Abstract
Background
Eosinophilic esophagitis (EoE) is a chronic Th2 inflammatory disease characterized by tissue remodeling that leads to esophageal strictures and food impactions. Effects of therapy on long term remodeling in pediatric eosinophil-associated diseases has not been previously described.
Objective
To understand the long term control of esophageal remodeling in EoE
Methods
We assessed endoscopic and histologic remodeling and TGFβ1 expression in esophageal biopsies from children (n=32) with EoE treated with topical corticosteroids (TCS) over 10 years (mean=4.5). We utilized standardized EoE scoring tools to gauge endoscopic and symptom features.
Results
738 biopsies from 246 endoscopic procedures were evaluated over 10 years. 486 biopsies had adequate lamina propria (LP) for evaluation of subepithelial remodeling. The severity of epithelial esophageal eosinophilia correlated with epithelial remodeling (basal zone hyperplasia, desquamation, and dilated intercellular spaces) (p<0.0001), LP eosinophilia (p<0.0001), and fibrosis (p<0.0001). Sixteen subjects were initial responders (<15 eosinophils per hpf) to TCS. Responders and non-responders spent 54% and 97% of their total disease duration with active EoE (p<0.001) and 23% and 53% (p<0.02) with maximal fibrosis scores, respectively. Responders had lower endoscopy scores during their disease duration (p=0.013). Having <15 eosinophils per hpf at any time correlated with lower fibrosis and endoscopic severity. TGFβ1 positive cells decreased in responders at the first biopsy but this was not sustained. Symptoms did not correlate with other disease features.
Conclusions
Children with EoE have substantial esophageal remodeling which associates with inflammation and that can improve in a sustainable manner with TCS. Although endoscopic features correspond to histologic features, symptoms did not correlate with inflammation or fibrosis.
Keywords: Eosinophilic esophagitis, remodeling, fibrosis, topical corticosteroid, TGFβ1
Introduction
Eosinophilic esophagitis (EoE) is a chronic, inflammatory Th2 disease of increasing prevalence and incidence that is associated with substantial tissue remodeling including fibrosis and angiogenesis (1, 2). Remodeling is believed to be the underpinning of EoE complications that include dysmotility, strictures, narrowing, and food bolus impactions (3, 4). Remodeling includes histologic changes of epithelial basal zone hyperplasia and dilated intercellular spaces as well as subepithelial angiogenesis, fibrosis, and smooth muscle hyperplasia/hypertrophy (5). Natural history studies in adults demonstrate that EoE is a progressive fibrostenotic disease which almost uniformly leads to stricture formation and increasing stricture risk is proportional to longer untreated disease duration (6, 7). In children and adults, EoE is a chronic disorder with spontaneous remission occurring in the minority of patients (8-10). Although longitudinal studies assessing endoscopic and symptomatic fibrostenosis in adults have been published (7, 11), there have been no long term, systematic histologic assessments of remodeling in EoE. Indeed eosinophil associated tissue remodeling and its clinical impacts have been difficult to study in the past due to a paucity of human tissue for analysis. In this regard, EoE offers a novel opportunity to understand the relationship between histologic and molecular tissue remodeling with clinical disease features.
Chronic management with either medications or food elimination is required to maintain EoE remission (9, 11). Disease control is important for patient quality of life as well as for decreasing complications (12, 13). Adult studies show that increasing use of esophageal topical corticosteroids (TCS) over a 5 year time period associates with fewer episodes of food impactions (11). While this type of data has not been published in the pediatric EoE population, it is clear that children also need prolonged dietary elimination and/or TCS (9, 14). In children, short term TCS and/or dietary elimination can control both inflammation and fibrosis (15-17). However, whether TCS can sustainably control remodeling is not clear. In addition, the duration and degree of inflammatory control to avoid the onset of fibrosis are not clear (18). The notion that prolonged fibrotic control implies control of esophageal narrowing is a hypothesis that has not been proven. These issues are particularly salient for children, many of whom will likely require lifelong therapy and for whom prevention of stricture over their lifetime is a key component of disease management.
In order to begin to understand the relationships between long-term TCS therapy, esophageal remodeling, and clinical course, we analyzed 738 biopsies from 32 pediatric EoE subjects over a maximum of 10 years. To our knowledge, this is the largest cohort of pediatric biopsies studied in the context of esophageal remodeling and fibrosis over time as well the first study to assess tissue remodeling in any eosinophil associated disease in children over long treatment times. Our current data suggest that control of epithelial eosinophilia correlates with control of LP eosinophilia and fibrosis in children and that endoscopic and histologic features are better controlled both in initial responders to esophageal TCS therapy and in those children who had epithelial eosinophilia of <15 per hpf at any given point in their disease course. While epithelial inflammation and endoscopic features correlated with fibrosis, symptoms do not. Given the number of years of follow up and the large number of endoscopic/histologic features studies, these data have potentially important clinical implications for EoE control and progression and point to the need for additional therapeutic strategies to control chronic esophageal eosinophilia.
Methods
EoE subjects/biopsies
Subjects were selected from the UCSD/RCHSD EoE database who met the following criteria: 1) EoE defined as ≥15 eosinophils per high power field (hpf) on hematoxylin/eosin (H&E) stain at 400x magnification on light microscopy; 2) the presence of typical symptom and endoscopic features; 3) semi-continuously prescribed esophageal TCS; 4) adequate (defined as at least 3 hpf) lamina propria (LP) in at least 3 specimens over time including the baseline biopsy; 5) followed at RCHSD/UCSD for a minimum of 12 months. A total of 32 subjects met criteria and were analyzed. Subject characteristics are listed in Table 1. All studies were approved under UCSD/RCHSD IRB.
Table 1.
Subject characteristics
| Age at diagnosis (years) | M/F | Total disease duration (years) | Duration of disease analyzed (years) | Total # of EGDs | EGDs that were analyzed | % of EGD's on topical corticosteroids | % of EGDs on proton-pump-inhibitor | Responder |
|---|---|---|---|---|---|---|---|---|
| 1.08 | M | 7.50 | 5.50 | 11 | 7 | 67 | 86 | Yes |
| 1.25 | M | 3.92 | 3.92 | 8 | 7 | 33 | 43 | No |
| 1.42 | M | 9.17 | 6.33 | 11 | 4 | 100 | 75 | Yes |
| 1.42 | M | 5.92 | 5.92 | 7 | 6 | 100 | 17 | Yes |
| 1.42 | M | 1.00 | 1.00 | 3 | 3 | 50 | 33 | No |
| 2.25 | M | 5.67 | 1.75 | 9 | 4 | 67 | 75 | No |
| 2.42 | M | 8.75 | 7.50 | 15 | 8 | 100 | 88 | No |
| 2.92 | M | 12.25 | 10.00 | 12 | 6 | 20 | 0 | No |
| 3.17 | M | 7.42 | 7.33 | 8 | 6 | 60 | 33 | No |
| 3.42 | M | 7.08 | 5.67 | 12 | 8 | 100 | 100 | Yes |
| 3.50 | M | 8.17 | 6.33 | 10 | 3 | 0 | 33 | Yes |
| 4.00 | F | 4.08 | 4.08 | 6 | 5 | 75 | 100 | Yes |
| 4.08 | M | 4.75 | 1.75 | 9 | 3 | 100 | 67 | Yes |
| 4.42 | F | 9.25 | 7.67 | 18 | 8 | 100 | 75 | No |
| 4.75 | M | 5.58 | 4.00 | 17 | 7 | 83 | 71 | Yes |
| 4.92 | M | 1.33 | 1.33 | 3 | 3 | 100 | 0 | Yes |
| 4.92 | M | 9.42 | 5.92 | 13 | 5 | 100 | 100 | No |
| 5.08 | M | 8.83 | 3.83 | 20 | 9 | 100 | 100 | No |
| 5.25 | M | 12.83 | 5.50 | 14 | 4 | 67 | 25 | Yes |
| 6.58 | M | 2.67 | 2.67 | 4 | 4 | 100 | 75 | No |
| 6.75 | M | 6.00 | 6.00 | 8 | 4 | 100 | 50 | Yes |
| 6.83 | F | 9.75 | 8.75 | 11 | 5 | 75 | 40 | No |
| 7.17 | M | 4.67 | 4.67 | 7 | 5 | 100 | 100 | Yes |
| 7.83 | M | 1.33 | 1.33 | 4 | 4 | 100 | 50 | Yes |
| 8.08 | M | 6.17 | 4.17 | 11 | 6 | 100 | 50 | No |
| 8.75 | M | 8.08 | 6.00 | 13 | 4 | 100 | 100 | Yes |
| 9.33 | M | 4.08 | 3.50 | 7 | 5 | 50 | 100 | Yes |
| 9.33 | M | 8.50 | 7.17 | 10 | 3 | 50 | 67 | Yes |
| 10.75 | M | 2.42 | 2.42 | 5 | 5 | 100 | 20 | No |
| 13.67 | M | 4.17 | 1.75 | 8 | 4 | 100 | 75 | No |
| 16.58 | M | 1.25 | 0.92 | 4 | 3 | 50 | 33 | No |
| 17.08 | M | 1.25 | 0.67 | 4 | 3 | 100 | 67 | No |
| 5.95 (mean) |
6.04 (mean) |
4.54 (mean) |
9.44 (mean) |
5.03 (mean) |
79.58 (mean) |
60.23 (mean) |
Immunostaining and histologic assessment
H&E stained, formalin fixed, paraffin embedded specimens from 3 esophageal levels (proximal, middle, and distal; 2-3 biopsies per level) were evaluated by a single pathologist blinded to the therapy (R.N.). The numbers of epithelial and LP eosinophils, severity of basal zone hyperplasia, and the presence/absence of dilated intercellular spaces, desquamation, and LP fibrosis severity were quantified using our previously published pathology scoring tool (19). The epithelial remodeling score was generated by adding basal zone hyperplasia severity (0-3) to the presence or absence of dilated intercellular spaces and desquamation (0=absent, 1=present). LP fibrosis was scored 0-3 based on the density of collagen bundles. The average epithelial remodeling score and LP fibrosis score (FS) is reported.
Tissue sections (5μM) were deparaffanized and hydrated and immunostained as previously described for anti-TGFβ1 antibody (1:400, Santa Cruz Biochemicals, Santa Cruz, California) (2). The mean of the peak numbers of TGFβ1 positive cells from 3-5 hpf were quantified and reported as cells per mm2 using ImagePro (ImagePro, Media Cybernetics, Bethesda MD). All images were analyzed under identical light microscopic conditions, including magnification, gain, camera position, and background illumination.
Endoscopy and symptom score
Endoscopy and symptom scores were generated as previously described (19). Features of pallor/lichenification, plaques, concentric rings/strictures/narrowing, and friability were graded as present/absent (0/1) at each esophageal level for total endoscopy score (maximum=12). Symptoms of heartburn/regurgitation, nausea/vomiting, dysphagia, abdominal pain, and nocturnal awakening were graded 0-3 by subjects and their parents for the total symptom score.
Statistical analysis
All statistical analyses and graphing were done using GraphPad Prism (San Diego, CA). Comparisons between two groups were done using an unpaired t-test for continuous/Gaussian variables; Mann-Whitney test was used for non-parametric variables. Comparisons within a group were done as paired t-test or Wilcoxon matched pairs test for continuous/Gaussian variables or non-continuous variables, respectively. p value <0.05 was considered significant.
Results
Clinical characteristics
One hundred and twelve subjects from the EoE database were screened for the presence of biopsies with adequate lamina propria to assess remodeling features in at least 3 instances when followed clinically for at least 12 months and prescribed semi-continuous topical corticosteroid therapy with fluticasone or oral viscous budesonide as routine clinical care. Thirty-two subjects met all of the required criteria. Three subjects, all non-responders, had their diagnostic biopsies done at an outside facility. Twenty-nine subjects (91%) were male, 26 (81%) were atopic (defined as positive serum IgE or skin prick test to foods or aeroallergen), and 26 (81%) were Caucasian. The mean age at diagnosis was 6 years (13 months-17 years). Twenty subjects (63%) failed PPI monotherapy. Nine of the subjects were diagnosed prior to the EoE consensus recommendations of PPI monotherapy and before our current understanding of the PPI-responsive esophageal eosinophilia (20-22).
We evaluated 246 endoscopic procedures with 738 biopsies in the study time period (Table 1). In total, 486 biopsies were evaluated for remodeling. The remainder was excluded due to lack of adequate LP. These biopsies covered a maximum of 10 years and a mean of 4.5 years of follow up. There were 4.7 procedures per subject done on TCS (range=3-8). Overall, 63% and 80% of biopsies were procured on reported adherence with prescribed PPI and TCS, respectively (Table 1). The initial dose of TCS was 110-220 mcg 2 puffs swallowed twice daily for fluticasone propionate or 0.5-2mg daily for oral viscous budesonide. Repeat biopsies were done in the subjects for changes in prescribed or self-instituted TCS dose, non-adherence to prescribed regimen, or for routine surveillance follow up.
Baseline histologic and endoscopic features
Baseline epithelial eosinophils per hpf in the total cohort was 81 (95% CI 66, 95). Our standardized histology scoring tool includes an epithelial remodeling score encompassing basal zone hyperplasia severity + the presence/absence of dilated intercellular spaces + presence/absence of desquamation (maximum=5). The baseline mean epithelial remodeling score was 3.2 (95% CI 2.8, 3.6). The mean number of LP eosinophils per hpf was 23 (95% CI 13, 32), fibrosis score (FS) was 2.7 (maximum=3) (95% CI 2.5, 2.9), and the mean number of TGFβ1 positive cells was 1332 per mm2 (95% CI 1127, 1537) (Table 2).
Table 2.
Baseline Histologic, Endoscopic and Symptom Scores in Responders versus Non-responders.
| Category | Age (years) |
Epithelial Eosinophils per hpf |
Epithelial Remodeling (range 0-5) |
LP Eosinophils per hpf |
Fibrosis (range 0-3) |
TGFβ1 + (Cells/mm2) |
Symptom Score (0-14) |
EGD Features |
EGD Score (range 0- 15) |
|---|---|---|---|---|---|---|---|---|---|
| Responder | 5.25 | 86 | 3 | 120 | 3 | 745 | 2 | Pa, F | 6 |
| 1.42 | 100 | 3.33 | 18 | 2.66 | 2032 | 0 | Pa, F, Pl | 8 | |
| 3.42 | 70 | 4 | 27 | 2.66 | 1650 | 0 | Pa, F | 4 | |
| 1.42 | 80 | 4 | 62 | 3 | 2284 | 2 | Pa, F, Pl | 9 | |
| 9.33 | 50 | 3 | 13 | 1 | 983 | 7 | Pa, F | 6 | |
| 1.08 | 50 | 4 | 26 | 2.33 | 1330 | 4 | Pa, F, Pl | 9 | |
| 3.50 | 16 | 1.33 | 40 | 2 | 946 | 2 | Normal | 0 | |
| 8.75 | 150 | 3 | 4 | 2.5 | 1386 | 2 | Normal | 0 | |
| 9.33 | 80 | 3.33 | 7 | 2.66 | 1416 | 2 | Pa, F | 6 | |
| 7.17 | 50 | 0 | 8 | 3 | 710 | 0 | Pa, R | 2 | |
| 6.75 | 28 | 3 | 4 | 3 | 535 | 5 | Pa, F | 6 | |
| 4.08 | 15 | 1.33 | 5 | 2.3 | 464 | 4 | Pa, F | 4 | |
| 4.75 | 120 | 3.67 | 15 | 2.66 | 481 | 4 | Pa, F, Pl | 9 | |
| 7.83 | 95 | 3 | 2 | 3 | 1494 | 1 | Pa, Pl | 6 | |
| 4.00 | 58 | 3.67 | 18 | 3 | 2062 | 7 | Pl | 2 | |
| 4.92 | 110 | 2.33 | 13 | 3 | 2437 | 5 | Pa, F, Pl | 7 | |
| Mean | 5.19 | 72.38 | 2.87 | 23.88 | 2.61 | 1309 | 2.94 | 5.25 | |
| Non-responder | 2.92 | 115 | 3.33 | 50 | 3 | 1651 | 4 | Pa, F, Pl | 9 |
| 13.67 | 54 | 4 | 25 | 2 | 905 | 2 | Pa, F, R, Pl, S | 15 | |
| 2.42 | 100 | 1.67 | 10 | 1.5 | 1573 | 2 | Pa, Pl | 2 | |
| 5.08 | 40 | ND | ND | ND | ND | ND | ND | ND | |
| 4.42 | 100 | 4.67 | 25 | 3 | 1252 | 0 | Pa, F | 6 | |
| 8.08 | 100 | 2.67 | 7 | 3 | 1443 | 0 | Pa, F, Pl | 9 | |
| 2.25 | 40 | 4 | 1 | 3 | 2093 | 9 | Normal | 0 | |
| 6.83 | 89 | 4 | 7 | 3 | 1154 | 2 | Pa, F, Pl | 9 | |
| 4.92 | 50 | 3.33 | 34 | 3 | 1187 | 4 | Pa, fF | 6 | |
| 3.17 | 100 | ND | ND | ND | ND | ND | ND | ND | |
| 10.75 | 80 | 4.33 | 45 | 3 | 816 | 2 | Pa, F, Pl | 9 | |
| 6.58 | 50 | 4 | 27 | 3 | 1317 | 4 | Pa, F | 6 | |
| 16.58 | 95 | 3.33 | 9 | 3 | 878 | 1 | Pa, F, Pl | 7 | |
| 17.08 | 150 | 2.67 | 1 | 3 | 1714 | 6 | Pa, F, R | 9 | |
| 1.25 | 60 | ND | ND | ND | ND | ND | ND | ND | |
| 1.42 | 200 | 4.67 | 35 | 3 | 1690 | 10 | Pl | 2 | |
| Mean | 6.71 | 88.9 | 3.59 | 21.23 | 2.81 | 1359 | 3.54 | 6.85 |
Pa: Pallor; F: Furrows; Pl: Plaques, R: Rings; S: Stricture; EGD: Endoscopy
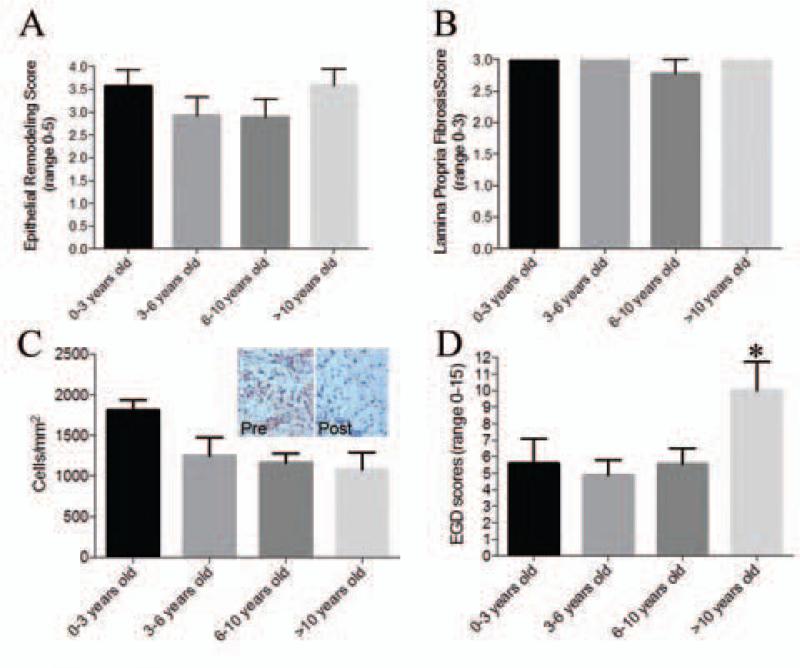
There were no differences in baseline histologic findings by age. Children 0-3 years old had epithelial remodeling, LP fibrosis and TGFβ1 positive cells that were similar to children in all of the older age groups (Figure 1a-c). However, there were significant differences in the baseline total EGD scores in children by age. Children who were > 10 years old had EGD scores that were almost twice as high (mean score=10, 95% CI 4.49, 15.51) as children who were <10 years old (mean EGD score=5.3, 95% CI 4.05, 6.6) (p=0.017) (Figure 1d).
Figure 1.
Esophageal remodeling features broken down by age. Epithelial remodeling (A), lamina propria fibrosis scores (B), TGFb1 positive cells (C), and EGD scores (D) by age groups
In adults, the validated endoscopic EREFS system uses concentric rings, narrowing, and strictures as esophageal remodeling features (23). EREFS is not validated in children. Our prior work suggests that, in children, LP remodeling features correlates with endoscopic linear furrows (19). Using rings, narrowing/strictures and/or furrows, 21 (65%) subjects had endoscopic remodeling with furrows in 21 (65%), rings in 3 (9%), and stricture in 1 (3%) (Table 2). Baseline endoscopic features reflective of inflammation (pallor, plaques) also occurred in the majority of subjects.
Longitudinal features in the total cohort
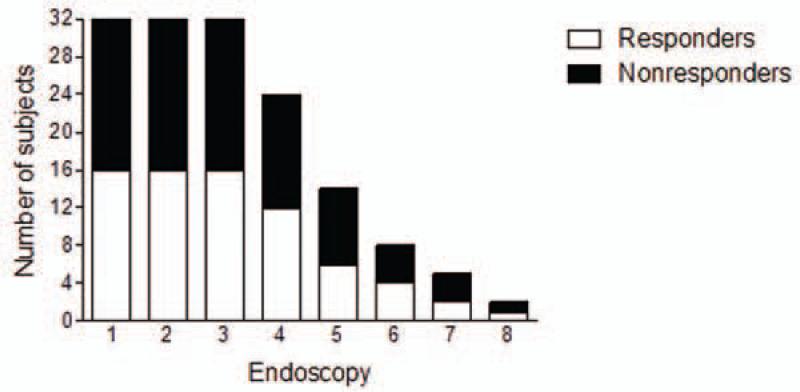
It is not possible to know exactly when esophageal inflammation reasonably started using symptom criteria in children. For this reason, we defined EoE initiation as the time of first histologic evidence of esophageal eosinophilia. All of the subjects could be evaluated through their 3rd endoscopy (2.6 years) and 44% could be evaluated through their 5th endoscopy (4.1 years) (Figure 2). In order to have sufficient numbers of subjects per group over time (Figure 2), we combined endoscopic procedures into instances such that endoscopies 4 and 5 were evaluated together (mean=4.1 years) and endoscopies 6-8 were evaluated together (mean=5.6 years).
Figure 2.
Number of study subjects by endoscopy. Clear bars represent responders, dark bars represent non-responders.
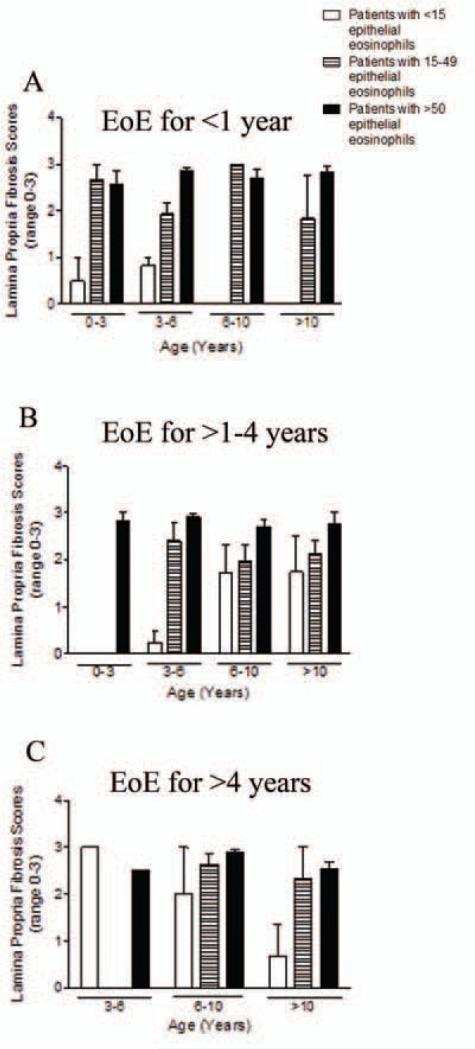
We analyzed the relationship between epithelial inflammation and histologic remodeling longitudinally over time. The severity of peak epithelial eosinophilia correlated positively with epithelial remodeling over the entire disease duration (r=0.76, p<0.0001) and in most instances (Table 3, “overall”=longitudinal over the entire disease, “instance”=at each endoscopy). Epithelial eosinophilia also correlated with LP fibrosis (r=0.53, p<0.001) and eosinophilia (r=0.54, p<0.001) both longitudinally and at multiple instances (Table 3). The severity of the mean epithelial eosinophilia correlated even more strongly with epithelial remodeling over time (Supplemental Table 1). Fibrotic severity depended on disease duration and inflammatory severity. Generally, subjects of all ages and disease durations had lower FS if they had <15 epithelial eosinophils (Figure 3a-c). The overall mean FS score over time in the children with eosinophils <15 per hpf was 1.03 as compared to a FS of 2.59 FS in children with >15 eosinophils per hpf (p<0.0001). TGFβ1 correlated with epithelial eosinophilia in the overall group only in the baseline biopsy (Table 3).
Table 3.
Correlation coefficients when comparing epithelial eosinophilia with epithelial features score, lamina propria eosinophil counts, lamina propria fibrosis score and TGFβ1 cell counts.
| Epithelial Remodeling | LP eosinophils | Fibrosis | TGFβ1+ Cells | |
|---|---|---|---|---|
| Time (years) | r (p) | r (p) | r (p) | r (p) |
| 0 | r=0.08 (p=0.68) | r= −0.03 (p=0.89) | r=0.19 (p=0.3) | r=0.38 (p=0.04) |
| 1.2 | r=0.85 (p<0.0001) | r=0.81 (p<0.0001) | r=0.77 (p<0.0001) | r=0.33 (p=0.06) |
| 2.6 | r=0.85 (p<0.0001) | r=0.65 (p=0.0002) | r=0.58 (p=0.002) | r=0.12 (p=0.50) |
| 4.1 | r=0.71 (p<0.0001) | r=0.46 (p=0.004) | r=0.42 (p=0.012) | r= −0.33 (p=0.045) |
| 5.6 | r=0.90 (p<0.0001) | r=0.61 (p=0.039) | r=0.62 (p=0.035) | r= −0.53 (p=0.041) |
| Overall | r=0.76 (p<0.0001) | r=0.54 (p<0.001) | r=0.53 (p<0.001) | r=0.06 (p=0.47) |
LP: Lamina Propria
Figure 3.
Fibrosis by age and disease duration. Fibrosis scores in children 0-3, >3-6, >6-10, and >10 years old by disease duration of <1 year (A) (there were no subjects in the 6-10 year old and >10 year old groups), >1-4 years (B) (there were no subjects in the <15 or 15-49 eosinophil per hpf groups), >4 years (C) (There were no subjects in the 3-6 yo 15-49 eosinophils per hpf group.
Longitudinal effects of therapies in addition to TCS
Since PPI therapy can alter esophageal eosinophilia and its effects on remodeling are unknown, we compared the degree of esophageal eosinophilia and remodeling between those subjects on or off PPI at any given instance (supplemental Figure 1). This analysis demonstrated that the presence of PPI did not have significant effects on eosinophilia or remodeling. We also completed a subgroup analysis using only those subjects (63%) who failed PPI monotherapy and found that this subgroup analysis was essentially identical to the analysis of the entire cohort longitudinally at each instance (left panel) and over the overall timeframe (right panel) (supplemental Figure 2).
We excluded time points in the study period when subjects were on oral corticosteroids (8 instances), dietary elimination (1 instance), or biologic therapy (4 instances). A secondary analysis that included these instances showed that overall epithelial eosinophilia and LP fibrosis was higher in those subjects who received other therapy in addition to TCS. This likely reflects the difficulty in controlling their disease. In addition, there were no substantial changes in these data when these additional instances were added (supplemental Figure 3).
Effects of topical corticosteroids on histologic features: responders versus non-responders
In order to compare subjects with active inflammation despite TCS therapy to those with controlled inflammation, we separated the subjects into “responders” and “non-responders” based on their initial histologic response of <15 eosinophils (responders) or ≥15 eosinophils (non-responders) per hpf. Based on these criteria, 16 subjects were responders and 16 were non-responders. Subjects were kept in their initial category for each instance evaluated over 10 years. No baseline symptom, histologic, or endoscopic features predicted initial responders from non-responders (Table 1, 2).
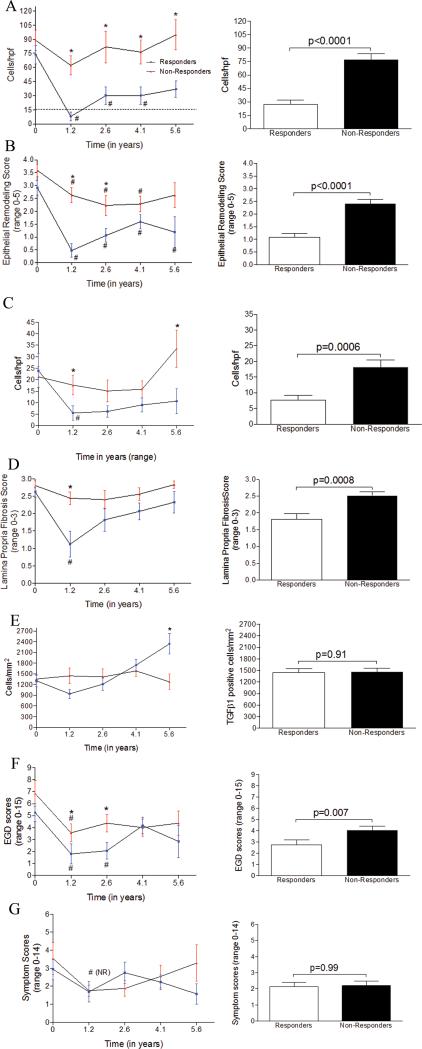
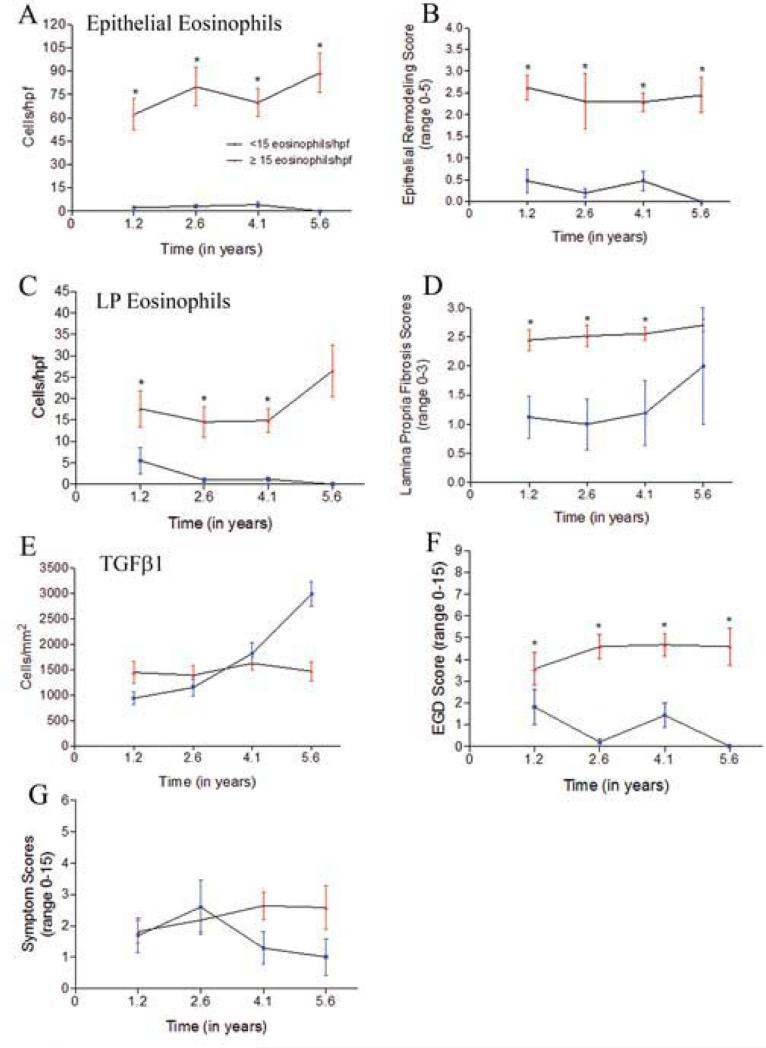
Responders and non-responders had equivalent numbers of biopsies to evaluate with 4.6 EGDs per subject (range=3-8) in responders over a mean of 4.7 years while nonresponders had 4.8 EGDs (range=3-8) per subject over a mean of 4.3 years. It is important to note that non-responders still had decreases in their eosinophil counts at a number of time points. They also had improvements in their symptoms and EGD scores (Figure 4).
Figure 4.
Histologic remodeling, endoscopic features, and subject symptoms over 10 years. Line graphs of responders (blue error bars) and non-responders (red error bars) over mean times of 1.2, 2.6, 4.1, and 5.6 years (left panels A-G) and cumulative findings over 10 years (right panels A-G) for epithelial eosinophils (A), epithelial remodeling (B), lamina propria eosinophils (C), lamina propria fibrosis (D), TGFβ1 positive cells (E), endoscopy scores (F), and symptoms (G). *: statistically significant between groups. #: statistically significant from baseline
Responders had significantly more substantial decreases in their epithelial eosinophilia and remodeling as compared with non-responders longitudinally over time (p<0.0001) (Figure 4a, b, right panel) and at multiple instances over the study period (Figure 4a, b, left panel). As a group, initial responders had fluctuating epithelial eosinophilia despite stated adherence to prescribed esophageal TCS (Figure 4a, left panel). Initial control of epithelial eosinophilia was maintained in 10 (62.5%) of responder subjects over 1.9 years when analyzing all of the biopsies even if LP was not available. The average time to recurrence of epithelial eosinophilia to >15 per hpf in responders was 2.8 years. Non-responders had non-significant improvements in their epithelial eosinophil counts initially (Figure 4a, left panel) and 4 time points from 4 individuals had epithelial eosinophils <15 per. As a group, the lowest epithelial eosinophil count per hpf in the non-responders was 62 per hpf which occurred at 1.21 years (Figure 4a). Responders and non-responders spent 46% and 2% of their disease duration, respectively, with histologic control of esophageal eosinophilia (p<0.0001). The reported rates of medication adherence were similar between responders (88%) and non-responders (84%) (p=0.47) over the 10 years, suggesting that medication use may not account for differences in therapeutic response. We also found that accounting for PPI use at any instance in responders and nonresponders did not significantly change any of the inflammatory or remodeling parameters except epithelial eosinophils at baseline in non-responders and LP eosinophils at year 4.1 in responders (data not shown).
At multiple instances over time and longitudinally over the study period, responders had fewer LP eosinophils (p=0.05) and lower FS (p=0.01) than non-responders (Figure 4c, d, left panel each instance, right panel overall). There was a significant correlation between LP eosinophils and fibrosis (r=0.54, p<0.0001) among responders. Responders and non-responders spent 23% and 54% of their time, respectively, at a maximal FS of 3 (p=0.025). A FS >2 correlated with epithelial eosinophil count of 78 per hpf and LP eosinophils of 20 per hpf while a FS <2 correlated with epithelial and LP eosinophils of 29 and 6 per hpf, respectively. Fibrosis went together with inflammation and seemed not to be significantly affected by TCS outside of inflammatory control since subjects on or off TCS ≥75% or <75% did not have significantly different FS and subjects with <50 eosinophils per hpf over time had significantly lower FS, (supplemental Figure 5). Consistent with this, subjects who had >50 eosinophils per hpf over their disease duration had significantly higher FS and EGD scores than subjects who had <15 or 15-50 eosinophils per hpf overall (supplemental Figure 5). The time to the first EGD was longer in this cohort of subjects than our previously published cohorts and, as such, the current data show that pediatric responder subjects can have control of many remodeling features including fibrosis for >1 year while successfully treated with TCS.
TGFβ1 expressing cells were lower at the first EGD following TCS in responders (p=0.05, Figure 4e) but not over the entire disease duration. TGFβ1 counts were lower in responders than non-responders for 2.6 years. Unexpectedly, TGFβ1 counts increased in responders to above that seen in non-responders at year 4.1 of therapy. In responders, the numbers of TGFβ1 positive cells correlated with the FS (r=0.32, p=0.01) but this was not the case in non-responders.
We also assessed the potential impact of atopy in our subjects. Of the 32 subjects, 84% had some atopic disease. Twenty-two subjects had allergic rhinitis (11 responders, 11 nonresponders), 9 had asthma (3 responders, 6 non-responders), 8 had eczema (3 responders, 5 non-responders), and 13 had food allergies (5 responders, 8 non-responders). Since there were more non-responders who had asthma and/or food allergies, we assessed potential differences in disease control in these subjects. Asthmatic subjects had significantly higher baseline epithelial eosinophils than non-asthmatics (supplemental Figure 6). There were no other differences in the inflammatory parameters in asthmatic or food allergic patients at baseline or overall (data not shown).
Effects of topical corticosteroids on endoscopy and symptoms
EGD scores decreased in proportion to the epithelial remodeling (r=0.73, p<0.0001) and the LP FS (r=0.58, p<0.0001) in responders. At multiple instances and over the disease duration, responders had significantly lower EGD scores than non-responders (mean 2.8 vs 4.0, p<0.01, Figure 4f). Using furrows, strictures, rings, or narrowing as endoscopic signs of remodeling, responders and non-responders spent 44% and 27%, respectively, of their disease duration with an endoscopically un-remodeled esophagus (p=0.04). Four children had strictures or rings, 3 of whom had these findings at baseline. One subject was a responder whose stricture reversed on therapy. The other 3 were non-responders and among them, 1 had improvement, 1 had no improvement, and 1 progressed to narrowing despite therapy. The 2 patients who had no improvement of progression to stricture spent 100% of their time during the study period at FS=3. Symptoms were not consistently different among the responders or nonresponders at any instances or overall (Figure 4g).
Results using shifting groups for responders and non-responders
For the purposes of this study, responders were defined as those subjects who responded initially to therapy. However, epithelial eosinophil counts did fluctuate from one biopsy to the next. For this reason, we re-analyzed the longitudinal data by allowing subjects to shift categories (that is, responder to non-responder and vice versa) at any given instance. Using this analysis, 62.5% of subjects were responders over the 10 years of follow up and it was evident that decreases in epithelial eosinophilia associated with decreases in epithelial remodeling, LP eosinophilia, and FS (Figure 5a-d). In subjects who had <15 eosinophils per hpf, the overall average epithelial remodeling score was 0.36, LP eosinophil count was 3, and FS was 1.2 as compared to 2.4 (p<0.0001), 17 (p<0.0001), and 2.5 (p<0.0001), respectively in the group with ≥15 eosinophils per hpf. TGFβ1 positive cells were lower in the group with <15 eosinophils per hpf for only the first two instances (Figure 5e). Esophageal epithelial eosinophil counts of <15 per hpf corresponded to lower endoscopy scores (Figure 5f). In contrast, symptoms did not reflect other disease features even in this analysis (Figure 5g).
Figure 5.
Histologic remodeling, endoscopic features, and symptoms among subjects with less than and ≥15 eosinophils per high power field at each time point. Line graphs of subjects with <15 (blue error bars) and >15 eosinophils per hpf (red error bars) over mean times of 1.2, 2.6, 4.1, and 5.6 years for epithelial eosinophils (A), epithelial remodeling (B), lamina propria eosinophils (C), lamina propria fibrosis (D), TGFβ1 positive cells (E), endoscopy scores (F), and symptom scores (G). *: statistically significant between groups.
Discussion
The concept of tissue remodeling and eosinophil associated fibrosis has been established in asthma, the hypereosinophilic syndrome, and, more recently, EoE (24). EoE allows a unique opportunity to study histologic remodeling in the context of clinical features over long time frames since management requires repeated tissue assessment (1). The natural history and potential of therapy to sustain fibrotic control in eosinophil associated diseases is not clear, especially in children. However, this is conceptually important since fibrosis appears to be an integral mechanism to strictures. Herein we report a number of novel findings based on data from >400 esophageal biopsy specimens obtained over 10 years in 32 children with EoE. First, we show that epithelial inflammation is concordant with epithelial remodeling, LP eosinophilia and fibrosis in children both longitudinally and at multiple time points over a maximum of 10 years of treatment. This information is important since fibrostenosis is essentially universal in untreated adults and since the theory that epithelial inflammatory control corresponds to fibrotic control has previously been only an assumption. Second, we demonstrate that histologic EoE control by TCS in pediatric EoE may help to mitigate disease complications of stricture and food impactions as only 1 subject progressed to esophageal narrowing. Third, our data suggest that there are likely to be intrinsic differences at the genetic/molecular level between patients who do and do not respond completely to therapy since responders and non-responders reported adherence to medications at the majority of instances. This underscores the need for additional data on maintenance regimens and additional or complementary therapeutic options in EoE.
The most severe disease complication in EoE is stricture formation which is driven by tissue remodeling (1, 6, 7, 10). In adults, risk factors for food impactions and strictures include esophageal rigidity, untreated disease duration, and less frequent use of TCS (3, 6, 7, 11). In our cohort of responders, those that used TCS ≥75% of the time trended toward lower FS. In adults, fibrosis can be difficult to reverse once established (25, 26). Children have more inflammation and less fibrostenosis and our data suggests that long term EoE control can keep fibrosis at a lower severity. Initially dropping eosinophils to <15 resulted in fibrosis score of 1 (normal using our FS). Since none of the responders had the onset of stricture while 2 non-responders had no improvement or progression of narrowing, our data suggest that the goal in children of preventing esophageal fibrostenosis may be accomplished when epithelial inflammation is controlled.
Our study also shows that there is recurrence of eosinophilia despite continued therapy. This aligns with recently published adult data from Kuchen et al that, despite chronic TCS therapy, most adult EoE subjects had histologic disease activity of >10 eosinophils per hpf over a 5 years treatment period (11) and underscores the importance of continuous follow-up in people with EoE. In our current study, 4 non-responders shifted to “responders” at 4 instances and 2 were due to increased medication dose or compliance. Responders had recurrence of esophageal eosinophilia; 6 stopped their medications but 9 had no change in their medications. The reason for recurrence of epithelial inflammation are not clear but could include intermittent medication adherence, worsening acid induced eosinophilia, accumulation of allergic triggers, seasonality, onset of TCS resistance, age and maturation of other systems such as hormonal axes during puberty, and/or differential penetration of TCS in the inflamed versus less inflamed esophagus. What does seem clear, however, is that there are distinct EoE phenotypes including persistent responders, transient responders, and perhaps even intermittent responders. Further understanding of the degree of inflammatory control that is necessary to prevent complications will be important in guiding management.
There are inherent limitations in our study. We did not study other treatments such as elimination diet and the limitation to TCS use could introduce potential bias in the population. However, we wanted to study a more homogenous population. Indeed, we had a very high percentage of male subjects (91%) and this also may help with the population homogeneity. Since this was not a prospective, randomized trial and since holding EoE therapy would not be medically advised, this study cannot include an untreated natural history cohort. As such, the persistent non-responder group functions as the closest approximation to a natural history cohort that we have available. Since non-responders still had some decreases in remodeling features and inflammation at certain instances, TCS is having a partial effect in this population. In addition, our overall cohort may be biased toward a more severe phenotype. In general, the presence of LP, a requirement for evaluation in this is study, was associated with higher epithelial eosinophils. In addition, it was rare to have a FS of 0-1 on the biopsies we were able to evaluate. As such, in children, the mere absence of LP in a biopsy with no or low epithelial eosinophilia may imply a non-fibrotic esophagus. This coupling of epithelial inflammation and LP fibrosis in children may allow for surrogate markers of fibrosis that occur in the epithelium. Despite the retrospective nature of our study, it is the first of its kind to assess longitudinal remodeling in children with EoE.
Although we saw alignment between epithelial inflammation and fibrosis as well as endoscopic features, found alignment with TGFβ1 except early in the disease course and only in responders. While there were initial decreases in TGFβ1 positive cells among responders, this was not sustained and, in fact, responders progressed to having more TGFβ1 positive cells than non-responders at later time points. These increases did associate with increasing FS but may also reflect a shift in the cellular compartment of cells that produce TGFβ1. For example, it is possible that early in the disease course inflammatory cells make TGFβ1 but that later in the disease TGFβ1 is produced by regulatory cells in responders. There may also be a shift in profibrotic molecules during the disease. For example, CXCL18 and FGF-9 have been reported to be elevated in adult EoE (25).
In conclusion, studies to date in pediatric and adult populations demonstrate that EoE is a chronic disease that recurs upon removal of therapy (8, 9, 26) but the history of tissue remodeling has not been systematically studied previously in adults or children. Our current study demonstrates that esophageal remodeling can be improved with long-term with TCS. Our data would suggest that childhood or shorter disease duration offers an important window for controlling fibrostenotic complications. Larger, longer studies are needed to clearly distinguish EoE phenotypes both on the therapeutic and molecular levels.
Supplementary Material
Clinical Implications.
Long term esophageal remodeling is associated with eosinophilia in pediatric EoE and can be improved following esophageal TCS therapy.
Capsule Summary.
Esophageal remodeling can remain improved in children with EoE treated with long-term esophageal TCS therapy and the severity of histologic remodeling correlates with the degree of eosinophilia over the 10 years studied.
Acknowledgements
This work was funded by NIH/NIAID AI 092135 (S.A), ART/APFED HOPE Award (S.A.), DOD FA100044 (D.H.B, S.A.), NIH/NIAID AI 107779 (D.H.B.), AI 70535 (D.H.B.), AI 72115 (D.H.B.), Hearst Foundation (R.D.) We thank Arjun Andrew Anilkumar, Tom Yang, and Loan Duong for technical assistance.
Abbreviations
- LP
Lamina Propria
- EoE
Eosinophilic esophagitis
- TCS
Topical corticosteroid
- TGFβ1
Transforming growth factor beta-1
- EGD
Esophagogastroduodenoscopy
- FS
Fibrosis score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts: SA and RD are co-inventors of oral viscous budesonide, patented by UCSD and hold stock options in Meritage Pharmaceutical
References
- 1.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. The Journal of allergy and clinical immunology. 2011 Jul;128(1):3–20. e6. doi: 10.1016/j.jaci.2011.02.040. quiz 1-2. PubMed PMID: 21477849. Epub 2011/04/12. eng. [DOI] [PubMed] [Google Scholar]
- 2.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. The Journal of allergy and clinical immunology. 2007 Jan;119(1):206–12. doi: 10.1016/j.jaci.2006.10.016. PubMed PMID: 17208603. Epub 2007/01/09. eng. [DOI] [PubMed] [Google Scholar]
- 3.Nicodeme F, Hirano I, Chen J, Robinson K, Lin Z, Xiao Y, et al. Esophageal Distensibility as a Measure of Disease Severity in Patients with Eosinophilic Esophagitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013 Apr 13; doi: 10.1016/j.cgh.2013.03.020. PubMed PMID: 23591279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirano I, Aceves SS. Clinical implications and pathogenesis of esophageal remodeling in eosinophilic esophagitis. Gastroenterology clinics of North America. 2014 Jun;43(2):297–316. doi: 10.1016/j.gtc.2014.02.015. PubMed PMID: 24813517. Pubmed Central PMCID: 4127387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aceves SS, Ackerman SJ. Relationships between eosinophilic inflammation, tissue remodeling, and fibrosis in eosinophilic esophagitis. Immunology and allergy clinics of North America. 2009 Feb;29(1):197–211. xiii–xiv. doi: 10.1016/j.iac.2008.10.003. PubMed PMID: 19141355. Pubmed Central PMCID: 2665721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoepfer AM, Safroneeva E, Bussmann C, Kuchen T, Portmann S, Simon HU, et al. Delay in Diagnosis of Eosinophilic Esophagitis Increases Risk for Stricture Formation, in a Time-Dependent Manner. Gastroenterology. 2013 Aug 13; doi: 10.1053/j.gastro.2013.08.015. PubMed PMID: 23954315. [DOI] [PubMed] [Google Scholar]
- 7.Dellon ES, Kim HP, Sperry SL, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointestinal endoscopy. 2013 Nov 23; doi: 10.1016/j.gie.2013.10.027. PubMed PMID: 24275329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assa'ad AH, Putnam PE, Collins MH, Akers RM, Jameson SC, Kirby CL, et al. Pediatric patients with eosinophilic esophagitis: an 8-year follow-up. The Journal of allergy and clinical immunology. 2007 Mar;119(3):731–8. doi: 10.1016/j.jaci.2006.10.044. PubMed PMID: 17258309. Epub 2007/01/30. eng. [DOI] [PubMed] [Google Scholar]
- 9.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, Franciosi J, Shuker M, Verma R, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. Journal of pediatric gastroenterology and nutrition. 2009 Jan;48(1):30–6. doi: 10.1097/MPG.0b013e3181788282. PubMed PMID: 19172120. Epub 2009/01/28. eng. [DOI] [PubMed] [Google Scholar]
- 10.Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology. 2003 Dec;125(6):1660–9. doi: 10.1053/j.gastro.2003.09.024. PubMed PMID: 14724818. [DOI] [PubMed] [Google Scholar]
- 11.Kuchen T, Straumann A, Safroneeva E, Romero Y, Bussmann C, Vavricka S, et al. Swallowed topical corticosteroids reduce the risk for long-lasting bolus impactions in eosinophilic esophagitis. Allergy. 2014 Jun 3; doi: 10.1111/all.12455. PubMed PMID: 24894658. [DOI] [PubMed] [Google Scholar]
- 12.Cortina S, McGraw K, Dealarcon A, Ahrens A, Rothenberg ME, Drotar D. Psychological Functioning of Children and Adolescents With Eosinophil-Associated Gastrointestinal Disorders. Children's health care : journal of the Association for the Care of Children's Health. 2010 Oct;39(4):266–78. doi: 10.1080/02739615.2010.515927. PubMed PMID: 21532963. Pubmed Central PMCID: 3082134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology. 2012 Jun;142(7):1451–9. e1. doi: 10.1053/j.gastro.2012.03.001. PubMed PMID: 22391333. Epub 2012/03/07. eng. [DOI] [PubMed] [Google Scholar]
- 14.Butz BK, Wen T, Gleich GJ, Furuta GT, Spergel J, King E, et al. Efficacy, Dose Reduction, and Resistance to High-Dose Fluticasone in Patients With Eosinophilic Esophagitis. Gastroenterology. 2014 Apr 22; doi: 10.1053/j.gastro.2014.04.019. PubMed PMID: 24768678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aceves SS, Newbury RO, Chen D, Mueller J, Dohil R, Hoffman H, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy. 2010 Jan;65(1):109–16. doi: 10.1111/j.1398-9995.2009.02142.x. PubMed PMID: 19796194. Pubmed Central PMCID: 2807896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lieberman JA, Morotti RA, Konstantinou GN, Yershov O, Chehade M. Dietary therapy can reverse esophageal subepithelial fibrosis in patients with eosinophilic esophagitis: a historical cohort. Allergy. 2012 Oct;67(10):1299–307. doi: 10.1111/j.1398-9995.2012.02881.x. PubMed PMID: 22913672. [DOI] [PubMed] [Google Scholar]
- 17.Abu-Sultaneh SM, Durst P, Maynard V, Elitsur Y. Fluticasone and food allergen elimination reverse sub-epithelial fibrosis in children with eosinophilic esophagitis. Digestive diseases and sciences. 2011 Jan;56(1):97–102. doi: 10.1007/s10620-010-1259-5. PubMed PMID: 20458625. Epub 2010/05/12. eng. [DOI] [PubMed] [Google Scholar]
- 18.DeBrosse CW, Franciosi JP, King EC, Butz BK, Greenberg AB, Collins MH, et al. Long-term outcomes in pediatric-onset esophageal eosinophilia. The Journal of allergy and clinical immunology. 2011 Jul;128(1):132–8. doi: 10.1016/j.jaci.2011.05.006. PubMed PMID: 21636117. Pubmed Central PMCID: 3130990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aceves SS, Newbury RO, Dohil MA, Bastian JF, Dohil R. A symptom scoring tool for identifying pediatric patients with eosinophilic esophagitis and correlating symptoms with inflammation. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2009 Nov;103(5):401–6. doi: 10.1016/S1081-1206(10)60359-6. PubMed PMID: 19927538. [DOI] [PubMed] [Google Scholar]
- 20.Wen T, Dellon EP, Moawad FJ, Furuta GT, Aceves SS, Rothenberg ME. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton pump-inhibitor reversible allergic inflammation. The Journal of allergy and clinical immunology. 2014 doi: 10.1016/j.jaci.2014.08.043. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dohil R, Newbury RO, Aceves S. Transient PPI responsive esophageal eosinophilia may be a clinical sub-phenotype of pediatric eosinophilic esophagitis. Digestive diseases and sciences. 2012 May;57(5):1413–9. doi: 10.1007/s10620-011-1991-5. PubMed PMID: 22134787. [DOI] [PubMed] [Google Scholar]
- 22.Dellon ES, Speck O, Woodward K, Gebhart JH, Madanick RD, Levinson S, et al. Clinical and Endoscopic Characteristics do Not Reliably Differentiate PPI-Responsive Esophageal Eosinophilia and Eosinophilic Esophagitis in Patients Undergoing Upper Endoscopy: A Prospective Cohort Study. The American journal of gastroenterology. 2013 Dec;108(12):1854–60. doi: 10.1038/ajg.2013.363. PubMed PMID: 24145677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrean C, Hirano I. Eosinophilic esophagitis: pathophysiology and optimal management. Current gastroenterology reports. 2009 Jun;11(3):175–81. doi: 10.1007/s11894-009-0028-0. PubMed PMID: 19463216. Epub 2009/05/26. eng. [DOI] [PubMed] [Google Scholar]
- 24.Klion A. Hypereosinophilic syndrome: current approach to diagnosis and treatment. Annual review of medicine. 2009;60:293–306. doi: 10.1146/annurev.med.60.062107.090340. PubMed PMID: 19630574. [DOI] [PubMed] [Google Scholar]
- 25.Lucendo AJ, Arias A, De Rezende LC, Yague-Compadre JL, Mota-Huertas T, Gonzalez-Castillo S, et al. Subepithelial collagen deposition, profibrogenic cytokine gene expression, and changes after prolonged fluticasone propionate treatment in adult eosinophilic esophagitis: a prospective study. The Journal of allergy and clinical immunology. 2011 Nov;128(5):1037–46. doi: 10.1016/j.jaci.2011.08.007. PubMed PMID: 21880354. [DOI] [PubMed] [Google Scholar]
- 26.Straumann A, Conus S, Degen L, Frei C, Bussmann C, Beglinger C, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011 May;9(5):400–9. e1. doi: 10.1016/j.cgh.2011.01.017. PubMed PMID: 21277394. Epub 2011/02/01. eng. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.