Abstract
The discovery of the TRPML subfamily of ion channels has created an exciting niche in the fields of membrane trafficking, signal transduction, autophagy, and metal homeostasis. The TRPML protein subfamily consist three members, TRPML1, -2, and -3, which are encoded by MCOLN1, -2, and -3 genes, respectively. They are non-selective cation channels with six predicted transmembrane domains, and intracellular amino- and carboxyl-terminus regions. They localize to the plasma membrane, endosomes, and lysosomes of cells. TRPML1 is associated with the human lysosomal storage disease known as Mucolipidosis type IV (MLIV), but TRPML2 and TRPML3 have not been linked with a human disease. Although TRPML1 is expressed in many tissues, TRPML3 is expressed in a varied but limited set of tissues, while TRPML2 has a more limited expression pattern where it is mostly detected in lymphoid and myeloid tissues. This review focuses on TRPML2 because it appears to play an important, yet unrecognized role in the immune system. While the evidence has been mostly indirect, we present and discuss relevant data that strengthen the connection of TRPML2 with cellular immunity. We also discuss the functional redundancy between the TRPML proteins, and how such features could be exploited as a potential therapeutic strategy for MLIV disease. We present evidence that TRPML2 expression may complement certain phenotypic alterations in MLIV cells, and briefly examine the challenges of functional complementation. In conclusion, the function of TRPML2 still remains obscure, but emerging data show that it may serve a critical role in immune cell development and inflammatory responses.
Keywords: B lymphocytes, Mucolipidosis IV, endosomes, lysosomes, MCOLN2, PAX5
Introduction
A subfamily of the transient receptor potential (TRP) superfamily known as the Mucolipins (TRPMLs) consists three proteins that share high sequence homology with each other, namely: TRPML1, -2 and -3 (encoded by the MCOLN1, -2 and -3 genes, respectively). The TRPMLs are non-selective cation channels that contain a predicted six transmembrane (TM) domain with cytosolic amino (N)- and carboxyl (C)-termini, as well as a channel pore located between TM5 and TM6 that is permeable to sodium (Na+) and calcium ions (Ca2+) [39, 40, 60, 115]. The TRPMLs exist as homomeric or heteromeric channels [24, 117], and are believed to play a role in endosome-lysosome fusion, scission of endo-lysosomal hybrid organelle, autophagy, vacuolar pH regulation, exocytosis, and metal homeostasis [17, 31, 53, 59, 64, 78, 87, 117]. TRPML1 was first identified due to its link with Mucolipidosis type IV (MLIV), a lysosomal storage disorder that mainly affects the cells of the brain, the eyes, and the stomach. TRPML1 (MCOLN1) is widely expressed in many tissues, while TRPML2 (MCOLN2) is highly expressed in lymphoid, and myeloid tissues [41, 89]. TRPML3 (MCOLN3) also has a tissue-specific expression, but appears to have a broader mRNA expression pattern than that of TRPML2. A case in point, TRPML3 expression has been detected in hair cells and strial cells of the inner ear, olfactory and vomeronasal sensory neurons [15], cells of the kidney, lung, and thymus, as well as skin melanocytes, and neonatal intestinal enterocytes [87]. Loss-of-function mutations or deletions in TRPML1 cause the human MLIV disease [7, 10, 100], while gain-of-function mutations in TRPML3 result in the varitint-waddler mouse phenotypes [26]. Interestingly, a recent report showed that the loss of function mutations in both TRPML1 and TRPML3 produce intestinal abnormalities and failure to thrive in neonatal mice [87]. So far, TRPML2 has neither been implicated in any human nor mouse disease phenotype. Despite the scarcity of research in the field, recent works suggest that TRPML2 may play an important role in the immune system. Here, we present and discuss relevant information that links immune cell maturation and function with TRPML2 (MCOLN2) expression. We also discuss the possibility of exploiting the functional redundancy between the TRPML proteins in the context of MLIV disease therapeutics. More specifically, we propose that a gene complementation approach may ameliorate some of the symptoms in MLIV and consider the challenges of using TRPML2 to functionally substitute for the missing TRPML1 in MLIV-affected cells.
The Transient Receptor Potential Mucolipin (TRPML) Ion Channel Subfamily
TRPML1 is, by and large, the most studied member of the TRPML protein subfamily, primarily due to its association with MLIV in which distinct point mutations or deletions in the MCOLN1 gene disables its normal function [7, 10, 100]. This autosomal recessive disorder is clinically manifested by developmental delays, cognitive and motor problems, insufficient gastric acid production, and progressive visual impairment due to cataract and retinal degeneration [1]. At the cellular level, MLIV disease is characterized by vacuolar formation and over-acidified lysosomes [97], as well as accumulation of lipids and proteins in these structures that vary in composition or feature depending on the specific cell type analyzed [5, 103]. TRPML1 is ubiquitously expressed across many tissues [41]. It is an inwardly-rectifying ion channel that is activated by phosphatidyl-inositol (3,5)-bisphosphate (PIP2) [29], and is permeable to sodium ions (Na+) as well as many divalent metal ions, but not trivalent metal ions [115]. It contains N- and C-termini di-leucine motifs necessary for lysosomal localization and adaptor protein-2 mediated internalization, respectively [86, 111]. Despite recent identification of several protein interactors for TRPML1 [21, 98, 109, 110], its cellular function remains poorly understood. What appears to be a common observation is that TRPML1 plays a role in metal ion homeostasis [21, 28, 31, 64], membrane trafficking [21, 41], and exocytosis of intracellular contents [66, 88] via the endosomal-lysosomal pathway. Recently, the forced induction of TRPML1 channel activity through an agonist or synthetic ligand (ML1-SA) has been shown to rescue phenotypes of various diseased models of Charcot-Marie-Tooth disease Type 4J and human immunodeficiency virus-induced dementia [6, 118]. Thus, the ability of TRPML1 to rescue disease phenotypes involving lysosomes add to a growing evidence of its importance in normal lysosomal physiology.
Both the TRPML2 and TRPML3 proteins were discovered using positional cloning during the search for the varitint-waddler (Va) mutation in mice [26]. While TRPML2 is largely detected in immune cells, TRPML3 is expressed by a wider variety of cell types of the skin, vomeronasal organ, as well as auditory and olfactory systems [15, 82]. TRPML3 is also detected in other tissues such as kidney, thymus, spleen, lung, intestine, and colon [23, 41, 87, 89]. TRPML3 is an inwardly rectifying channel that heteromerizes with other members of the TRPML subfamily [24] but in a limited manner, since it was reported to only partially co-localize with its relatives in terms of its presence in the lysosomes and other vesicular compartments [116]. The TRPML3-associated mouse Va mutation is an alanine to proline substitution at amino acid (AA) position 419 (TRPML3-A419P) [26], which causes abnormal coat pigmentation, hearing loss and vestibular dysfunction in mice. This is because melanocytes that specifically express the TRPML3-A419P protein in their melanosomes die via a calcium ion (Ca2+) overload [39, 58, 82] possibly due to a constitutively open and expanded channel pore [60] induced by the proline kink in the TM5 domain of TRPML3-A419P protein (see Ref. [23] for a review). Adding weight to the proline-induced kink hypothesis is the observation of constitutive channel activity that results in cytotoxicity when a critical region of the TM5 domain of TRPML1, TRPML2, TRP Vanilloid 5 (TRPV5), and TRPV6 ion channels is replaced with a proline amino acid residue [39, 58, 60, 68, 69, 115]. In contrast, genetic approaches to prevent the pore from opening [23] or from reaching the plasma membrane (PM) by mis-localization [39], as well as precluding calcium entry via a loss-of-function mutation targeting the acidic residues of the selectivity filter near the pore [58, 115] have all been successful in rescuing the cell phenotype of the Va mutation. Interestingly, a spontaneous mutation in the Va mice in which an isoleucine became substituted by a threonine at position 362 (TRPML3-I362T/A419P) conferred a milder disease phenotype in the animal that might be due to mis-localization of the double mutant protein when heterologously expressed in human embryonic kidney (HEK)-293 cells [39]. It is yet to be shown, however, that such mis-localization of the TRPML3-I362T/A419P ion channels in vivo is the main reason for the milder Va mouse phenotype.
TRPML2 appears to be somewhat neglected since it is the least studied TRPML subfamily member, due in part to its lack of direct link to a disease phenotype. Unlike TRPML1 and TRPML3, TRPML2 is also predominantly expressed in lymphoid and myeloid tissues, and possibly in certain cell types of the kidney [41, 89]. The structural similarities between TRPML1 and TRPML2 enable the formation of functional heteromeric complexes in the lysosomal membrane [24], but similar to TRPML3, it only partially co-localizes with its relatives with respect to its distribution in lysosomal and extra-lysosomal compartments [116]. Calcium imaging and electrophysiological studies of heterologously expressed TRPML2 mouse homologue containing the Va mutation showed that TRPML1 and TRPML2 mutant proteins have relatively more similar channel features than TRPML3 (Fig. 1) [89]. Like its relatives, TRPML2 is an inwardly rectifying ion channel [24, 28]. It works as a Ca2+-permeable cation channel and is regulated by extracytosolic proton (H+), but not by Ca2+ levels [28, 69], or linoleic acid, which is known to activate other TRP channels [69]. Recent studies, however, show that like TRPML1 and TRPML3, TRPML2 is also activated by PIP2 [29]. Two isoforms of mouse TRPML2 exist, a short variant and a long variant, but only the short variant shows markedly high expression levels in lymphoid organs and the kidney [89]. Some expression has also been indicated in the lateral wall of the cochlea, vestibular sensory cells, and spiral ganglion cells [101]. TRPML2 has been observed to localize in the long tubular recycling endosome, lysosomes and PM [53, 69, 107]. Meanwhile, the presence of TRPML2 in the PM has been confirmed through electrophysiological recordings of channel activity in cells following exposure to specific small molecule agonists such as SF-21 (4-chloro-N-(2-morpholin-4-ylcyclohexyl)benzenesulfonamide), SF-41 (1-(2,4-dimethylphenyl)-4-piperidin-1-ylsulfonylpiperazine), and SF-81 (4,6-dimethyl-3-(2-methylphenyl) sulfonyl-1-propan-2-ylpyridin-2-one) [40, 41]. Additionally, homomeric TRPML2 ion channels within the PM have unique channel properties that could serve a completely different function than heteromeric TRPML2-TRPML1 channels possibly be detected in the lysosomes of certain cells of the immune system [33, 35, 116].
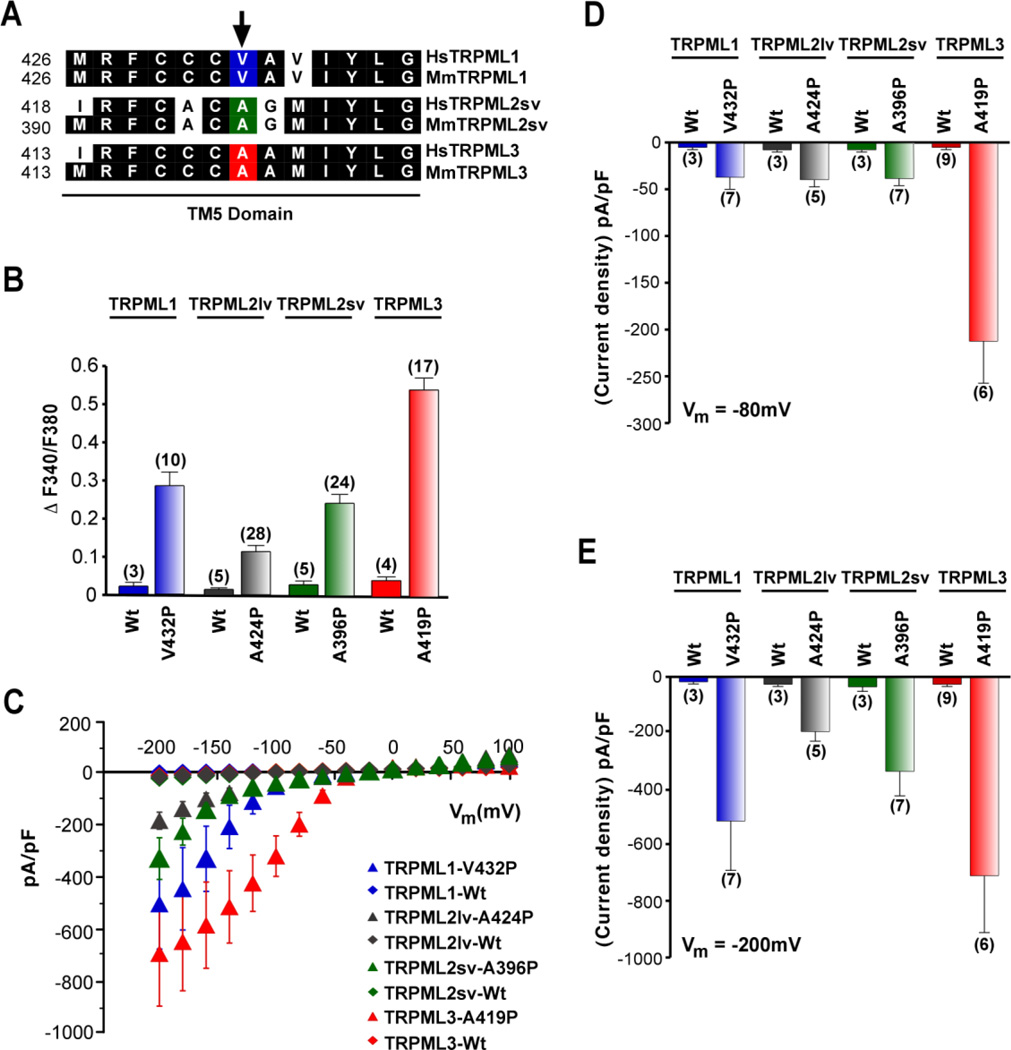
Figure 1. Constitutive activity of TRPML varitint-waddler (Va) mutant isoforms.
(A) Protein sequence alignment of the inner half of TM5 of human and mouse TRPML channels. Conserved amino-acid residues are highlighted in black. Arrow indicates position of the varitint-waddler mutation A419P in TRPML3 and equivalent positions in TRPML1 and TRPML2. (B) Ca2+-imaging experiments showing intracellular Ca2+ levels of HEK-293 cells expressing either wild-type (WT) mouse TRPML1, TRPML2lv (long variant), TRPML2sv (short variant), or TRPML3, and TRPML mutant isoforms TRPML1-V432P, TRPML2lv-A424P, TRPML2sv-A396P, and TRPML3-A419P. The TRPML1 and TRPML2 mutant isoforms contain a proline substitution on TM5 that is equivalent to A419P of the varitint-waddler mutant of TRPML3. All experiments were performed 15–20 hours after transfection. Mean values ± SEM, n = number in parenthesis. (C) Steady-state current-voltage plots of constitutively active whole-cell currents elicited by TRPML1-V432P, TRPML2sv-A396P, TRPML2lv-A424P as well as TRPML3-A419P mutant compared to their respective WT isoforms in response to 10 ms voltage steps from a holding potential (Hp) of +10 mV between −200 mV and +100 mV in 20 mV incremental steps, normalized by cell capacitance (pF). (D) and (E) Average inward current densities of the TRPML-Va mutant and WT isoforms shown in C tested at −80 mV and −200 mV shown in D and E, respectively. All values were normalized by cell capacitance (pF). Mean values ± SEM, n = numbers in parenthesis. Reprinted with permission from Ref. [89]: Samie et al., (2009), Pflugers Archiv – European Journal of Physiology, 459, 79–91. Copyright 2009 Springer-Verlag.
Development and Function of Specific Immune Cells: A Brief Overview
B Cell Lymphocytes
Plasma and memory B cells are essential components of the adaptive immune system. These cells are heavily regulated and required to pass extensive checkpoints through their development to differentiate into plasma B cells that are capable of aiding in the destruction of foreign antigen [79]. B cells are derived from hematopoietic stem cells, and once committed, the lymphoid precursor cell can enter the different phases in B cell development that are distinguished by activation of specific transcription factors and expression of protein markers (e.g. the pre-B cell receptor [BCR]) [2, 18, 25, 77]. Interestingly, B cell Specific Activator Protein (BSAP, also known as paired box 5 [PAX5]) has been identified as an essential transcription factor in the maintenance of the B cell lineage [18, 47, 85]. Indeed, abrogation of BSAP/PAX5 protein expression during a critical period of differentiation or upon B cell maturation results in de-differentiation of the mature B cells into pro-B cell stage with the potential to become T cells [18]. Thus, BSAP/PAX5 is not only important for B cell progenitor commitment, but also for the maintenance of B cell identity [47].
The signaling cascade activated by the pre-BCR is suggested to be identical to the signaling pathway in BCRs of mature B cells [42, 46]. BCRs are activated upon the binding of foreign antigen, but at the pre-B cell stage antigen binding to pre-BCR is detrimental, which implies that the pre-B cells must have other ligands bound to their receptors. Nevertheless, the binding of the pre-BCR ligand to the receptor leads to the activation of Yamaguchi sarcoma virus related homolog (LYN) (Fig. 2), a protein tyrosine kinase that phosphorylates the immunoreceptor tyrosine-based activation motifs (ITAM) found on the Igα and Igβ heterodimers [46]. The spleen tyrosine kinase (SYK) protein recognizes the phosphorylated ITAMs that in turn form docking sites where SYK can physically associate with the scaffolding heterodimers. This interaction causes the auto-phosphorylation of SYK, and the activated SYK can now phosphorylate other components of the signaling network, such as the scaffold protein B cell linker (BLNK), which is phosphorylated at multiple tyrosine residues. Once activated, BLNK forms docking sites for two proteins, namely, Bruton’s tyrosine kinase (BTK) and phospholipase C gamma 2 (PLCγ2). BTK is a non-receptor tyrosine kinase involved in innate and adaptive immunity [12] in which upon association with BLNK, becomes phosphorylated by SYK. The close proximity between the BTK and PLCγ2 made possible by the BLNK adaptor protein is necessary for the activation of the BTK pathway through the phosphorylation of PLCγ2; however, BTK could also be phosphorylated by LYN while tethered by phosphatidyl-inositol (3,4,5)-trisphosphate (PIP3) [81]. Hence, in the pre-B cell stage, the activation of the pre-BCR is the critical checkpoint in B cell maturation, which could follow two separate signaling cascades. The first signaling event being the activation of phosphoinositide-3-kinase (PI3K) pathway, which inhibits further heavy chain recombination, and the down-regulation of expression of distinct genes that specify and commit lymphoid progenitors to early B cell progenitors (e.g. Rag1, Rag2, E2A/TCF3, EBF1, and BSAP/PAX5) [2]. The PI3K activity also initiates the rearrangement of the light chain while promoting cell proliferation – an essential step in the pre-B cell stage [46]. The second signaling event is the activation of the BTK pathway, which promotes the down-regulation of the surrogate light chain, the recombination of the light chain immunoglobulin gene segments (Igκ), and ultimately cell differentiation, which permits the pre-B cell to enter the next phase in its development – the immature B cell stage [46]. The immature B cell undergoes negative selection to eradicate self-reactive cells prior to leaving the bone marrow [84]. Subsequently, once fully mature, the BCR signaling of B cells allows them to differentiate into plasma cells that play an essential role in adaptive immunity [80].
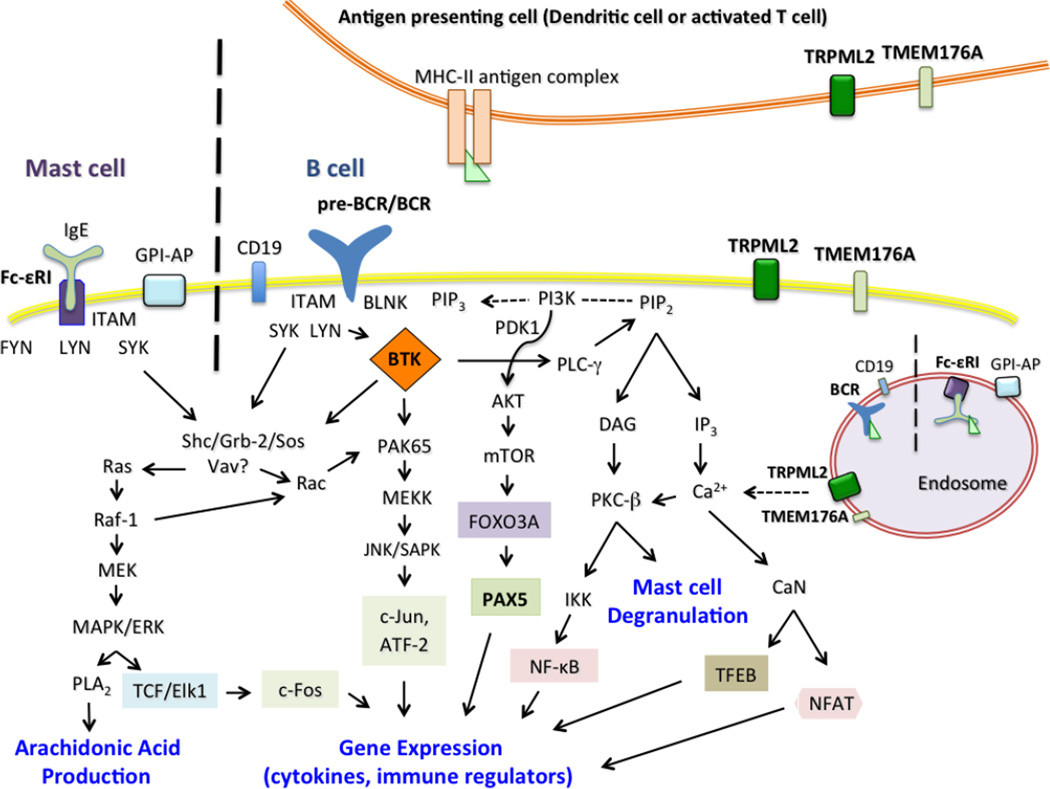
Figure 2. Convergent signaling pathways of specific immune cells implicate a role for TRPML2 ion channel in trafficking and signal transduction.
For simplicity, only the most relevant proteins in the signal transduction pathways are shown on this illustration. Proteins that are associated with TRPML2 (MCOLN2) expression are in bold text. In this model, TRPML2 has been shown to play a role in the ARF6-dependent trafficking of GPI-APs, and we propose that it serves an important function in the internalization of cell surface receptors in mast cells and B cells such as the Fc-εRI and pre-BCR/BCR, respectively. Finally, the interaction of TRPML2 with TMEM176A could potentially influence the maturation and activation of dendritic cells.
Mast Cells
Mast cells are critical effectors of type I hypersensitivity or allergic reaction [11]. These cells express the crystallizable fragment-epsilon receptor I (Fc-εRI) on their plasma membrane, and feature a considerable amount of cytoplasmic granules containing histamine, heparin, and other cytokines necessary for the inflammatory response associated with allergy [112]. The Fc-εRI protein is a high-affinity receptor of the immunoglobulin type E (IgE) ligand. IgEs are secreted by plasma cells, whereby exposure to certain types of allergens causes the production of more IgE molecules. Even though an increase in IgE secretion could instigate severe allergic reactions, it has been shown that the survival of mast cells is regulated by IgE interaction with Fc-εRI [4]. Ultimately, the cross-linking of IgE with the Fc-εRI activates a signaling cascade that subsequently results in the degranulation of the mast cell and a targeted cytokine gene expression, leading to acute or chronic inflammatory response [52].
Dendritic Cells
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) that are crucial in immune tolerance and maturation of naïve T cell lymphocytes through the activation of pattern recognition receptors such as the toll-like receptors (TLRs) and release of co-stimulatory molecules like cluster of differentiation (CD)-40, CD80, and CD86 [99]. DCs are classified into two distinct lineages according to their morphology and cell surface receptors. Classical or conventional DCs (cDCs) are different from plasmacytoid DC type (pDCs) in that they have high levels of major histocompatibility complex (MHC)-II expression and unique cell surface proteins from pDCs [99]. The pDCs have molecular and morphological characteristics that are similar to lymphocytes, in which they produce high levels of type-I interferon (IFN) following virus-mediated TLR activation [74]. In a 2010 study by Condamine and co-workers, rodent transmembrane (TMEM)-176A and TMEM176B proteins were reported to interact as evidenced by a yeast two-hybrid interaction assay [20]. They then showed that the mRNA expression levels of TMEM176A (or TMEM176B) were significantly reduced within 24 hours following maturation of DCs exposed to lipopolysaccharide (LPS; a TLR4 ligand) or poly-I:C (a TLR3 ligand). In addition, RNA interference (RNAi)-induced knockdown of TMEM176A (or TMEM176B) mRNA expression resulted in DC maturation, significant production of co-stimulatory molecules (CD40 and CD86), and activation of cultured allogeneic T cells. Conversely, over-expression (OE) of TMEM176A and TMEM176B transcripts rendered cells resistant to maturation by LPS, essentially keeping DCs in an immature state [20]. Thus, these results suggest that the TMEM176A and TMEM176B proteins play a key role in immune tolerance. One caveat about these observations is that the study only looked at changes in TMEM176A (or TMEM176B) mRNA levels and not the protein levels per se. In 2012, our laboratory confirmed the interaction between TMEM176A and TMEM176B using both biochemical and microscopy techniques [22]. Moreover, we were able to correlate abnormal accumulations of human TMEM176A and TMEM176B proteins in lymphoma, further hinting at a possible role for both proteins in immune cell tolerance, or cancer cell evasion of the immune system.
Human TMEM176A has a predicted four TM domain with extracellular or luminal N- and C-terminus regions [20]. It is highly expressed in cDCs, but not in pDCs [36]. The protein was initially discovered based on its elevated expression levels in human liver cancer [113], and kidney disease [83]. Our laboratory also found that TMEM176A protein levels were elevated in human lung cancer tissues [22]. Meanwhile, TMEM176B (also known as LR8) was first identified in human lung fibroblasts [76], and has been shown to be highly expressed in small cell lung cancer [37]. TMEM176A and TMEM176B have low or undetectable transcript levels in subsets of B lymphocyte [120]. They share 16% amino acid sequence similarity and have been classified as members of the membrane-spanning 4-domain subfamily A (MS4A) proteins [75]. Phylogenetic analyses suggest that the MS4A and TMEM176 subfamilies are evolutionary linked and are associated with the adaptive immune system [119]. Noteworthy is that the MS4A family is also related to other Fc-εRI receptors [71] such as CD20 (MS4A1; reported to mediate calcium signaling in B-cells [13], and Fc-εRIβ (MS4A2; a signal amplifier of the Fc-εRI tetrameric complex [72]).
Despite genetic [20] and biochemical [22] evidences showing that TMEM176A physically interacts with TMEM176B, the function of either protein remains poorly understood. Likewise, it is not clear how the heteromeric interaction between the two proteins modifies or influences their individual function in cells. Nevertheless, the current knowledge on their ability to block the maturation of DCs through the inhibition of co-stimulatory molecules necessary for T cell activation such as CD40, CD80, and CD86 proteins [20], make them critical players in organ transplantation and tissue tolerance. Thus, based on their evolutionary relationship with the MS4A/Fc-εRI receptor family, we propose that TMEM176A and TMEM176B play a role in immune cell tolerance by modulating the response of APCs to antigens and their maturation, thereby limiting APC interaction with T lymphocytes and consequently hindering a proper immune response. It would indeed be very interesting to determine whether TMEM176A and TMEM176B are members of an immune receptor complex, in which they could possibly serve as a scaffolding function, mediator of trafficking, or a signal amplifier just like the observed role of Fc-εRIβ subunit following Fc-εRI/IgE engagement [62].
TRPML2 and its Potential Role in Immune Cell Development, Function, and Signal Transduction Process
TRPML2 and Antigen Processing in Immune Cells
Antigen presentation is a fundamental process of the immune system. APCs are specialized cells that display MHC-II bound antigen on their surface to signal the immune system of foreign pathogens. Once a foreign antigen is internalized via phagocytosis or receptor-mediated endocytosis, it is processed by the endosome-lysosome pathway. The lysosome contains specific pH-dependent enzymes that cleave the antigen into small peptides. The antigenic peptide is then transferred to the MHC-II protein and the complex exits the endocytic pathway to allow the antigen to be displayed on the surface of the cell. Thus, the acidity of the lysosomal compartment plays a pivotal role in the processing and binding of the antigen to the MHC-II receptor. Subcellular localization analysis of mouse macrophages has shown that TRPML1 co-localizes with MHC-II [104], and by virtue of its heteromeric interactions with TRPML2 [24, 117], it is quite possible that TRPML2 also contributes to MHC-II/antigen complex formation. Notwithstanding, TRPML2 has been observed to co-localize with MHC-I [53], which makes it a potential key player in the recycling of MHC-I receptor back to the PM, and thus, antigen presentation that warns the immune system of an infection.
It is worth noting that no observable lysosomal defects in circulating lymphocytes have been reported from MLIV patients [8], which suggests that TRPML2 might functionally complement the loss of TRPML1 in the specialized compartments of these specific immune cells. In line with this observation, chicken DT-40 B lymphocytes have an intact and functional lysosome when TRPML1 expression is knocked out [96]. Song et al., (2006) were the first to suggest that the lack of MLIV-like disease phenotype in chicken DT-40 B lymphocytes knocked out of TRPML1 could be due to the presence and functional complementation conferred by TRPML2. Indeed, when both TRPML1 and TRPML2 were then knocked out, an MLIV-like disease phenotype was observed in these DT-40 B lymphocytes [96]. This finding parallels a similar phenotype defect in cultured HeLa cells when TRPML2 (or TRPML3) protein was knocked out [116]. Taken together, these data suggest that a complete loss of TRPML1 and TRPML2 functionality interferes with the normal physiological function of the lysosomes in cells. Future research on double knockouts of TRPML1 and TRPML2, or TRPML1 and TRPML3 should reveal whether phenotypic deficits are evident on the immune system of animals.
Convergence between TRPML2, Toll-like Receptors (TLR), and Interferon (IFN) Function
The TLR family consists ten proteins that specialize in the recognition of microbial and viral antigenic peptides [9]. When TLRs encounter a foreign antigen, these receptors are activated and a signaling cascade is initiated that results in the transcription of specific genes involved in innate and adaptive immunity [114]. TLR3, TLR7, TLR8 and TLR9 are strictly found in the endosomal-lysosomal membrane and are important in recognition of foreign nucleic acid [54]. TLR1, TLR2, TLR4, TLR5 and TLR6 are found on the plasma membrane, but enter the endosomal-lysosomal pathway upon receptor activation [9]. Although no evidence yet has been shown for an involvement of TRPML2 in TLR function, TRPML1 was recently implicated in immune cell function through TLR7-mediated nucleic acid (single-stranded RNA) processing and trafficking in the lysosomes of DCs [70].
IFN is part of an antiviral protective response in the immune system. A cell infected with a virus can warn nearby cells of a possible viral infection by the release of IFN. The binding of IFN to specific receptors on the cell surface of neighboring cells activates the IFN signaling network that leads to the expression of interferon-stimulated genes (ISGs), which inhibit certain cycles of viral replication to protect nearby cells from infection. The expression of these ISGs is suggested to help limit or abolish viral replication, but this is not always the case. Schoggins and his colleagues in 2011 identified several ISGs that actually enhanced viral replication. Among those genes, the expression of MCOLN2 enhanced viral replication in human signal transducers and activators of transcription 1 (STAT1)-deficient fibroblasts that were infected with yellow fever virus (YFV) or Venezuelan equine encephalitis virus (VEEV). In addition, when MCOLN2 was heterologously co-expressed with the LY6E gene (another ISG that increases viral replication) the enhancement of viral replication was even more intensified [92]. Whereas when MCOLN2 was heterologously co-expressed with C6orf150 gene (an ISG identified to thwart viral replication), the inhibitory gene (C6orf150) prevailed and reduced viral replication [92]. The release of IFN is a defense mechanism against viral infection, but in this case not all the gene targets of IFN helped diminish the infection. It would be interesting to know if the ability of TRPML2 protein to affect membrane trafficking or vesicle fusion is the mediator of enhanced YFV and VEEV replication among infected cells.
Regulation of MCOLN2 Gene Expression by the Bruton’s Tyrosine Kinase (BTK) Signaling Cascade
Patients with X-linked agammaglobulinemia (XLA), suffer from a B cell deficiency even though they have functional and normal levels of T cells [32]. These patients experience defective B cell development in which they possess B cells at the pre-B cell stage, but are unable to produce mature B and plasma cells due to failure in differentiation. XLA patients are thus susceptible to recurring bouts of infection. The XLA pathology is caused by mutations in the BTK gene [32, 81]. Interestingly, the murine X-linked immunodeficiency (Xid) mutation in mice is similar to XLA, but causes a less severe phenotype [56, 57]. Unlike XLA, the Xid mutation yields about half the number of normal circulating B cells despite also having an abnormal BTK function [57]. Although BTK loss does not affect DC maturation [34], there have been reports that its loss in XLA impairs TLR activation in DCs [95, 102]. Nevertheless, the BTK protein is an important factor for normal B cell differentiation and maturation, and partly crucial in DC function.
The BTK signaling cascade activates several downstream activation pathways (Fig. 2) upon antigen engagement of the BCR [81], or activation of TLR2, -3, -4, -8 or -9 [30, 38, 50, 67]. It is also activated upon ligation of IgE-allergen complex with the Fc-εRI complex [43]. The loss of BTK in mice impairs mast cell degranulation, increases serum IgE levels, and reduces cytokine secretion downstream of Fc-εRI activation [49]. Within the BTK signaling pathway, activation of protein kinase C (PKC) in turn triggers the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and nuclear factor of activated T cells (NFAT) transcription factors to induce gene expression [3]. NF-κB provides a feedback mechanism that induces the expression of the BTK gene [81]. Interestingly, MCOLN2 is among several genes targeted by the activation of the BTK pathway [73] and may very well play a role in the tight regulation of B cell development, since the MCOLN2 mRNA is detectable at different types of B lymphocyte cell line, as well as in a particular mast cell line [73] (Table 1). The expression of MCOLN2 mRNA was exclusively found in B cell lines expressing a functional pre-BCR or BCR such as the pre-B cell, mature B cell, or plasma cell stage [73]. Furthermore, Lindvall and colleagues showed that wild-type (WT) whole primary splenic B cells express MCOLN2 mRNA (Table 2), and that there was a significant increase in MCOLN2 expression in these cells upon stimulation with anti-IgM, which activates the BCR, or with phorbol 12-myristate 13-acetate (PMA) plus ionomycin (compounds that activate PKC). On the other hand, BTK-defective B cells showed a four-fold decrease in the amount of MCOLN2 mRNA relative to WT splenic cells [73]. Note, however, that when BTK-deficient B cells are stimulated with anti-IgM, or PMA plus ionomycin, an up-regulation of the MCOLN2 gene is also detected. Even though both WT and BTK deficient cells showed an up-regulation of the MCOLN2 mRNA expression, the amount of detectable transcripts was substantially lower in the BTK-deficient cells. Thus, the crux of the study illustrates that the disruption of the BTK pathway significantly affects the expression of the MCOLN2 gene. Future research should investigate the relationship and mechanism underlying BCR activation and TRPML2 protein expression. It would also be interesting to elucidate whether or not TRPML2 plays a critical role in B cell differentiation using RNAi-induced knockdown, or clustered regulatory interspersed sequence palindromic repeats (CRISPR)-Cas9 mediated knockout of MCOLN2 gene expression.
Table 1. MCOLN2 mRNA expression levels in mouse hematopoietic cell lines.
Adapted from Lindvall et al., 2005 [73].
| Cell line | TRPML2 Expression |
|---|---|
| A20 (Mature B-cells) | ++ |
| Yac-1 (T-cells) | − |
| NIH 3T3 (Fibroblast) | − |
| N2A (Neuroblastoma) | − |
| 5T33 (Myeloma) | +++ |
| EL-4 (T-cell) | +++ |
| P815 (Mastocytoma) | + |
| Negative control | − |
+++, High levels; ++, Intermediate levels; +, Low levels; − not detected
Table 2. MCOLN2 mRNA expression levels in wild-type and BTK-defective mice.
Adapted from Lindvall et al., 2005 [73].
| Cell type | Unstimulated | Anti-IgM | PMA + Ionomycin |
|---|---|---|---|
| Primary splenic B cells (Wild-type CBA mouse) | ++ | +++ | +++ |
| Primary splenic B-cells (BTK-defective mouse) | + | ++ | +++ |
| Negative control | − | − | − |
+++, High levels; ++, Intermediate levels; +, Low levels; − Not detected
B cell-Specific Activator Protein (BSAP) Activates MCOLN2 Gene Expression
Our research laboratory is especially interested in characterizing the transcriptional activation and regulation of MCOLN2 and MCOLN3 genes in an effort to better understand their tissue expression, genomic sequence structure, and therapeutic potential to complement the non-functional MCOLN1 gene in MLIV disease. Our analysis of the chromosome 1 region where both MCOLN2 and MCOLN3 are located shows that both genes are relatively very close to each other with predicted CpG islands (Fig. 3A). Note that the physical location of both genes suggests that they may have been duplicated from unequal crossing over rather than whole-genome duplications that created most paralogue genes in vertebrates [33, 35]. We refer the reader to two recent excellent reviews on the evolution of the TRPMLs, with a particular emphasis on TRPML2 protein [33, 35]. Focusing on MCOLN2, our analysis of its genomic DNA spanning the 5’ untranslated region (UTR) and putative promoter region upstream of the transcriptional start site (TSS) revealed two predicted CpG islands: a 630 basepair (bp) long sequence located at −963 bp upstream of the TSS, and 1,182 bp long sequence located at −780 bp that also flanks the 5’-UTR, and parts of the gene (Fig. 3A) [106]. We identified three GC boxes but no TATA box within the putative promoter region, and our subsequent dual-luciferase reporter assays showed the core promoter region to be between −79 and −52 bp upstream of the TSS [106]. Using the JASPAR and TRANSFAC online databases, we narrowed down five transcription factors (TFs) predicted to bind the DNA sequence spanning −79 and −52 bp, namely, CTCF, KLF4, MZF1, NF-κB1, and BSAP (also known as PAX5) (Fig. 3B). Real-time reverse-transcription quantitative polymerase chain reaction (RT-qPCR) and Western blot (WB) analyses indicated that BSAP/PAX5 increased the endogenous MCOLN2 mRNA and TRPML2 protein levels, respectively, upon heterologous expression of the TF in HEK-293 cells (Figs. 3C–3E). We therefore conclude that BSAP/PAX5, the TF responsible for the commitment and regulation of B cell development [18, 47, 85], is also the protein necessary for MCOLN2 gene activation [106].
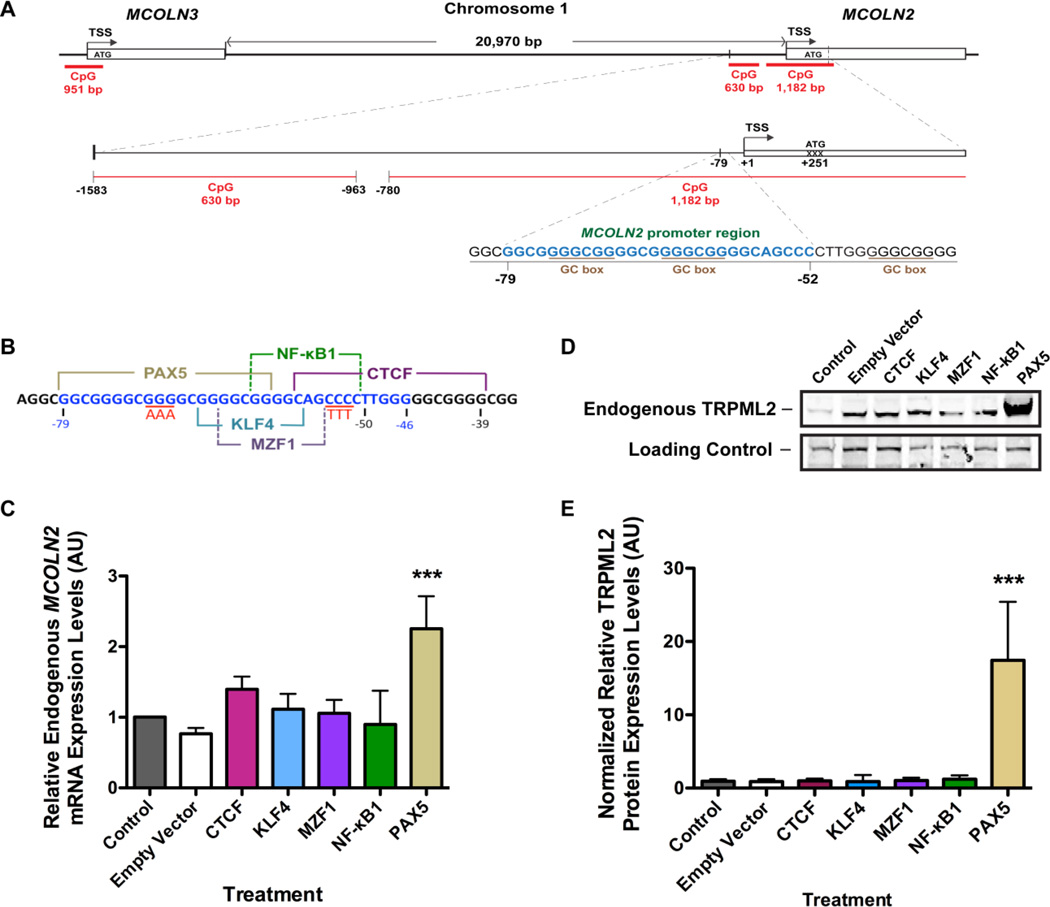
Figure 3. Identification of the promoter region and transcription factor responsible for MCOLN2 gene activation.
A) Schematic diagram of Chromosome 1 showing the position and orientation of MCOLN2 and MCOLN3 genes. A predicted CpG island 951 bp in length is shown for MCOLN3, while two predicted CpG islands 1,182 bp and 630 bp in lengths are shown for MCOLN2. B) A map of the core promoter region of MCOLN2 illustrating the putative binding sites of five immune-related transcription factors (CTCF, MZF1, KLF4, NF-κB and PAX5) identified by cross-referencing the JASPAR and TRANSFAC databases. C) Real-time RT-qPCR analysis of HEK-293 cells transiently transfected with the corresponding TF proteins. Endogenous MCOLN2 mRNA levels were quantified 48 hours post-transfection. The RT-qPCR data were analyzed using 18S rRNA as the normalizer, while the untreated control was used as the calibrator (value = 1). ***p-value < 0.001; ANOVA followed by Bonferroni’s multiple comparison post-hoc test, n > 3 independent trials. D) Representative WB image of endogenous TRPML2 protein expression upon transient transfection of candidate TFs. The top panel is TRPML2 blotted with anti-TRPML2 polyclonal antibody (pAb). The lower panel is the loading control taken from the same blot where the bands in each individual lane represented non-specific binding of the anti-TRPML2 pAb. E) Semi-quantitative analysis of endogenous TRPML2 protein bands upon heterologous expression of candidate TF proteins using IDV analysis (NIH ImageJ). PAX5 significantly increased the TRPML2 protein levels compared with control and other transcription factors. ***p-value < 0.001; ANOVA followed by Bonferroni’s multiple comparison post-hoc test, n > 3 independent trials. Reprinted with permission from Ref. [106]: Valadez and Cuajungco (2015), Gene, 555, 194–202. Copyright 2015 Elsevier.
Despite having shown that PAX5 regulates MCOLN2 gene expression, our experimental data and bioinformatics analysis of the ENCODE database indicate that CTCF also binds to the MCOLN2 promoter. Furthermore, heterologous expression of CTCF in HEK-293 cells also conferred a modest increase in relative MCOLN2 transcript levels [106]. We did not, however, observe a significant parallel increase in TRPML2 protein levels upon treatment with CTCF [106]. Therefore, additional work needs to be done to determine if CTCF, in cooperation with PAX5, may contribute to the regulation of MCOLN2 gene expression.
TRPML2 Involvement in ARF6-dependent Subcellular Sorting Pathway of Specific Cell Types
The ADP-ribosylation factor (ARF)-6 pathway in cells regulates actin remodeling and the clathrin-independent endocytic membrane trafficking of specific transmembrane proteins, and in particular, the recycling of glycosylphosphatidylinositol-anchored proteins (GPI-APs) [27]. During post-translational modification, the GPI moiety is attached to certain proteins to anchor them on the PM and facilitate signal transduction processes, cytokine secretion, Ca2+ influx, and intracellular trafficking [16]. The importance of GPI-APs in immune function is exemplified by the observation that mouse mast cells devoid of a gene necessary for their synthesis have disrupted Fc-εRI subunit membrane sorting and defective intra-subunit interaction, leading to abnormal IgE-induced receptor activation and degranulation [45]. GPI-APs such as CD58, CD59, CD73, LY6, BIG1, and Fc-γRIIIb have been implicated in immune cell function [16] in which TRPML2 may play some role in their trafficking and recycling via the ARF6 pathway [53]. Indeed, it was found that heterologously expressed TRPML2 distributes within long tubular endosomes of HeLa cells and co-localizes with MHC-I and CD59 proteins [53]. Regulation of CD59 membrane trafficking by TRPML2 [53] is especially important, since CD59 is crucial for the recognition of “self” to prevent an autoimmune attack [61]. Meanwhile, heterologous over-expression of TRPML2 increases ARF6 protein activity by seven-fold, and augments CD59 internalization [53]. Conversely, a dominant-negative double mutation in TRPML2 (D463K/D464K) confers an inhibitory effect on CD59 recycling [53]. It is important to note that increased ARF6 activity as a consequence of TRPML2 over-expression also results in enhanced vesicle fusion and formation of large vesicles in HeLa cells [53], which could explain the increase in CD59 trafficking via the ARF6-dependent pathway in this context. Overall, TRPML2 seems to play an important function in the activation of ARF6, and thus, the clathrin-independent internalization of GPI-APs.
TRPML2 in Mast Cell Signaling
The Fc-εRI consist of a transmembrane alpha (α), a beta (β), and two gamma (γ) disulfide-bonded subunits, which exists as either a tetrameric (αβγγ) or trimeric (αγγ) complex [62]. The α subunit has two binding motifs for the IgE antibody. The β subunit is predicted to have four TM domains, and serves as the stabilizer and signal amplifier. The β subunit also promotes the trafficking of the Fc-εRI receptor complex. Meanwhile, the γ subunit contains the ITAM and is the signal transducer of the receptor complex [62]. The stimulation of the trimeric Fc-εRI receptor complex in mast cells and basophils is central to degranulation and arachidonic acid synthesis [43, 63]. Specifically, the cross-linking of the IgE/Fc-εRI receptor complex activates the β subunit-associated LYN kinase, which then phosphorylates the tyrosine residues of the β- and γ-subunit ITAMs [43]; SYK then recognizes the γ-subunit phosphorylated ITAM and once associated, it auto-phosphorylates itself (Fig. 2). Upon activation of the Fc-εRI receptor, BTK becomes membrane-bound [55], where its pleckstrin homology (PH) domain interacts with PIP3. Similar to the BCR signaling events, BTK becomes phosphorylated, which then phosphorylates PLCγ. Activated PLCγ then cleaves PIP2 into inositol trisphosphate (IP3) and diacylglycerol (DAG) (Fig. 2). IP3 travels to the endoplasmic reticulum and causes the release of intracellular Ca2+ stores while DAG activates PKCβ [43]. Ca2+ plays a pivotal role in microtubule formation, microfilament contraction, and together with PKC, it aids in the degranulation of the mast cell (Fig. 2). In addition, an increase in intracellular Ca2+ activates calcineurin (CaN), which in turn dephosphorylates cytoplasmic NFAT and allows its translocation to the nucleus, where it regulates the transcription of its target genes. Furthermore, BTK is very important for cytokine gene expression in mast cells [81] such that murine bone marrow-derived mast cells knocked out of BTK exhibit defects in degranulation and the production of cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin 2 (IL-2) [44]. Where might TRPML2 play a role in the signaling pathway of mast cell activation? We propose that TRPML2 participates in the release of Ca2+ ions that is crucial to: (a) the process of degranulation; (b) vesicle fusion; (c) ARF6-dependent trafficking of immune-related GPI-APs; and quite possibly, (d) the trafficking of activated Fc-εRI/IgE complex. Future research in this area should specifically determine the effects of knocking down or knocking out the TRPML2 protein on the internalization of Fc-εRI/IgE complex, degranulation, and cytokine gene expression.
TRPML2 in Dendritic Cell Signaling via its Interaction with TMEM176A Protein
Our laboratory recently demonstrated that TMEM176A interacts with the TRPML2 ion channel [21]. The interaction may seem logical considering the two proteins both localize to the PM and vesicular compartments of the cell [22, 53]. However, it is unclear how and whether the TMEM176A-TRPML2 interaction actually influences the maturation of DCs. What appears to be a common denominator from various studies is that the maturation of B cells and DCs may be dependent on the function of the TRPML2 ion channel. Figure 2 illustrates a convergent pathway where TRPML2 may be crucial in cell surface protein internalization and recycling, as well as vesicle fusion. Future investigations should focus on why TRPML2 and TMEM176A interact with each other, and whether TRPML2 plays a role in the trafficking of TMEM176A. Does TRPML2 through its interaction with TMEM176A mediate the trafficking of an immune receptor complex, which is similar to the role of Fc-εRIβ subunit [62]? Is TRPML2 necessary for the internalization of TMEM176A or the receptor complex it may be associated with? These are some of the questions that need to be answered to fully understand the functional relevance of TRPML2 and TMEM176A interaction. Another point of interest is to determine whether TRPML2 also interacts with TMEM176B, since TMEM176A and TMEM176B interact with one another.
Exploiting the Functional Redundancy or Similarity Between the TRPML Proteins as a Potential Therapy for MLIV
Previous studies have suggested a functional redundancy between the TRPML subfamily. A study by Song and colleagues (2006) revealed that disrupting the function of TRPML2 or TRPML1 protein by addition of a green fluorescent protein (GFP) at the C-terminus (TRPML2-GFP or TRPML1-GFP, respectively) resulted in an enlargement of cellular lysosomes in DT-40 B-lymphocytes and HEK-293 cells [96], much like the MLIV disease phenotype. Furthermore, TRPML2-GFP or TRPML1-GFP expression resulted in the disruption of cellular transport in B-lymphocytes. Specifically, endocytosed BCRs were delivered to the MLIV-like enlarged lysosomes, further demonstrating a role for the two TRPMLs in the regulation of cellular transport to the lysosomal compartments of B cells [96]. Additionally, the study found that simply knocking down TRPML1 expression alone did not induce an MLIV-like phenotype [96]. Both TRPML1 and TRPML2 were found to localize to the lysosomes of B-lymphocytes, upon which the authors hypothesized that the overlapping function of TRPML2 could compensate for the loss of TRPML1 and thus, the ensuing lack of MLIV-like phenotype. Another study by Zeevi et al. (2009) demonstrated that knock down of TRPML2 or TRPML3 in HeLa cells also produce the characteristic MLIV phenotype of vacuole formation and enlargement of lysosomes [116], suggesting a common function for the TRPMLs in lysosomal regulation. Furthermore, genetic complementation of C. elegans Cup-5−/− gene knockout (the MCOLN1 gene orthologue) using either human TRPML1 or TRPML3 confers phenotypic rescue [105], which suggests that the TRPMLs across species have a similar or overlapping function. Finally, a more compelling evidence for functional redundancy between the TRPMLs is the recent observation that neonatal mouse enterocytes knocked out of both Mcoln1 and Mcoln3 genes develop vacuoles that result in growth delay from birth up until weaning [87].
We and others have successfully recapitulated MLIV phenotype in several types of cultured cells using RNAi [19, 31, 64, 116]. Recently, RNAi-mediated knock down (KD) of TRPML1 in cultured retinal pigmented epithelial (RPE) cells have been found to mimic mitochondrial dysfunction [19] that is typically observed in MLIV cells [51]. TRPML1-KD also yields oxidative stress in cells [19, 108], which could possibly be explained by recent observations of intracellular zinc [21, 31, 64] and iron dyshomeostasis in MLIV-affected cells [28]. Noteworthy is that zinc accumulation, much like iron accumulation, is known to disrupt mitochondrial function, which subsequently produces reactive oxygen species (ROS) [93, 94]. In their report, Coblentz et al. (2014) found that transient iron exposure of TRPML1-KD RPE cells results in lipid peroxidation and loss of mitochondrial membrane permeability through increased production of ROS. The increase of ROS was mitigated by the addition of α-Tocopherol (vitamin E, a ROS scavenger) [19]. These observations suggest that the underlying pathology in MLIV maybe partly or wholly caused by metal dyshomeostasis and oxidative stress.
Recall that MLIV patients exhibit abnormally low gastric acid production [1], and that oxidative stress is a cellular characteristic typically seen in tissues or cells of several MLIV disease paradigms [19, 108]. Using these two disease markers, we explored the potential of TRPML2 to alter their manifestation in a cell culture model of MLIV. We obtained HGT-1 cells stably expressing a short-hairpin RNA that knocks down TRPML1 expression (a kind gift of Dr. Ehud Goldin, NIH/NHGRI). The HGT-1 cell line is ideal to model the MLIV disease because it exhibits many characteristics found in primary parietal cells such as the presence of omeprazole-inhibited H+/K+-ATPase, calcium-activated potassium channels, cAMP-activated chloride channels, histamine receptors, and protein transporters involved in acid secretion [14, 65, 90, 91]. Indeed, cultured HGT-1 cells secrete gastric acid in its milieu upon exposure to histamine and/or 3-isobutyl-1-methylxanthine (IBMX, a phosphodiesterase inhibitor) [14]. To confirm the effect of RNAi, we used Western blot analysis and confirmed a modest reduction of TRPML1 protein levels in TRPML1-KD HGT-1 cells compared with WT (Fig. 4A). To test the possibility that TRPML2 OE could influence the secretion of gastric acid in HGT-1 cells, or in this case, the effect of TRPML1 protein reduction on HGT-1 cells ability to secrete gastric acid (which have been reported to be abnormally low in MLIV patients), we heterologously expressed TRPML2 in both WT and TRPML1-KD HGT-1 cells. Using the membrane impermeant pH-sensitive fluorescent dye, 8-hydroxypyrene-1,3,6-trisulphonic acid trisodium salt (HPTS), we analyzed gastric acid secretion in both WT and TRPML1-KD HGT-1 cells after three hours of exposure to histamine plus IBMX. We found that TRPML2 OE significantly increased acid secretion not only in TRPML1-KD, but also in WT HGT-1 cells when compared with untreated and negative controls (Fig. 4B). With respect to oxidative stress caused by elevated ROS production (a cell physiological marker that has been reported in cell culture models of MLIV disease), we observed that TRPML2 OE significantly reduced ROS production in TRPML1-KD HGT-1 cells compared to controls using the CellRox Orange fluorescent dye (Fig. 4C). Note, however, that our data indicate that the RNAi-induced reduction of TRPML1 protein levels was not sufficient to either markedly reduce the levels of acid secretion or the concomitant increase in ROS production in TRPML1-KD HGT-1 cells. These observations convey to us that RNAi, at least for the TRPML1-KD HGT-1 cells used in our study, might not be a reliable cell culture model of MLIV disease. We thus propose the use of CRISPR-Cas9 technique to knock out TRPML1 as the best cell culture paradigm owing to its effectiveness and low cost. Overall, the results of our pilot study indicate that TRPML2 could have a therapeutic potential to reverse certain disease phenotypes in MLIV cells by reducing oxidative stress and increasing the production and secretion of gastric acid.
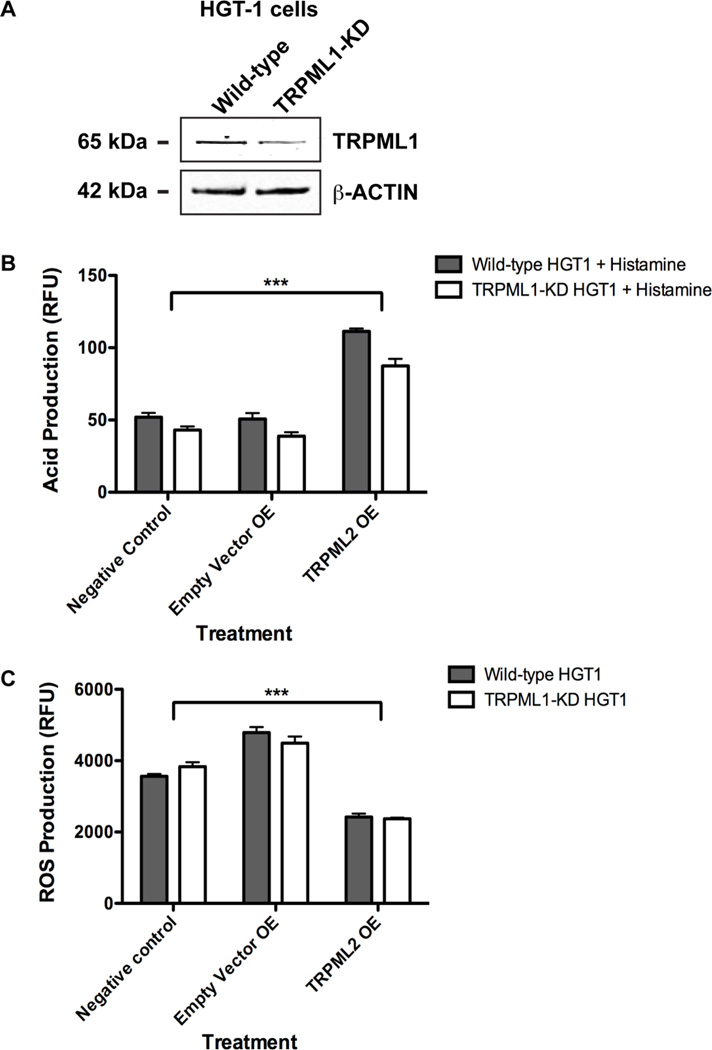
Figure 4. Heterologous expression of TRPML2 in wild type HGT-1 cells and TRPML1 knocked down HGT-1 cells.
(A) Representative image of WB illustrating TRPML1 protein expression levels in HGT-1 WT cells and HGT-1 cells knocked down of TRPML1 (TRPML1-KD). The blot was probed with human anti-TRPML1 monoclonal antibody (mAb) and anti-β-ACTIN pAb, and visualized using a LICOR Sa™ infrared imaging scanner. (B) Relative acid production between WT and TRPML1-KD HGT-1 cells with or without heterologously over-expressed TRPML2 (TRPML2 OE). The cells were plated in triplicate wells, transfected with TRPML2, and cultured for 48 hours. The cells were then incubated with 100 µM histamine and 100 µM IBMX for 3 hours, washed with phosphate-buffered saline (PBS), incubated with HPTS for 1 hour, and then assayed for relative fluorescence. Data shown were obtained at 450 nm excitation wavelength (emission at 510 nm wavelength), since the fluorescence excitation at 405 nm produced saturation due to continued acid secretion in the culture media. ***p-value < 0.001, ANOVA followed by Dunnett’s multiple comparison post-hoc test (n = 3 independent trials). (C) Relative ROS production between WT and TRPML1-KD HGT-1 cells in the presence or absence of TRPML2 OE. The cells were plated in triplicate wells, transfected with TRPML2, and cultured for 48 hours. The cells were then incubated with 5 µM CellRox Orange, washed with PBS, and assayed for relative fluorescence. ***p-value < 0.001, ANOVA followed by Dunnett’s multiple comparison post-hoc test (n = 3 independent trials).
Challenges to Gene Therapy or Functional Complementation Approach for MLIV
Gene correction therapy works by substituting a mutant nucleotide with the WT sequence. This current mainstream approach uses viral vectors such as adeno-associated virus (AAV) to deliver the therapy, but it has had many setbacks in the past due to potential complications or immunogenic reactions in patients. Should gene correction or replacement therapy be used for MLIV, it needs to be performed very early in life (or ideally in utero), because the malady is congenital and begins even before the patient is born. The cost of developing such therapy is less conducive for many pharmaceutical industries because MLIV is a rare disease with less than 100 patients worldwide. The CRISPR-Cas9 technique may be a cost-effective gene therapeutic option for MLIV disease; however, experts in the field have recently advocated for a moratorium in research using human germ line cells (eggs, sperms, and zygotes) until it is proven to be an efficient and safe technique [48]. Since no treatment exists to alleviate symptoms or even cure MLIV, a potential therapeutic approach that our laboratory is focusing on is to exploit the functional redundancy between the TRPML proteins. The use of TRPML2 or TRPML3 to functionally complement the loss of TRPML1 is a proof-of-principle approach that could accomplish what gene correction therapy could not do. One advantage of gene complementation is that endogenous MCOLN2 and -3 genes are present in every cell and it is just a matter of activating the expression of one or both genes. Herein lies the challenge: How could we induce the expression of MCOLN2 or -3 gene if they are barely detected or completely absent in brain cells? Despite knowing the transcription factor for MCOLN2, would heterologous expression of PAX5 be sufficient to induce TRPML2 expression in MLIV-affected brain cells? What potential side effects might PAX5 give when over-expressed in brain cells? On the other hand, would screening for and identifying a drug compound that specifically expresses endogenous TRPML2 or -3 protein be sufficient to functionally complement the missing TRPML1 in MLIV-affected brain tissue? If such a drug were identified, how would the drug be administered? How could potential side effects of this drug be prevented or minimized? These are just a few questions that need to be addressed before any possible pre-clinical studies on gene complementation could be done using the existing MLIV mouse model. Clearly, more research is needed to determine if gene complementation is a viable option for children and young adults suffering from a devastating genetic disease like MLIV.
Concluding Remarks
In this review, we have highlighted several significant studies on TRPML2, discussed its possible links to immune cell processes, and underscored its apparently crucial role in normal immune function. Yet, due to the limited amount of research conducted on TRPML2, many questions remain unanswered, and as of today, the cellular roles of TRPML2 and its two subfamily members, remains poorly understood. Despite this, recent evidence suggests that TRPML2 is essential in endosome-lysosome interaction, the recycling of GPI-APs, and possibly the trafficking of immune-associated proteins such as MHC-I, TLRs, and Fc-εRI to name a few. In addition to its possible role in adaptive immunity through proper B cell differentiation and maturation, TRPML2 may also be involved in type I hypersensitivity. While many aspects of the function of TRPML2 remain to be revealed, future research will undoubtedly shed light on its importance in cells and perhaps even unlock its potential to complement for the functional loss of TRPML1 protein in MLIV disease.
Acknowledgments
We are very grateful to Dr. Sean Murray (CSU Northridge) for reading and critiquing this manuscript. We thank Dr. Ehud Goldin (NIH/NHGRI) for providing WT and TRPML1-KD HGT-1 gastric adenocarcinoma cells. JV acknowledges research awards from CSUF EPOCHS Program, CSUF’s RCATT Office, and the CSU Statewide Student Research Competition. MPC and JS acknowledge support from the National Institutes of Health (NIH) Maximizing Access to Research Careers (MARC) U*STAR Program (NIH T34-GM008612-20) and the Louis Stokes Alliance for Minority Participation (NSF HRD-0802628) research fellowship.
Funding: This work was funded by grants to MPC from the NIH AREA R15-NS070774-01, NIH MARC U*STAR Program T34-GM008612-20, National Science Foundation MCB-0920127, and Cal State Fullerton Intramural Grants program.
Footnotes
Conflict of Interest: The authors declare no conflict of interest concerning this work.
References
- 1.Amir N, Zlotogora J, Bach G. Mucolipidosis type IV: clinical spectrum and natural history. Pediatrics. 1987;79:953–959. [PubMed] [Google Scholar]
- 2.Anbazhagan K, Rabbind Singh A, Isabelle P, Stella I, Celine AD, Bissac E, Bertrand B, Remy N, Naomi T, Vincent F, Rochette J, Lassoued K. Human pre-B cell receptor signal transduction: evidence for distinct roles of PI3kinase and MAP-kinase signalling pathways. Immun Inflamm Dis. 2013;1:26–36. doi: 10.1002/iid3.4. PMID: 4217539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antony P, Petro JB, Carlesso G, Shinners NP, Lowe J, Khan WN. B cell receptor directs the activation of NFAT and NF-kappaB via distinct molecular mechanisms. Exp Cell Res. 2003;291:11–24. doi: 10.1016/s0014-4827(03)00338-0. [DOI] [PubMed] [Google Scholar]
- 4.Asai K, Kitaura J, Kawakami Y, Yamagata N, Tsai M, Carbone DP, Liu FT, Galli SJ, Kawakami T. Regulation of mast cell survival by IgE. Immunity. 2001;14:791–800. doi: 10.1016/s1074-7613(01)00157-1. [DOI] [PubMed] [Google Scholar]
- 5.Bach G, Cohen MM, Kohn G. Abnormal ganglioside accumulation in cultured fibroblasts from patients with mucolipidosis IV. Biochem Biophys Res Commun. 1975;66:1483–1490. doi: 10.1016/0006-291x(75)90526-4. [DOI] [PubMed] [Google Scholar]
- 6.Bae M, Patel N, Xu H, Lee M, Tominaga-Yamanaka K, Nath A, Geiger J, Gorospe M, Mattson MP, Haughey NJ. Activation of TRPML1 clears intraneuronal Abeta in preclinical models of HIV infection. J Neurosci. 2014;34:11485–11503. doi: 10.1523/JNEUROSCI.0210-14.2014. PMID: 4138351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bargal R, Avidan N, Ben-Asher E, Olender Z, Zeigler M, Frumkin A, Raas-Rothschild A, Glusman G, Lancet D, Bach G. Identification of the gene causing mucolipidosis type IV. Nat Genet. 2000;26:118–123. doi: 10.1038/79095. [DOI] [PubMed] [Google Scholar]
- 8.Bargal R, Goebel HH, Latta E, Bach G. Mucolipidosis IV: novel mutation and diverse ultrastructural spectrum in the skin. Neuropediatrics. 2002;33:199–202. doi: 10.1055/s-2002-34496. [DOI] [PubMed] [Google Scholar]
- 9.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. PMID: Pmc3934928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassi MT, Manzoni M, Monti E, Pizzo MT, Ballabio A, Borsani G. Cloning of the gene encoding a novel integral membrane protein, mucolipidin-and identification of the two major founder mutations causing mucolipidosis type IV. Am J Hum Genet. 2000;67:1110–1120. doi: 10.1016/s0002-9297(07)62941-3. DOI: S0002-9297(07)62941-3 [pii] 10.1016/S0002-9297(07)62941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 12.Brunner C, Muller B, Wirth T. Bruton's Tyrosine Kinase is involved in innate and adaptive immunity. Histol Histopathol. 2005;20:945–955. doi: 10.14670/HH-20.945. [DOI] [PubMed] [Google Scholar]
- 13.Bubien JK, Zhou LJ, Bell PD, Frizzell RA, Tedder TF. Transfection of the CD20 cell surface molecule into ectopic cell types generates a Ca2+ conductance found constitutively in B lymphocytes. J Cell Biol. 1993;121:1121–1132. doi: 10.1083/jcb.121.5.1121. DOI: 2119683 PMID: 2119683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmosino M, Procino G, Casavola V, Svelto M, Valenti G. The cultured human gastric cells HGT-1 express the principal transporters involved in acid secretion. Pflugers Arch. 2000;440:871–880. doi: 10.1007/s004240000363. [DOI] [PubMed] [Google Scholar]
- 15.Castiglioni AJ, Remis NN, Flores EN, Garcia-Anoveros J. Expression and vesicular localization of mouse Trpml3 in stria vascularis, hair cells, and vomeronasal and olfactory receptor neurons. J Comp Neurol. 2011;519:1095–1114. doi: 10.1002/cne.22554. PMID: 4105223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee S, Mayor S. The GPI-anchor and protein sorting. Cell Mol Life Sci. 2001;58:1969–1987. doi: 10.1007/PL00000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi S, Kim HJ. The Ca2+ channel TRPML3 specifically interacts with the mammalian ATG8 homologue GATE16 to regulate autophagy. Biochem Biophys Res Commun. 2014;443:56–61. doi: 10.1016/j.bbrc.2013.11.044. [DOI] [PubMed] [Google Scholar]
- 18.Cobaleda C, Schebesta A, Delogu A, Busslinger M. Pax5: the guardian of B cell identity and function. Nat Immunol. 2007;8:463–470. doi: 10.1038/ni1454. [DOI] [PubMed] [Google Scholar]
- 19.Coblentz J, St Croix C, Kiselyov K. Loss of TRPML1 promotes production of reactive oxygen species: is oxidative damage a factor in mucolipidosis type IV? Biochem J. 2014;457:361–368. doi: 10.1042/BJ20130647. [DOI] [PubMed] [Google Scholar]
- 20.Condamine T, Le Texier L, Howie D, Lavault A, Hill M, Halary F, Cobbold S, Waldmann H, Cuturi MC, Chiffoleau E. Tmem176B and Tmem176A are associated with the immature state of dendritic cells. J Leukoc Biol. 2010;88:507–515. doi: 10.1189/jlb.1109738. [DOI] [PubMed] [Google Scholar]
- 21.Cuajungco MP, Basilio LC, Silva J, Hart T, Tringali J, Chen CC, Biel M, Grimm C. Cellular zinc levels are modulated by TRPML1-TMEM163 interaction. Traffic. 2014;15:1247–1265. doi: 10.1111/tra.12205. PMID: 4205267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuajungco MP, Podevin W, Valluri VK, Bui Q, Nguyen VH, Taylor K. Abnormal accumulation of human transmembrane (TMEM)-176A and 176B proteins is associated with cancer pathology. Acta Histochem. 2012;114:705–712. doi: 10.1016/j.acthis.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuajungco MP, Samie MA. The varitint-waddler mouse phenotypes and the TRPML3 ion channel mutation: cause and consequence. Pflugers Arch. 2008;457:463–473. doi: 10.1007/s00424-008-0523-4. [DOI] [PubMed] [Google Scholar]
- 24.Curcio-Morelli C, Zhang P, Venugopal B, Charles FA, Browning MF, Cantiello HF, Slaugenhaupt SA. Functional multimerization of mucolipin channel proteins. J Cell Physiol. 2010;222:328–335. doi: 10.1002/jcp.21956. [DOI] [PubMed] [Google Scholar]
- 25.Decker T, Pasca di Magliano M, McManus S, Sun Q, Bonifer C, Tagoh H, Busslinger M. Stepwise activation of enhancer and promoter regions of the B cell commitment gene Pax5 in early lymphopoiesis. Immunity. 2009;30:508–520. doi: 10.1016/j.immuni.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Di Palma F, Belyantseva IA, Kim HJ, Vogt TF, Kachar B, Noben-Trauth K. Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc Natl Acad Sci U S A. 2002;99:14994–14999. doi: 10.1073/pnas.222425399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donaldson JG. Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane. J Biol Chem. 2003;278:41573–41576. doi: 10.1074/jbc.R300026200. DOI: 10.1074/jbc.R300026200 [pii] R300026200. [DOI] [PubMed] [Google Scholar]
- 28.Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. DOI: nature07311 [pii] 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun. 2010;1:38. doi: 10.1038/ncomms1037. PMID: 2928581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doyle SL, Jefferies CA, Feighery C, O'Neill LA. Signaling by Toll-like receptors 8 and 9 requires Bruton's tyrosine kinase. J Biol Chem. 2007;282:36953–36960. doi: 10.1074/jbc.M707682200. [DOI] [PubMed] [Google Scholar]
- 31.Eichelsdoerfer JL, Evans JA, Slaugenhaupt SA, Cuajungco MP. Zinc dyshomeostasis is linked with the loss of mucolipidosis IV-associated TRPML1 ion channel. J Biol Chem. 2010;285:34304–34308. doi: 10.1074/jbc.C110.165480. PMID: 2966043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiorini M, Franceschini R, Soresina A, Schumacher RF, Ugazio AG, Rossi P, Plebani A, Notarangelo LD. BTK:22 novel and 25 recurrent mutations in European patients with X-linked agammaglobulinemia. Hum Mutat. 2004;23:286. doi: 10.1002/humu.9219. [DOI] [PubMed] [Google Scholar]
- 33.Flores EN, Garcia-Anoveros J. TRPML2 and the evolution of mucolipins. Adv Exp Med Biol. 2011;704:221–228. doi: 10.1007/978-94-007-0265-3_12. [DOI] [PubMed] [Google Scholar]
- 34.Gagliardi MC, Finocchi A, Orlandi P, Cursi L, Cancrini C, Moschese V, Miyawaki T, Rossi P. Bruton's tyrosine kinase defect in dendritic cells from X-linked agammaglobulinaemia patients does not influence their differentiation, maturation and antigen-presenting cell function. Clin Exp Immunol. 2003;133:115–122. doi: 10.1046/j.1365-2249.2003.t01-1-02178.x. PMID: 1808743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Anoveros J, Wiwatpanit T. TRPML2 and mucolipin evolution. Handb Exp Pharmacol. 2014;222:647–658. doi: 10.1007/978-3-642-54215-2_25. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh HS, Cisse B, Bunin A, Lewis KL, Reizis B. Continuous expression of the transcription factor e2-2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity. 2010;33:905–916. doi: 10.1016/j.immuni.2010.11.023. PMID: 3010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottschling S, Jauch A, Kuner R, Herpel E, Mueller-Decker K, Schnabel PA, Xu EC, Muley T, Sultmann H, Bender C, Granzow M, Efferth T, Hoffmann H, Dienemann H, Herth FJ, Meister M. Establishment and comparative characterization of novel squamous cell non-small cell lung cancer cell lines and their corresponding tumor tissue. Lung Cancer. 2012;75:45–57. doi: 10.1016/j.lungcan.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Gray P, Dunne A, Brikos C, Jefferies CA, Doyle SL, O'Neill LA. MyD88 adapter-like (Mal) is phosphorylated by Bruton's tyrosine kinase during TLR2 and TLR4 signal transduction. J Biol Chem. 2006;281:10489–10495. doi: 10.1074/jbc.M508892200. [DOI] [PubMed] [Google Scholar]
- 39.Grimm C, Cuajungco MP, van Aken AF, Schnee M, Jors S, Kros CJ, Ricci AJ, Heller S. A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc Natl Acad Sci U S A. 2007;104:19583–19588. doi: 10.1073/pnas.0709846104. DOI: 0709846104 [pii] 10.1073/pnas.0709846104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grimm C, Jors S, Guo Z, Obukhov AG, Heller S. Constitutive activity of TRPML2 and TRPML3 channels versus activation by low extracellular sodium and small molecules. J Biol Chem. 2012;287:22701–22708. doi: 10.1074/jbc.M112.368876. PMID: 3391124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grimm C, Jors S, Saldanha SA, Obukhov AG, Pan B, Oshima K, Cuajungco MP, Chase P, Hodder P, Heller S. Small molecule activators of TRPML3. Chem Biol. 2010;17:135–148. doi: 10.1016/j.chembiol.2009.12.016. PMID: 2834294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo B, Kato RM, Garcia-Lloret M, Wahl MI, Rawlings DJ. Engagement of the human pre-B cell receptor generates a lipid raft-dependent calcium signaling complex. Immunity. 2000;13:243–253. doi: 10.1016/s1074-7613(00)00024-8. DOI: [DOI] [PubMed] [Google Scholar]
- 43.Hata D, Kawakami Y, Inagaki N, Lantz CS, Kitamura T, Khan WN, Maeda-Yamamoto M, Miura T, Han W, Hartman SE, Yao L, Nagai H, Goldfeld AE, Alt FW, Galli SJ, Witte ON, Kawakami T. Involvement of Bruton's tyrosine kinase in FcepsilonRI-dependent mast cell degranulation and cytokine production. J Exp Med. 1998;187:1235–1247. doi: 10.1084/jem.187.8.1235. PMID: 2212237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hata D, Kitaura J, Hartman SE, Kawakami Y, Yokota T, Kawakami T. Bruton's tyrosine kinase-mediated interleukin-2 gene activation in mast cells. Dependence on the c-Jun N-terminal kinase activation pathway. J Biol Chem. 1998;273:10979–10987. doi: 10.1074/jbc.273.18.10979. [DOI] [PubMed] [Google Scholar]
- 45.Hazenbos WL, Wu P, Eastham-Anderson J, Kinoshita T, Brown EJ. Impaired FcepsilonRI stability, signaling, and effector functions in murine mast cells lacking glycosylphosphatidylinositol-anchored proteins. Blood. 2011;118:4377–4383. doi: 10.1182/blood-2011-02-338053. PMID: 3204909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat Rev Immunol. 2009;9:195–205. doi: 10.1038/nri2491. [DOI] [PubMed] [Google Scholar]
- 47.Holmes ML, Pridans C, Nutt SL. The regulation of the B-cell gene expression programme by Pax5. Immunol Cell Biol. 2008;86:47–53. doi: 10.1038/sj.icb.7100134. [DOI] [PubMed] [Google Scholar]
- 48.Ishii T. Germline genome-editing research and its socioethical implications. Trends Mol Med. 2015;21:473–481. doi: 10.1016/j.molmed.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Iyer AS, Morales JL, Huang W, Ojo F, Ning G, Wills E, Baines JD, August A. Absence of Tec family kinases interleukin-2 inducible T cell kinase (Itk) and Bruton's tyrosine kinase (Btk) severely impairs Fc epsilonRI-dependent mast cell responses. J Biol Chem. 2011;286:9503–9513. doi: 10.1074/jbc.M110.165613. PMID: 3059023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jefferies CA, Doyle S, Brunner C, Dunne A, Brint E, Wietek C, Walch E, Wirth T, O'Neill LA. Bruton's tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor kappaB activation by Toll-like receptor 4. J Biol Chem. 2003;278:26258–26264. doi: 10.1074/jbc.M301484200. [DOI] [PubMed] [Google Scholar]
- 51.Jennings JJ, Jr, Zhu JH, Rbaibi Y, Luo X, Chu CT, Kiselyov K. Mitochondrial aberrations in mucolipidosis Type IV. J Biol Chem. 2006;281:39041–39050. doi: 10.1074/jbc.M607982200. [DOI] [PubMed] [Google Scholar]
- 52.Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, Krystal G. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001;14:801–811. doi: 10.1016/s1074-7613(01)00159-5. [DOI] [PubMed] [Google Scholar]
- 53.Karacsonyi C, Miguel AS, Puertollano R. Mucolipin-2 localizes to the Arf6-associated pathway and regulates recycling of GPI-APs. Traffic. 2007;8:1404–1414. doi: 10.1111/j.1600-0854.2007.00619.x. DOI: TRA619 [pii] 10.1111/j.1600-0854.2007.00619.x. [DOI] [PubMed] [Google Scholar]
- 54.Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, Terai K, Matsuda M, Inoue J, Uematsu S, Takeuchi O, Akira S. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 55.Kawakami Y, Yao L, Miura T, Tsukada S, Witte ON, Kawakami T. Tyrosine phosphorylation and activation of Bruton tyrosine kinase upon Fc epsilon RI cross-linking. Mol Cell Biol. 1994;14:5108–5113. doi: 10.1128/mcb.14.8.5108. PMID: Pmc359029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kerner JD, Appleby MW, Mohr RN, Chien S, Rawlings DJ, Maliszewski CR, Witte ON, Perlmutter RM. Impaired expansion of mouse B cell progenitors lacking Btk. Immunity. 1995;3:301–312. doi: 10.1016/1074-7613(95)90115-9. [DOI] [PubMed] [Google Scholar]
- 57.Khan WN, Alt FW, Gerstein RM, Malynn BA, Larsson I, Rathbun G, Davidson L, Muller S, Kantor AB, Herzenberg LA, et al. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 58.Kim HJ, Li Q, Tjon-Kon-Sang S, So I, Kiselyov K, Muallem S. Gain-of-function mutation in TRPML3 causes the mouse Varitint-Waddler phenotype. J Biol Chem. 2007;282:36138–36142. doi: 10.1074/jbc.C700190200. [DOI] [PubMed] [Google Scholar]
- 59.Kim HJ, Soyombo AA, Tjon-Kon-Sang S, So I, Muallem S. The Ca(2+) channel TRPML3 regulates membrane trafficking and autophagy. Traffic. 2009;10:1157–1167. doi: 10.1111/j.1600-0854.2009.00924.x. PMID: 2993507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim HJ, Yamaguchi S, Li Q, So I, Muallem S. Properties of the TRPML3 channel pore and its stable expansion by the Varitint-Waddler-causing mutation. J Biol Chem. 2010;285:16513–16520. doi: 10.1074/jbc.M109.078204. PMID: 2878031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kimberley FC, Sivasankar B, Paul Morgan B. Alternative roles for CD59. Mol Immunol. 2007;44:73–81. doi: 10.1016/j.molimm.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 62.Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 63.Kuehn HS, Swindle EJ, Kim MS, Beaven MA, Metcalfe DD, Gilfillan AM. The phosphoinositide 3-kinase-dependent activation of Btk is required for optimal eicosanoid production and generation of reactive oxygen species in antigen-stimulated mast cells. J Immunol. 2008;181:7706–7712. doi: 10.4049/jimmunol.181.11.7706. PMID: 2709775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kukic I, Lee JK, Coblentz J, Kelleher SL, Kiselyov K. Zinc-dependent lysosomal enlargement in TRPML1-deficient cells involves MTF-1 transcription factor and ZnT4 (Slc30a4) transporter. Biochem J. 2013;451:155–163. doi: 10.1042/BJ20121506. PMID: 3654546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laboisse CL, Augeron C, Couturier-Turpin MH, Gespach C, Cheret AM, Potet F. Characterization of a newly established human gastric cancer cell line HGT-1 bearing histamine H2-receptors. Cancer Res. 1982;42:1541–1548. [PubMed] [Google Scholar]
- 66.LaPlante JM, Sun M, Falardeau J, Dai D, Brown EM, Slaugenhaupt SA, Vassilev PM. Lysosomal exocytosis is impaired in mucolipidosis type IV. Mol Genet Metab. 2006;89:339–348. doi: 10.1016/j.ymgme.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 67.Lee KG, Xu S, Kang ZH, Huo J, Huang M, Liu D, Takeuchi O, Akira S, Lam KP. Bruton's tyrosine kinase phosphorylates Toll-like receptor 3 to initiate antiviral response. Proc Natl Acad Sci U S A. 2012;109:5791–5796. doi: 10.1073/pnas.1119238109. PMID: 3326448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee KP, Nair AV, Grimm C, van Zeeland F, Heller S, Bindels RJ, Hoenderop JG. A helix-breaking mutation in the epithelial Ca(2+) channel TRPV5 leads to reduced Ca(2+)-dependent inactivation. Cell Calcium. 2010;48:275–287. doi: 10.1016/j.ceca.2010.09.007. PMID: 3780571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lev S, Zeevi DA, Frumkin A, Offen-Glasner V, Bach G, Minke B. Constitutive activity of the human TRPML2 channel induces cell degeneration. J Biol Chem. 2010;285:2771–2782. doi: 10.1074/jbc.M109.046508. PMID: 2807332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li X, Saitoh SI, Shibata T, Tanimura N, Fukui R, Miyake K. Mucolipin 1 positively regulates TLR7 responses in dendritic cells by facilitating RNA transportation to lysosomes. Int Immunol. 2014;27:83–94. doi: 10.1093/intimm/dxu086. [DOI] [PubMed] [Google Scholar]
- 71.Liang Y, Tedder TF. Identification of a CD20-, FcepsilonRIbeta-, and HTm4-related gene family: sixteen new MS4A family members expressed in human and mouse. Genomics. 2001;72:119–127. doi: 10.1006/geno.2000.6472. [DOI] [PubMed] [Google Scholar]
- 72.Lin S, Cicala C, Scharenberg AM, Kinet JP. The Fc(epsilon)RIbeta subunit functions as an amplifier of Fc(epsilon)RIgamma-mediated cell activation signals. Cell. 1996;85:985–995. doi: 10.1016/s0092-8674(00)81300-8. [DOI] [PubMed] [Google Scholar]
- 73.Lindvall JM, Blomberg KE, Wennborg A, Smith CI. Differential expression and molecular characterisation of Lmo7, Myo1e, Sash1, and Mcoln2 genes in Btk-defective B-cells. Cell Immunol. 2005;235:46–55. doi: 10.1016/j.cellimm.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 74.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 75.Louvet C, Chiffoleau E, Heslan M, Tesson L, Heslan JM, Brion R, Beriou G, Guillonneau C, Khalife J, Anegon I, Cuturi MC. Identification of a new member of the CD20/FcepsilonRIbeta family overexpressed in tolerated allografts. Am J Transplant. 2005;5:2143–2153. doi: 10.1111/j.1600-6143.2005.01007.x. [DOI] [PubMed] [Google Scholar]
- 76.Lurton J, Rose TM, Raghu G, Narayanan AS. Isolation of a gene product expressed by a subpopulation of human lung fibroblasts by differential display. Am J Respir Cell Mol Biol. 1999;20:327–331. doi: 10.1165/ajrcmb.20.2.3368. [DOI] [PubMed] [Google Scholar]
- 77.Mandel EM, Grosschedl R. Transcription control of early B cell differentiation. Curr Opin Immunol. 2010;22:161–167. doi: 10.1016/j.coi.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 78.Martina JA, Lelouvier B, Puertollano R. The calcium channel mucolipin-3 is a novel regulator of trafficking along the endosomal pathway. Traffic. 2009;10:1143–1156. doi: 10.1111/j.1600-0854.2009.00935.x. PMID: 2955859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McHeyzer-Williams LJ, Malherbe LP, McHeyzer-Williams MG. Checkpoints in memory B-cell evolution. Immunol Rev. 2006;211:255–268. doi: 10.1111/j.0105-2896.2006.00397.x. [DOI] [PubMed] [Google Scholar]
- 80.McHeyzer-Williams M, Okitsu S, Wang N, McHeyzer-Williams L. Molecular programming of B cell memory. Nat Rev Immunol. 2012;12:24–34. doi: 10.1038/nri3128. PMID: 3947622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohamed AJ, Yu L, Backesjo CM, Vargas L, Faryal R, Aints A, Christensson B, Berglof A, Vihinen M, Nore BF, Smith CI. Bruton's tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol Rev. 2009;228:58–73. doi: 10.1111/j.1600-065X.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 82.Nagata K, Zheng L, Madathany T, Castiglioni AJ, Bartles JR, Garcia-Anoveros J. The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration. Proc Natl Acad Sci U S A. 2008;105:353–358. doi: 10.1073/pnas.0707963105. PMID: 2224216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakajima H, Takenaka M, Kaimori JY, Nagasawa Y, Kosugi A, Kawamoto S, Imai E, Hori M, Okubo K. Gene expression profile of renal proximal tubules regulated by proteinuria. Kidney Int. 2002;61:1577–1587. doi: 10.1046/j.1523-1755.2002.00300.x. [DOI] [PubMed] [Google Scholar]
- 84.Nussenzweig MC. Immune receptor editing: revise and select. Cell. 1998;95:875–878. doi: 10.1016/s0092-8674(00)81711-0. [DOI] [PubMed] [Google Scholar]
- 85.Nutt SL, Heavey B, Rolink AG, Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401:556–562. doi: 10.1038/44076. [DOI] [PubMed] [Google Scholar]
- 86.Pryor PR, Reimann F, Gribble FM, Luzio JP. Mucolipin-1 is a lysosomal membrane protein required for intracellular lactosylceramide traffic. Traffic. 2006;7:1388–1398. doi: 10.1111/j.1600-0854.2006.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Remis NN, Wiwatpanit T, Castiglioni AJ, Flores EN, Cantu JA, Garcia-Anoveros J. Mucolipin co-deficiency causes accelerated endolysosomal vacuolation of enterocytes and failure-to-thrive from birth to weaning. PLoS Genet. 2014;10:e1004833. doi: 10.1371/journal.pgen.1004833. PMID: 4270466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Samie M, Wang X, Zhang X, Goschka A, Li X, Cheng X, Gregg E, Azar M, Zhuo Y, Garrity AG, Gao Q, Slaugenhaupt S, Pickel J, Zolov SN, Weisman LS, Lenk GM, Titus S, Bryant-Genevier M, Southall N, Juan M, Ferrer M, Xu H. A TRP channel in the lysosome regulates large particle phagocytosis via focal exocytosis. Dev Cell. 2013;26:511–524. doi: 10.1016/j.devcel.2013.08.003. PMID: 3794471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Samie MA, Grimm C, Evans JA, Curcio-Morelli C, Heller S, Slaugenhaupt SA, Cuajungco MP. The tissue-specific expression of TRPML2 (MCOLN-2) gene is influenced by the presence of TRPML1. Pflugers Arch. 2009;459:79–91. doi: 10.1007/s00424-009-0716-5. PMID: 2913554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sandle GI, Fraser G, Fogg K, Warhurst G. Properties of a potassium channel in cultured human gastric cells (HGT-1) possessing specific omeprazole binding sites. Gut. 1993;34:1331–1338. doi: 10.1136/gut.34.10.1331. DOI: 1374536 PMID: 1374536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sandle GI, Fraser G, Long S, Warhurst G. A cAMP-activated chloride channel in the plasma membrane of cultured human gastric cells (HGT-1) Pflugers Arch. 1990;417:259–263. doi: 10.1007/BF00370990. [DOI] [PubMed] [Google Scholar]
- 92.Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. PMID: 3409588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sensi SL, Ton-That D, Sullivan PG, Jonas EA, Gee KR, Kaczmarek LK, Weiss JH. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc Natl Acad Sci U S A. 2003;100:6157–6162. doi: 10.1073/pnas.1031598100. DOI: 10.1073/pnas.1031598100 [pii] 1031598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci U S A. 1999;96:2414–2419. doi: 10.1073/pnas.96.5.2414. DOI: 26798 PMID: 26798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sochorova K, Horvath R, Rozkova D, Litzman J, Bartunkova J, Sediva A, Spisek R. Impaired Toll-like receptor 8-mediated IL-6 and TNF-alpha production in antigen-presenting cells from patients with X-linked agammaglobulinemia. Blood. 2007;109:2553–2556. doi: 10.1182/blood-2006-07-037960. [DOI] [PubMed] [Google Scholar]
- 96.Song Y, Dayalu R, Matthews SA, Scharenberg AM. TRPML cation channels regulate the specialized lysosomal compartment of vertebrate B-lymphocytes. Eur J Cell Biol. 2006;85:1253–1264. doi: 10.1016/j.ejcb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 97.Soyombo AA, Tjon-Kon-Sang S, Rbaibi Y, Bashllari E, Bisceglia J, Muallem S, Kiselyov K. TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J Biol Chem. 2006;281:7294–7301. doi: 10.1074/jbc.M508211200. [DOI] [PubMed] [Google Scholar]
- 98.Spooner E, McLaughlin BM, Lepow T, Durns TA, Randall J, Upchurch C, Miller K, Campbell EM, Fares H. Systematic screens for proteins that interact with the mucolipidosis type IV protein TRPML1. PLoS One. 2013;8:e56780. doi: 10.1371/journal.pone.0056780. PMID: 3572064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010;234:5–17. doi: 10.1111/j.0105-2896.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- 100.Sun M, Goldin E, Stahl S, Falardeau JL, Kennedy JC, Acierno JS, Jr, Bove C, Kaneski CR, Nagle J, Bromley MC, Colman M, Schiffmann R, Slaugenhaupt SA. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet. 2000;9:2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- 101.Takumida M, Anniko M. Expression of transient receptor potential channel mucolipin (TRPML) and polycystine (TRPP) in the mouse inner ear. Acta Otolaryngol. 2010;130:196–203. doi: 10.3109/00016480903013593. [DOI] [PubMed] [Google Scholar]
- 102.Taneichi H, Kanegane H, Sira MM, Futatani T, Agematsu K, Sako M, Kaneko H, Kondo N, Kaisho T, Miyawaki T. Toll-like receptor signaling is impaired in dendritic cells from patients with X-linked agammaglobulinemia. Clin Immunol. 2008;126:148–154. doi: 10.1016/j.clim.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 103.Tellez-Nagel I, Rapin I, Iwamoto T, Johnson AB, Norton WT, Nitowsky H. Mucolipidosis IV. Clinical, ultrastructural, histochemical, and chemical studies of a case, including a brain biopsy. Arch Neurol. 1976;33:828–835. doi: 10.1001/archneur.1976.00500120032005. [DOI] [PubMed] [Google Scholar]
- 104.Thompson EG, Schaheen L, Dang H, Fares H. Lysosomal trafficking functions of mucolipin-1 in murine macrophages. BMC Cell Biol. 2007;8:54. doi: 10.1186/1471-2121-8-54. DOI: 1471-2121-8-54 [pii] 10.1186/1471-2121-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Treusch S, Knuth S, Slaugenhaupt SA, Goldin E, Grant BD, Fares H. Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis. Proc Natl Acad Sci U S A. 2004;101:4483–4488. doi: 10.1073/pnas.0400709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Valadez JA, Cuajungco MP. PAX5 is the transcriptional activator of mucolipin-2 (MCOLN2) gene. Gene. 2015;555:194–202. doi: 10.1016/j.gene.2014.11.003. PMID: 4276718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Venkatachalam K, Hofmann T, Montell C. Lysosomal localization of TRPML3 depends on TRPML2 and the mucolipidosis-associated protein TRPML1. J Biol Chem. 2006;281:17517–17527. doi: 10.1074/jbc.M600807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Venkatachalam K, Long AA, Elsaesser R, Nikolaeva D, Broadie K, Montell C. Motor deficit in a Drosophila model of mucolipidosis type IV due to defective clearance of apoptotic cells. Cell. 2008;135:838–851. doi: 10.1016/j.cell.2008.09.041. DOI: S0092-8674(08)01198-7 [pii] 10.1016/j.cell.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vergarajauregui S, Martina JA, Puertollano R. Identification of the penta-EF-hand protein ALG-2 as a Ca2+-dependent interactor of mucolipin-1. J Biol Chem. 2009;284:36357–36366. doi: 10.1074/jbc.M109.047241. PMID: 2794751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vergarajauregui S, Martina JA, Puertollano R. LAPTMs regulate lysosomal function and interact with mucolipin 1: new clues for understanding mucolipidosis type IV. J Cell Sci. 2011;124:459–468. doi: 10.1242/jcs.076240. PMID: 3022000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vergarajauregui S, Puertollano R. Two di-leucine motifs regulate trafficking of mucolipin-1 to lysosomes. Traffic. 2006;7:337–353. doi: 10.1111/j.1600-0854.2006.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.von Bubnoff D, Novak N, Kraft S, Bieber T. The central role of FcepsilonRI in allergy. Clin Exp Dermatol. 2003;28:184–187. doi: 10.1046/j.1365-2230.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- 113.Wang Y, Han KJ, Pang XW, Vaughan HA, Qu W, Dong XY, Peng JR, Zhao HT, Rui JA, Leng XS, Cebon J, Burgess AW, Chen WF. Large scale identification of human hepatocellular carcinoma-associated antigens by autoantibodies. J Immunol. 2002;169:1102–1109. doi: 10.4049/jimmunol.169.2.1102. [DOI] [PubMed] [Google Scholar]
- 114.Werling D, Jungi TW. TOLL-like receptors linking innate and adaptive immune response. Vet Immunol Immunopathol. 2003;91:1–12. doi: 10.1016/s0165-2427(02)00228-3. [DOI] [PubMed] [Google Scholar]
- 115.Xu H, Delling M, Li L, Dong X, Clapham DE. Activating mutation in a mucolipin transient receptor potential channel leads to melanocyte loss in varitint-waddler mice. Proc Natl Acad Sci U S A. 2007;104:18321–18326. doi: 10.1073/pnas.0709096104. DOI: 0709096104 [pii] 10.1073/pnas.0709096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zeevi DA, Frumkin A, Offen-Glasner V, Kogot-Levin A, Bach G. A potentially dynamic lysosomal role for the endogenous TRPML proteins. J Pathol. 2009;219:153–162. doi: 10.1002/path.2587. [DOI] [PubMed] [Google Scholar]
- 117.Zeevi DA, Lev S, Frumkin A, Minke B, Bach G. Heteromultimeric TRPML channel assemblies play a crucial role in the regulation of cell viability models and starvation-induced autophagy. J Cell Sci. 2010;123:3112–3124. doi: 10.1242/jcs.067330. PMID: 2931605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zou J, Hu B, Arpag S, Yan Q, Hamilton A, Zeng YS, Vanoye CG, Li J. Reactivation of Lysosomal Ca2+ Efflux Rescues Abnormal Lysosomal Storage in FIG4-Deficient Cells. J Neurosci. 2015;35:6801–6812. doi: 10.1523/JNEUROSCI.4442-14.2015. PMID: 4412898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zuccolo J, Bau J, Childs SJ, Goss GG, Sensen CW, Deans JP. Phylogenetic analysis of the MS4A and TMEM176 gene families. PLoS One. 2010;5:e9369. doi: 10.1371/journal.pone.0009369. PMID: 2826416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zuccolo J, Deng L, Unruh TL, Sanyal R, Bau JA, Storek J, Demetrick DJ, Luider JM, Auer-Grzesiak IA, Mansoor A, Deans JP. Expression of MS4A and TMEM176 Genes in Human B Lymphocytes. Front Immunol. 2013;4:195. doi: 10.3389/fimmu.2013.00195. PMID: 3711070. [DOI] [PMC free article] [PubMed] [Google Scholar]