Abstract
Neutrophil extracellular traps (NETs) contribute to innate immunity as well as numerous diseases processes such as deep vein thrombosis, myocardial ischemia, and autoimmune disease. To date, most knowledge on NETs formation has been gathered via the qualitative microscopic examination of individual neutrophils in vitro, or aggregate structures in vivo.
Here we describe a novel flow cytometry (FLOW)-based assay to identify and quantify NETs using antibodies against key NETs constituents, specifically DNA, modified histones and granular enzymes. This method is applicable to both murine and human samples for the assessment of induced NETs in vitro, or detection of NETosis in vivo in blood samples.
This FLOW-based method was validated by comparison with the well-established microscopy assay using two genetic mouse models previously demonstrated to show defective NETosis. It was then used on healthy human neutrophils for detection of ex vivo induced NETs and on blood samples from patients with sepsis for direct assessment of in vivo NET-forming neutrophils.
This new methodology allows rapid and robust assessment of several thousand cells per sample and is independent of potential observer-bias, the two main limitations of the microscopic quantification. Using this new technology facilitates the direct detection of in vivo circulating NETs in blood samples and purification of NETting neutrophils by fluorescence-activated cell sorting (FACS) for further analysis.
Keywords: Biological Markers, Flow Cytometry, Methods, Neutrophils, Sepsis
INTRODUCTION
Neutrophils release neutrophil extracellular traps (NETs) after specific agonist induction as a form of non-apoptotic death in a stepwise process termed NETosis[1]. NETosis requires chromatin decondensation followed by nuclear membrane rupture that allows chromatin to mix with granule contents such as neutrophil elastase or myeloperoxidase (MPO). Ultimately the preformed NETs are released into the extracellular space.[1] Histone citrullination (H3Cit) that entails the conversion of arginine to citrulline on histone tails by peptidylarginine deaminase 4 (PAD4) promotes chromatin decondensation and is considered a key marker of early NETosis.[2-4] NETosis is also dependent on the production of reactive oxygen species (ROS) by the neutrophil NADPH oxidase complex[1], of which the Rho GTPase Rac is a component. It has been shown that PAD4- and Rac-deficient neutrophils have defective NETosis.[2-5]
NETs were initially described as component of innate immunity as they are able to bind microorganisms and ensure high local concentrations of antimicrobial agents.[6] Subsequently, NETs were shown to contribute to autoimmune diseases[7], cancer[8, 9], myocardial ischemia/reperfusion injury[10] and deep vein thrombosis.[11] Thus, a better understanding of NETosis may be a key to the development of novel therapeutic interventions for a wide array of clinical situations.
We have developed a flow cytometric-based protocol that is observer-independent and allows direct quantification of large numbers of cells. We validated this assay using mice previously shown to have defective NETosis due to genetic mutations of Rac and PAD4. The FLOW-based method was then used to detect and quantify NETs in human blood samples from healthy individuals after ex vivo induction of NETs and on blood samples from patients with sepsis for direct assessment of in vivo circulating NET-forming neutrophils. The FLOW-based method, based on immunological detection of NETs components, facilitates the detection and FACS-sorting of NETs in clinical settings and thus will facilitate new avenues of clinical and biological investigations.
METHODS
Microscopy-based NETs quantification assay
Mouse peripheral blood (PB) polymorphonuclear neutrophil (PMNs) isolation, stimulation and staining were performed as previously described.[3]
Flow-based assay to detect NETs
Purified mouse PB PMNs from Rac2−/− [12] or WT mice were suspended in RPMI1640 (Invitrogen, Carlsbad, CA). Peripheral blood from Pad4−/− [4] or WT mice underwent red blood cell sedimentation with Hetastarch (6% hydroxyethyl starch, HES) in 0.9% NaCl solution (Hospira, Inc., Lake Forest, IL) at 1:4 v/v dilution at 37°C and then was resuspended in RPMI1640. The flow-based assay described herein is performed entirely in suspension. Cells were stimulated with 100nM Phorbol-1-myristate-13-acetate, (PMA) (Sigma, St Louis, MO) or 4 μM Ionomycin (Life Technologies, Carlsbad, CA) for 4h at 37°C in 5%CO2, then fixed in 2% paraformaldehyde, blocked for 30 min with 2% bovine serum albumin (BSA, Sigma) in Dulbecco's phosphate-buffered saline (DPBS, Life Technologies) at 37°C and, without a permeabilization step, incubated sequentially with the primary anti-histone H3 antibody (citrulline 2,8,17, ab5103; Abcam, Cambridge, MA) at 1:300 dilution, Alexa Fluor700-conjugated secondary antibody (A-21038, Thermo Scientific, Rockford, IL) at 1:300 dilution and FITC-conjugated anti-myeloperoxidase antibody (mouse: ab90812, 1:50 dilution; human: ab11729, 1:10 dilution, Abcam). Each incubation was followed by a wash with 2% BSA in DPBS and centrifugation at 16,400 rpm at 4°C for 20 minutes. Samples were then resuspended in 2% BSA in DPBS containing Hoechst 33342, trihydrochloride trihydrate, (Thermo Scientific, Rockford, IL) at 1:5000 dilution and analyzed by flow-cytometry according to the gating strategy detailed in Figure S1. There is no selection on the FSC/SSC properties, as those are very heterogenous for NETs in suspension. A minimum of 10,000 cells per condition were analyzed in duplicates.
Human samples
Discarded whole blood samples from patients at Boston Children's Hospital (BCH) or Beth Israel Deaconess Medical Center (BIDMC) were obtained under an Institutional Review Board-approved protocol (04-02-017R at BCH and 2005P000116 at BIDMC). Samples were anticoagulated with ethylenediaminetetraacetic acid (EDTA) and processed as described above within 3 hours of collection. Control samples were stimulated ex vivo with 4 μM Ionomycin for 30 min for qualitative analysis (Figure 2.A) and quantification (Figure 2.B.). For in vivo NETs quantification, cases fulfilling sepsis criteria of the American College of Chest Physicians (ACCP)[13] and controls, without recent infection or leukocytosis of any etiology, were selected.
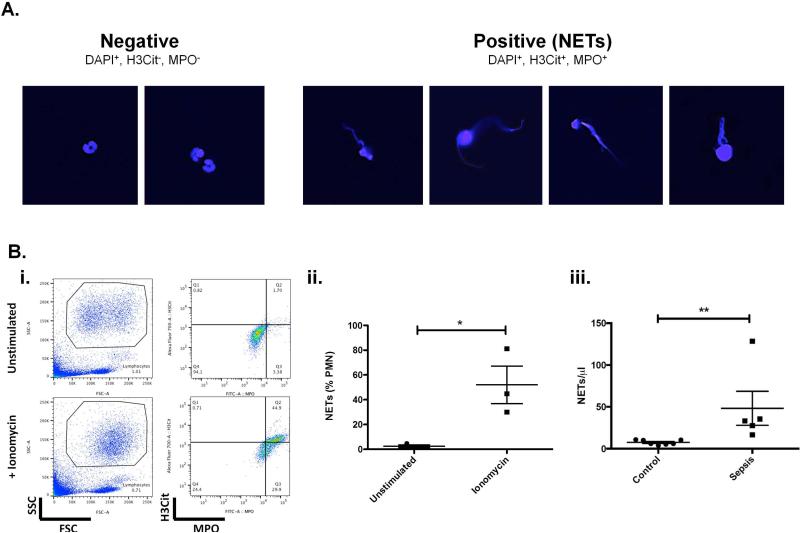
Figure 2. Validation and analysis of flow assay on human samples.
A. Separation of NETs from resting neutrophils. Neutrophils were purified from peripheral blood of control individuals. NETs were induced by ionomycin for 4h and unstimulated PMNs from the same donor were kept in parallel. Both samples were then mixed 1:1 before fixation, staining, and sorting onto glass slides. Photomicrographs demonstrate the correlation between sorted DAPI+, H3Cit+ and MPO+ NETs population with the characteristic shooting star morphology of extracellular fibrous DNA used to identify them by microscopy (right panel). Sorted intact PMNs defined as DAPI+, H3Cit−, MPO− exhibit typical multi-lobulated shaped nuclei of resting human neutrophils (left panel). These images are representative from one of two independent experiments. B. Detection of NETs formed in human samples ex vivo or in vivo. Human EDTA-anticoagulated whole blood was processed as described. i. NETs were induced in PB from healthy donors and compared to the unstimulated sample. Representative flow data showing the initial FSC/SSC blot, and NETs detected as H3Cit and MPO double positive cells. ii. Quantitative analysis of (N=3), iii. Quantification of in vivo circulating NETs in PB samples from healthy controls (N=7) and patients with sepsis (N=5). Mann-Whitney test: **P<0.01.
Statistics
Data are presented as means ± SEM and were analyzed with two-sided Student or Mann-Whitney test (Figure 2B) using GraphPad Prism software (Version 5.0, La Jolla, CA). Results were considered significant at P<0.05.
RESULTS AND DISCUSSION
Development and validation of the flow assay on murine samples
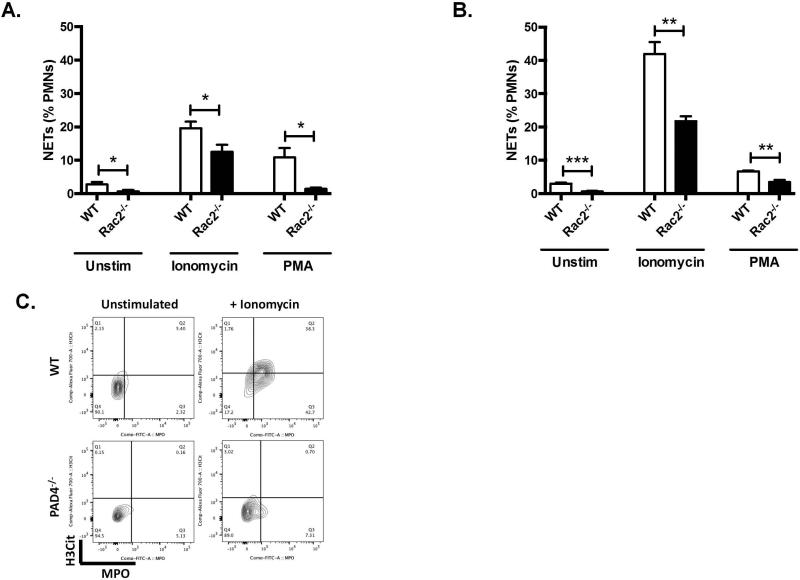
Rac2 is required for ROS-dependent NETosis in vitro[5]. We used neutrophils from Rac2−/− mice to compare the flow-based method with the well-established microscopic assay previously described[3, 5, 14]. Stimulated neutrophils from mouse blood were assessed for NETs by microscopy (Figure 1A) and by the flow method (Figure 1B) yielding similar qualitative and quantitative results. As expected, Rac2−/− neutrophils had reduced spontaneous NETosis in vitro. After 4 hours of treatment with PMA or ionomycin, induced NETosis was also significantly impaired in Rac2−/− PMNs using both methods of analysis (quantification in Table S1). In addition, these data indicate multiple levels of interaction between Rac2 signaling and NETosis. To further strengthen the validity of our method, we compared WT and Pad4−/− neutrophils, which are characterized by severe impairment of NETosis [3, 4]. Following ionomycin treatment, WT neutrophils show massive NET formation while these are virtually undetectable in the Pad4−/− sample (Figure 1C).
Figure 1. Development and validation of flow assay on mouse samples.
A. and B. Validation of flow-based assay to measure NETs. Neutrophils were isolated from peripheral blood of Rac2−/− mice or WT controls and stimulated with ionomycin (4 μM), PMA (100 nM) or the same volume of RPMI (Unstim) for 4 hours. Cells were then fixed and stained for NETs, by either microscopy-based assay (A); or by a flow-based assay (B) (N= 4 each). T-test: *P<0.05, **P<0.01, ***P<0.001, versus control (WT Unstim) unless otherwise specified. (C) NETs were detected in PB from WT mice stimulated with ionomycin, but not in PB from Pad4−/− mice or from unstimulated WT controls. Representative flow data showing H3Cit-positive and MPO-positive cells defined as NETs.
Human neutrophils forming NETs can be purified by fluorescence activated cell sorting
We also compared flow and microscopic methods qualitatively. Neutrophils purified from peripheral blood of control individuals were treated by ionomycin and mixed 1:1 with unstimulated PMNs from the same donor. The cell mixture was then sorted onto glass slides. Representative photomicrographs (Figure 2A) demonstrate morphologic correlation between sorted DAPI+, H3Cit+ and MPO+NETs population with the characteristic shooting star morphology of extracellular fibrous DNA used to identify them by microscopy (right panel). Sorted intact PMNs, defined as DAPI+, H3Cit−, MPO−, exhibit typical multi-lobulated shaped nuclei of resting human neutrophils (left panel).
Detection of NETs in human blood
We next determined if the flow method could detect NETs in human blood samples. NETs were induced ex vivo with ionomycin in PB from controls, then quantified (Figure 2 i and ii). We show significant NET formation in these conditions (unstimulated 2.49±1.11% NETs, ionomycin 52.0±15.2% NETs, P = 0.031).
Quantification of in vivo NETs in patient or clinical blood samples
The flow method facilitates analysis of clinical blood samples. In vivo circulating NETs were quantified for 7 control individuals and 5 patients diagnosed with sepsis. We observed low NET counts in control samples (7.3±1.4 mean ± SEM, range 3.9-10.8 NETs/μl) (Figure 2B). All sepsis samples showed significantly increased NETs (65.7±31.4 mean ± SEM, range 16.6-128.4, P<0.01 vs. control), consistent with previously described increase in NET surrogate markers observed in septic patients.[15, 16] These data indicate for the first time that NETs can be directly quantified in blood samples using the flow method.
In conclusion, we describe and validate in both murine and human samples a novel method to quantify NETs. This methodology circumvents two main limitations of the well-established microscopic assay in that it is observer-independent and allows robust and rapid assessment of a much larger number of cells per sample. This original method is also a potential tool to investigate conditions associated with in vivo circulating NETs as a biomarker and to FACS-sort NETs for further biological analysis.
Supplementary Material
KEY POINTS.
We describe a novel, convenient, observer-independent method of NETs quantification based on the flow cytometric detection of key NET components.
This method allows the direct quantification of in vivo circulating NETs in blood samples.
ACKNOWLEDGEMENTS
The authors are thankful to Ronald Mathieu for helpful discussions and technical assistance. We also thank Prof. Carlo Brugnara of the BCH Clinical Hematology Laboratory for his logistical support. This study was supported by grants from the National Institutes of Health (5R01DK062757 [D.A.W.], NHLBI R01 HL102101 [D.D.W]), NHLBI T32 HL066987 (KM), the Department of Medicine, Boston Children's Hospital (D.A.W. and D.D.W.), and the Swiss National Science Foundation (PBLAP3-140046 [M.G.]).
Footnotes
AUTHORSHIP CONTRIBUTIONS
M.G. designed and performed experiments, analyzed data, and wrote the paper; K.M. designed and performed experiments and analyzed data; R.R. assisted in handling human samples, assisted in designing and performing experiments, analyzed data, N.I.S. provided human samples, D.D.W. assisted with study design and supervised the study; D.A.W; supervised the study, designed experiments, analyzed data, and wrote the paper.
DISCLOSURE OF CONFLICTS OF INTEREST
The authors declare no competing financial interests.
REFERENCES
- 1.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. The Journal of cell biology. 2007;176:231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. The Journal of cell biology. 2009;184:205–13. doi: 10.1083/jcb.200806072. 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M, Hu J, Wang Y, Wagner DD. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:8674–9. doi: 10.1073/pnas.1301059110. 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. The Journal of experimental medicine. 2010;207:1853–62. doi: 10.1084/jem.20100239. 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim MB, Kuiper JW, Katchky A, Goldberg H, Glogauer M. Rac2 is required for the formation of neutrophil extracellular traps. J Leukoc Biol. 2011;90:771–6. doi: 10.1189/jlb.1010549. 10.1189/jlb.1010549. [DOI] [PubMed] [Google Scholar]
- 6.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 7.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone HJ, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–5. doi: 10.1038/nm.1959. 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, Wagner DD. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13076–81. doi: 10.1073/pnas.1200419109. 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013 doi: 10.1172/JCI67484. 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savchenko AS, Borissoff JI, Martinod K, De Meyer SF, Gallant M, Erpenbeck L, Brill A, Wang Y, Wagner DD. VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood. 2014;123:141–8. doi: 10.1182/blood-2013-07-514992. 10.1182/blood-2013-07-514992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood. 2014;123:2768–76. doi: 10.1182/blood-2013-10-463646. 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, Atkinson SJ, Dinauer MC, Williams DA. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–96. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 13.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. International Sepsis Definitions Conference. Crit Care Med. 2003. 31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. Sccm/Esicm/Accp/Ats/Sis. 2001 SCCM/ESICM/ACCP/ATS/SIS 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 14.Chen G, Zhang D, Fuchs TA, Manwani D, Wagner DD, Frenette PS. Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood. 2014;123:3818–27. doi: 10.1182/blood-2013-10-529982. 10.1182/blood-2013-10-529982 blood-2013-10-529982 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margraf S, Logters T, Reipen J, Altrichter J, Scholz M, Windolf J. Neutrophil-derived circulating free DNA (cf-DNA/NETs): a potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock. 2008;30:352–8. doi: 10.1097/SHK.0b013e31816a6bb1. 10.1097/SHK.0b013e31816a6bb1. [DOI] [PubMed] [Google Scholar]
- 16.Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–94. doi: 10.1182/blood-2013-04-457671. 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.