Abstract
Acrolein, an endogenous aldehyde, has been shown to be involved in sensory hypersensitivity after rat SCI, for which the pathogenesis is unclear. Acrolein can directly activate a pro-algesic transient receptor protein ankyrin 1 (TRPA1) channel that exists in sensory neurons. Both acrolein and TRPA1 mRNA are elevated post SCI, which contributes to the activation of TRPA1 by acrolein and consequently, neuropathic pain. In the current study, we further showed that, post-SCI elevation of TRPA1 mRNA exists not only in DRGs but also in both peripheral (paw skin) and central endings of primary afferent nerves (dorsal horn of spinal cord). This is the first indication that pain signaling can be over-amplified in the peripheral skin by elevated expressions of TRPA1 following SCI, in addition over-amplification previously seen in the spinal cord and DRG. Furthermore, we show that acrolein alone, in the absence of physical trauma, could lead to the elevation of TRPA1 mRNA at various locations when injected to the spinal cord. Additionally, post-SCI elevation of TRPA1 mRNA could be mitigated using acrolein scavengers. Both of these attributes support the critical role of acrolein in elevating TRPA1 expression through gene regulation. Taken together, these data indicate that acrolein is likely a critical causal factor in heightening pain sensation post-SCI, through both the direct binding of TRPA1 receptor, and also by boosting the expression of TRPA1. Finally, our data also further supports the notion that acrolein scavenging may be an effective therapeutic approach to alleviate neuropathic pain after SCI.
Keywords: lipid peroxidation, aldehyde, hydralazine, proalgesic, hyperreflexia
Introduction
Spinal cord injury (SCI)–induced neuropathic pain remains refractory to therapeutic treatment despite recent years of intense research (Hulsebosch et al. 2009). This pain, stemming from unknown origins, greatly hinders the quality of life for SCI victims (Hulsebosch et al. 2009). Recent advances in the understanding of secondary injury after SCI have triggered the investigation of the role of unsaturated aldehydes, especially acrolein, in sensory dysfunction after SCI (Due et al. 2014, Park et al. 2014b). Acrolein has been identified as both a product and initiator of lipid peroxidation (LPO), and therefore a key perpetuator of oxidative stress, a hallmark of post-SCI secondary injury (Shi & Luo 2006, Hamann & Shi 2009, Shi et al. 2015, Park et al. 2014a, Luo et al. 2005, Hamann et al. 2008, Uchida et al. 1998, Uchida 1999). More importantly, acrolein has been identified as a specific agonist of a transient receptor potential ankyrin 1 (TRPA1) cation channel in nociceptors (Bandell et al. 2004, Bautista et al. 2006). TRPA1 is a sub member of the TRP family and is expressed in sensory neurons such as unmyelinated primary sensory neurons located in the dorsal root ganglia (DRG) and trigeminal ganglia (Bautista et al. 2013, Nilius et al. 2012, Patapoutian & Macpherson 2006, Patapoutian et al. 2009). TRPA1 is a polymodal receptor, responding to mechanical and thermal stimuli in addition to chemical stimuli including acrolein.
In a previous study (Due et al. 2014), we have provided evidence of the involvement of acrolein in sensory hypersensitivity following spinal cord injury in rats. We demonstrated that in addition to the elevation of endogenous acrolein, TRPA1 gene expression levels at the DRG were also increased following SCI. Both these factors appeared to work synergistically to contribute to sensory hypersensitivity. However, it is still not known whether acrolein also contributes to the up regulation of TRPA1. Therefore, this study was designed to investigate the possibility that, in addition the direct binding and activation of TRPA1, acrolein can also stimulate the up regulation of TRPA1 receptor to further intensify acrolein-instigated neuropathic pain.
It is known that in addition to the cell body of sensory neurons (within DRG), TRPA1 is also expressed in the primary sensory cells in its central (dorsal horn of spinal cord) and peripheral terminals, such as those at skin (Anand et al. 2008, Atoyan et al. 2009). Therefore it is possible that TRPA1 in these locations may also be up-regulated post-SCI, and such up regulation of TRPA1 may even be influenced by acrolein, which could play a role in the generation and maintenance of exaggerated pain signaling following SCI, a hypothesis we also intend to test here.
In this study, we have found that TRPA1 mRNA levels were indeed significantly elevated in both peripheral and central endings of primary afferent nerves after SCI, which was associated with mechanical and thermal hypersensitivity. These data suggest that in addition to spinal cord, peripheral skin is also a location where pain hypersensitivity resulted from spinal cord trauma. Interestingly, such post-SCI up-regulation of TRPA1 can be mitigated by daily IP injection of hydralazine, an acrolein scavenger, suggesting that acrolein is a stimulator of TRPA1 expression, and therefore an algesic causal factor. Such notion is further supported by current finding that the post-SCI elevation of TRPA1 mRNA, along with mechanical and thermal pain, can be mimicked by the direct injection of acrolein to the thoracic spinal cord. This indicates that acrolein plays a critical role in heightened pain sensation post-SCI, not only through the direct binding of TRPA1 receptor, but also by encouraging the up-regulation of TRPA1, in cell bodies as well as central and peripheral terminals.
Experimental procedures
Experimental Animal
sMale Sprague-Dawley rats (200-220 g) were used at the time of surgery. Rats were obtained from Harlan Laboratory (Indianapolis, IN, USA). All animals were handled and housed in accordance with Purdue Animal Care and Use Committee approved protocols (PACUC), and in compliance with the ARRIVE guidelines. For acclimation, the rats were kept at least one week before surgery.
Spinal cord contusion injury rat model
All surgical procedures were carried out aseptically. An adequate level of anesthesia was induced by a ketamine (80mg/kg) and xylazine (10mg/kg) mixture via intraperitoneal (IP) injection. Complete anesthesia was considered when there was no withdrawal response to a foot pinch. Contused spinal cord injury (SCI) models were induced by using a New York University (NYU)-style impactor with a 10 gram rod (Gruner 1992). Briefly, a dorsal laminectomy was performed to expose the dorsal surface of T-10 level of the spinal cord. After stabilizing the animal’s vertebrae, the impactor weight rod was centered directly above the spinal cord T-10 level, then was dropped from a height of 25 mm on the intact dura matter. A sham operation was carried out using only a laminectomy at the T-10 vertebra without a spinal cord injury. After surgery, animals recovered on a heating pad. Manual bladder expression was performed two times daily until the return of reflexive control of bladder function was observed. Saline (3 mL) was administrated through subcutaneous injections for one week post SCI to prevent from dehydration.
Hydralazine treatment
Hydralazine hydrochloride (Sigma, St. Louis, Mo, USA) was dissolved in phosphate buffered saline then sterilized via a filter. A final dose of 5 mg/kg of hydralazine solution was administrated through IP injection. To investigate hydralazine’s effect of suppressing acrolein, hydralazine injections were administered twice: once within three minutes following SCI, and then again 24-h post-SCI. Hydralazine was administrated once daily for two weeks after SCI for experiments involving behavioral assessments. Hydralazine treatment occurred once daily for one week after SCI for experiments using RT-PCR for gene expression analysis.
Isolation of spinal cord and paw skin
The animals were properly anesthetized with a ketamine (80mg/kg) and xylazine (10mg/kg) mixture through an IP injection. Then they were perfused with cold oxygenated Kreb’s solution (124 mmol/L NaCl, 2 mmol/L KCl, 1.24 mmol/L KH2PO4, 26 mmol/L NaHCO3, and 10 mmol/L ascorbic acid, 1.3 mmol/L MgSO4, 1.2 mmol/L CaCl2, and 10 mmol/L glucose). The whole vertebral column was rapidly removed then a dorsal laminectomy was carried out along the vertebral column to remove the spinal cord. A 1 cm segment, including the injury site, was cut from the spinal cord and used for immunoblotting and western blotting experiments.
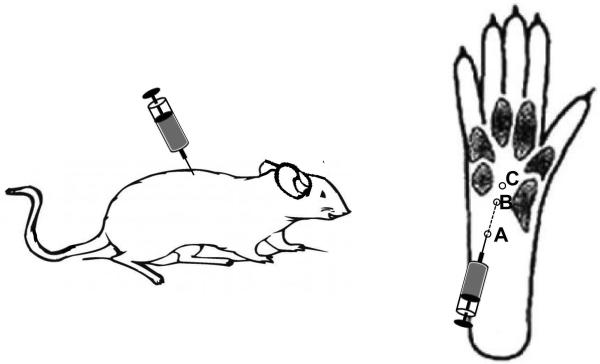
The extraction of paw skin is similar to the procedures described in a previous study (Moran et al. 2011). TRPA1 channels, which are expressed on keratinocytes, transmit pain sensations to the higher order neurons (Moran et al. 2011). In this study, we collected basal layer of the skin epithelium of the hind limb paw (0.5 cm × 0.5 cm) including the von frey filament application site (figure 1 right C).
Figure 1.
Sites for Acrolein Injection and Mechanical Allodynia Von Frey Test Left. Injection of acrolein directly to T10 of rat spinal cord. 40 nmol acrolein was injected into the right side of the cord while saline was injected into the left side as a control. Right. For the acrolein injection into the paw, a 30 gauge needle was inserted at the site of A (heel of the rat’s hindlimb foot), and advanced to site B, where an acrolein solution (25 μl, 625nmol) was injected intradermally. Acrolein-induced mechanical hypersensitivity was assessed by applying Von Frey filaments at site C.
Artificial acrolein standard preparation
An artificial acrolein standard was prepared as a control for immunoblotting. Initially, rat albumin (0.5 ml of 2mg/ml, CalBioChem) solution and acrolein (0.5ml of 100 mM, Sigma) solution were mixed together, which was incubated for four hours at 37°C. Using 0.1% BSA, serial dilutions up to 10000 fold were performed with the acrolein concentration ranging from 5 μM (or 100ng/ml) to 25 nM (or 0.5ng/ml). The standard samples were loaded and measured, and analyzed by using Image J (NIH). Based on the band densities, a calibration acrolein standard curve was generated, allowing the measurement of acrolein levels in the samples of interest.
Immunoblotting
Spinal cord segments, which included the damage site, were incubated with a 1% Triton solution with the corresponding amount of Protease Inhibitor Cocktails (Sigma Aldrich), then homogenized with a sonicator for 10 sec. The homogenized tissue suspension was incubated in ice for one hour then, centrifuged at 4 °C at 13500g for 30 mins. Using a BCA protein assay kit, the total protein concentration from each sample was measured for equal sample loading. A portion (200 μg) of each sample was transferred to the nitrocellulose membrane by using a Bio-Dot SF Microfiltration Apparatus (Bio-Rad, Hercules, CA, USA). Next, this membrane was blocked in 0.2% casein and 0.1% Tween 20 in a PBS blocking buffer for one hour and incubated with a monoclonal mouse anti-acrolein antibody (ABCAM) for at least 18 hours at 4 °C. The anti-acrolein antibody was diluted at a ratio of 1:1000 in blocking buffer with 2% goat serum and 0.025% sodium azide. The membrane was washed four times in the blocking buffer for 4 minutes and then the membrane was transferred to 1:10000 alkaline phosphatase conjugated goat anti-mouse IgG solution for another hour (VECTASTAIN ABC-AmP Kit). For the final washes, the blocking buffer and 0.1% Tween 20 in Tris-buffered saline were used, and then the membrane was exposed to the substrate of an ABC-AMP kit and visualized by chemiluminescence. The band density was assessed by Image J (NIH), and an arbitrary unit for the expression was used.
Urine collection in animals
To collect the urine of the animals, standard metabolic cages were used. Saline (0.3 mL) was administrated through peritoneum to induce urination. During the urine collection time, regular food and water were supplied. Water sources were carefully separated to prevent urine dilution by water. Occasionally, manual bladder expression was performed to collect urine.
Creatinine assay
Creatinine measurements were carried out using with a creatinine (urinary) assay kit (Cayman Chemical Company, MI, USA). Briefly, creatinine standards, 12x, and 24x diluted urine samples were incubated with an alkaline picrate solution for approximately 20 minutes in 96 well plates. For the initial reading, the absorbance at 490-500nm was detected with standard spectrophotometry. Next, 5 uL of an acid solution was added to each of the samples and the plate was incubated on a shaker for 20 minutes. For the final measurement, the absorbance at 490-500nm was again recorded with a standard spectrophotometry and the difference between the initial and final reading were used for quantitative analysis. The creatinine standard curve was prepared according to the assay manual.
3-hydroxypropyl mercapturic acid (3-HPMA) quantification in urine
3-HPMA was measured based on the previous study (Eckert et al. 2010). Briefly, ENV+ cartridges (Biotage, Charlotte, NC) were used to prepare solid phase extraction before LC/MS/MS analysis. Each cartridge was conditioned with 1 mL of methanol, followed by 1 mL of water, and finally 1 mL of 0.1% formic acid in water. A volume of 500 uL of urine was spiked with 200 ng of deuterated 3-HPMA (d3-3-HPMA) (Toronto Research Chemicals Inc., New York, Ontario) and mixed with 500 uL of 50 mM ammonium formate and 10 uL of undiluted formic acid. Each cartridge was washed twice with 1 mL of 0.1% formic acid, then followed by 1 mL of 10% methanol/90% of 0.1% formic acid in water. The cartridges were dried with nitrogen gas for 30min and eluted with 600uL methanol plus 2% formic acid three times. The eluates were dried with an evaporation centrifuge before being reconstituted in 100uL of 0.1% formic acid before LC/MS/MS analysis. An Agilent 1200 Rapid Resolution liquid chromatography (LC) system coupled to an Agilent 6460 series QQQ mass spectrometer (MS) was used to analyze 3-HPMA in each sample (Zheng et al. 2013).
Neuropathic pain behavior assessment
Mechanical hypersensitivity
Behavioral testing for mechanical allodynia was performed one day before SCI surgery to establish base line withdrawal threshold for the animals. Mechanical allodynia was assessed by the hind paw withdrawal threshold in response to a series of calibrated von Frey filaments (range: 0.4, 0.6, 1.0, 2.0, 4.0, 6.0, 8.0, and 15.0 grams, Stoelting, Wood Dale, IL, USA). Briefly, the animals were placed on a metal mesh and covered by a transparent plastic box. They were left alone at least 10 min for acclimation before testing. Testing was initiated with the filament that bending force was 2.0 grams. The filament was applied to the plantar surface of each paw perpendicularly with a sufficient bending force for three to five seconds. Mechanical stimuli were applied at a frequency of one per minute. The 50% withdrawal threshold was determined using the up-down method (Chaplan et al. 1994). During the paw injection of acrolein experiments, mechanical hypersensitivity behavior was measured by the frequency of paw withdrawal to a fixed value of filament (15 grams). The filament was applied 10 times to the plantar paw one hour after acrolein injection at a frequency of one per minute.
Cold hypersensitivity
Cold hypersensitivity was tested using the 100% acetone-generated evaporative cooling effect. Similar to the mechanical hypersensitivity testing, 100% acetone (0.05mL) was applied from a distance of two millimeters from the plantar surface of the hind paw. The acetone was applied five times to each paw at intervals of three minutes. Brisk paw withdrawals with or without licking and biting were considered positive reactions. To minimize the stress to the animals, the order of the behavior tests was the von Frey filament test first, followed by the 100% acetone application assay. The order of pain behavior tests was kept the same throughout the experimental period for all animals. In between different behavioral tests, the animals were allowed to rest for at least 20 minutes.
Locomotor function assessment (BBB)
Hind limb locomotor function was assessed using the Basso, Beattie and Bresnahan (BBB) open field rating scale (Basso et al. 1996). The hind limb motor function abilities were determined one day after SCI and every other day until two weeks. The animals were observed in an open field for five minutes and each hind limb was scored form 0 (no movement) to 21 (normal gait). The score was obtained by taking an average value of both hindlimbs.
Gene expression analysis using Real-time PCR
The DRG cells (L1-L6), spinal dorsal horn, and paw skin of sham and SCI rats were homogenized using Trizol reagent (Sigma-Aldrich, St. Louis, MO, USA). RNA isolation was followed by chloroform extraction and isopropanol precipitation. RNA concentration was determined using NanoDrop 2000c (Thermo Scientific, DE, USA). The extracted RNA was dissolved in 50uL of RNase-free distilled water and stored at −80°C deep freezer until in use. cDNA was synthesized by the iScript™ cDNA Synthesis kit manual guide (BIO-RAD,170-8890,USA). Primers were designed for RT-PCR based on a previous study (Due et al. 2014). To recognize the TRPA1 channel, the primers 5′-TCCTATACTGGAAGCAGCGA-3′, and 5′-CTCCTGATTGCCATCGACT-3′; 18S were used as an internal control against 5′-CGGCTACCACATCCAAGGAA-3′ and 5′-GCTGGAATTACCGCGGCT-3′. The accumulation of PCR products was measured by the level of iQ™SYBR Green Supermix (BIO-RAD, 170-8880, USA) fluorescence following the manufacturer’s manual. The gene expression level was normalized by the level of 18S expression. Relative quantification was calculated as X = 2 – ΔΔCt, where ΔΔCt = ΔE−ΔCt and ΔE = Ct exp – Ct18s, ΔCt = Ct control – Ct 18 s (Livak & Schmittgen 2001). Data were then normalized to the average of the control group.
Acrolein micro injection into the spinal cord
After adequate anesthesia, as described earlier, a laminectomy was performed at the T-10 level to expose the intact spinal cord. Micro-pipettes were pulled and beveled for the insertion into the spinal cord. A micro-pipette was loaded with saline for a control and acrolein in saline for an experimental group. The solution was injected using a three-way valve coupled with a syringe under negative pressure. For the injection, a PMI-100 pressure micro-injector (Dagan Corp., Minneapolis, MN) was utilized to inject 1.6 μl of saline into the left side of the spinal cord as a control and the same volume of acrolein (40nmol, 1.6 μl) was injected into the right side (Figure 1). Specifically, each solution was injected 0.6 mm lateral to the spinal cord mid-line and 1.2 mm ventral to the spinal cord surface at the T-10 level. For post-surgical care, rats were placed on a heating pad and anti-biotic ointment was applied to the incision site.
Acrolein injection into plantar paw
A volume of 25 of acrolein (625 nmol) was injected into the plantar region of the right hind foot in sham control and SCI groups (Figure 1). For the acrolein injection, a 30 gauge needle attached to a Hamilton Syringe was used. A successful injection was considered if there was about a two millimeter in diameter bleb formation at the injection site. The needle was also left inserted for one minute after the injection to prevent from leakage of acrolein solution after removal of the needle. Mechanical hypersensitivity tests began one hour after acrolein injection.
Statistical analysis
All data were presented as mean ± standard error of the mean (SEM). One-way ANOVA and Tukey post hoc test and student’s t test were used for the statistical analysis. The statistical significance level was set at p < 0.05.
Results
Spinal cord injury induces neuropathic pain mechanical allodynia and motor deficits
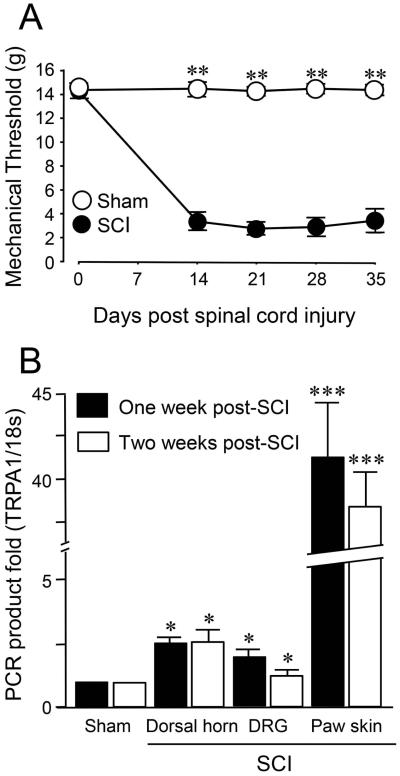
In a previous investigation, we demonstrated that spinal cord injury leads to neuropathic pain behaviors that correlate to the severity of injury in an animal model of SCI (Due et al. 2014, Park et al. 2014b). Here, it is again confirmed that NYU impactor-induced contusion injury at the T-10 level resulted in neuropathic pain mechanical allodynia (Figure 2A). Specifically, two to five weeks after SCI, the mechanical withdrawal threshold to Von Frey filaments after SCI was significantly lower than that of sham-injured controls (P<0.01). In addition, SCI rats also displayed motor deficits based on BBB locomotor rating scale. The BBB scores of pre-surgery, immediately post-surgery, 7, 14, 21, and 28 days post-surgery were, 21 ± 0, 0, 5.8 ± 1.4, 9.3 ± 1.1, 11.9 ± 1.3, and 12.6 ± 1.1 respectively. The severity of motor deficits is comparable to that observed previously using the same model (Park et al. 2014b).
Figure 2.
Sensory and behavioral hypersensitivity following spinal cord injury.
A) Mechanical sensory hypersensitivity after moderate contusive spinal cord injury. Mechanical sensory status was determined using Von Frey filament testing performed before the injury, and weekly post injury starting two weeks post SCI for up to 5 weeks. Note that there were no difference in mechanical threshold in sham injury and SCI groups before the injury. However, starting from 14 days post injury, the average values of the mechanical threshold in SCI group were significantly reduced compared to sham group in the 2-5 weeks post SCI. N=10~13 in each group. **P < 0.01. One-way ANOVA and Tukey test.
B) Elevation of TRPA1 mRNA level 1 week and 2 weeks after spinal cord injury. Bar graph indicates the RT-PCR relative quantification of TRPA1 mRNA examined 1 and 2 weeks post SCI at three tissue types: Dorsal horn (1 cm long including T10), DRG (L1-L6), and paw skin (1 cm × 1cm). Gene expression was normalized by the expression of 18 s and compared to the cycle threshold value for 18 s of tissue mRNA post SCI. There was no difference of the RT-PCR mRNA level of TRPA1 between the two time points (1 week vs 2 weeks) in each tissue type. However, significant differences were detected regarding the RT-PCR mRNA level of TRPA1 between SCI and sham groups in all three tissue types at both 1 week and 2 weeks. Note the near forty fold increase of TRPA1 mRNA in paw skin presented 1 and 2 weeks post SCI. * P < 0.05, *** P < 0.005 when compared to sham. One-way ANOVA and Tukey test. All values in A and B were expressed as mean ± SEM.
Changes in TRPA1 gene expression level after SCI
It has been previously shown that TRPA mRNA gene expression levels increased in DRG from the lumbar area one week after SCI (Due et al. 2014). In this study, TRPA1 gene expression level was assessed within various tissues such as DRG, dorsal horn of the spinal cord and hind limb paw skin at different time points after injury. Utilizing RT-PCR, DRG cells from L1-L6, dorsal horn of spinal cord including the T-10 injury site, and hind-limb paw skin were collected from the sham-injured controls and SCI groups. As Figure 2B indicates, the TRPA1 gene expression level was significantly increased in all tissues one and two weeks after SCI compared to sham-injured controls in the spinal dorsal horn, DRG, and paw skin. However, there was no change in the TRPA1 mRNA expression between one week and two weeks after SCI in any of the tissues investigated. Specifically, the TRPA1 gene expression level in the spinal dorsal horn was 2.58 ± 0.16 for one week and 2.57 ± 0.43 two weeks after SCI (both significant when compared to sham, *p<0.05). A similar elevation of TRPA1 mRNA at one and two weeks post-SCI were also observed in DRG (2.03 ± 0.22 and 1.87±0.21, *p<0.05 compared to sham), and in paw skin (41.45 ± 2.99 and 38.48±2.02, ***p<0.005 compared to sham). The dramatic, forty fold increase in TRPA1 gene expression in the paw skin should be especially noted here. This data indicates that TRPA1 gene expression level increase at three locations: dorsal horn of spinal cord, DRGs, paw skins, after SCI and its elevation persisted at least two weeks after SCI.
Elevation of acrolein-lysine adduct and acrolein metabolite 3-HPMA level after traumatic SCI
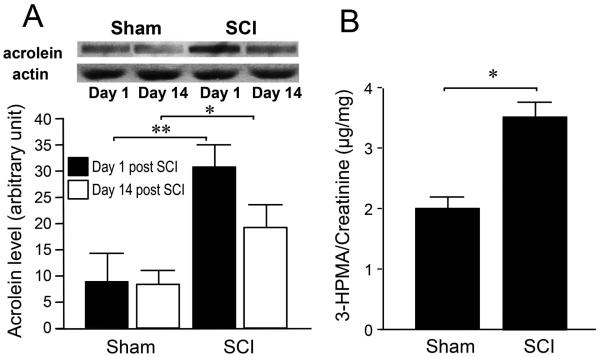
Acrolein levels after SCI were measured in tissue by immunoblotting (Figure 3A) and in urine utilizing LC-MS-MS to detect the acrolein metabolite, 3-HPMA (Figure 3B). The immunoblotting data have shown that acrolein-lysine adduct levels were significantly increased after SCI compared to the sham-injured controls one day and 14 days after surgery. Specifically, one day after SCI, the level of acrolein-lysine adducts in the sham and SCI groups were 9.15.31, and 31.14.02, respectively (** P < 0.01). At 14 days post-SCI, persistent elevation of the acrolein adduct levels was observed. The arbitrary unit of acrolein adducts in sham and SCI were 8.482.71, and 19.434.32, repectively (* P < 0.05). To detect the endogenous acrolein metabolite 3-HPMA, urine samples were analyzed after SCI. As indicated in Figure 3B, 3-HPMA levels in the SCI group (3.5 ± 0.24 μg/mg) were significantly elevated compared to the sham group (1.9±0.21 μg/mg, *P < 0.05).
Figure 3.
Elevation of acrolein-lysine adducts in spinal cord tissue and 3-HPMA in urine after spinal cord injury. A) Bar graph depicts the elevation of acrolein-lysine adduct levels in spinal cord tissue after contusive spinal cord injury (SCI) at 1 day and 2 weeks post injury. Measurements through dot immunoblotting indicated that SCI was associated with significant elevations of acrolein-lysine adducts 1 day following SCI compared to the sham injury group. Persistent elevations of acrolein-lysine adducts were observed for at least 2 weeks after SCI. Top: Representative blots for each condition. Bottom: Bar graph depicts the quantification of the bands density by Image J. N=6~8 for each group. * P < 0.05, ** P < 0.01. One-way ANOVA and Tukey test. B) Elevated level of 3-HPMA, an acrolein metabolite, in urine after SCI in rats (3.5 ± 0.24 μg/mg), when compared to sham injured group (1.9±0.21 μg/mg). Rat urine samples were collected one day after SCI to determine 3-HPMA level. N=4~6 in each group. * P < 0.05. unpaired t-test. All values were expressed as mean ± SEM.
The acrolein scavenger, hydralazine, can suppress TRPA1 gene expression levels in various tissues one and two weeks following SCI
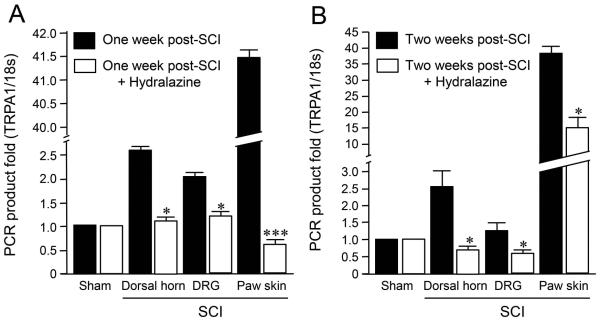
Hydralazine (5mg/kg) was injected once daily for one and two weeks after SCI to investigate the effect of the acrolein scavenger on TRPA1 channel gene expression in various tissues after SCI. Figure 4A depicts the gene expression level one week after SCI in dorsal horn, DRG, and paw skin with and without hydralazine injections. The data indicates that systemic application of hydralazine for one week after SCI could attenuate the elevated TRPA1 gene expression levels in all three tissues investigated. Specifically, the magnitude of the gene expression level increase was reduced from 2.6 ± 0.15 to 1.0 ± 0.13 fold in dorsal horn of spinal cord, from 2.0 ± 0.22 to 1.2 ± 0.20 fold in DRG, and from 41.4 ± 2.98 to 0.60 ± 0.11 fold in hind limb paw skin. In addition, the data in Figure 4B indicates that hydralazine also significantly reduced TRPA1 gene expression levels two weeks after SCI. Specifically, with the daily application of hydralazine, TRPA1 gene expression two weeks after SCI was significantly reduced from 2.57 ± 0.45 to 0.71 ± 0.10 in dorsal horn (*P < 0.05), from 1.27 ± 0.22 to 0.59 ± 0.01 in DRG (*P < 0.05), and paw skin from 38.48 ± 2.0 to 15.25 ± 3.12 (*P < 0.05).
Figure 4.
Suppression of TRPA1 mRNA levels by the acrolein scavenger, hydralazine, in various tissues 1 and 2 weeks after injury. A) Suppression of TRPA1 mRNA levels by hydralazine 1 week after injury. Spinal dorsal horn (1 cm long including T10), dorsal root ganglia (DRG, L1-L6), and paw skin were assessed one week after SCI. Specifically, the TRPA1 mRNA levels were significantly increased in dorsal horn, DRG, and paw skin following SCI (P < 0.05 in dorsal horn and DRG, and P < 0.005 in paw skin group when compared to sham). However, this elevated TRPA1 mRNA was significantly attenuated in all three tissue types with the continuous daily IP injection of hydralazine for a week post SCI. (* P<0.05, *** P<0.005 when compared to SCI alone, One-way ANOVA and Tukey test). N=4~8 in each group. All values were expressed as mean ±SEM. B) Suppression of TRPA1 mRNA levels by hydralazine 2 weeks after injury. Specifically, the TRPA1 mRNA levels were significantly increased 2 weeks post SCI in dorsal horn, DRG, and paw skin following SCI (P < 0.05 in dorsal horn, DRG, and P < 0.005 in paw skin group when compared to sham). However, this elevated TRPA1 mRNA was significantly attenuated in all three tissue types with the continuous daily IP injection of hydralazine for 2 weeks post SCI. (* P<0.05, One-way ANOVA and Tukey test). N=4~8 in each group. All values were expressed as mean ±SEM.
Micro-injected acrolein into the spinal cord increases TRPA1 gene expression level
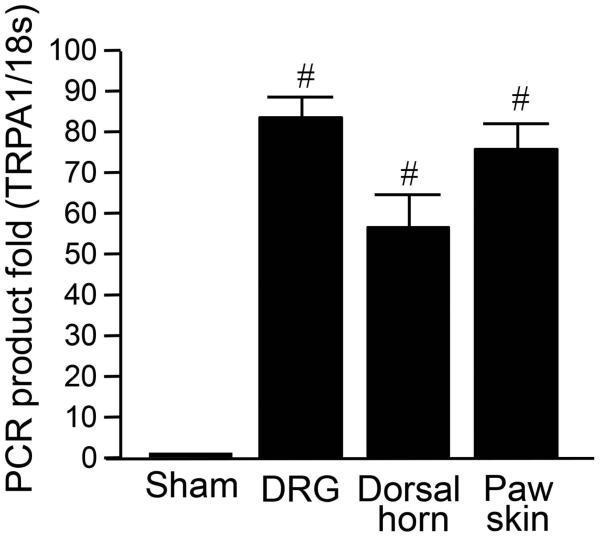
In the absence of traumatic injury, acrolein promoted the elevation of TRPA1 gene expression in the dorsal horn of spinal cord, DRG, and paw skin via a micro-injection into the hind paw of rats (Figure 5). The gene expression level was assessed using RT-PCR and tissues samples were collected one week after acrolein injection. Figure 5 shows the result of relative TRPA1 mRNA gene expression levels in DRG, spinal cord dorsal horn, and paw skin. As the data indicates, TRPA1 gene expression levels were significantly increased in all tissues one week after acrolein micro-injection. Specifically, the magnitudes of increase in gene expression level were 83.4 ± 5.9 fold (DRG), 56.9 ± 8.4 fold (spinal dorsal horn) and 76.3 ± 6.4 fold (paw skin). This data demonstrates that acrolein, in the absence of mechanical trauma, is sufficient to cause the elevation of TRPA1 gene expression level.
Figure 5.
Elevation of TRPA1 mRNA expression levels in DRG, spinal dorsal horn, and paw skin after directly injecting acrolein into spinal cord. Post-injection TRPA1 mRNA levels were increased in all groups compared to sham injury group. N=4 in each group. (# P < 0.001, One-way ANOVA and Tukey test). All values were expressed as mean ± SEM.
Micro-injected acrolein into the spinal cord produces neuropathic pain behaviors
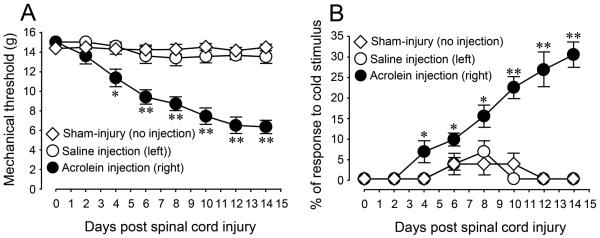
To further investigate the influence of acrolein as a pro-nociceptive factor, the effect of micro-injections to the spinal cord on neuropathic pain behaviors was quantified. Mechanical (Figure 6A) and cold allodynia (Figure 6B) neuropathic pain tests were performed after acrolein micro-injection every two days until 14 days post injection. The data indicates that acrolein injection into the spinal cord produced significant mechanical and cold hypersensitivity on the injection side (P<0.05 or P<0.01), whereas saline injections to the contralateral side did not result in hypersensitivity to mechanical and cold stimuli (P>0.05). Furthermore, micro-injected acrolein-induced neuropathic pain behaviors were maintained for at least two weeks. This data further supports the notion that acrolein can generate mechanical and cold allodynia without any mechanical injury to the spinal cord.
Figure 6.
Mechanical and cold sensory hypersensitivity after microinjection of acrolein directly into the spinal cord (T10). Mechanical and cold sensitivity changes associated with saline (left side) and acrolein (right side) injections into thoracic spinal cord were assayed over time using mechanical (a) and cold hyperreflexia (b) up to 14 days. Acrolein (40 nmol, 1.6 μL) was injected into the right side of dorsal aspect of spinal cord at T10 level and an equal volume of saline was injected into the left side of the cord. (* p < 0.05; ** P < 0.01, when compared to control group, One-way ANOVA and Tukey’s test, n = 4 in all groups). All data were expressed as mean SEM.
Acrolein promotes motor function deficit without traumatic SCI
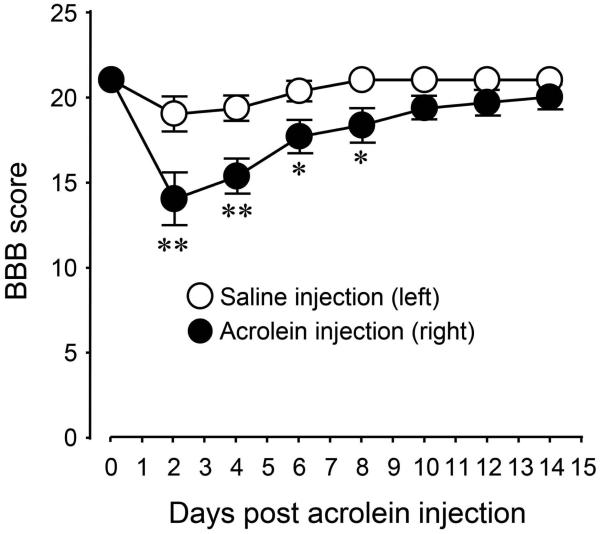
In addition to the ability of acrolein to cause sensory abnormalities, its role in promoting locomotor dysfunction without any mechanical injury was also studied. The animals’ hind limb locomotor ability was evaluated every two days for 14 days post acrolein micro-injection. As shown in Figure 7, micro-injections of acrolein produced significant locomotor malfunction on the side of acrolein injection, while there was little change in BBB score saline injection. This data suggests that acrolein alone could induce the motor deficits with no physical insult.
Figure 7.
Changes of Basso, Beattie and Bresnahan (BBB) scores for acrolein and saline injections over the course of 2 weeks. Notice the significant reduction of the BBB score on the side of acrolein injection compared to the contralateral or saline injected side. **p < 0.01, *P < 0.05 for comparison between right side (acrolein injected) and left side (saline injected). One-way ANOVA then Tukey test. n = 6 in each group. The values were expressed as mean ± SEM.
Acrolein increases the peripheral terminal sensitivity following SCI
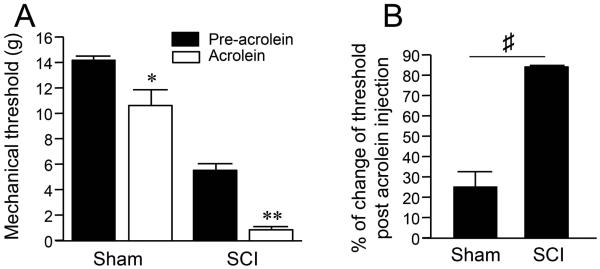
Finally, the ability of acrolein to increase peripheral terminal sensitivity after SCI was evaluated. Initially, for this study, a volume of 25 μl of acrolein (625 nmol) was injected into the intradermal plantar region of the right hind foot in sham-injured control and SCI groups (Figure 1). As shown in Figure 8A, intradermally injected acrolein resulted in the reduction of mechanical pain thresholds in the sham-injured control group (from 14.26 ± 0.28 g to 10.65 ± 1.22 g, *P < 0.05). However, similar acrolein injections produced a more prominent reduction of mechanical pain threshold in spinal cord injured rats (5.42 ± 0.41 g to 0.82 ± 0.24 g, **P < 0.01). Specifically, the percentage of reduction in mechanical threshold after acrolein injection was 25.53 ± 10.21% in the sham control group and 82.44±1.21% in the SCI group (Figure 8B, P<0.0001). This data demonstrates that acrolein-mediated pain sensation was significantly greater in SCI rats than those with no injury (P < 0.0001).
Figure 8.
Neuropathic pain-like behavior after peripheral acrolein injection and its potentiation after SCI. Mechanical sensitivity was assessed using Von Frey filaments before and after the injection of acrolein the intraplantar paw. (A) Bar graph shows that mechanical threshold in both sham and SCI groups can be reduced significantly following acrolein injection (* P < 0.05, ** P < 0.01, when compared to pre-acrolein, One-way ANOVA then Tukey test). (B) The percent of change of mechanical threshold in SCI following acrolein injection was significantly greater compared that in sham group (♯ P < 0.0001) N = 5-8 in all groups. Unpaired t-test. The values were expressed as mean ± SEM.
Discussion
In this study, our data strongly supports the hypothesis that acrolein plays a key role in post-SCI establishment and maintenance of sensory hypersensitivity, in part by stimulating the up-regulation of TRPA1 channel. Coupled with our previous findings (Due et al., 2014; Park et al., 2014), it appears that acrolein contributes to neuropathic pain through at least two mechanisms, by directly binding and activating TRPA1, and by stimulating the up-regulation of TRPA1 gene expression, which heightens the sensitivity of sensory neurons to acrolein.
One significant finding of this study is that we observed a marked elevation of TRPA1 in peripheral terminal in addition to the central terminals post-SCI. Specifically, RT-PCR analysis of TRPA1 gene expression revealed an elevated production of mRNA not only in the DRG, where the cell bodies of sensory neurons reside, but also in the dorsal horn of the spinal cord (central terminal of primary sensory neurons), and perhaps more interestingly and unexpectedly, in the paw skin (peripheral terminal) that the primary afferents innervate. In addition, such wide spread up-regulation of TRPA1 was shown to last at least two weeks following injury, when post-SCI neuropathic pain is firmly established (Due et al. 2014). To our knowledge, this is the first report that the up-regulation of TRPA1 and, consequently pain sensitization, is not just limited to the vicinity of the primary injury such as the spinal cord tissue and local DRGs, but also in the loci of peripheral terminal such as the skin of the paw. These findings are also consistent with our observations that the percentage of decrease in paw withdrawal thresholds, resulting from acrolein injections at the paw skin, is significantly greater in SCI rats compared to control rats, indicating a higher magnitude of ion channel activation (likely TRPA1) by acrolein in SCI rats. The heightened acrolein-mediated activation of TRPA1 in paw skin is also in agreement with the notion that higher levels of mRNA leads to the elevated level of TRPA1 channel protein translation. In addition, in a previous study reported by our group, we have shown that acrolein can induce DRG hyperactivity when extracted from SCI rats compared to that from uninjured rats, further suggesting a higher density of TRPA1 expressed at DRG cells in SCI rats. Taken together, this suggests that in addition to sensory dysfunction that is known to induce hypersensitivity at the spinal cord at the injury site, the amplification of sensory stimulation first occurs in the periphery following spinal injury. Such information may further our understanding of the mechanisms of post-SCI neuropathic pain and perhaps, more importantly, guide our efforts to prevent and treat this condition.
We have shown in this study that acrolein, an aldehyde that has been linked to neuropathic pain in our prior study, can be attributed to TRPA1 up-regulation, further strengthening its pro-algesic role. Previously, the elevation of acrolein was found to coincide with the establishment of neuropathic pain, which anti-acrolein treatments were able to suppress, and also the injection of acrolein was shown to cause heightened pain sensation (Due et al. 2014, Park et al. 2014b). In the current study, we have shown that micro-injections of acrolein directly into the spinal cord of otherwise healthy rats has resulted in up-regulation of TRPA1 not only in the spinal cord dorsal horn and DRGs at the level of injection, but also in paw skin where the axons of the sensory neurons are terminated. These studies have further strengthened the notion of acrolein being a pro-algesic culprit in neuropathic pain development after SCI. The fact that acrolein alone could lead to sensory hypersensitivity in the absence of mechanical damage suggests that acrolein is not only critical, but sufficient to produce neuropathic pain. In fact, it has been shown that neuropathic pain following spinal cord injury can develop not just at the level of injury, but above and below the spinal cord segment which suffered the original physical insult (Gwak & Hulsebosch 2009, Ondarza et al. 2003). Similarly, we have shown that post-SCI acrolein elevation could be detected well beyond the original injury site, spreading in both caudal and rostral directions (Luo et al. 2005). These data suggest that acrolein has the capability to cause wide spread sensory hypersensitivity and likely plays an essential role in post-SCI diffusive neuropathic pain.
In this and prior investigations, we have shown that acrolein elevation peaks 24 hours post SCI, and persists for at least 2 weeks (Due et al. 2014, Luo et al. 2005). It is likely that the early surge (hours post-SCI) of acrolein contributes to acute sensory hypersensitivity by directly activating TRPA1 receptors while the continued elevated acrolein stimulates the up-regulation of TRPA1 which was detected 1 and 2 weeks post SCI. Such acrolein-mediated up-regulation of TRPA1 may contribute more to subacute and chronic neuropathic pain even when the acrolein level is normalized: a hypothesis that remains to be tested.
The mechanism of acrolein-mediated TRPA1 up-regulation has not yet been established. However, several pieces of evidence may offer some clues that could guide future investigation. For example, TRPA1 expression has been shown to be up-regulated by tumor necrosis factor-α (TNFα) and interleukin-1 α (IL1α) via transcriptional factor hypoxia-inducible factor-1α (HIF1α) and NF-kB pathways (Hatano et al. 2012). On the other hand, it is also known that acrolein has the ability to stimulate the production of TNFα and IL1α, and both are factors shown to be elevated in SCI (Beck et al. 2010, Borchers et al. 2008, Esterbauer et al. 1991, Facchinetti et al. 2007, Kawabata et al. 2010, Kehrer & Biswal 2000, Luo et al. 2005, Park et al. 2014b, Sato et al. 1999, Stevens & Maier 2008). Therefore, it is possible that acrolein-instigated downstream pro-inflammatory cellular events could enhance the elevated production of TRPA1. The interactions of the aforementioned cytokines with HIF1α and NF-kB remain to be elucidated in the context of TRPA1 up-regulation.
Another interesting question stemming from our observation is the mechanism by which the peripheral terminal increase of TRPA1 is achieved. One possibility is that additional TRPA1 is synthesized at the cell body within the DRGs and then transported to the peripheral terminals through axonal transport. Another possibility is that TRPA1 protein is synthesized locally at the axons near the terminal which requires the transmission of an initial signal to the axonal terminal from the cell body where acrolein, or other unidentified critical signaling molecules, are elevated. Since the protein synthesis machinery is known to exist at both cell bodies and axons, both options remain as viable possibilities (Kandel et al. 2000).
Regarding the observation of an elevation of TRPA1 in the skin of post-SCI rats, necessary caution needs to be taken when interpreting the data in that our skin preparation also contains keratinocytes that is known to also express TRPA1 (Anand et al. 2008, Andre et al. 2009, Nilius et al. 2007). Therefore, we cannot conclude that the axon is sorely responsible for the up-regulation of TRPA1 in our skin samples. However, considering the cell body of these axons are located in the DRG which is near the spinal cord where the injury is suffered and the relevant biochemical and structural changes are detected, it is likely that TRPA1 changes in axons play a major, if not exclusive, role in the observed up-regulation of TRPA1 in skin. One possible strategy to gain insight to this question is to directly observe and compare the change of TRPA1 labeling in both axonal terminals and keratinocytes in the skin preparation using histological methods, in both control and SCI.
Based on current and prior related studies, we conclude that acrolein could be a promising therapeutic target for neuropathic pain treatment after SCI. This supposition is supported by its ability to stimulate the up-regulation of TRAP1, as well as serve as a direct agonist of the TRPA1 channel. But perhaps more importantly, acrolein scavenging strategies can reduce TRPA1 production and alleviate mechanical and cold allodynia following SCI. The translational power of this novel analgesic intervention is supported by the fact that hydralazine, which was used in our current and previous studies, is an FDA approved hypertension medication (Due et al. 2014, Park et al. 2014b, Khan 1953, Pandit 1984). Further, the dosage (5 mg/kg) of hydralazine used in this and other prior studies to lower the acrolein has been shown to cause no significant change of blood pressure in rats (Zheng et al. 2013). Taken together, we expect that our study will lead to the development of new analgesic therapies in SCI that has a highly likelihood of translating to a clinical setting.
Conclusion
As a specific TRPA1 channel agonist and an inflammatory factor, acrolein appears to play an important role in establishment and maintenance of neuropathic pain pathogenesis after SCI, likely through the activation of TRPA1 receptor as well as augmentation of TRPA1 channel expression in both central and peripheral locations. As such, acrolein constitutes an effective therapeutic target for novel analgesic approaches. Further, anti-acrolein treatments using FDA-approved medication is an effective, feasible analgesic approach in rat SCI model, and therefore a viable candidate as a new analgesic therapy that could reduce pain and improve the quality for SCI victims. It is well known that pathogenesis of neuropathic pain is complex and multimodal (Yu et al. 2013, Hulsebosch et al. 2009, Costigan et al. 2009). Consequently, anti-acrolein treatment can likely be combined with other potential therapies to achieve maximal analgesic effect which could ultimately alleviate the pain not just in SCI patients, but many other pathological conditions.
Acknowledgments
This work was supported by the Indiana State Department of Health (Grant # 204200 to RS), National Institutes of Health (Grant # NS073636 to RS), Indiana CTSI Collaboration in Biomedical Translational Research (CBR/CTR) Pilot Program Grant (Grant # RR025761 to RS), and Project Development Teams pilot grant (Grant #TR000006 to RS). Riyi Shi is the co-founder of Neuro Vigor, a star-up company with business interests of developing effective therapies for CNS neurodegenerative diseases and trauma.
Abbreviations
- SCI
Spinal cord injury
- TRPA1
transient receptor potential ankyrin 1
- LPO
lipid peroxidation
- DRG
dorsal root ganglia
- IP
intraperitoneal
- 3-HPMA
3-hydroxypropyl mercapturic acid
- TNFα
necrosis factor-α
- IL1α
Interleukin-1 α
- HIF1α
transcriptional factor hypoxia-inducible factor-1α
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B cells
Footnotes
ARRIVE guidelines have been followed:
Yes
=> if No, skip complete sentence
=> if Yes, insert "All experiments were conducted in compliance with the ARRIVE guidelines."
Conflicts of interest: Riyi Shi is the co-founder of Neuro Vigor, a star-up company with business interests of developing effective therapies for CNS neurodegenerative diseases and trauma.
=> if 'none', insert "The authors have no conflict of interest to declare."
=> otherwise insert info unless it is already included
References
- Anand U, Otto WR, Facer P, et al. TRPA1 receptor localisation in the human peripheral nervous system and functional studies in cultured human and rat sensory neurons. Neurosci Lett. 2008;438:221–227. doi: 10.1016/j.neulet.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Andre E, Gatti R, Trevisani M, Preti D, Baraldi PG, Patacchini R, Geppetti P. Transient receptor potential ankyrin receptor 1 is a novel target for pro-tussive agents. Br J Pharmacol. 2009;158:1621–1628. doi: 10.1111/j.1476-5381.2009.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atoyan R, Shander D, Botchkareva NV. Non-neuronal expression of transient receptor potential type A1 (TRPA1) in human skin. J Invest Dermatol. 2009;129:2312–2315. doi: 10.1038/jid.2009.58. [DOI] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: A gatekeeper for inflammation. Annu Rev Physiol. 2013;75:181–200. doi: 10.1146/annurev-physiol-030212-183811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133:433–447. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers MT, Wesselkamper SC, Eppert BL, Motz GT, Sartor MA, Tomlinson CR, Medvedovic M, Tichelaar JW. Nonredundant functions of alphabeta and gammadelta T cells in acrolein-induced pulmonary pathology. Toxicol Sci. 2008;105:188–199. doi: 10.1093/toxsci/kfn106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due MR, Park J, Zheng L, Walls M, Allette YM, White FA, Shi R. Acrolein involvement in sensory and behavioral hypersensitivity following spinal cord injury in the rat. J Neurochem. 2014;128:776–786. doi: 10.1111/jnc.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert E, Drexler H, Goen T. Determination of six hydroxyalkyl mercapturic acids in human urine using hydrophilic interaction liquid chromatography with tandem mass spectrometry (HILIC-ESI-MS/MS) J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2506–2514. doi: 10.1016/j.jchromb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology & Medicine. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Amadei F, Geppetti P, et al. Alpha,beta-unsaturated aldehydes in cigarette smoke release inflammatory mediators from human macrophages. Am J Respir Cell Mol Biol. 2007;37:617–623. doi: 10.1165/rcmb.2007-0130OC. [DOI] [PubMed] [Google Scholar]
- Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience. 2009;161:895–903. doi: 10.1016/j.neuroscience.2009.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann K, Durkes A, Ouyang H, Uchida K, Pond A, Shi R. Critical role of acrolein in secondary injury following ex vivo spinal cord trauma. J Neurochem. 2008;107:712–721. doi: 10.1111/j.1471-4159.2008.05622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann K, Shi R. Acrolein scavenging: a potential novel mechanism of attenuating oxidative stress following spinal cord injury. J Neurochem. 2009;111:1348–1356. doi: 10.1111/j.1471-4159.2009.06395.x. [DOI] [PubMed] [Google Scholar]
- Hatano N, Itoh Y, Suzuki H, Muraki Y, Hayashi H, Onozaki K, Wood IC, Beech DJ, Muraki K. Hypoxia-inducible factor-1alpha (HIF1alpha) switches on transient receptor potential ankyrin repeat 1 (TRPA1) gene expression via a hypoxia response element-like motif to modulate cytokine release. J Biol Chem. 2012;287:31962–31972. doi: 10.1074/jbc.M112.361139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. McGraw-hill; 2000. [Google Scholar]
- Kawabata H, Setoguchi T, Yone K, Souda M, Yoshida H, Kawahara K, Maruyama I, Komiya S. High mobility group box 1 is upregulated after spinal cord injury and is associated with neuronal cell apoptosis. Spine (Phila Pa 1976) 2010;35:1109–1115. doi: 10.1097/BRS.0b013e3181bd14b6. [DOI] [PubMed] [Google Scholar]
- Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicological Sciences. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- Khan MA. Effect of hydralazine in hypertension. Br Med J. 1953;1:27–29. doi: 10.1136/bmj.1.4800.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo J, Uchida K, Shi R. Accumulation of acrolein-protein adducts after traumatic spinal cord injury. Neurochem Res. 2005;30:291–295. doi: 10.1007/s11064-005-2602-7. [DOI] [PubMed] [Google Scholar]
- Moran MM, McAlexander MA, Biro T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nature reviews. Drug discovery. 2011;10:601–620. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch. 2012;464:425–458. doi: 10.1007/s00424-012-1158-z. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Ondarza AB, Ye Z, Hulsebosch CE. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp Neurol. 2003;184:373–380. doi: 10.1016/j.expneurol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Pandit RB. Long term propranolol and hydralazine in hypertension. J Assoc Physicians India. 1984;32:199–202. [PubMed] [Google Scholar]
- Park J, Muratori B, Shi R. Acrolein as a novel therapeutic target for motor and sensory deficits in spinal cord injury. Neural regeneration research. 2014a;9:677–683. doi: 10.4103/1673-5374.131564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Zheng L, Marquis A, et al. Neuroprotective role of hydralazine in rat spinal cord injury-attenuation of acrolein-mediated damage. J Neurochem. 2014b;129:339–349. doi: 10.1111/jnc.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Macpherson L. Channeling pain. Nat Med. 2006;12:506–507. doi: 10.1038/nm0506-506. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nature reviews. Drug discovery. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato E, Koyama S, Takamizawa A, Masubuchi T, Kubo K, Robbins RA, Nagai S, Izumi T. Smoke extract stimulates lung fibroblasts to release neutrophil and monocyte chemotactic activities. Am J Physiol. 1999;277:L1149–1157. doi: 10.1152/ajplung.1999.277.6.L1149. [DOI] [PubMed] [Google Scholar]
- Shi R, Luo L. The role of acrolein in spinal cord injury. Applied Neurology. 2006;2:22–27. [Google Scholar]
- Shi R, Page JC, Tully M. Molecular mechanisms of acrolein-mediated myelin destruction in CNS trauma and disease. Free Radic Res. 2015;49:888–895. doi: 10.3109/10715762.2015.1021696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K. Current status of acrolein as a lipid peroxidation product. Trends in Cardiovascular Medicine. 1999;9:109–113. doi: 10.1016/s1050-1738(99)00016-x. [DOI] [PubMed] [Google Scholar]
- Uchida K, Kanematsu M, Sakai K, et al. Protein-bound acrolein: potential markers for oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4882–4887. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Thakor DK, Han I, Ropper AE, Haragopal H, Sidman RL, Zafonte R, Schachter SC, Teng YD. Alleviation of chronic pain following rat spinal cord compression injury with multimodal actions of huperzine A. Proc Natl Acad Sci U S A. 2013;110:E746–755. doi: 10.1073/pnas.1300083110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Park J, Walls M, Tully M, Jannasch A, Cooper B, Shi R. Determination of Urine 3-HPMA, a Stable Acrolein Metabolite in a Rat Model of Spinal Cord Injury. J Neurotrauma. 2013;30:1334–1341. doi: 10.1089/neu.2013.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]