Abstract
Thirteen-lined ground squirrels (Ictidomys tridecemlineatus) have an extraordinary capacity to withstand prolonged and profound reductions of blood flow and oxygen delivery to brain without incurring any cellular damage. As such, the hibernation torpor of I. tridecemlineatus provides a valuable model of tolerance to ischemic stress. Herein, we report that during hibernation torpor, a marked reduction in the phosphorylation of the ribosomal protein S6 (rpS6) occurs within the brains of I. tridecemlineatus. Of note, rpS6 phosphorylation was shown to increase in the brains of rats that underwent an occlusion of the middle cerebral artery. However, such an increase was attenuated after the implementation of an ischemic preconditioning paradigm. In addition, cultured cortical neurons treated with the rpS6 kinase (S6K) inhibitors, D-glucosamine or PF4708671, displayed a decrease in rpS6 phosphorylation and a subsequent increase in tolerance to oxygen/glucose deprivation, an in vitro model of ischemic stroke. Collectively, such evidence suggests that the down regulation of rpS6 signal transduction may account for a substantial part of the observed increase in cellular tolerance to brain ischemia that occurs during hibernation torpor and after ischemic preconditioning. Further identification and characterization of the mechanisms used by hibernating species to increase ischemic tolerance may eventually clarify how the loss of homeostatic control that occurs during and after cerebral ischemia in the clinic can ultimately be minimized and/or prevented.
Introduction
The ability to hibernate is arguably one of the most dramatic examples of phenotypic plasticity (Carey et al. 2003). Hibernators undergo a complex series of physiological and behavioral transformations in response to periods of high-energy demand that are coupled with reductions in energy availability within their respective environments (Carey et al. 2003). During the course of hibernation torpor whole-body metabolism drops to a mere fraction of its basal level (i.e. ~ < 5% of normal) (Storey 2003). In line with such a profound overall reduction, cerebral blood flow (CBF) has been noted to fall to severely ischemic levels during torpor (Frerichs et al. 1994). Despite these intense changes, hibernators are capable of blunting the otherwise destructive effects of ischemia and, in so doing, repeatedly return to the euthermic state without any evidence of damage to tissue.
Beyond the natural ischemic tolerance displayed by hibernators during the torpor phase, tolerance within the brain can also be induced by a variety of stimuli through the implementation of preconditioning. For example, sub-lethal ischemic challenges (Chen & Simon 1997, Barone et al. 1998), the administration of a low dose of lipopolysaccharide (LPS) (Tasaki et al. 1997, Dawson et al. 1999), or hypothermic treatment (Lee et al. 2014) have been shown to be protective in in vivo models of focal ischemia. While the mechanisms governing the aforementioned have yet to be fully elucidated, it is likely that overlap exists between those mechanisms mediating protection during hibernation torpor and those that increase resistance to ischemia after preconditioning.
One of the common mechanisms underlying tolerance to ischemia may be the inhibition of protein synthesis. Reduction in the capacity of protein synthesis by knocking down translational components has been shown to increase longevity in both yeast and worms (Kaeberlein & Kennedy 2007). Critically, it has been shown that hibernating animals display a marked reduction in protein synthesis (Frerichs et al. 1998) and further that the administration of protein synthesis inhibitors (e.g. cycloheximide) reduces infarct volume after a focal ischemia in rats (Snider et al. 2001). During hibernation torpor, both the initiation and elongation steps of protein synthesis are suppressed in the brain (Frerichs et al. 1998).
Ribosomal protein S6 (rpS6) is one of thirty-three 40S ribosomal proteins (Wool et al. 1996) and has been implicated as a key regulator in the biogenesis of translational components (Fumagalli & Thomase 2000). Phosphorylation sites within the rpS6 have been mapped to five clustered residues (Ser 235, Ser 236, Ser 240, Ser 244, and Ser 247) (Krieg et al. 1988), which are conserved from drosophila to higher mammals (Krieg et al. 1988, Fumagalli & Thomase). Early studies demonstrated a temporal correlation between rpS6 phosphorylation and the initiation of protein synthesis following mitogenic/nutritional stimuli (Kruppa & Clemens 1984). This model was further supported by a series of experiments which demonstrated the intimate spatial relationship of rpS6 with tRNA, initiation factors and mRNA (Nygard & Nilsson 1990). Of note, attempts to establish a casual relationship between rpS6 phosphorylation and protein synthesis have yielded conflicting results and as such the precise physiologic role of rpS6 phosphorylation continues to remain controversial (reviewed in (Meyuhas 2008).
Herein we show that rpS6 is hypophosphorylated in the neuronal cells of I. tridecemlineatus during hibernation torpor. We also demonstrate that rats, which have undergone ischemic preconditioning, display suppressed rpS6 phosphorylation under prolonged/severe focal ischemia. Further, we show that cultured cortical neurons display an increase in ischemic tolerance to oxygen/glucose deprivation (OGD) when their rpS6 phosphorylation was reduced via treatment with known S6K inhibitors (i.e. D-glucosamine and PF-4708671). Collectively, these results strongly suggest that the hypophosphorylation of the rpS6 occurs in the brains of animals capable of tolerating an otherwise destructive ischemic insult thereby suggesting that the focused investigation of such biological network may be able to help guide new strategies for preventing and/or treating ischemic stroke.
Materials and Methods
Animal preparation
Thirteen-lined ground squirrel (I. tridecemlineatus)
Thirteen-lined ground squirrels were captured by USDA-licensed trappers (TLS Research, Bartlett, IL). All ensuing animal experiments were approved by the NINDS Animal Care and Use Committee. Both male and female squirrels were used equally for this study, and all animals were between one and three years of age, but because the animals were caught from the wild there is no way to know the exact age of the animals. We acknowledge that our study have a limitation without knowing the exact ages of the hibernating animals. The squirrels were housed, fed and induced to hibernate as has been previously described (Frerichs et al. 1994, Lee et al. 2007). Animals in 7 different phases of the hibernation cycle were differentiated via body temperature (Tb), time, and respiratory rate as previously described (Frerichs et al. 1994, Lee et al. 2007). ART designates active animals housed in a room with an ambient temperature of 21°C (Tb = 34–37°C). ACR designates active animals that had not entered hibernation torpor housed in the cold chamber for 4–5 days (Tb = 34–37°C). EN designates animals, which were in the entrance phase of hibernation (31°C > Tb > 12°C). Torpor animals were selected after they had been in the torpor phase for more than 5 days, aroused and then re-entered the torpor phase for either 1 or 2 additional periods. EH designates animals in early torpor and was defined as 1 day after entrance into torpor (Tb = 5–8°C). LH designates torpor animals that have remained in the torpor state for more than 5 days, but had not yet begun to undergo periodic arousal (Tb = 5–8°C). AR designates animals that were arousing spontaneously without any stress and/or signals (e.g. touching or warming); these animals were characterized by an increased respiratory rate of > 60 breaths/minute accompanied by the persistent of a low body temperature (Tb = 9–12°C). IA designates aroused interbout animals that have been in an active state inside the hibernaculum for at least 7 hours (Tb = 34–37°C). Animals were euthanized via decapitation under anesthesia (5% isoflurane in 30% O2/70% N2O). Brain, heart, skeletal muscle, kidney, liver, thymus, spleen were immediately removed, washed with chilled 0.15M NaCl, frozen instantly using 2-methylbutane and were subsequently stored at −80°C.
Rats
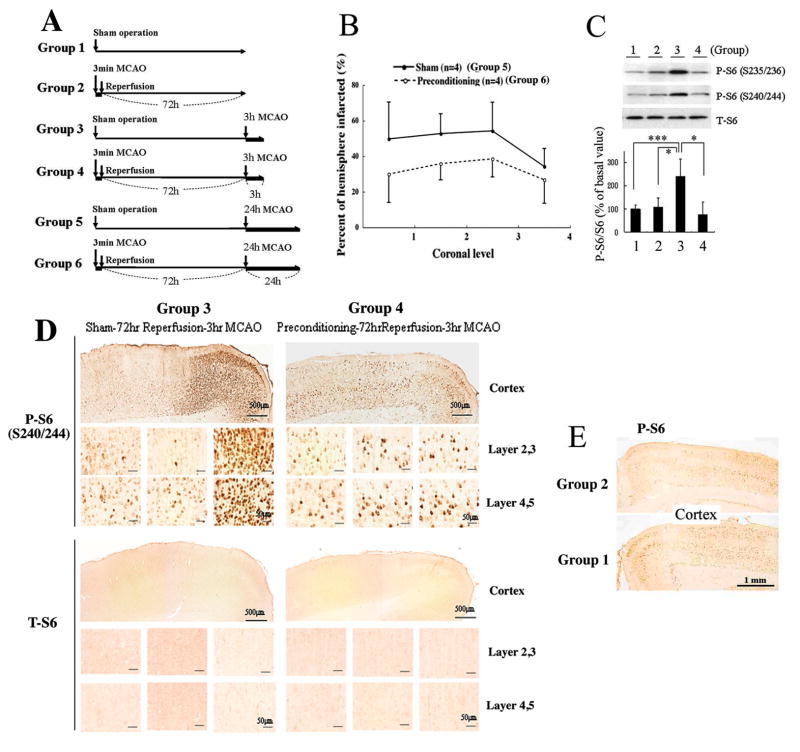
Male Sprague-Dawley rats (Taconic, NY, USA) weighing between 270–370g were used for the in vivo preconditioning study. Animals were divided into 6 groups. Group 1 (n=4) underwent sham surgery only, while group 2 (n=4) underwent preconditioning. Both groups 1 and 2 were sacrificed 72 hours later. Group 3 (n=4) underwent sham surgery and group 4 (n=4) underwent preconditioning yet 72 hours later both groups underwent 3 hours of middle cerebral artery occlusion (MCAO). Group 5 (n=4) underwent sham surgery and group 6 (n=4) underwent preconditioning. Again, both of them were followed 72 hours later by 24 hours of MCAO.
Intraluminal occlusion of middle cerebral artery (MCA)
Rats were anesthetized with 5% isoflurane for induction and 1.5% isoflurane for maintenance of anesthesia respectively in 30%O2/70%N2O. The core body temperature was monitored and maintained at 37.0 +/− 0.5°C using a heating pad and a lamp. The left femoral artery was cannulated with PE-50 catheter (Becton Dickinson) to monitor the mean blood pressure. Blood Focal cerebral ischemia was induced by the reversible intraluminal MCAO method described by Longa et al. (Longa et al. 1989), with slight modifications. We confirmed by laser-Doppler flowmetry that 75~90% CBF reduction was seen immediately upon MCAO, and CBF was restored fully after removing the suture.
Focal ischemia for in vivo preconditioning
Briefly, the right common carotid artery (CCA) was exposed via a midline cervical incision. The occipital branch of the external carotid artery (ECA) was ligated at its origin using a 6-0 silk suture and coagulated distally; other branches of the ECA were also coagulated if present. The internal carotid artery (ICA) was isolated and carefully separated from vagus nerve. The pterygopalatine artery was ligated with 6-0 silk suture close to its origin. 5-0 silk sutures were tied loosely around the CCA and the ICA adjacent to the ECA to avoid the regurgitation from the ECA stump and an arteriotomy was made in the ECA slightly proximal to the ligation. An ~ 5 cm length of a 4-0 silicone (RTV1556, Rhodia) coated nylon monofilament was introduced into the ECA stump and advanced into the ICA 18–20 mm past the carotid artery bifurcation. At this point, the intraluminal filament occluded the origin of the MCA. Three minutes later, the filament was gently withdrawn and the silk suture around the CCA removed to allow reperfusion. Finally, the origin of ECA stump was ligated with 6-0 suture. 3 minutes of temporary MCAO was selected based on a series of pilot studies (data not shown). Sham surgeries were performed in an identical manner to those undertaken during preconditioning except that the origin of the MCA was never occluded.
Prolonged focal ischemia
To evaluate the effects of preconditioning, prolonged MCAO was induced 72 hr after either a 3-minute period of temporary MCAO or sham surgery. The procedure employed to induce prolonged MCAO was similar to that used for preconditioning except that the intraluminal filament was inserted into the small puncture of the CCA. Briefly, before puncturing, the CCA was double-ligated proximally with a 5-0 silk suture, and the ICA was tied loosely with 5-0 silk suture to avoid hemorrhage. The CCA containing the filament was ligated distally with 6-0 sutures, and then the 5-0 silk suture around the ICA was removed. Finally, the silicon coated nylon filament was advanced to same point (18–20 mm past the carotid artery bifurcation) employed during preconditioning ischemia.
Measurement of infarct size
24 hr after prolonged MCAO animals in groups 5 and 6 were anesthetized with sodium pentobarbital (100 mg/kg, intraperitoneal), perfused transcardially with 0.01 M phosphate buffer (PB), and finally fixed using 4% paraformaldehyde in 0.1 M PB (pH 7.4). The brains were post-fixed for an additional 12 hours in 4% paraformaldehyde in 0.1 M PB (pH 7.4), and stored in 20% sucrose in 0.1 M PB (pH 7.4). The sections (20 μm thick) were stained with cresyl violet and were analyzed using Image J (NIH, Bethesda, MD) at four coronal levels (bregma +1.00, −0.60, −2.20, −3.80). Infarct sizes were calculated and corrected for brain edema (Swanson et al. 1990). The persons who measured and analyzed the infarction volumes are completely blinded. Data are presented as mean ± SD. Differences among the groups with regard to infarct sizes were determined by using analysis of variance (ANOVA) followed by Bonferroni correction. A p<0.05 value was considered significant.
Cloning of the small ribosomal protein S6 cDNA from the 13-lined ground squirrel
To obtain the 13-lined ground squirrel rpS6 cDNA fragment, primers were designed as follows; 5′-atgaagctgaatatctccttccc-3′ and 5′-tttttgactggactcagatttagaag-3′. Polymerase chain reaction (PCR) amplification using a 13-lined ground squirrel brain cDNA library as a template was performed and the amplified DNA fragments were subcloned into pGEM-T vector (Promega) and sequenced.
Isolation of rat cortical neurons and cortical neuronal culture
Embryos of Sprague-Dawley rats were used to prepare cortical neuronal cultures. Cortices were dissected from rat embryos (E18) and dissociated with papain (Worthington Biochemicals, Lakewood, NJ, USA), and plated out at 1,000,000 cells per well on poly-L-lysine-coated 6 well plates in Neurobasal/B27 media (Lee et al. 2009). Cells were used after 7 days in culture.
Oxygen/glucose deprivation (OGD), restoration of oxygen/glucose (ROG) and cell death assessments
OGD experiments on cortical neuronal cultures were performed as has been previously described (Heldmaier et al. 2004) (Lee et al. 2007). After 5 hr of OGD, plates were taken out of the hypoxia chamber (Billups-Rothenberg, Inc.), media was replaced with Neurobasal/B27, and the cultures were incubated further for 16 hr allowing for ROG. Of note, cells were preincubated with S6K inhibitors (D-glucosamine or PF-4708671) at various concentrations (0–10 mM, and 0–10 μM, respectively) before being subjected to OGD. These drugs were also added during OGD/ROG in order to maximize any effect. Stock solutions of D-glucosamine were made in H2O and those of PF-4708671 in DMSO. Cell death after OGD/ROG was measured via LDH release (Abcam) according to the manufacturer’s instructions.
Western blotting
All antibodies with the exception of protein phosphatase 2A catalytic subunit (PP2A/c) (Calbiochem) and beta-actin (Sigma) were purchased from Cell Signaling Technology. Briefly, crushed frozen brains were added to a lysis buffer containing 2% SDS, 60 mM Tris-HCl buffer (pH 6.8), 50 mM EDTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1mM PMSF, 1 μM leupeptin and homogenized on ice. Homogenates were then sonicated for 10–15 seconds and boiled for 5 minutes at 95°C. Samples were then centrifuged at 15,000xg for 10 min at 4°C. 30 μg of protein were electrophoresed in a SDS–polyacrylamide gel and transferred onto a PVDF membrane (Invitrogen). The membrane was incubated with a blocking buffer (5% nonfat dried milk in PBS containing 0.05% Tween 20 (PBST)) for 1hr at room temperature, followed by incubation with a primary antibody for 1hr at room temperature or overnight at 4°C. The primary antibodies were used 1:1000 dilutions, unless stated otherwise. The membrane was then washed four times in PBST, probed with the secondary HRP-linked antibody (1:5000) in blocking buffer for 1hr at room temperature. Detection of the signal was performed using ECL Plus Western blotting reagents (Amersham Pharmacia Biotech) or Immobilon Western (Millipore). A t-test was used for statistical analysis and a p value of < 0.05 was considered significant.
Immunohistochemistry
20 μm thick brain sections were cut on a cryostat. Brain sections (free float) were incubated overnight with the following antibodies: rpS6 (1:500) and phospho (Ser 240 and 244) rpS6 (1:500). The sections were subsequently incubated with a biotinylated anti-mouse IgG (Vector Laboratories, Burlingame, CA,) at 1:200 for 1 hr and then incubated with an avidin-biotin peroxidase complex solution (Vector Laboratories, Burlingame, CA) at 1:100 for 1hr. After each incubation, the sections were rinsed for 15 min with PBS containing 0.3% Triton X-100. Finally, the products of the immunoreaction were visualized using diaminobenzidine (DAB) as the substrate (Vector Laboratories, Burlingame, CA). To evaluate the ischemic core, the transition area and peri-infarct area, sections stained with cresyl violet were compared to those that were immunostained.
Measurement of S6K activity
Briefly, crushed frozen brains were added to a lysis buffer containing 20 mM MOPS, 5 mM EGTA, 2 mM EDTA, 1% NP40, 1 mM dithiothreitol, 1 mM benzamidine, 1 mM PMSF, and 10 μg/mL leupeptin/aprotinin, 50 mM β-glycerolphosphate, 50 mM sodium fluoride, and 1 mM sodium vanadate. Benzamidine, leupeptin/aprotinin, β-glycerolphosphate, sodium fluoride, and sodium vanadate were supplied via the reconstitution of a lyophilized powder (i.e. MS-SAFE Protease and Phosphatase Inhibitor Cocktail [Sigma-Aldrich]). Samples were homogenized three times on ice for 20, 10, and 10 seconds, then sonicated for 10 seconds on ice. Samples were subsequently centrifuged at 15,000xg for 30 minutes at 4°C. Protein concentration in the supernatant was determined using the Pierce BCA Protein Assay (Life Technologies) and an equal concentration of protein from each sample was used for the assay. The p70S6 kinase activity assay was carried out according to the manufacturer’s instructions (Enzo Life Sciences, ADI-EKS-470).
Statistical analysis
Statistic analyses between two groups were done by the student’s t-test. Repeated measure ANOVA followed by Bonferroni correction was applied to the analyses of infarct volumes at four coronal levels (Fig. 7B). All reported p-values are two-sided and are considered as significant if the p-values are less than 0.05.
Figure 7. Rat ischemic tolerance model induced by non-lethal 3 min MCAO followed by 72 h reperfusion (in vivo preconditioning).
(A) Outline of general procedure for 6 different groups as descried in the Materials and Methods section; four animals (n=4) within each group. (B) Infarct sizes in the brains from Group 5 and Group 6 animals analyzed at four coronal levels (bregma +1.00, −0.60, −2.20, −3.80). The infarct sizes of preconditioned animals (Group 6) were significantly smaller than the sham-operated animals (Group 5). p=0.0482 by repeated measure ANOVA. (C) Effect of preconditioning on rpS6 phosphorylation. Proteins from cortical samples (MCA perfused area) were extracted and rpS6 phosphorylation (S235/236 or S240/244) and total rpS6 levels were measured. Upper panel: A representative immunoblot; lower panel: densities of phosphorylated rpS6 (S240/244) were measured in each group, normalized by the corresponding total rpS6 densities, and shown as relative to the average of Group 1 (basal level). Results represent the mean ±SD of 4 animals; *P<0.05, ***P<0.005 by the student’s t-test. (D) Immunohistchemistry by phospho-rpS6 antibody (S240/244) and total rpS6 antibody. rpS6 phosphorylation levels along with total rpS6 levels in cerebral cortex of Group 3 and 4 animals were examined and shown at two different magnifications. Scale bars show 500 and 50 μm. Ischemic core (left), transition area (middle), and the peri-infarct area (right) were shown. (E) rpS6 phosphorylation levels before prolonged ischemia with (Group 2) or without (Group 1) preconditioning. Scale bars show 1.0 mm.
Results
Cloning and sequence analysis of the 13-lined ground squirrel rpS6
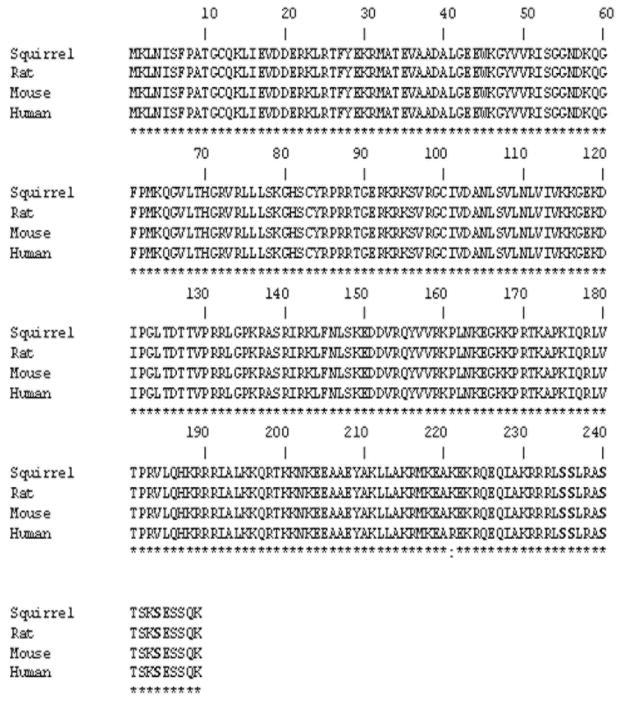
We searched the GenBank database to find conserved regions within the rpS6 cDNA sequences between humans, rats and mice. Conserved areas in the 5′ and 3′ regions were found and as such a cDNA fragment, which contained the coding region, was amplified via PCR using a 13-lined ground squirrel cDNA brain library as a template. The coding cDNA sequence (submitted to GenBank: Acc # KP453774) displayed a high similarity with human (91.73%), rat (89.73%), mouse (87.87%) rpS6. The putative amino acid sequence of the 13-lined ground squirrel rpS6 was essentially homologous with that of rat (100%), mouse (100%) and human (99.6%). Of note, the amino acid sequences recognized by anti-phospho-rpS6 antibody (S235/S236, S240/S244) and total-rpS6 antibody (residues 185–205 of the rpS6 protein) were completely conserved thereby allowing for an accurate comparison to unfold (Fig. 1).
Figure 1. rpS6 amino acid sequence alignment: 13-lined ground squirrel vs. rat, mouse and human.
Identical amino acids are illustrated with (*). Highly similar amino acids are illustrated with (:). Phosphorylation sites, which play an important role in activity of rpS6, are noted via a “P”.
rpS6 phosphorylation in the brains of 13-lined ground squirrels was significantly decreased during hibernation torpor
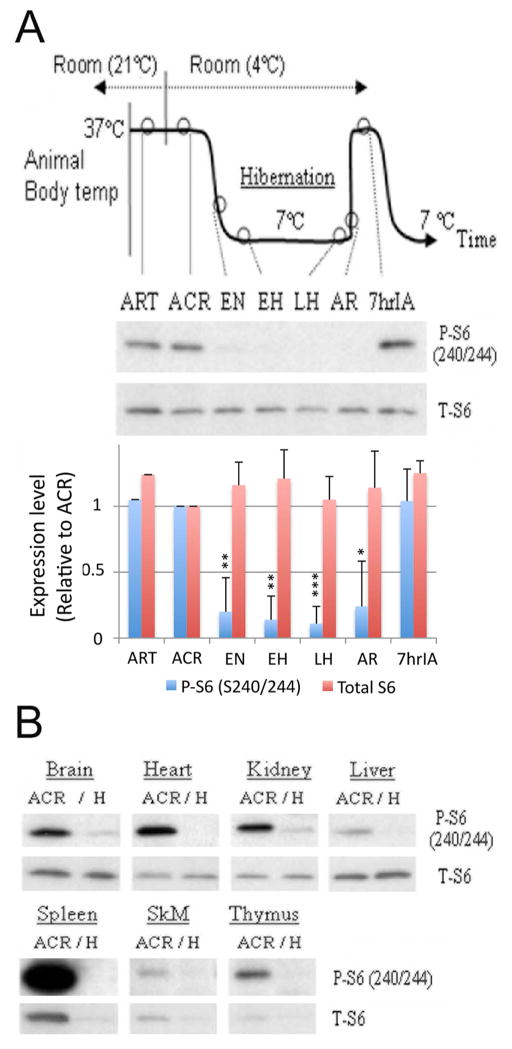
Thirteen-lined ground squirrels were captured during summer, housed individually at 21°C and induced to undergo hibernation during the fall/winter. A hibernation bout may last for weeks and consists of entry into, maintenance of, and arousal from torpor. Animals in 7 different phases of the hibernation bout were differentiated by body temperature, time, and respiratory rate (Fig. 2A). rpS6 phosphorylation in the brain across the 7 different phases was examined via immunoblotting with the anti-phospho-rpS6 (S240/S244) and anti-total-rpS6 antibodies. rpS6 phosphorylation significantly decreased within the brain from EN to AR as compared with the active states, which included ART, ACR and 7hIA (Fig. 2A). Such a decrease in rpS6 phosphorylation was also witnessed in the heart, kidney and liver during hibernation torpor (Fig. 2B). Of note, the total amount of rpS6 protein did not change significantly within the brain (Fig. 2A), heart, kidney and liver but did decrease in the spleen, skeletal muscle and thymus during hibernation torpor (Fig. 2B).
Figure 2. Hibernation bout and rpS6 phosphorylation.
(A) Diagram of hibernation bout (upper panel), representative immunoblots of phospho- and total-rpS6 in the squirrel brains at 7 different phases of the hibernation bout (middle panel), and their quantification summary which are shown as mean ± standard deviation (SD) of three animals (lower panel) are presented. *p<0.05, **p<0.01, ***p<0.001 compared to ACR level by the student’s t-test. ART: active in room temperature; ACR: active in cold room; EN: entrance; EH: early hibernation torpor; LH: late hibernation torpor; AR: arousal; 7hrIA: 7h after interbout started. (B) Comparison of rpS6 phosphorylation in different tissues between active (ACR) and torpor phase (H) squirrels. SkM: Skeletal muscle.
Signal transduction upstream of the rpS6 protein within the brain cortices of 13-lined ground squirrels during hibernation
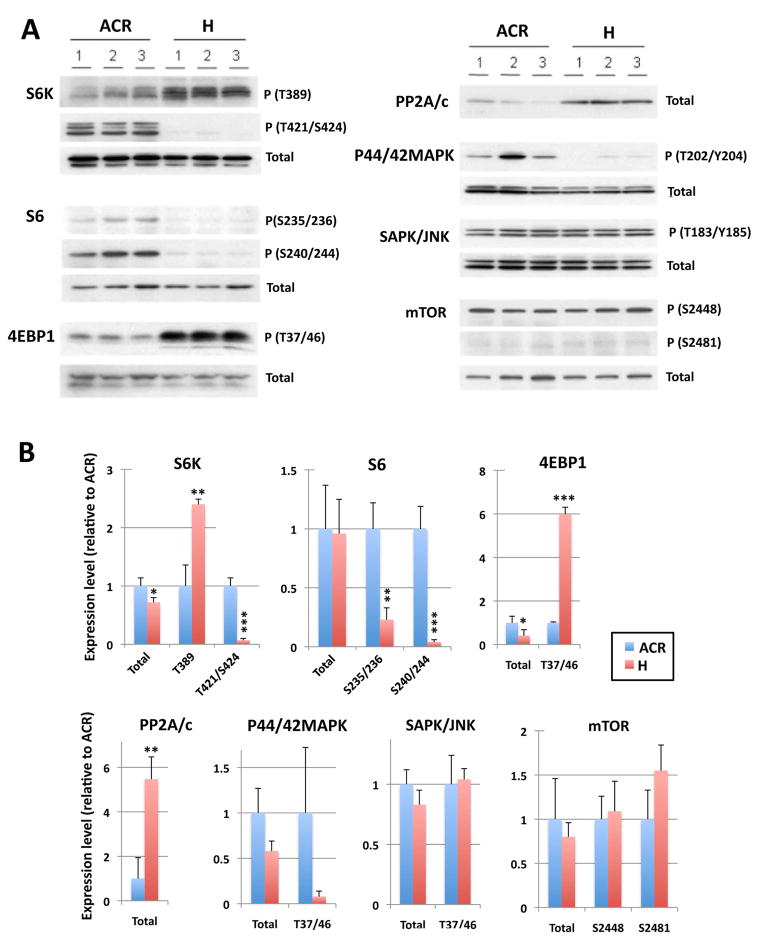
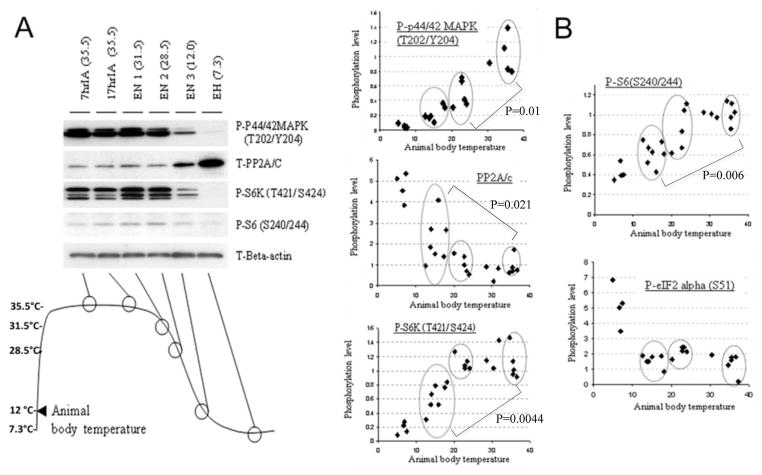
Phosphorylations of rpS6 and its upstream kinases in cortical brain sections were examined via immunoblotting. Representative immunoblots are shown in Fig. 3A, and their quantitative data are shown in Fig. 3B. Brain cortex samples from torpor animals (H) (n=3) were compared with those, which were deemed to be active in the cold room animals (ACR) (n=3). First we examined the phosphorylation of S6K, which is a kinase that targets the rpS6 protein (Fumagalli & Thomase 2000). Interestingly, S6K phosphorylation on residues T421/S424, which are supposed to be phosphorylated by p44/42 MAPK, (Dufner & Thomas 1999, Page et al. 2006) decreased during hibernation torpor. In contrast, phosphorylation on T389 (a substrate of mTOR) (Dufner & Thomas 1999) increased during torpor. The total amount of S6K protein did not change. S6K activity categorically requires the phosphorylation of the T389 residue (reviewed in (Magnuson et al. 2012) which was increased during hibernation torpor. However, for full activation, S6K should also be phosphorylated on it’s autoinhibitory domain (i.e. residues T421/S424) (Han et al. 1995). These data suggested that the activity of S6K within the brains of torpor animals may in fact be inhibited/diminished. Next, we examined the activation state of the extracellular signal-regulated kinase (ERK) family of mitogen-activated protein (MAP) kinase p44/42 MAPK, which is responsible for the phosphorylation of residues T421/S424 on S6K. As expected, the phosphorylation of T202/Y204 on the p44/42 MAPK was decreased in torpor brains. However, the phosphorylation status of T183/Y185 of another MAPK, the SAPK/JNK (stress-activated protein kinases/Jun amino-terminal kinases) was unchanged. Of note, p44/42 MAPK and SAPK/JNK are activated through the phosphorylation of activation loop residues (i.e. T202/Y204 and T185/Y187, respectively). We also examined levels of protein phosphatase 2A (PP2A) which has been reported to bind to S6K in the brain (Westphal et al. 1999) and inhibit its activity (Ballou et al. 1988). Consistent with our previous report (Chen et al. 2001), the expression of the PP2A catalytic subunit (PP2A/c) was increased in the brain cortex during hibernation torpor. These results suggested that S6K activity was greatly inhibited by p44/42 MAPK inactivation (leading to hypophosphorylation within the C-terminal domain [T421/S424]) and/or via the induction of PP2A/c expression. We next examined the activation/expression levels of mTOR, which is a major kinase that targets the T389 residue of S6K. It is known that mTOR is phosphorylated at S2448 via the PI3 kinase/AKT signaling pathway and is autophosphorylated at S2481 (Nave et al. 1999, Peterson et al. 2000). Phosphorylation levels at both sites were unchanged during hibernation bout. Further, the total amount of mTOR protein remained the same. While we did not detect any change in phosphorylation or protein levels of mTOR, its kinase activity may have increased within torpor brains being that the phosphorylation of the T389 residue on S6K and T37/T46 residues on 4EBP1, both of which are known substrates of mTOR, increased during hibernation torpor (Fig. 3).
Figure 3. Upstream signal transduction of rpS6 during torpor.
A: Upstream kinases and phosphatases of rpS6 in the brain cortex from 3 animals each of active (ACR) and torpor (H) phases were examined by immunoblotting. Antibodies against phospho- and total- S6K, rpS6, 4EBP1, PP2A/c, p44/42MAPK, SAPK/JNK and mTOR were used. B: Quantative analyses of the immunoblots. Densities of each protein band(s) (including multiple bands which we consider isoforms) were measured, normalized with corresponding actin levels and shown as relative to control (average of three ACR samples). Data are shown as the mean ± SD of three samples. *P<0.05, **P<0.01, ***P<0.001 compared to ACR by the student’s t-test.
Regulation of signal transduction upstream of the rpS6 protein within the brain cortices of 13-lined ground squirrels during hibernation entrance and hibernation torpor
In order to gain insight into the specific timing of critical regulators upstream of rpS6 during the entrance phases into hibernation, the phosphorylation levels of p44/42 MAPK, S6K, rpS6, eIF2alpha, and the protein levels of PP2A/c within the brain cortex were examined via immunoblotting (representative image was shown in Fig. 4A). Squirrels in entrance phase of hibernation were isolated and their core body temperatures were measured prior to their being euthanized by decapitation. 3 groups (Tb=15–20°C, 20–25°C, >34°C) shown in circles were chosen for statistical analyses. Reductions of phosphorylation on residues T421/S424 of S6K and residues S240/S244 of rpS6, and the induction of PP2A/c expression were profound after squirrel body temperature declined to < 20° (p=0.0044, p=0.006, p=0.021, respectively when Tb=15–20°C group was compared to >34°C group) (Fig. 4B). The concordant timing of the dephosphorylation of S6K/rpS6 and the increase of PP2A/c protein levels suggests that down regulation of rpS6 signal transduction (S6K-rpS6) may indeed correlate with an increase of PP2A/c protein levels. Interestingly, the phosphorylation of p44/42MAPK was significantly decreased even before animal body temperature reached < 20°C (p=0.01, the 20–25°C group was compared to the >34°C group) (Fig. 4B), ahead of the dephosphorylation of the T421/S424 residues of S6K, which supports the inference that p44/42MAPK is responsible for phosphorylation of S6K (T421/S424). The initiation factor, eIF2-alpha, is a translational regulator and phosphorylation on S51 has been suggested to be involved in the inhibition of global protein syntheses (Pathak et al. 1988). Critically, the level of phosphorylation on the residue S51 of eIF2-alpha did not change at all during entrance phase (24°C < Tb < 12°C) (Fig. 4B). Collectively, these data suggest that the down regulation of rpS6 signal transduction (S6K-rpS6) during the hibernation entrance phase occurs after the inactivation of p44/42MAPK, but before global protein synthesis inhibition via eIF2-alpha phosphorylation.
Figure 4. Relationship between protein phosphorylation (upstream of rpS6 signal transduction) and squirrel body temperature during entrance into hibernation torpor.
Squirrels at an interbout stage usually enter into torpor within 24 h after arousal. Animals which start to re-enter torpor were identified, their body temperatures measured and they were subsequently sacrificed. Protein extracts from their brain cortex were made, the phosphorylation of various proteins upstream of rpS6 signal transduction were then examined by Western blot analysis. (A) Representative images of immunoblots. (B) Dot plots of body temperature vs phosphorylation levels in individual animals. Each dot represents an animal, and 24 animals altogether were analyzed. The phosphorylation levels were shown as relative to control (the average level of 7hrIA). Three groups (Tb=15–20°C, 20–25°C, >34°C) were chosen (shown in circles) for statistical analysis (student’s t-test).
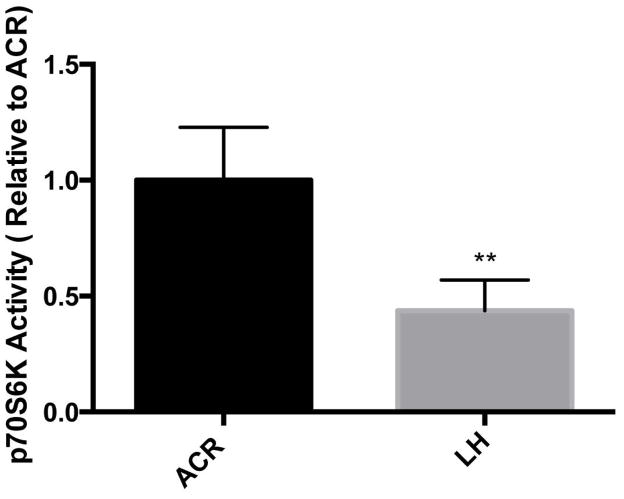
S6K activity is depressed in the brains of torpor phase 13-lined ground squirrels
During hibernation torpor, S6K is highly phosphorylated at residue T389, but hypophosphorylated at residues T421/S424. We thus wondered whether this differentially phosphorylated S6K was in fact less active. We therefore examined the S6K activity in the brain extracts from active (ACR) and late torpor phase (LH) of hibernating squirrels via the use of a solid state ELISA S6K activity kit (Enzo Life Sciences, ADI-EKS-470). As shown in Fig. 5, S6K activity within the brain during the torpor phase of hibernation was significantly lower than that which is present in the active phase of the hibernation bout. This result again suggested that S6K is not fully active even though the T389 residue is phosphorylated unless the C-terminal domain residues (T421/S424) are also phosphorylated. Of note, we still cannot exclude, the confounding effects of PP2A present in the brain extracts despite the inclusion of phosphatase inhibitors in preparatory buffers used to make the brain tissue extracts.
Figure 5. S6K activities in the brain extracts from active and torpor phase 13-lined ground squirrels.
p70S6K activity was assessed by measuring the intensity of color after exposure to 3,3′,5,5′-Tetramethylbenzidine (TMB) at 450nm. Results are expressed as a relative measure to animal in the ACR phase of hibernation (i.e. the first column in the figure). Data are shown as the average of 5 animals from either and ACR or LH phase of hibernation with standard deviations. **p<0.01, by the student’s t-test (unpaired).
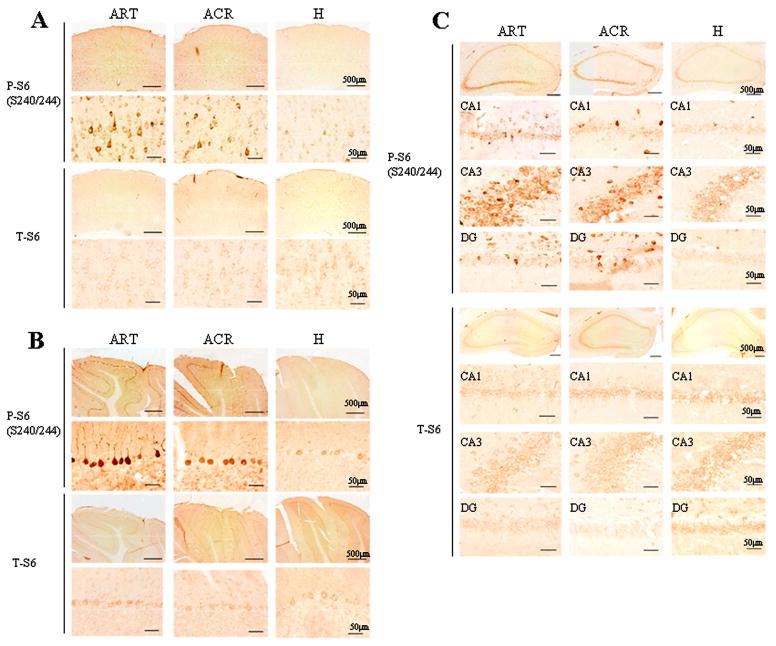
Dephosphorylation of rpS6 occurs within the brains of 13-lined ground squirrels during hibernation torpor
The distribution of phosphorylated rpS6 (P-S6) or total rpS6 (T-S6) within the brains of 13-lined ground squirrels during the various stages of hibernation were examined using immunohistochemistry. Of note, the majority of rpS6 immunoreactive cells located in cerebral cortex, hippocampus and cerebellum appeared to be neurons. In active animals (ART and ACR), P-S6 was clearly detectable in medium to large-sized cells (~ 20μm in diameter) with large nuclei (i.e. cells that are morphologically typical of neuronal cells) in cortex (Fig. 6A), and in Purkinje/granule cells within the cerebellar cortex (Fig. 6B). Further, granule cells within the dentate gyrus and pyramidal cells throughout the CA1 to CA3 subfields of the hippocampus were shown to be immunopositive (Fig. 6C). In contrast, very little P-S6 was observed in the brain of torpor phase (H) animals. Of note, as was suggested via immunoblotting, the intensity of T-S6 immunoreactivity in the brain was not different between the active (ART and ACR) and torpor phase (H) animals.
Figure 6. Distribution of rpS6 phosphorylation in squirrel brain during hibernation.
Immunoreactivity of phospho-rpS6 antibody (S240/244) and total rpS6 antibody in the cerebral cortex (A), cerebellum (B) and hippocampus (C) were compared between ART, ACR and torpor state (H) animals. Scale bar show 50 or 500μm.
Effect of ischemic preconditioning on rpS6 phosphorylation and ischemic tolerance in the rat MCAO model
As shown in Fig. 7A, we divided animals (rats) into six groups (4 animals each) in order to evaluate the effects of preconditioning on rpS6 phosphorylation levels and ischemic tolerance in the brain. Three minutes of brief MCAO was used as an ischemic preconditioning (Groups 2, 4 and 6). Sham operated animals (i.e. no 3 min MCAO) were used as controls (Groups 1, 3 and 5). After 72 hr, the animals were then challenged with either 3 hr (Groups 3 and 4) or 24 hr (Groups 5 and 6) of prolonged (severe) MCAO. First, we measured and compared infarct volumes in the brain from Groups 5 and 6 animals. As shown in Fig. 7B, infarct sizes in the animals which had been preconditioned (Group 6) were significantly smaller at all coronal levels we measured compared to the sham-operated group (Group 5) (p = 0.0482). Next, we measured rpS6 phosphorylation levels in the brain tissue collected from the MCA perfused areas of Groups 1, 2, 3 and 4 animals. rpS6 phosphorylation on both the S235/S236 and the S240/S244 sites were significantly increased in the samples from Group 3 animals which were subjected to a 3 hr MCAO without preconditioning (Fig. 7C). However, no increase was detected in the samples from Group 4, which had been preconditioned prior to the 3hr MCAO (Fig. 7C). We also examined the distribution of the phosphorylated rpS6 (P-S6) in the brain cortices from these animals by immunohistochemisty. Much higher P-S6 levels were detected in the brain cortex in Group 3 animals than, as expected, in the brain cortex from Group 4 animals (Fig. 7D), and Groups 1 and 2 animals (Fig. 7E) supporting the immunoblots discussed above (Fig. 7C). It is important to note that there was no difference in the total amount of rpS6 (T-S6) between the two groups (Group 3 and Group 4) (Fig. 7C, D).
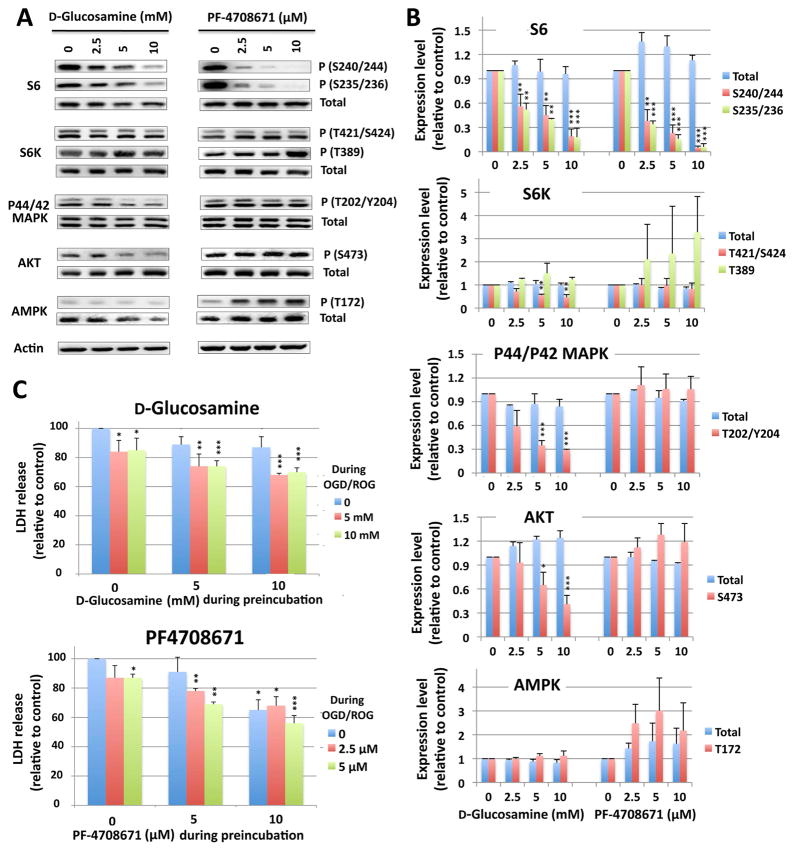
Cultured cortical neurons display an increased tolerance to oxygen/glucose deprivation (OGD) when rpS6 phosphorylation is reduced via the S6K inhibitors, D-glucosamine and PF-4708671
To establish a direct/translational connection between states of ischemic tolerance and rpS6 hypophosphorylation, we used cortical neurons derived from E18 rat embryos and two known S6K inhibitors, D-glucosamine (Oh et al. 2007) and PF-4708671 (Pearce et al. 2010). First, we confirmed that these inhibitors indeed inhibited the phosphorylation of rpS6. As shown in Fig. 8A (representative immunoblots) and 8B (quantification of the immunoblots) when cells were treated with 2.5~10 mM D-glucosamine or with 2.5~10 μM PF-4708671 overnight, the phosphorylated forms of rpS6 (both S240/244 and S235/236 sites) were diminished. As for S6K, the phosphorylation at T421/S424 sites was inhibited especially in D-glucosamine-treated cells, but phosphorylation at the T389 site (i.e. the mTOR target site) was not. Interestingly, the phosphorylation of P44/42 MAPK and AKT (S473) were also reduced in D-glucosamine treated cells (Fig. 8A, B), which mirrors the situation in hibernating squirrels as per the abovementioned (Figs. 3,4) and which has been reported previously (Cai et al. 2004). Recently, PF-4708671 has been reported to activate AMP-activated protein kinase (AMPK) in addition to a direct inactivation of S6K activity (Vainer et al. 2014). We examined the activation (phosphorylation at T172) of AMPK in rat cortical neurons treated with PF-4708671. As shown in Fig. 8A, PF-4708671 (but not D-glucosamine) indeed activated AMPK in a dose dependent way. Since activated AMPK inhibits mTORC1 and thus S6K activity (Bolster et al. 2002), the activation of AMPK by PF-4708671 may amplify the direct inhibition of S6K by the compound. We next examined the effect of these inhibitors on OGD/ROG-induced cell death. These inhibitors were used during a preincubation (16 hr) and/or during OGD/ROG at two different concentrations (5 and 10 mM for D-glucosamine, 2.5 μM and 5 μM for PF-4708671). As Fig. 8C demonstrate, OGD/ROG-induced cell death in cortical neurons was significantly reduced via exposure to either of these drugs. Of note, pretreatment with D-glucosamine alone did not provide much protection, but including it during OGD/ROG yielded a significant reduction in cell death (Fig. 8C upper panel). In the case of PF-4708671, pretreatment with a 5 μM dose alone was already effective (Fig. 8C lower panel).
Figure 8. Effect of the S6K inhibitors, D-glucosamine and PF-4708671, on phosphorylation of S6K-rpS6 signaling proteins, and OGD/ROG-induced cell death in rat cortical neurons.
(A) Rat cortical neurons were incubated with glucosamine at 0, 2.5, 5 and 10 mM (left panels) or with PF-4708671 at 0, 2.5, 5, 10 μM (right panels) for 16 h; total cell lysates were analyzed for the total and phosphorylation levels of rpS6, S6K, p44/42MAPK, AKT and AMPK by Western blot. Actin was used for loading controls. (B) Quantification of protein (total and/or phosphorylated) levels. Densities of each protein band(s) were measured, normalized with corresponding actin levels and shown as relative to control (no drugs). Data are shown as the mean ± SD of three independent experiments. *P<0.05, **P<0.01, ***P<0.001 compared to control by the student’s-test. (C) Upper panel: cells were preincubated without, with 5 mM or 10 mM D-glucosamine for 16 h, and challenged to OGD (5h) followed by ROG (16 h) in the absence or presence of 5 or 10 mM D-glucosamine. Lower panel: cells were preincubated without, with 2.5 μM, or 5 μM PF-4708671 for 16 h, and challenged to OGD/ROG in the absence, or presence of 2.5 or 5 μM PF-4708671. Cell death was assessed by measuring the released LDH at the end of the procedure. Results are displayed in relation to the amount of released LDH in the control sample (no exposure of the drug neither during preincubation nor during OGD/ROG) (i.e. the first column in each figure). Data are shown as the average of four (D-glucosamine) or three (PF-4708671) independent experiments with standard deviations. *p<0.05, **p<0.01, ***p<0.001 by the student’s t-test compared to the control (no exposure to the drug at all, the first lane of each graph).
Discussion
Our results demonstrate that the phosphorylation of rpS6 within the brains of 13-lined ground squirrels significantly decreases during hibernation torpor. The timing of such dephosphorylation was shown to occur after the animal’s body temperature decreased to less than 20°C (i.e. during entrance into hibernation) (Fig. 4). It is relevant to note that heart rate falls prior to a reduction in an animal’s body temperature during entrance into hibernation and reduces to a rate ~ 1/5 of the active state at 20°C Tb (Frerichs et al. 1994). Such physiology reflects a CBF within the brain at Tb < 20°C that is approaching oligemic levels (Frerichs et al. 1994). Critically, within this manuscript we have also reported that rpS6 phosphorylation in the brain peri-infarct regions of rats that had undergone in vivo ischemic preconditioning was not increased in response to prolonged MCAO; having shown concurrently that without preconditioning, a marked elevation of rpS6 phosphorylation does occur within peri-infarct areas (Fig. 7D, F). Of note, the temporal cellular depolarizations that occur within the peri-infarct area have been suggested to activate rpS6 signal transduction directly, as calcium influx via KCl-induced depolarization does increase rpS6 phosphorylation in cultured neurons (Lenz & Avruch 2005).
Eukaryotic initiation factor, eIF2-alpha, is a translational regulator and the phosphorylation of residue S51 inhibits global protein synthesis (Pathak et al. 1988). Therefore, the increase in eIF2-alpha phosphorylation appreciated in the brains of I. tridecemlineatus during hibernation torpor represents yet another key mechanism that may reduce the rate of protein synthesis (Frerichs et al. 1998). Interestingly, previous work has demonstrated that hibernators in the entrance phase (Tb decreased from 19.0 °C to 7.5 °C) already displayed as low a rate of protein synthesis as those hibernators in the deep torpor phase (Frerichs et al. 1998). Here we show that eIF2-alpha phosphorylation did not occur in the brain during entrance phase at 24°C > Tb > 12°C (Fig. 4B), suggesting that the marked reduction of protein synthesis during entrance phase may occur independently of eIF2-alpha phosphorylation. Since the role of rpS6 phosphorylation in regulation of protein synthesis is still controversial (reviewed in (Meyuhas 2008), we cannot conclude that the hypophosphorylation of rpS6 is solely responsible for the low rate of protein synthesis during hibernation entrance/torpor. Of note, the phosphorylation of the C-terminal residues (T421/S424) of S6K were also reduced during the entrance phase mimicking the kinetics of rpS6 dephosphorylation (Fig. 4B). While rpS6 is the major target, activated S6K is known to phosphorylate two other proteins that may affect the ultimate rate of protein synthesis (i.e. elongation factor 2 [EF2] kinase (Wang et al. 2001) and initiation factor eIF4B (Raught et al. 2004)). Inhibition of protein synthesis during hibernation entrance/torpor may therefore be caused by S6K inactivation and/or partial inhibition (dephosphorylation of C-terminus residues), thereby leading to the collective inactivation of these proteins (i.e. rpS6, EF2K, eIF4B).
S6K1 isoform p70 (p70-S6K1) is the major and the most studied rpS6 kinase amongst several others (S6K2, RSK1-RSK2) (reviewed in (Meyuhas 2008), (Magnuson et al. 2012), and is regulated by a series of complex multi-site phosphorylations. S6K1 activity absolutely requires the phosphorylation of 3 critical sites: the T-loop site on the activation loop (T229) by PDK1, the TM site within the linker domain (S371) and the HM site also in the linker domain (T389) by mTOR (reviewed in (Magnuson et al. 2012). In addition, 4 residues in C-terminal pseudosubstrate domain (S411, S418, T421, S424) are known to be phosphorylated in fully activated S6K1 (reviewed in (Magnuson et al. 2012) (Ferrari et al. 1992). Two models have thus been proposed for the stepwise activation of S6K1, both of which require phosphorylation of C-terminal pseudosubstrate domain residues prior to those of the HM (T389) and T-loop (T229) phosphorylation (Weng et al. 1998) (Keshwani et al. 2011). Interestingly, during hibernation torpor, S6K within the brain was highly phosphorylated at the T389 residue despite the fact that the C-terminal residues (T421/S424) were dephosphorylated (Fig. 3), a finding that does not fit with either of the canonical activation models that have thus far been proposed. The C-terminus residues of S6K (T421/S424) are most likely phosphorylated by p44/42MAPK (Dufner & Thomas 1999, Page et al. 2006), which we found to be dephosphorylated during hibernation entrance/torpor (Fig. 3 and 4). Interestingly, the decrease in p44/42MAPK phosphorylation during hibernation torpor has also been seen in Arctic ground squirrels (i.e. another hibernating species) (Zhu et al. 2005).
Beyond the hibernation torpor of the 13-lined ground squirrel as a model of natural ischemic tolerance and/or the rat model of induced cellular tolerance via ischemic preconditioning, we sought to further confirm the relationship between states of ischemic tolerance and rpS6/S6K hypophosphorylation via the implementation of a reductionist experimental paradigm. By means of an in vitro model of ischemia, in which cortical neurons derived from E18 rat embryos were exposed to the known endogenous S6K inhibitor D-glucosamine (Oh et al. 2007) and the highly specific S6K inhibitor PF-4708671 (Pearce et al. 2010), we confirmed that these molecules indeed inhibited the phosphorylation of rpS6/S6K and that this was capable of providing protection from the effects of OGD/ROG-induced cell death.
In line with the aforementioned, knockdown of the C. elegans S6K homolog (rsk-1) and rpS6 homolog (rps-6) increase the lifespan of the worm and induce resistance to thermal-stress (Kaeberlein & Kennedy 2007). Lifespan extension has also been observed in worms with reduced expression of translation initiation factors and other ribosomal proteins (Kaeberlein & Kennedy 2007). Similarly, mammalian hibernation torpor represents a physiological state characterized by a marked decrease in protein synthesis (Frerichs et al. 1998) and a substantial portion of hibernators display an increase in longevity (Lyman et al. 1981). Interestingly, the endogenous S6K inhibitor D-glucosamine whose effects mimic hibernation torpor (Fig. 8A) was reported to extend the life span of C.elegans and aged mice (Weimer et al. 2014) as well.
In the present study we present data that support the hypothesis that down-regulation of rpS6 signal transduction plays an important role in various forms of ischemic tolerance (i.e. hibernation and ischemic preconditioning). Although we have yet fully understand the network dynamics of rpS6 signal transduction in ischemic tolerance, the elucidation of both upstream and downstream contributors within the rpS6 pathway may be critical to ultimately understanding the governing controllers of ischemic tolerance. It is the authors’ hope that such work may eventually guide new strategies for both the prevention and treatment of ischemic stroke.
Acknowledgments
This work was supported by the Intramural Research Program of the NINDS/NIH.
Abbreviation
- rpS6
ribosomal protein S6
- S6K
ribosomal protein S6 kinase
- CBF
cerebral blood flow
- ART
active animals at room temperature
- ACR
active animals in cold room
- EN
animals in the entrance phase of hibernation
- EH
animals in early torpor phase
- LH
animals in late torpor phase
- IA
aroused interbout animals
- 4EBP1
Eukaryotic translation initiation factor 4E-binding protein 1
- p44/42MAPK
extracellular signal-regulated kinase family of mitogen-activated protein (MAP) kinase p44/42
- PP2A
protein phosphatase 2A
- MCAO
middle cerebral artery occlusion
- CBF
cerebral blood flow
- OGD
oxygen/glucose deprivation
- ROG
restoration of OGD
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- Ballou LM, Jeno P, Thomas G. Protein phosphatase 2A inactivates the mitogen-stimulated S6 kinase from Swiss mouse 3T3 cells. J Biol Chem. 1988;263:1188–1194. [PubMed] [Google Scholar]
- Barone FC, White RF, Spera PA, Ellison J, Currie RW, Wang X, Feuerstein GZ. Ischemic preconditioning and brain tolerance: temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke. 1998;29:1937–1950. doi: 10.1161/01.str.29.9.1937. discussion 1950–1931. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Cai D, McCarron RM, Yu EZ, Li Y, Hallenbeck J. Akt phosphorylation and kinase activity are down-regulated during hibernation in the 13-lined ground squirrel. Brain Res. 2004;1014:14–21. doi: 10.1016/j.brainres.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Chen J, Simon R. Ischemic tolerance in the brain. Neurology. 1997;48:306–311. doi: 10.1212/wnl.48.2.306. [DOI] [PubMed] [Google Scholar]
- Chen Y, Matsushita M, Nairn AC, Damuni Z, Cai D, Frerichs KU, Hallenbeck JM. Mechanisms for increased levels of phosphorylation of elongation factor-2 during hibernation in ground squirrels. Biochemistry. 2001;40:11565–11570. doi: 10.1021/bi010649w. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Furuya K, Gotoh J, Nakao Y, Hallenbeck JM. Cerebrovascular hemodynamics and ischemic tolerance: lipopolysaccharide-induced resistance to focal cerebral ischemia is not due to changes in severity of the initial ischemic insult, but is associated with preservation of microvascular perfusion. J Cereb Blood Flow Metab. 1999;19:616–623. doi: 10.1097/00004647-199906000-00004. [DOI] [PubMed] [Google Scholar]
- Dufner A, Thomas G. Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res. 1999;253:100–109. doi: 10.1006/excr.1999.4683. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Bannwarth W, Morley SJ, Totty NF, Thomas G. Activation of p70s6k is associated with phosphorylation of four clustered sites displaying Ser/Thr-Pro motifs. Proc Natl Acad Sci U S A. 1992;89:7282–7286. doi: 10.1073/pnas.89.15.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM. Local cerebral blood flow during hibernation, a model of natural tolerance to “cerebral ischemia”. J Cereb Blood Flow Metab. 1994;14:193–205. doi: 10.1038/jcbfm.1994.26. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Smith CB, Brenner M, DeGracia DJ, Krause GS, Marrone L, Dever TE, Hallenbeck JM. Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation. Proc Natl Acad Sci U S A. 1998;95:14511–14516. doi: 10.1073/pnas.95.24.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli S, Thomase G. S6 Phosphorylation and Signal Transduction. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression, Cold Spring Harbor mongraph series 39. Cold Spring Harbor Press; Cold Spring Harbor, Plainview, NY: 2000. pp. 695–717. [Google Scholar]
- Han JW, Pearson RB, Dennis PB, Thomas G. Rapamycin, wortmannin, and the methylxanthine SQ20006 inactivate p70s6k by inducing dephosphorylation of the same subset of sites. J Biol Chem. 1995;270:21396–21403. doi: 10.1074/jbc.270.36.21396. [DOI] [PubMed] [Google Scholar]
- Heldmaier G, Ortmann S, Elvert R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol. 2004;141:317–329. doi: 10.1016/j.resp.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Kennedy BK. Protein translation, 2007. Aging cell. 2007;6:731–734. doi: 10.1111/j.1474-9726.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- Keshwani MM, von Daake S, Newton AC, Harris TK, Taylor SS. Hydrophobic motif phosphorylation is not required for activation loop phosphorylation of p70 ribosomal protein S6 kinase 1 (S6K1) J Biol Chem. 2011;286:23552–23558. doi: 10.1074/jbc.M111.258004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg J, Hofsteenge J, Thomas G. Identification of the 40 S ribosomal protein S6 phosphorylation sites induced by cycloheximide. J Biol Chem. 1988;263:11473–11477. [PubMed] [Google Scholar]
- Kruppa J, Clemens MJ. Differential kinetics of changes in the state of phosphorylation of ribosomal protein S6 and in the rate of protein synthesis in MPC 11 cells during tonicity shifts. Embo J. 1984;3:95–100. doi: 10.1002/j.1460-2075.1984.tb01767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Castri P, Bembry J, Maric D, Auh S, Hallenbeck JM. SUMOylation participates in induction of ischemic tolerance. J Neurochem. 2009;109:257–267. doi: 10.1111/j.1471-4159.2009.05957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Miyake S, Wakita H, McMullen DC, Azuma Y, Auh S, Hallenbeck JM. Protein SUMOylation is massively increased in hibernation torpor and is critical for the cytoprotection provided by ischemic preconditioning and hypothermia in SHSY5Y cells. J Cereb Blood Flow Metab. 2007;27:950–962. doi: 10.1038/sj.jcbfm.9600395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Mou Y, Klimanis D, Bernstock JD, Hallenbeck JM. Global SUMOylation is a molecular mechanism underlying hypothermia-induced ischemic tolerance. Frontiers in cellular neuroscience. 2014;8:416. doi: 10.3389/fncel.2014.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz G, Avruch J. Glutamatergic regulation of the p70S6 kinase in primary mouse neurons. J Biol Chem. 2005;280:38121–38124. doi: 10.1074/jbc.C500363200. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Lyman CP, O’Brien RC, Greene GC, Papafrangos ED. Hibernation and longevity in the Turkish hamster Mesocricetus brandti. Science. 1981;212:668–670. doi: 10.1126/science.7221552. [DOI] [PubMed] [Google Scholar]
- Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- Meyuhas O. Physiological roles of ribosomal protein S6: one of its kind. International review of cell and molecular biology. 2008;268:1–37. doi: 10.1016/S1937-6448(08)00801-0. [DOI] [PubMed] [Google Scholar]
- Nave BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344(Pt 2):427–431. [PMC free article] [PubMed] [Google Scholar]
- Nygard O, Nilsson L. Translational dynamics. Interactions between the translational factors, tRNA and ribosomes during eukaryotic protein synthesis. Eur J Biochem. 1990;191:1–17. doi: 10.1111/j.1432-1033.1990.tb19087.x. [DOI] [PubMed] [Google Scholar]
- Oh HJ, Lee JS, Song DK, Shin DH, Jang BC, Suh SI, Park JW, Suh MH, Baek WK. D-glucosamine inhibits proliferation of human cancer cells through inhibition of p70S6K. Biochem Biophys Res Commun. 2007;360:840–845. doi: 10.1016/j.bbrc.2007.06.137. [DOI] [PubMed] [Google Scholar]
- Page G, Khidir FA, Pain S, Barrier L, Fauconneau B, Guillard O, Piriou A, Hugon J. Group I metabotropic glutamate receptors activate the p70S6 kinase via both mammalian target of rapamycin (mTOR) and extracellular signal-regulated kinase (ERK 1/2) signaling pathways in rat striatal and hippocampal synaptoneurosomes. Neurochemistry international. 2006;49:413–421. doi: 10.1016/j.neuint.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Pathak VK, Schindler D, Hershey JW. Generation of a mutant form of protein synthesis initiation factor eIF-2 lacking the site of phosphorylation by eIF-2 kinases. Mol Cell Biol. 1988;8:993–995. doi: 10.1128/mcb.8.2.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce LR, Alton GR, Richter DT, Kath JC, Lingardo L, Chapman J, Hwang C, Alessi DR. Characterization of PF-4708671, a novel and highly specific inhibitor of p70 ribosomal S6 kinase (S6K1) Biochem J. 2010;431:245–255. doi: 10.1042/BJ20101024. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Beal PA, Comb MJ, Schreiber SL. FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem. 2000;275:7416–7423. doi: 10.1074/jbc.275.10.7416. [DOI] [PubMed] [Google Scholar]
- Raught B, Peiretti F, Gingras AC, Livingstone M, Shahbazian D, Mayeur GL, Polakiewicz RD, Sonenberg N, Hershey JW. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. Embo J. 2004;23:1761–1769. doi: 10.1038/sj.emboj.7600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider BJ, Du C, Wei L, Choi DW. Cycloheximide reduces infarct volume when administered up to 6 h after mild focal ischemia in rats. Brain Res. 2001;917:147–157. doi: 10.1016/s0006-8993(01)02822-0. [DOI] [PubMed] [Google Scholar]
- Storey KB. Mammalian hibernation. Transcriptional and translational controls. Adv Exp Med Biol. 2003;543:21–38. [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Tasaki K, Ruetzler CA, Ohtsuki T, Martin D, Nawashiro H, Hallenbeck JM. Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res. 1997;748:267–270. doi: 10.1016/s0006-8993(96)01383-2. [DOI] [PubMed] [Google Scholar]
- Vainer GW, Saada A, Kania-Almog J, Amartely A, Bar-Tana J, Hertz R. PF-4708671 activates AMPK independently of p70S6K1 inhibition. PLoS One. 2014;9:e107364. doi: 10.1371/journal.pone.0107364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. Embo J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer S, Priebs J, Kuhlow D, et al. D-Glucosamine supplementation extends life span of nematodes and of ageing mice. Nature communications. 2014;5:3563. doi: 10.1038/ncomms4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng QP, Kozlowski M, Belham C, Zhang A, Comb MJ, Avruch J. Regulation of the p70 S6 kinase by phosphorylation in vivo. Analysis using site-specific anti-phosphopeptide antibodies. J Biol Chem. 1998;273:16621–16629. doi: 10.1074/jbc.273.26.16621. [DOI] [PubMed] [Google Scholar]
- Westphal RS, Coffee RL, Jr, Marotta A, Pelech SL, Wadzinski BE. Identification of kinase-phosphatase signaling modules composed of p70 S6 kinase-protein phosphatase 2A (PP2A) and p21-activated kinase-PP2A. J Biol Chem. 1999;274:687–692. doi: 10.1074/jbc.274.2.687. [DOI] [PubMed] [Google Scholar]
- Wool IG, Chan Y, Land AG. Mammalian Ribosomes: The Structure and the Evolution of the Proteins. In: Hershey JWB, Mathews MB, Sonenberg N, editors. Translational control, Cold Spring Harbor monograph series. Cold Spring Harbor Press, Cold Spring Harbor Laboratory; 1996. pp. 685–732. [Google Scholar]
- Zhu X, Smith MA, Perry G, Wang Y, Ross AP, Zhao HW, Lamanna JC, Drew KL. MAPKs are differentially modulated in arctic ground squirrels during hibernation. J Neurosci Res. 2005;80:862–868. doi: 10.1002/jnr.20526. [DOI] [PubMed] [Google Scholar]