Abstract
Children with sickle cell anemia (SCA) and conditional transcranial Doppler (TCD) ultrasound velocities (170-199 cm/sec) may develop stroke. However, with limited available clinical data, the current standard of care for conditional TCD velocities is observation. The efficacy of hydroxyurea in preventing conversion from conditional to abnormal TCD (≥200 cm/sec), which confers a higher stroke risk, has not been studied prospectively in a randomized trial. Sparing Conversion to Abnormal TCD Elevation (SCATE #NCT01531387) was an NHLBI-funded Phase III multicenter international clinical trial comparing alternative therapy (hydroxyurea) to standard care (observation) to prevent conversion from conditional to abnormal TCD velocity in children with SCA. SCATE enrolled 38 children from the United States, Jamaica, and Brazil [HbSS (36), HbSβ0-thalassemia (1), and HbSD (1), median age 5.4 years (range, 2.7-9.8)]. Due to slow patient accrual and administrative delays, SCATE was terminated early. In an intention-to-treat analysis, the cumulative incidence of abnormal conversion was 9% (95% CI 0 to 35%) in the hydroxyurea arm and 47% (95% CI 6 to 81%) in observation arm at 15 months (p=0.16). In post-hoc analysis according to treatment received, significantly fewer children on hydroxyurea converted to abnormal TCD velocities, compared to observation (0% versus 50%, p=0.02). After a mean of 10.1 months, a significant change in mean TCD velocity was observed with hydroxyurea treatment (−15.5 versus +10.2 cm/sec, p=0.02). No stroke events occurred in either arm. Hydroxyurea reduces TCD velocities in children with SCA and conditional velocities.
Keywords: Conditional TCD, stroke, sickle cell disease, SCATE, TAMV
INTRODUCTION
Since the implementation of screening programs with transcranial Doppler examinations, the number of first strokes in children with sickle cell anemia (SCA) has significantly decreased. Identification of high risk patients (abnormal time-averaged mean velocity, TAMV ≥ 200 cm/sec) followed by chronic blood transfusion therapy, has effectively prevented most primary strokes.(1;2) Patients with abnormal TCD velocities are at highest risk for developing cerebrovascular accidents; however, stroke events can also occur among children whose last screening TCD examinations document conditional velocities (TAMV 170 - 199 cm/sec).(3;4) Although their risk of primary stroke is lower relative to patients with abnormal TCD velocities (2 - 5% versus 9% per year), there are more patients with conditional than abnormal TCD velocities (prevalence 17% versus 9%), and the absolute number of children who might develop stroke is roughly equivalent between these two screening categories.(3;5-7)
Although conditional TCD velocities represent a moderate risk for developing primary stroke,(3;6) the current approach is clinical observation without specific disease-modifying therapy. However, the risk of TCD conversion from conditional to abnormal velocities is substantial and ranges from 15-55%.(7) Once conversion occurs, the established treatment is chronic transfusion therapy,(4) posing a substantial burden to patients and families, especially in resource-limited nations with an inadequate or less safe blood supply.
There is evidence that hydroxyurea can reduce TCD velocities in children with SCA,(8-13) but further investigation is necessary to define the efficacy of hydroxyurea for preventing conversion from conditional to abnormal TCD velocities, thereby preventing the transition from moderate to high stroke risk category. Successfully preventing conversion to abnormal TCD velocities with hydroxyurea would avoid exposure to prolonged blood transfusion and its known complications (e.g., iron overload, alloimmunization, and infections). Prevention of abnormal TCD conversion would, therefore, represent a significant improvement in the medical management of children with SCA at moderate risk for stroke, and ultimately reduce medical costs while simultaneously providing an established effective preventive treatment for other sickle-related medical complications (e.g., painful events).
Sparing Conversion to Abnormal TCD Elevation (SCATE, Clinical trials.gov NCT01531387) was a National Heart, Lung, and Blood Institute (NHLBI)-funded Phase III multicenter international randomized clinical trial that compared alternative therapy (hydroxyurea) to standard care (observation) to prevent conversion of TCD velocities from the conditional to the abnormal range in children with SCA. SCATE enrolled children from the United States (US), Jamaica, and Brazil. This study was terminated early due to slow patient accrual and a low likelihood of reaching the primary study endpoint. However, even with challenges of low patient accrual and regulatory difficulties that limited the number of participants who actually received hydroxyurea therapy, a significant reduction in TCD velocities and abnormal TCD conversion were observed in the hydroxyurea-treated group. These findings suggest that hydroxyurea represents a potential treatment choice for children with conditional TCD velocities, to help reduce the risk of first stroke.
METHODS
Patient Enrollment
Children 2-11 years of age with SCA (HbSS, HbSβ0-thalassmia, HbSOArab, or HbSD) and conditional TCD velocities were recruited between May 2012 and August 2013 at three sites: St. Jude Children’s Research Hospital (St. Jude), Memphis, TN, US; Sickle Cell Unit, Tropical Medicine Research Institute (TMRI), University of the West Indies, Kingston, Jamaica; and Instituto de Hematologia Arthur Siqueira Cavalcanti (HEMORIO), Rio de Janeiro, RJ, Brazil (recruitment brochure provided as supplemental material). To meet study eligibility, a screening TCD exam with conditional TCD velocity was required within 3 months of enrollment. Participants were excluded for prior history of abnormal TCD velocities or clinical stroke, red blood cell transfusion within 2 months of enrollment, concurrent use of another anti-sickling medication, or contra-indication to hydroxyurea therapy (allergy, pregnancy, renal insufficiency). In addition, patients were temporarily excluded from enrollment for profound anemia and/or cytopenias [hemoglobin (Hb) concentration <6 g/dL, absolute reticulocyte count (ARC) <100 × 109/L with Hb concentration <8.0 gm/dL, white blood cell count (WBC) <3.0 × 109/L, absolute neutrophil count (ANC) <1.0 × 109/L, or platelet count <100 × 109/L]. All participants received standard age-appropriate care for SCA (penicillin prophylaxis, pneumococcal immunization, and disease education) as per local standards of care.
TCD Exam Procedure and Study Coordination
SCATE was a Phase III, randomized, open label, partially masked, multi-center, international, prospective trial of hydroxyurea versus observation for children with SCA and centrally confirmed conditional TCD velocities. The study was partially masked in the following fashion: (1) all site TCD examiners and central TCD reviewers were masked to the assigned treatment arm; and (2) all site clinicians were masked to the participants’ serial TCD results.
All three sites used a standardized TCD examination protocol with identical equipment (Sonaratek TCD system, software version 6.68, Middleton, WI). The TCD examiners from each site received standardized training, and all were required to pass a certification process to ensure proficiency in performing the examinations according to the SCATE protocol. The TAMV was obtained bilaterally in five arteries: middle cerebral artery (MCA), anterior cerebral artery (ACA), posterior cerebral artery (PCA), internal carotid artery (ICA), and internal carotid bifurcation (Bif). All exams were scored locally and sent electronically to the Data Coordinating Center (DCC) by secure data transfer, and then de-identified and uploaded to the TCD Core for review and scoring.
Pre-enrollment screening TCD exams were performed at each clinical site as per normal practice. When a conditional TCD velocity was identified, the child and family were approached regarding enrollment in the trial. After informed consent, this exam was reviewed centrally to confirm the conditional velocity. Additional TCD exams were then performed to document a second conditional velocity. Two independent exams with conditional velocities were required within 6 months to proceed to randomization. Once randomized, follow-up TCD exams were performed every 3 months thereafter (supplemental Figure 1). All TCD exams were evaluated by the TCD Core and masked to study site, subject demographics, and clinical status.
The SCATE study used a remote internet-based data capture system with secure data entry and storage in a central database maintained by the DCC. Hydroxyurea was provided in capsules of different sizes (300 mg, 400 mg, and 500mg) to facilitate dosing and were purchased from Bristol-Myers Squibb (New York, NY). Hydroxyurea powder was obtained from UPM Pharmaceuticals (Baltimore, MD) and used for reconstitution as a liquid formulation (100 mg/mL).(14) A Central Pharmacy distributed the study drug (capsules and powder) to all sites and monitored drug expiration.
The Medical Coordinating Center (MCC) ensured quality of the data, timely data entry, guidance to sites in following the protocol with regards to hydroxyurea dosing, and ongoing monitoring of adverse events. The study was approved by the Institutional Review Board of all participating institutions, and informed consent was signed by all guardians of the children, prior to any study-related activities. An independent Data Safety Monitoring Board evaluated the unblinded study results for safety and study endpoints.
Study Endpoints
The primary endpoint of the SCATE trial was the cumulative incidence of conversion to abnormal maximum TAMV velocities (in any of the 10 vessels measured). Secondary endpoints included: (1) changes in serial TCD velocities; (2) cumulative incidence of neurological and non-neurological acute events including stroke; and (3) health-related quality of life (HRQOL). The MCC informed the site clinicians once subjects met the primary endpoint of the study (conversion to abnormal maximum TAMV velocities), at which point the subject was removed from the study.
Study Procedures and Evaluation
After randomization, participants received hydroxyurea treatment or observation, planned for a total of 30 months. Monthly physical exams and laboratory tests (monthly complete blood counts and reticulocyte counts and bi-monthly chemistries) were performed locally. Hb identification using high performance liquid chromatography with quantitation of HbA, HbS, and fetal Hb (HbF) was obtained quarterly and centrally analyzed. For subjects randomized to the alternative (hydroxyurea) arm, the initial drug dose was 20 mg/kg/day and escalated to maximum tolerated dose (MTD) at 5 mg/kg/day increments to a dose not to exceed 35 mg/kg/day.(15) MTD was defined as the daily dose leading to mild neutropenia (ANC 2.0 to 4.0 × 109/L), without reaching pre-defined thresholds of hematological toxicity, defined as ANC <1.0 × 109/L, Hb concentration <7.5 gm/dL with absolute reticulocyte count <100 × 109/L, platelets <80 × 109/L, or ARC <80 × 109/L with hemoglobin concentration <8.5 gm/dL. Hydroxyurea dose was held for one week if hematological toxicity occurred, and restarted at the same dose or with a 10% reduction if toxicity recurred. Hydroxyurea adherence (%) was assessed through calculation of returned doses, divided by the dispensed amount, at every study visit.
Statistical Considerations
The SCATE study was designed to have a 5% chance (α = 0.05) of falsely concluding that hydroxyurea reduces the cumulative incidence of conversion to abnormal TCD velocity, when in fact there is no difference between the hydroxyurea and standard treatment groups; and at least 80% (= 1 − ß) power to correctly conclude that hydroxyurea reduces the cumulative incidence of conversion to abnormal TCD velocity, given the true effect is at least a three-fold reduction in cumulative incidence after 30-months. Assuming a 5% per year dropout rate, 100 randomized participants were required to demonstrate that hydroxyurea reduces the cumulative incidence of conversion to abnormal TCD velocity. Children were randomized at a 1 to 1 ratio and used an adaptive blocked randomization algorithm. The intention-to-treat principle was followed and all eligible, randomized participants were analyzed as randomized, in the primary analysis. As a secondary post-hoc analysis, participants were analyzed according to the actual treatment received (hydroxyurea versus observation), since two children were randomized but did not receive treatment with hydroxyurea due to unavailability of study drug at one site. The exact log-rank test (1-sided) was used to compare the cumulative incidence of conversion to abnormal TCD velocity. The exact Wilcoxon-Mann-Whitney test was used to compare the other secondary outcomes of longitudinal TCD velocity changes, acute events, and standardized z-scores for weight, height, and body mass index (BMI).
RESULTS
Participant Characteristics and Treatment Allocation
During the period of study enrollment from May 2012 to August 2013, a total of 1234 children received TCD screening across all three participating sites. There were 1062 (86%) children with normal, 115 (9%) with conditional, and 24 (2%) with abnormal TCD velocities, while 33 (3%) were technical failures (Figure 1).
Figure 1. Consort diagram of the SCATE study.
* These 12 subjects were either not yet confirmed or already centrally confirmed as conditional TCD, but not randomized due to early study suspension and later study closure. **Two subjects did not receive hydroxyurea due to drug unavailability at one of the sites.
Among the 115 children with conditional TCD exams identified during screening, 38 enrolled (18 from Jamaica, 13 from Brazil, and 7 from the US) and 22 were randomized to either hydroxyurea or observation. There were 4 children who dropped out during screening (due to not having a confirmatory TCD), while 12 were in screening at the time of study closure (Figure 1). The median age at enrollment was 5.4 years (range 2.7 to 9.8 years). Most participants were female (25/38) and their self-reported races were 28 black and 10 white, reflecting the demographics of the international clinical sites. Genotypes included HbSS (36), HbSβ0-thalassemia (1), and HbSD (1). The demographics and laboratory findings at baseline are shown in Table 1.
Table 1.
Patient Characteristics at Baseline.
| Variable | Randomization arm | |
|---|---|---|
| Hydroxyurea (N=11) | Observation (N=11) | |
| Age (years) | ||
| 6.2 (2.4) | 6.6 (1.5) | |
| Gender (n,%) | ||
| Males | 4 (36) | 4 (36) |
| Females | 7 (64) | 7 (64) |
| Ethnicity (n,%) | ||
| Latino | 2 (18) | 3 (27) |
| Non-Hispanic | 9 (82) | 8 (73) |
| Race (n,%) | ||
| Black | 10 (91) | 9 (82) |
| White | 1 (9) | 2 (18) |
| Genotype (n,%) | ||
| HbSS | 11 (100) | 10 (91) |
| HbSβ0thalassemia | 0 (0) | 1 (9) |
| Hematologic indices | ||
| Hb (g/dL) | 7.5 (0.9) | 7.7 (1.0) |
| MCV (fL) | 85.9 (5.0) | 84.9 (10.8) |
| ARC (×109/L) | 316.8 (73.22) | 381.3 (139.73) |
| WBC (×109/L) | 14.2 (4.0) | 13.1 (4.9) |
| ANC (×109/L) | 5.9 (2.0) | 4.9 (1.9) |
| Platelets (×109/L) | 474.2 (126.2) | 388.1 (151.8) |
| HbF (%) | 9.9 (6.7) | 9.4 (6.1) |
| TCD Values | ||
| Overall TAMV (cm/sec) | 183.5 (8.5) | 181.8 (6.4) |
| Right side TAMV (cm/sec) | 167.0 (15.1) | 172.7 (14.9) |
| Left side TAMV (cm/sec) | 175.3 (3.9) | 174.5 (15.6) |
Notes: ANC: absolute neutrophil count, Hb: hemoglobin, MCV: mean corpuscular volume, ARC: absolute reticulocyte count, WBC: white blood cell count, TAMV: time-averaged mean velocity. Results presented as mean (SD), unless otherwise specified. There were no significant differences between both arms.
Premature Study Termination
There was a regulatory delay of 18 months preventing importation of the study drug (hydroxyurea) at one site (HEMORIO). This delay was due to numerous administrative difficulties in obtaining formal approval by ANVISA (Agência Nacional de Vigilância Sanitária), the Brazilian government agency responsible for authorizing the entry of study drugs into the country. The SCATE study opened for enrollment in May 2012, and in August 2013 the study was suspended for new enrollments and randomization by the sponsor for slow patient accrual. The study was terminated in January 2014 due to slow accrual and the unlikelihood of meeting the study’s enrollment target and primary endpoint within the timeframe of funding. Due to the administrative delay in obtaining drug approval in Brazil, 2 of the 11 children randomly allocated to the treatment arm (hydroxyurea) did not initiate study drug as per protocol.
Hematological Effects of Hydroxyurea
The median MTD for the 9 participants who received hydroxyurea was 25 mg/kg/day (range 20 – 35 mg/kg/day). Calculated % adherence, based on data from 92 of 112 total study visits for those who were randomized and received hydroxyurea, was 100% (for both mean and median). Exit versus baseline median differences in hematological indices for children receiving hydroxyurea showed expected significant increases in Hb concentration, HbF, and MCV, along with significant reductions in WBC and ANC in comparison with those who did not receive hydroxyurea (Table 2). There were no significant differences in renal or hepatic function tests between the treatment arms.
Table 2.
Comparisons between Entry and Exit Variables between Arms as Randomized.
| Hydroxyurea Arm (N=11) | Observation Arm (N=11) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Last Follow-up | Change* | Baseline | Last Follow-up | Change* | ||||||||||
| Variable | Median | Min | Max | Median | Min | Max | Median | Median | Min | Max | Median | Min | Max | Median | P-Value |
|
Hb
(g/dL) |
7.41 | 6.4 | 9.4 | 8.4 | 7.0 | 11.7 | 1.6 | 7.5 | 6.7 | 10.2 | 7.0 | 5.0 | 9.5 | −0.5 | <0.0001 |
|
MCV
(fL) |
86.5 | 77.9 | 93.2 | 95.7 | 83.2 | 102.0 | 8.7 | 84.8 | 66.2 | 106.5 | 84.0 | 70.0 | 102.0 | 1.0 | 0.0014 |
|
ARC
(×109/L) |
312.6 | 209.7 | 473.0 | 316.7 | 186.1 | 414.0 | 22.7 | 373.0 | 147.2 | 600.2 | 326.7 | 261.0 | 617.0 | −33.2 | 0.76 |
|
WBC
(×109/L) |
11.9 | 9.6 | 20.4 | 6.7 | 3.8 | 29.0 | −4.6 | 12.4 | 5.6 | 23.9 | 13.2 | 6.8 | 19.0 | 1.3 | 0.0652 |
|
ANC
(×109/L) |
6.1 | 3.7 | 10.7 | 2.7 | 1.3 | 7056.0 | −2.2 | 4.5 | 2.5 | 8.3 | 7.4 | 3.9 | 5762.0 | 1.4 | 0.0473 |
|
Platelets
(×109/L) |
460.0 | 256.0 | 723.0 | 410.0 | 179.0 | 642.0 | −76.0 | 411.0 | 111.0 | 634.0 | 376.0 | 127.0 | 469.0 | −35.0 | 0.56 |
|
HbF
(%) |
11.8 | 0.7 | 20.4 | 18.8 | 3.0 | 34.0 | 8.9 | 9.8 | 1.1 | 19.7 | 9.2 | 2.3 | 19.3 | 0.3 | 0.002 |
|
Weight
(Kg) |
20.6 | 13.2 | 25.3 | 22.2 | 15.1 | 28.3 | 2.5 | 19.5 | 16.2 | 30.5 | 21.9 | 17.0 | 37.8 | 1.8 | 0.51 |
|
Height
(cm) |
113.6 | 91.1 | 135.2 | 121.1 | 102.0 | 142.8 | 6.8 | 121.3 | 103.6 | 130.5 | 125.0 | 107.0 | 137.3 | 3.8 | 0.22 |
Note: P-values are calculated with the exact Wilcoxon-Mann-Whitney test comparing the exit versus entry differences between median values in the hydroxyurea and observation arms.
Change refers to last follow up minus baseline value. Comparison were also made between subjects who actually received hydroxyurea (n=9) versus those who did not (n=13), and the median change for the following variables showed significant difference: Hb (p<0.0001), MCV (p=0.003), WBC (p=0.03), ANC (p=0.003), and HbF (p < 0.0001).
Primary Study Endpoint
The cumulative incidences of conversion from conditional to abnormal TAMV were 9% (95% CI 0 to 35%) and 47% (95% CI 6 to 81%) in the hydroxyurea and observation arms after 15 months, respectively (p = 0.16, Figure 2A). However, none of the participants assigned to the hydroxyurea arm who actually received the drug converted to abnormal TCD velocities, whereas three in the observational arm did (supplemental Table 1). In post-hoc analysis according to treatment received, hydroxyurea treatment was associated with a significantly lower risk of conversion to abnormal TAMV after 15 months, 0% versus 50% (95% CI 9 to 82%), (p = 0.02, Figure 2B).
Figure 2. Cumulative incidence of conversion to abnormal TCD.
B> A. In the intention-to-treat analysis, conversion to abnormal TCD velocity was higher in the observation arm, but not statistically significant (p=0.17). B. In the post-hoc as-treated analysis, conversion to abnormal TCD velocity was significantly higher in the observation arm (p=0.02).
Secondary Endpoints
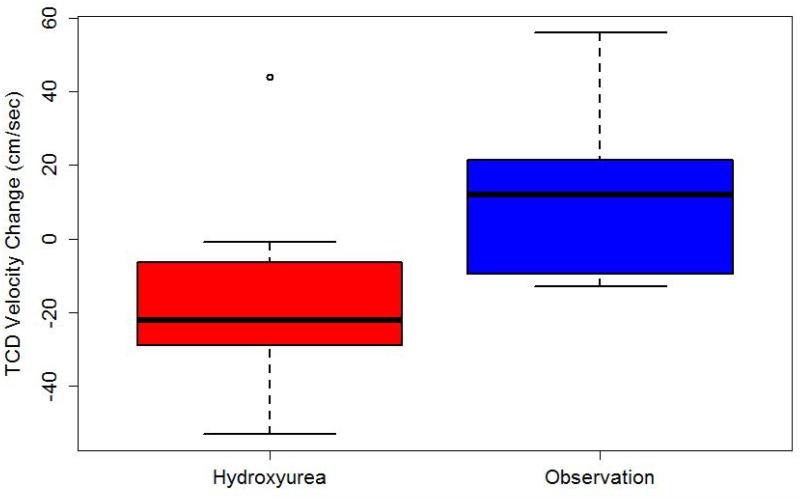
All secondary endpoints were evaluated using the intention-to-treat analysis and compared from randomization to last observation. After a mean of 10.1 months (median 8.7, range 0.5 to 17 months), participants randomized to the hydroxyurea arm had a mean reduction of TAMV values of 15.5 cm/sec (95% CI -32.0 to 1.1 cm/sec), whereas those in the observation arm had a mean increase in velocity of 10.2 cm/sec (95% CI -4.8 to 25.2 cm/sec) (p = 0.02, Figure 3).
Figure 3. Change in TCD velocity according to treatment.
B> Children randomized to hydroxyurea had a mean reduction of 15.5 cm/sec, whereas those on the observation arm had a mean increase of 10.2 cm/sec (p=0.02).
No strokes or transient ischemic attacks occurred. The only three new neurological events were dizziness (one event in the hydroxyurea arm) and headaches (one in each arm), with no significant difference between the two arms. Vaso-occlusive events (pain and acute chest syndrome) were more common in the observation arm than in the hydroxyurea arm (5 versus 2, respectively), but not significantly different. Two cases of acute splenic sequestration occurred, one in each arm.
Due to the early study termination, HRQOL was not sufficiently collected at study exit, therefore due to insufficient data points, no analyses could be performed regarding changes in HRQOL during the study.
Growth parameters of children from all 3 sites plotted within normal limits throughout the study when using the World Health Organization Child Growth Standards (http://www.who.int/childgrowth). There was no significant difference between the hydroxyurea and observation arms in the z-scores for weight, height, and BMI at baseline and last follow-up (Supplemental Table 2).
Adverse Events and Drug Toxicity
One subject on the hydroxyurea arm had one episode of transient neutropenia (ANC <1.0 × 109/L), vs. none on the observation arm. There were two cases of reticulocytopenia with worsened anemia (one in each arm), and no thrombocytopenia. There were two cases of parasite infestation (both hookworm), one in each arm of the study, both treated and with complete resolution of symptoms. Among those randomized to hydroxyurea who actually received the drug, only one required blood transfusion (due to an episode of worsened anemia during influenza B infection marrow suppression), and three subjects not treated with hydroxyurea received erythrocyte transfusions (two secondary to acute chest syndrome and one due to exacerbated anemia in the setting of a viral illness) (p=NS).
DISCUSSION
To our knowledge, SCATE is the first international NHLBI-sponsored Phase III randomized multi-center study in pediatric SCA involving limited-resource countries. Because the study was closed early by the sponsor despite active patient accrual and screening, there were insufficient data to demonstrate a significant reduction in the cumulative incidence of conversion from conditional to abnormal TCD velocities using intention-to-treat analysis. When considering only those who received hydroxyurea (post-hoc analysis), however, there was a significant difference in cumulative incidence of conversion from conditional to abnormal TCD velocities. In addition, we observed that hydroxyurea treatment significantly reduced conditional TCD velocities, an important finding suggested by retrospective and single-arm observational studies.(8-13) SCATE extends these prior observations by demonstrating prospectively in a randomized phase III trial that hydroxyurea reduces TCD velocities and may, thereby, lower the risk of primary stroke. By sparing the commonly observed conversion to abnormal TCD elevation, hydroxyurea therapy for conditional TCD velocities could obviate the need for chronic and indefinite transfusion therapy in many young patients with SCA, which represents an important cost-savings to the medical systems of both low- and high-resource countries.
While hydroxyurea is the only commercially available drug for the treatment of SCA, and can now be offered to most adults and children 9 months and older,(16) it is far from the standard of care. Hydroxyurea is still largely unavailable in many countries and thus underutilized, especially in resource-poor areas of the world. To help the impact of hydroxyurea treatment be fully realized by the global sickle cell disease community, it is important to gather prospective data regarding its safety and efficacy in more geographical locations and clinical settings, which will ultimately define its future indications and motivate its broader use.
Children with conditional TCD velocities represent a group of patients for whom treatment is not formally recommended (as opposed to those with abnormal TCD velocities) and therefore remain untreated despite an elevated risk for a primary stroke. The cumulative conversion rate in our patient sample was higher than in prior reports (30 and 50% versus 10 and 23% at 12 and 18 months, respectively).(5) This difference likely reflects the smaller sample size of this study, but also the fact that our populations derive from different countries, possibly adding genetic variability that contributed to the higher cumulative conversion rates.
Hydroxyurea induces fetal hemoglobin production and decreases intracellular HbS polymers within sickle erythrocytes.(17) Hydroxyurea has a number of additional beneficial effects besides induction of HbF, including decreased neutrophil and reticulocyte counts caused by marrow cytotoxicity, interference with red blood cell and leukocyte adhesion to the endothelium,(18;19) reduction of inflammatory cytokines,(20) and increased release of the vasodilator, nitric oxide.(21) Clinical benefits of hydroxyurea have been shown in both children and adults through reduction of frequency of pain and acute chest syndrome episodes.(12;22;23) Similar to prior studies in children with SCA, laboratory benefits from hydroxyurea in the SCATE trial also included increases in Hb concentration, HbF, MCV, and reduction in ANC.(12;24) The beneficial effect of increasing the Hb concentration in the hydroxyurea-treated arm may have contributed to the observed TAMV reduction. The use of hydroxyurea in SCA is especially attractive for low-resource settings due to its ease of oral administration, potential for reducing medical cost, and safety profile.(25) In the SCATE study, toxicities from hydroxyurea were uncommon and no different than reported in other pediatric populations (<10% severe neutropenia and reticulocytopenia).(26-29) Other adverse events during the study (neurological and non-neurological) were also rare and not observed in any greater frequency than prior studies.(24;26)
The SCATE study explored a new paradigm for collaborative multi-center clinical research in SCA. Although prematurely interrupted, SCATE highlighted the challenges and opportunities for international collaboration. High quality and important scientific questions can and should be answered without limitation of geographical borders or economic status of a nation. International collaboration facilitates SCA clinical trials for which large sample sizes are required, and not easily attained with the relatively smaller number of patients in North America and Europe. Inclusion of countries in Africa, South and Central America may significantly increase the pool of study participants, although the complexity of such trials may also increase.(30) Furthermore, in developing nations, where the burden of SCA is far greater than in the developed world, collaborative research participation provides overall benefits from improved standards of care, which are likely to impact care beyond the objectives of the study.
International collaborative research involving developing countries is an important goal of the NHLBI and the American Society of Hematology, particularly for research in SCA.(31;32) Collaborative research between resource-rich and resource-limited countries brings many opportunities to further research expertise and to improve clinical care in underprivileged areas of the world.(33) This study had many unique design features and challenges, among which was the enrollment of children from three different regions of the world with different laws and regulatory governance. This was an ambitious study that included centers with large numbers of children with SCA from both developed and developing countries. SCATE followed the same rigorous standards of research in all participating countries and scientifically investigated a potential new indication for hydroxyurea therapy.
Our study, although important in addressing the feasibility of conducting rigorously designed international clinical trials, had a limited final sample size for analysis, which hindered our ability to reach the primary study endpoint. Of the 115 targeted enrollment for SCATE, only 38 had enrolled (33%) at the time of study closure. Barriers to study enrollment are multi-factorial, including investigator-patient mistrust, trial design, and imbalance of perceived risks and benefits.(34-36) In SCATE, there were added complexities related to study design, language differences, and prolonged government and regulatory approvals of the protocol and drug importation, which hindered the ability to provide study treatment. Wider use of hydroxyurea also played a role at the North American site, reducing the pool of eligible patients. Because reduction in the TAMV was observed in subjects from only 2 sites, generalizability of our findings is limited. Despite its limitations, an important strength of SCATE includes the establishment of an infrastructure that allowed high-quality research for this trial but also future trials, using the highest scientific standards. Global efforts, such as that of the Global Sickle Cell Disease network (http://www.globalsicklecelldisease.org) should build on the experience of the SCATE trial to create future successful international studies that will answer relevant questions for individuals living with SCA worldwide.
In summary, we demonstrated in a phase III randomized clinical trial that hydroxyurea reduces TAMV in children with SCA and conditional TCD velocities. Hydroxyurea should be considered for treatment of children with conditional TCD velocities. Future international multicenter studies should build on the lessons learned through SCATE to develop additional and relevant clinical trials for people living with SCA.
Supplementary Material
ACKNOWLEDGMENTS
Funding for the SCATE study was provided by the National Hearth Lung and Blood Institute (5R01HL098239). We acknowledge the SCATE participants and their families for their trust, time, and efforts to the study. The authors are indebted to the following people at the participating Study Cores and Clinical Sites for their tireless efforts in building and maintaining the infrastructure and operation of the SCATE trial: Medical Coordinating Center (Susan Stuber, MA, Nicole A. Mortier, MHS PA-C, William H. Schultz, MHS PA-C), Data Coordinating Center (Joseph Moen, MS, Stacey Richardson, MSHS, Siminder Kaur, MS, Aregahegn, PhD, Chris Smith, MS, Emily Carps, MBA), TCD Core (Gail Fortner, RN), HEMORIO (Ana Claudia Leite, MD, Erica Pires, RN, Thais Oliveira, RN), TMRI (Margaret Wisdom-Phipps RN, Annabelle Nunes-Pearce RN, Dr Arlene Thorbourne), St. Jude (Alyssa Cotton, FNP, Jennifer Larkin, MS, Teresa Carr, RN, BS, Terri Davis, BS), NHLBI (Erin Smith, BS, Harvey Luksenburg, MD).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors have no conflict of interest to disclose.
AUTHORSHIP CONTRIBUTIONS
Jane S. Hankins, MD, MS: Designed study, collected data, coordinated the study, interpreted the results, and wrote the manuscript, including its first version.
M. Beth McCarville, MD: Designed study, performed blinded TCD exams, directed the TCD core, interpreted the results, and edited the manuscript.
Angela Rankine-Mullings, MD: enrolled participants, collected data, edited the manuscript.
Marvin E. Reid, MBBS, PhD: enrolled participants, collected data, edited the manuscript.
Clarisse L.C. Lobo, MD: enrolled participants, collected data, edited the manuscript.
Patricia G. Moura, MD: enrolled participants, collected data, edited the manuscript.
Susanna Ali, MD: performed TCD exams, edited the manuscript.
Deanne Soares, MD: performed TCD exams, edited the manuscript
Karen Aldred, MSc: performed TCD exams, edited the manuscript
Dennis Jay, MD: performed central fetal hemoglobin measurements.
Banu Aygun, MD: monitored adverse events, interpreted the results, and edited the manuscript.
John Bennett, PhD: performed blinded TCD exams, and edited the manuscript.
Guolian Kang, PhD: performed statistical analysis, interpreted data, and edited the manuscript.
Jonathan C. Goldsmith, MD: Designed study, interpreted the results, and edited the manuscript.
Matthew P. Smeltzer, PhD, MS: performed statistical analysis, interpreted data, and edited the manuscript.
James M. Boyett, PhD: Designed study, supervised statistical analysis, coordinated the DCC, interpreted the results, and edited the manuscript.
Russell E. Ware, MD, PhD: Designed study, collected data, coordinated the study, interpreted the results, and wrote the manuscript.
Supplemental document - Recruitment brochure provided as supplemental material in a PDF file.
REFERENCES
- (1).McCarville MB, Goodin GS, Fortner G, et al. Evaluation of a comprehensive transcranial doppler screening program for children with sickle cell anemia. Pediatr Blood Cancer. 2008 Apr;50(4):818–21. doi: 10.1002/pbc.21430. [DOI] [PubMed] [Google Scholar]
- (2).Hussain S, Nichols F, Bowman L, et al. Implementation of transcranial Doppler ultrasonography screening and primary stroke prevention in urban and rural sickle cell disease populations. Pediatr Blood Cancer. 2014 Nov 8;(62):219–223. doi: 10.1002/pbc.25306. [DOI] [PubMed] [Google Scholar]
- (3).Adams RJ, McKie VC, Carl EM, et al. Long-term stroke risk in children with sickle cell disease screened with transcranial Doppler. Ann Neurol. 1997 Nov;42(5):699–704. doi: 10.1002/ana.410420505. [DOI] [PubMed] [Google Scholar]
- (4).Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998 Jul 2;339(1):5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- (5).Hankins JS, Fortner GL, McCarville MB, et al. The Natural History of Conditional Transcranial Doppler Flow Velocities in Children with Sickle Cell Anaemia. Br J Haematol. 2008 Jul;142(1):94–9. doi: 10.1111/j.1365-2141.2008.07167.x. PMCID: 18477038. [DOI] [PubMed] [Google Scholar]
- (6).Adams R, McKie V, Nichols F, et al. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med 1992 February. 27;326(9):605–10. doi: 10.1056/NEJM199202273260905. [DOI] [PubMed] [Google Scholar]
- (7).Adams RJ, Brambilla DJ, Granger S, et al. Stroke and conversion to high risk in children screened with transcranial Doppler ultrasound during the STOP study. Blood. 2004 May 15;103(10):3689–94. doi: 10.1182/blood-2003-08-2733. [DOI] [PubMed] [Google Scholar]
- (8).Gulbis B, Haberman D, Dufour D, et al. Hydroxyurea for sickle cell disease in children and for prevention of cerebrovascular events: the Belgian experience. Blood. 2005 Apr 1;105(7):2685–90. doi: 10.1182/blood-2004-07-2704. [DOI] [PubMed] [Google Scholar]
- (9).Kratovil T, Bulas D, Driscoll MC, et al. Hydroxyurea therapy lowers TCD velocities in children with sickle cell disease. Pediatr Blood Cancer. 2006 Dec;47(7):894–900. doi: 10.1002/pbc.20819. [DOI] [PubMed] [Google Scholar]
- (10).Lagunju I, Brown BJ, Sodeinde O. Hydroxyurea lowers transcranial Doppler flow velocities in children with sickle cell anaemia in a Nigerian cohort. Pediatr Blood Cancer. 2015 Apr 1;62(9):1587–91. doi: 10.1002/pbc.25529. [DOI] [PubMed] [Google Scholar]
- (11).Lefevre N, Dufour D, Gulbis B, et al. Use of hydroxyurea in prevention of stroke in children with sickle cell disease. Blood. 2008 Jan 15;111(2):963–4. doi: 10.1182/blood-2007-08-102244. [DOI] [PubMed] [Google Scholar]
- (12).Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG) Lancet. 2011 May 14;377(9778):1663–72. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Zimmerman SA, Schultz WH, Burgett S, et al. Hydroxyurea therapy lowers transcranial Doppler flow velocities in children with sickle cell anemia. Blood. 2007 Aug 1;110(3):1043–7. doi: 10.1182/blood-2006-11-057893. [DOI] [PubMed] [Google Scholar]
- (14).Heeney MM, Whorton MR, Howard TA, et al. Chemical and functional analysis of hydroxyurea oral solutions. J Pediatr Hematol Oncol. 2004 Mar;26(3):179–84. doi: 10.1097/00043426-200403000-00007. [DOI] [PubMed] [Google Scholar]
- (15).Heeney MM, Ware RE. Hydroxyurea for children with sickle cell disease. Pediatr Clin North Am. 2008 Apr;55(2):483–501. doi: 10.1016/j.pcl.2008.02.003. PMCID: 18381097. [DOI] [PubMed] [Google Scholar]
- (16).Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014 Sep 10;312(10):1033–48. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- (17).Platt OS, Orkin SH, Dover G, et al. Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. J Clin Invest. 1984 Aug;74(2):652–6. doi: 10.1172/JCI111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Hillery CA, Du MC, Wang WC, Scott JP. Hydroxyurea therapy decreases the in vitro adhesion of sickle erythrocytes to thrombospondin and laminin. Br J Haematol. 2000 May;109(2):322–7. doi: 10.1046/j.1365-2141.2000.02040.x. [DOI] [PubMed] [Google Scholar]
- (19).Almeida CB, Scheiermann C, Jang JE, et al. Hydroxyurea and a cGMP-amplifying agent have immediate benefits on acute vaso-occlusive events in sickle cell disease mice. Blood. 2012 Oct 4;120(14):2879–88. doi: 10.1182/blood-2012-02-409524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Lanaro C, Franco-Penteado CF, Albuqueque DM, et al. Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydroxyurea therapy. J Leukoc Biol. 2009 Feb;85(2):235–42. doi: 10.1189/jlb.0708445. [DOI] [PubMed] [Google Scholar]
- (21).Gladwin MT, Shelhamer JH, Ognibene FP, et al. Nitric oxide donor properties of hydroxyurea in patients with sickle cell disease. Br J Haematol. 2002 Feb;116(2):436–44. doi: 10.1046/j.1365-2141.2002.03274.x. [DOI] [PubMed] [Google Scholar]
- (22).Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995 May 18;332(20):1317–22. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- (23).Scott JP, Hillery CA, Brown ER, et al. Hydroxyurea therapy in children severely affected with sickle cell disease. J Pediatr. 1996 Jun;128(6):820–8. doi: 10.1016/s0022-3476(96)70335-9. [DOI] [PubMed] [Google Scholar]
- (24).Zimmerman SA, Schultz WH, Davis JS, et al. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103(6):2039–45. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- (25).Wang WC, Oyeku SO, Luo Z, et al. Hydroxyurea is associated with lower costs of care of young children with sickle cell anemia. Pediatrics. 2013 Oct;132(4):677–83. doi: 10.1542/peds.2013-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Thornburg CD, Files BA, Luo Z, et al. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood. 2012 Nov 22;120(22):4304–10. doi: 10.1182/blood-2012-03-419879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Dehury S, Purohit P, Patel S, et al. Low and fixed dose of hydroxyurea is effective and safe in patients with HbSbeta thalassemia with IVS1-5(G-->C) mutation. Pediatr Blood Cancer. 2014 Dec 24;62(6):1017–23. doi: 10.1002/pbc.25391. [DOI] [PubMed] [Google Scholar]
- (28).Silva-Pinto AC, Angulo IL, Brunetta DM, et al. Clinical and hematological effects of hydroxyurea therapy in sickle cell patients: a single-center experience in Brazil. Sao Paulo Med J. 2013;131(4):238–43. doi: 10.1590/1516-3180.2013.1314467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).de MM, Brousse V, Elie C, et al. Long-term hydroxyurea treatment in children with sickle cell disease: tolerance and clinical outcomes. Haematologica. 2006 Jan;91(1):125–8. [PubMed] [Google Scholar]
- (30).McGann PT, Tshilolo L, Santos B, et al. Hydroxyurea therapy for children with sickle cell anemia in sub-Saharan Africa: Rationale and design of the REACH trial. Pediatr Blood Cancer. 2015 Aug;:14. doi: 10.1002/pbc.25705. PMID:26275071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hoots WK, Shurin SB. Future directions of sickle cell disease research: the NIH perspective. Pediatr Blood Cancer. 2012 Aug;59(2):353–7. doi: 10.1002/pbc.24180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Williams DA. Gotlib J, editor. A Call to Action on Sickle Cell Disease. The Hematologist, ASH News and Reports. 4-29-2015. Last accessed: July-6-2015. [Google Scholar]
- (33).Aygun B, Odame I. A global perspective on sickle cell disease. Pediatr Blood Cancer. 2012 Aug;59(2):386–90. doi: 10.1002/pbc.24175. [DOI] [PubMed] [Google Scholar]
- (34).Patterson CA, Chavez V, Mondestin V, et al. Clinical Trial Decision Making in Pediatric Sickle Cell Disease: A Qualitative Study of Perceived Benefits and Barriers to Participation. J Pediatr Hematol Oncol. 2015 Aug;37(6):415–22. doi: 10.1097/MPH.0000000000000216. [DOI] [PubMed] [Google Scholar]
- (35).Lebensburger JD, Sidonio RF, DeBaun MR, et al. Exploring barriers and facilitators to clinical trial enrollment in the context of sickle cell anemia and hydroxyurea. Pediatr Blood Cancer. 2013 Aug;60(8):1333–7. doi: 10.1002/pbc.24486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Peters-Lawrence MH, Bell MC, Hsu LL, et al. Clinical trial implementation and recruitment: lessons learned from the early closure of a randomized clinical trial. Contemp Clin Trials. 2012 Mar;33(2):291–7. doi: 10.1016/j.cct.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.