Abstract
We aimed to label tubastatin A (1) with carbon-11 (t1/2 = 20.4 min) in the hydroxamic acid site to provide a potential radiotracer for imaging histone deacetylase 6 (HDAC6) in vivo with positron emission tomography (PET). Initial attempts at a one-pot Pd-mediated insertion of [11C]carbon monoxide between the aryl iodide (2) and hydroxylamine gave low radiochemical yields (< 5%) of [11C]1. Labeling was achieved in useful radiochemical yields (16.1 ± 5.6%, n = 4) through a two-step process based on Pd-mediated insertion of [11C]carbon monoxide between the aryl iodide (2) and p-nitrophenol to give the [11C]p-nitrophenyl ester ([11C]5), followed by ultrasound-assisted hydroxyaminolysis of the activated ester with excess hydroxylamine in DMSO/THF mixture in the presence of a strong phosphazene base P1-t-Bu. However, the success in labeling the hydroxamic acid group of [11C]tubastatin A was not transferable to the labeling of three other model hydroxamic acids.
Keywords: [11C]tubastatin A, HDAC6, [11C]Carbon monoxide, Carbonylation, Hydroxyaminolysis
1 Introduction
Transcriptional dysfunction and imbalance in protein acetylation, in which histone deacetylases 6 (HDAC6)1 play a significant role,2 may be important factors in several neurodegenerative disorders3, including Huntington’s disease, Alzheimer’s disease, Parkinson’s disease, mood disorders4, and autoimmune diseases5. In addition to neurological disorders, HDAC6 is also heavily implicated in cancer-relevant processes, such as cell migration, metastasis and angiogenesis, and inhibitors show promise for cancer treatment.6,7 Consequently, radioligands for imaging HDAC6 density with PET, based on high-affinity inhibitors, could be useful for research of HDAC6 in neurology, psychiatry, oncology, and also for drug discovery and development.
The hydroxamic acid moiety is prominently featured in many HDAC inhibitors due to the strong interaction between this group and the zinc(II) ion at the enzyme active site.2,8 A method for labeling hydroxamic acids with no-carrier-added (NCA) carbon-11 (t1/2 = 20.4 min) could be useful for developing PET radioligands for HDAC8, or other zinc(II)-dependent enzymes of molecular imaging interest, such as the matrix metalloproteinases,9,10 However, there is no precedent for labeling the hydroxamic acid group with carbon-11.
Tubastatin A (1) is a potent (IC50 = 15 nM) and selective (57-fold over HDAC8, and > 1000 fold over all other HDAC isoforms) second generation HDAC6 inhibitor.11 In order to develop a HDAC6 selective PET radioligand, we set out to label 1 with carbon-11 in the hydroxamic acid group. The radiolabeling was ultimately achieved through a two-step process from [11C]carbon monoxide via Pd-mediated synthesis of a [11C]p-nitrophenyl ester followed by hydroxyaminolysis. Control reactions and attempted application of this labeling chemistry to simple model compounds are also discussed.
2. Results and Discussion
2.1. Basics of Pd-mediated [11C]CO insertion
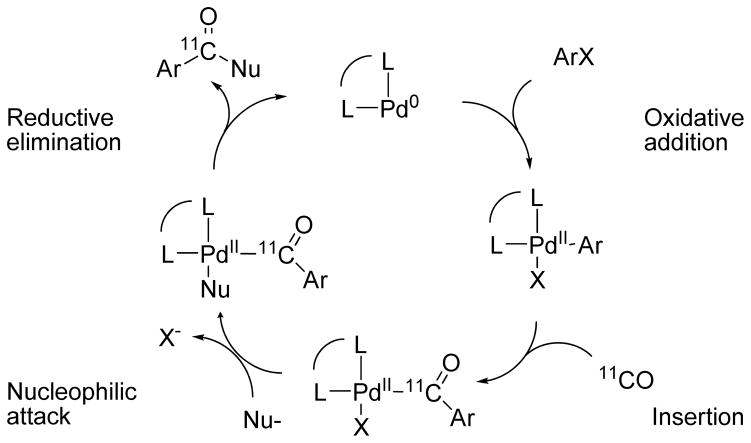
No-carrier-added (NCA) [11C]carbon monoxide is increasingly important as a labeling agent because of: i) its ready and efficient production from cyclotron-produced [11C]carbon dioxide;12,13 ii) practical advances for efficient utilization in solution chemistry; 14–18 and iii) the development of methods for performing a variety of Pd-promoted [11C]carbon monoxide insertion reactions leading to radiotracers with a labeled carbonyl group.17–19 Often such labeling can be achieved without the need to protect functional groups remote from the reaction center. Mechanistically, for substrates ArX, where X may be, for example, Cl, Br, I or triflate, these reactions are generally thought to proceed sequentially through oxidative addition of Pd(0), insertion of [11C]carbon monoxide, attack by a nucleophile (Nu-) and reductive elimination to give the labeled product, Ar11CONu (Scheme 1). Ligands (L) added for chelation to the Pd(0) center may strongly influence reaction outcome.19 On a macroscale, such reactions may run with catalytic amounts of Pd, usually derived from a Pd(II) compound. In NCA 11C-radiochemistry the Pd is in excess and is therefore not necessarily acting catalytically. Several nucleophiles, including the hydroxide ion,20 alcohols,21,22 and amines,23,24 have been shown to be effective for producing compounds of the type Ar11CONu, We initially considered that hydroxylamine might serve as a nucleophile in this process and directly deliver [11C]1, especially in view of a report describing the successful Pd(0)-catalyzed hydroxylamination of allyl esters to produce N-allylhydroxylamines.25
Scheme 1.
General process for Pd-mediated 11C-carbonylation reactions leading to aryl 11C-carbonyl compounds.
2.2. Attempted labeling using one-pot process
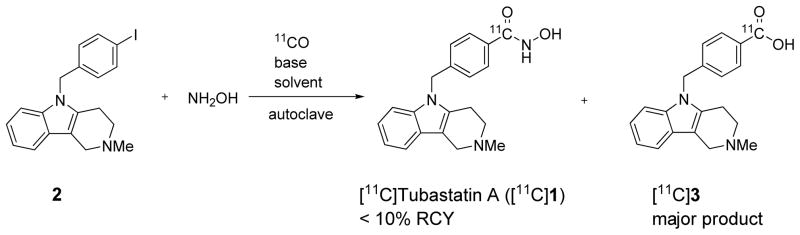
Initially, we attempted to prepare [11C]1 through direct Pd-mediated insertion of [11C]carbon monoxide between aryl iodide 2 and hydroxylamine. For this purpose, hydroxylamine was generated in situ from its commercially available hydrochloride salt and DMAP. Reactions using a 20 molar ratio of hydroxylamine to 2 in DMF in the presence of Pd(PPh3)4 gave [11C]1 in <5% decay-corrected radiochemical yield (RCY) from cyclotron-produced [11C]carbon dioxide. The acid [11C]3 (tR = 6–8 min, m/z = 321 for M++H) was obtained as the main labeled product. Solvents and reagents, although used under as much inert gas protection as possible, were the likely sources of trace moisture. Moreover practical difficulties ensued. Thus, DMAP·hydrochloride had to be filtered off to avoid clogging the micro-autoclave, and a large excess of DMAP had to be removed during HPLC separation of labeled product. Use of triethylamine in place of DMAP did not overcome these difficulties and gave even lower yield of [11C]1 (1–2%). Replacement of DMF with THF and Pd(PPh3)4 with Pd2(dba)3 plus Xantphos, gave more consistent but still low yields of [11C]1 (<10% from [11C]carbon dioxide). The average time for one-pot radiosynthesis was 40 min.
As an ambident nucleophile, hydroxylamine has two possible paths for attack on the hypothesized ArPd11COI intermediate, potentially reducing the yield of [11C]1. Use of-non-ambident O-trityl-protected hydroxylamine as nucleophile in the insertion reaction proved, however, unpromising. We noted that the nucleophilicity of hydroxylamine is appreciably lower than that of methylamine in reaction with benzhydrilium ions,26 and also that special measures had to be taken to increase the weak nucleophilicity of methylamine for the preparation of [carbonyl-11C]N-methyl-amides by similar Pd-mediated [11C]carbon monoxide insertion.27,28 Hence, given our observations and these reports, we suspect hydroxylamine is a weaker nucleophile than water and does not react adequately with the postulated radioactive transition metal intermediate. We therefore sought a different approach for the radiosynthesis of [11C]1.
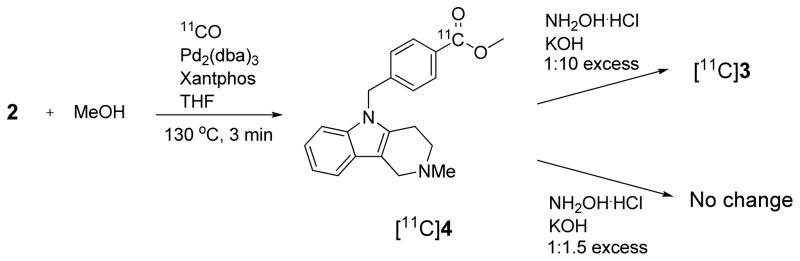
2.3. Labeling of [11C]tubastatin A ([11C]1) using hydroxyaminolysis of an activated ester ([11C]4)
The aminolysis of esters is a well-known method for producing hydroxamic acids.29 Indeed, 1 has itself been synthesized by treating the methyl ester 4 with hydroxylamine hydrochloride in the presence of base (KOH or NaOMe) in methanol.30 [carbonyl-11C]Esters are readily prepared from [11C]carbon monoxide through Pd-promoted reactions.19 Therefore, we next considered a two-step process based on the synthesis of an appropriate 11C-carbonyl-labeled ester followed by aminolysis with hydroxylamine (Scheme 3). The methyl ester [11C]4 (tR = 13.1 min, m/z = 335 for M++H) was readily obtained in moderate yield (18.5 ± 6.2%, n = 11) from [11C]carbon monoxide and the aryl iodide 2, in the presence of Pd2(dba)3/Xantphos and methanol with THF as the solvent. However, attempts to convert [11C]4 into [11C]1 with hydroxylamine hydrochloride in the presence of a slight excess of potassium hydroxide in MeOH as a base (1.5 eq.) were ineffective. At room temperature or moderately elevated temperature (≤ 70 °C), with base used in a ten-fold molar ratio to 2, only the labeled acid [11C]3 was produced, showing that hydroxyaminolysis of the methyl ester [11C]4 was uncompetitive with hydrolysis.
Scheme 3.
Aminolysis of the intermediate methyl ester [11C]4 with hydroxylamine did not yield [carbonyl-11C]tubastatin A ([11C]1).
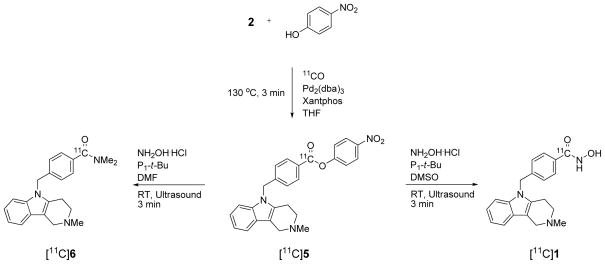
The negative results from methyl ester prompted us to consider that a labeled ester with greater susceptibility towards aminolysis would be needed. We expected that the corresponding p-nitrophenyl ester [11C]5 would meet this need due to conjugation of the carbonyl group to the aromatic nucleus bearing an electron-withdrawing nitro group.31,32
Gratifyingly, [11C]5 (tR = 19.1 min, m/z = 442 for M++H) was readily obtained through Pd-mediated insertion of [11C]carbon monoxide between 2 and p-nitrophenol (Scheme 4). In particular, use of Pd2(dba)3 and Xantphos in THF at 130 °C for 3 min gave [11C]5 in optimal high yield (54.6 ± 8.0%, n = 3).
Scheme 4.
Synthesis of [11C]tubastatin A ([11C]1) via ultrasound-assisted hydroxyaminolysis of the p-nitrophenyl ester intermediate [11C]5 in the presence of phosphazene base P1-t-Bu.
For hydroxyminoloysis, the reaction mixture was directed to a crimp-sealed fluoropolymer vessel33 which was pre-loaded with a mixture of NH2OH·HCl and a selected base in DMSO or DMF. The vessel was sonicated at room temperature for 3 to 5 min. The reaction mixture was diluted with aqueous potassium hydroxide solution and [11C]1 was separated from the p-nitrophenoxide ion and other material by reverse phase HPLC. Separated [11C]1 was identified by its comobility with 1 in analytical reverse phase HPLC and by LC-MS of associated carrier ([M+H]+ = m/z 336). Yields depended on the amount of hydroxylamine, choice of base and solvent (Table 1). Thus, excess hydroxylamine in the presence of BaO or DBU gave unsatisfactory results. Excess hydroxylamine in DMSO in the presence of DMAP gave [11C]1 in 8.0 ± 2.1% (n = 4) from [11C]carbon dioxide. However, a large amount of DMAP co-eluted with [11C]1 during HPLC separation. Replacement of DMAP with a lower molar amount of the strong phosphazene base P1-t-Bu34 gave [11C]1 in higher overall yield (16.1 ± 5.6%, n = 4; Table 1). The specific activity of a sample of [11C]1 was 221 mCi/μmol at 61 min from EOB. Use of DMF under these conditions gave the unexpected [carbonyl-11C]N,N-dimethylamido product [11C]6 (tR = 14.2 min, m/z = 348 for M++H in quantitative yield (Scheme 4 and Table 1) and in 15.2 ± 5.1% overall yield (n = 6) from cyclotron-produced [11C]carbon dioxide. This result again exemplifies the ability of DMF to act as a dimethylamine donor under basic conditions.35,36 This type of process is potentially useful for preparing [carbonyl-11C]N,N-dimethyl-arylamides.
Table 1.
Synthesis of [11C]1 via hydroxyaminolysis of [11C]5.
| Entry | Base | Solvent | RCY (%)a |
|---|---|---|---|
| 1 | BaO | DMSO/THF | 2.1 (n = 1) |
| 2 | BaO | DMF/THF | 2.3 (n = 1) |
| 3 | DBU | DMSO/THF | 5.1 ± 0.5 (n = 4) |
| 4 | DMAP | DMSO/THF | 8.0 ± 2.1 (n = 4) |
| 5 | P1-t-Bu | DMSO/THF | 16.1 ± 5.6 (n = 4) |
| 6 | P1-t-Bu | DMF/THF | 0 (n = 6)b |
RCY is based on the activity of the collected fraction of [11C]1 from HPLC, decay-corrected from starting [11C]CO2. The average radiosynthesis time was 45 min.
[11C]6 was obtained in 15.2 ± 5.1% RCY.
The possibility that the O-acylhydroxylamine isomer of [11C]1 might arise as a result of the ambident nucleophilicity of hydroxylamine must be considered.37,38 However, studies indicate that although such O-acylhydroxylamines may form initially in the treatment of esters with hydroxylamine, they are readily converted with excess hydroxylamine into the desired hydroxamic acids.29,37,38 Furthermore, O-acylhydroxylamines have been easily separated by reverse phase HPLC and silica gel TLC from their hydroxamic acid isomers.39 Correct identification of [11C]1 therefore seems assured.
This reaction also appeared less sensitive to the presence of water than expected. Under the optimized condition, [11C]1 and [11C]3 were normally produced in 3.3: 1 ratio. When three equivalent H2O was added to the reaction, [11C]1 and [11C]3 were produced in equal amount. When the second step reaction was carried out with H2O only, ~70% of the radioactivity was the intact [11C]5 while ~30% was hydrolyzed to [11C]3 (Table 2).
Table 2.
Influence of H2O on hydroxyaminolysis/hydrolysis of [11C]5.
| Entry | Reagentsa (μmol) | Product ratiob | |
|---|---|---|---|
| 1 | NH2OH (34) | 3.3 ([11C]1) | 1.0 ([11C]3) |
| 2 | NH2OH (34) + H2O (111) | 1.0 ([11C]1) | 1.0 ([11C]3) |
| 3 | H2O (111) | 1.0 ([11C]3) | 2.5 ([11C]5) |
Other conditions: P1-t-Bu, RT, sonication, 3 min.
As measured by HPLC peak integration in the radio-chromatogram of the reaction mixture.
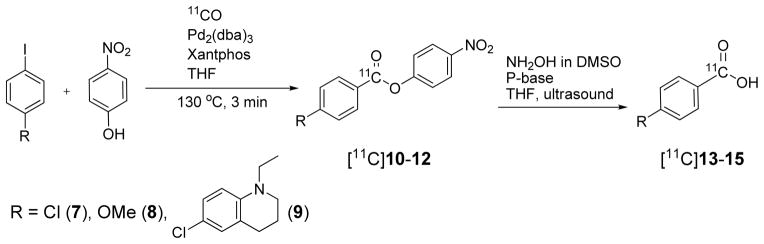
Attempts to apply this labeling method to three simple model aryl iodides (7–9) produced the expected [11C]nitrophenyl esters ([11C]10–12) in good radiochemical yields. However, the second step hydroxyaminolysis only yielded the respective [11C]acids ([11C]13–15) (Scheme 5). In view of the control reaction illustrated in Table 2, the presence of moisture may not be solely responsible for the failure of forming hydroxamic acids. Some as yet ungleaned structural influence of the tubastatin A molecule may play a role.
Scheme 5.
Simple model compounds only produced the respective [11C]acids under ultrasound-assisted hydroxyaminolysis of the appropriate [11C]p-nitrophenyl ester in the presence of phosphazene base P1-t-Bu.
In summary, [11C]tubastatin A ([11C]1) was labeled in the hydroxamic group via preparation of a 11C-labeled p-nitrophenyl ester, itself prepared from [11C]carbon monoxide. However, application of this approach to the labeling of other hydroxamic acids with carbon-11 to show generic value was unsuccessful.
3. Experimental
All reagents and solvents were ACS grade or higher and used without further purification. Hydroxylamine hydrochloride, O-trityl-hydroxylamine, Pd(PPh3)4, Pd2(dba)3, Xantphos (4,5-bis(diphenylphosphino)-9,9-dimethylxanthene), phosphazene base P1-t-Bu (tert-butylimino-tris(dimethylamino)phosphorane), and other chemicals were purchased from Sigma-Aldrich (Milwaukee, WI). Precursors were synthesized as previously described.11 Radiosynthesis was performed in a lead-shielded hot-cell for personnel protection with a modified Synthia radiosynthesis platform40 coupled to a high-pressure autoclave for 11C-carbonylation.22 Radioactive products were separated with HPLC on an apparatus comprising a solvent module (System Gold 126; Beckman Coulter, CA) coupled with an absorbance detector operating at 274 nm (Model 166; Beckman Coulter) and a sodium iodide radioactivity detector (Bioscan, Washington DC). The HPLC apparatus for reaction analysis comprised a solvent module (System Gold 126; Beckman Coulter) coupled with an absorbance detector operating at 274 nm (Model 168; Beckman Coulter) and a radioactivity detector (PMT, Flow-count; Bioscan). Radioactivity was measured with a calibrated dose ionization chamber (Atomlab 300; Biodex Medical Systems Inc., Shirley, NY). LC-MS was performed on a LCQ Deca instrument (Thermo Fisher Scientific Corp.; Waltham, MA) equipped with a reversed-phase HPLC column (Luna C18, 3 μm, 50 mm × 2 mm; Phenomenex, Torrance, CA), eluted at 200 μL/min with a mixture of A (H2O–MeOH–AcOH, 90: 10: 0.5 by vol.) and B (MeOH AcOH, 100: 0.5 v/v), initially composed of 20% B and linearly reaching 80% B in 3 min. An industrial ultrasonic processor instrument (UIS250L, 250 W, 24 kHz; for a description, see: http://downloads.german-pavilion.com/downloads/pdf/exhibitor_19738.pdf, last accessed on 9/16/2013), was used to agitate heterogeneous radioactive reaction mixtures in closed vials. These vials (1 mL volume) and their caps were custom-made from inert fluoropolymers. The reaction vial was designed to fit snugly into the port of the instrument so that ultrasound was efficiently transmitted to the reaction mixture. In order to allow reagents to be added and reaction mixtures to be sampled multiple times, the septum liner of the vial cap was designed to withstand multiple needle punctures without leaking. The chemical identities of compounds 3–6 were inferred from LC-MS data and not from independent reference compounds.
Production of [11C]carbon monoxide
No-carrier-added (NCA) [11C]carbon dioxide (~ 300 mCi) was prepared by the 14N(p,α)11C nuclear reaction in a nitrogen-1% oxygen gas target (initial pressure, 150 p.s.i.) bombarded for 10 min with a 10 μA beam of 16 MeV protons from a PETtrace cyclotron (GE Medical Systems, Milwaukee, WI). [11C]Carbon dioxide was converted into [11C]carbon monoxide with ~ 74% efficiency by single pass in helium carrier gas at 10 mL/min through a quartz column filled with 8.25 g molybdenum wire heated at 875 °C. The generated [11C]carbon monoxide was concentrated on a silica gel trap, cooled in liquid nitrogen.
One-pot direct insertion
5-(4-Iodobenzyl)-2-methyl-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole (2; 2.3 μmol) Pd(PPh3)4, (1.25 μmol), DMAP (54 μmol) and hydroxylamine hydrochloride (50 μmol) were in turn dissolved in DMF (80 μL). In some instances, a stock solution of DMAP and hydroxylamine hydrochloride in DMF was prepared first. White solids were filtered off from the final mixture with a SPEC TP C18AR filter and the solution loaded into the autoclave of the radiosynthesis apparatus. [11C]Carbon monoxide was then released into the autoclave by passing helium gas through the silica gel trap during heating of the trap to ~70 °C with a lamp. The autoclave was sealed and heated at 120–150 °C for 3–6 min. Then, the reaction mixture was flushed out with THF (0.7 mL) into a V-vial (5-mL), diluted with water (1.4 mL) and loaded onto a HPLC column (Luna C18, 5 μm, 250 × 10 mm) eluted with a mixture of MeCN (B) and aqueous ammonium formate (25 mM) (A) at 4 mL/min. The mobile phase composition began at 25% B for 5 min, was increased to 65% B over 4 min and then maintained at 65% B until the end of the separation.
In some experiments, Pd(PPh3)4 was replaced with Pd2(dba)3/Xantphos, DMAP was replaced with Et3N, DMF was replaced with THF, or NH2OH·HCl was replaced with O-trityl hydroxamine.
Synthesis of [11C]tubastatin A via [11C]p-nitrophenyl ester ([11C]5)
Tris(dibenzylidene-acetone)dipalladium(0) [Pd2(dba)3, 0.6 μmol], Xantphos (1.2 μmol), 5-(4-iodobenzyl)-2-methyl-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole (2; 2.4 μmol) and p-nitrophenol (5.0 μmol) were mixed with THF (80 μL) and loaded into the autoclave of the radiosynthesis apparatus. [11C]Carbon monoxide was then released into the autoclave by passing helium gas through the silica gel trap during heating to ~70 °C with a lamp. The autoclave was sealed and heated at 130 °C for 3 min. The reaction mixture was then flushed out with THF (0.7 mL) into a crimp-sealed vial (1-mL, fluoropolymer)33 that had been charged with NH2OH·HCl (32 μmol) and phosphazene base P1-t-Bu (38 μmol) in DMSO (33 μL). The mixture was sonicated at RT for 3 min, diluted with water (3 mL), treated with aqueous KOH (1.8 M, 200 μL), and separated by reverse phase HPLC on a Luna C18 column (10 μm, 250 × 10 mm) eluted at 5 mL/min with a mixture of MeCN (B) and 25 mM aqueous ammonium formate (A), with mobile phase composition kept at 25% B for 5 min, increased to 65% B in 6 min and kept at 65% B until the end of separation. The radioactive fraction having the same retention time as 1 was collected (tR = 10–11 min) and analyzed with HPLC, and with LC-MS-MS. BaO, DBU and DMAP replaced phosphazene base in some experiments.
Supplementary Material
Scheme 2.
One-pot direct insertion of [11C]CO between aryl iodide 2 and hydroxylamine gave [carbonyl-11C]tubastatin A ([11C]1) in low RCY.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health (NIMH, ZIA-MH002793). The authors are grateful to the NIH Clinical Center PET Department (Chief, Dr. Peter Herscovitch) for the production of carbon-11 and Dr. Jinsoo Hong for assistance on operation and maintenance of the radiochemistry apparatus.
References
- 1.Gray SG, Ekström TJ. Exp Cell Res. 2001;262:75–78. doi: 10.1006/excr.2000.5080. [DOI] [PubMed] [Google Scholar]
- 2.De Ruijter AJM, Van Gennip AH, Caron HN, Kemp S, Van Kuilenburg ABP. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Trends Neurosci. 2009;32:591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hobara T, Uchida S, Otsuki K, Matsubara T, Funato H, Matsuo K, Suetsugi M, Watanabe Y. J Psychiatric Res. 2010;44:263–270. doi: 10.1016/j.jpsychires.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Hancock WW, Akimova T, Beier UH, Liu Y, Wang L. Ann Rheum Dis. 2012;71(Supp II):i46–i54. doi: 10.1136/annrheumdis-2011-200593. [DOI] [PubMed] [Google Scholar]
- 6.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Proc Natl Acad Sci, USA. 2003;100:4389–4394. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalavalle S, Pisano C, Zunino F. Biochem Pharmacol. 2012;84:756–765. doi: 10.1016/j.bcp.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Eessalu TE, Barth VN, Mitch CH, Wagner FF, Hong Y, Neelamegam R, Schroeder FA, Holson EB, Haggarty SJ, Hooker JM. Am J Nucl Med Mol Imaging. 2014;4:29–38. [PMC free article] [PubMed] [Google Scholar]
- 9.Matusiak N, Van Waarde A, Bischoff R, Oltenfreiter R, Van de Wiele C, Dierckx RAJO, Elsinga PH. Curr Radiopharm Design. 2013;19:4647–4672. doi: 10.2174/1381612811319250011. [DOI] [PubMed] [Google Scholar]
- 10.Di Fulvio S, Azakir BA, Therrien C, Sinnreich M. PLoS ONE. 2012;6:e28563. doi: 10.1371/journal.pone.0028563. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Butler KV, Kalin J, Brochier C, Vistoli G, Langley B, Kozikowski AP. J Am Chem Soc. 2010;132:10842–10846. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christman DR, Finn RD, Karlström K, Wolf AP. Int J Appl Radiat Isot. 1975;26:435–442. [Google Scholar]
- 13.Zeisler SK, Nader M, Theobald A, Oberdorfer F. Appl Radiat Isot. 1997;48:1091–1095. [Google Scholar]
- 14.Lidström P, Kihlberg T, Långström B. J Chem Soc Perkin Trans. 1997;1:2701–2706. [Google Scholar]
- 15.Kihlberg T, Långström B. WO 2002102711 A1 Pct Intl Appl. 2002
- 16.Itsenko O, Kihlberg T, Långström B. Nature Protocols. 2006;1:798–802. doi: 10.1038/nprot.2006.112. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson J, van den Hoeck J, Windhorst AD. J Label Compd Radiopharm. 2012;55:223–228. [Google Scholar]
- 18.Dahl K, Schou M, Amini N, Halldin C. Eur J Org Chem. 2013:1228–1231. [Google Scholar]
- 19.Långström B, Itsenko O, Rahman O. J Label Compd Radiopharm. 2007;50:794–810. [Google Scholar]
- 20.Karimi F, Långström B. J Chem Soc Perkin Trans. 2002;1:2256–2259. [Google Scholar]
- 21.Itsenko O, Kihlberg T, Långström B. Eur J Org Chem. 2005:3830–3834. [Google Scholar]
- 22.Lu S, Hong J, Itoh T, Fujita M, Inoue O, Innis RB, Pike VW. J Label Compd Radiopharm. 2010;53:548–551. doi: 10.1002/jlcr.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karimi F, Långström B. J Chem Soc Perkin Trans. 2002;1:2111–2116. [Google Scholar]
- 24.Kealey S, Plisson C, Collier TL, Long NJ, Husbands SM, Martarello L, Gee AD. Org Biomol Chem. 2011;9:3313–3319. doi: 10.1039/c0ob00631a. [DOI] [PubMed] [Google Scholar]
- 25.Murahashi SI, Imada Y, Taniguchi Y, Kodera Y. Tetrahedron Lett. 1988;29:2973–2976. [Google Scholar]
- 26.Karimi F, Långström B. Org Biomol Chem. 2003;1:541–546. doi: 10.1039/b209553j. [DOI] [PubMed] [Google Scholar]
- 27.Karimi F, Långström B. Eur J Org Chem. 2003:2132–2157. [Google Scholar]
- 28.Nigst TA, Antipova A, Mayr H. J Org Chem. 2012;77:8142–8155. doi: 10.1021/jo301497g. [DOI] [PubMed] [Google Scholar]
- 29.Bauer L, Exner O. Angew Chem Intl Ed. 1974;13:376–384. [Google Scholar]
- 30.Porcheddu A, Giacomelli G. Patai’s Chemistry of Functional Groups. John Wiley and Sons; 2010. pp. 1–69. [Google Scholar]
- 31.Jencks WP. J Am Chem Soc. 1958;80:4585–4588. [Google Scholar]
- 32.Martinelli JR, Clark TP, Watson DA, Munday RH, Buchwald SL. Angew Chem Int Ed. 2007;46:8460–8463. doi: 10.1002/anie.200702943. [DOI] [PubMed] [Google Scholar]
- 33.Cai L, Xu R, Guo X, Pike VW. Eur J Org Chem. 2012:1303–1310. doi: 10.1002/ejoc.201101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwesinger R, Schlemper H. Angew Chem Intl Ed. 1987;26:1167–1169. [Google Scholar]
- 35.Petersen TP, Larsen AF, Ritzén A, Ulven T. J Org Chem. 2013;78:4190–4195. doi: 10.1021/jo400390t. [DOI] [PubMed] [Google Scholar]
- 36.Toffano M, Legros J-Y, Fiaud J-C. Tetrahedron Lett. 1997;38:77–80. [Google Scholar]
- 37.Jencks WP. J Am Chem Soc. 1958;80:4581–4584. [Google Scholar]
- 38.Mazera DJ, Gesser JC, Pliego JR. Arkivoc. 2007;xv:199–214. [Google Scholar]
- 39.Sen VD, Shilov GV, Golubev VA. Russ J Org Chem. 2009;45:1189–1199. [Google Scholar]
- 40.Bjurling P, Reineck R, Westerburg G, Gee AD, Sutcliffe J, Långström B. Proc. VIth Workshop on Targetry and Target Chemistry. TRIUMF. 1995:282–284. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.