Abstract
A new group of “clickable” and brightly emissive metalloporphyrins has been developed for the visualization of oxygenation under ambient light by eye. These alkynyl-terminated compounds permit the rapid and facile synthesis of oxygen-sensing dendrimers through azide-alkyne click chemistry. With absorption maxima overlapping with the wavelengths of common commercial laser sources, they are readily applicable to biomedical imaging of tissue oxygenation. An efficient synthetic methodology, featuring the stable trimethylacetyl (pivaloyl) protecting group, is described for their preparation. A paint-on liquid bandage containing a new, click-synthesized porphyrin dendrimer has been used to map oxygenation across an ex vivo porcine skin burn model. A group of easy-to-use, mass-producible sensors should spur major advances in both clinical use and basic research in the field of oxygen sensing.
Keywords: synthesis (org.), luminescence, dendrimers, sensors, biotechnology
Proper oxygen supply and consumption are essential to cellular function; thus, the measurement of oxygen partial pressures (pO2) is of great importance in the study of disease pathophysiology. Molecular oxygen has a known, quantifiable effect on the phosphorescence lifetime and intensity of certain molecules, described by the Stern-Volmer equation.[1,2] Due to their ability to map oxygen concentrations with high spatial resolution, optical methods based on oxygen-dependent phosphorescence quenching are under active development.[3–6] Out of the many known oxygen-responsive materials,[7–11] metalloporphyrins have been extensively explored as oxygen sensors[10,12–18] due to their room-temperature phosphorescence, structural versatility, and tunable photophysical properties.
Nonetheless, the phosphorescence of most porphyrins is very dim and thus hard to detect under ambient light conditions. In addition, their absorption maxima often lie far from the wavelengths of common laser sources, thereby requiring complex optical setups for both excitation and detection. Lastly, bare porphyrins tend to exhibit poor behavior in biological systems, necessitating modification with biocompatible moieties. As a result, there is an ongoing need for bright, readily functionalizable oxygen-sensing porphyrins that can be excited by commercial laser lines and LED sources.
To address these needs, we report the development of a new group of metallated, meso-unsubstituted porphyrins and benzoporphyrins bearing surface alkyne groups. The terminal alkynes encourage modification through the Huisgen 1,3-dipolar cycloaddition, a fast and highly efficient azide-alkyne click reaction.[19] These new compounds, named “Clickaphors” following the convention of Wilson and colleagues,[20] allow for the rapid, modular synthesis of a vast array of brightly-emitting dendritic oxygen sensors.
The Clickaphors were designed from meso-unsubstituted porphyrins,[21] which are generally brighter than their bulkier, meso-substituted analogues. As a result, the cyclohexenyl metalloporphyrins described in this work exhibit phosphorescence so strong that they are visible by eye, and were evaluated in the mapping of transdermal oxygenation in an ex vivo porcine skin burn model.
The synthesis of oxygen-sensing metalloporphyrins 7 through 12, presented in Scheme 1, was achieved from the common intermediate 6, whose precursors have been synthesized by adapting previously developed chemistry.[22,23] Briefly, the intermediate 6 was prepared by a Lindsey-type condensation of the pivaloyl-protected tetrahydroisoindole 5. Insertion of platinum or palladium yielded the metallated cyclohexenyl porphyrins 7 and 8. Subsequent aromatization led to the Pt- or Pd-benzoporphyrins 9 and 10, respectively. After removal of the pivaloyl groups, a Williamson-type alkylation yielded the final alkynyl-terminated clickable metalloporphyrins 11 and 12, hereafter referred to as “Clickaphor Red” and “Clickaphor Blue,” based on the colors of the respective purified solids. The route to free-base porphyrin 6 has been successfully reproduced by an outside manufacturer on a multi-gram scale. Its high yield of approximately 20% is the product of extensive optimization. The use of the pivaloyl ptotecting group provided stability that allowed the synthesis of substituted tetrahydroisoindole 5 with a yield of 52%, even though isoindole derivatives are known to be unstable,[21] Furthermore, our use of the reported optimal concentration of 10−3 M in the Lindsey-type condensation[24] to form 6 gave a yield of 69%. We found that metal insertion prior to aromatization afforded significantly higher yields and shorter reaction times than inserting metal into the poorly soluble benzoporphyrins, though the two steps are transposable. Attempts to forgo the use of protecting groups resulted in unwanted polymerization products when an alkynyl-terminated free base was subjected to metal insertion or aromatization conditions.
Scheme 1.
Synthesis of alkynyl-terminated porphyrins. Reagents and conditions: (a) K2OsO4, K3Fe(CN)6, K2CO3, CH3SO2NH2, tBuOH/H2O (1:1), r.t., overnight, 78%; (b) (CH3)3CCOCl, 4-Dimethylaminopyridine, pyridine, CH2Cl2, r.t., 18 h, 85%; (c) CNCH2CO2tBu, tBuOK, THF, r.t., 4h, 81%; (d) TFA, r.t. 30min, 52%; (e) p-TsOH(monohydr.), formaldehyde (37% in water), benzene, reflux (Dean-Stark condenser), 8 h, 69%; (f) PtCl2, PhCN, reflux, 6–8 h, 65%; (g) PdCl2, benzonitrile, reflux, 15–20 min, 85%; (h) DDQ, dry 1,4-Dioxane, reflux, 8–10 h, 60%; (i) DIBAL-H, dry CH2Cl2, −78°C-r.t., 18 h; (j) NaH (60%), BrCH2CCH, dry DMF, 18 h, 0°C-r.t., 35% (2-step yield).
Prior efforts to aromatize cyclohexenyl porphyrins with acetyl protecting groups using DDQ proceeded only after elimination of four of the eight acetyl groups.[23] The pivaloyl groups used in our synthesis, on the other hand, tolerated these conditions and permitted formation of the octa-pivaloxy, metallated benzoporphyrins 9 and 10. The presence of eight attachment points enables the synthesis of dendrimer layers denser than typical Oxyphor-type dendrimers. Limited elimination and aromatization byproducts, formed during both the metal insertion and aromatization steps, were easily removed by column chromatography.
Since benzoporphyrins 9 and 10 dissolve appreciably only in dichloromethane, the less common, dichloromethane-soluble diisobutylaluminum hydride (DIBAL-H) was found to be ideal for the deprotection of the pivaloyl esters, offering near-quantitative yields with all four porphyrins.
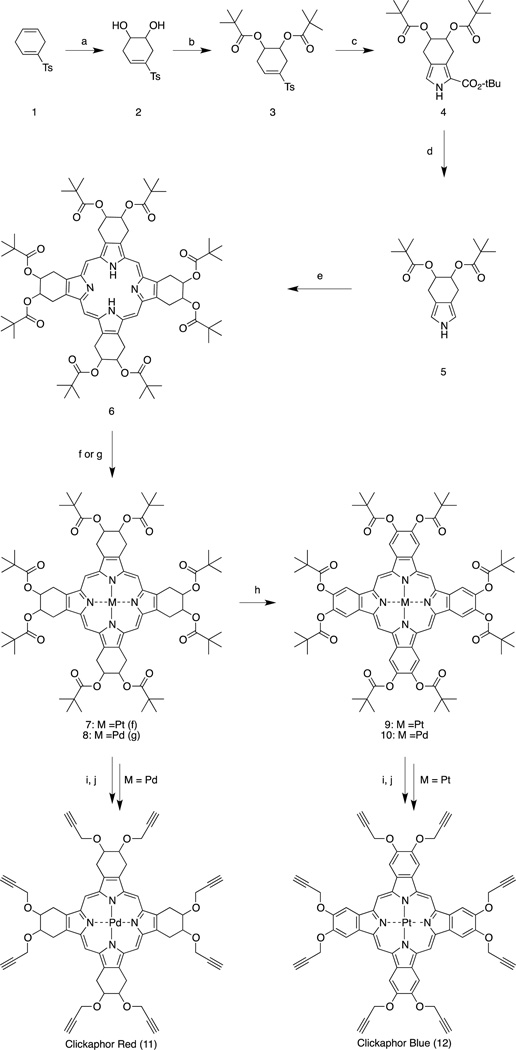
The four pivaloyl-protected metalloporphyrins have strong and distinctive absorption bands throughout the UV and visible regions (Figure 1). They exhibit bright room-temperature phosphorescence with high quantum yields in the absence of oxygen. Most importantly, the quantum yields of the alkynyl derivatives were found identical to those of their parent, pivaloyl-protected porphyrins (Table 1). The lower quantum yields of 9 and 10 may be due to aggregation-induced self-quenching under the measurement conditions.
Figure 1.
Absorption (top) and emission (bottom) spectra of pivaloyl-protected porphyrins 7, 8 (in DMF as solvent) and benzoporphyrins 9, 10 (in dichloromethane). Spectral profiles have been normalized to display similar absorbances at the Soret bands and similar emission intensities.
Table 1.
Photophysical properties of porphyrins 7 through 12.[a]
| Compound | Absorption λmax (nm) (logε) |
Emission λmax (nm) |
τ (µs) |
ϕ | “Brightness” (Soret; Q) |
|---|---|---|---|---|---|
| 7 | 377 (5.40), 531 (4.74) | 645 | 108 | 0.41 | 102,000; 22,000 |
| 8 | 392 (4.98), 543 (4.38) | 661 | 484 | 0.17 | 16,000; 4,000 |
| 9 | 396 (4.98), 592 (4.95) | 744 | 99 | 0.20 | 20,000; 19,000 |
| 10 | 410 (5.18), 605 (4.98) | 774 | 412 | 0.10 | 15,000; 9,500 |
| 11 | 392 (4.97), 544 (4.26) | 660 | 0.15 | 14,000; 3,000 |
|
| 12 | 398 (4.95), 595 (4.88) | 745 | 0.20 | 18,000; 15,000 |
Absorption and emission spectra acquired in N,N-dimethylformamide for 7, 8 and 11, and dichloromethane for 9 and 10. Quantum yields were measured in the corresponding deoxygenated solution, purged with argon gas. For detailed protocols and the instrumentation used for photophysical measurements and lifetime determination, see Supporting Information. The “brightness” metric is defined as the product of the molar absorption coefficient and the quantum yield. As a comparison, the “brightness” metric for the commercially available phosphor Oxyphor G2 is 6,000 at the Q-band.
These phosphors, with their substituents linked to the central porphyrin via an oxygen instead of a carbon atom, were designed such that their absorption peaks exist at or very near the wavelengths of common commercial laser sources, including the Helium-Neon laser lines at 543 and 594 nm, as well as the doubled output of most Nd-based lasers at 532 nm.
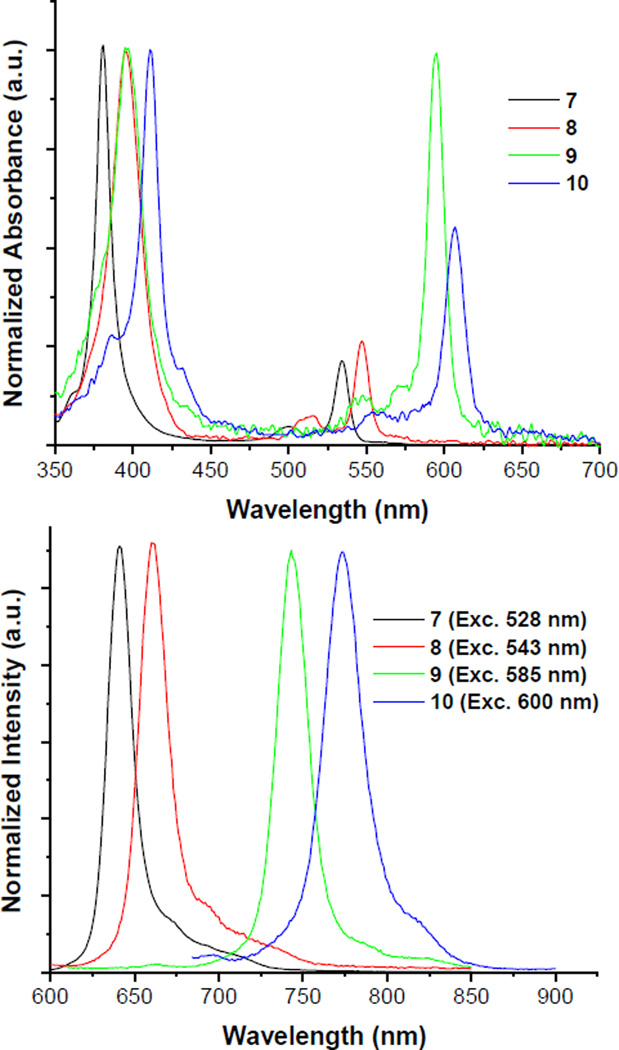
To demonstrate the visible response of the sensor to oxygenation changes, the phosphorescence of a deoxygenated solution of platinum porphyrin 7 was captured under ambient light with a regular smartphone camera (Figure 2b). The phosphor was also embedded in a liquid bandage formulation, along with Coumarin 500 (λexc,max=392nm; λem,max=495nm), a green, oxygen-insensitive fluorophore. The intensity of the phosphor’s red emission, visible under ambient light, increases upon deoxygenation, turning the apparent color of the film from green to red (Figure 2c).
Figure 2.
Sensor response to different oxygen levels. a) A solution of platinum porphyrin 7 in dichloromethane under room air (left) and in the absence of oxygen (right); the porphyrin was excited with a handheld LED flashlight. Image was acquired using a smartphone camera. b) Palladium porphyrin 8 was embedded in a liquid bandage formulation along with a green-emitting, oxygen-insensitive fluorophore, and the film’s emission was recorded with a commodity color camera equipped with filters, under room air (left) and in the absence of oxygen (right).
To show the ease of dendrimer synthesis, a glutamic dendrimer was built by simply clicking eight azido-terminated, second-generation glutamic dendrons onto Clickaphor Red. The resulting dendritic oxygen sensor, Clickaphor Red G2 (see Supporting Information for structure), and Coumarin 500 were embedded in a liquid bandage matrix formulation. Once applied to a wound, the bandage dries into a thin, solid, airtight film that conforms to the skin.
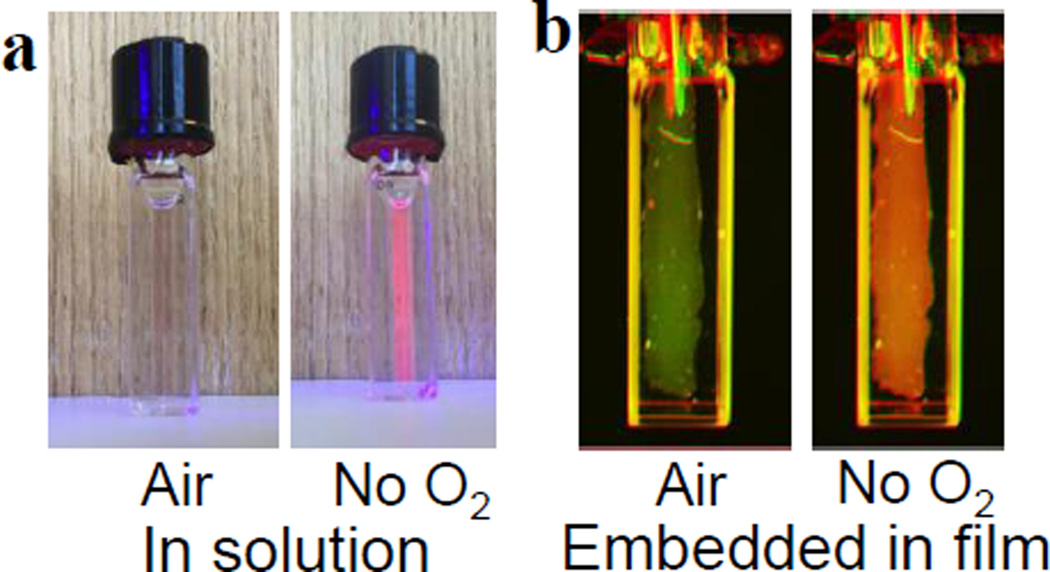
Following the procedure of Li et al.,[18] this bandage was used to visualize and quantify tissue oxygenation of full thickness burns in fresh ex vivo porcine skin. The brightness of Clickaphor Red G2 allowed for the rapid acquisition of tissue oxygen tension images using a custom camera (see SI). Collected in both intensity and lifetime modes, the oxygen consumption maps obtained with the Clickaphor Red G2 bandage (Figure 3) clearly show the extent of burned skin, which displays reduced oxygen consumption due to necrosis. The burn can be readily identified as a clear, dark, non-O2-consuming region within the tissue.
Figure 3.
Oxygen consumption maps, expressed as normalized percent oxygen consumption, obtained with Clickaphor Red G2 in burned tissue on freshly excised porcine skin. Images shown for (a,b) circular burn and control skin imaged using the intensity-based ratiometric approach; (c,d) circular burn and control skin imaged using the lifetime approach; R/G: red-to-green ratio; %Con: Percentage oxygen consumption. The information presented on the images were obtained from Matlab analysis of the raw data acquired with a custom-modified camera.[18]
The “clickability“ and bright emission of this new group of oxygen sensors enable both the modular assembly of biocompatible, oxygen-sensing dendrimers and, more importantly, the straightforward detection of pO2 by eye under ordinary room lighting conditions. The synthetic route reported here, a marked improvement upon earlier porphyrin syntheses, provides eight sites for functionalization. The convenient absorption and emission profiles of these probes eliminate the need for complex optical setups. Lastly, the ability to mass produce both the porphyrin sensors and the bandage formulation should make large-scale oxygen-sensing applications, such as the non-invasive monitoring of wounds and grafts, readily available to a broad pool of clinicians and researchers.
Supplementary Material
Footnotes
This work was funded by the Air Force Office for Scientific Research (FA9550-13-1- 0068) and the National Institutes of Health through the NIH Director’s New Innovator Award Program, Grant No. 1 DP2 OD007096-1. Information on the New Innovator Award Program can be found at http://nihroadmap.nih.gov/newinnovator/. A.J.N. would like to acknowledge the support of an NSF Graduate Research Fellowship and an NDSEG Fellowship. The authors thank Dr. Gabriela Apiou-Sbirlea and Dr. Reginald Birngruber for their contributions to the development of the oxygen-sensing bandage. We acknowledge Ryan Caulfield and John L. Beagle from the Knight Surgical Facility at Massachusetts General Hospital for obtaining the excised porcine skin. Finally, we thank Dr. Lauren A. Austin, Sam Osseiran, Stefano Belfiore, Taylor M. Cannon and Emily J. Keeley for their help in editing this manuscript
Contributor Information
Emmanuel Roussakis, Wellman Center for Photomedicine, Massachuetts General Hospital, CNY 149-3210, 13th Street, Charlestown, MA 02129 (USA).
Zongxi Li, Wellman Center for Photomedicine, Massachuetts General Hospital, CNY 149-3210, 13th Street, Charlestown, MA 02129 (USA).
Nicholas H. Nowell, Wellman Center for Photomedicine, Massachuetts General Hospital, CNY 149-3210, 13th Street, Charlestown, MA 02129 (USA)
Alexander J. Nichols, Wellman Center for Photomedicine, Massachuetts General Hospital, CNY 149-3210, 13th Street, Charlestown, MA 02129 (USA) Harvard University Program in Biophysics, Building C2, Room 112, 240 Longwood Avenue, Boston, MA 02115 (USA); Harvard-MIT Division of Health Sciences and Technology, 77 Massachusetts Avenue E25-519, Cambridge, MA 02139 (USA).
Conor L. Evans, Email: evans.conor@mgh.harvard.edu, Wellman Center for Photomedicine, Massachuetts General Hospital, CNY 149-3210, 13th Street, Charlestown, MA 02129 (USA); Harvard University Program in Biophysics, Building C2, Room 112, 240 Longwood Avenue, Boston, MA 02115 (USA).
References
- 1.Papkovsky DB, Dmitriev RI. Chem. Soc. Rev. 2013;42:8700–8732. doi: 10.1039/c3cs60131e. [DOI] [PubMed] [Google Scholar]
- 2.Roussakis E, Li Z, Nichols AJ, Evans CL. Angew. Chem. Int. Ed. 2015;54:8340–8362. doi: 10.1002/anie.201410646. [DOI] [PubMed] [Google Scholar]
- 3.Vinogradov SA, Wilson DF. In: Designing Dendrimers. Campagna S, Ceroni P, Puntoriero F, editors. Vol. 1. New York: Wiley; 1996. pp. 463–503. [Google Scholar]
- 4.Wang X-d, Wolfbeis OS. Chem. Soc. Rev. 2014;43:3666–3761. doi: 10.1039/c4cs00039k. [DOI] [PubMed] [Google Scholar]
- 5.Rumsey WL, Vanderkooi JM, Wilson DF. Science. 1988;241:1649–1651. doi: 10.1126/science.241.4873.1649. [DOI] [PubMed] [Google Scholar]
- 6.Vanderkooi JM, Maniara G, Green TJ, Wilson DF. J. Biol. Chem. 1987;262:5476–5482. [PubMed] [Google Scholar]
- 7.Esipova TV, Vinogradov SA. J. Org. Chem. 2014;79:8812–8825. doi: 10.1021/jo501521x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemon CM, Karnaas E, Bawendi MG, Nocera DG. Inorg. Chem. 2013;52:10394–10406. doi: 10.1021/ic4011168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaurin EJ, Greytak AB, Bawendi MG, Nocera DG. J. Am. Chem. Soc. 2009;131:12994–13001. doi: 10.1021/ja902712b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roussakis E, Spencer JA, Lin CP, Vinogradov SA. Anal. Chem. 2014;86:5937–5945. doi: 10.1021/ac501028m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshihara T, Yamaguchi Y, Hosaka M, Takeuchi T, Tobita S. Angew. Chem. Int. Ed. 2012;51:4148–4151. doi: 10.1002/anie.201107557. [DOI] [PubMed] [Google Scholar]
- 12.Finikova OS, Artem Y, Lebedev AA, Troxler T, Gao F, Garnacho C, Muro S, Hochstrasser RM, Vinogradov SA. Chem Phys Chem. 2008;9:1673–1679. doi: 10.1002/cphc.200800296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esipova TV, Karagodov A, Miller J, Wilson DF, Busch TM, Vinogradov SA. Anal. Chem. 2011;83:8756–8765. doi: 10.1021/ac2022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols AJ, Roussakis E, Klein OJ, Evans CL. Angew. Chem. Int. Ed. 2014;53:3671–3674. doi: 10.1002/anie.201311303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meier RJ, Schreml S, Wang X-d, Landthaler M, Babilas P, Wolfbeis OS. Angew. Chem. Int. Ed. 2011;50:10893–10896. doi: 10.1002/anie.201104530. [DOI] [PubMed] [Google Scholar]
- 16.Schreml S, Meier RJ, Wolbeis OS, Maisch T, Szeimies RM, Landthaler M, Regensburger J, Santarelli F, Klimant I, Babilas P. Exp. Dermatol. 2011;20:550–554. doi: 10.1111/j.1600-0625.2011.01263.x. [DOI] [PubMed] [Google Scholar]
- 17.Schreml S, Meier RJ, Kirschbaum M, Kong SC, Gehmert S, Felthaus O, Küchler S, Sharpe JR, Wöltje K, Weiß KT, Albert M, Seidl U, Schröder J, Morsczeck C, Prantl L, Duschl C, Pedersen SF, Gosau M, Berneburg M, Wolfbeis OS, Landthaler M, Babilas P. Theranostics. 2014;4:721–735. doi: 10.7150/thno.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Roussakis E, Koolen PGL, Ibrahim AMS, Kim K, Rose LF, Wu J, Nichols AJ, Baek Y, Birngruber R, Apiou-Sbirlea G, Matyal R, Huang T, Chan R, Lin SJ, Evans CL. Biomed. Opt. Express. 2014;5:3748–3764. doi: 10.1364/BOE.5.003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolb HC, Finn MG, Sharpless KB. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Dunphy I, Vinogradov SA, Wilson DF. Anal. Biochem. 2002;310:191–198. doi: 10.1016/s0003-2697(02)00384-6. [DOI] [PubMed] [Google Scholar]
- 21.Finikova OS, Cheprakov AV, Vinogradov SA. J. Org. Chem. 2005;70:9562–9572. doi: 10.1021/jo051580r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filatov MA, Cheprakov AV, Beletskaya IP. Eur. J. Org. Chem. 2007;2007:3468–3475. [Google Scholar]
- 23.Cheprakov AV, Filatov MA. J. Porphyrins Phthalocyanines. 2009;13:291–303. [Google Scholar]
- 24.Lindsey JS, Schreiman IC, Hsu HC, Kearney PC, Marguerettaz AM. J. Org. Chem. 1987;52:827–836. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.