Abstract
Objectives
To investigate whether an imaging measure of corticospinal tract (CST) injury in the acute phase can predict motor outcome at 3 month in comparison to clinical assessment of initial motor impairment.
Methods
A two-site prospective cohort study followed up a group of first-ever ischemic stroke patients using the Upper-Extremity Fugl-Meyer (UE-FM) Scale to measure the motor impairment in the acute phase and at 3 months. A weighted CST lesion load (wCST-LL) was calculated by overlaying the patient’s lesion map on MRI with a probabilistic CST constructed from healthy control subjects. Regression models were fit to assess the predictive value of wCST-LL and compared with initial motor impairment.
Results
76 patients (37 from cohort 1 and 39 from cohort 2) completed the study. wCST-LL correlated motor impairment at 3 months measured by UE-FM scale, similar to the clinical assessment of initial motor impairment in both cohort 1 (R2=0.69 vs. R2=0.67, p=0.43) and cohort 2 (R2=0.69 vs. R2=0.62, p=0.25). In the severely impaired subgroup (defined as UE-FM ≤10 at baseline), wCST-LL correlated outcomes significantly better than clinical assessment (R2=0.47 vs. R2=0.11, p=0.03). In the non-severely impaired subgroup, stroke patients recovered approximately 70% of their maximal recovery potential. All stroke patients in both cohorts had poor motor outcomes at 3 months (defined as UE-FM≤25) when wCST-LL was ≥7.0 cc (positive predictive value is 100%).
Interpretation
wCST-LL, a potential imaging biomarker from the acute phase, can predict post-stroke motor outcomes at 3 months, especially in patients with severe impairment at baseline.
Keywords: Corticospinal tract, Lesion load, Outcome, Stroke recovery
INTRODUCTION
Motor impairment is the most common complication after stroke, negatively affecting quality of life. Making accurate predictions about motor outcome and recovery potential continues to be challenging for stroke clinicians. Several factors may influence post-stroke motor recovery including: age,1 gender,2 intensity of therapy,3 initial motor impairment,4–6 lesion volume,7, 8 and degree of injury to the corticospinal tract (CST).9–11
Previous research has explored various ways to assess the degree of injury to the CST and use it to predict motor recovery. Most simply, clinical assessment of motor impairment in the acute phase has been shown to be prognostic of motor impairment in the chronic phase.12, 13 For example, the ability to perform finger extension tasks three or more days post-stroke has been shown to predict hand function at 3 months and beyond.4, 6 However, bedside clinical assessments have limitations, particularly in the group of patients with severe initial motor impairment, who often show significant inter-individual variability in recovery.12
Neuroimaging and/or neurophysiological measures are able to uncover the pathophysiological basis of an injury and might better reveal a patient’s recovery potential, especially for those with severe impairment acutely. The absence or presence of motor evoked potentials (MEPs) induced by Transcranial Magnetic Stimulation (TMS) has been used to determine the injury of the CST and can predict motor outcome to some degree. Although MEPs have a high sensitivity,14, 15 their specificity is low; i.e., absence of MEPs does not necessarily mean poor recovery.16, 17 Neuroimaging can also be used to determine to what extent and by what mechanism recovery can be achieved.18 Fractional anisotropy (FA) values derived from Diffusion Tensor Imaging (DTI) of the posterior limb of the internal capsule (PLIC)10 have been associated with motor recovery in chronic stroke patients. However, in the acute phase, FA does not seem to be significantly altered9 likely due to the fact that Wallerian degeneration takes time to manifest. In addition, measuring FA is subject to the confounding influence of tissue edema secondary to acute injury. Alternatively, task-related brain activation using functional magnetic resonance imaging (fMRI), which might be related to the integrity of corticospinal tract,19 has been correlated with motor recovery.13, 20 However, fMRI is difficult to implement and standardize in the acute phase, particularly in patients with hemiplegia, global aphasia, or neglect. Approaches to combine clinical assessment of the initial motor impairment with imaging or electrophysiological tools have been statistically susceptible to colinearity issues. Our group has recently developed a new imaging marker – the weighted CST lesion load (wCST-LL)11 – which was shown to highly correlate with motor impairment in chronic stroke patients. Thus, the aims of this study were: (1) to test if the wCST-LL, calculated by overlaying lesion maps derived from the stroke patients’ diffusion-weighted images (DWI) in the acute phase, with a canonical CST tract derived from healthy elderly control subjects, predicts motor outcome at 3 months; (2) to replicate the results in a second cohort at another site; and (3) to test whether wCST-LL leads to better outcome predictions than the clinical assessment of the initial motor impairment.
SUBJECTS and METHODS
Study Subjects
This is a two-site prospective cohort study consisting of patients with first-ever acute ischemic stroke with various degrees of unilateral motor impairment at baseline. They were assessed at 2–7 days after stroke onset and followed up for 3 months post-stroke (90 days ± 15 days). It was conducted at two academic stroke centers (Beth Israel Deaconess Medical Center as cohort 1 or derivation cohort and Medical University of South Carolina as cohort 2 or validation cohort) in the US. Inclusion criteria were as follows: greater than 18 years-old of any ethnicity, first ever acute ischemic stroke with unilateral limb weakness, and Upper Extremity Fugl-Meyer (UE-FM) score <60 at baseline (to avoid ceiling effects), assessed between 2–7 days after stroke onset, and brain MRI obtained in the acute phase as a part of routine clinical care. Exclusion criteria were as follows: bihemispheric strokes; history of previous stroke documented either on imaging or medical history, any concomitant neurological disorder causing motor impairment, and documented history of severe dementia or medication uncontrolled depression either prior to stroke or after stroke. A patient who suffered a recurrent stroke before his/her follow-up visit would be discontinued from the study.
The following variables were assessed: age, gender, ethnicity, handedness, stroke subtype based on TOAST criteria,21 reperfusion therapy (yes or no), days of therapy (the total number of physical and/or occupational therapy days that the patient received between the day of hospital admission and the 3 month follow-up visit) as a surrogate measure of the dosage of rehabilitation therapy, level of education (high school or less, some college, college degree or above).
In addition to the stroke patients, 12 healthy subjects were recruited from our imaging database as an age-matched control group (9 male; mean age: 56.5 ± 14.8 years). This study was approved by the Institutional Review Board at both sites.
Outcome Measures
Upper Extremity Fugl-Meyer (UE-FM) scale22 and the National Institute of Health Stroke Scale (NIHSS) were collected at baseline (between 2–7 days after onset of stroke symptoms) and again at 3 months (90 ± 15 days) post-stroke. The UE-FM assessment,22 a validated impairment scale with excellent inter- and intra-rater reliability23 was the primary outcome variable (max score is 66). The NIHSS24 is a 42-point scale that quantifies global neurologic deficits in 11 categories. The NIHSS arm motor score is the score from the item of arm function (ranges from 0 to 4).
Image Processing and Lesion Mapping
The methods for lesion mapping and calculation of the lesion load of the CST are detailed in a previous publication.11 In the this study, the wCST-LL was determined in the acute stroke phase using the lesion maps drawn on spatially normalized DWI obtained as part of the standard-of-care stroke work-up. The DWI provides the strongest contrast between the ischemic lesion and normal tissue. Lesion maps were manually drawn on the normalized DWI in MRIcro25 by a rater who was blind to the behavioral assessment, and overlaid with the canonical CST to determine the wCST-LL for each patient. The wCST-LL was calculated by weighting each slice for overlap with the CST by the ratio of the maximum cross-sectional area of the CST over the cross-sectional area of that specific slice. This weighing option corrects for the narrowing of the CST descending into the PLIC from the motor cortex.
In contrast to the previous study11, the canonical tract was determined by a probabilistic fiber tracing approach using FSL 3.1.2 (http://www.fmrib.ox.ac.uk). Preprocessing steps include correction for eddy current effects, skull stripping, as well as estimation and fitting of diffusion parameters. Single slice regions of interest (ROIs) were drawn on the FA images in the pons, PLIC, and the white matter underlying the posterior part of the precentral gyrus. Exclusion ROIs were drawn on the superior and middle cerebellar peduncle to exclude fibers to the cerebellum, as well as the middle sagittal region covering the brain stem and corpus callosum to exclude trans-hemispheric fibers. Probtrackx (http://www.fmrib.ox.ac.uk/fsl/fdt/fdt_probtrackx.html) was run to track fibers from the pons ROI as the seeding region. Tracts were normalized to the SPM5 T2 template from SPM5 (Wellcome, Department of Neurology, London, UK) implemented in MATLAB (The Mathworks, Inc., Natick, MA), which was achieved by normalizing the DWI image to the SPM 5 T2 template, and then applying the normalization parameter to each CST tract. A 50th fractional anisotropy (FA) percentile threshold was applied to each CST fiber, and then the twelve tracts were each binarized and summed to create the canonical CST.
The DWI of the patients were normalized to a skull-stripped T1-weighted SPM5 brain template with isotropic 2×2×2 mm voxels. Skull stripping was achieved using BET implemented in the FSL 4.1.4 software package. The skull-stripped T1 template was found to be the most appropriate template because the T1-weighted images and the Diffusion trace images have dark signals representing the CSF compartment. For 14 patients, the large hyperintense lesion on the DWI images distorted the normalization process and an alternate two-step normalization process was applied. The two-step normalization process consisted of normalizing the apparent diffusion coefficient (ADC) images of the DWI sequences to the SPM T2-weighted template first, and then applying those normalization parameters to the T2-weighted diffusion trace images. A visual inspection of normalization was done by using well-known anatomical landmarks (e.g., anterior and posterior commissure, corpus callosum extents, frontal horns of the lateral ventricles, location of the central sulcus, outer contour of the brain) to determine whether or not the normalization was adequate. To find more objective measures and to standardize the process of normalization in lesioned brains, we came up with a way of quantitatively describing the precision of the normalization process. First, we realized that even in brains that showed distortions and warping after normalization, typically the outer contour was still well normalized. It was more the inner structures, close to the midline and above the anterior commissure that ended up showing distortions. Therefore, we created a bounding box (x=37–41, y=43–59, z=24–39) that spanned around the anterior and posterior commissure, included 5 sagittal slices centered around the midline (inter-hemispheric fissure) and 15 slices dorsal from a horizontal line connecting the AC and PC. All voxels and their values were extracted from the SPM template brain and each patient’s brain after the one-step normalization process (DWI-trace to SPM5 T1-template). In the next step, we regressed each voxel value of this bounding box from the SPM template with each patient’s voxel value from the bounding box. The brains that were visually determined to have a satisfactory normalization had median r-value of 0.49 (SD 0.09), while the brains that were visually determined to be unsatisfactory had median r-value of 0.28 (SD 0.15). These two groups differed significantly from each other (p<0.001). Subjecting the “badly” normalized brains to the two-step process described above, improved their r-value to 0.49 (SD 0.15). There were significant differences between the “badly normalized brains and the two-step normalized brains (p<0.001) while the previously “badly” normalized brains did not significantly differ from the one-step normalized brains anymore after the “badly” normalized brains had undergone the 2-step process. This two-step process worked well for these patients with large lesions.
Statistical Analysis
The primary outcome variable was the UE-FM scale at 3 months. Secondary outcome variables included the NIHSS arm motor score and the NIHSS total score at 3 months. A univariate regression analysis was conducted to assess the amount of variance (R2) explained by wCST-LL or initial motor impairment with regard to UE-FM scores at 3 months. The regression model diagnostics included model fit, influence diagnosis, and multi-colinearity diagnostic. Fisher r-to-z test was used to test the statistical difference of R2. Cohort 1 was treated as a derivation cohort, the coefficient and intercept from the regression analysis based on cohort 1 were applied to cohort 2 to calculate predicted R2 in cohort 2. A subsequent regression analysis was conducted in the combined severely impaired subgroup (defined as UE-FM score ≤10 at baseline). Regression analysis was also applied to the NIHSS arm score as an alternative outcome variable and the NIHSS total score as a global outcome variable. Lastly, a multivariate regression was fit with variables acute UE-FM, wCST-LL, and additional variables with significant correlation with outcome variables. A backward elimination procedure for variable selection (p=0.05) was used to obtain a more parsimonious model.
UE-FM score ≤ 25 at 3 months was arbitrarily defined as poor motor outcomes in our analysis (all patients with UE-FM score ≤ 25 at 3 months in the combined cohort had modified Rankin Scale >3 which is considered as poor outcome). Receiver Operating Characteristic (ROC) Curve was generated by logistic regression by modeling the poor motor outcomes in cohort 1. Specificity, sensitivity, positive predictive value (PPV), negative predictive value (NPV) and accuracy were calculated with regards to different cut-off values of wCST-LL. These cut-off values of wCST-LL were applied to cohort 2 (validation cohort) to calculate the specificity, sensitivity, PPV, NPV and accuracy. The main interests are specificity and positive predictive value.
A proportional recovery score12 was calculated by relating the actual change score in the UE-FM between baseline and 3 months to the maximal recovery potential which was defined as the difference between the maximal UE-FM score (66) minus the baseline UE-FM score.
All statistical analyses were performed using SAS V9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Patients’ characteristics
76 Patients (37 in cohort 1, and 39 in cohort 2) completed both baseline and 3-month follow-up assessment. The two groups were largely comparable but did have differences in some demographics as shown in Table 1. Cohort 2 had more African Americans (35.9% vs. 8.1%), was slightly younger (56.9 vs. 60.7 years old), had fewer subjects who attended college or higher education (13.6% vs. 47.2%), and had fewer subjects who were discharged to an inpatient rehabilitation facility (71.8% vs. 91.9%).
Table 1.
Patients’ Demographic and Clinical Characteristics
| Cohort 1 (N=37) |
Cohort 2 (N=39) |
Combined (N=76) |
|
|---|---|---|---|
| Demographic | |||
| Age (yrs) | 60.7 (16.4) | 56.9 (11.2) | 58.8 (14.0) |
| Male (%) | 64.9% | 56.4% | 60.5% |
| Ethnicity | |||
| Caucasian | 81.1% | 64.1% | 72.4% |
| African American | 8.1% | 35.9% | 22.4% |
| Others | 10.8% | 0% | 5.3% |
| Education (College or higher) | 47.2% | 13.6% | 30.1% |
| Lesion side (Right) | 62.1% | 69.2% | 65.4% |
| Right Handed | 91.9% | 97.4% | 96.1% |
| tPA or Reperfusion Therapy | 37.8% | 26.8% | 31.6% |
| Stroke subtype | |||
| Small vessel disease | 18.9% | 28.2% | 23.7% |
| Cardioembolism | 32.4% | 18.0% | 25.0% |
| Large vessel atherosclerotic disease | 18.9% | 33.3% | 26.3% |
| Other or unknown etiology | 29.7% | 20.5% | 25.0% |
| Disposition | |||
| Length of stay (days) | 5.8 (3.3) | 6.6 (6.0) | 6.2 (4.9) |
| Days between onset of stroke symptom and the first assessment | 2.9 (1.5) | 2.0 (1.2) | 2.4 (1.5) |
| Days between stroke admission and follow up | 92.6 (14.4) | 94.3 (10.5) | 93.5(13.4) |
| Days of rehabilitation therapy | 39.8 (18.8) | 29.8 (17.5) | 34.5 (18.7) |
| Acute rehabilitation facility (%) | 91.9% | 71.8% | 81.6% |
| Behavioral Assessment | |||
| NIHSS at baseline | 9.2 (6.4) | 8.7 (5.2) | 9.0 (5.8) |
| NIHSS at 3 months | 4.4 (5.1) | 3.9 (3.5) | 4.2 (4.3) |
| UE-FM at baseline | 24.8 (19.7) | 25.1 (19.6) | 25.0 (19.5) |
| UE-FM at 3 months | 42.5 (23.8) | 42.3 (23.3) | 42.4 (23.4) |
| mRS | 2.3 (1.5) | 2.5 (1.3) | 2.4 (1.4) |
| Imaging Information | |||
| wCST-LL | 4.16 (3.06) | 3.74 (3.21) | 394 (3.12) |
| Lesion Volume | 43.33 (59.78) | 42.90(49.95) | 43.11(54.58) |
| Outcome Prediction (R2) | |||
| Clinical assessment (initial motor impairment) | 0.67 | 0.62 | 0.64 |
| Imaging assessment (wCST-LL) | 0.69 | 0.69 | 0.69 |
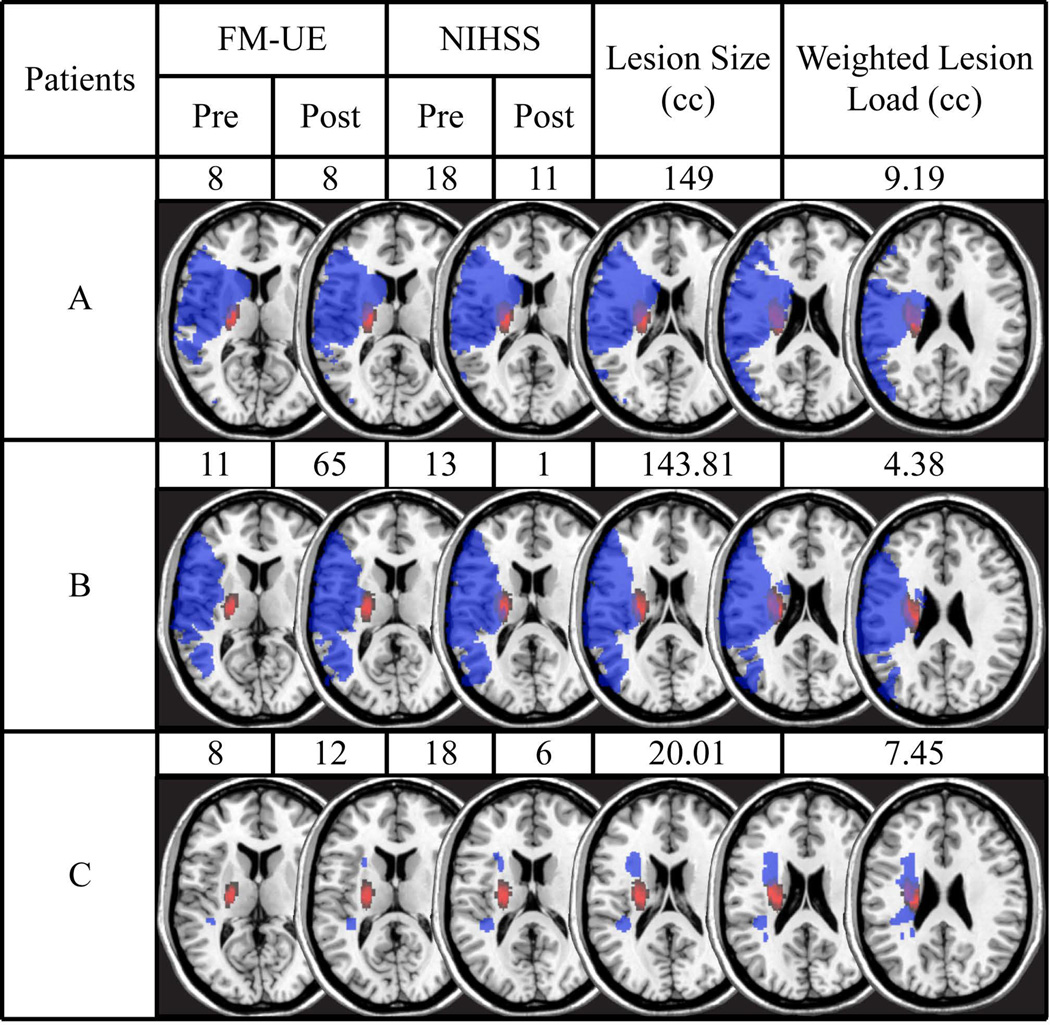
Overall, patients were assessed at 2.4 ± 1.5 days after onset of stroke symptoms and average length of hospital stay was 6.4 ± 4.9 days. 81.6% of patients were discharged to an acute rehabilitation facility and the average days of therapy that a patient received were 34.5 ± 18.7 days. The follow-up visit occurred at 93.5 ± 13.4 days after stroke admission. The mean UE-FM score was 25.0 ± 19.5 points at baseline and 42.4 ± 23.4 points at 3 months. The mean NIHSS score at baseline was 9.0 ± 5.8 points at baseline and 4.2 ± 4.3 points at 3 months. The average modified Rankin Scale was 2.4 ± 1.4 at 3 months. The overall lesion volume was 43.11 ± 54.58 cc and the wCST-LL was 3.94 ± 3.12 cc. Figure 1 shows an example of three patients with different lesion pattern with their UE-FM and NIHSS scores (at baseline and 3 months), lesion volumes, and wCST-LL value with regard to recovery.
Figure 1.
shows examples of 3 patients with their UE-FM and NIHSS scores (at baseline and 3 months post-stroke) their lesion maps (blue) overlaid onto the probabilistic fiber map (red) as well as their lesion volume and weighted CST lesion load (wCST-LL). The overlap between lesion and CST is displayed in purple. The axial slices depicted correspond to Z= 0, 4, 8, 10, 20, and 28 in Talairach space. A comparison of Patients A and B shows that two similarly sized lesions can have markedly different wCST-LL and, accordingly, results in very different levels of motor impairment both at baseline and 3 months post-stroke. A comparison of Patients A and C shows that two patients have similar wCST-LL and motor recovery, but drastically different lesion volumes.
Regression Analysis
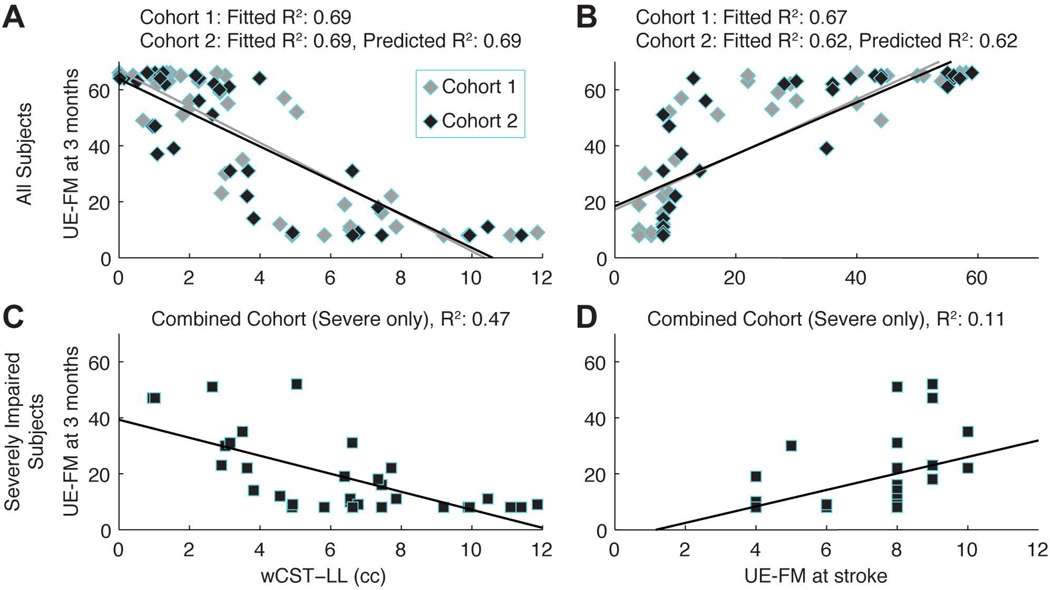
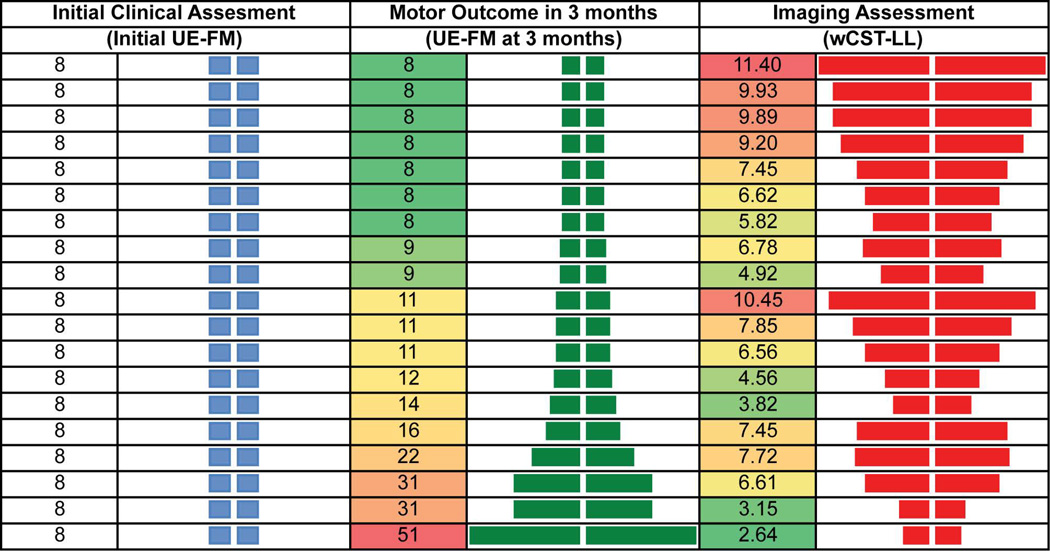
5 outliers were excluded from the analysis. They were firstly identified by regression model diagnostics. By reviewing clinical characteristics of these outliers, various reasons were revealed, including very large lesion, unusually excessive amount of rehabilitation therapy, possibility of a recurrent stroke, etc. The fitted R2 (0.69) and predicted R2 (0.69) in cohort 2 were the same for wCST-LL with respect to UE-FM at 3 months. Similarly, the fitted R2 (0.67) and predicted R2 (0.67) in cohort 2 were the same for initial motor impairment with respect to UE-FM at 3 months. R2 for wCST-LL and for initial motor impairment were statistically equivalent (R2=0.69 vs. R2 =0.67, p=0.43) with respect to motor outcomes at 3 months for cohort 1 as well as for cohort 2. (R2=0.69 vs. R2 =0.62, p=0.25). By pooling data from both cohorts, wCST-LL correlated with motor outcomes at 3 months significantly better than the initial motor impairment (R2=0.47 vs. R2=0.11, p=0.03, Figure 2) in the subgroup of patients with severe motor impairment at baseline (UE-FM ≤ 10). This is further illustrated (Figure 3) in a group of stroke patients who presented with the exact same motor impairment at baseline (i.e., UE-FM score = 8), but recovered to different levels at 3 months (UE-FM score ranges from 8 to 51). Although all these patients had the same low UE-FM score initially, the wCST-LL was able to differentiate the degree of injury to the corticospinal tract (CST) and correlated with motor outcomes at 3 months (R2=0.47) significantly better than the initial UE-FM which had a very low R2 of 0.11. Specifically, a higher wCST-LL value indicated a more injured CST and a greater likelihood that a patient would have a poor motor outcome at 3 months.
Figure 2.
demonstrates scatter plot and correlation for wCST vs. Initial motor impairment (A): For cohort 1, fitted R2 for wCST-LL is 0.69; for cohort 2, the fitted R2 for wCST-LL is 0.69 and predicted R2 is 0.69; (B): For cohort 1, fitted R2 for initial motor impairment is 0.67; for cohort 2, the fitted R2 for initial motor impairment is 0.62 and predicted R2 is 0.62; (C): R2 for wCST-LL is 0.47 for severely impaired subgroup; (D): R2 for initial motor impairment is 0.11 for severely impaired subgroup.
Figure 3.
shows the relationship between initial motor impairment, wCST-LL and motor outcomes at 3 months. Despite the fact that all patients presented with the same initial motor impairment by clinical assessment, those patients with smaller weighted CST lesion load recovered better at 3 months.
When the NIHSS arm motor score, as an alternative motor outcome variable, was modeled, wCST-LL had an equivalent correlations with the 3 months NIHSS arm motor score (R2=0.58 vs. R2=0.55, p=0.39) as compared to the initial NIHSS arm motor score. But when a measurement of global outcome - total NIHSS score, was modeled, the initial NIHSS score was found to correlate with NIHSS score at 3 months significantly better than the wCST-LL (R2=0.71 vs. R2=0.45, p=0.01, Table 2). This provides strong evidence that wCST-LL, an imaging measure of CST injury, is specialized at predicting motor outcome only.
Table 2.
Comparison of Predictive Value of wCST-LL vs. initial impairment with Regards to Different Outcome Variables
| Outcome Variable | Predictive Value (R2) | P value | |
|---|---|---|---|
| UE-FM Scores at 3 Months | Initial UE-FM score R2=0.69 |
wCST-LL R2=0.64 |
0.30 |
| NIHSS Arm Motor Scores at 3 months | Initial NIHSS Motor Score R2=0.55 |
wCST-LL R2=0.58 |
0.39 |
| NIHSS Total Scores at 3 months | Initial NIHSS score R2=0.71 |
wCST-LL R2=0.45 |
0.01 |
wCST-LL Threshold Analysis
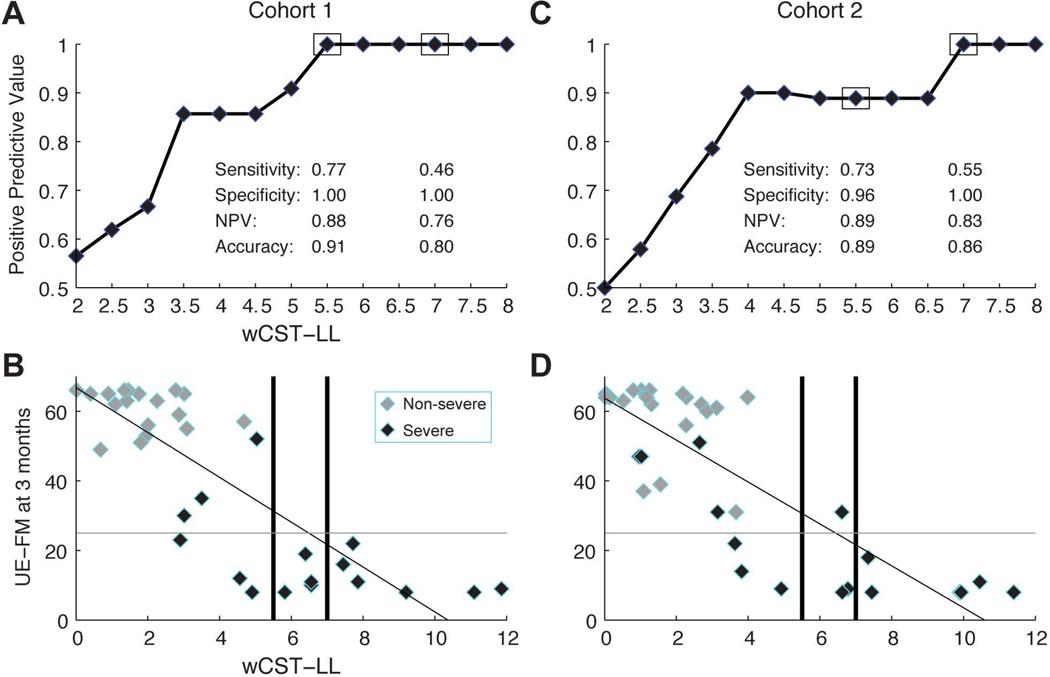
In cohort 1, a wCST-LL cutoff of 5.5 cc had sensitivity of 77%, specificity of 100% and positive predictive value of 100% (i.e., when the wCST was ≥ 5.5 cc in the acute phase, the chance of a patient to have poor motor outcome, defined as UE-FM≤25 at 3 months, is 100%). If wCST-LL cutoff was increased, for example, ≥ 7.0 cc, sensitivity decreased to 46% but specificity remained at 100% and PPV remained at 100% as well. A high specificity and PPV are important in making a prediction of poor motor outcomes in the acute phase in order not to misclassify patients as having a poor outcome and to potentially miss out on any rehabilitation opportunity. a wCST-LL of 7.0 cc threshold was validated in cohort 2 with a specificity of 100% and PPV of 100%.
Multivariate Analysis
In a multivariate regression analysis with initial motor impairment (UE-FM), wCST-LL, age, race, gender, days of therapy, reperfusion therapy and lesion volume, only two variables (wCST-LL and initial UE-FM) remained in the model by the backward elimination procedure. These two variables together explained 81% of the variance in outcome at 3 months; however, there was some colinearity between the initial UE-FM and the wCST-LL and both variable correlated significantly with each other (r=0.65 and P<0.0001). This suggests that clinical assessment (initial UE-FM) and imaging assessment (wCST-LL) both reflect the degree of injury to the corticospinal tract.
Proportional Recovery
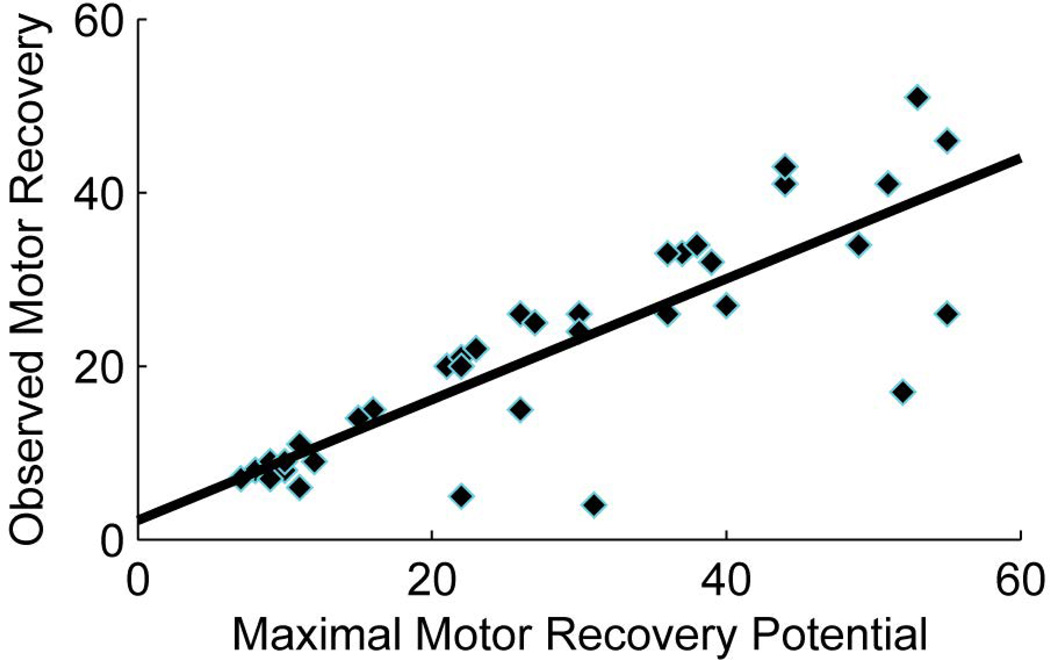
Prabhakaran and colleagues12 first discovered that stroke patients with mild to moderate initial impairments show an almost fixed proportional upper extremity motor recovery when tested again around 3 months. This phenomenon was confirmed later by the other study.26 We examined whether this proportional recovery rule was also true for our sample of stroke patients. Similar to these studies,12, 26 proportional recovery was not obvious when all patients were included. After excluding a subgroup of severely impaired patients (UE-FM ≤10 at baseline), the remaining patients indeed showed a recovery pattern of about 70% of their maximal recovery potential at 3 months (Figure 5).
Figure 5.
demonstrates “the proportional recovery” in a subgroup of patients with less severe motor impairment in the acute phase (UE-FM>10). Patients recovered approximately 70% of their maximal recovery potential.
DISCUSSION
Our study demonstrates that either the wCST-LL by imaging assessment or the initial motor impairment by clinical assessment (UE-FM) is well correlated with motor impairment measured at 3 months after stroke. Although the overall R2 value regarding motor outcome using wCST-LL and UE-FM is equivalent, the initial motor impairment assessment had limited predictive value in the subgroup of patients with severe motor impairment (UE-FM score ≤10 at baseline) while the wCST-LL was a significantly better predictor in this subgroup. Furthermore, a wCST-LL of ≥7.0 cc implies severe injury to the corticospinal tract to such a degree that poor motor outcome at 3 months (i.e., UE-FM≤25) cannot be avoided. Similar to other studies, we found evidence for a proportional recovery rule12, 26 in the non-severely impaired group, i.e., most patients with an initial UE-FM of >10 can recover approximately 70% of their maximal recovery potential at 3 months.
Consistent with previous studies,11, 27 the overall lesion volume was not found to be correlated with motor impairment at 3 months, suggesting that information about which relevant anatomic structures (i.e., the CST) are affected by a stroke lesion is necessary to increase correlations with outcomes or the predictive power of an imaging variable, as illustrated in Figure 1, 3 and 4.
Figure 4.
shows Positive Predictive Value of poor motor outcomes (defined as UE-FM≤25 at 3 months) at different cut-off value of wCST-LL in both cohort 1 and cohort 2. When wCST-LL in the acute phase exceeds 7.0 cc, all stroke patients in our cohort have poor motor outcomes at 3 months in both cohorts (i.e., UE-FM≤25).
The wCST-LL also correlates with another motor outcome measure – the NIHSS arm motor score, but less well with a global outcome (NIHSS total score). This specificity of the wCST-LL variable suggests that it is a unique imaging marker for post-stroke motor outcome prediction. Our results include an effective replication in two separate cohorts collected in two academic centers. While similar, the two cohorts do have some differences in race, age, educational level and disposition. Nevertheless, the wCST-LL imaging variable still effectively correlated with motor outcomes with an equivalent R2 per regression model, suggesting the wCST-LL is a robust motor outcome predictor. Stroke motor recovery depends on the degree of injury to the CST. In this study, we were able to demonstrate that once the lesion cumulated to a certain total volume in the acute phase, i.e. ≥7.0 cc, patients were highly likely to have poor motor outcomes at 3 months. This might have important clinical as well as economic implications. It can help set an appropriate expectation for the clinician, patient and caregiver at the very early stage after a stroke. Additionally, it might give the clinician the opportunity to triage a patient with predicted poor outcome to different rehabilitation modalities with more appropriate focus. A similar approach to early patient rehabilitation planning has been investigated.28
As a potential imaging biomarker of post-stroke motor outcome, the wCST-LL has several advantages over other methods. The most obvious advantage is that it only requires a clinical MRI scan, which most stroke patients will have as a part of the standard of care in the majority of hospitals in the US, making the components to determine wCST-LL are widely available and easy to implement. Other methods, such as functional MRI and TMS, are difficult to do in the acute stroke phase, are not available in most medical centers, and may not yield useful information in stroke patients with severe impairment. Diffusion tensor imaging in the acute phase may provide information about the integrity of CST, however, studies have shown that a tract distal to the lesion might still appear as structurally intact up to several days after an infarct because Wallerian degeneration takes times to develop and manifest as an imaging abnormality.9
This study also observed a similar recovery pattern as outlined by other studies12, 26 that most stroke patients, except those with severe motor impairment, recover in a proportional manner that is roughly 70% of their maximal potential recovery potential (Figure 5). This proportional recovery pattern was not seen in the subgroup of patients with severe impairment, who had a much greater inter-individual variability in terms of recovery potential posing a challenge for motor outcome predictions. For example, out of many patients with the same severe initial motor impairment (i.e., UE-FM score= 8), some reached a UE-FM score in the 50s at 3 months (Figure 3). While the motor assessment (a behavioral measure) shows limited correlation in these severely impaired patients in the acute phase (as first pointed out and emphasized by Prabhakaran and colleagues in 200812 suggesting that a non-behavioral measure was needed for better correlation of motor outcomes in this group), it is the wCST-LL (an imaging measure) that better reflects the differential injury to the CST and better correlates with motor outcome at 3 months in this subgroup. Since wCST-LL is superior to the clinical assessments in predicting outcome in this subgroup, it could also serve as a stratification variable in experimental stroke recovery studies, particularly for cases in which clinical assessment is not a good predictor of outcome or of response to interventions.
Days-of-therapy (DoT), a surrogate measure for rehabilitation dosage, was shown3 to correlate with stroke outcome. It was significantly related to UE-FM at 3 months in the univariate analysis. However, it did not survive as a covariate in the multivariate regression analysis. Our study revealed that the more severely impaired at baseline, the more DoT a patient likely received. But there was no linear relationship between DoT and the degree of motor improvement. The DoT variable is a complex variable, since it does not always indicate the amount or intensity of therapy that is dedicated towards the affected limbs especially in severely impaired patients; rehabilitation therapy may also focus on training compensatory activities by working on the non-affected limb. Additionally, the DoT variable is vulnerable to external factors such as insurance status and other personal factors. A note of caution is that our measure of DoT may not capture the exact amount of therapy that the patients should have or actually received as it was based on self-report. Future studies should obtain more detailed information on activities done during rehabilitation sessions.
Reperfusion therapy did not survive as a covariate in the multivariate regression analysis. There are several explanations for this. Patients were assessed between 2–6 days (average 2.4 days) after the onset of stroke symptoms and our assessments might have captured a new baseline after the immediate effects of a reperfusion therapy.
There is still some variance that remains to be explained. One source of variability could be factors that were not measured in this study and have not been proven to play a strong role, such as genetic predisposition.29, 30 Another source of variability could be the effect of post-stroke depression and use of antidepressant, which have been shown to have an effect on stroke motor recovery.31 However, in our study, the use of antidepressants after a stroke was not a significant predictor in the univariate analysis. Finally, the integrity of alternative motor fibers such as the cortico-rubral or cortico-tegmental tracts32, 33 with crossed and uncrossed connections to alpha-motor neurons in the spinal cord could have an influence on motor recovery and their influence on recovery should be considered in future studies.
Our study and approach do have some limitations. First, spatially normalized brain images of acute stroke patients may contain distortions due to large ventricles in elderly patients and/or very large lesions with edema. While we have developed solutions to improve spatial normalization even in brains with large lesions, CST location could potentially be more inaccurate in brains with large lesions than with smaller lesions. Second, we only included first-ever acute ischemic stroke patients in this cohort and excluded patients with their second or third stroke; this could affect somewhat the generalization of the study results. Thirdly, although the wCST-LL of cut off 7.0 cc was validated by two independent cohorts, the sample sizes are still relatively small (especially the subgroup with severe impairment at baseline), this wCST-LL threshold needs to be further defined and validated in a new large cohort before it can be used as a biomarker in experimental trials or clinical practice.
In summary, the wCST-LL, a potential imaging biomarker obtained in the acute stroke phase is well correlated with post-stroke motor outcomes at 3 months in two independent cohorts, especially in a subgroup of patients with severe impairment at baseline. Further validation of this imaging biomarker in another cohort with large sample size is required, and automation of the quantification process is actively pursued to establish wCST-LL as a tool for clinical stroke outcome predictions and as a stratification variable for future stroke recovery trials.
Acknowledgements
The Authors acknowledge grant support from American Heart Association Scientist Development Grant 14SDG1829003 (WF) and the South Carolina Clinical & Translational Research Institute/Medical University of South Carolina, through NIH Grant Numbers UL1 RR029882 and UL1 TR000062 (WF), NIH P20GM109040 (WF, PYC and SK), Rehabilitation Research & Development Service of the Department of Veterans Affairs (SK), NIH 1R01 DC008796 (GS), R01 DC009823-01 (GS), the Mary Crown and William Ellis Fund (GS), the Richard and Rosalyn Slifka Family Fund (GS), and the Tom and Suzanne McManmon Family Fund (GS) and the Doris Duke Charitable Foundation (CD).
We would also express thanks to Drs. Evgeny Sidorov, Magdy Selim, Sandeep Kumar, Robert Adams, Lindsay Perry and Ilya Lipkovich for their generous input and assistance while conducting the study and preparing the manuscript.
Footnotes
Authorship Contribution:
WF, DL, SK and GS contributed to concept and design of this study; WF, JW, PYC, CD, DL, VAL, and GS participated in the data acquisition and analysis; WF, JW, PYC, CD, DL, VAL, SK and GS drafted the manuscript or figures.
Potential Conflict of Interest: None.
REFERENCE
- 1.Bagg S, Pombo AP, Hopman W. Effect of age on functional outcomes after stroke rehabilitation. Stroke. 2002;33:179–185. doi: 10.1161/hs0102.101224. [DOI] [PubMed] [Google Scholar]
- 2.Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, et al. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in europe: Data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–1119. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 3.Cooke EV, Mares K, Clark A, Tallis RC, Pomeroy VM. The effects of increased dose of exercise-based therapies to enhance motor recovery after stroke: A systematic review and meta-analysis. BMC Med. 2010;8:60. doi: 10.1186/1741-7015-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nijland RH, van Wegen EE, Harmeling-van der Wel BC, Kwakkel G. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: Early prediction of functional outcome after stroke: The epos cohort study. Stroke. 2010;41:745–750. doi: 10.1161/STROKEAHA.109.572065. [DOI] [PubMed] [Google Scholar]
- 5.Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke. 1992;23:1084–1089. doi: 10.1161/01.str.23.8.1084. [DOI] [PubMed] [Google Scholar]
- 6.Smania N, Paolucci S, Tinazzi M, Borghero A, Manganotti P, Fiaschi A, et al. Active finger extension: A simple movement predicting recovery of arm function in patients with acute stroke. Stroke. 2007;38:1088–1090. doi: 10.1161/01.STR.0000258077.88064.a3. [DOI] [PubMed] [Google Scholar]
- 7.Mak W, Cheng TS, Chan KH, Cheung RT, Ho SL. A possible explanation for the racial difference in distribution of large-arterial cerebrovascular disease: Ancestral european settlers evolved genetic resistance to atherosclerosis, but confined to the intracranial arteries. Med Hypotheses. 2005;65:637–648. doi: 10.1016/j.mehy.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Chen CL, Tang FT, Chen HC, Chung CY, Wong MK. Brain lesion size and location: Effects on motor recovery and functional outcome in stroke patients. Arch Phys Med Rehabil. 2000;81:447–452. doi: 10.1053/mr.2000.3837. [DOI] [PubMed] [Google Scholar]
- 9.Puig J, Pedraza S, Blasco G, Daunis IEJ, Prados F, Remollo S, et al. Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR Am J Neuroradiol. 2011;32:857–863. doi: 10.3174/ajnr.A2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 11.Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41:910–915. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prabhakaran S, Zarahn E, Riley C, Speizer A, Chong JY, Lazar RM, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22:64–71. doi: 10.1177/1545968307305302. [DOI] [PubMed] [Google Scholar]
- 13.Zarahn E, Alon L, Ryan SL, Lazar RM, Vry MS, Weiller C, et al. Prediction of motor recovery using initial impairment and fmri 48 h poststroke. Cereb Cortex. 2011;21:2712–2721. doi: 10.1093/cercor/bhr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Escudero JV, Sancho J, Bautista D, Escudero M, Lopez-Trigo J. Prognostic value of motor evoked potential obtained by transcranial magnetic brain stimulation in motor function recovery in patients with acute ischemic stroke. Stroke. 1998;29:1854–1859. doi: 10.1161/01.str.29.9.1854. [DOI] [PubMed] [Google Scholar]
- 15.Dachy B, Biltiau E, Bouillot E, Dan B, Deltenre P. Facilitation of motor evoked potentials in ischemic stroke patients: Prognostic value and neurophysiologic correlations. Clin Neurophysiol. 2003;114:2370–2375. doi: 10.1016/s1388-2457(03)00252-9. [DOI] [PubMed] [Google Scholar]
- 16.Arac N, Sagduyu A, Binai S, Ertekin C. Prognostic value of transcranial magnetic stimulation in acute stroke. Stroke. 1994;25:2183–2186. doi: 10.1161/01.str.25.11.2183. [DOI] [PubMed] [Google Scholar]
- 17.Catano A, Houa M, Caroyer JM, Ducarne H, Noel P. Magnetic transcranial stimulation in acute stroke: Early excitation threshold and functional prognosis. Electroencephalogr Clin Neurophysiol. 1996;101:233–239. doi: 10.1016/0924-980x(96)95656-8. [DOI] [PubMed] [Google Scholar]
- 18.Seitz RJ, Donnan GA. Role of neuroimaging in promoting long-term recovery from ischemic stroke. J Magn Reson Imaging. 2010;32:756–772. doi: 10.1002/jmri.22315. [DOI] [PubMed] [Google Scholar]
- 19.Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, et al. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129:809–819. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall RS, Zarahn E, Alon L, Minzer B, Lazar RM, Krakauer JW. Early imaging correlates of subsequent motor recovery after stroke. Ann Neurol. 2009;65:596–602. doi: 10.1002/ana.21636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient: A method for evaluation of physical performance. Scandanavian Journal of Rehabilitation Medicine. 1975;7:13–31. [PubMed] [Google Scholar]
- 23.Duncan PW, Propst M, Nelson SG. Reliability of the fugl-meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606–1610. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- 24.Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, et al. Improved reliability of the nih stroke scale using video training. Ninds tpa stroke study group. Stroke. 1994;25:2220–2226. doi: 10.1161/01.str.25.11.2220. [DOI] [PubMed] [Google Scholar]
- 25.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- 26.Winters C, van Wegen EE, Daffertshofer A, Kwakkel G. Generalizability of the proportional recovery model for the upper extremity after an ischemic stroke. Neurorehabil Neural Repair. 2014 doi: 10.1177/1545968314562115. [DOI] [PubMed] [Google Scholar]
- 27.Mark VW, Taub E, Perkins C, Gauthier L, Uswatte G. Mri infarction load and ci therapy outcomes for chronic post-stroke hemiparesis. Restor Neurol Neurosci. 2008;26:13–33. [PubMed] [Google Scholar]
- 28.Stinear CM, Barber PA, Petoe M, Anwar S, Byblow WD. The prep algorithm predicts potential for upper limb recovery after stroke. Brain. 2012;135:2527–2535. doi: 10.1093/brain/aws146. [DOI] [PubMed] [Google Scholar]
- 29.Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, et al. Bdnf val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9:735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- 30.Cramer SC, Procaccio V. Correlation between genetic polymorphisms and stroke recovery: Analysis of the gain americas and gain international studies. Eur J Neurol. 2012;19:718–724. doi: 10.1111/j.1468-1331.2011.03615.x. [DOI] [PubMed] [Google Scholar]
- 31.Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C, et al. Fluoxetine for motor recovery after acute ischaemic stroke (flame): A randomised placebo-controlled trial. Lancet Neurol. 2011;10:123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- 32.Lindenberg R, Zhu LL, Ruber T, Schlaug G. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum Brain Mapp. 2012;33:1040–1051. doi: 10.1002/hbm.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain : a journal of neurology. 2012 doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]