Abstract
Poor sleep and low social support have each been associated with mortality and morbidity from chronic illness and a small body of research suggests that the two interact to influence systemic inflammation, whereby good social relationships may buffer the relationship between poor sleep and increased inflammation. The current study investigated interactions between sleep and social support in the prediction of inflammation in a clinical population (prehypertensive and hypertensive individuals) at high risk for the development of cardiovascular disease. Using a standardized subjective measure of sleep quality, we found that social support moderated the association between sleep and circulating levels of both IL-6 and CRP, such that poor sleep appeared to confer a risk of increased inflammation only in those participants who also reported low social support. In women, the same relationship was observed for TNF-α. These results extend previous findings into a clinical population and also demonstrate that sleep quality and social support interact in the prediction of two previously uninvestigated clinically relevant inflammatory markers (CRP and TNF-α). High levels of perceived social support may compensate for the negative health impact of poor sleep quality and vice versa.
Keywords: sleep quality, social support, interleukin 6, C-reactive protein, tumor necrosis factor-α
Chronically elevated levels of inflammatory proteins have been associated with myriad negative health outcomes (Pradhan, Manson, Rifai, Buring, & Ridker, 2001). For example, levels of C-reactive protein (CRP) are predictive of the development of cardiovascular disease (CVD), and have been linked to ischaemic stroke, vascular mortality, and death (Danesh et al., 2004; Pradhan et al., 2001). Additionally, in large, population-based studies, relatively high levels of the inflammatory markers interleukin-6 (IL-6) and tumor necrosis factor (TNF)-α are predictive of morbidity and mortality (Aggarwal, Gupta, & Kim, 2012; Bruunsgaard et al., 2003; Harris et al., 1999). There is a degree of inconsistency in the literature regarding associations between individual markers and health outcomes, indicating the potential influence of moderating factors.
Individuals with pre-hypertension and hypertension are at high risk for developing cardiovascular disease (CVD), and the population has established levels of low-grade systematic inflammation, which contributes to disease development (Blake, Rifai, Buring, & Ridker, 2003). Due to the high risk for individuals with hypertension/pre-hypertension to progress to more serious cardiovascular problems, understanding modifiable variables that affect known risk factors, such as inflammation, is important.
Levels of circulating inflammatory proteins are influenced by a number of biopsychosocial factors, including sleep. Disturbed sleep, ranging from chronic insomnia to experimental sleep deprivation, along with objectively measured sleep indices has been robustly associated with elevated inflammation (Grandner, Sands-Lincoln, Pak, & Garland, 2013; Motivala, 2011). Poor subjectively assessed sleep quality, characterized by tiredness on waking and through the day, not feeling rested and restored on waking, and/or experiencing numerous nighttime awakenings (Harvey, Stinson, Whitaker, Moskovitz, & Virk, 2008) has also been associated with inflammation, such that worse sleep quality has been linked with higher circulating CRP, IL-6 and increased IL-6 production in response to stimulation with lipopolysaccharide (Friedman, 2011; Friedman et al., 2005; McDade, Hawkley, & Cacioppo, 2006; Okun, Hall, & Coussons-Read, 2007; Okun, Roberts, Marsland, & Hall, 2009; Prather et al., 2009). Increased systemic inflammation, resulting from poor sleep, has been hypothesized to be a mechanism through which poor sleep increases risk of morbidity and mortality from CVD (Grandner et al., 2013; Simpson & Dinges, 2007).
While experiencing poor sleep quality appears to increase markers of systemic inflammation and confer risk of morbidity and mortality, a number of studies have shown that social support, or “the perception or experience that one is loved and cared for by others, esteemed and valued, and part of a social network of mutual assistance and obligations” (Wills, 1991) confers an opposite, protective effect (Holt-Lunstad, Smith, Baker, Harris, & Stephenson, 2015; Holt-Lunstad, Smith, & Layton, 2010). Individuals lacking in social ties or social integration have higher risk of mortality from numerous diseases, including CVD (Holt-Lunstad et al., 2015, 2010). Low levels of social support have been associated with elevations in inflammatory proteins (Kiecolt-Glaser, Gouin, & Hantsoo, 2010). Social support not only has direct associations with health, but also appears to work as a moderator of the relationship between stress and health, such that high levels of social support have been shown to buffer the negative impact of stressors on health outcomes (Bowen et al., 2013; Steptoe, 2000). There is some evidence that social support also moderates the relationship between poor sleep and inflammation (Friedman, 2011; Friedman et al., 2005; O’Connor et al., 2009). For example, in a study of women aged 61 to 90, Friedman et al. (2005) found that participants who reported poorer sleep efficiency had higher levels of IL-6, but social support buffered the relationship between poor sleep and inflammation. In fact, the only women with elevated IL-6 in the study reported both poor sleep quality and low social engagement (Friedman et al., 2005). These findings were recently extended to 1,229 participants from a national sample of adult Americans. Again, social engagement moderated the association between sleep complaints with both IL-6 and the soluble adhesion molecule E-selectin (Friedman, 2011).
Gender is another potential moderator in the complicated relationship between sleep, social support and inflammation. A body of work suggests that disrupted sleep may be more robustly related to inflammation in women (Irwin, Carrillo, & Olmstead, 2010; M. A. Miller et al., 2009; Suarez, 2008). Additionally, laboratory research suggests that in response to social negativity (e.g., marital conflict) women have stronger neuroendocrine and cardiovascular responses than men, suggesting that potentially the relationship between social support and inflammation may also be stronger in women (Kiecolt-Glaser & Newton, 2001; Pietromonaco, Uchino, & Dunkel Schetter, 2013). However, there are some contradictory findings concerning the interaction between gender, sleep and social support on the outcome of inflammation. For example, research from Friedman et al. (2011) suggested that the interaction between sleep quality and social support on IL-6 was stronger in men than in women.
The hypothalamic-pituitary-adrenal (HPA) axis is a system that could hypothetically underlie the complicated relationship between sleep, social support and inflammatory processes.
The HPA axis plays an important role in regulating and controlling immune responses (Spies, Straub, Cutolo, & Buttgereit, 2014). Sleep and social support both have relationships with HPA function and worsening sleep quality and poor social support are both associated with small but meaningful changes in HPA activation (Balbo, Leproult, & Van Cauter, 2010; Cole, 2008). Potentially, either good sleep or good social support may promote balance in the HPA system and reduce levels of systematic inflammation.
In this study, we investigated if social support and sleep quality interacted to predict inflammation in a clinical population with hypertension/pre-hypertension. Consistent with research examining relationships between social support and inflammation, we examined the potential moderating impact of gender (Kiecolt-Glaser & Newton, 2001; O’Connor et al., 2009). In addition to levels of IL-6, we also investigated two outcome variables (TNF-α and CRP) that have not been previously studied. CRP is particularly important due to its clinical utility as a predictive variable for the development of CVD (Ridker, 2003).
We hypothesized that in this sample (1) worse subjective sleep quality would be associated with elevated levels of inflammatory biomarkers and that (2) the relationships would be moderated by levels of perceived social support, such that higher social support would attenuate the relationship between sleep quality and inflammation. Given conflicting findings concerning a potential three-way interaction between gender, social support, and sleep quality in the prediction of inflammation, we conducted exploratory analyses of a potential moderating role of gender, but did not make an a priori hypothesis about the nature of this effect.
Method
Participants
Participants were 67 sedentary men (N = 37) and women (N = 30) with a diagnosis of prehypertension or hypertension. Data are from the baseline assessment of a larger trial investigating the impact of exercise on blood pressure (BP) in a sedentary population with prehypertension/hypertension (Clinical Trial NCT00338572). Other than having a diagnosis of prehypertension (120 – 139 mmHg systolic BP / 80 – 89 mmHg diastolic BP) or stage 1/stage 2 hypertension (140 – 170 mmHg systolic BP / 90 – 105 mmHg diastolic BP) (Chobanian et al., 2003) participants were otherwise healthy.
Recruitment of individuals with prehypertension or hypertension occurred through advertisements, word of mouth referral and medical practice referrals in the San Diego area between August 16, 2006 and July 1, 2010. After an initial phone screening, all participants met with a physician at the University of California, San Diego (UCSD), who performed a medical examination to ensure study eligibility. Inclusion criteria included having a diagnosis of prehypertension or hypertension, a sedentary lifestyle, being free from any chronic illness with the exception of hypertension and the ability to read/write in English. The Leisure Time Exercise Questionnaire (LTEQ) (Godin & Shephard, 1985) was used to determine physical inactivity according to its established validity and reliability (D. J. Miller, Freedson, & Kline, 1994; Sallis, Buono, Roby, Micale, & Nelson, 1993). Individuals scoring less than 40 on the LTEQ were considered physically inactive based on normative data and were included in the study (Godin & Shephard, 1985; Stewart et al., 1994). Participants whose ECG screening showed evidence of a condition that might increase their risk for harm or compromise data collection (e.g., arrhythmia) were excluded. Under medical supervision, four participants who were taking medication for hypertension were gradually weaned off their medication three weeks prior to participation in the study. At the time of the baseline assessment, no participants were taking antihypertensive medication.
Baseline data were collected before randomization to the larger trial. A trained technician measured weight, height, and assessed resting BP; prehypertensive or hypertensive status was confirmed during this assessment. Blood was drawn between 11:30am and 1:00pm, after a 30-minute rest period. Blood was collected with a 19-gauge catheter, inserted into an antecubital vein by a registered nurse at the UCSD Clinical and Translational Research Institute (CTRI). Participants were asked to abstain from eating for 2 hours and from drinking caffeine for 12 hours prior to the visit. Questionnaires were administered during the visit.
The UCSD Human Subjects Committee approved the project. All participants signed a written informed consent form before participation in the study commenced.
Measures
Inflammatory markers
Blood for the IL-6, CRP, and TNF-α assays was dispensed in EDTA tubes and spun at 3000 g for 10 minutes at 4–8°C. Obtained plasma was immediately stored in plastic tubes at −80°C until analyzed. Plasma levels of IL-6 and TNF-α were measured by a high-sensitivity immunoassay kit (Quantikine, R&D Systems, Minneapolis, MN). Measurement of plasma CRP levels was taken by using the High Sensitive CRP Reagent Set (DiaSorin, Stillwater, MN) using the Roche Cobas Mira Plus analyzer (Roche, Palo Alto, CA). Intra- and inter-assay coefficients of variation were <5% for all analyses.
Blood pressure (BP)
BP was measured at rest while participants were seated in a comfortable chair. An appropriately sized BP cuff from an automated blood pressure monitor (Dinamap 1846SX) was attached to the non-dominant arm and BP was assessed six times. Mean Arterial Pressure (MAP) was calculated using the mean systolic and diastolic BP values (MAP = [(2 × diastolic) + systolic]/3).
Body mass index (BMI)
BMI was calculated as the ratio of body weight (measured to the nearest 0.1 kg) divided by the height in meters squared (to the nearest 0.1 cm).
The Pittsburg Sleep Quality Index (PSQI)
Subjective sleep quality was assessed with the PSQI, a 19-item self-rated questionnaire that assesses sleep quality and sleep disturbances over a one-month time interval (Buysse, F., Monk, Berman, & Kupfer, 1989). The PSQI assesses seven clinically derived domains of sleep difficulties: sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction (Buysse et al., 1989). The combination of these domains yields a global PSQI score; higher scores are indicative of worse subjective sleep quality. Cronbach’s alpha in this sample was 0.73.
RAND Medical Outcomes Study (MOS) Social Support Survey
A 9-item version of the RAND MOS Social Support Survey was used to assess social support. The scale is a self-report measure designed to identify a person’s perceived availability of social support. Participants were instructed to answer “How often is each of the following kinds of support available to you if you need it?” Example questions include, “Someone to love you and make you feel wanted” and “Someone to help you with daily chores if you are sick.” Questions were rated on a 5-point Likert scale, ranging from “None of the time” to “All of the time.” Total scores range from 9 to 45, with higher scores indicating more perceived social support (Sherbourne & Stewart, 1991). Cronbach’s alpha in this sample was 0.93.
Center for Epidemiologic Studies Depression Scale (CES-D)
The CES-D is a 20-item self-report scale that assesses symptoms of depression. The scale has high internal consistency and reliability across numerous demographic groups (Radloff, 1977). Due to concerns about collinearity in the predictor and outcome variables, one question that assesses sleep difficulties was excluded from the total CES-D. Although this is in line with previous approaches, it should be noted that removing the sleep question from the CES-D impacts the construct validity of the measure. Cronbach’s alpha for the 19-item version of the CES-D was 0.90.
Data Analysis
Statistical analyses were performed using SPSS statistical software package (IBM SPSS, Version 20). Data were investigated for normality and outliers. One significant outlier (greater than 3SD from the mean) was removed from the IL-6 data. Log transformations were performed on CRP, IL-6, and CES-D data, due to positively skewed distributions. Correlational analyses were used to investigate relationships between sleep, social support, inflammation, and covariates. Hierarchical regression analyses were used to investigate the main effects of PSQI scores and interactions with other variables in the prediction of individual inflammatory markers CRP, TNF-α, and IL-6. In each set of regression analyses, the model examined direct and interactive associations between subjective sleep quality and social support, adjusting for covariates with known associations to inflammation (i.e., age, gender, BMI, mean arterial pressure [MAP], and depression) (O’Connor et al., 2009). Covariates were held constant on step 1, continuous predictor variables were centered and held constant on step 2, and the interaction term(s) were entered on step 3. Non-significant interaction terms were removed from the model. Assumptions of regression were assessed and met for each model presented.
Results
Demographic and Health Characteristics
Demographic and health characteristics of the sample are presented in Table 1. On average, participants were middle aged, obese, and hypertensive. The majority of participants were Caucasian (66%) or African American (22.5%). Remaining participants identified as Asian, Native Hawaiian, or other Pacific Islander (11.5%). Mean CES-D, before removal of the sleep question, was 10.7 (SD = 8.62), indicating that the majority of participants were not suffering from clinically significant symptoms of depression (Radloff, 1977). Mean CRP = 4.4 (SD = 5.2) mg/L; >3 mg/L is considered high-risk for cardiovascular disease (Yeh, 2005). Mean TNF-α = 2.2 (SD = 1.3) pg/ml and mean IL-6 = 2.4 (SD = 1.9) pg/ml.
Table 1.
Sample Characteristics
| Valid N | Percentage | M ± SD | |
|---|---|---|---|
| Gender (male) | 67 | 55% | - |
| Age | 46.3 ± 9.6 | ||
| BMI (kg/M2) | 30.7 ± 4.0 | ||
| SBP (mmHg) | 142.34 ± 10.70 | ||
| DBP (mmHg) | 84.96 ± 8.40 | ||
| MAP (mmHg) | 104.10 ± 8.23 | ||
| CRP (mg/L) | 4.4 ± 5.2 | ||
| IL-6 (pg/mL) | 2.4 ± 1.9 | ||
| TNF-α (pg/mL) | 2.2 ± 1.3 | ||
| CES-D (removed sleep question) | 9.77 ± 8.73 | ||
| SSQ | 37.6 ± 7.4 | ||
| PSQI Global Score | 4.6 ± 2.9 |
BMI = body mass index; MAP = mean arterial pressure; CES-D = center for epidemiologic studies depression (scale); SSQ = social support questionnaire; CRP = C-reactive protein; TNF-α = tumour necrosis factor alpha; IL-6 = interleukin 6; PSQI = Pittsburgh Sleep Quality Index;
CRP and IL-6 values are presented in their non-transformed state. All analyses were conducted with logged values
Table 2 shows the simple correlations between study variables. Of note, PSQI scores were positively associated with TNF-α and MAP. No associations were observed between social support and inflammatory markers. Gender was associated with CRP, such that men in the sample had higher levels. IL-6 and CRP were significantly correlated with BMI.
Table 2.
Correlations between Study Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gender | – | .16 | −.04 | −.04 | −.01 | .30* | 0.20 | .05 | <.01 | .01 |
| 2. Age | – | .05 | .19 | −.05 | .16 | .21 | −.15 | .12 | .11 | |
| 3. BMI | – | .09 | .06 | .27* | .38** | .04 | −.04 | .22 | ||
| 4. MAP | – | −.07 | .08 | .13 | −.08 | .31** | .02 | |||
| 5. TNF-α | – | .08 | .02 | .14 | .27* | .03 | ||||
| 6. CRP | – | .40** | .07 | .12 | .02 | |||||
| 7. IL-6 | – | −.27* | .10 | .08 | ||||||
| 8. CES-D | – | −.02 | −.07 | |||||||
| 9. PSQI | – | .14 | ||||||||
| 10. Social Support | – |
p < .05 (2-tailed);
p < .01 (2-tailed)
Gender, males were coded as 0 and females coded as 1.
TNF-α = tumour necrosis factor alpha; IL-6 = interleukin 6; CRP = C-reactive protein; PSQI = Pittsburgh Sleep Quality Index; CES-D = center for epidemiologic studies depression (with sleep question removed); MAP = mean arterial pressure; BMI = body mass index
Inflammation has been implicated in the progression of hypertension (Pauletto & Rattazzi, 2015). We therefore conducted t-tests to examine if there were significant differences between participants with pre-hypertension and hypertension on key study variables, including age, BMI, social support, subjective sleep quality, symptoms of depression, IL-6, CRP, and TNA-α. Analyses revealed no significant differences between groups on any variable (ps > .09) and so participants with pre-hypertension and hypertension were analyzed as one group.
Hierarchical Regression Analyses
IL-6
See Table 3. After controlling for covariates, neither the subjective sleep quality nor social support was directly associated with IL-6 (ps > .05); however, the two variables interacted to predict IL-6 (see Figure 1). A three-way interaction between gender, subjective sleep quality, and social support was not observed and the interaction was removed from the final model (β = 0.002, p = 0.55).
Table 3.
PSQI global scores interact with social support to predict IL-6
| Step | Predictor | B | SE | p | R2 | ΔR2 |
|---|---|---|---|---|---|---|
| 1 | Age | .002 | .002 | .37 | .25 | |
| Gender | .05 | .05 | .26 | |||
| BMI | .02 | .01 | .001 | |||
| MAP | .001 | .003 | .69 | |||
| CES-D (removed sleep question) | −.01 | .003 | .03 | |||
| 2 | PSQI | .003 | .008 | .69 | .25 | < .01 |
| Social Support | −.01 | .004 | .15 | |||
| 3 | PSQI X Social Support | −.003 | .001 | .05 | .30 | .05 |
SE = standard error; BMI = body mass index; MAP = mean arterial pressure; CES-D = center for epidemiologic studies depression (with sleep question removed); IL-6 = interleukin 6; PSQI = Pittsburgh Sleep Quality Index.
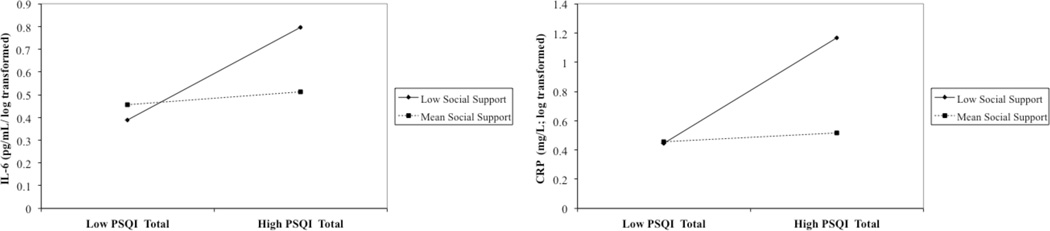
Figure 1.
PSQI global scores predicting IL-6 and CRP. The statistical interaction between PSQI and social support (Tables 3 and 4) is shown here using “Mean Social Support” and “Low Social Support” modeled at 1 SD below the mean of the sample.
IL-6 = Interleukin-6; CRP = C-reactive protein; PSQI = Pittsburgh Sleep Quality Index
Simple slopes of the effect of subjective sleep quality on IL-6 were tested for the mean and ±1 SD of social support (Hayes & Matthes, 2009). Simple slopes were significant at 1 SD below the mean of social support (β = 0.026, p < .05) but not at the mean (β = 0.004, p = .64) or at 1 SD above the mean (β = −.02, p = .15). In participants who reported low social support, there was a significant association between subjective sleep quality and IL-6, such that poor subjective sleep quality was associated with higher IL-6. Conversely, in participants who had mean or higher levels of social support, there was no association between self-reported sleep quality and IL-6.
CRP
See Table 4. After controlling for covariates, neither subjective sleep quality nor social support were directly associated with CRP (ps > .05). A significant interaction was observed between subjective sleep quality and social support in the prediction of CRP (see Figure 1). A three-way interaction with gender was not observed and the interaction was removed from the final model (β = −0.004, p = 0.40).
Table 4.
PSQI global scores interact with social support to predict CRP
| Step | Predictor | B | SE | p | R2 | ΔR2 |
|---|---|---|---|---|---|---|
| 1 | Age | .01 | .004 | .11 | .17 | |
| Gender | .14 | .08 | .09 | |||
| BMI | .02 | .01 | .03 | |||
| MAP | < −.01 | .004 | .81 | |||
| CES-D (removed sleep question) | .002 | .10 | .73 | |||
| 2 | PSQI | .01 | .01 | .53 | .18 | < .01 |
| Social Support | −.008 | .01 | .21 | |||
| 3 | PSQI X Social Support | −.01 | .002 | .04 | .24 | .06 |
SE = standard error; BMI = body mass index; MAP = mean arterial pressure; CES-D = center for epidemiologic studies depression (with sleep question removed); CRP = C-Reactive Protein; PSQI = Pittsburgh Sleep Quality Index
Again, simple slopes of the effect of subjective sleep quality on CRP were tested at the mean and ±1 SD of social support. Similar to IL-6, simple slopes were significant at 1 SD below the mean of social support (β = 0.05, p < .05), but not at the mean (β = 0.01, p = .61) or at 1 SD above the mean (β = −.03, p = .18). In participants who reported low social support, poor subjective sleep quality was associated with higher levels of CRP. In participants who had mean or higher levels of social support, there was no association between subjective sleep quality and inflammatory markers.
TNF-α
After controlling for covariates, subjective sleep quality was directly associated with TNF-α, such that worse sleep quality was associated with higher TNF-α (β = 0.15, p < .01). There was a significant three-way interaction between gender, subjective sleep quality, and social support (β = 0.02, p = .01, ∆R2 = 0.09). Results of the regression analyses are therefore presented separately by gender in Table 5. Follow-up analyses showed that subjective sleep quality significantly interacted with social support to predict TNF-α in women (p < .05) but not in men (p = .07). In men, there was a trend suggesting a main effect of PSQI scores on TNF-α (p = .06).
Table 5.
PSQI global scores interact with social support to predict TNF-α
| Step | Predictor | B | SE | p | R2 | ΔR2 | |
|---|---|---|---|---|---|---|---|
| Men | |||||||
| 1 | Age | −.04 | .02 | .06 | .06 | ||
| BMI | −.001 | .05 | .99 | ||||
| MAP | −.001 | .03 | .98 | ||||
| CES-D | .02 | .03 | .20 | ||||
| 2 | PSQI | .13 | .07 | .06 | .24 | .18 | |
| Social Support | .05 | .03 | .10 | ||||
| 3 | PSQI X Social Support | .03 | .02 | .07 | .32 | .08 | |
| Women | |||||||
| 1 | Age | .02 | .02 | .31 | .04 | ||
| BMI | .07 | .06 | .22 | ||||
| MAP | −.05 | .02 | .07 | ||||
| CES-D | .01 | .02 | .77 | ||||
| 2 | PSQI | .12 | .08 | .13 | .20 | .15 | |
| Social Support | −.08 | .03 | .03 | ||||
| 3 | PSQI X Social Support | −.02 | .01 | .05 | .34 | .14 | |
SE = standard error; BMI = body mass index; MAP = mean arterial pressure; CES-D = center for epidemiologic studies depression (with sleep question removed); TNF-α = tumour necrosis factor alpha; PSQI = Pittsburgh Sleep Quality Index.
There was a significant three-way interaction between gender, subjective sleep quality, and social support (β = 0.02, p = .01, ΔR2 = 0.09). Results are presented separately for men and women to aid in interpretation of findings
Women
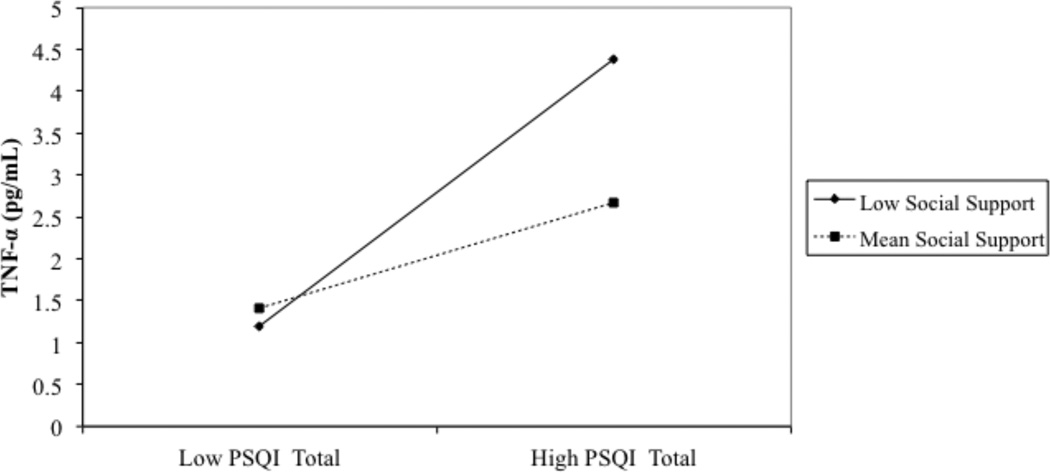
Simple slopes of the effect of subjective sleep quality on TNF-α were tested at the mean and ±1 SD of social support. Simple slopes were significant at 1 SD below the mean of social support (β = 0.29, p < .01), but not at the mean (β = 0.11, p = .17) or at 1 SD above the mean (β = −0.06, p = .63). In women who reported low social support, poor subjective sleep quality was associated with higher levels of TNF-α. Conversely, in participants who had mean or higher levels of social support there was no association between self-reported sleep quality and TNF-α (see Figure 2).
Figure 2.
PSQI global scores predicting TNF-α in women. The statistical interaction between PSQI and social support (Table 5) is shown here using “Mean Social Support” modeled at the mean of the sample and “Low Social Support” modeled at 1 SD below the mean of the sample.
Discussion
The primary hypothesis of the current study was that subjective sleep quality would interact with social support to predict levels of inflammatory biomarkers in this sample of sedentary adults with prehypertension and hypertension. This hypothesis was supported: subjective sleep quality interacted with social support to predict levels of IL-6 and CRP such that in individuals who reported low levels of social support, poor subjective sleep quality was associated with higher CRP and IL-6. However, in individuals with mean or high social support, no such relationship was detected. In women, the same pattern was observed in the prediction of TNF-α, whereby social support moderated the association between poor sleep quality and elevated TNF-α. These findings partially support those reported by Friedman et al. (2005), which indicated the relationship between poor sleep quality and higher inflammation was attenuated in individuals who reported good social relationships (Friedman, 2011; Friedman et al., 2005). Our findings also extend the identification of an interaction between subjective sleep quality and social support on inflammation into a clinical population at high risk for developing CVD. Finally, we show that the interaction between subjective sleep quality and social support is associated with not only IL-6 and TNF-α, but also the clinically relevant inflammatory protein, CRP.
Sleep Quality and Inflammation
Poor sleep quality has been linked to numerous adverse health outcomes including cardiovascular disease (CVD) and mortality (Cappuccio, D’Elia, Strazzullo, & Miller, 2010; Hoevenaar-Blom, Spijkerman, Kromhout, van den Berg, & Verschuren, 2011; Jennings, Muldoon, & Hall, 2007; Mallon, Broman, & Hetta, 2002). The mechanisms linking sleep and CVD have yet to be fully explained, but a proposed mediator in the relationship is systemic inflammation (Grandner et al., 2013; Simpson & Dinges, 2007). Although poor subjective sleep quality has been associated higher circulating CRP and IL-6 in some samples (e.g., McDade et al., 2006; Okun, Hanusa, Hall, & Wisner, 2009) findings from this, and other studies, increasingly suggest that the relationship between sleep and inflammation is moderated by variables, such as social support. In this sample, we did not observe a direct relationship between subjective sleep quality and IL-6 or CRP. Failing to consider social support as a moderator in our models would have resulted in the erroneous conclusion that subjective sleep quality was unrelated to IL-6 or CRP in this population. Potentially, studies that have concluded that sleep is not associated with inflammatory markers may have failed to observe relationship because of underlying moderation effects (e.g., Dowd, Goldman, & Weinstein, 2011).
The Moderating Role of Social Support
Social isolation and perception of loneliness have each been associated with mortality in epidemiological studies (Holwerda et al., 2012; Steptoe, Shankar, Demakakos, & Wardle, 2013); conversely, self-reported social support appears to provide a buffer against the health related effects of stress (Barth, Schneider, & von Kanel, 2010; Krause, 2005). In our sample, we did not observe direct associations between social support and inflammatory markers; instead, social support was associated with inflammation only when participants also reported poor subjective sleep quality. Core components in the definition of social support include the provision of instrumental and emotional support, and theoretically, the importance of high levels of social support for health may only be salient or observable under conditions of stress, such as poor sleep quality and factors that promote poor sleep, when individuals are in need of support (Cohen & Wills, 1985).
Both low social support and feelings of loneliness have been linked with worse subjective and objective sleep (Åkerstedt et al., 2002; Allaert & Urbinelli, 2004; Brummett et al., 2006; Cacioppo et al., 2002; Hawkley, Preacher, & Cacioppo, 2010; Jacobs, Cohen, Hammerman-Rozenberg, & Stessman, 2006; Mahon, 1994). Interestingly, high social support appears to be associated with better objective sleep, even in populations diagnosed with insomnia (Troxel et al., 2012). In a study of individuals with insomnia compared to those without insomnia, higher social support was associated with less wake after sleep onset (WASO) in both groups; additionally, an interaction was observed whereby participants with insomnia who reported high social support had objectively shorter sleep latency than participants with insomnia and low social support (Troxel et al., 2012). This finding suggests that even in individuals suffering from clinically significant sleep problems, social support may confer a better objective quality of sleep, which could hypothetically decrease levels of inflammation.
Gender
The relationships between social isolation, sleep, and mortality may differ by gender (Holwerda et al., 2012) and the extent to which men and women receive a benefit from social support differs across studies. We did not observe a three-way interaction between gender, social support and sleep in our group for the outcome variables IL-6 or CRP; however, we observed a three-way interaction in the prediction of TNF-α, such that women who reported poor sleep quality and low social support had higher levels of TNF-α than women with poor sleep quality and high social support. Gender differences have been observed in response to social stress, such that women appear more physiologically reactive to social rejection challenges than men (Stroud, Salovey, & Epel, 2002). Women tend to show higher neuroendocrine and cardiovascular reactions during conflict, when compared to men (Kiecolt-Glaser & Newton, 2001; Pietromonaco et al., 2013). It is possible that similar gender differences exist in the relationship between sleep quality and social support in the prediction of IL-6 and CRP, but that given the modest sample of our study, we were unable to detect them. Further investigation in this population, with larger sample sizes, is warranted.
Clinical Implications
The interaction between sleep and social support in the prediction of inflammation is particularly relevant in a hypertensive population, given the importance of inflammation at all stages of atherosclerosis development (Libby, Ridker, & Maseri, 2002; Libby & Ridker, 2004). A better understanding of the risk profile of inflammatory markers in individuals with pre-hypertension and hypertension may lead to the development of targeted psychosocial interventions aimed at improving both psychological and physical outcomes. For example, understanding the risk of poor subjective sleep quality on inflammation could speak to an increased need to screen and treat hypertension patients for sleep problems using evidence based sleep interventions, such as cognitive behavioral therapy for insomnia (CBT-I), especially in those patients also endorsing low levels of social support. Indeed, treatment of insomnia using CBT-I has been associated with reductions in CRP (Irwin et al., 2014). Additionally, given the important role of social support for health, those hypertensive patients with low social support and poor sleep could be screened and targeted for social interventions. Although social support was not related directly to inflammation in the current study, our findings suggest that ensuring adequate social support is important because it becomes a salient resource under times of stress or challenge (e.g., poor sleep) in protecting health.
Limitations
Conclusions from this study should be tempered by several limitations. First, information about sleep was drawn from self-report surveys. Although cohort studies have shown reliable associations between polysomnography and self-report indices of sleep, there is variation between the measures (Lauderdale, Knutson, Yan, Liu, & Rathouz, 2008; Van Den Berg et al., 2008; Vitiello, Larsen, & Moe, 2004). Second, the analyses were cross-sectional, so casual interpretations cannot be made. This is particularly important when considering the relationship between sleep and inflammation, which is likely bidirectional, with poor sleep resulting from diseases which include increased inflammation, and reduced / disrupted sleep directly inducing inflammation (Ranjbaran, Keefer, Stepanski, Farhadi, & Keshavarzian, 2007). Similarly, as social, psychological, physiological, and behavioral health variables tend to inter-relate in complex manners (e.g., Umberson, Crosnoe, & Reczek, 2010), it is possible that inflammation feeds back to exert influence on social support and sleep (e.g., via behaviors aimed at reducing aversive physiological states). Third, the sample size was modest—increasing the risk of Type I and II errors. The diminished ability to identify significant relationships between variables was of particular concern when examining interactions, particularly three-way interactions including gender, in which cell sizes across analyses were small. Fourth, there are known associations between obstructive sleep apnea (OSA) and inflammation, and undiagnosed OSA may have influenced findings from this study (Nadeem et al., 2013). Finally, we had only self-report information regarding menopausal status. Inclusion of menopausal status in analyses of the relationship between subjective sleep quality and social support predicting inflammation in women did not significantly change the findings; however, we may have been limited by the small sample of menopausal women and self-report data.
Conclusions
Despite these limitations, the current study extends the literature on sleep and inflammation and highlights the importance of examining the influence of perceived social support when examining relationships between sleep and other variables. In this study, there were important relationships between sleep and inflammatory markers that were initially not detected by simple correlational analyses or tests of main effects—it was not until we took into account the impact of social support on the relationship that we better understood the links between sleep and inflammation. Had we not examined the buffering role of social support in the relationship we would have incorrectly concluded that sleep was not related to IL-6 and CRP. The study also extends earlier work on the associations between sleep and social support into a population at high risk for developing cardiovascular disease. Importantly, these findings highlight the need to examine interactions between protective psychological variables that may buffer the risk conferred by other deleterious health variables (e.g., smoking, sleep problems).
Acknowledgements
This work was supported by the Alberta Children’s Hospital Research Institute (L.T.), NIH Grants HL44915 (to PJM), University of California San Diego Clinical and Translational Science Award UL1TR000100, and P60 MD00220 (San Diego EXPORT Center Grant). Thanks to Delaine Ammaturo and Kathleen Wilson for their help in preparing the data.
Footnotes
Drs. Tomfohr, Edwards, Madsen & Mills declare that they have no conflict of interest.
References
- Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119(3):651–665. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åkerstedt T, Knutsson A, Westerholm P, Theorell T, Alfredsson L, Kecklund G. Sleep disturbances, work stress and work hours: a cross-sectional study. Journal of Psychosomatic Research. 2002;53(3):741–748. doi: 10.1016/s0022-3999(02)00333-1. [DOI] [PubMed] [Google Scholar]
- Allaert F-A, Urbinelli R. Sociodemographic profile of insomniac patients across national surveys. CNS Drugs. 2004;18(1):3–7. doi: 10.2165/00023210-200418001-00003. [DOI] [PubMed] [Google Scholar]
- Balbo M, Leproult R, Van Cauter E. Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. International Journal of Endocrinology. 2010;2010:759234. doi: 10.1155/2010/759234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth J, Schneider S, von Kanel R. Lack of social support in the etiology and the prognosis of coronary heart disease: a systematic review and meta-analysis. Psychosomatic Medicine. 2010;72(3):229–238. doi: 10.1097/PSY.0b013e3181d01611. [DOI] [PubMed] [Google Scholar]
- Blake GJ, Rifai N, Buring JE, Ridker PM. Blood pressure, C-reactive protein, and risk of future cardiovascular events. Circulation. 2003;108(24):2993–2999. doi: 10.1161/01.CIR.0000104566.10178.AF. [DOI] [PubMed] [Google Scholar]
- Bowen KS, Uchino BN, Birmingham W, Carlisle M, Smith TW, Light KC. The stress-buffering effects of functional social support on ambulatory blood pressure. Health Psychology. 2013;33(11):1440–1443. doi: 10.1037/hea0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett BH, Babyak MA, Siegler IC, Vitaliano PP, Ballard EL, Gwyther LP, Williams RB. Associations among perceptions of social support, negative affect, and quality of sleep in caregivers and noncaregivers. Health Psychology. 2006;25(2):220–225. doi: 10.1037/0278-6133.25.2.220. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jørgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clinical & Experimental Immunology. 2003;132(1):24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJF, 3rd, Reynolds C, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Berntson GG, Ernst JM, Gibbs AC, Stickgold R, Hobson JA. Do lonely days invade the nights? Potential social modulation of sleep efficiency. Psychological Science. 2002;13(4):384–387. doi: 10.1111/1467-9280.00469. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Jr, J LI, Jr, J TW. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. Journal of the American Medical Association. 2003;289(19):2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98(2):310–357. [PubMed] [Google Scholar]
- Cole SW. Social regulation of leukocyte homeostasis: The role of glucocorticoid sensitivity. Brain, Behavior, and Immunity. 2008;22(7):1049–1055. doi: 10.1016/j.bbi.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. New England Journal of Medicine. 2004;350(14):1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Annals of Epidemiology. 2011;21(11):799–806. doi: 10.1016/j.annepidem.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM. Sleep quality, social well-being, gender, and inflammation: an integrative analysis in a national sample. Annals of the New York Academy of Sciences. 2011;1231(1):23–34. doi: 10.1111/j.1749-6632.2011.06040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Hayney MS, Love GD, Urry HL, Rosenkranz MA, Davidson RJ, Ryff CD. Social relationships, sleep quality, and interleukin-6 in aging women. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(51):18757–18762. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian Journal of Applied Sport sciences.Journal Canadien Des Sciences Appliquees Au Sport. 1985;10(3):141–146. [PubMed] [Google Scholar]
- Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nature and Science of Sleep. 2013;5:93–107. doi: 10.2147/NSS.S31063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Jr, W HE, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. The American Journal of Medicine. 1999;106(5):506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Stinson K, Whitaker KL, Moskovitz D, Virk H. The subjective meaning of sleep quality: a comparison of individuals with and without insomnia. Sleep. 2008;31(3):383–393. doi: 10.1093/sleep/31.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Preacher KJ, Cacioppo JT. Loneliness impairs daytime functioning but not sleep duration. Health Psychology. 2010;29(2):124–129. doi: 10.1037/a0018646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavior Research Methods. 2009;41(3):924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren WM. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34(11):1487–1492. doi: 10.5665/sleep.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. Loneliness and Social Isolation as Risk Factors for Mortality: A Meta-Analytic Review. Perspectives on Psychological Science. 2015;10(2):227–237. doi: 10.1177/1745691614568352. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Medicine. 2010;7(7) doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain, Behavior, and Immunity. 2010;24(1):54–57. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Breen E, Witarama T, Nicassio P. Cognitive behavioral therapy vs. tai chi for late life insomnia and inflammation: A randomized controlled comparative efficacy trial. Sleep. 2014;37(9):1543–1552. doi: 10.5665/sleep.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JM, Cohen A, Hammerman-Rozenberg R, Stessman J. Global sleep satisfaction of older people: The Jerusalem Cohort Study. Journal of the American Geriatrics Society. 2006;54(2):325–329. doi: 10.1111/j.1532-5415.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Muldoon MF, Hall M. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30(2):219–223. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin J-P, Hantsoo L. Close relationships, inflammation, and health. Neuroscience & Biobehavioral Reviews. 2010;35(1):33–38. doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychological Bulletin. 2001;127(4):472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Krause N. Exploring age differences in the stress-buffering function of social support. Psychology and Aging. 2005;20(4):714. doi: 10.1037/0882-7974.20.4.714. [DOI] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19(6):838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. The American Journal of Medicine. 2004;116(6):9–16. doi: 10.1016/j.amjmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Mahon NE. Loneliness and sleep during adolescence. Perceptual and Motor Skills. 1994;78(1):227–231. doi: 10.2466/pms.1994.78.1.227. [DOI] [PubMed] [Google Scholar]
- Mallon L, Broman J, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. Journal of Internal Medicine. 2002;251(3):207–216. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosomatic Medicine. 2006;68(3):376–381. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Freedson PS, Kline GM. Comparison of activity levels using the Caltrac accelerometer and five questionnaires. Medicine and Science in Sports and Exercise. 1994;26(3):376–382. [PubMed] [Google Scholar]
- Miller MA, Kandala NB, Kivimaki M, Kumari M, Brunner EJ, Lowe GD, Cappuccio FP. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 2009;32(7):857–864. [PMC free article] [PubMed] [Google Scholar]
- Motivala SJ. Sleep and inflammation: psychoneuroimmunology in the context of cardiovascular disease. Annals of Behavioral Medicine. 2011;42(2):141–152. doi: 10.1007/s12160-011-9280-2. [DOI] [PubMed] [Google Scholar]
- Nadeem R, Molnar J, Madbouly EM, Nida M, Aggarwal S, Sajid H, Loomba R. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. Journal of Clinical Sleep Medicine. 2013;9(10):1003–1012. doi: 10.5664/jcsm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M-F, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Sloan EK. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain, Behavior, and Immunity. 2009;23(7):887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Hall M, Coussons-Read ME. Sleep disturbances increase interleukin-6 production during pregnancy: implications for pregnancy complications. Reproductive Sciences. 2007;14(6):560–567. doi: 10.1177/1933719107307647. [DOI] [PubMed] [Google Scholar]
- Okun ML, Hanusa BH, Hall M, Wisner KL. Sleep complaints in late pregnancy and the recurrence of postpartum depression. Behavioral Sleep Medicine. 2009;7(2):106–117. doi: 10.1080/15402000902762394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Roberts JM, Marsland AL, Hall M. How disturbed sleep may be a risk factor for adverse pregnancy outcomes. Obstetrical & Gynecological Survey. 2009;64(4):273–280. doi: 10.1097/OGX.0b013e318195160e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauletto P, Rattazzi M. Inflammation and Hypertension. In: Weir MR, Lerma EV, editors. Chronic Kidney Disease and Hypertension. New York, NY: Springer New York; 2015. pp. 141–156. [Google Scholar]
- Pietromonaco PR, Uchino B, Dunkel Schetter C. Close relationship processes and health: Implications of attachment theory for health and disease. Health Psychology. 2013;32(5):499–513. doi: 10.1037/a0029349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. The Journal of the American Medical Association. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Prather AA, Marsland AL, Hall M, Neumann SA, Muldoon MF, Manuck SB. Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biological Psychology. 2009;82(1):12–17. doi: 10.1016/j.biopsycho.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbaran Z, Keefer L, Stepanski E, Farhadi A, Keshavarzian A. The relevance of sleep abnormalities to chronic inflammatory conditions. Inflamm Res. 2007;(2):51–57. doi: 10.1007/s00011-006-6067-1. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Clinical application of C-Reactive Protein for cardiovascular disease detection and prevention. Circulation. 2003;(107):363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Buono MJ, Roby JJ, Micale FG, Nelson JA. Seven-day recall and other physical activity self-reports in children and adolescents. Medicine and Science in Sports and Exercise. 1993;25(1):99–108. doi: 10.1249/00005768-199301000-00014. [DOI] [PubMed] [Google Scholar]
- Simpson N, Dinges DF. Sleep and inflammation. Nutrition Reviews. 2007;65(s3):S244–S252. doi: 10.1111/j.1753-4887.2007.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Spies CM, Straub RH, Cutolo M, Buttgereit F. Circadian rhythms in rheumatology – a glucocorticoid perspective. Arthritis Research and Therapy. 2014;16(Suppl 2):1–8. doi: 10.1186/ar4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A. Stress, social support and cardiovascular activity over the working day. International Journal of Psychophysiology. 2000;37(3):299–308. doi: 10.1016/s0167-8760(00)00109-4. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Hays RD, Wells KB, Rogers WH, Spritzer KL, Greenfield S. Long-term functioning and well-being outcomes associated with physical activity and exercise in patients with chronic conditions in the Medical Outcomes Study. Journal of Clinical Epidemiology. 1994;47(7):719–730. doi: 10.1016/0895-4356(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel E. Sex differences in stress responses: social rejection versus achievement stress. Biological Psychiatry. 2002;52(4):318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain, Behavior, and Immunity. 2008;22(6):960–968. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel WM, Kupfer DJ, Reynolds Charles F, 3rd, Frank E, Thase ME, Miewald JM, Buysse DJ. Insomnia and objectively measured sleep disturbances predict treatment outcome in depressed patients treated with psychotherapy or psychotherapy-pharmacotherapy combinations. The Journal of Clinical Psychiatry. 2012;73(4):478–485. doi: 10.4088/JCP.11m07184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberson D, Crosnoe R, Reczek C. Social Relationships and Health Behavior Across Life Course. Annual Review of Sociology. 2010;36:139–157. doi: 10.1146/annurev-soc-070308-120011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Berg JF, Rooij FJA, Van Vos H, Tulen JHM, Hofman A, Miedema HME, Tiemeier H. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons*. Journal of Sleep Research. 2008;17(3):295–302. doi: 10.1111/j.1365-2869.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Larsen LH, Moe KE. Age-related sleep change: gender and estrogen effects on the subjective–objective sleep quality relationships of healthy, noncomplaining older men and women. Journal of Psychosomatic Research. 2004;56(5):503–510. doi: 10.1016/S0022-3999(04)00023-6. [DOI] [PubMed] [Google Scholar]
- Wills TA. Social support and interpersonal relationships. In: Clark MS, editor. Prosocial behavior. Review of personality and social psychology. Thousand Oaks, CA, US: Sage Publications, Inc.; 1991. pp. 265–289. [Google Scholar]