Abstract
Purpose
As stereotactic body radiation therapy (SBRT) has emerged as a quick, effective, and well-tolerated treatment for early stage non-small cell lung carcinoma (NSCLC), it can be difficult to convince patients to quit smoking in follow-up. We evaluated whether there was a survival benefit to smoking cessation after SBRT.
Methods and Materials
Patients with early stage NSCLC treated from 2004–2013 who were still smoking tobacco at the time of SBRT were identified from a prospective institutional review board approved registry. Peripheral tumors were treated to 54 in 3 fractions and central tumors to 50 Gy in 5 fractions. Patients were reviewed for overall survival (OS) and disease progression. The log-rank test and Cox regression were used to identify factors predictive of OS.
Results
Thirty-two patients (27%) quit smoking after SBRT, and 87 (73%) continued smoking. Median follow-up was 22 months (range 2–87). On multivariate analysis, smoking status (HR 2.1, 95% CI: 1.02–4.2, p=0.045), increasing age-adjusted Charlson comorbidity score, and larger tumor size were predictive of worse OS. The prior number of cigarette pack-years was not significant (p=0.62). In a Kaplan-Meier comparison, smoking cessation after SBRT was associated with improved 2-year OS, 78% vs. 69% (p=0.014). There was no significant difference in 2-year progression free survival (75% vs. 55%, p=0.23) or local control (97% vs. 88%, p=0.63).
Conclusion
Overall survival is significantly improved in patients who stop smoking after SBRT for early stage NSCLC, no matter their previous smoking history. Encouraging smoking cessation should be an important part of every post-treatment visit.
Introduction
Lung cancer is the most common cause of cancer death worldwide, and tobacco smoking is responsible for approximately 70% of these lung cancer deaths (1). This risk of death from lung cancer increases proportionally with both the duration and intensity of smoking (2). In addition, smoking has a number of other negative effects beyond the development of cancer itself. Once lung cancer has been diagnosed, continued smoking further depresses the quality of life of patients (3). In those with metastatic disease, continued smoking increases resistance to systemic therapies (4–5). In those with limited stage small cell lung carcinoma treated with combined chemotherapy and radiation therapy, continued smoking decreases overall survival (6). Likewise in those with early stage non-small cell carcinoma (NSCLC) treated with surgical resection, continued smoking also decreases overall survival (7–8).
However, the impact of smoking cessation after treatment of early stage NSCLC with modern radiation is unknown. The benefit of cessation after surgery cannot be extrapolated from surgical patients as they typically have a higher performance status and less comorbidities than those typically referred for definitive radiation therapy (9). Older studies of definitive radiation cannot be applied, as the use of 1–5 large fractions of stereotactic body radiation therapy (SBRT) has recently supplanted 5–7 weeks of conventional fractionated radiation therapy due to SBRT’s improvement in local control and survival (10). With the increasing adaptation of screening via low-dose computed tomography and the suggestion of parity between resection and SBRT in recent prospective randomized trials (11), we are certain to see a continued increase in its utilization in the years to come.
Unlike the alternative treatment of surgical resection, treatment with SBRT is noninvasive and does not require hospitalization. SBRT treatments are generally well tolerated and involve at worst mild discomfort during immobilization. The predicted high local control rate (12) and ease of treatment with SBRT add little impetus for patients to go through the physically and psychologically difficult process of quitting smoking, which is often required of patients being offered surgical resection. The importance of smoking cessation after SBRT therefore needs to be quantified to motivate not just the increasing number of future treated patients, but also the treating physicians as it is well described that only approximately a third of those diagnosed with lung cancer are ever formally counseled to quit by medical professionals (13).
Materials and Methods
Patients
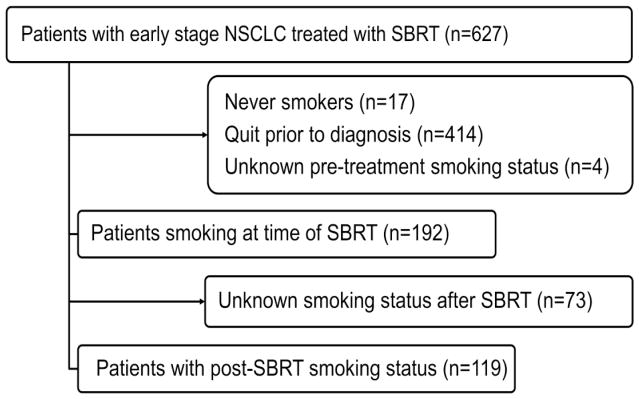
The records of patients who received lung SBRT from June 2004 to June 2013 at our institution were reviewed. Prior to treatment, all patients signed consent for enrollment on an IRB-approved prospective registry. Those included in this study had to have either pathologically proven non-small cell carcinoma or a growing lesion with a positive pre-treatment positron emission tomography (PET) scan if biopsy was felt too risky after multidisciplinary review. All were free of extra thoracic disease by PET, treated with curative intent, and had at least two months of follow-up. Patients who received adjuvant systemic or surgical therapy were excluded. All required documentation of continued smoking. Prior to consult with a physician, all patients completed a self-reported questionnaire that includes tobacco history, and all patients were assessed by nurses about smoking status. All patients in this study also required additional documentation of smoking status at any follow-up visit with surgical, medical, or radiation oncology. A total of 119 patients met inclusion criteria (Figure 1).
Figure 1.
Patients analyzed for the study.
Demographic data collected included age, gender, prior number of smoking pack years, Karnofsky performance status (KPS), and body-mass index (BMI). Patient comorbidities were scored according to the age-adjusted Charlson comorbidity index. Though history of a prior cancer is included in this index, a history of a prior cancer was also included as a separate variable for analysis. Tumor characteristics collected included the greatest diameter of the treated lesion, stage of the cancer according to American Joint Committee on Cancer guide version 7, maximum standardized uptake value (SUVmax) of the lesion on PET, tumor location and whether a biopsy was performed. Tumor locations were classified as peripheral if they were more than 2 cm from the mediastinum and proximal bronchial tree; else, the tumors were classified as central. If a biopsy was performed, histology was divided between squamous and non-squamous carcinoma.
Treatment
All patients were immobilized for simulation and treatment. Abdominal compression was applied if the tumor moved more than 1.0 cm in any direction on four-dimensional computed tomography (4D CT). Maximum intensity projection images from this 4D scan were used to delineate an internal target volume (ITV) that comprised the gross tumor and its complete motion throughout the respiratory cycle. The planning target volume was a 0.5–1.0 cm expansion of the ITV in all directions. Treatment plans were then generated with 7–11 non-opposing beams. Heterogeneity corrections were applied using the super-position/convolution algorithm. Dose was generally prescribed to the 80% isodose line (range 60–90%) and covered greater than 95% of the PTV. Treatment was performed over 3–21 days (median 8 days). The prescribed dose was generally 54 in 3 fractions to peripheral tumors and 50 Gy in 5 fractions for central tumors, both with heterogeneity corrections.
Follow-up consisted of physical exam and chest CT scan every 3 months for the first 2 years, and chest CT scans every 6 months thereafter. PET/CT was performed if there was clinical suspicion for recurrence. Patients were not uniformly asked about smoking status in follow-up, and the treating radiation oncologists did not receive any specialized training in smoking cessation. When patients expressed an interest in quitting smoking however, they were given business cards for the state department of health’s free telephone based counseling service. After obtaining verbal consent, they were also referred by phone or by email to our cancer center’s free smoking cessation program. After an initial consultation with a trained counselor in the cancer center, patients would be referred back to the original physician with support and prescription recommendations. Those who were interested also would be enrolled in an additional six week program covering behavior modification, stress reduction, and relapse prevention.
Statistics
Overall survival (OS), progression-free survival (PFS), and local control duration were calculated from the date of first SBRT fraction to the date of last follow-up, death, or progression. Patients who did not have a recurrence were censored at either the date of last follow-up or death. Summary statistics were provided with frequency count and percentage for categorical variables, and mean, standard deviation, median, and range for continuous variables. Fisher’s exact test was used to assess associations between two categorical variables. A two-sided Wilcoxon rank-sum was used to evaluate the difference in a continuous variable between patient groups. The Kaplan-Meier method was used to estimate overall survival, progression-free survival, and local control duration. The log-rank test was used to evaluate differences in time-to-event outcomes between those who quit smoking and those who continued smoking. Two sided p values were used, and these were classified as statistically significant if <0.05. Cox proportional hazard analysis was used to calculate differences between groups with 95% confidence intervals. Analysis was done using SAS version 9.4.
Results
A total of 627 patients with NSCLC treated with SBRT with definitive intent between 2004 and 2013 were reviewed. Four hundred fourteen (66.0%) were former smokers and 17 (2.7%) were never smokers. Of the 192 (30.6%) still smoking at the time of treatment, 119 patients had information about post-treatment smoking status in follow-up. These 119 were included in the analysis are detailed in Table 1. The majority were female (n=70, 59%). The median age of the entire group was 67 years (range: 51–85). The median number of pack years smoked was 50 (interquartile range: 40–75 pack years). Most patients (n=93, 78%) had biopsy proven disease. Most had peripheral NSCLC lesions (n=87, 73%) that were treated in three fractions to 54Gy. The median follow-up was 22 months (range 2–87 months).
Table 1.
Patient characteristics.
| Characteristic | All | Smoking | Quit | P |
|---|---|---|---|---|
| 119 (100%) | 87 (73%) | 32 (27%) | ||
| Age | 0.42 | |||
| Median | 67 | 68 | 67 | |
| Range | 51–85 | 51–85 | 56–80 | |
| Sex | 0.41 | |||
| Female | 70 (59%) | 49 (56%) | 21 (66%) | |
| Male | 49 (41%) | 38 (44%) | 11 (34%) | |
| BMI | 0.15 | |||
| Underweight (<18.5) | 15 (13%) | 11 (13%) | 4 (13%) | |
| Normal weight | 42 (35%) | 28 (32%) | 14 (44%) | |
| Overweight (>25) | 53 (44%) | 41 (47%) | 12 (38%) | |
| Unknown | 9 (8%) | 7 (8%) | 2 (6%) | |
| Smoking history (pack-years) | 0.32 | |||
| 1–25 | 17 (14%) | 11 (13%) | 6 (19%) | |
| 26–50 | 43 (36%) | 29 (33%) | 14 (44%) | |
| 51–75 | 34 (29%) | 28 (32%) | 6 (19%) | |
| 76–100 | 20 (17%) | 14 (16%) | 6 (19%) | |
| >100 | 3 (2%) | 3 (3%) | 0 | |
| Unknown | 2 (2%) | 2 (2%) | 0 | |
| KPS | 0.07 | |||
| 90–100 | 29 (24%) | 22 (25%) | 7 (22%) | |
| 70–89 | 64 (54%) | 50 (57%) | 14 (44%) | |
| <70 | 21 (18%) | 12 (14%) | 9 (28%) | |
| Unknown | 5 (4%) | 3 (4%) | 2 (6%) | |
| Previous cancer | 0.04 | |||
| No | 62 (52%) | 40 (46%) | 22 (69%) | |
| Yes | 57 (48%) | 47 (54%) | 10 (31%) | |
| CCI (age adjusted) | 0.20 | |||
| 2–3 | 15 (13%) | 10 (11%) | 5 (16%) | |
| 4–5 | 50 (42%) | 36 (41%) | 15 (47%) | |
| 6–7 | 34 (29%) | 26 (30%) | 8 (25%) | |
| >7 | 20 (17%) | 15 (17%) | 4 (13%) | |
| Histology | 0.63 | |||
| Squamous | 29 (24%) | 20 (23%) | 9 (28%) | |
| Non-squamous | 64 (54%) | 47 (54%) | 17 (53%) | |
| Unbiopsied | 26 (22%) | 20 (23%) | 6 (19%) | |
| Tumor Stage | 0.86 | |||
| T1a | 60 (50%) | 41 (47%) | 19 (59%) | |
| T1b | 41 (35%) | 33 (38%) | 8 (25%) | |
| T2a | 17 (14%) | 12 (14%) | 5 (16%) | |
| Tumor location | 0.39 | |||
| Central | 17 (14%) | 11 (13%) | 6 (19%) | |
| Peripheral | 102 (86%) | 76 (87%) | 26 (81%) | |
| Treatment Dose | 0.36 | |||
| 50 Gy in 5 fractions | 26 (22%) | 19 (22%) | 7 (22%) | |
| 54 Gy in 3 fractions | 87 (73%) | 64 (74%) | 23 (72%) | |
| 55 Gy in 5 fractions | 6 (5%) | 4 (5%) | 2 (6%) |
Abbreviations: BMI, body mass index; KPS, Karnofsky performance status; CCI, Charlson comorbidity index (age-adjusted); Gy, gray.
Of the 119 patients smoking during definitive SBRT, 32 (27%) quit smoking after SBRT, and 87 (73%) continued smoking. Patients being treated for their first malignancy were twice as likely to quit smoking after SBRT as those with a prior malignancy, 35% vs. 18% (p=0.038). There were no other significant differences between those who continued smoking after treatment and those who quit smoking, including intensity of smoking prior to treatment.
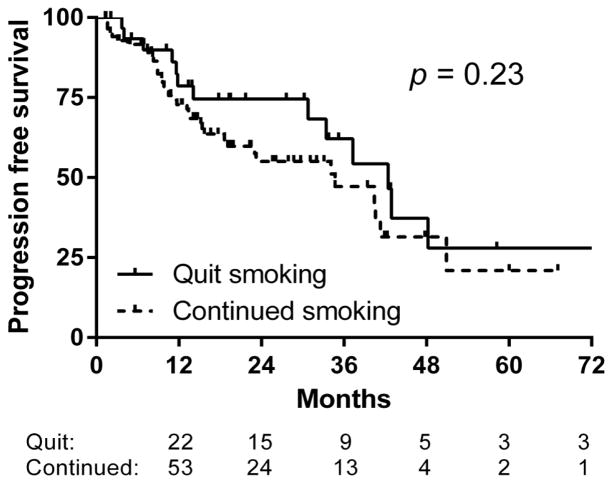
The 2-year OS for all patients smoking during SBRT was 72%. On univariate analysis of factors significant for OS, smoking status after treatment, age-adjusted Charlson comorbidity score, BMI, tumor size, and squamous histology were predictive of worse OS (Table 2). No other factors approached statistical significance, including the prior number of cigarette pack-years in this population of patients still smoking at the time of treatment (p=0.62). In a Kaplan-Meier comparison, smoking cessation after SBRT was associated with an increase in 2-year OS, 78% vs. 69% (p=0.014) (Figure 2). There was no difference in 2-year progression free survival (75% vs. 55%, p=0.23) or local control (97% vs. 88%, p=0.63) (Figures 3 & 4).
Table 2.
Univariate analysis of factors predictive of overall survival among patients smoking at the time of SBRT.
| Characteristic | HR | 95% CI | P |
|---|---|---|---|
| Smoking after SBRT | 2.34 | 1.17–4.67 | 0.02 |
| Age | 1.03 | 0.99–1.07 | 0.14 |
| Sex (Male) | 1.56 | 0.90–2.69 | 0.11 |
| BMI | 1.05 | 1.00–1.09 | 0.03 |
| Pack years | 1.00 | 0.99–1.01 | 0.62 |
| KPS | 0.99 | 0.97–1.01 | 0.30 |
| Previous Cancer | 1.45 | 0.84–2.48 | 0.18 |
| Charlson comorbidty index | 1.16 | 1.00–1.35 | 0.05 |
| Biopsy | 1.41 | 0.69–2.88 | 0.35 |
| Squamous carcinoma | 1.86 | 1.01–3.44 | 0.048 |
| Tumor size | 1.36 | 1.08–1.73 | 0.01 |
| Central location | 1.21 | 0.54–2.73 | 0.63 |
| Dose | 0.94 | 0.86–1.04 | 0.24 |
Abbreviations: SBRT, stereotactic body radiation therapy; HR, hazard ratio; 95% CI, 95% confidence interval.
Figure 2.
Smoking cessation is associated with a significant improvement in overall survival at two years after SBRT, 78% vs. 69% (p=0.014).
Figure 3.
Smoking cessation does not lead to an improvement in progression-free survival after SBRT.
Figure 4.
Smoking cessation does not affect local control after SBRT.
On multivariate analysis of those factors significant for OS on univariate analysis, only smoking status (HR 2.1, 95% CI: 1.02–4.2, p=0.045), age-adjusted Charlson comorbidity score (HR 1.20, 95% CI: 1.06–1.71, p=0.015), and tumor size (HR 1.35 per cm, 95% CI: 1.06–1.71, p=0.021) remained significant predictors of worse OS.
Discussion
The association between smoking and early death is well established. Compared to those who have never smoked, smokers have at least 15 times the risk of death from lung cancer and lose at least one decade of life expectancy (2). Early cessation can reduce this risk of death by as much as 90%, but the reduction in risk with late cessation at more advanced ages and after the diagnosis of lung cancer is less established. To our knowledge, this is the first study to look at the benefit to cessation after treatment of early stage NSCLC with stereotactic body radiation therapy. Despite an average age of nearly 70 and multiple comorbidities, patients in this study who were able to quit smoking after receiving definitive SBRT had a 9% improvement in overall survival at 2 years. This is more than the 5% improvement seen with cessation prior to treatment for small cell lung carcinoma, but the median OS for early stage NSCLC is greater, allowing more time for the benefits of cessation to appear. Also, while the hazard ratio of 2.1 associated with continued smoking after SBRT for early stage NSCLC is less than that of 2.9 observed in a meta-analysis of surgically treated early stage NSCLC patients (8), our patients were largely medically inoperable and had more competing causes for mortality, therefore likely lowering the beneficial effect of cessation. Our hazard ratio of 2.1 for continued smoking is slightly higher than the 1.7 recently reported by Roswell Park (14), but their population was heterogeneous and included patients with more advanced stage disease treated with combination therapies. Overall though, the benefit associated with smoking cessation after SBRT is consistent with the benefit reported in these other groups of lung cancer patients.
The observed reduction in all-cause mortality with smoking cessation after SBRT is likely multifactorial. Medically, cessation improves outcomes in those with chronic pulmonary (15) and cardiovascular disease (16), all of which are common comorbidities in medically inoperable SBRT patients. Psychologically, continued smoking after treatment for NSCLC is associated with lower quality of life (17), higher pain scores (18–19), and depression (20). Continued smoking has been associated with more severe toxicity from SBRT including worse damage to the chest wall (21). On a molecular level, continued stimulation by nicotine in tobacco has been shown to increase tumor growth and neovascularization through synergy with cell membrane receptors for epidermal growth factor, vascular endothelial growth factor, and insulin-like growth factor (22). Nicotine also inhibits apoptosis of carcinoma through prevention of opening of mitochondrial permeability transition pores (23). Together, these effects are all likely responsible for the improvement in overall survival in those patients who are able to quit smoking after treatment. However given that progression free survival and local control did not differ between those who quit smoking and those who continued in this cohort, the most important effect is likely due to interactions with chronic comorbid illnesses.
The rate of smoking after treatment was high at 73% in this study, which is within the wide range (6–83%) reported by studies of surgically treated early stage NSCLC(8,24)and comparable to the rate seen in patients treated for other cancers in United States population-based studies at 69% (25). Yet our observed rate of continued smoking is significantly higher than the rate of 15% recently reported in lung cancer survivors in the American Cancer Society’s Study of Cancer Survivors (SCS-I). However, SCS-I was the result of smoking surveys taken on average nine years after diagnosis (26). Given the survival benefit to smoking cessation seen at just two years in our study of SBRT and in other surgical series, it is likely that the population in SCS-I surveyed a decade after diagnosis is biased towards non-smokers.
The fact that any patients continue smoking after treatment is nonetheless discouraging. Many in this study had in fact been diagnosed with prior malignancies yet continued to smoke during SBRT. These patients were only half as likely to quit later. Due to competing concerns and perhaps a feeling of futility shared by both providers and lung cancer patients, cessation counseling is not addressed as often as it should. Self-reported surveys of oncologists suggest rates of counseling are above 80% at initial consultation, but less than 30% in follow-up (27). Lack of confidence in counseling abilities, discomfort with interventions, and frustration with lack of patient motivation were common barriers cited by these oncologists (28). Despite these barriers, medical professionals should be encouraged that surveys of cancer survivors consistently cite medical advice as an important motivating factor for quitting (20). A Cochrane review also concluded that a brief advice intervention by a physician can double the unassisted quit rate (29). The most effective physician advice interventions are those that use “gain framed” statements (30) such as our finding that smokers who can quit after SBRT can double their chances of survival.
This study is limited largely by its retrospective nature. However, it was an analysis of a prospective cohort in which all patients consented for inclusion pre-treatment, and there will not likely be randomized trials funded to look at the benefit of smoking cessation after treatment. Another limitation is the reliability of documentation of smoking status, both before and after treatment. Smoking status was determined though through a multidisciplinary review though, namely dictated physician notes, nursing notes, and referrals to cessation counselors within our cancer center. The median follow-up at just under two years is also relatively short. However, while the median survival for the cohort has not yet been reached, a statistically significant survival benefit was already apparent. While these limitations might affect the exact magnitude of the survival benefit, they should not detract from the utility of smoking cessation counseling by oncologists and all other medical providers in lung cancer survivors.
Conclusion
No matter their previous smoking history, current smokers treated with SBRT for early stage NSCLC appear to enjoy an overall survival advantage if they are able to quit after treatment. While this population needs further study, smoking cessation should be a focus of follow-up care for all patients with lung cancer.
Table 3.
Multivariate analysis of factors predictive of overall survival among patients smoking at the time of SBRT.
| Characteristic | HR | 95% CI | P |
|---|---|---|---|
| Smoking after SBRT | 2.07 | 1.02–4.20 | 0.045 |
| Tumor size (cm) | 1.35 | 1.06–1.71 | 0.015 |
| Charlson comorbidity index | 1.2 | 1.03–1.41 | 0.021 |
Abbreviations: SBRT, stereotactic body radiation therapy; HR, hazard ratio; 95% CI, 95% confidence interval.
Acknowledgments
Source of Funding: Supported by Clinical and Translational Science Award (CTSA) Grant [UL1TR00448] and Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842.
Footnotes
Conflicts of interest notification: there are no significant conflicts of interest relevant to this publication to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stewart BW, Wild CP. World Cancer Report 2014. International Agency for Research on Cancer, World Health Organization; 2014. [Google Scholar]
- 2.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–50. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 3.Garces YI, Yang P, Parkinson J, et al. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest. 2004;126:1733–1741. doi: 10.1378/chest.126.6.1733. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Kamdar O, Le W, et al. Nicotine induces resistance to chemotherapy by modulating mitochondrial signaling in lung cancer. Am J Respir Cell Mol Biol. 2009;40:36–45. doi: 10.1165/rcmb.2007-0277OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes AN, O’Brien ME, Petty WJ, et al. Overcoming CYP1A1/1A2 mediated induction of metabolism by escalating erlotinib dose in current smokers. J Clin Oncol. 2009;27:1220–1226. doi: 10.1200/JCO.2008.19.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Videtic GM, Stitt LW, Dar AR, et al. Continued cigarette smoking by patients receiving concurrent chemoradiotherapy for limited-stage small-cell lung cancer is associated with decreased survival. J Clin Oncol. 2003;21:1544–9. doi: 10.1200/JCO.2003.10.089. [DOI] [PubMed] [Google Scholar]
- 7.Hung JJ, Jeng WJ, Hsu WH, et al. Predictors of death, local recurrence, and distant metastasis in completely resected pathological stage-I non-small-cell lung cancer. J Thorac Oncol. 2012;7:1115–1123. doi: 10.1097/JTO.0b013e31824cbad8. [DOI] [PubMed] [Google Scholar]
- 8.Parsons A, Daley A, Begh R, et al. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: Systemic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson CG, DeWees TA, El Naga IM, et al. Patterns of failure after stereotactic body radiation therapy or lobar resection for clinical stage I non-small cell lung cancer. J Thorac Oncol. 2013;8:192–201. doi: 10.1097/JTO.0b013e31827ce361. [DOI] [PubMed] [Google Scholar]
- 10.Koshy M, Malik R, Mahmood U, et al. Stereotactic body radiotherapy and treatment at a high volume facility is associated with improved survival in patients with inoperable stage I non-small cell lung cancer. Radiother Oncol. 2015;114:148–54. doi: 10.1016/j.radonc.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomized trials. Lancet Oncol. doi: 10.1016/S1470-2045(15)70168-3. Published online May 13, 2015 ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–76. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hildebrand JR, Sastry S. Stop smoking! Do we say it enough? J Oncol Pract. 2013:230–2. doi: 10.1200/JOP.2013.000890. [DOI] [PubMed] [Google Scholar]
- 14.Amato KD, Hyland A, Reed R, et al. Tobacco cessation may improve lung cancer patient survival. J Thorac Oncol. 2015;10:1014–9. doi: 10.1097/JTO.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiménez-Ruiz CA, Andreas S, Lewis KE, et al. Statement on smoking cessation in COPD and other pulmonary diseases and in smokers with comorbidities who find it difficult to quit. Eur Respir J. 2015 Apr 16; doi: 10.1183/09031936.00092614. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Clair C, Rigotti NA, Porneala B, et al. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA. 2013;309:1014–21. doi: 10.1001/jama.2013.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poghosyan H, Sheldon LK, Leveille SG, Cooley ME. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: a systematic review. Lung Cancer. 2013;81:11–26. doi: 10.1016/j.lungcan.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Daniel M, Keefe FJ, Lyna P, et al. Persistent smoking after the diagnosis of lung cancer is associated with higher reported pain levels. J Pain. 2009;10:323–328. doi: 10.1016/j.jpain.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ditre JW, Gonzalez BD, Simmons VN, et al. Associations between pain and current smoking status among cancer patients. Pain. 2011;152(1):60–65. doi: 10.1016/j.pain.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg CJ, Thomas AN, Mertens AC, et al. Correlates of continued smoking versus cessation among survivors of smoking-related cancers. Psychooncology. 2013;22:799–806. doi: 10.1002/pon.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephens KL, Djemil T, Tendulkar RD, et al. Prediction of chest wall toxicity from lung stereotactic body radiotherapy (SBRT) Int J Radiat Oncol Biol Phys. 2012;82:974–80. doi: 10.1016/j.ijrobp.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Sobus SL, Warren GW. The biologic effects of cigarette smoke on cancer cells. Cancer. 2014;120:3617–26. doi: 10.1002/cncr.28904. [DOI] [PubMed] [Google Scholar]
- 23.Chernyavsky AI, Shchepotin IB, Galitovkiy V, Grando SA. Mechanisms of tumor-promoting activities of nicotine in lung cancer: synergistic effects of cell membrane and mitochondrial nicotinic acetylcholine receptors. BMC Cancer. 2015;15:152. doi: 10.1186/s12885-015-1158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park ER, Japuntich S, Temel J, et al. A smoking cessation intervention for thoracic surgery and oncology clinics: a pilot trial. J Thorac Oncol. 2011;6:1059–65. doi: 10.1097/JTO.0b013e318215a4dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westmaas JL, Newton CC, Stevens VL. Does a recent cancer diagnosis predict smoking cessation? An analysis from a large prospective US cohort. J Clin Oncol. 2015;33:1647–52. doi: 10.1200/JCO.2014.58.3088. [DOI] [PubMed] [Google Scholar]
- 26.Westmaas JL, Alcaraz KI, Berg CJ, Stein KD. Prevalence and correlates of smoking and cessation-related behavior among survivors of ten cancers: findings from a nationwide survey nine years after diagnosis. Cancer Epidemiol Biomarkers Prev. 2014;23:1783–92. doi: 10.1158/1055-9965.EPI-14-0046. [DOI] [PubMed] [Google Scholar]
- 27.Warren GW, Marshall JR, Cummings KM, et al. Practice patterns and perceptions of thoracic oncology providers on tobacco use and cessation in cancer patients. J Thorac Oncol. 2013;8:543–8. doi: 10.1097/JTO.0b013e318288dc96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weaver KE, Danhauer SC, Tooze JA, et al. Smoking cessation counseling beliefs and behaviors of outpatient oncology providers. Oncologist. 2012;17:455–62. doi: 10.1634/theoncologist.2011-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stead LF, Buitrago D, Preciado N, et al. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013;5:CD000165. doi: 10.1002/14651858.CD000165.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toll BA, Rojewski AM, Duncan LR, et al. “Quitting smoking will benefit your health”: the evolution of clinician messaging to encourage tobacco cessation. Clin Cancer Res. 2014;20:301–9. doi: 10.1158/1078-0432.CCR-13-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]