and colleagues of intramuscular Erwinia asparaginase at 25,000 IU/m2/dose on a Monday/Wednesday/Friday schedule, 92.7% of patients achieved NSAA ≥0.10 IU/ml at 48 hr.10 In our study, with intravenous administration, 83% of evaluable patients achieved NSAA levels ≥0.10 IU/ml 48 hr post‐dose 5. Similar findings were consistently noted 48 hr post‐dosing at other time‐points throughout Cycle 1. However, we found that 72 hr post‐dose 6, 43% of patients achieved NSAA levels ≥0.10 IU/m. This is in contrast to the report from Salzer and colleagues of 88.4% of patients achieving this level 72 hr after intramuscular administration.8 Formal comparison with the report of Salzer and colleagues was not pursued due to the limitations of sample size in our study.
These findings have potential implications for the monitoring of patients receiving intravenous Erwinia asparaginase. The apparently lower NSAA levels at 72 hr with intravenous compared with intramuscular Erwinia asparaginase, despite identical dosing and schedule, suggest that intravenous and intramuscular administration of Erwinia asparaginase have different pharmacokinetics. Consistent with this observation, Albertsen and colleagues previously showed that intravenously administered Erwinia asparaginase was associated with higher peak asparaginase activity and lower NSAA compared with intramuscular Erwinia asparaginase in a pharmacokinetic modeling study.17 Our results support the monitoring of NSAA levels with intravenous Erwinia administration. With therapeutic drug monitoring, changing the frequency of intravenous administration to every 48 hr or switching to intramuscular administration could be considered in patients with low 72 hr NSAA levels. Indeed, in our study, the protocol was amended for patients continuing on the trial beyond Cycle 1 to receive IV‐Erwinia dosing every 48 hr for seven doses rather than on a Monday/Wednesday/Friday schedule. In the two patients who continued on the trial with every‐other‐day dosing, all subsequent NSAA levels measured at 48 hr after dosing remained above 0.10 IU/ml.
Overall, toxicities associated with intravenously administered Erwinia asparaginase appeared consistent with previous reports in children treated with intramuscular Erwinia asparaginase after E. coli‐asparaginase allergy, although the overall frequency of hypersensitivity reactions with intravenous administration reported here may be higher than previously reported with intramuscular administration.9, 10, 11 It is notable, however, that in our study, there were no reported episodes of anaphylaxis. With the exception of a single grade 3 occurrence of urticaria, all other reported hypersensitivity reactions were grade 1–2. Of all patients, 87% completed Cycle 1, and approximately half (53%) completed their full course of planned asparaginase with the switch from pegaspargase to intravenous Erwinia asparaginase. In a compassionate‐use trial evaluating the safety of Erwinia asparaginase in 940 patients who had developed hypersensitivity reaction to E. coli‐derived asparaginase, 13.6% of patients developed hypersensitivity reaction with Erwinia asparaginase; the majority of patients (91%) in that study had received Erwinia asparaginase intramuscularly.11 In a subgroup analysis of the 29 patients who had received Erwinia asparaginase intravenously, 17.2% of patients developed hypersensitivity reaction with Erwinia asparaginase and 58.6% of patients receiving intravenous Erwinia asparaginase completed their planned asparaginase treatment. Tong and colleagues reported a lower frequency of allergy (3%) with intravenous Erwinia asparaginase administration (with dosing of 20,000 IU/m2 2–3 times per week).6
The reasons for the differences in the reported rates of hypersensitivity with Erwinia asparaginase are unclear, but could be influenced by the small sample sizes or differences in underlying treatment programs, such as the dosing and schedule of asparaginase, or the schedule of other agents including corticosteroids. It is also possible that some of the reactions classified as hypersensitivity reactions in this study reflected non‐allergic infusion reactions, which can be clinically difficult to distinguish from true hypersensitivity reactions.18, 19 Any clinically significant infusion reaction was classified as hypersensitivity and acted upon as such. However, it is possible that some reactions, including those early in Cycle 1 when hypersensitivity to Erwinia would not have been expected, did not reflect true allergy. Future investigations could aim to better distinguish between true hypersensitivity and non‐allergic infusion reactions. Three of 11 patients reported with a hypersensitivity reaction were confirmed positive for the presence of anti‐asparaginase antibodies (however, the antibody isotype was not determined). Regarding the eight patients with reported hypersensitivity and without detected anti‐asparaginase antibody, reactions could have represented non‐allergic infusion reactions or antibodies may not have been identified. In addition, the correlation between the presence of anti‐asparaginase antibodies and allergy (and silent inactivation) is imperfect in previous studies, with some patients developing reactions without antibody detection and some with antibody development without reactions or inactivation.4, 6
There are several important limitations of our study, including limitations of sample size. This study aimed primarily to assess the proportion of patients with therapeutically effective NSAA with intravenously administered Erwinia asparaginase, with toxicity assessment as a secondary end‐point. Toxicity data must be interpreted within the context of the small number of patients. In addition, due to the limited number of positive antibody samples, the relationship between antibody status and serum asparaginase activity could not be fully assessed in this cohort. Finally, in this multi‐center study, patients were not receiving Erwinia asparaginase in the context of uniform treatment regimens and the agents administered at the same time were not collected for analysis. Information on the number of previous doses of pegaspargase was also not collected.
In conclusion, intravenous administration of Erwinia asparaginase in children and adolescents with ALL or LBL with hypersensitivity to pegaspargase was feasible and tolerable. A therapeutically effective serum asparaginase level (defined as ≥0.10 IU/ml) was achieved in the majority of patients 48 hr after dosing, but in fewer than half of patients at 72 hr after dosing. These results support the monitoring of NSAA levels with intravenous Erwinia administration. Changing the frequency of intravenous administration to every 48 hr or switching to intramuscular administration could be considered in patients with low 72 hr NSAA levels. Further investigation could focus on individualizing Erwinia asparaginase dosing based upon monitoring of NSAA levels as well as determining whether a 72 hr NSAA of 0.10 IU/ml is required for optimal therapeutic effect.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Table S1. Timing of Positive Anti‐Asparaginase Antibody Determinations.
ACKNOWLEDGMENTS
Editorial assistance was provided by Jessica Mendoza, PhD, an employee of Jazz Pharmaceuticals plc, funded by Jazz Pharmaceuticals plc.
Conflict of interest: L.M.V. served on an advisory board for Jazz Pharmaceuticals; N.H. served as a consultant for Sigma Tau and for Jazz Pharmaceuticals; P.B. served on an advisory board for Jazz Pharmaceuticals; Y.H.M. served on an advisory board for Jazz Pharmaceuticals; Y.L. is an employee of Jazz Pharmaceuticals, Inc., who in the course of this employment has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc; P.V.P. was an employee of Jazz Pharmaceuticals, Inc., who in the course of this employment has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc; L.B.S. served on an advisory board for Jazz Pharmaceuticals. Remaining authors: Nothing to declare.
Clinical trial registration identifier: NCT01643408, ClinicalTrials.gov.
Presented in abstract form at the 55th Annual Meeting of the American Society of Hematology, New Orleans, LA, December 7–10, 2013.
[The copyright line for this article was corrected in Jan 2016 after original online publication.]
REFERENCES
- 1. Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y, Schorin MA, Arkin S, Declerck L, Cohen HJ, Sallan SE. Improved outcome for children with acute lymphoblastic leukemia: Results of Dana‐Farber Consortium Protocol 91‐01. Blood 2001; 97:1211–1218. [DOI] [PubMed] [Google Scholar]
- 2. Woo MH, Hak LJ, Storm MC, Sandlund JT, Ribeiro RC, Rivera GK, Rubnitz JE, Harrison PL, Wang B, Evans WE, Pui CH, Relling MV. Hypersensitivity or development of antibodies to asparaginase does not impact treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol 2000; 18:1525–1532. [DOI] [PubMed] [Google Scholar]
- 3. Muller HJ, Beier R, Loning L, Blutters‐Sawatzki R, Dorffel W, Maass E, Muller‐Weihrich S, Scheel‐Walter HG, Scherer F, Stahnke K, Schrappe M, Horn A, Lumkemann K, Boos J. Pharmacokinetics of native Escherichia coli asparaginase (Asparaginase medac) and hypersensitivity reactions in ALL‐BFM 95 reinduction treatment. Br J Haematol 2001; 114:794–799. [DOI] [PubMed] [Google Scholar]
- 4. Panosyan EH, Seibel NL, Martin‐Aragon S, Gaynon PS, Avramis IA, Sather H, Franklin J, Nachman J, Ettinger LJ, La M, Steinherz P, Cohen LJ, Siegel SE, Avramis VI. Asparaginase antibody and asparaginase activity in children with higher‐risk acute lymphoblastic leukemia: Children's Cancer Group Study CCG‐1961. J Pediatr Hematol Oncol 2004; 26:217–226. [DOI] [PubMed] [Google Scholar]
- 5. Zalewska‐Szewczyk B, Andrzejewski W, Mlynarski W, Jedrychowska‐Danska K, Witas H, Bodalski J. The anti‐asparagines antibodies correlate with L‐asparagines activity and may affect clinical outcome of childhood acute lymphoblastic leukemia. Leuk Lymphoma 2007; 48:931–936. [DOI] [PubMed] [Google Scholar]
- 6. Tong WH, Pieters R, Kaspers GJ, te Loo DM, Bierings MB, van den Bos C, Kollen WJ, Hop WC, Lanvers‐Kaminsky C, Relling MV, Tissing WJ, van der Sluis IM. A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood 2014; 123:2026–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang B, Relling MV, Storm MC, Woo MH, Ribeiro R, Pui CH, Hak LJ. Evaluation of immunologic crossreaction of antiasparaginase antibodies in acute lymphoblastic leukemia (ALL) and lymphoma patients. Leukemia 2003; 17:1583–1588. [DOI] [PubMed] [Google Scholar]
- 8. Asselin BL, Whitin JC, Coppola DJ, Rupp IP, Sallan SE, Cohen HJ. Comparative pharmacokinetic studies of three asparaginase preparations. J Clin Oncol 1993; 11:1780–1786. [DOI] [PubMed] [Google Scholar]
- 9. Vrooman LM, Supko JG, Neuberg DS, Asselin BL, Athale UH, Clavell L, Kelly KM, Laverdiere C, Michon B, Schorin M, Cohen HJ, Sallan SE, Silverman LB. Erwinia asparaginase after allergy to E. coli asparaginase in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 2010; 54:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salzer WL, Asselin B, Supko JG, Devidas M, Kaiser NA, Plourde P, Winick NJ, Reaman GH, Raetz E, Carroll WL, Hunger SP. Erwinia asparaginase achieves therapeutic activity after pegaspargase allergy: A report from the Children's Oncology Group. Blood 2013; 122:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plourde PV, Jeha S, Hijiya N, Keller FG, Silverman LB, Rheingold SR, Dreyer ZE, Dahl GV, Mercedes T, Lai C, Corn T. Safety profile of asparaginase Erwinia chrysanthemi in a large compassionate‐use trial. Pediatr Blood Cancer 2014; 61:1232–1238. [DOI] [PubMed] [Google Scholar]
- 12. Strullu M, Corradini N, Audrain M, Orsonneau JL, Bouige D, Thomare P, Vermot‐Desroches C, Mansuy A, Legrand A, Roze JC, Mohty M, Mechinaud F. Silent hypersensitivity to Escherichia coli asparaginase in children with acute lymphoblastic leukemia. Leuk Lymphoma 2010; 51:1464–1472. [DOI] [PubMed] [Google Scholar]
- 13. Avramis VI, Martin‐Aragon S, Avramis EV, Asselin BL. Pharmacoanalytical assays of Erwinia asparaginase (erwinase) and pharmacokinetic results in high‐risk acute lymphoblastic leukemia (HR ALL) patients: Simulations of erwinase population PK‐PD models. Anticancer Res 2007; 27:2561–2572. [PubMed] [Google Scholar]
- 14. Avramis VI, Sencer S, Periclou AP, Sather H, Bostrom BC, Cohen LJ, Ettinger AG, Ettinger LJ, Franklin J, Gaynon PS, Hilden JM, Lange B, Majlessipour F, Mathew P, Needle M, Neglia J, Reaman G, Holcenberg JS, Stork L. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard‐risk acute lymphoblastic leukemia: A Children's Cancer Group study. Blood 2002; 99:1986–1994. [DOI] [PubMed] [Google Scholar]
- 15. Riccardi R, Holcenberg JS, Glaubiger DL, Wood JH, Poplack DG. L‐asparaginase pharmacokinetics and asparagine levels in cerebrospinal fluid of rhesus monkeys and humans. Cancer Res 1981; 41:4554–4558. [PubMed] [Google Scholar]
- 16. Boos J, Werber G, Ahlke E, Schulze‐Westhoff P, Nowak‐Gottl U, Wurthwein G, Verspohl EJ, Ritter J, Jurgens H. Monitoring of asparaginase activity and asparagine levels in children on different asparaginase preparations. Eur J Cancer 1996; 32A:1544–1550. [DOI] [PubMed] [Google Scholar]
- 17. Albertsen BK, Jakobsen P, Schroder H, Schmiegelow K, Carlsen NT. Pharmacokinetics of Erwinia asparaginase after intravenous and intramuscular administration. Cancer Chemother Pharmacol 2001; 48:77–82. [DOI] [PubMed] [Google Scholar]
- 18. Vogel WH. Infusion reactions: Diagnosis, assessment, and management. Clin J Oncol Nurs 2010; 14:E10–E21. [DOI] [PubMed] [Google Scholar]
- 19. Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist 2007; 12:601–609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Table S1. Timing of Positive Anti‐Asparaginase Antibody Determinations.


