SUMMARY
Introduction
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common human enzyme defect caused by G6PD gene mutations. This study aimed to develop a cost-effective, multiplex, genotyping method for detecting common mutations in the G6PD gene.
Methods
We used a SNaPshot approach to genotype multiple G6PD mutations that are common to human populations in Southeast Asia. This assay is based on multiplex PCR coupled with primer extension reactions. Different G6PD gene mutations were determined by peak retention time and colors of the primer extension products.
Results
We designed PCR primers for multiplex amplification of the G6PD gene fragments and for primer extension reactions to genotype 11 G6PD mutations. DNA samples from a total of 120 unrelated G6PD-deficient individuals from the China-Myanmar border area were used to establish and validate this method. Direct sequencing of the PCR products demonstrated 100% concordance between the SNaPshot and the sequencing results.
Conclusion
The SNaPshot method offers a specific and sensitive alternative for simultaneously interrogating multiple G6PD mutations.
Keywords: Glucose-6-phosphate dehydrogenase deficiency, mutation detection, SNaPshot assay
INTRODUCTION
Hereditary deficiency in glucose-6-phosphate dehydrogenase (G6PD) is estimated to affect about 400 million people worldwide. The highest prevalence rates are found in tropical Africa, the Middle East, and tropical and sub-tropical Asia.[1]. G6PD deficiency is associated with several clinical disorders including neonatal jaundice, hemolytic anemia following infection by certain pathogens and favism [2]. G6PD deficiency is caused by mutations in the G6PD gene, which is located on the Xq28 region with a length of ~18.5 kb comprising 13 exons and 12 introns and encoding 515 amino acids [3]. Because G6PD deficiency is X-linked and recessive, expression of phenotypic deficiency occurs more frequently in males than females. Heterozygous females may be either phenotypically deficient or normal depending on the relative proportion of deficient and non-deficient red cells, because females exhibit mosaicism in expression of G6PD [4]. There are over 180 recorded G6PD gene mutations, many of which have characteristic distributions in different geographical regions and among different ethnic groups [5, 6]. Most G6PD deficiencies are caused by a point mutation resulting in an amino acid substitution [5]. Mutations in the G6PD gene may disrupt the normal structure and function of the enzyme or reduce the amount of the enzyme in cells, resulting in different levels of enzymatic activity. The global distribution of mutations correlates with historically recorded distributions of the disease [1, 7].
Infections with the malaria parasites Plasmodium vivax and Plasmodium ovale require the administration of primaquine, which is the only registered drug used to eliminate the dormant liver stages and achieve radical cure of the disease [8]. However, this drug may cause severe hemolysis in G6PD-deficient patients, thus limiting its use in vivax-endemic populations [9]. Thus, prior screening for G6PD deficiency in vivax patients is required for delivering primaquine treatment. The commonly used diagnostic methods for G6PD deficiency either measure the enzymatic activity or rely on the detection of the causative mutations. Several methods for the detection of G6PD mutations have been reported. These include PCR-restriction length polymorphism technique [10, 11], amplification refractory mutation system [12, 13], denaturing high performance liquid chromatography, single-stranded conformation polymorphism analysis [14, 15], PCR high-resolution melting assay [16, 17], PCR TaqMan assay [18], denaturing gradient gel electrophoresis [19] and DNA sequencing [20]. However, these methods are of low throughput and incur relative high test costs. In recent years, several methods for multiplexing detection of G6PD mutant alleles have been developed. The iPlex genotyping assay allows the highest level of multiplicity by including 68 G6PD SNPs [21], but this assay is limited by instrumentation requirement. Gold nanoparticle technology was also validated for designing a multiplex detection method for G6PD genotyping, but it includes a rather sophisticated probe synthesis step [22]. A reverse dot blot assay first developed to detect six G6PD mutations [23], and it has been expanded recently to detect a total of 20 G6PD mutations [24].
In this study, we adapted a multiplex SNaPshot technique and designed primers to allow simultaneous interrogation of multiple common G6PD mutations. The SNaPshot method, first developed by Smith et al. [25], is a minisequencing or primer extension technique. With this approach, multiplex extension primers with different lengths and labeled with four fluorescently labeled dNTPs (A, T, G and C) of four colors (green, red, blue and black) allow the detection of different single nucleotide polymorphisms (SNPs) shown as single-peak fluorescence wave forms in an electropherogram on an automated sequencer. It has been utilized to screen SNPs with high accuracy and effectiveness and enables rapid detection of mutations in both homozygotes and heterozygotes [26–31]. Here we designed primers for the detection of 11 G6PD SNPs commonly present in Southeast Asian human populations. We validated this multiplexing method for genotyping G6PD using G6PD deficient samples identified by a fluorescent spot test (FST). Finally, the accuracy of this assay was tested by direct sequencing analysis of target G6PD mutant alleles.
MATERIALS AND METHODS
Study subjects and ethical clearance
In an effort to screen for G6PD deficiency in a vivax malaria endemic region in northeast Kachin State of Myanmar along the China-Myanmar border, we identified 120 unrelated individuals (70 females) with G6PD deficiency, which was measured using a FST. Briefly, 0.1 mL of finger-prick blood of 120 samples was collected from each individual into an EDTA anti-coagulation tube. The detection of G6PD deficiency was performed using a FST kit following the manufacturer’s instructions (Micky Ltd, Guangzhou, China). Fluorescence was visualized under a UV light (365 nm) and samples showing weak or no fluorescence after incubation at 37°C for 30 min were considered G6PD deficient. Blood samples from G6PD deficient individuals were preserved on Whatman 3 filter paper at −20°C for DNA extraction. Recruitment of the study population was approved by the Institutional Review Board of Kunming Medical University (IRB approval #KMC2011-01). All participants provided written informed consent. For minors 15–18 years old, written assent from each participant and consent from his/her parent or guardian were obtained.
DNA extraction
Genomic DNA was extracted from ~100 μL of blood preserved on filter paper with the TaKaRa Genomic DNA Kit (TaKaRa Biotechnology) according to the manufacturer’s instructions. DNA was eluted in 50 μL of water and used for PCR. All 120 samples were used to establish the SNaPshot assay, while 48 randomly selected samples were used in direct DNA sequencing analysis to validate the assay results.
Design of the SNaPshot multiplex primers
The primers for PCR amplification (Tables 1) and SNaPshot extension reactions (Table 2) were designed using Primer Premier 5 and synthesized by Generay Biotechnology (Generay, Shanghai). BLAST searches were performed with each of the primer sequences to ensure no non-specific binding of the primers. The extension primers were designed to anneal to the DNA strand immediately adjacent to the mutations site. Each primer was synthesized with a different length of poly (dT) tail to allow separation of SNaPshot products on the basis of differing sizes. All extension primers were purified by polyacrylamide gel electrophoresis. Amplification of the 11 target sites in the G6PD gene was done in three multiplex PCR groups. The first group includes four SNP sites (487 G>A, 592 C>T, 871 G>A, and 1024 C>T); the second group includes five SNP sites (93 T>C, 1311 C>T, 1360 C>T, 1376 G>T and 1388 G>A); and the third group include 95 A>G and 392 G>T.
Table 1.
Primer sequences for PCR amplification of the target regions in G6PD gene
| Mutations | Substitutions | dbSNP Ref. ID | Primer sequence (5′ → 3′) | Size (bp) | |
|---|---|---|---|---|---|
| Group A | Mahidol | 487 G >A | rs137852314 | TGAATGATGCAGCTCTGATCC | 293 |
| Coimbr | 592 C>T | rs137852330 | CCAGGTGAGGCTCCTGAGTAC | ||
|
| |||||
| Viangchan | 871 G>A | rs137852327 | CCAACTCAACACCCAAGGAGC | 280 | |
| Chinese | 1024 C>T | rs137852342 | GGCATGCCCAGTTCTGCCTTG | ||
|
| |||||
| Group B | Canton | 1376 G >T | rs72554665 | ||
| Union | 1360C>T | rs398123546 | TGGCATCAGCAAGACACTCTC | 384 | |
| Kaiping | 1388 G>A | rs72554664 | GGAGAGGCATGAGGTAGCTCC | ||
| IVS1193 T >C | rs2071429 | ||||
| 1311 C>T | rs2230037 | ||||
|
| |||||
| Group C | Gaohe | 95 A>G | rs137852340 | TGGCAGAGCAGGTGGCCCTGA | 178 |
| GCTGAGGCATGGAGCAGGCAC | |||||
| Chinese | 392 G>T | rs137852341 | AACTCCTATGTGGCTGGCCAG | 164 | |
| CTCATGCAGGACTCGTGAATG | |||||
Table 2.
Primer sequences for the SNaPshot extension reactions.
| Mutations | Extension primer sequence (5′ → 3′) | Size (bp) | |
|---|---|---|---|
| Group 1 | 487 G>A | GCAGCTCCGGGCTCCCAGCAGA | 22 |
| 592 C>T | TTTTTTGTTCCGTGAGGACCAGATCTAC | 28 | |
| 871 G>A | TTTTTTTTCTTGGCTTTCTCTCAGGTCAAG | 30 | |
| 1024 C>T | TTTTTTTTTTTTCGCCACTTTTGCAGCCGTCGTC | 34 | |
| Group 2 | 1376 G>T | TGTCCCCTCAGCGACGAGCTCC | 22 |
| 1360C>T | TTCCGGCAGCTGGGCCTCACCTGC | 24 | |
| 1388 G> A | TTTTTTGTGCAGCAGTGGGGTGAAAATA | 28 | |
| IVS 93 T>C | TTTTTTTTTTTTTTCCACCGGCCTCCCAAGCCATAC | 36 | |
| 1311 C>T | TTTTTTTTTTTTTTTTAGACGTCCAGGATGAGGCGCTC | 38 | |
| Group 3 | 95 A>G | GCCTTCCATCAGTCGGATACAC | 22 |
| 392 G>T | TTTTTTTTCACATGAATGCCCTCCACCTGG | 30 |
Multiplex PCR amplification
Three multiplex PCR reactions were designed and optimized so that each of the 11 mutation positions of the G6PD gene was amplified in 1–2 fragments, with sizes ranging from 164 bp to 384 bp encompassing each mutation (Table 1). The 15 μL PCR reaction consisted of 20 ng of genomic DNA, 200 μmol/L dNTP, 200 pmol/L each primer, 2 U of Platinum Taq DNA polymerase (Fermentas, Canada), 2 mM MgCl2, and 10×PCR buffer (Mg2+ free) provided by the manufacturer. Amplification was performed using an EDC-810 PCR Thermal Cycler (Eastwin Life Sciences, Inc) under the following conditions: an initial denaturation step at 95°C for 3 min; 11 cycles of 94°C for 15 s, 62°C for 15 s decreasing 0.5 °C every cycle, and 72°C for 30 s; 24 cycles of 94°C for 15 s, 56°C for 15 s, and 72°C for 30 s; followed by 3 min of final extension at 72°C. After amplification, 5 μL of the PCR product was analyzed on a 1.5% agarose gel to check for product integrity.
SNaPshot primer extension reactions
The multiplex PCR products were purified to remove remaining primers and dNTPs through the following procedure. A 7 μL purification reaction consisted of 3 μL of PCR product, 0.8 μL of 1 U/μL of FastAP (Fermentas), and 0.2 μL of 20 u/μL ExoI (Fermentas). The reaction was incubated at 37°C for 15 min, then at 80°C for 15 min to inactivate the enzymes. The extension reaction was performed using the SNaPshot mix in an EDC-810 PCR Thermal Cycler with 1 μL of SNaPshot ready reaction mix, 0.1 μL of extension primer for each mutation and 2 μL of purified PCR products in a 6 μL total volume. Extension reaction was performed for 30 cycles of 96°C for 10 s, 52°C for 5 s, and 60°C for 30 s. Genotyping of the G6PD mutations relies on four different fluorochromes and controlled extension products sizes (final extension product sizes ranged between 22 and 38 bp). After extension, 1 μL of product was mixed with 9.5 μL of Hi-Di™ formamide and 0.5 μL of GeneScan 120 LIZ size standard (Applied Biosystems, USA). This mixture was denatured at 95°C for 3 min and chilled immediately on ice. The fluorescently labeled products were separated by capillary electrophoresis on an ABI PRISM 3730 DNA Sequencer. Sizes of the extension products were analyzed by using the GeneScan Analysis Software version 3.7 (Applied Biosystems).
RESULTS AND DISCUSSION
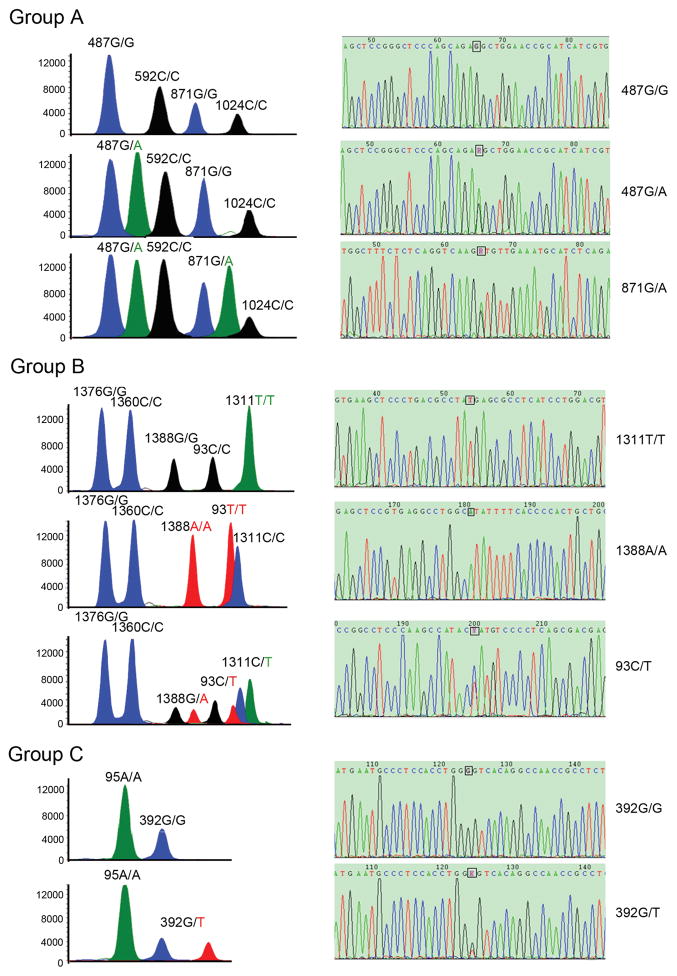
Traditional methods for genotyping G6PD mutations normally analyze one mutation at a time. To increase throughput, we adapted the SNaPshot assay using three multiplex PCR coupled with primer extension reaction to interrogate 11 G6PD mutation sites at the same time. The assignment of the alleles was based on both migration and color scheme of the extension products. Figure 1 shows representative electropherogram of the extension products of female participants in order to show the capability of this method to distinguish homozygous versus heterozygous alleles. The assay clearly distinguished female homozygotes from heterozygotes. For example, in a group A reaction, the individual shown in the top graph is homozygous for all four nucleotide positions of 487G/G, 592C/C, 871G/G, and 1024C/C. In comparison, the 487G/A heterozygote appeared as two adjacent peaks of different colors (group A, middle and bottom graphs). Interestingly, an individual in group A (bottom graph) was heterozygous at both 487G>A and 871G>A. The results demonstrated that different genotypes of the G6PD mutations could be unequivocally identified by easily interpreted peak retention time and colors.
Figure 1.
Electropherograms of the SNaPshot assay obtained from the three multiplex primer extension groups (left panels) and direct sequencing of the PCR products of the target alleles (right panels). In the left panels, G6PD mutations were identified on the basis of peak retention time and colors. The A, G, T, and C alleles are represented by the green, blue, black, and red colors, respectively. The homozygous alleles yield only one peak, whereas heterozygous alleles yield double peaks. The right panels show direct sequencing validation of the SNaPshot G6PD gene mutations. The location of each SNP site is boxed, which corresponds to the single and double sequencing peaks in homozygous and the heterozygous individuals, respectively.
To evaluate the specificity and sensitivity of the SNaPshot assay for the detection of G6PD mutations, we carried out direct sequencing analysis of the PCR products in a blind study. Sequencing analysis of 48 randomly selected samples for direct sequencing of the PCR products revealed 100% concordance between the sequencing analysis and the SNaPshot assay. Representative sequencing chromatograms are shown in Figure 1 (right panels).
In conclusion, the SNaPshot assay described here will be a useful method for screening G6PD mutations at a higher throughput. It allows simultaneous detection of multiple G6PD point mutations in both homozygous and heterozygous individuals with high accuracy. Moreover, it is simple to design and can easily include additional G6PD mutation sites present in other geographic regions. It can be combined with enzyme-based tests such as the FST to quickly establish the genetic basis of G6PD deficiency in a large human population.
Acknowledgments
This work was supported by National Natural Science Foundation of China (# 31260264), High talent introduction project of Yunnan province (# 2013HA026), and National Institutes of Health, USA (U19AI089672).
Footnotes
Yongshu He: Department of Cell Biology and Medical Genetics, Kunming Medical University, 1168 West Chunrong Road, Kunming, Yunnan Province 650500, China.
AUTHOR CONTRIBUTIONS
LZ and YY performed most of the research. RL, QL, FY, and LM participated in the data analysis and validation. HL, XC, and ZY contributed essential reagents and sample collections. LC and YH designed the research and wrote the paper.
References
- 1.Beutler E. G6PD: population genetics and clinical manifestations. Blood Rev. 1996;10:45–52. doi: 10.1016/s0268-960x(96)90019-3. [DOI] [PubMed] [Google Scholar]
- 2.Beutler E. G6PD deficiency. Blood. 1994;84:3613–36. [PubMed] [Google Scholar]
- 3.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 4.Peters AL, Van Noorden CJ. Glucose-6-phosphate dehydrogenase deficiency and malaria: cytochemical detection of heterozygous G6PD deficiency in women. J Histochem Cytochem. 2009;57:1003–11. doi: 10.1369/jhc.2009.953828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutler E, Vulliamy TJ. Hematologically important mutations: glucose-6-phosphate dehydrogenase. Blood Cells Mol Dis. 2002;28:93–103. doi: 10.1006/bcmd.2002.0490. [DOI] [PubMed] [Google Scholar]
- 6.Minucci A, Moradkhani K, Hwang MJ, Zuppi C, Giardina B, Capoluongo E. Glucose-6-phosphate dehydrogenase (G6PD) mutations database: Review of the “old” and update of the new mutations. Blood Cells Mol Dis. 2012;48:154–65. doi: 10.1016/j.bcmd.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Frank JE. Diagnosis and management of G6PD deficiency. Am Family Phy. 2005;72:1277–82. [PubMed] [Google Scholar]
- 8.White NJ. Primaquine to prevent transmission of falciparum malaria. Lancet Infect Dis. 2013;13:175–81. doi: 10.1016/S1473-3099(12)70198-6. [DOI] [PubMed] [Google Scholar]
- 9.Baird JK. Primaquine toxicity forestalls effective therapeutic management of the endemic malarias. Int J Parasitol. 2012;42:1049–54. doi: 10.1016/j.ijpara.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Al-Musawi BM, Al-Allawi N, Abdul-Majeed BA, Eissa AA, Jubrael JM, Hamamy H. Molecular characterization of glucose-6-phosphate dehydrogenase deficient variants in Baghdad city-Iraq. BMC Hematol. 2012;12:4. doi: 10.1186/1471-2326-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laouini N, Bibi A, Ammar H, Kazdaghli K, Ouali F, Othmani R, et al. Glucose-6-phosphate dehydrogenase deficiency in Tunisia: molecular data and phenotype-genotype association. Mol Biol Rep. 2013;40:851–6. doi: 10.1007/s11033-012-2124-8. [DOI] [PubMed] [Google Scholar]
- 12.Ren X, He Y, Du C, Jiang W, Chen L, Lin Q. A novel mis-sense mutation (G1381A) in the G6PD gene identified in a Chinese man. Chin Med J. 2001;114:399–401. [PubMed] [Google Scholar]
- 13.Maffi D, Pasquino MT, Caprari P, Caforio MP, Cianciulli P, Sorrentino F, et al. Identification of G6PD Mediterranean mutation by amplification refractory mutation system. Clin Chim Acta. 2002;321:43–7. doi: 10.1016/s0009-8981(02)00098-0. [DOI] [PubMed] [Google Scholar]
- 14.Calabro V, Mason P, Filosa S, Civitelli D, Cittadella R, Tagarelli A, et al. Genetic heterogeneity of glucose-6-phosphate dehydrogenase deficiency revealed by single-strand conformation and sequence analysis. Am J Hum Genet. 1993;52:527. [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng C-P, Huang C-L, Chong K-Y, Hung I-J, Chiu DT-Y. Rapid detection of glucose-6-phosphate dehydrogenase gene mutations by denaturing high-performance liquid chromatography. Clin Biochem. 2005;38:973–80. doi: 10.1016/j.clinbiochem.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Yan J-b, Xu H-p, Xiong C, Ren Z-r, Tian G-l, Zeng F, et al. Rapid and reliable detection of glucose-6-phosphate dehydrogenase (G6PD) gene mutations in han chinese using high-resolution melting analysis. J Mol Diagn. 2010;12:305–11. doi: 10.2353/jmoldx.2010.090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan M, Lin M, Yang L, Wu J, Zhan X, Zhao Y, et al. Glucose-6-phosphate dehydrogenase (G6PD) gene mutations detection by improved high-resolution DNA melting assay. Mol Biol Rep. 2013;40:3073–82. doi: 10.1007/s11033-012-2381-6. [DOI] [PubMed] [Google Scholar]
- 18.Hsu J, Fink D, Langer E, Carter ML, Bengo D, Ndidde S, et al. PCR-based allelic discrimination for glucose-6-phosphate dehydrogenase (G6PD) deficiency in Ugandan umbilical cord blood. Ped Hematol Oncol. 2014;31:68–75. doi: 10.3109/08880018.2013.860649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam VM, Huang W, Lam ST, Yeung CY, Johnson PH. Rapid detection of common Chinese glucose-6-phosphate dehydrogenase (G6PD) mutations by denaturing gradient gel electrophoresis (DGGE) Genetic Anal Biomol Eng. 1996;12:201–6. [PubMed] [Google Scholar]
- 20.Matsuoka H, Wang J, Hirai M, Arai M, Yoshida S, Kobayashi T, et al. Glucose-6-phosphate dehydrogenase (G6PD) mutations in Myanmar: G6PD Mahidol (487G> A) is the most common variant in the Myanmar population. J Hum Genet. 2004;49:544–7. doi: 10.1007/s10038-004-0187-7. [DOI] [PubMed] [Google Scholar]
- 21.Maiga B, Dolo A, Campino S, Sepulveda N, Corran P, Rockett KA, et al. Glucose-6-phosphate dehydrogenase polymorphisms and susceptibility to mild malaria in Dogon and Fulani, Mali. Malar J. 2014;13:270. doi: 10.1186/1475-2875-13-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seow N, Lai PS, Yung LY. Gold nanostructures for the multiplex detection of glucose-6-phosphate dehydrogenase gene mutations. Anal Biochem. 2014;451:56–62. doi: 10.1016/j.ab.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Lu X, Hua L, Zhang T, Li S, Fan X, Peng Q, et al. A reverse dot blot assay for the expanded screening of eleven Chinese G6PD mutations. Clin Chim Acta. 2013;418:45–9. doi: 10.1016/j.cca.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 24.Peng Q, Li S, Ma K, Li W, Ma Q, He X, et al. Large cohort screening of G6PD deficiency and the mutational spectrum in the Dongguan District in Southern China. PloS One. 2015;10:e0120683. doi: 10.1371/journal.pone.0120683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith WM, Van Orsouw NJ, Fox EA, Kolodner RD, Vijg J, Eng C. Accurate, high-throughput “snapshot” detection of hMLH1 mutations by two-dimensional DNA electrophoresis. Genet Test. 1998;2:43–53. doi: 10.1089/gte.1998.2.43. [DOI] [PubMed] [Google Scholar]
- 26.Esteves LM, Bulhões SM, Brilhante MJ, Mota-Vieira L. Three multiplex snapshot assays for SNP genotyping in candidate innate immune genes. BMC research notes. 2013;6:54. doi: 10.1186/1756-0500-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sagong B, Baek J-I, Oh S-K, Na KJ, Bae JW, Choi SY, et al. A rapid method for simultaneous screening of multi-gene mutations associated with hearing loss in the Korean population. PLoS One. 2013;8:e57237. doi: 10.1371/journal.pone.0057237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertoncini S, Blanco-Rojo R, Baeza C, Arroyo-Pardo E, Vaquero MP, López-Parra AM. A novel SNaPshot assay to detect genetic mutations related to iron metabolism. Genet Test Mol Biomar. 2011;15:173–9. doi: 10.1089/gtmb.2010.0140. [DOI] [PubMed] [Google Scholar]
- 29.Edelmann J, Schumann S, Nastainczyk M, Husser-Bollmann D, Lessig R. Long QT syndrome mutation detection by SNaPshot technique. Int J Legal Med. 2012;126:969–73. doi: 10.1007/s00414-011-0598-x. [DOI] [PubMed] [Google Scholar]
- 30.Ekici AB, Hackenbeck T, Morinière V, Pannes A, Buettner M, Uebe S, et al. Renal fibrosis is the common feature of Autosomal Dominant Tubulointerstitial Kidney Diseases caused by mutations in mucin 1 or uromodulin. Kidney Int. 2014;86:589–599. doi: 10.1038/ki.2014.72. [DOI] [PubMed] [Google Scholar]
- 31.Lai G, Zhang W, Tang H, Zhao T, Wei L, Tao Y, et al. A SNaPshot assay for the rapid and simple detection of hepatitis B virus genotypes. Mol Med Rep. 2014;10:1245–51. doi: 10.3892/mmr.2014.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]