Abstract
We determined whether time-domain P3 amplitude and time-frequency principal component (TF-PC) reductions could serve as stable and predictive developmental endophenotypes of externalizing psychopathology. Participants from the Minnesota Twin Family Study were assessed at age 17 and again at age 29 for lifetime externalizing (EXT) disorders. Comparisons of P3 amplitude and TF-PCs at delta and theta frequencies were made between EXT and unaffected comparison subjects. P3 amplitude and all five extracted TF-PCs were significantly reduced in those presenting lifetime EXT disorders at both ages 17 and 29 and showed substantial 12-year rank-order stability. P3 amplitude and delta TF-PCs measured at age 17 also predicted subsequent development of EXT by age 29, with every 1-microvolt decrease in age 17 amplitude associated with an approximately 5% increase in risk for an EXT diagnosis by age 29. Overall, results from this study further confirm that these P3-derived brain measures maintain their potential as putative EXT endophenotypes through the third decade of life.
Keywords: P3 amplitude, time-frequency principal components, longitudinal, substance dependence, externalizing disorders, developmental endophenotype
Converging evidence suggests that visual P3 brain event-related potential measures, including both time-domain amplitude and time-frequency components, are candidate endophenotypes for externalizing (EXT) spectrum disorders. These disorders are characterized by behavioral disinhibition (see review by Iacono, Malone, & McGue, 2008) and include childhood disruptive, antisocial, and substance use disorders. Endophenotypes like P3 amplitude are heritable laboratory-based measures associated with psychiatric disorders such as those in the EXT spectrum (Gottesman & Gould, 2003). Because they are presumed to tap a narrow component of genetic liability, they should be genetically less complex than psychiatric phenotypes. In addition, because they are linked to neural processes, they are presumed to be more proximal to the effects of genes. Given this combination of features, finding genetic variants for endophenotypes would be tractable if the variants have large effect sizes that are detectable with relatively small samples. This is an important consideration because amassing very large samples required to study the genetic architecture of complex traits is both lab and resource intensive.
However, concerns have been raised that endophenotypes are genetically no less complex than clinical disorders (Anokhin, 2014; Cannon & Keller, 2006; de Geus, 2010; Flint & Munafo, 2007; Glahn et al., 2014; Munafo & Flint, 2014), and that their value as a tool for gene discovery may limited. Of note, a series of papers exploring the molecular genetic basis of 17 psychophysiological endophenotypes, including P3 amplitude, using candidate gene, genome wide association, exome chip, and whole genome sequencing analyses on a sample of nearly 5,000 individuals, found that these measures were massively polygenic (Iacono, Vaidyanathan, Vrieze, & Malone, 2014; Malone, Burwell, et al., 2014; Malone, Vaidyanathan, et al., 2014; Vaidyanathan, Isen, et al., 2014; Vaidyanathan, Malone, Donnelly, et al., 2014; Vaidyanathan, Malone, Miller, McGue, & Iacono, 2014; Vrieze, Malone, Pankratz, et al., 2014; Vrieze, Malone, Vaidyanathan, et al., 2014). Like the clinical disorders with which they are associated, their genetic etiology is complex, involving many variants contributing small effects at a population level. Given that psychophysiological endophenotypes maybe be limited regarding their potential to facilitate gene finding, it is important to consider the other ways in which they add value to psychiatric research.
Iacono and Malone (2011) noted that endophenotypes have important developmental properties that are infrequently emphasized. Because they identify gene carriers, they should be detectable in unaffected individuals prior to the manifest development of psychiatric disorder, and they should forecast increased risk for disorder development. Providing evidence for the developmental significance of endophenotypes is difficult, however, because it requires prospective research to show that unaffected individuals with an endophenotype develop disorders as they pass through the age of risk. It also requires an understanding of how the endophenotype changes with development. The present investigation was designed to investigate the developmental properties of P3 amplitude and its constituent time-frequency components in male participants first assessed for EXT and P3 amplitude at age 17 and last assessed at age 29.
P3 amplitude reflects the apex of the large positive-going brainwave associated with information-processing events. Depending on the task, either a P3a or P3b peak can be produced, in either the auditory or visual modality. P3a is detected after the presentation of unexpected, rare nontarget stimuli, while P3b follows cognitive processing of rare target stimuli embedded within frequently presented nontargets (see review by Polich, J, 2007). In line with previous reports from our laboratory and that of others, the current study investigated the visual P3b in relation to EXT. P3 amplitude reduction (P3AR) is posited to tap into a core neurobiological substrate for externalizing disorders characterized by behavioral disinhibition (Iacono, Malone, & McGue, 2003), including attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), conduct disorder (CD), adult antisocial behavior (AAB, the symptoms of antisocial personality disorder that onset after age 15), and alcohol, nicotine, and illicit drug use disorders. The covariance among these frequently comorbid disorders, which reflect the inability to inhibit socially-inappropriate behaviors, can be modeled as a highly heritable latent externalizing factor (Bornovalova, Hicks, Iacono, & McGue, 2010; Krueger et al., 2002; Matt McGue, Iacono, & Krueger, 2006). P3 amplitude constitutes a facet of this factor (Patrick et al., 2006) due to shared genetic effects (Hicks et al., 2007). Furthermore, genetically informed studies demonstrate that time-domain P3 amplitude displays robust heritability (Malone, Vaidyanathan, et al., 2014; van Beijsterveldt & van Baal, 2002; Yoon, Iacono, Malone, & McGue, 2006) and that P3AR indexes both lifetime morbidity (Iacono et al., 2008; Yoon, Malone, Burwell, Bernat, & Iacono, 2013) as well as premorbid risk (Euser et al., 2012) for disorders in the EXT spectrum. Although visual P3 amplitude becomes smaller as individuals pass through adolescence into adulthood, P3 shows rank-order stability when measured repeatedly in young adulthood (Carlson & Iacono, 2006). Additionally, in follow-up studies of youths, those engaging in substance misuse have been found to have reduced P3 amplitude when measured earlier in life, prior to significant use (Berman, Whipple, Fitch, & Noble, 1993; Carlson, Iacono, & McGue, 2004; Carlson, McLarnon, & Iacono, 2007; Habeych, Charles, Sclabassi, Kirisci, & Tarter, 2005; Hill, Steinhauer, Locke-Wellman, & Ulrich, 2009; Iacono, Carlson, Malone, & McGue, 2002; Perlman, Markin, & Iacono, 2013). However, this effect may not continue through young adulthood because prior research has shown that with brain maturation and the accompanying reduction seen in P3 amplitude, differences in P3 amplitude between those at high and low risk for disorders in the EXT spectrum appear to diminish (Carlson et al., 2007; Hill et al., 1999).
The event-related potential (ERP) response, consisting of averaged voltage changes over time, can be represented in the time-frequency domain, which reveal moment by moment, frequency-specific changes in ongoing EEG activity (Basar, Basar-Eroglu, Karakas, & Schurmann, 1999b). EXT research has focused predominantly on delta (0–3 Hz) and theta (3–7 Hz) since these frequencies dominate the ERP time course, thus contributing substantially to the composition of the P3 waveform (Basar-Eroglu, Basar, Demiralp, & Schurmann, 1992; Bernat, Malone, Williams, Patrick, & Iacono, 2007; Jones et al., 2006). These two components are thought to reflect features of cognitive processing with delta associated with signal detection, decision-making (Basar, Basar-Eroglu, Karakas, & Schurmann, 1999a) and consciousness (Karakas, Erzengin, & Basar, 2000a), and theta with selective attention (Basar-Eroglu, Basar, Demiralp, & Schurmann, 1992), orienting (Basar, Rahn, Demiralp, & Schurmann, 1998), and associative processes that include the encoding of new information (Karakas, Erzengin, & Basar, 2000b). Relevantly, these TF measures may also serve as markers of genetic risk, in effect constituting possibly simpler endophenotypes for a more complex measure (see Miller & Rockstroh, 2013).
TF transforms of the ERP can be fruitfully decomposed into principal components (TF-PCs; Bernat, Williams, & Gehring, 2005). In male participants, such TF-PCs have been used to successfully discriminate each disorder in the EXT spectrum from healthy comparison subjects, with some TF-PCs accounting for variance in externalizing not accounted for by P3 amplitude alone (Gilmore, Malone, Bernat, & Iacono, 2010). One component in particular provided incremental validity over P3 amplitude in differentiating groups. This component was only detected via PCA; it did not reflect activity that one would necessarily think was relevant to EXT. Results of multivariate modeling have shown that both time-domain P3 amplitude and certain TF-PCs show significant phenotypic and genetic correlations with the latent EXT factor (Gilmore, Malone, Bernat, et al., 2010), indicating that shared genetic effects account in part for the phenotypic correlations. Yoon et al. (2013) have extended these Gilmore reports, which were based on study participants at age 17, by showing that TF-PCs are associated with EXT in men at age 29. Other investigators have shown that TF components are reduced in men at familial risk for developing alcoholism (Rangaswamy et al., 2007). Collectively, these studies point to the value of examining TF-PC endophenotypes in addition to P3 amplitude. The present investigation serves as the first longitudinal study to examine the developmental stability of TF-PC endophenotypes and how their association with EXT changes over the course of young adulthood.
The current study had three goals important to the evaluation of P3 as a developmental endophenotype. The first was to evaluate the rank-order stability of P3-derived time-domain and time-frequency measures over a 12-year span covering the period from late adolescence through young adulthood. This is the longest period of time over which the stability of P3 has been investigated, and it covers an important developmental stage during which the high risk period for the development of substance use and antisocial disorders peaks and begins to wane (Vrieze, Hicks, Iacono, & McGue, 2012). The second was to examine the strength of association of each measure with EXT spectrum disorders at both time points to determine if the effect became weaker with age. Doing so helps further address whether P3AR ceases to be effective as an EXT endophenotype due to the diminishment of P3 amplitude that occurs with brain maturation during this developmental period. The third aim was to study the predictive utility of P3AR. Although such studies have been undertaken previously, this is the first report to investigate the period encompassing the transition from adolescence through emerging adulthood, and is the first to examine both time-domain and time-frequency features. Participants constituted an unselected, community representative sample evaluated first at study intake (age 17) and again at age 29. At both ages, participants were assessed for lifetime EXT disorders including ADHD, ODD, CD, AAB, and substance dependence (alcohol, nicotine, illicit street drugs). EEG activity was recorded at site-Pz, and ERP data at each assessment were decomposed using a novel PCA-based time-frequency method (Bernat, Malone, Williams, Patrick, & Iacono, 2007). The rank-order stability of these P3-derived measures was ascertained by determining their 12-year correlations and by observing the magnitude of effect sizes associated with the EXT groups at each age. Furthermore, the predictive utility of P3 amplitude was determined by focusing on participants identified as unaffected comparison subjects during the intake assessment and determining whether, despite their apparent low risk status, those in this group who went onto develop EXT showed significant P3AR at age 17. We hypothesized that, although the P3-derived measures (i.e., amplitude and time-frequency components) would be expected to diminish normally with age, they would display significant rank-order stability from age 17 to age 29. Also, we anticipated that the EXT group comparisons would produce diminished effects at age 29. We further hypothesized that these P3-derived measures would predict the development of new cases of EXT emerging between ages 17 and 29.
Method
Participants
Participants comprised 578 male youths who were first assessed at age 17 as part of the Minnesota Twin Family Study (MTFS), a community-based longitudinal investigation of the development of substance use and related disorders. Subjects were identified through public records of twin births in Minnesota between January 1, 1972 and December 31, 1978, and recruited through mail and phone contact using similar procedures in the year they turned 17 and again in the year they turned 29. A more complete description of the MTFS can be found in Iacono and McGue (2002). Overall, the MTFS sample is representative of the population of Minnesota with respect to self-reported mental health and socioeconomic background. The vast majority of participants are Caucasian (99%), consistent with the makeup of the state at the time the twins were born. All participants and their parents provided written informed assent and consent. Subjects who underwent EEG assessment at both the age 17 and 29 assessments were included in the current study.
Interview and Assessment Procedure
When visiting together (as at the intake assessment, by design), members of twin pairs were interviewed simultaneously, each in a separate room, by a different interviewer. Because the third revised edition of the Diagnostic and Statistical Manual (DSM-III-R; American Psychiatric Association, 1987) was the diagnostic system in place when the study was initiated, it was also used for the age 29 assessment to maintain continuity. Lifetime diagnoses for childhood disruptive disorders (ADHD, ODD, and CD) were determined during intake evaluation at approximately age 17. Twins reported on symptoms of ADHD and ODD during clinical interviews using the revised version of the Diagnostic Interview for Children and Adolescents (DICA-R; Reich, 2000; Welner, Reich, Herjanic, Jung, & Amado, 1987). They reported on symptoms of CD during interviews using the Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II; Spitzer, Williams, Gibbon, & First, 1987). Diagnoses for AAB were also determined by means of the SCID-II at all assessment phases and assigned to participants who met criteria for ASPD except for the CD requirement (40% of participants with AAB did not meet criteria for CD; cf. Elkins, Iacono, & Doyle, 1997). Twins were further assessed for substance use disorders using the expanded Substance Abuse Module (Robins, Babor, & Cottler, 1987) developed as a supplement to the World Health Organization’s Composite International Diagnostic Interview (Robins et al., 1988). Substance use diagnostic criteria were assessed for both licit (alcohol, nicotine) and illicit (amphetamines, cannabis, cocaine, hallucinogens, inhalants, opioids, phencyclidine, and sedatives) psychoactive substances. Furthermore, although participants were instructed not to use alcohol, illicit drugs, or stimulant medications within 24-hours of their lab visit, an abbreviated checklist from the Substance Abuse Module was used to determine whether participants used any substance within the past 24-hours that might influence the EEG recordings (i.e., coffee, soda, tobacco/cigarettes, prescription drugs, or any other drugs). These self-report data were further supplemented by breathalyzer analysis administered prior to EEG testing.
For the age 17 intake assessment, the mother or other caregiver of the twins was interviewed with the DICA-R-Parent version regarding the same diagnoses. All questions asked of the twins were also asked of the mother as they pertained to the twins. To establish diagnoses for each childhood disruptive disorder, a “best-estimate” approach was used combining twin- and mother-reports (Kosten & Rounsaville, 1992; Leckman, Scholomskas, Thompson, Belanger, & Weisman, 1982). Although the childhood disruptive disorder diagnoses were not re-assessed after age 17, the lifetime assessments of SUDs and AAB were updated at age 29 using the same structured clinical interviews carried out with the twins at age 17 (Hamdi & Iacono, 2014).
Clinical interviews were reviewed by at least two individuals with advanced clinical training who coded, by consensus, every relevant DSM-III-R symptom and diagnostic criterion. We required that all diagnostic criteria be satisfied for all disorders. Cohen kappa reliability coefficients for the disorders assessed in the current study all exceeded .71 (Iacono, Carlson, Taylor, Elkins, & McGue, 1999).
Diagnostic and control groups
Similar to the reports by Iacono et al. (2002), Gilmore, Malone, Bernat, et al. (2010), and Yoon et al. (2013), seven groups were formed reflecting whether participants met lifetime diagnoses for any EXT disorder(s) by their age 29 assessment within two broad classes: 1) Disinhibitory Behavioral Disorders: participants who met diagnostic criteria for any childhood disruptive disorder (ADHD, ODD, CD), or adult antisocial behavior (AAB). 2) Substance Dependence: participants who satisfied criteria for alcohol, nicotine, or illicit drug dependence.
Individuals were assigned to these seven diagnostic groups without regard for possible comorbid diagnoses to produce representative samples of individuals with these conditions; they were thus not mutually exclusive. To simplify secondary analyses and presentation of some of the results, an additional composite group (‘Any-EXT’) was created reflecting those participants who met diagnosis for any of the seven EXT disorders (i.e., ADHD, ODD, CD, AAB, dependence to alcohol, nicotine, or illicit drugs). Finally, a Control group was formed consisting of subjects who had none of the seven EXT disorders, including possible dependence for any licit or illicit substances.
Psychophysiological Assessment
ERP data were acquired as part of a battery of psychophysiological tasks administered to twin participants during at ages 17 and 29. During the 12-year interval elapsing between assessments, the psychophysiological laboratory instrumentation was upgraded as described below.
EEG procedures common to both age 17 and age 29
During both assessments, the rotated-heads oddball paradigm (Begleiter, Porjesz, Bihari, & Kissin, 1984) was used to elicit the P3 response. In-house software written in Pascal was used to control stimulus delivery at the age 17 assessment, whereas at the age 29 assessment stimulus delivery was achieved by means of a script written in E-Prime 1.1 software (Psychology Software Tools, Pittsburgh, PA). During recording, subjects were instructed to respond as quickly and accurately as possible by pressing either a left or right button when target stimuli appeared on a computer monitor. Targets consisted of infrequently occurring schematics of heads with a nose pointed vertically up or down on the screen and only one ear represented on either the left or right side. “Easy” targets (n = 40) consisted of heads with noses pointed towards the top of the screen with the left or right ear appearing directly on the side corresponding to the correct response button. In contrast, “hard” targets (n = 40) consisted of heads rotated 180° so that the nose pointed downward and with either left or right ear appearing on the head corresponding to the opposite response button. Subjects were also instructed to ignore frequently-occurring, nontarget stimuli which consisted of ovals (n = 160) interspersed between target trials. All stimuli were displayed for 100 ms, with intertrial intervals randomly varying between 1 and 2 s following a 1.5-s response period.
Age 17 EEG procedure
EEG activity for each participant was recorded from three parietal electrodes using an electrode cap: one over each hemisphere (P3 and P4) and one at the midline scalp (i.e., site-Pz). Linked earlobes served as reference while an electrode on the right shin served as ground. Electrooculographic (EOG) data were recorded from a pair of electrodes: one at the supraorbital ridge and one on the outer canthus of the eye. Impedances were kept below 5 kilohms for scalp electrodes and below 10 kilohms for EOG recordings. All data were acquired using a Grass Model 12A Neurodata system and filtered with a passband of 0.01 to 30 Hz (half-amplitude). Data were digitized with 12 bits of resolution at a rate of 256 samples per second.
Age 29 EEG procedure
EEG was recorded using the ActiveTwo BioSemi electrode system (BioSemi, Amsterdam, the Netherlands) from 61 scalp electrodes, including the three parietal electrodes assessed at age 17 (i.e., P3, Pz, P4). The data were digitized at 1024 Hz with a pass-band from DC to 205 Hz. In addition, four monopolar leads recorded EOG activity to derive horizontal and vertical bipolar channels indexing eye movements and blinks: two leads placed on the outer canthi of each eye and two placed above and below the right eye. Referencing and grounding arrangements for the Biosemi system involves a Common Mode Sense active electrode and a Driven Right Leg passive electrode which form a feedback loop. After data acquisition, all data were re-referenced to the average activity at the two ears.
ERP data processing
Age 17 assessment
EEG waveforms were filtered using a 7.5-Hz lowpass zero-phase digital filter (3 dB down) in order to reduce high frequency artifacts not eliminated by online (analog) filters. Corrections for eye blinks as well as other ocular artifacts were made offline using standard regression-based procedures (Gratton, Coles, & Donchin, 1983). ERP data were carefully checked for outliers based on distributional inspections of P3 latency and amplitude values as well as extreme task performance errors. If P3 data could not be re-scored in cases where data were anomalous (e.g., 4 standard deviations from group mean), these subjects were dropped from the study. A total of five subjects were dropped for these reasons (three controls, two subjects with EXT).
Age 29 assessment
EEG was processed offline in Matlab (version 6.5, Mathworks) using functions contained in the EEGLAB toolbox (Delorme & Makeig, 2004) and in-house scripts. Continuous data were downsampled to 256 Hz and highpass filtered at 0.1 Hz (6 dB down) using a finite impulse response filter with a Kaiser window of order 1286 and 0.0001 dB maximum passband ripple using the firfilt plugin to EEGLAB. Trial-level, averaged, and overall spectral qualities for each subject electrode were visually inspected and scalp data were replaced through spherical-spline interpolation (Perrin, Pernier, Bertrand, & Echallier, 1989) if deemed not to be EEG. Blink artifacts were identified with a custom routine based on temporal and spatial cross-correlations. For this, Infomax independent components analysis was performed on each dataset, and independent components (ICs) were classified as blink-related and subtracted from the data if 1) correlations between bipolar vertical EOG and IC time-series exceeded a threshold of 0.70, and 2) correlations between IC electrode inverse-weights (scalp maps) and a spatial template exceeded 0.75. Epochs spanning 1-s before stimulus onset to 2-s afterward were extracted, and the mean of a 200-ms baseline period immediately preceding stimulus onset was subtracted from the raw data. Whole epochs with too much contamination from muscle or other transient causes (cap movement, “pops”, etc.) were detected by means of deviant spectral qualities or kurtosis, respectively (Delorme, Sejnowski, & Makeig, 2007) and removed from the dataset. Prior to averaging, epochs were lowpass filtered at 55 Hz and individual electrode-epochs were excluded if values exceeded a ±75 μV threshold within the region of interest (−200 to 1000 ms).
Time-domain P3 amplitude peak identification
For the present investigation, ERP data from the site-Pz electrode recorded at both the age 17 and age 29 assessments were chosen for analysis because of its importance in the P3 literature (Hill, Muka, Steinhauer, & Locke, 1995; Hill, Steinhauer, Lowers, & Locke, 1995; Hill & Steinhauer, 1993; Porjesz & Begleiter, 1990; Steinhauer & Hill, 1993) and because it allowed for comparisons at a common electrode site between the two EEG systems at the two assessments. P3 amplitude peak was identified algorithmically using a “peak-in-window” approach specifying the positive apex within a specified time range (from 275–625 ms post-stimulus onset) P3 latency was defined as the time interval between stimulus onset to waveform apex in milliseconds. Although a variety of P3 amplitude identification procedures exist, the current approach has been used extensively in numerous studies examining disinhibitory disorders, including those using MTFS samples (e.g., Iacono et al., 2002; Yoon et al., 2013).
Time-frequency PCA
ERPs were decomposed into time-frequency (TF) energy distributions according to techniques outlined in Bernat et al. (2005; Bernat et al., 2007). TF techniques permit characterizing ERP waveform morphology in terms of joint frequency and energy components, as opposed to analyzing these signals in time or frequency alone. In particular, in the present investigation, we computed TF distributions (TFDs) from averaged ERP waveforms, which facilitate representation of those TF features most consistently phase-locked to the experimental event (i.e., stimulus onset). To derive these surfaces, we used the Reduced Interference Distribution (or RID) transform from Cohen’s class of transforms (Williams, 2001), which produces uniform resolution in both time and frequency and demonstrates clear advantages in this respect over other TF transforms (cf. Bernat et al., 2005). To reduce the dimensionality of TF data, we conducted principal components analysis (PCA) of the TFDs (i.e., TF-PCA) on a specified window (1 to 1000 ms, DC to 12 Hz) to reduce them to a few dimensions (detailed in Bernat et al., 2005). PCA was applied to the covariance matrix followed by a varimax rotation to maximize simple structure. To determine the TF-PC energy at site-Pz for each subject, element-wise multiplication of the loadings from the TF-PCs and original TF surfaces were carried out with each of the resulting surfaces being a weighted matrix for a given component of the same dimensionality as its input. These extracted TF components were then compared between participants with EXT disorders and controls using component scores representing peak energy on the weighted TF data surface (i.e., the TF point with the highest energy). This method was similarly used by Gilmore et al. (2010) in an investigation of EXT disorders and demonstrated that it allows for evaluations between the TF (peak energy) and time (peak P3 amplitude) domains.
Task performance
Previous investigations with this sample at both age 17 (Gilmore, Malone, Bernat, et al., 2010; Iacono et al., 2002) and age 29 (Yoon et al., 2013) found that task performance measures did not differentiate those with EXT disorder from controls. Nevertheless, we re-evaluated these measures for the subsample with data at both ages included in the current investigation. Three task performance measures were evaluated: False alarms constituted the number of nontarget stimuli (160 ovals) incorrectly identified as targets. Reaction time was defined as the average time subjects took to make a button press to identify target stimuli. Total hits reflected the number of both easy and hard targets correctly identified from the 80 presented.
In line with the report from Yoon et al. (2013), the effect of the most comprehensive diagnostic grouping variable (i.e., Any-EXT by age 29) on the three task performance measures was assessed in an ANOVA with group as the sole fixed effect. These task performance measures were skewed. Thus, prior to analyses, false alarm and reaction time data were log transformed whereas the proportion of hits data underwent arcsine transformation. Data were missing for one participant.
Participants Available for EEG Analyses
Of the 578 male twin participants first assessed at age 17, 501 participants had usable EEG data at that age (Iacono et al., 2002). Among this group with usable EEG data 443 (88%) returned for an in-person assessment at approximately age 29, with the remainder participating by phone because they no longer resided within easy driving distance to our laboratory. Among these 443 visiting participants, 39 subjects had to be dropped from analyses at the time of this investigation due to technical or equipment problems (e.g., common mode ground electrode out of range, EEG recording equipment failure), three subjects had incomplete diagnostic data, and 15 subjects at the age 29 assessment were dropped due to excessive artifacts whereby more than 10% of channels required interpolation. Furthermore, due to the nature of our sample criteria, 60 subjects were not assigned to either the diagnostic or control groups. These subjects were either only one symptom short of meeting DSM criteria for a disorder or met criteria for substance abuse but not dependence. Hence they failed to meet our criteria for any of the EXT groups, and given their elevated symptom status, were also considered ineligible for the Control group. Finally, 67 participants were excluded because they did not have EEG data at both the age 17 and age 29 assessments. Thus, 259 (58%) of the 443 available participants with both lifetime diagnostic and EEG data were included for final analysis.
To determine if these 259 participants differed from those who were not in the initial age 17 (intake) dataset, comparisons were made on two intake variables: P3 amplitude and EXT diagnosis. P3 amplitude data was obtained for 501 participants at study intake at site-Pz using the same ERP eliciting task described previously and elsewhere (cf. Iacono et al., 2002). Among those with age 17 P3 data, 259 participants who were included in the current study were compared to the 242 who were not included. Furthermore, to evaluate the possibility that those who were included and excluded in the current study differed in their likelihood of ever having been diagnosed with an EXT disorder at intake, the proportions of subjects who were ever diagnosed in the two groups were also compared.
The effect of the grouping variable (participating vs. non-participating subjects) on intake P3, was assessed using an ANOVA with group as the sole fixed effect. A chi-square analysis was performed to determine whether the proportions of EXT diagnoses varied between the two groups.
Statistical analyses
To index the rank-order stability of the P3-derived measures, correlations (Pearson’s r) were computed for P3 amplitude and for each of the analogous TF-PCs measured at the age 17 and age 29 assessments. For group comparisons, a series of separate 2 (Age [17, 20]) × 2 (Group [each of seven EXT groups and the Any-EXT composite group, controls]) ANOVAs were conducted using linear mixed models (LMM) as implemented in PROC MIXED in SAS. The dependent measures were all P3-derived measures (i.e., P3 amplitude, P3 latency, and the various TF-PCs). Each LMM included two random effects in addition to the fixed effects just described. A random effect of assessment age was modeled at the individual subject level, resulting in a 2 × 2 covariance matrix of freely estimated random effects, corresponding to a MANOVA model. A random effect of assessment age was also modeled at the twin-pair level, which accounts for the correlation between twins in each dependent measure as well as twin similarity in stability over time. In addition to allowing us to accommodate non-independence represented by twins, the LMM approach uses all available data, unlike standard repeated measures ANOVA, an approach that is appropriate when data are missing at random (Little, 2002).
All univariate comparisons (participation analyses, task performance, etc.) were also performed through a series of ANOVAs, treated as LMMs, with a random intercept to account for the correlation between members of a twin pair with respect to each dependent measure. A significance criterion of alpha < .05 was set for every test. In order to obtain effect sizes, we converted the mean difference between controls and comparison group for each of the two assessment ages examined in the MANOVAs into statistics analogous to Cohen’s d, using a standard formula for converting t-statistics to d (Rosenthal, 1991).
In order to determine whether time-domain P3 amplitude and associated TF-PCs observed at age 17 predicted the development of new instances of EXT by age 29, participants who were identified as psychiatrically healthy controls (i.e., they had no EXT disorder) at the age 17 intake assessment were divided into those who continued to remain disorder-free (n = 74) and those who subsequently met diagnosis for an EXT disorder by age 29 (n = 73). Univariate comparisons between these two groups were made using the ERP measures at age 17 to determine if the subsequently affected group had already evidenced anomalies at age 17. A similar analysis was carried out using age 29 data to determine how the two groups compared when the outcome diagnosis was known. In addition, the equivalent of logistic regression was conducted by means of generalized estimating equations (GEE) in SAS PROC GENMOD in order to quantify the degree to which a reduction in P3 amplitude or TF-PC energy at age 17 predicted EXT status at age 29, while accounting for the correlation between twins.
Results
Task performance
Consistent with expectations from past (cf. Iacono et al., 2002) as well as more recent reports (Yoon et al., 2013), results revealed that none of the three task performance measures (false alarms, reaction time, total hits) differed significantly between controls and those with any EXT disorder (all Fs < 0.68, all p-values > .409).
Participation effects
ANOVA results indicated that intake P3 amplitude did not significantly differ between participants who were included versus excluded in this study: F(1, 292) = 0.50, p = .480. Furthermore, the percentages of participants with any of the seven lifetime EXT diagnoses in each of the two groups were 47.6% (excluded) and 52.4% (included), respectively, a non-significant difference, χ2 (1, N = 485) = 0.3, p = .607. Overall, these analyses suggest that included participants did not differ from nonparticipants on key measures, and thus the participating group does not appear to represent a biased subset of the intake sample.
Time-Domain
Table 1 displays descriptive statistics (means, standard deviations) associated with both the age 17 and age 29 P3 amplitude peak/latency values at site-Pz. The omnibus F- and p-values associated with statistical comparisons for the various EXT groups, including those diagnosed with any EXT disorder by age 29 (i.e., Any-EXT), are also presented in this table. Figure 1 (top) displays, among other features, the grand average waveforms at ages 17 and 29. The effect sizes corresponding to the mean comparisons for each of the EXT groups as well as the composite Any-EXT group versus controls are displayed Figure 2. Lastly, for summary purposes, the grand average P3 waveform associated with the Any-EXT and control groups at each age are displayed in Figure 3.
Table 1.
Mean (SD) associated with time domain P3 peak amplitude (μV), and P3 latency (ms) by assessment age, and results of statistical comparisons
| P3 Amplitude & Latency by Assessment Age | ANOVA results | |||||
|---|---|---|---|---|---|---|
| Age-17 Mean (SD) |
Age-29 Mean (SD) |
Group (G) | Age (A) | G x A | ||
| p-values | ||||||
| Controls (n = 74) | Amplitude | 27.14 (7.22) | 16.05 (3.73) | ________ | ________ | ________ |
| Latency | 455.01 (57.93) | 394.26 (38.18) | ________ | ________ | ________ | |
| ADHD (n = 19) | Amplitude | 24.43 (5.18) | 12.10 (3.84) | .089 | <.001 | .413 |
| Latency | 450.21 (60.79) | 419.03 (56.05) | .455 | <.001 | .048 | |
| ODD (n = 30) | Amplitude | 22.74 (7.65) | 12.80 (3.63) | .002 | <.001 | .565 |
| Latency | 444.61 (60.23) | 398.08 (38.73) | .955 | <.001 | .279 | |
| CD (n = 62) | Amplitude | 22.25 (7.82) | 12.97 (5.02) | <.001 | <.001 | .166 |
| Latency | 446.13 (65.73) | 396.52 (42.22) | .617 | <.001 | .333 | |
| AAB (n = 45) | Amplitude | 21.36 (6.68) | 12.53 (3.96) | <.001 | <.001 | .069 |
| Latency | 442.56 (72.46) | 393.61 (40.18) | .437 | <.001 | .302 | |
| Alcohol (n = 115) | Amplitude | 23.59 (7.37) | 13.74 (3.97) | <.001 | <.001 | .205 |
| Latency | 450.07 (61.73) | 396.97 (43.24) | .937 | <.001 | .461 | |
| Nicotine (n = 113) | Amplitude | 23.33 (7.13) | 13.31 (4.40) | <.001 | <.001 | .386 |
| Latency | 454.56 (66.26) | 398.02 (44.61) | .723 | <.001 | .859 | |
| Illicit Drugs (n = 45) | Amplitude | 23.50 (7.24) | 13.34 (3.98) | <.001 | <.001 | .453 |
| Latency | 452.22 (69.06) | 396.91 (41.64) | .649 | <.001 | .599 | |
| Any-EXT (n = 185) | Amplitude | 23.57 (7.19) | 13.56 (4.29) | <.001 | <.001 | .302 |
| Latency | 454.91 (62.59) | 399.25 (43.95) | .407 | <.001 | .784 | |
Note: The reported sample sizes for the various groups reflect lifetime diagnostic status by age 29. ADHD = Attention-Deficit Hyperactivity Disorder; ODD = Oppositional Defiant Disorder; CD = Conduct Disorder; AAB = Adult Antisocial Behavior; Alcohol/Nicotine/Illicit Drugs = meeting lifetime dependence for these substances by age 29; Any-EXT = participants who met lifetime diagnosis for any of the seven EXT disorders in the study by age 29.
Figure 1.
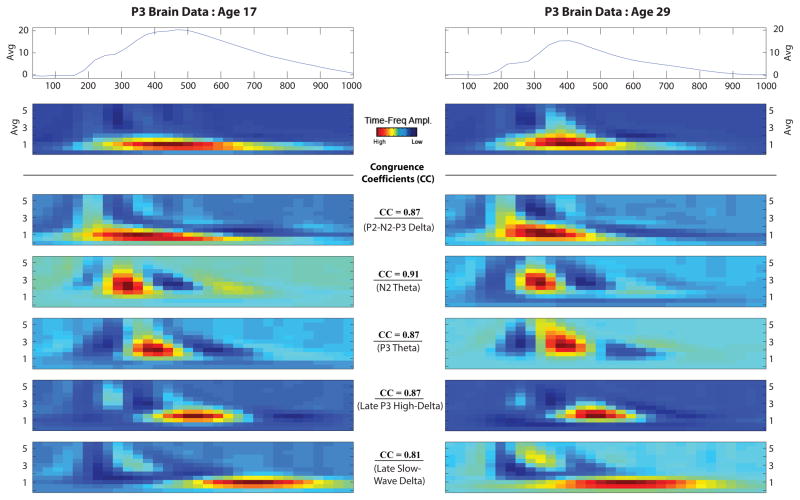
Grand average waveforms and time-frequency component alignment from the age 17 and age 29 decompositions. Grand-averaged time-domain and time-frequency (Avg) plots derived from participant data (N = 259) are presented at the top and displayed corresponding to assessment age. The five time-frequency components retained from the principal components analysis decomposition are presented below the grand averages and are arranged by ERP time course for presentation purposes. For all time-frequency plots, x-axis is time from stimulus onset (0 ms) to 1000 ms, and y-axes range from 0 – 5.75 Hz. Analogous TF-PCs derived at age 17 (left panel) and age 29 (right panel) were aligned based on patterns of TF-PC peak correlations and cross-factor loadings. Congruence coefficients (CC) associated with the TF-PC pairings are also indicated.
Figure 2.
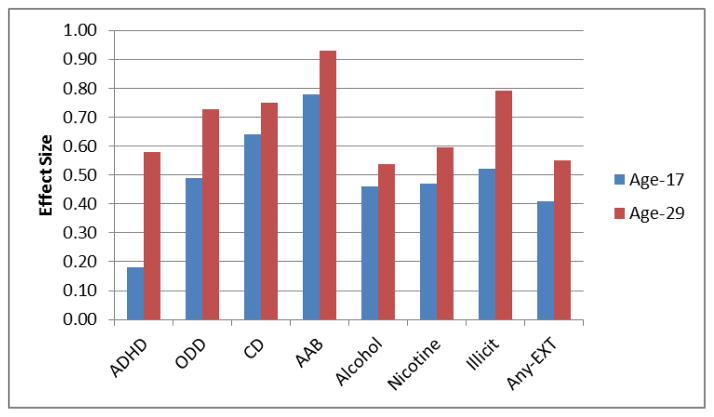
Effect size graph for each EXT group and the composite Any-EXT comparisons on time-domain P3. Effect sizes corresponding to the P3 amplitude comparisons between each of the seven EXT disorder groups as well as the composite Any-EXT group versus controls are displayed. Effect sizes were obtained by converting the mean microvolt (μV) difference between controls and the EXT groups for each of the two assessment ages examined in the MANOVAs into statistics analogous to Cohen’s d, using a standard formula for converting t-statistics to d (Rosenthal, 1991). Illicit refers to any participant meeting lifetime diagnosis for dependence to any of the following: amphetamines, cannabis, cocaine, hallucinogens, inhalants, opioids, phencyclidine, and sedatives. Any-EXT refers to any participant meeting lifetime diagnosis for an EXT disorder by age 29.
Figure 3.
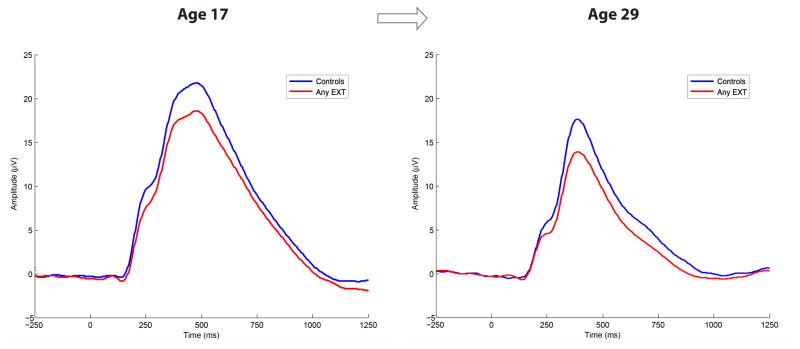
Grand average waveforms for time-domain P3 coinciding with the composite Any-EXT group (n = 185) and controls (n = 74) at the age 17 and age 29 assessments.
Grand average waveform activity associated with target trials for any participant meeting lifetime diagnosis for an EXT disorder by age 29 (i.e., Any-EXT; n = 185) and control groups (n = 74) are displayed corresponding to both the age 17 and age 29 assessments.
Amplitude
Although P3 amplitude was smaller at age 29 than at age 17, P3 amplitude showed strong rank-order stability over this 12-year span (r = 0.50, p < .01).Table 1 indicates that peak amplitude significantly declined by age 29 across all groups, all Fs > 204.26, all ps < .001. Table 1 additionally reveals that, with the exception of ADHD, which trended in the hypothesized direction, F(1, 86) = 3.19, p = .089, significant group differences in P3 amplitude were seen between all EXT groups and controls, all Fs > 10.80, all ps < .001. Figure 3 illustrates the reduction evident in the grand average waveform associated with the Any-EXT group compared to controls at each assessment. Figure 2 illustrates that although the magnitude of effects related to P3 differences between the EXT groups and controls may be comparable over the approximate twelve-year assessment span, there were consistently stronger effects observed at age 29. Finally, there were no significant interactions detected from the Group x Age comparison, all Fs < 3.37, all ps > .069 (Table 1).
Latency
P3 latency did not significantly differ between the EXT groups and controls, all Fs < 3.58, all ps > .069. In line with previous observations with this sample (e.g., Katsanis, Iacono, & McGue, 1996) significant main effects for age were detected across all comparisons, all Fs > 44.66, all ps < .001, indicating that latencies decreased from age 17 to age 29. Finally, with the exception of the ADHD group, F(1, 81.7) = 4.02, p = .048, there were no significant Group x Age interactions, all Fs < 1.19, all ps > .279. The nature of the ADHD x Age interaction was further explored by inspecting group means, profile plots, as well as by conducting univariate ANOVAs that compared the groups at each assessment age. Latencies decreased in both groups from age 17 to age 20. However, this decrease was more pronounced for the Control participants (Mlatency at 17 = 457.19 ms; Mlatency at 29 = 394.24 ms) than those with ADHD (Mlatency at 17 = 450.21 ms; Mlatency at 29 = 419.03 ms). Furthermore, results of the univariate analyses indicated that although the latencies did not differ significantly between participants with ADHD and controls at age 17, F(1, 159) = 0.13, p = .717, this difference trended towards significance by age 29, F(1, 86.1) = 3.90, p = .052.
Time-Frequency PCA/component analyses
The TF-PCA conducted on the participants’ intake data resulted in a five component solution that accounted for approximately 77% of the variance. This component solution was chosen by scree plot where visual inspection determined a break in singular values that indicated a point at which the amount of explanatory variance became negligible. Similar TF components accounting for approximately 83% of the variance were also retained from participants’ age 29 data. Figure 1 displays the paired age 17 and age 29 PC decompositions arranged by ERP time course for presentation purposes. These pairings were based on patterns of TF-PC peak correlations and cross-factor loadings. The correlations for TF-PC energy for each pairing were all significant (ps < .001) and greater than 0.50 (median correlation = 0.55). The absolute values of the associated congruence coefficients (CCs; presented in Figure 1) were large for those pairs (all CCs greater than 0.81; median CC = 0.87).
Time-frequency group analyses
With time-domain results revealing broad and significant reductions for P3 amplitude across the EXT groups, time-frequency comparisons were made using the composite Any-EXT group for ease of presentation. Table 2 provides descriptive statistics (means, standard deviations) for the mean weighted energy unit values for the TF pairs at each age, and the p-values associated with comparisons between Any-EXT and controls. Effect sizes for these comparisons are provided in Figure 4.
Table 2.
Mean (SD) for weighted energy units for analogous time-frequency components between age-17 and age-29, and results of statistical comparisons between Controls (n = 74) and the Any-EXT group (n = 185) on those components.
| Weighted energy units for analogous TF-PCs by age | ANOVA results | |||||
|---|---|---|---|---|---|---|
| Age-17 Mean (SD) |
Age-29 Mean (SD) |
Group (G) | Age (A) | G x A | ||
| p-values | ||||||
| P2-N2-P3 Delta | Controls | 54.61 (29.14) | 27.66 (14.44) | <.001 | <.001 | .079 |
| Any-EXT | 40.82 (23.70) | 19.63 (13.01) | ||||
| N2 Theta | Controls | 29.76 (17.45) | 19.23 (12.36) | <.001 | <.001 | .638 |
| Any-EXT | 23.66 (14.57) | 14.04 (10.86) | ||||
| P3 Theta | Controls | 39.84 (24.35) | 21.38 (11.45) | <.001 | <.001 | .398 |
| Any-EXT | 31.65 (19.23) | 15.36 (10.43) | ||||
| Late P3 High-Delta | Controls | 52.19 (28.42) | 24.92 (13.06) | <.001 | <.001 | .031 |
| Any-EXT | 40.04 (22.82) | 19.29 (12.72) | ||||
| Late Slow-Wave Delta | Controls | 39.84 (21.97) | 18.71 (11.19) | .002 | <.001 | .104 |
| Any-EXT | 31.11 (20.29) | 14.15 (9.56) | ||||
Note. Any-EXT = participants who met lifetime diagnosis for any of the seven EXT disorders in the study by age 29.
Figure 4.
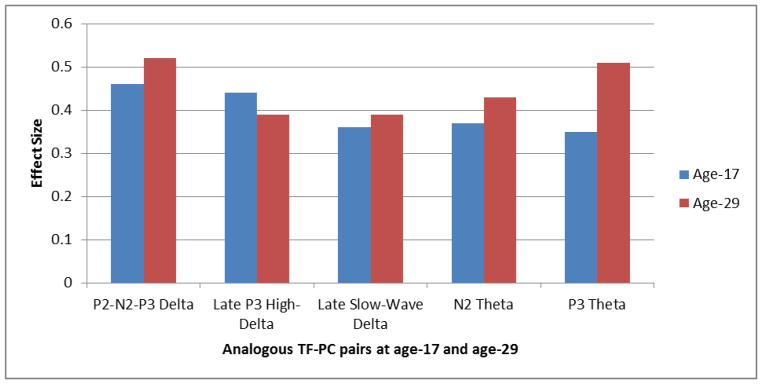
Effect size graph for the Any-EXT vs. Control comparisons on the analogous TF-PC pairs at age 17 and age 29. Effect sizes corresponding to the composite Any-EXT group (i.e., any participant meeting lifetime diagnosis for an EXT disorder by age 29; n = 185) versus Controls (n = 74) are displayed for analogous TF-PC pairs at age 17 and age 29. Effect sizes were obtained by converting the mean weighted energy unit difference between controls and the Any-EXT group for each of two assessment ages examined in the MANOVAs into statistics analogous to Cohen’s d, using a standard formula for converting t-statistics to d (Rosenthal, 1991).
A significant effect for age was detected across all comparisons for all TF-PCs, (all Fs > 91.77, all ps < .001). Similar to the time-domain results, TF signals generally decreased over age (Table 2). Table 2 also shows that, in line with the time-domain findings, group comparisons were consistent with significant reductions observed for all five TF-PCs in the Any-EXT group across the assessment periods, all Fs > 10.31, all ps < .002. The age-related effect sizes across the TF-PCs were in line with the patterns observed in the time-domain, with increasing effects generally observed from age 17 to age 29: median effect size (d) by assessment: age 17 = 0.37 (range: 0.35 – 0.46); age 29 = 0.43 (range: 0.39 – 0.52). The exception to this trend was the Late P3 High-Delta component in which a slightly larger effect size was observed at age 17 (d = 0.44) than at age 29 (d = 0.39). Overall, the largest effects were observed for two frequency components at age 29 coinciding with the P3-ERP activity: P2-N2-P3 Delta (d = 0.52), P3 Theta (d = 0.51), respectively reflecting a 12% and 31% increase in effect from age 17.
Finally, although most comparisons assessing the interaction between the Any-EXT Group x Age were not significant, all Fs < 3.12, all ps > .079 (Table 2), a significant interaction pertaining to the Late P3 High-Delta component was detected, F(1, 235) = 4.72, p = .031 indicating that the weighted energy units for both controls and the Any-EXT groups decreased between age 17 and age 29. However, the group mean differences at age 17 (mean difference = 22.99) were greater than at age 29 (mean difference = 18.01), coinciding with the unique feature of this component in terms of effect sizes (Figure 4) which indicated a stronger effect at age 17 (d = 0.44) than at age 29 (d = 0.39).
Predicting New Cases of EXT in Age 17 Non-EXT Controls who Developed EXT by Age 29
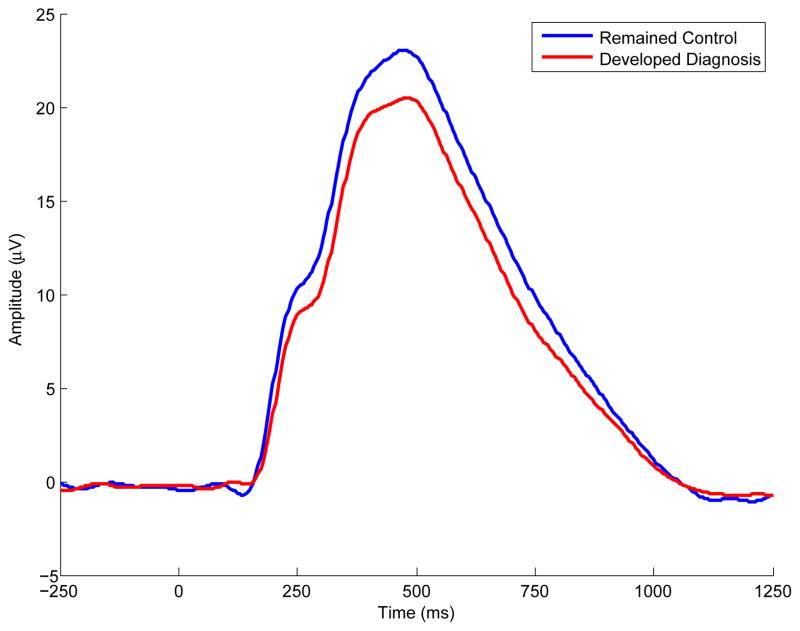
Table 3 presents descriptive statistics (mean, standard deviation) for P3 amplitude and the TF-PCs associated with age 17 control participants who developed EXT (n = 74) or remained EXT-free (n = 73) by age 29. Results of the t-tests for P3 amplitude confirmed that despite their healthy status as members of the control group at intake (age 17), significant reduction in P3 was already apparent at age 17 in those eventually meeting diagnosis for any EXT disorder by age 29 compared to participants who remained free of any EXT disorder, F(1, 142) = 4.56, p = .034 (ES = 0.36). The age 17 results are highlighted in Figure 5 which illustrates the reduction in the grand average waveform associated with developing EXT subsequent to the age 17 assessment. Furthermore, Table 3 reveals that significant reductions at age 17 were also observed for two time-frequency components at the delta range between participants developing an EXT disorder or remaining EXT-free by age 29, Fs > 4.47, ps < .036. Figure 1 depicts these two components which coincided with the P2-N2-P3 complex (P2-N2-P3 Delta) and the downslope of the ERP waveform (Late Slow-Wave Delta).
Table 3.
Descriptive statistics and t-test results comparing intake (age 17) control cases who did or did not develop EXT by age 29 on both time-domain and time-frequency brain components.
| Identified as Controls at Age-17 and by Age-29… | Results of logistic regression analyses using age 17 brain components to predict EXT status by age 29 | |||||||
|---|---|---|---|---|---|---|---|---|
| Age-17 Brain Component | Remained Controls (n = 74) | Developed an EXT disorder (n = 73) | t-test Results | Effect Size | ||||
| Mean (SD) | Mean (SD) | p-values | Odds Ratio (OR) | OR 95% CI | p-value | |||
| P3 Amplitude | 27.14 (7.22) | 24.46 (7.61) | .034 | .359 | 1.050 | 1.004 | 1.098 | .032 |
| P2-N2-P3 Delta | 54.61 (29.14) | 44.68 (27.88) | .036 | .354 | 1.012 | 1.001 | 1.024 | .039 |
| Late P3 High-Delta | 52.19 (28.42) | 43.58 (25.69) | .060 | .326 | 1.012 | 0.999 | 1.025 | .059 |
| Late Slow-Wave Delta | 39.84 (21.97) | 31.68 (19.58) | .022 | .392 | 1.019 | 1.003 | 1.036 | .022 |
| N2 Theta | 29.76 (17.45) | 25.57 (17.14) | .130 | .253 | 1.014 | 0.995 | 1.034 | .146 |
| P3 Theta | 39.84 (24.35) | 35.05 (23.87) | .217 | .206 | 1.008 | 0.995 | 1.022 | .231 |
Note. The odds ratios (OR) represent the increase in odds of developing an EXT disorder for every unit decrease in the particular brain response. Thus, for P3 amplitude (OR = 1.050) every microvolt (μV) decrease was associated with a 5% increase for developing EXT.
Figure 5.
Grand average waveforms for controls at age 17 who either remained controls or developed EXT by age 29. Grand average waveforms associated with target trials measured during intake (age 17) assessment are presented. The two waveforms coincide with participants identified as controls at age 17 who either eventually met diagnosis for any EXT disorder, or, remained free of any EXT disorder approximately twelve years later.
Results of the GEE analysis provide a complementary characterization of these significant effects pertaining to P3 amplitude (χ2(1, N = 147) = 4.59, p = .032), P2-N2-P3 Delta (χ2(1, N = 147) = 4.27, p = .039), and Late Slow-Wave Delta (χ2(1, N = 147) = 5.29, p = .022) by quantifying the increase in odds of developing an EXT disorder per unit of decrease in the P3-derived measure. For instance, the P3 amplitude results (Table 3) produced an odds ratio (OR) of 1.050 (95% CI [1.004, 1.098]), indicating that a one microvolt (μV) decrease in P3 amplitude was associated with a 5% increase in the odds of developing an EXT disorder. Furthermore, an approximate 1–2% increase in odds for developing EXT were noted for each weighted energy unit reduction associated with the P2-N2-P3 Delta and Late Slow-Wave Delta components, respectively.
Discussion
This study presented a longitudinal assessment of P3-derived brain measures in a male community sample at uniform age to evaluate their potential utility as developmental endophenotypes for EXT through adulthood. The results of this study extend our previous report (Yoon et al., 2013) as well as those from our colleagues (Carlson et al., 2007; Gilmore, Malone, Bernat, et al., 2010; Iacono et al., 2002) to show that both time-domain P3 amplitude as well as TF-PCs at the delta and theta ranges reflect stable brain indices related to EXT spectrum disorders. These measures yielded robust correlations across the 12-year assessment span and successfully differentiated EXT groups from controls at both age 17 and age 29, with stronger group effects generally found at age 29. Furthermore, this study was unique in evaluating and quantifying the predictive utility of P3 amplitude as well as TF-PCs as markers of EXT when first assessed during adolescence and again 12 years into adulthood. Overall, these findings lend further support to the notion that these neurophysiological measures are tapping into a neural substrate underlying behavioral disinhibition.
Time-domain results
P3 continues to be a psychometrically-sound brain measure that shows developmental stability and criterion validity for lifetime EXT disorders. For instance, the 12-year rank order stability correlation for P3 amplitude was significant and robust (r = 0.50, p < .001), especially notable given that brain data were gathered using two different recording EEG systems at age 17 and age 29. Results further showed that despite an apparent decrease in mean P3 amplitude between age 17 and age 29, P3AR effectively discriminated between participants with lifetime EXT disorder compared to controls. The one exception was the comparison for ADHD participants (n = 19) where the results trended in the expected direction but did not meet statistical significance (p = .089). However, for all group comparisons, effect sizes were larger in magnitude at age 29 than seen at age 17 (see Figure 2). This observation was contrary to our hypothesis which was informed in part by a meta-analysis of high-risk studies which suggested that larger effect sizes are typically seen in younger (< 18) compared to older subjects (Polich, Pollock, & Bloom, 1994).
In light of these results, this study does not support the findings by Hill et al., 1999 which suggest that P3AR is a developmentally-limited indicator of EXT that shows diminishing utility past late childhood. Furthermore, the P3-EXT associations presented in our study were not likely due to the neurotoxic effects of substance use which has been suggested by other investigations, particularly if P3 reduction is observed in later ages (Hill et al., 1999; Hill et al., 2009). Previous reports from our lab (Iacono et al., 2002; Malone, Iacono, & McGue, 2001), including a prospective study (Perlman et al., 2013) and a study examining whether exposure to alcohol moderated the heritbaility of P3 (Perlman et al., 2009), did not provide evidence indicating that substance use per se causes reduced P3. Most relevant to the current report, Yoon et al. (2013) evaluated a larger sample of men from which participants from the current investigation were drawn, and found that neither recent (past 24-hour) nor cumulative use of psychoactive substances (alcohol, nicotine, illicit street drugs) likely accounted for time-domain/time-frequency differences observed in participants diagnosed with any EXT disorder by age 29. Collectively, these studies suggest that significant P3 reduction does not appear to reflect effects of substance exposure but rather to reflect familial risk for substance misuse, which is consistent with previous investigations (Costa et al., 2000; O’Connor, Hesselbrock, & Tasman, 1986; Pfefferbaum, Ford, White, & Mathalon, 1991). Instead, time-domain P3AR continues to characterize participants assessed through adulthood; indexing a range of lifetime EXT disorders, including diagnoses made during childhood (i.e., ADHD, ODD, CD). Our findings further support the view that P3AR is a proxy for genetic risk for EXT in participants who have not yet fully passed the age of risk for substance use disorders. Perhaps the strongest evidence for this assertion is the finding that subjects initially classified at age 17 as non-EXT controls who went on to receive a diagnosis by age 29 displayed significant P3AR at age 17 compared to those who remained free of EXT (see Table 3 and Figure 5).
Time-frequency results
Time-frequency decomposition of the ERP waveform produced findings that complemented those observed for time-domain P3 amplitude. TF-PCs in both the delta and theta frequency ranges successfully indexed EXT cases from late adolescence to age 29, the end of what might be considered early adulthood. Such observations support and extend the cross-sectional evaluation of this sample at age 17 (Gilmore, Malone, Bernat, et al., 2010) and at age 29 (Yoon et al., 2013) by showing that these TF-PCs reflect stable (median r = 0.55) neurophysiological measures between those ages. In line with those reports, we demonstrated that delta components coinciding with the later peak of P3 (Late P3 high-delta) and the P2-N2-P3 complex (P2-N2-P3 delta) both served as broad indices of EXT. In particular, the P2-N2-P3 delta comparisons were associated with the largest effects observed across all TF-PCs (Figure 4), and comparable to the time-domain peak effects observed for the Any-EXT group (see Figure 2). Furthermore, this component, along with another delta component coinciding with the late slow-wave, displayed predictive utility with significant reduction characterizing those receiving a diagnosis for an EXT disorder by adulthood despite being characterized as a control participant over a decade earlier.
In addition to the TF-PCs in the theta range which further provided effective indices of EXT (P3-theta, N2-theta), the components evaluated in this study collectively canvassed much of the ERP waveform, including the N1 through the late slow-wave, indicating that the decomposition techniques offered further assessment of the potential neurophysiological abnormalities associated with EXT.
P3 amplitude as a developmental endophenotype
The results of this study provide further supporting evidence that P3AR is a developmental endophenotype for disorders characterized by behavioral disinhibition by expanding both the phenotypic and developmental scope of previous longitudinal P3-EXT prediction studies. For instance, although one investigation (Carlson et al., 2007) determined that P3AR was predictive of EXT broadly defined when assessed between age 17 through 24, most studies have focused on various substance use phenotypes such as age of drinking onset (Hill, Shen, Lowers, & Locke, 2000), a composite measure of substance use (Berman et al., 1993), as well as the subsequent development of substance use disorders (Carlson et al., 2004; Carlson et al., 2007; Habeych et al., 2005; Hill, Steinhauer, et al., 1995; Hill et al., 2009; Iacono et al., 2002; Perlman et al., 2013). Furthermore, the current study provides one of the longest assessment spans in a P3 investigation of EXT. Although Hill et al. (2009) provided a longitudinal P3 investigation with a comparable assessment span averaging 11.2 years, they evaluated SUD outcomes using a high-risk sample of mixed-aged participants. Thus, the finding that P3AR provides a predictive measure of EXT over a 12-year span into adulthood using a community-based and uniformed-aged sample expands the developmental scope of previous reports that have collectively documented these associations from childhood to adolescence (Hill et al., 2000) up through young adulthood (Habeych et al., 2005; Hill, Steinhauer, et al., 1995; Hill et al., 2009), or from preadolescence to adolescence (Berman et al., 1993; Perlman et al., 2013), and from adolescence to young adulthood (Carlson et al., 2004; Carlson et al., 2007; Iacono et al., 2002). Taken together, these investigations indicate that P3AR appears to reflect a consistent and stable brain feature of EXT risk across development.
Limitations
Although we noted robust and significant correlations among the P3 measures between age 17 and age 29, the group effects associated with these measures were nevertheless obtained using two different recording procedures between those ages. Thus, the P3 effects associated with development are confounded by a change in laboratory recording procedures over this same period. However, as noted in Tables 1 and 2, the interactions between the various EXT groups by assessment age were generally non-significant. Moreover, the observed reductions in P3 amplitude from age 17 to 29 were consistent with expectation given the existing literature on the effects of maturation on visual P3 amplitude. Also, any potential effects attributable to changing the EEG recording procedure do not detract from the general key findings that the P3 measures serve as broad indices of EXT at both ages. Another potential concern is that the P3 measurements reported in this study were obtained from a single posterior lead (site-Pz). Some studies suggest that P3 displays a posterior to frontal shift in topographic distribution during late adolescence (Bauer & Hesselbrock, 2001, 2003) and that P3 assessment at the frontal leads may offer more sensitive measures of EXT, especially in older subjects (Bauer, 2001; Bauer, O’Connor, & Hesselbrock, 1994; Costa et al., 2000; Kamarajan et al., 2005). However, a topographic assessment of participants drawn from the same cohort as the current investigation found that P3 and its time-frequency components displayed stronger association with EXT at age 29 when measured at parietal as opposed to frontal regions (Yoon et al., 2013). Also, with a newer diagnostic system available (DSM-5), concerns may be raised as to whether results from the current study, which defines EXT cases using DSM-III-R, are comparable. Although some differences exist, the symptom criteria for the various EXT disorders largely overlap suggesting that results would be similar under the two systems. Furthermore, although this longitudinal, community-based sample offers unique research opportunities, it is comprised of predominantly Caucasian twin participants from Minnesota, potentially limiting generalizability to samples similarly composed.
Finally, although the present study helps establish the utility of P3-derived measures as developmental endophenotypes, the utility of endophenotypes as tools to simplify gene finding is uncertain given the series of reports from the MTFS indicating that psychophysiological measures are themselves vastly polygenic, with many genetic variants each contributing very small effects (see review Iacono et al., 2014). Nonetheless, the finding that these brain indices serve as reasonably stable, consistently robust, and predictive markers of EXT risk across development helps better position these measures as supplementary tools that can inform insights into pathophysiology once disorder-relevant genetic variants have been found (de Geus, 2010, 2014; Munafo & Flint, 2014). Furthermore, with their enduring endophenotypic properties, these P3 measures are well-positioned to offer more refined analyses with respect to the disorders that comprise the EXT spectrum, and provide biological validity to alternative EXT-relevant phenotypes across development. For instance, P3AR has been used to refine which disorders and behaviors share variance with the EXT factor. Yoon, Iacono, Malone, Bernat, and McGue, (2008) noted that 11-year-old participants with ADHD combined with conduct disorder or oppositional defiant disorder displayed P3AR and thus were at potentially greater risk for EXT compared to youth with ADHD alone who did not display such P3AR. Others have shown that P3AR exhibits utility as an index of EXT severity, with greater EXT disorder comorbidity associated with larger decreases in amplitude (e.g., Perlman et al., 2013; Yoon et al., 2013). Furthermore, P3AR has been successfully used to uncover alternative phenotypes that reflect risk for disinhibitory disorders. For instance, reduced amplitude characterized those who engaged in the early behavioral correlates of disinhibition prior to age 15, such as sexual intercourse, being contacted by the police (Iacono & McGue, 2005), or having an earlier age of first drink (McGue, Iacono, Legrand, Malone, & Elkins, 2001). P3AR also characterized adolescent participants who reported excessive misuse of substances such as binge drinking, or the frequent use of cigarettes or cannabis (Yoon et al., 2006). Overall, these findings make apparent that P3 will be useful as a developmentally-based measure that will continue to inform EXT research.
Conclusions
This study presented a time-domain and time-frequency assessment of stimulus-evoked brain activity in an unselected, community-based sample of participants assessed uniformly at age 17 and again at age 29. We demonstrated that these P3-derived measures provided consistent, stable, predictive measures of disorders in the EXT spectrum through an important developmental period when brain maturation is more likely complete, and when participants have largely passed through the high risk period for the development of substance use and antisocial disorders. The unique features of this investigation included the longest assessment span over which the stability and utility of P3 has been investigated, the first longitudinal evaluation of TF-PCs as stable developmental endophenotypes for EXT, and the novel approach of quantifying the predictive utility of these measures by comparing those who did or did not develop an EXT disorder from their initial categorization in the control group at age 17. Collectively, this and other reports from the MTFS suggest that these measures serve as effective developmental endophenotypes (Gilmore, Malone, & Iacono, 2010; Iacono, Carlson, & Malone, 2000) into adulthood, indexing continued genetic liability for the development of EXT, with potential to contribute insight into the neural processes associated with behavioral disinhibition (Iacono et al., 2008).
Acknowledgments
This work was supported in part by grants R01 DA5147 (Iacono), and K01 AA015621 (Malone).
Contributor Information
Henry H. Yoon, Augsburg College
Stephen M. Malone, University of Minnesota, Twin Cities
William G. Iacono, University of Minnesota, Twin Cities
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, D.C: Author; 1987. rev. [Google Scholar]
- Anokhin AP. Genetic psychophysiology: advances, problems, and future directions. International Journal of Psychophysiology. 2014;93:173–197. doi: 10.1016/j.ijpsycho.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neuroscience Letters. 1999a;259:165–168. doi: 10.1016/S0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Oscillatory brain theory: a new trend in neuroscience. IEEE Engineering Medicine Biology Magazine. 1999b;18:56–66. doi: 10.1109/51.765190. [DOI] [PubMed] [Google Scholar]
- Basar E, Rahn E, Demiralp T, Schurmann M. Spontaneous EEG theta activity controls frontal visual evoked potential amplitudes. Electroencephalography and Clinical Neurophysiology. 1998;108:101–109. doi: 10.1016/S0168-5597(97)00039-7. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Basar E, Demiralp T, Schurmann M. P300-response: possible psychophysiological correlates in delta and theta frequency channels. A review. International Journal of Psychophysiology. 1992;13:161–179. doi: 10.1016/0167-8760(92)90055-G. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Antisocial personality disorder and cocaine dependence: their effects on behavioral and electroencephalographic measures of time estimation. Drug & Alcohol Dependence. 2001;63:87–95. doi: 10.1016/S0376-8716(00)00195-2. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. CSD/BEM localization of P300 sources in adolescents “at-risk”: evidence of frontal cortex dysfunction in conduct disorder. Biological Psychiatry. 2001;50:600–608. doi: 10.1016/S0006-3223(01)01066-6. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Brain maturation and subtypes of conduct disorder: interactive effects on p300 amplitude and topography in male adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:106–115. doi: 10.1097/00004583-200301000-00017. [DOI] [PubMed] [Google Scholar]
- Bauer LO, O’Connor S, Hesselbrock VM. Frontal P300 decrements in antisocial personality disorder. Alcoholism: Clinical and Experimental Research. 1994;18:1300–1305. doi: 10.1111/j.1530-0277.1994.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Berman SM, Whipple SC, Fitch RJ, Noble EP. P3 in young boys as a predictor of adolescent substance use. Alcohol. 1993;10:69–76. doi: 10.1016/0741-8329(93)90055-S. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Malone SM, Williams WJ, Patrick CJ, Iacono WG. Decomposing delta, theta, and alpha time-frequency ERP activity from a visual oddball task using PCA. International Journal of Psychophysiology. 2007;64:62–74. doi: 10.1016/j.ijpsycho.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Williams WJ, Gehring WJ. Decomposing ERP time-frequency energy using PCA. Clinical Neurophysiology. 2005;116:1314–1334. doi: 10.1016/j.clinph.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Hicks BM, Iacono WG, McGue M. Familial transmission and heritability of childhood disruptive disorders. American Journal of Psychiatry. 2010;167:1066–1074. doi: 10.1176/appi.ajp.2010.09091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Keller MC. Endophenotypes in the genetic analyses of mental disorders. Annual Review of Clinical Psychology. 2006;2:267–290. doi: 10.1146/annurev.clinpsy.2.022305.095232. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG. Heritability of P300 amplitude development from adolescence to adulthood. Psychophysiology. 2006;43:470–480. doi: 10.1111/j.1469-8986.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG, McGue M. P300 amplitude in nonalcoholic adolescent twin pairs who become discordant for alcoholism as adults. Psychophysiology. 2004;41:841–844. doi: 10.1111/j.0048-5772.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- Carlson SR, McLarnon ME, Iacono WG. P300 amplitude, externalizing psychopathology, and earlier- versus later-onset substance-use disorder. Journal of Abnormal Psychology. 2007;116:565–577. doi: 10.1037/0021-843X.116.3.565. [DOI] [PubMed] [Google Scholar]
- Costa L, Bauer L, Kuperman S, Porjesz B, O’Connor S, Hesselbrock V, Begleiter H. Frontal P300 decrements, alcohol dependence, and antisocial personality disorder. Biological Psychiatry. 2000;47:1064–1071. doi: 10.1016/S0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- de Geus EJ. From genotype to EEG endophenotype: a route for post-genomic understanding of complex psychiatric disease? Genome Medicine. 2010;2:63. doi: 10.1186/gm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Geus EJ. Molecular genetic psychophysiology: a perspective on the Minnesota contribution. Psychophysiology. 2014;51:1203–1204. doi: 10.1111/psyp.12341. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage. 2007;34:1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, Iacono WG, Doyle AE. Characteristics associated with the persistence of antisocial behavior: Results from recent longitudinal research. Aggression & Violent Behavior. 1997;2:101–124. doi: 10.1016/S1359-1789(96)00013-4. [DOI] [Google Scholar]
- Euser AS, Arends LR, Evans BE, Greaves-Lord K, Huizink AC, Franken IH. The P300 event-related brain potential as a neurobiological endophenotype for substance use disorders: a meta-analytic investigation. Neuroscience & Biobehavioral Reviews. 2012;36:572–603. doi: 10.1016/j.neubiorev.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychological Medicine. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, Bernat EM, Iacono WG. Relationship between the P3 event-related potential, its associated time-frequency components, and externalizing psychopathology. Psychophysiology. 2010;47:123–132. doi: 10.1111/j.1469-8986.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, Iacono WG. Brain electrophysiological endophenotypes for externalizing psychopathology: a multivariate approach. Behavioral Genetics. 2010;40:186–200. doi: 10.1007/s10519-010-9343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Knowles EE, McKay DR, Sprooten E, Raventos H, Blangero J, Almasy L. Arguments for the sake of endophenotypes: examining common misconceptions about the use of endophenotypes in psychiatric genetics. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics. 2014;165B:122–130. doi: 10.1002/ajmg.b.32221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophsyiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Habeych ME, Charles PJ, Sclabassi RJ, Kirisci L, Tarter RE. Direct and mediated associations between P300 amplitude in childhood and substance use disorders outcome in young adulthood. Biological Psychiatry. 2005;57:76–82. doi: 10.1016/j.biopsych.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Hamdi NR, Iacono WG. Lifetime prevalence and co-morbidity of externalizing disorders and depression in prospective assessment. Psychological Medicine. 2014;44:315–324. doi: 10.1017/S0033291713000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muka D, Steinhauer S, Locke J. P300 amplitude decrements in children from families of alcoholic female probands. Biological Psychiatry. 1995;38:622–632. doi: 10.1016/0006-3223(94)00384-7. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, Connolly J. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biological Psychiatry. 1999;46:970–981. doi: 10.1016/S0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke J. Factors predicting the onset of adolescent drinking in families at high risk for developing alcoholism. Biological Psychiatry. 2000;48:265–275. doi: 10.1016/S0006-3223(00)00841-6. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer S, Lowers L, Locke J. Eight-year longitudinal follow-up of P300 and clinical outcome in children from high-risk for alcoholism families. Biological Psychiatry. 1995;37:823–827. doi: 10.1016/0006-3223(95)00041-E. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR. Event-related potentials in women at risk for alcoholism. Alcohol. 1993;10:349–354. doi: 10.1016/0741-8329(93)90019-K. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Locke-Wellman J, Ulrich R. Childhood risk factors for young adult substance dependence outcome in offspring from multiplex alcohol dependence families: a prospective study. Biological Psychiatry. 2009;66:750–757. doi: 10.1016/j.biopsych.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM. Identifying a multivariate endophenotype for substance use disorders using psychophysiological measures. International Journal of Psychophysiology. 2000;38:81–96. doi: 10.1016/S0167-8760(00)00132-X. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Archives of General Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM. Developmental Endophenotypes: Indexing Genetic Risk for Substance Abuse with the P300 Brain Event-Related Potential. Child Development Perspectives. 2011;5:239–247. doi: 10.1111/j.1750-8606.2011.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. International Journal of Psychophysiology. 2003;48:147–178. doi: 10.1016/S0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Iacono WG, McGue M. Minnesota Twin Family Study. Twin Research. 2002;5:482–487. doi: 10.1375/136905202320906327. [DOI] [PubMed] [Google Scholar]
- Iacono WG, McGue M. Association between P3 Event-Related Brain Potential Amplitude and Adolescent Problem Behavior. Psychophysiology. 2006;43:465–469. doi: 10.1111/j.1469-8986.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Vaidyanathan U, Vrieze SI, Malone SM. Knowns and unknowns for psychophysiological endophenotypes: integration and response to commentaries. Psychophysiology. 2014;51:1339–1347. doi: 10.1111/psyp.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Chorlian D, Rangaswamy M, Kamarajan C, Padmanabhapillai A, Stimus A, Begleiter H. S-transform time-frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clinical Neurophysiology. 2006;117:2128–2143. doi: 10.1016/j.clinph.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Begleiter H. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biological Psychology. 2005;69:353–373. doi: 10.1016/j.biopsycho.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E. The genesis of human event-related responses explained through the theory of oscillatory neural assemblies. Neuroscience Letters. 2000a;285:45–48. doi: 10.1016/S0304-3940(00)01022-3. [DOI] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clinical Neurophysiology. 2000b;111:1719–1732. doi: 10.1016/S1388-2457(00)00418-1. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, McGue MK. The association between P300 and age from preadolescence to early adulthood. International Journal of Psychophysiology. 1996;24:213–221. doi: 10.1016/S0167-8760(96)00063-3. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Rounsaville BJ. Sensitivity of psychiatric diagnosis based on the best estimate procedure. American Journal of Psychiatry. 1992;149:1225–1227. doi: 10.1176/ajp.149.9.1225. http://dx.doi.org/10.1176/ajp.149.9.1225. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. http://dx.doi.org/10.1037/0021-843X.111.3.411. [PubMed] [Google Scholar]
- Leckman JF, Scholomskas D, Thompson WD, Belanger A, Weisman MM. Best estimate of lifetime psychiatric diagnosis: A methodological study. Archives of General Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. 2. Hoboken, NJ: John Wiley & Sons, Inc; 2002. [Google Scholar]
- Malone SM, Burwell SJ, Vaidyanathan U, Miller MB, McGue M, Iacono WG. Heritability and molecular-genetic basis of resting EEG activity: a genome-wide association study. Psychophysiology. 2014;51:1225–1245. doi: 10.1111/psyp.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone SM, Iacono WG, McGue M. Event-related potentials and comorbidity in alcohol-dependent adult males. Psychophysiology. 2001;38:367–376. doi: 10.1111/1469-8986.3830367. [DOI] [PubMed] [Google Scholar]
- Malone SM, Vaidyanathan U, Basu S, Miller MB, McGue M, Iacono WG. Heritability and molecular-genetic basis of the P3 event-related brain potential: a genome-wide association study. Psychophysiology. 2014;51:1246–1258. doi: 10.1111/psyp.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Krueger R. The association of early adolescent problem behavior and adult psychopathology: a multivariate behavioral genetic perspective. Behavior Genetics. 2006;36:591–602. doi: 10.1007/s10519-006-9061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcoholism: Clinical and Experimental Research. 2001;25:1156–1165. doi: 10.1111/j.1530-0277.2001.tb02330.x. [DOI] [PubMed] [Google Scholar]
- Miller GA, Rockstroh B. Endophenotypes in psychopathology research: where do we stand? Annual Review of Clinical Psychology. 2013;9:177–213. doi: 10.1146/annurev-clinpsy-050212-185540. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Flint J. The genetic architecture of psychophysiological phenotypes. Psychophysiology. 2014;51:1331–1332. doi: 10.1111/psyp.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor S, Hesselbrock V, Tasman A. Correlates of increased risk for alcoholism in young men. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1986;10:211–218. doi: 10.1016/0278-5846(86)90075-8. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman G, Johnson W, Iacono WG. The heritability of P300 amplitude in 18-year-olds is robust to adolescent alcohol use. Psychophysiology. 2009;46:962–969. doi: 10.1111/j.1469-8986.2009.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman G, Markin A, Iacono WG. P300 amplitude reduction is associated with early-onset and late-onset pathological substance use in a prospectively studied cohort of 14-year-old adolescents. Psychophysiology. 2013 doi: 10.1111/psyp.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Mathalon D. Event-related potentials in alcoholic men: P3 amplitude reflects family history but not alcohol consumption. Alcoholism: Clinical and Experimental Research. 1991;15:839–850. doi: 10.1111/j.1530-0277.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychological Bulletin. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. http://dx.doi.org/10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Event-related potentials in individuals at risk for alcoholism. Alcohol. 1990;7:465–469. doi: 10.1016/0741-8329(90)90033-9. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, Begleiter H. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. International Journal of Psychophysiology. 2007;63:3–15. doi: 10.1016/j.ijpsycho.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W. Diagnostic interview for children and adolescents (DICA) Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Robins LN, Babor TF, Cottler LB. Composite International Diagnostic Interview: Expanded Substance Abuse Module. St. Louis: Authors; 1987. [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Meta-analytic procedures for social research. Vol. 6. Sage; 1991. [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II) New York: New York State Psychiatric Institute, Biometrics Research; 1987. [Google Scholar]
- Steinhauer SR, Hill SY. Auditory event-related potentials in children at high risk for alcoholism. Journal of Studies on Alcohol. 1993;54:408–421. doi: 10.15288/jsa.1993.54.408. http://dx.doi.org/10.15288/jsa.1993.54.408. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan U, Isen JD, Malone SM, Miller MB, McGue M, Iacono WG. Heritability and molecular genetic basis of electrodermal activity: a genome-wide association study. Psychophysiology. 2014;51:1259–1271. doi: 10.1111/psyp.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Malone SM, Donnelly JM, Hammer MA, Miller MB, McGue M, Iacono WG. Heritability and molecular genetic basis of antisaccade eye tracking error rate: a genome-wide association study. Psychophysiology. 2014;51:1272–1284. doi: 10.1111/psyp.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Malone SM, Miller MB, McGue M, Iacono WG. Heritability and molecular genetic basis of acoustic startle eye blink and affectively modulated startle response: a genome-wide association study. Psychophysiology. 2014;51:1285–1299. doi: 10.1111/psyp.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beijsterveldt CE, van Baal GC. Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biological Psychology. 2002;61:111–138. doi: 10.1016/S0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- Vrieze SI, Hicks BM, Iacono WG, McGue M. Decline in genetic influence on the co-occurrence of alcohol, marijuana, and nicotine dependence symptoms from age 14 to 29. American Journal of Psychiatry. 2012;169:1073–1081. doi: 10.1176/appi.ajp.2012.11081268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze SI, Malone SM, Pankratz N, Vaidyanathan U, Miller MB, Kang HM, Iacono WG. Genetic associations of nonsynonymous exonic variants with psychophysiological endophenotypes. Psychophysiology. 2014;51:1300–1308. doi: 10.1111/psyp.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze SI, Malone SM, Vaidyanathan U, Kwong A, Kang HM, Zhan X, Iacono WG. In search of rare variants: preliminary results from whole genome sequencing of 1,325 individuals with psychophysiological endophenotypes. [Research Support, N.I.H., Extramural] Psychophysiology. 2014;51:1309–1320. doi: 10.1111/psyp.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welner Z, Reich W, Herjanic B, Jung K, Amado H. Reliability, validity, and parent-child agreement studies of the Diagnostic Interview for Children and Adolescents (DICA) Journal of the Academy of Child and Adolescent Psychiatry. 1987;26:649–653. doi: 10.1097/00004583-198709000-00007. [DOI] [PubMed] [Google Scholar]
- Williams WJ. Reduced interference time-frequency distributions: Scaled decompositions and interpretations. In: Debnath L, editor. Wavelet transforms and time-frequency signal analysis. Boston: Birkhauser; 2001. pp. 381–417. [Google Scholar]
- Yoon HH, Iacono WG, Malone SM, Bernat EM, McGue M. The effects of childhood disruptive disorder comorbidity on P3 event-related brain potentials in preadolescents with ADHD. Biological Psychology. 2008;79:329–336. doi: 10.1016/j.biopsycho.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HH, Iacono WG, Malone SM, McGue M. Using the brain P300 response to identify novel phenotypes reflecting genetic vulnerability for adolescent substance misuse. Addictive Behaviors. 2006;31:1067–1087. doi: 10.1016/j.addbeh.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Yoon HH, Malone SM, Burwell SJ, Bernat EM, Iacono WG. Association between P3 event-related potential amplitude and externalizing disorders: a time-domain and time-frequency investigation of 29-year-old adults. Psychophysiology. 2013;50:595–609. doi: 10.1111/psyp.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]