Abstract
Background
Early stage breast cancer patients face a series of complex treatment decisions, with the first typically being choice of locoregional treatment. There is a need for tools to support patients in this decision-making process.
Methods
We developed an innovative online locoregional treatment tool based on International Patient Decision Aids Standards (IPDAS) criteria. We evaluated its impact on patient knowledge about treatment and appraisal of decision making in a pilot study using a clinical sample of newly diagnosed breast cancer patients who were randomized to view the decision aid website first or complete a survey prior to viewing the decision aid. Differences in knowledge and decision appraisal between the two groups were compared using t-tests and chi-square tests. Computer-generated preferences for treatment were compared to patients’ stated preferences using chi-square tests.
Results
101 newly diagnosed patients were randomized to view the website first or take a survey first. Women who viewed the website first had slightly higher, though not significantly, knowledge about surgery (P=0.29) and reconstruction (P=0.10) than the survey-first group. Those who viewed the website first also appraised their decision process significantly more favorably than did those who took the survey first (P<0.05 for most decision outcomes). There was very good concordance between computer-suggested and stated treatment preferences.
Conclusion
This pilot study suggests that an interactive decision tool shows promise for supporting early stage breast cancer patients with complicated treatment decision-making.
Keywords: decision-making, locoregional treatment, breast cancer, quality
1. Introduction
Patients newly diagnosed with early stage breast cancer face a series of complex decisions across the continuum of their care. The first, and often most difficult, decision these patients make involves their locoregional treatment planning. For most women with early stage breast cancer, this decision requires a choice between mastectomy (with or without reconstruction) and lumpectomy with radiation (breast conservation therapy, or BCT) [1–4]. There is strong professional consensus that most women with early stage breast cancer are candidates for BCT [5–6], and many surgeons endorse BCT in these patients [7–8]. However, a substantial, though still minority, number of women choose mastectomy based on factors that are prioritized by patients, such as fear of recurrence or avoiding radiation [9–11]. In addition, data shows that rates of mastectomy (including contralateral prophylactic mastectomy) are on the rise [12–15].
This trend has raised concerns that patients are not adequately informed about treatment choices [16–18]. Research suggests that these decisions may not meet the criteria of a high quality decision, defined as one that is informed and consistent with the decision-maker’s underlying values [19]. While patients do make choices based on factors important to them, it remains unclear if these choices are truly based on accurate understanding of the treatment risks and benefits. Though some research shows that women who choose mastectomy are well informed [11], other studies have found a strikingly low level of knowledge about surgical treatment risks and benefits, even among patients who were recently treated [16–17, 20]. Further, many women do not receive their preferred amount of information in the decision-making process [22–22] or believe that their preferences are incorporated into treatment decision-making [23–26]. This complex interplay between knowledge and patient values underscores the importance of decision tools for helping women with both components of a high quality decision.
Decision aids focusing on surgical treatment for breast cancer patients have been developed and evaluated, including a decision board [27], an audiobooklet [28], an interactive CD-ROM [29], and tailored websites [11, 30]. While these aids were associated with improvements in knowledge about surgical treatment options, their impact on treatment choice was mixed. Other decision aids focused on early stage breast cancer treatment have been developed but to date not evaluated for impact on decision appraisal or treatment utilization [31]. A recent observational study found that an online breast cancer treatment decision aid (i.e, BresDEX) was associated with greater readiness to make a decision about surgery, though not with surgery type [32]. Despite the recommendation of the International Patient Decision Aids Standards (IPDAS) committee that decision aids should include preference clarification exercises [33], few breast cancer treatment decision aids have done so, and none have provided women with feedback with which to make their decisions. Furthermore, although research has shown that women should be made aware of the option of breast reconstruction prior to the initial surgical treatment decision [34], existing decision aids either do not describe reconstruction options or addressed it in a limited way.
We therefore conducted a pilot study to develop and evaluate a web-based decision aid focused on locoregional breast cancer treatment that is unique from existing breast cancer patient information or decision aids in three ways: 1) provision of comprehensible information about treatment risks and benefits, 2) an interactive values clarification exercise with feedback, and 3) guidance for communicating with clinicians. We sought to determine whether this tool could improve the quality of local treatment decisions (by improving knowledge about treatment and appraisal of the decision process) through a randomized study among newly diagnosed early stage breast cancer patients at two cancer centers. We hypothesized that patients who were randomized to view the website first (“intervention”) would have higher knowledge scores and more decision satisfaction than patients who were randomized to complete a survey prior to viewing the website (“control”).
This study was approved by the Institutional Review Board of the University of Michigan and Karmanos Cancer Institute.
2. Methods
2.1 Development of the decision aid
We worked with the NCI-funded Center of Excellence in Cancer Communication Research at our institution to develop our decision aid, following the criteria outlined by IPDAS [35]. A key feature was of our tool, as recommended by IPDAS [33], was the inclusion of an interactive values clarification exercise. To accomplish this, we used conjoint analysis (CA) to assess patients’ preferences for treatment attributes and provide them with feedback in real time. Conjoint analysis has been used in marketing and mathematical psychology research to assess preferences for products, and is recommended when consumers have to trade off certain product attributes for others (i.e., size for cost) [36–38]. It has been increasingly used to assess preferences for medical treatments [39–41].
Conjoint analysis based preference elicitation exercise: From the literature, a potential set of 3 attributes related to women’s choices for mastectomy (with or without reconstruction) or lumpectomy with radiation was initially generated (risk of the cancer coming back, likelihood of needing radiation therapy, whether or not the natural breast is retained) [17–18, 21, 42]. We then conducted interviews with 5 surgeons and 4 oncologists to further develop a potential attribute list (these interviews also provided content for the locoregional and chemotherapy sections of the decision aid). These interviews generated an additional 6 attributes that physicians felt might be considered by patients in their treatment decisions (cosmesis/how the breast(s) looks after surgery, recovery time, need for a second surgery, sensation following surgery, whether or not implants were needed). These 9 attributes were then shared with 10 breast cancer survivors who had received different treatments for their cancer, with the goal of narrowing the attribute set to 4–6 attributes, each with 2–3 levels, to ensure that the final exercise could be completed by the target population. Each survivor was asked to rate and rank the attributes. Among the 10 survivors interviewed, all ranked the same four among their five “most important.” Interviewers described each of the 9 attributes with a set of “levels” and survivors provided feedback regarding how to phrase the levels in a way that they thought newly diagnosed patients would understand. From these interviews, the top attributes and associated levels were determined.
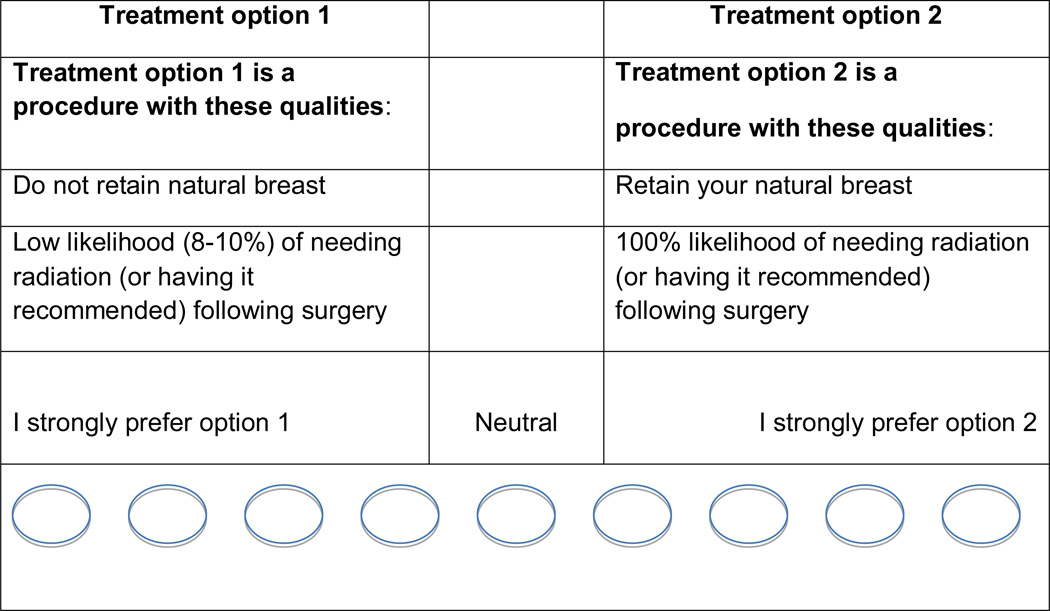
This process resulted in a set of 4 key treatment attributes, each having 2 levels (descriptors of each attribute): 1) risk of the cancer coming back [5% risk of the cancer coming back in 10 years, 8% chance of cancer coming back in 10 years], 2) likelihood of needing radiation therapy [very high chance (close to 100%), low chance (8–10%), which is the case for some women who receive mastectomy], 3) whether or not one’s natural breast is retained [yes, no], and 4) how the breast looks after surgery [very good chance breasts will look similar, no chance breasts will look similar]. For the final exercise, patients were presented with 16 scenarios in the tool based on combinations of these attributes and levels. An example of a screen shot for one scenario is provided in Figure 1. Using responses from each patient, a “best fit” treatment was determined and provided to the patient as feedback using the following language, “Based on the responses you just gave, the treatment that seems to be the best fit for you is (mastectomy, mastectomy with reconstruction, lumpectomy with radiation).”
Figure 1.
Example of one screenshot of interactive values clarification exercise using conjoint analysis
Informational components: A primary purpose of the decision aid was to educate women about their disease and treatment options. Information content of our decision tool was developed based on existing information given to newly diagnosed patients at each site, with input from the same 5 surgeons, and 4 medical oncologists as for the attribute development. We also obtained content feedback from 2 radiation oncologists and 2 plastic surgeons. The following sections were offered: 1) about breast cancer, 2) treatment options for breast cancer (including information on initial surgical treatment, radiation treatment, reconstruction, genetic testing, and systemic treatment), 3) talking to your doctors (including questions and tips for making sure patients obtained their desired amount of involvement in the decision), and 4) your preferences (the CA-based exercise). The content was reviewed by our NCI Center of Excellence in Cancer Communication and revised where needed to ensure an 8th grade or below reading level.
2.2 Pre-test
We conducted a pre-test of both the overall content of the decision tool, and of the values clarification exercise specifically with separate groups of breast cancer survivors. Upon completion of an initial version of the content of the website, 5 breast cancer survivors reviewed it in the presence of a research associate and provided feedback on the comprehensiveness of the content and on the flow of the information. Based on this feedback, the content was then revised, including simplifying the language, adding some images to enhance the content, and providing a printable comparison table of treatment options for patients.
To specifically determine whether the CA-based approach for values clarification was more difficult than a more simple approach [45], we conducted a separate pre-test comparing our method to a rating/ranking method. Eleven breast cancer survivors who had completed treatment (different from those who reviewed the content) were randomized to complete the conjoint exercise or a rating/ranking task first, and the other method afterward. They were interviewed to determine which method they preferred and why. Of the 11 participants, 10 preferred the conjoint method because they thought it was more useful in helping them think through the factors important in their decision. Although 6 thought the conjoint method was more difficult, all still felt it was better in helping with decision-making. We therefore decided to retain the conjoint method for preference elicitation in our decision aid.
2.3 Measures
There were two primary outcomes for the evaluation, both components of a high quality decision: 1) knowledge about initial surgical treatment and knowledge about breast reconstruction, and 2) participant appraisal of the decision process, including her perceived concordance between the treatment received (or leaning toward) and her values and her overall satisfaction with decision making. The surgical knowledge measure included 3 true/false questions asking about the risk and benefits of mastectomy vs. BCT. A score of 3 indicated perfect surgical treatment knowledge and a score of 0 indicated no knowledge. Reconstruction knowledge included 2 true/false questions (range 0–2). We evaluated patient appraisal of the decision process through two measures; decision satisfaction and perceived values concordance. Perceived values concordance was assessed by asking, “The surgical treatment decision matches my values” (strongly agree to strongly disagree). We assessed decision satisfaction using an adapted version of the decision satisfaction scale [46].
2.4. Preparation for evaluation
To prepare for the clinic-based evaluation, we conducted three meetings with the breast cancer clinicians and/or their clinical teams (nurses, nurse practitioners, physician assistants and administrators) of the first study site to ensure that the study, as well as any follow up dissemination efforts, would fit within the clinical workflow. These meetings informed the approach used to recruit newly diagnosed patients to the study, as outlined below. All clinicians and staff strongly endorsed the decision tool, and agreed that newly diagnosed patients would benefit from being offered the opportunity to view it prior to making their final treatment decision. In fact, because of the support for the decision tool, we developed a modified RCT where patients were randomized to view the website first (“intervention”), or to take a survey prior to viewing the website (“control”), as this allowed all eligible patients a chance to view the website. We were further advised that because of the overwhelming amount of information patients received prior to their appointment, the tool should be offered to patients at the time of their surgical consult.
2.4 Evaluation of the decision aid
Participants were eligible if they were newly diagnosed with early stage breast cancer (stage 0, I or II), age 25–80 and receiving care at either cancer center from 11/2009 through 2/2011, who had not yet made their locoregional treatment decision. Because stage was not always known at the time of recruitment, patients were eligible for the study if the surgeon determined that they were making a choice between mastectomy and BCT. Patients with cancer recurrence, metastatic disease, receiving neoadjuvant chemotherapy, or otherwise unable to make a choice between mastectomy and BCT (i.e., being recommended one of the procedures by their surgeon) were not eligible.
As noted above, the recruitment and enrollment process was informed by the clinical staff and adapted to the workflow. Potential participants were identified by the administrative staff or the surgeon at each site. Upon arrival, potentially eligible patients were directed to the study coordinator who was based in the clinic. The coordinator briefly described the study to them as “a website that is designed to help women with breast cancer make treatment decisions” and was designed for those who had not yet made a locoregional treatment decision. Participants were told they would complete a short survey before or after being given the log-in information. Those interested and willing completed informed consent and were randomized in the clinic to complete the survey or given the log-in information with a survey to mail back after viewing the website. Randomization was done simply by assigning every other consented patient to website first or survey first, and coordinators were not blinded to the condition. The website could be viewed in the clinic or from their home. Participants randomized to complete the survey first (“control”) were given log-in information after the survey was returned to the coordinator. Patients then proceeded to meet with their surgeon and other breast clinicians as needed. All subjects were given a $50 gift card to a local retail store.
Surveys were identified with a study ID and an “W” for website first and “S” for survey first on the covering page. Completed de-identified surveys were entered into Microsoft access by the study team, and then transferred into STATA 11.0 for analysis [47].
2.5 Analysis
We first described the sample of patients who enrolled into the study, as well as the distribution of responses to the knowledge, values concordance, and decision satisfaction measures. To test our hypothesis that patients who viewed the website first would have higher knowledge scores and more decision satisfaction than patients who completed the survey first, we compared the responses of patients who viewed the website prior to taking the survey (“intervention”) to those who completed the survey prior to viewing the website (“control”). We compared mean scores for women in each group using t-tests.
We then conducted exploratory analyses of the results of the preference elicitation exercise among those who chose to view that portion of the website (89%). First we tabulated the most important attributes across the sample. We then conducted comparisons between the treatment generated by the computer program to the treatment that the patient received or was leaning toward using chi-square tests. We did these comparisons overall and for individual patients.
3. Results
3.1 Study sample
A total of 110 patients were recruited (86 from study site A and 24 from study site B). Because of the recruitment method that required interested patients to approach the study team, we cannot accurately determine the participation rate for this study among all potentially eligible patients. However, we were able to estimate from data on numbers of new potentially eligible patients at each site, that approximately 50% of them approached the study team at each site. Of those who approached the study team (total N=137), 80.2% consented to be in the study, which resulted in the N of 110. The majority (N=105, 95%) viewed the website from home. Complete data including both log-in and survey data was obtained from 101, and was relatively equally divided between website first (N=51) and survey first (N=50) groups. Of these, the mean age was 53 (range: 30–80). Eighty-six percent were white and 14% were black. Two-thirds (66%) had educational attainment of some college or more.
3.2 Knowledge
We found that overall surgical knowledge was moderate (mean = 3.0, range: 0–4), and that overall reconstruction knowledge was low (mean = 1.4, range: 0–3). Although not statistically significant, patients who viewed the website first had slightly higher scores on both surgery and reconstruction knowledge; 3.1 vs. 2.9 for surgical knowledge (P=0.29) and 1.6 vs. 1.2 for reconstruction knowledge (P=0.10) (Table 1).
Table 1.
Treatment Knowledge and Appraisal of Decision Making among Study Participants (N=101)
| Website first (N=51)1 | Survey first (N=50)2 | |
|---|---|---|
|
Treatment Knowledge Surgical knowledge (Range: 0–4) |
3.1 | 2.9 |
| Reconstruction knowledge (Range: 0–3) | 1.6 | 1.2^ |
|
Decision Appraisal (5-pt scale from 1: strongly agree to 5:strongly disagree) |
||
| I know the benefits of mastectomy | 1.3 | 1.7^ |
| I know the risks of mastectomy | 1.6 | 2.0^ |
| I know the benefits of lumpectomy w radiation | 1.6 | 1.8 |
| I know the risks of lumpectomy w radiation | 1.6 | 2.1^ |
| I’m aware of the choices I have for treatment | 1.6 | 1.8 |
| It’s clear what choice is best for me | 2.0 | 2.3^ |
| I feel I’ve made an informed choice | 1.4 | 1.7* |
| I am satisfied with my treatment decision | 1.4 | 1.8^ |
|
Perceived values concordance (5-pt scale from 1: strongly agree to 5: strongly disagree) The surgical treatment decision matches my values |
1.6 | 1.9^ |
Participants viewed the decision tool website prior to completing the survey
Participants completed the survey prior to viewing the decision tool website
P<1.0,
P<0.05
3.3 Appraisal of decision-making
Patients who viewed the website first scored more favorably on all decision satisfaction items than did women who took the survey first. Website-first patients were more likely to disagree that they felt the treatment decision was hard to make and that they were unsure what decision to make compared to survey first patients, although not all differences were statistically significant. They more often agreed that they understood the risk and benefits associated with different surgical options, that they were aware of their choices, and that they were satisfied with their treatment (Table 2). In addition, patients viewing the website first significantly more often agreed that “the treatment decision matches my values” (1.61 vs. 1.85, P<0.05) (Table 1).
Table 2. Concordance between Computer-suggested Preferences and Preferences Stated by Participants (N=92).
Concordance between computer suggested and stated treatment preferences (N=92)1
| Treatment | Computer- suggested |
Stated |
|---|---|---|
| Lumpectomy with radiation | 45% | 47% |
| Mastectomy with reconstruction | 49% | 37% |
| Mastectomy considering reconstruction2 | NA | 8% |
| Mastectomy without reconstruction | 6% | 8% |
Overall, for 96% (N=88) patients, there was concordance between computer-suggested and stated preferences, including a stated preference for “mastectomy considering reconstruction” to be concordant with a computer-suggested preference for “mastectomy with reconstruction”
The computer was not able to make a suggestion to “consider reconstruction,” however participants were given this option when entering their stated preference
3.4 Concordance between computer-suggested and stated treatment preferences
Overall, among the subsample who engaged with the interactive exercise (N=92), the most important attributes across all participants were: 1) avoiding cancer coming back, 2) avoiding radiation, and 3) retaining one’s natural breast. There was good concordance between the treatment that the computer suggested (based on patient input), and the treatment that patient indicated she actually received or was planning to receive. The computer suggested lumpectomy with radiation in 45% of patients who completed the exercise and 47% of patients reported receiving or planning to have this treatment. The computer suggested mastectomy with reconstruction for 49% of patients. While 37% of patients indicated they had received or were leaning toward mastectomy with reconstruction, an additional 8% reported “considering reconstruction,” raising the total to 45%. Finally, the computer suggested mastectomy without reconstruction in 6% of women, while 8% reported getting or leaning toward this option (Table 2). When we compared computer-suggested to stated preferences at the individual patient levels, we found concordance for 96% (N=88) patients, if we considered “mastectomy considering reconstruction” to be concordant with mastectomy with reconstruction.
3.5 Participant reactions to the website
In addition to the quantitative results, just under a quarter (23%) of participants provided written comments in the survey. These comments were overwhelmingly positive. For example,
“Very thorough…this was my first time in a hospital/decision making situation and this was so helpful.”
“I can't begin to convey the thanks for having the information your website provided and the ease of the format and way it was set up. …”
4. Discussion and Conclusion
4.1 Discussion
We developed an interactive web-based breast cancer treatment decision tool intended to help newly diagnosed patients with their locoregional treatment decision and evaluated its impact on decision making through a pilot study. The results suggest that our decision tool holds promise for improving the quality of decisions for early stage breast cancer patients, by potentially raising their knowledge about treatments improving appraisal of the process of decision making.
Prior studies of breast cancer treatment decision making have found that surgical treatment decisions are often made without sufficient knowledge, and may be made prematurely [12, 20–22]. Patients have reported making decisions for more extensive treatment, such as mastectomy or double mastectomy, for “peace of mind” [42–44]. Yet whether these decisions are based on accurate understanding of treatment risks and benefits is unclear. The need to improve decision quality, by improving both knowledge and concordance between the patient’s underlying values and the treatment received, has been highlighted [19]. Few existing breast cancer treatment decision aids have included an interactive values clarification exercise, and none have done so and provided patients with feedback in real time. A key innovative component of our web-based tool was the inclusion of such an exercise using conjoint analysis. To our knowledge, this is the first decision tool that has utilized a conjoint analysis based values clarification exercise with real time feedback to breast cancer patients about the treatment that may be the best fit for them. The exercise was well received by patients, and we found that the exercise generated an accurate mapping of the treatment attributes most important to individual patients to the treatments they had planned to receive.
Though our tool produced positive, though not statistically significant, results in two academic treatment centers, implementation studies suggest that it will be challenging to deploy such tools in community practices [48]. Research indicates there is considerable variation in the delivery of breast cancer treatment information, including diagnosis and initial surgical consultation, in community practices [49–50], and that not all breast surgeons endorse integrating decision tools into their fast-paced and often overwhelming clinical environment. [51] Yet these are often the very sites that do not routinely use patient-facing decision tools in the delivery of breast cancer treatment information [50]. Considerable preparation and engagement of key clinical stakeholders, including surgeons, nurses, and administrative staff, at each site ensured our tool was well integrated into the clinical workflow. Yet a downside of this integration was the inability to offer to the tool to patients prior to their surgical consult, which may have prepared them better for decision-making. More research is needed to assess integration and utilization in various practice settings in order to ensure that interventions such as our decision tool are optimized for patients treated across the broad landscape of breast cancer treatment delivery.
Our study also highlights the fact that decision tools cannot remain static over time; they must evolve and reflect advances in oncologic treatment. The interactive tool used in this study is more relevant to contemporary breast cancer treatment planning because it included information regarding reconstructive surgery. Future tools will require modification to reflect the downstream effects of the BCT vs. mastectomy decision on the potential axillary surgery needs, and to incorporate these considerations into initial treatment planning. Patients found to harbor limited volume metastatic disease in the sentinel lymph nodes will usually be able to avoid undergoing the complete axillary lymph node dissection [52–53], potentially offering a meaningful advantage to patients because of the corresponding diminished risk of experiencing lymphedema. Postmastectomy radiation needs can also influence immediate reconstruction eligibility. Thus the extent to which a patient prioritizes avoiding lymphedema as well as having immediate reconstruction represent another set of appropriate factors that should be incorporated into future breast cancer treatment decision tools.
Some limitations to this study should be noted. Perhaps most importantly, this was a relatively small pilot study, making it difficult to have sufficient power to detect differences between groups and the findings need to be replicated in a large, more well powered randomized trial. Although we were able to recruit newly diagnosed patients prior to making their surgical treatment decisions, the results cannot be generalized to different settings particularly non-academic medical centers. Moreover, we cannot determine exactly when the treatment decision was made. While we desired for women to view the decision aid prior to their surgical consult, this approach did not work within the clinical workflow. Understanding how to integrate decision aids into the faced paced nature of surgical decision-making is an area in need of further research. The conjoint analysis based values clarification exercise was limited by the attributes selected, the use of an adaptive versus choice-based approach, as well as by the subjective linking of the results of trade-off preferences to actual treatments. Future work is needed to refine these types of exercises in decision aids. We were not able to fully assess potential associations between socioeconomic factors and attitudes toward treatment, which may have impacted how women interacted with the decision aid. Finally, we did not have information on treatment received for all patients, making it difficult to fully assess the impact of the tool on treatment utilization and on concordance between what the computer suggested and what patients actually received.
4.3 Conclusion
Despite these limitations, our pilot study of 101 newly diagnosed patients from two academic medical settings provides important results upon which to base further work to improve the quality of breast cancer treatment decisions. Our findings indicate that a treatment decision tool implemented at the time of the surgical consult offers patients information that is useful in their decision-making. These results provide the basis on which to base hypotheses for future, larger-scale RCTs of breast cancer treatment decision aids. Our tool was both well utilized and positively appraised by patients making these decisions in two academic teaching practices. Further work to develop and evaluate decision tools across the continuum of breast cancer care, including systemic treatment and survivorship care planning, and across varied clinical practice settings is needed.
Key Points.
Breast cancer patients found an interactive decision tool focused on surgical treatment to be helpful in their decision making
An interactive values clarification exercise was useful to patients in understanding trade-offs associated with surgical treatment options
Challenges to implementing decision tools into surgical practices remain
Acknowledgments
Sarah T. Hawley, Jennifer J. Griggs, Lisa Newman and Steven J. Katz conceptualized the study. These authors plus Mary Ann Kosir designed and implemented the study. Sarah T. Hawley conducted the data analysis. All authors assisted in interpreting the results. The complete first draft of the paper was written by Sarah T. Hawley, with assistance from Jennifer J. Griggs and Steven J. Katz. All authors commented on the complete first draft as well as subsequent drafts and revisions. All authors approved the final version. The authors acknowledge the assistance of Rebecca Morrison in editing and formatting versions of the manuscript. Sarah T. Hawley acts as guarantor of for the manuscript.
Funding: This work was funded by grant number R21 CA129859 to the University of Michigan.
Footnotes
Disclosures:
Conflict of interest: Sarah T. Hawley, Jennifer J. Griggs, Lisa Newman, Mary Ann Kosir and Steven J. Katz declare that they have no conflict of interest.
Ethical approval: “All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
References
- 1.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E. Twenty year follow up of a randomized study of breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 2.(EBCTCG) EBCTCG. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Morrow M. Rational Local Therapy for Breast Cancer. N Engl J Med. 2002;347(16):1270–1271. doi: 10.1056/NEJMe020112. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 5.Clinical Practice Guidelines. Mastectomy or lumpectomy? The choice of operation for clinical stages I and II breast cancer. CMAJ. 1998;158(Suppl 3):S15–S21. [PubMed] [Google Scholar]
- 6.Morrow M, Strom EA, Bassett LW, Dershaw DD, Fowble B, Giuliano A, Harris JR, O'Malley F, Schnitt SJ, Singletary SE, Winchester DP. Standard for breast conservation therapy in the management of invasive breast carcinoma. CA Cancer J Clin. 2002;52(5):277–300. doi: 10.3322/canjclin.52.5.277. [DOI] [PubMed] [Google Scholar]
- 7. Katz surgeons. [Google Scholar]
- 8. Opatt surgeons. [Google Scholar]
- 9.Molenaar S, Oort F, Sprangers M, Rutgers E, Luiten E, Mulder J, de Haes H. Predictors of patients' choices for breast-conserving therapy or mastectomy: a prospective study. Br J Cancer. 2004;90(11):2123–2130. doi: 10.1038/sj.bjc.6601835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawley ST, Griggs JJ, Hamilton AS, Graff JJ, Janz NK, Morrow M, Jagsi R, Salem B, Katz SJ. Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst. 2009;101(19):1337–1347. doi: 10.1093/jnci/djp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins ED, Moore CP, Clay KF, Kearing SA, O'Connor AM, Llewellyn-Thomas HA, Barth RJ, Jr, Sepucha KR. Can women with early-stage breast cancer make an informed decision for mastectomy? J Clin Oncol. 2009 Feb 1;27(4):519–525. doi: 10.1200/JCO.2008.16.6215. [DOI] [PubMed] [Google Scholar]
- 12.Tuttle TM, Abbott A, Arrington A, Rueth N. The increasing use of prophylactic mastectomy in the prevention of breast cancer. Curr Oncol Rep. 2010;12(1):16–21. doi: 10.1007/s11912-009-0070-y. [DOI] [PubMed] [Google Scholar]
- 13.Kurian AW, Lichtensztajn DY, Keegan TH, Nelson DO, Clarke CA, Gomez SL. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998–2011. JAMA. 2014;312(9):902–914. doi: 10.1001/jama.2014.10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14].Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in Mastectomy for Early Stage Breast Cancer. JAMA Surgery. 2015;150(1):9–16. doi: 10.1001/jamasurg.2014.2895. [DOI] [PubMed] [Google Scholar]
- 15.Pesce CE, Liederbach E, Czechura T, Winchester DJ, Yao Km. Changing surgical trends in young patients with early stage breast cancer, 2003 to 2010: a report from the National Cancer Data Base. J Am Coll Surg. 2014;219(1):19–28. doi: 10.1016/j.jamcollsurg.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 16.Fagerlin A, Lakhani I, Lantz PM, Janz NK, Morrow M, Schwartz K, Deapen D, Salem B, Liu L, Katz SJ. An informed decision? Breast cancer patients and their knowledge about treatment. Patient Educ Couns. 2006;64(1–3):303–312. doi: 10.1016/j.pec.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Covelli AM, Baxter NN, Fitch MI, McCready DR, Wright FC. 'Taking Control of Cancer': Understanding Women's Choice for Mastectomy. Ann Surg Oncol. 2015;22(2):383–391. doi: 10.1245/s10434-014-4033-7. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg SM, Partridge AH. Contralateral Prophylactic Mastectomy: An Opportunity for Shared Decision Making. JAMA Surg. 2014 doi: 10.1001/jamasurg.2013.5713. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sepucha KR, Belkora JK, Chang Y, Cosenza C, Levin CA, Moy B, Partridge A, Lee CN. Measuring decision quality: psychometric evaluation of a new instrument for breast cancer surgery. BMC Med Inform Decis Mak. 2012;12:51. doi: 10.1186/1472-6947-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawley ST, Fagerlin A, Janz NK, Lantz PM, Katz SJ. Racial/Ethnic Disparities in Knowledge about Risks and Benefits of Breast Cancer Treatment: Does It Matter Where You Go? Health Serv Res. 2008;43(4):1366–1387. doi: 10.1111/j.1475-6773.2008.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nold RJ, Beamer RL, Helmer SD, McBoyle MF. Factors influencing a woman's choice to undergo breast conserving surgery versus modified radical mastectomy. Am J Surg. 2000;180(6):413–418. doi: 10.1016/s0002-9610(00)00501-8. [DOI] [PubMed] [Google Scholar]
- 22.O'Leary KA, Estabrooks CA, Olson K, Cumming C. Information acquisition for women facing surgical treatment for breast cancer: influencing factors and selected outcomes. Patient Educ Couns. 2007;69(1–3):5–19. doi: 10.1016/j.pec.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Janz NK, Wren PA, Copeland LA, Lowery JC, Goldfarb SL, Wilkins EG. Patient-physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol. 2004;22(15):3091–3098. doi: 10.1200/JCO.2004.09.069. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien MA, Charles C, Whelan TJ, Ellis PM, Gafni A, Lovrics P. Women's perceptions of their involvement in treatment decision making for early stage breast cancer. Support Care Cancer. 2013;21(6):1717–1723. doi: 10.1007/s00520-013-1718-6. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien MA, Ellis PM, Whelan TJ, Charles C, Gafni A, Lovrics P, Mukherjee SD, Hodgson N. Physician-related facilitators and barriers to patient involvement in treatment decision making in early stage breast cancer: perspectives of physicians and patients. Health Expect. 2013;21(6):1717–1723. doi: 10.1111/j.1369-7625.2011.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keating NL, Guadagnoli E, Landrum MB, Borbas C, Weeks JC. Treatment decision making in early-stage breast cancer: should surgeons match patients' desired level of involvement? J Clin Oncol. 2002;20(6):1473–1479. doi: 10.1200/JCO.2002.20.6.1473. [DOI] [PubMed] [Google Scholar]
- 27.Whelan T, Levine M, Willan A, Gafni A, Sanders K, Mirsky D, Chambers S, O'Brien MA, Reid S, Dubois S. Effect of a decision aid on knowledge and treatment decision making for breast cancer surgery: a randomized trial. JAMA. 2004;292(4):435–441. doi: 10.1001/jama.292.4.435. [DOI] [PubMed] [Google Scholar]
- 28.Goel V, Sawka CA, Thiel EC, Gort EH, O'Connor AM. Randomized trial of a patient decision aid for choice of surgical treatment for breast cancer. Med Decis Making. 2001;21(1):1–6. doi: 10.1177/0272989X0102100101. [DOI] [PubMed] [Google Scholar]
- 29.Molenaar S, Sprangers MA, Rutgers EJ, Luiten EJ, Mulder J, Bossuyt PM, van Everdingen JJ, Oosterveld P, de Haes HC. Decision support for patients with early-stage breast cancer: effects of an interactive breast cancer CDROM on treatment decision, satisfaction, and quality of life. J Clin Oncol. 2001;19(6):1676–1687. doi: 10.1200/JCO.2001.19.6.1676. [DOI] [PubMed] [Google Scholar]
- 30.Jibaja-Wess M, Volk RJ, Granchi TS, Neff NE, Robinson EK, Spann SJ, Aoki N, Friedman LC, Beck JR. Entertainment education for breast cancer surgery decisions: a randomized trial among patients with low health literacy. Patient Educ Couns. 2011 Jul;84(1):41–48. doi: 10.1016/j.pec.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Ottawa Hospital Research Institute: Catalogue of Decision Aids. [accessed on 5/26/2015]; Available at http://decisionaid.ohri.ca/index.html. [Google Scholar]
- 32.Sivell S, Edwards A, Manstead AS, Reed MW, Caldon L, Collins K, Clements A, Elwyn G, BresDex Group Increasing readiness to decide and strengthening behavioral intentions: evaluating the impact of a web-based patient decision aid for breast cancer treatment options (BresDex: www.bresdex.com) Patient Educ Couns. 2012;88(2):209–217. doi: 10.1016/j.pec.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Elwyn G, O'Connor A, Stacey D, Volk R, Edwards A, Coulter A, Thomson R, Barratt A, Barry M, Bernstein S, Butow P, Clarke A, Entwistle V, Feldman-Stewart D, Holmes-Rovner M, Llewellyn-Thomas H, Moumjid N, Mulley A, Ruland C, Sepucha K, Sykes A, Whelan T International Patient Decision Aids Standards (IPDAS) Collaboration. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333(7565):417. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alderman AK, Hawley ST, Waljee J, Mujahid M, Morrow M, Katz SJ. Understanding the impact of breast reconstruction on the surgical decision-making process for breast cancer. Cancer. 2008;112(3):489–494. doi: 10.1002/cncr.23214. [DOI] [PubMed] [Google Scholar]
- 35.Green PE, Rao VR. Conjoint measurement for quantifying judgemental data. Journal of Marketing Research. 1971;8:355–363. [Google Scholar]
- 36.Ramirez Jose Manuel. Measuring: from Conjoint Analysis to Integrated Conjoint Experiments. Journal of Quantitative Methods for Economics and Business Administration. 2009;9:28–43. [Google Scholar]
- 37.Ryan M, Bate A, Eastmond CJ, Ludbrook A. Use of discrete choice experiments to elicit preferences. Qual Health Care. 2001;10(Suppl 1):i55–i60. doi: 10.1136/qhc.0100055... [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, Johnson FR, Mauskopf J. Conjoint analysis applications in health--a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Wouters H1, Maatman GA, Van Dijk L, Bouvy ML, Vree R, Van Geffen EC, Nortier JW, Stiggelbout AM. Trade-off preferences regarding adjuvant endocrine therapy among women with estrogen receptor-positive breast cancer. Ann Oncol. 2013 Sep;24(9):2324–2329. doi: 10.1093/annonc/mdt195. [DOI] [PubMed] [Google Scholar]
- 40.Phillips KA, Maddala T, Johnson FR. Measuring preferences for health care interventions using conjoint analysis: an application to HIV testing. Health Serv Res. 2002;37(6):1681–1705. doi: 10.1111/1475-6773.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryan M. Using conjoint analysis to take account of patient preferences and go beyond health outcomes: an application to in vitro fertilization. Soc Sci Med. 1999;48(4):535–546. doi: 10.1016/s0277-9536(98)00374-8. [DOI] [PubMed] [Google Scholar]
- 42.Mac Bride MB, Neal L, Dilaveri CA, Sandhu NP, Hieken TJ, Ghosh K, Wahner-Roedler DL. Factors associated with surgical decision making in women with early-stage breast cancer: a literature review. J Womens Health (Larchmt) 2013;22(3):236–242. doi: 10.1089/jwh.2012.3969. [DOI] [PubMed] [Google Scholar]
- 42.Rendle KA, Halley MC, May SG, Frosch DL. Redefining Risk and Benefit: Understanding the Decision to Undergo Contralateral Prophylactic Mastectomy. Qual Health Res. 2014 doi: 10.1177/1049732314557085. pii: 1049732314557085 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Hamelinck VC, Bastiaannet E, Pieterse AH, Jannink I, van de Velde CJ, Liefers GJ, Stiggelbout AM. Patients' preferences for surgical and adjuvant systemic treatment in early breast cancer: a systematic review. Cancer Treat Rev. 2014;40(8):1005–1018. doi: 10.1016/j.ctrv.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Pignone MP, Brenner AT, Hawley ST, Sheridan SL, Lewis CL, Jonas DE, Howard K. Conjoint Analysis Versus Rating and Ranking for Values Elicitation and Clarification in Colorectal Cancer Screening. J Gen Intern Med. 2012;27(1):45–50. doi: 10.1007/s11606-011-1837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holmes-Rovner M, Kroll J, Schmitt N, Rovner DR, Breer ML, Rothert ML, Padonu G, Talarczyk G. Patient satisfaction with health care decisions: the satisfaction with decision scale. Med Decis Making. 1996;16(1):58–64. doi: 10.1177/0272989X9601600114. [DOI] [PubMed] [Google Scholar]
- 47.StataCorp. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 48.Elwyn G, Scholl I, Tietbohl C, Mann M, Edwards AG, Clay C, Légaré F, van der Weijden T, Lewis CL, Wexler RM, Frosch DL. "Many miles to go …": a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13(Suppl 2):S14. doi: 10.1186/1472-6947-13-S2-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The Challenge of Individualizing Treatment for Breast Cancer, National Cancer Institute P01 (P01 CA 163233) S. Katz, PI
- 50.Katz SJ, Hawley ST, Morrow M, Griggs JJ, Jagsi R, Hamilton AS, Graff JJ, Friese CR, Hofer TP. Coordinating cancer care: patient and practice management processes among surgeons who treat breast cancer. Med Care. 2010;48(1):45–51. doi: 10.1097/MLR.0b013e3181bd49ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caldon LJ1, Collins KA, Reed MW, Sivell S, Austoker J, Clements AM, Patnick J, Elwyn G, BresDex Group Clinicians' concerns about decision support interventions for patients facing breast cancer surgery options: understanding the challenge of implementing shared decision-making. Health Expect. 2011 Jun;14(2):133–146. doi: 10.1111/j.1369-7625.2010.00633.x. Epub 2010 Oct 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guiliano AE, Chung AP. Long term follow up confirms the oncologic safely of sentinel node biopsy without axillary dissetion in node negative breast cancer patients. Ann Surg. 2010;251(4):601–603. doi: 10.1097/SLA.0b013e3181d6115f. [DOI] [PubMed] [Google Scholar]
- 53.Recht A, Edge SB, Solin LJ, Robinson DS, Estabrook A, Fine RE, Fleming GF, Formenti S, Hudis C, Kirshner JJ, Krause DA, Kuske RR, Langer AS, Sledge GW, Jr, Whelan TJ, Pfister DG, American Society of Clinical Oncology Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19(5):1539–1569. doi: 10.1200/JCO.2001.19.5.1539. [DOI] [PubMed] [Google Scholar]