Abstract
Renal cell carcinoma (RCC) is resistant to traditional cancer therapies, and metastatic RCC (mRCC) is incurable. The shortcomings in current therapeutic options for patients with mRCC provide the rationale for the development of novel treatment protocols. TNF-related apoptosis-inducing ligand (TRAIL) has proven to be a potent inducer of tumor cell death in vitro and in vivo, and a number of TRAIL death receptor agonists (recombinant TRAIL or TRAIL death receptor-specific mAb) has been developed and tested clinically. Unfortunately the clinical efficacy of TRAIL has been underwhelming and is likely due to a number of possible mechanisms that render tumors resistant to TRAIL, prompting the search for drugs that increase tumor cell susceptibility to TRAIL. The objective of this study was to determine the effectiveness of combining the diterpene triepoxide triptolide, or its water-soluble prodrug, Minnelide, with TRAIL receptor agonists against RCC in vitro or in vivo, respectively. TRAIL-induced apoptotic death of human RCC cells was increased in the presence of triptolide. The triptolide-induced sensitization was accompanied by increased TRAIL-R2 (DR5) and decreased HSP70 expression. In vivo treatment of mice bearing orthotopic RCC (Renca) tumors showed the combination of Minnelide and agonistic anti-DR5 mAb significantly decreased tumor burden and increased animal survival compared to either therapy alone. Our data suggest triptolide/Minnelide sensitizes RCC cells to TRAIL-induced apoptosis through altered TRAIL death receptor and heat shock protein expression.
Keywords: TRAIL, renal cell carcinoma, triptolide, apoptosis, death receptor
Introduction
Renal cell carcinoma (RCC) represents 2-3% of all malignant disease in adults, resulting in an estimated 209,000 new cases and 102,000 deaths annually worldwide [1]. Treatment options for RCC vary according to the staging at the time of diagnosis. Partial or radical nephrectomy with surgical resection of metastatic lesions is standard-of-care for localized RCC tumors with no evidence of metastasis or a single metastasis, resulting in high 5- and 10-year survival rates [2, 3]. However, ~30% of RCC patients have multiple metastases at initial diagnosis. Metastatic RCC (mRCC) is considered incurable, with a median survival of ~18 months [4]. Treatment for mRCC includes cytokine-based immunotherapies (IFN-α or IL-2), multikinase inhibitors (including sunitinib or sorafenib), or vascular endothelial growth factor (VEGF) inhibitors [5]. These treatments are palliative options for mRCC, as remission rates and 5-year survival of patients receiving these therapies are both low. The limited efficacy of current therapeutic options underscores the need to identify novel treatment options for mRCC patients.
The tumor necrosis factor (TNF) family member TNF-related apoptosis-inducing ligand (TRAIL) is among the proteins and compounds identified in recent years with potent tumoricidal activity. TRAIL generated considerable excitement as a potential cancer therapeutic because of its ability to induce apoptotic death in a wide variety of tumor types while having little-to-no cytotoxic activity on normal cells and tissues [6-8]. Despite the therapeutic efficacy of TRAIL death receptor agonists (e.g., recombinant soluble versions of the TRAIL protein or agonistic TRAIL-R1 or –R2-specific mAb) in a variety of preclinical tumor models, testing of these agents in clinical trials has failed to demonstrate the same potency [9]. This lack of clinical success might relate to the fact that ~50% of the human tumor cell lines and the majority of primary tumor isolates are resistant to TRAIL-induced death [10]. Multiple factors can contribute to suboptimal clinical responses to TRAIL-based therapies, and many studies have been conducted to identify agents that will circumvent the various mechanisms of resistance to sensitize tumor cells to TRAIL and improve therapeutic efficacy and clinical outcome.
Triptolide, a diterpenoid triepoxide isolated from the medicinal vine Tripterygium wilfordii, effectively induces autophagic and apoptotic death of a variety of cancer types, including neuroblastoma [11, 12], cholangiocarcinoma [13], colon cancer [14], and pancreatic cancer [15, 16], through the inhibition of heat shock protein 70 (HSP70) [11, 14-16]. A water-soluble analog of triptolide, Minnelide, was designed to remedy issues with solubility that restrict in vivo administration of triptolide [17]. Importantly, Minnelide promotes apoptotic cell death in both in vitro and in vivo models of pancreatic cancer [17], osteosarcoma [18], and small cell lung carcinoma [19], suggesting it could be an effective therapeutic alternative in clinical settings. Beyond its efficacy as a stand-alone therapy, triptolide has also been used in combination with chemotherapeutics (including curcumin [20], indarubicin [20], and cisplatin [21]) or irradiation [22, 23] to enhance antitumor treatments. Other combination therapies have included the in vitro treatment of cholangiocarcinoma or pancreatic cancer cells with triptolide and TRAIL [13, 24]. Though investigations of novel therapies for RCC have included both TRAIL [25-27] and triptolide [28] individually, using these two molecules in combination – especially in vivo – has not yet been examined.
In the present study, we investigated the tumoricidal activity of triptolide and TRAIL receptor agonists against human and mouse RCC lines in vitro, and in vivo using an orthotopic immunocompetent mouse model. Our data demonstrate the combination of triptolide with recombinant TRAIL (rTRAIL) protein effectively induces apoptotic cell death of human RCC lines in vitro, and the increased apoptosis is associated with triptolide-induced modulation of HSP70 expression and increased TRAIL-R2 (DR5) expression. Similar modulation of HSP70 and sensitization to TRAIL receptor agonist-induced apoptosis was observed in the murine renal adenocarcinoma cell line Renca after triptolide exposure in vitro and in vivo. When mice bearing established orthotopic RCC tumors were treated with Minnelide and agonistic anti-DR5 mAb, those mice receiving combination therapy had increased tumor cell apoptosis, decreased tumor burden, and increased survival compared to either agent alone. Our results provide evidence for the use of combination therapy consisting of triptolide and TRAIL receptor agonists in the treatment of metastatic RCC. The implications of these findings will be discussed.
Results
Triptolide enhances TRAIL-induced death of RCC cells
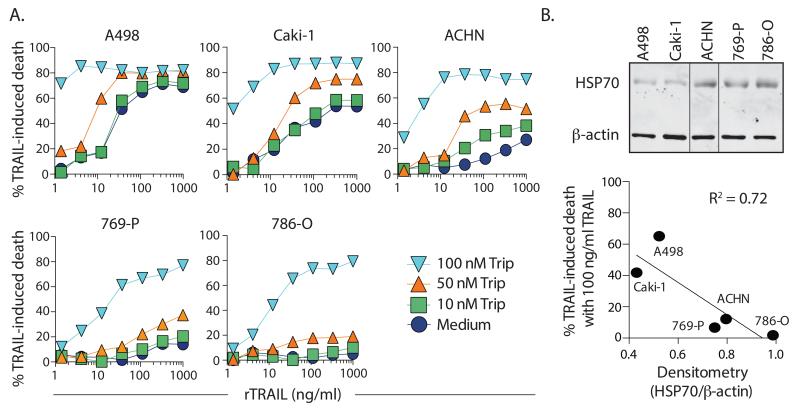
To evaluate the potential benefit of using triptolide to sensitize RCC tumor cells to TRAIL, we initially examined a panel of human RCC cell lines with differing sensitivity to TRAIL alone. In our hands, the hierarchy of TRAIL sensitivity for the cell lines tested was A498 > Caki-1 > ACHN > 769-P >786-O (Figure 1A; open symbols). When these cells were treated with increasing concentrations (10, 50, or 100 nM) of triptolide, there was a dose-dependent augmentation in TRAIL-induced cell death observed for each line. Increased expression of HSP70 can render tumor cells resistant to TRAIL [29], and elevated HSP70 expression has been detected in RCC cell lines and tumor lesions [30, 31]. Moreover, triptolide inhibits the heat shock response and suppresses HSP70 expression [32]. Thus, we assessed native HSP70 expression in each of these RCC cell lines, and found an inverse correlation between HSP70 expression and TRAIL sensitivity (Figure 1B). Since ACHN was in the middle of the TRAIL sensitivity hierarchy and showed augmentation in cell death with several doses of triptolide over the range of TRAIL concentrations, we concentrated our subsequent analyses to examine the triptolide-induced changes in this RCC cell line.
Figure 1. Triptolide sensitizes human RCC cells to TRAIL-induced death.
A. The effect of triptolide and recombinant soluble TRAIL (rTRAIL) on human RCC cell viability. Cell lines were treated with the indicated doses of triptolide and recombinant TRAIL. Cell death was measured after 24 h by crystal violet staining. Data presented are representative of at least 2 independent experiments with each cell line. B. HSP70 and β-actin expression in the 5 human RCC lines was determined by western blotting. The sequence of the samples was changed in the blot to match the sequence in (A). Vertical lines denote splicing. Band intensities were determined by densitometry (using ImageJ) to calculate the ratio of HSP70/ β-actin expression. This number was then compared to the percentage of death induced by 100 ng/ml TRAIL to demonstrate an inverse correlation between HSP70 expression and TRAIL sensitivity.
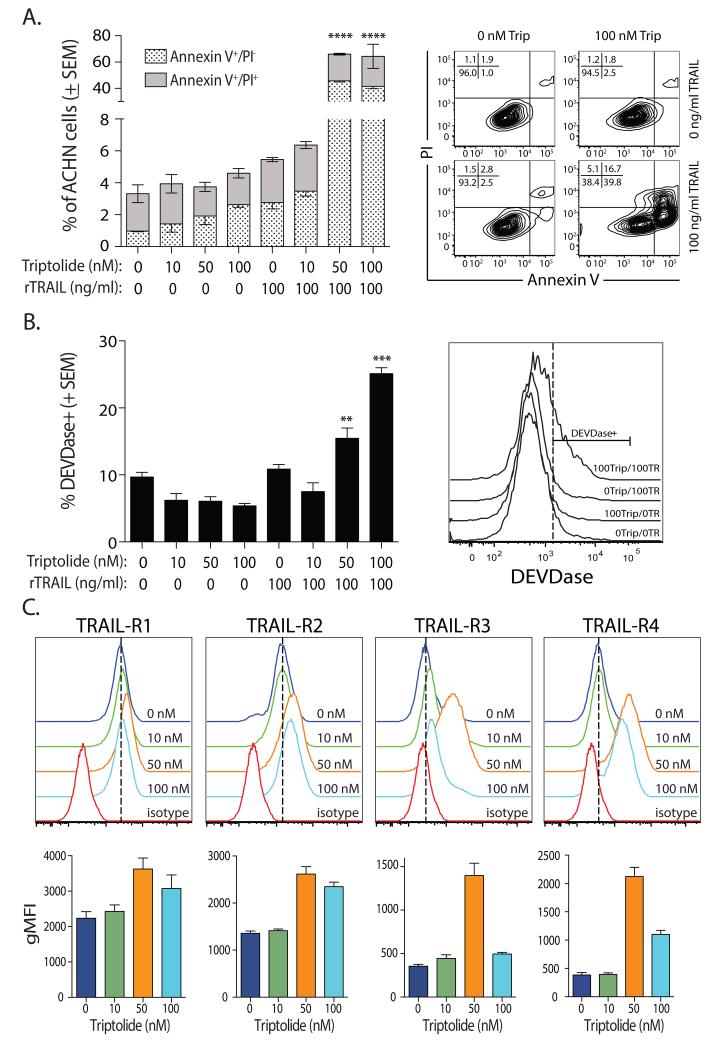
TRAIL-induced cell death occurs primarily by an apoptotic mechanism [6, 8, 33]. Since the assay used in Figure 1A is not specific for a particular mechanism of death [34], we confirmed the addition of triptolide increased TRAIL-induced apoptosis by Annexin V/PI staining and DEVDase activity in ACHN cells. ACHN cells were incubated with triptolide (10, 50, or 100 nM) and/or TRAIL (100 ng/ml) for 8 h. Treatment with escalating doses of triptolide did not substantially alter the frequency of Annexin V+PI− staining or induce DEVDase activation in ACHN cells compared to untreated cells (Figure 2A-B), while treatment with TRAIL alone lead to minimal increases in the frequency of Annexin V+PI− or DEVDase+ cells. However, there were significant increases in the frequencies of Annexin V+PI− or DEVDase+ cells after combination treatment with triptolide (50 and 100 nM) and TRAIL (100 ng/ml).
Figure 2. TRAIL-induced apoptosis is augmented by triptolide-induced upregulation of TRAIL-R2 expression on human RCC cells.
A-B. Increased frequency of (A) Annexin V+ PI− /Annexin V+ PI+ and (B) DEVDase+ ACHN cells with combination triptolide and TRAIL treatment. ACHN cells were treated with the indicated doses or triptolide and/or TRAIL for 8 h. The frequency of Annexin V+ PI− /Annexin V+ PI+ and DEVDase+ cells was then determined by flow cytometry. Representative plots for each analysis is shown, with the frequency of cells in each quadrant indicated. C. Triptolide increases TRAIL receptor expression on ACHN cells. Cells were treated with the indicated doses of triptolide for 24 h. TRAIL-R1, -R2, -R3, and -R4 expression was then determined by flow cytometry. Statistical significance was determined using group-wise, one-way ANOVA with multiple-testing correction using the Holm-Sidak method, and α = 0.05. ** p < 0.01, *** p < 0.005. Data presented are representative of at least 3 independent experiments. Each bar is the average + SE of at least 3 individual replicates per experiment.
One mechanism by which tumor cells can be sensitized to TRAIL-induced apoptosis is through TRAIL death receptor (TRAIL-R1/DR4 and –R2/DR5) modulation, and previous data suggest triptolide can increase TRAIL-R2 expression on tumor cells [35, 36]. Consistent with the observed increase in TRAIL sensitivity, treating ACHN cells with triptolide increased TRAIL-R1 and -R2 expression (Figure 2C). ACHN normally does not express detectable levels of TRAIL-R3 and only minimal TRAIL-R4 [37], but there was an upregulation of TRAIL-R3 and -R4 on these cells after triptolide treatment. Analysis of the other 4 human RCC lines used in Figure 1 revealed a similar increase in TRAIL death receptor and decoy receptor expression after triptolide treatment (data not shown). This surprising result of increased TRAIL-R3 and –R4 expression after triptolide treatment suggests the expression of TRAIL decoy receptors on these cells has little-to-no effect in governing TRAIL sensitivity. Collectively, the data in Figures 1 and 2 suggest triptolide increases sensitivity to TRAIL, in part, by augmenting TRAIL death receptor expression.
Triptolide decreases HSP70 expression in human RCC cells
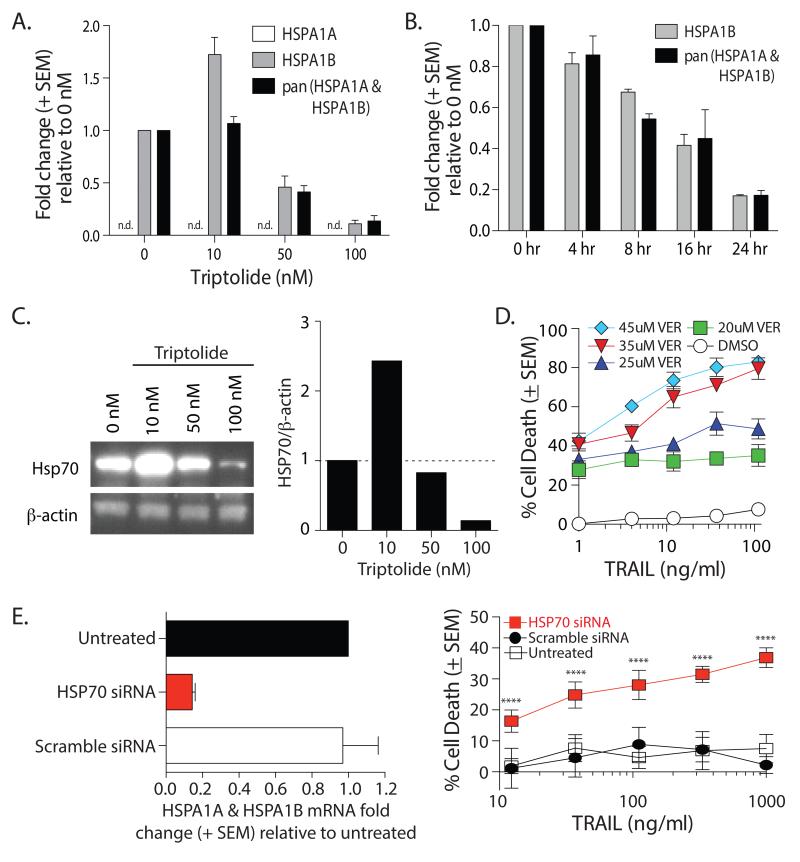
While alterations in TRAIL receptor expression can change tumor cell susceptibility to TRAIL-induced apoptosis, changes in the expression of intracellular proteins that regulate apoptosis can also control tumor cell responsiveness to TRAIL. One consequence of triptolide treatment is a reduction in HSP70 expression [14, 16, 38]. To determine the extent to which HSP70 modulation affects ACHN sensitivity to TRAIL, we first examined HSP70 mRNA expression in ACHN after 24 h treatment with different concentrations of triptolide. Our assessment of HSP70 expression took into account the fact that it is encoded by two genes, HSPA1A and HSPA1B [39]. HSPA1B mRNA expression increased when ACHN was treated with 10 nM triptolide, which was not surprising since HSP70 expression is induced during cellular stress [16, 40]. However, HSPA1B mRNA decreased at higher triptolide concentrations (50nM and 100nM) compared to untreated cells (Figure 3A). We did not detect any HSPA1A mRNA in these cells. Similar modulation was seen when examining the abundance of HSP27 and HSF1 mRNA (data not shown). We then examined changes in HSPA1A and HSPA1B mRNA expression in ACHN cells treated with a single concentration of triptolide (100 nM) over time. We detected a decrease in these mRNA species as early as 4 h, which continued to fall over the 24 h period (Figure 3B). Concurrent with the changes in mRNA, ACHN cells treated with 10 nM triptolide had increased HSP70 protein expression, which decreased when higher triptolide doses were used (Figure 3C). To determine the extent to which the observed loss of HSP70 expression influenced the sensitivity of ACHN cells to TRAIL-induced apoptosis, we treated ACHN cells with TRAIL in the presence or absence of the HSP70 inhibitor VER-155008, which targets the ATPase binding domain of HSP70 [41]. Incubation with VER-155008 alone induced ~25-40% cell death (Figure 3D). When ACHN cells were treated with VER-155008 and TRAIL, there was a dose-dependent increase in sensitivity of ACHN cells to TRAIL (Figure 3D) – similar to the increased sensitivity after treatment with triptolide. Additional data supporting the importance of HSP70 in the resistance of ACHN cells to TRAIL-mediated death was obtained after transfecting the cells with siRNA oligonucleotides specific for HSP70 or a scramble control. After 48 h, total mRNA was harvested to confirm siRNA-mediated knockdown (Figure 3E, left panel). As the half-life of HSP70 protein is 1-2 h [42, 43] ACHN cells transfected with HSP70 siRNA were significantly more sensitive to TRAIL compared to cells transfected with the scramble control siRNA (Figure 3E, right panel). Together, these data suggest the triptolide-mediated decrease in HSP70 expression in ACHN cells also contributes to the increased susceptibility to TRAIL.
Figure 3. Triptolide decreases HSP70 expression in ACHN cells.
A-B. ACHN cells were treated with (A) increasing doses of triptolide for 24 h or (B) 100 nM triptolide for 4, 8, 16, or 24 h. Total RNA was isolated and expression of HSPA1A, HSPA1B, and pan HSPA1A/1B was assessed by qRT-PCR. C. ACHN cells were treated with increasing doses of triptolide for 24 h. Cell lysates were prepared and HSP70 expression was assessed by western blot (left). Densitometry analyses of each band normalized to β-actin were calculated (right). D. Addition of the HSP70 inhibitor VER-155008 (VER) sensitized ACHN cells to TRAIL-induced death. Cells were incubated with the indicated concentrations of VER-155008 and/or TRAIL (DMSO). TRAIL-induced cell death was determined after 24 h. E. siRNA knockdown of HSP70 increases ACHN sensitivity to TRAIL. ACHN cells were treated with HSP70-specific or scramble siRNA. After 48 h, total RNA was isolated and expression of pan HSPA1A/1B was assessed by qRT-PCR (left panel) or TRAIL sensitivity was measured. Statistical significance was determined using group-wise, one-way ANOVA with multiple-testing correction using the Holm-Sidak method, and α = 0.05. **** p < 0.001. Data presented (average + SEM) are representative of at least 2 independent experiments consisting of at least 3 replicates in each group.
Triptolide sensitizes Renca cells to TRAIL-induced apoptosis and decreases HSP70 expression
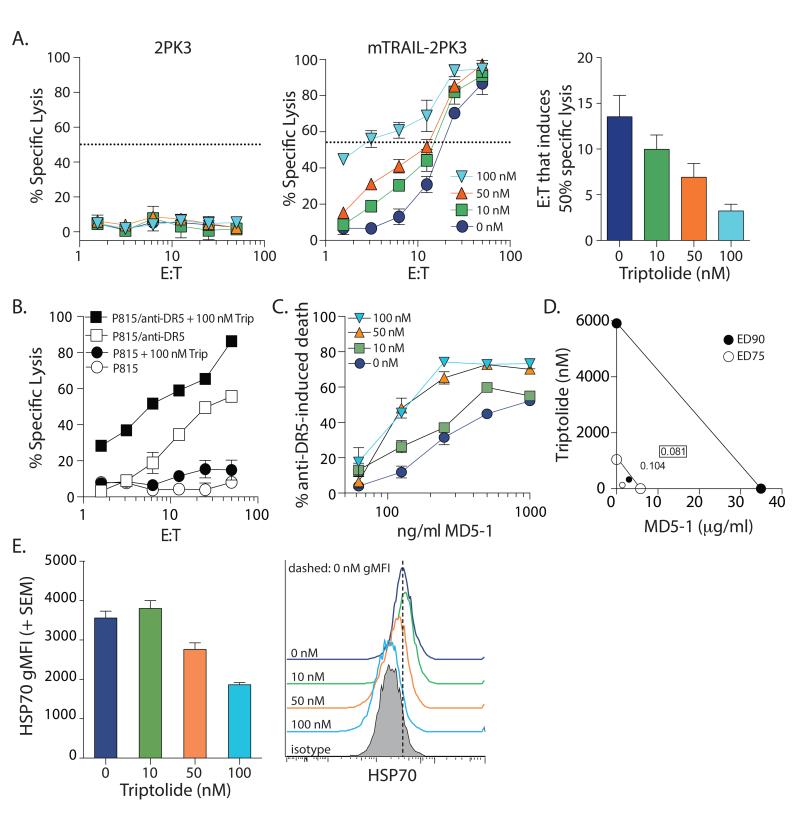
The data showing triptolide increased the in vitro sensitivity of human RCC cells to TRAIL-induced apoptosis suggests the potential of using this drug combination as a therapy for RCC. Thus, we next wanted to determine the extent to which these in vitro results could be translated in vivo using a mouse model of RCC where the murine renal cell carcinoma cell line Renca is implanted orthotopically into immunocompetent BALB/c mice [25]. In addition to recombinant soluble TRAIL protein, an established in vivo therapy uses agonistic mAb specific for TRAIL-R2/DR5 [44-48]. However, the efficacy of agonistic anti-DR5 mAb monotherapy has been suboptimal in controlling tumor outgrowth [49-51]. We started by determining the extent to which combination therapy consisting of triptolide and mouse TRAIL receptor agonists induced Renca cell death in vitro. Similar to the human RCC lines, the sensitivity of Renca cells to various TRAIL receptor agonists [TRAIL-expressing effector cells (mTRAIL-2PK3; Figure 4A), cell-bound anti-DR5 mAb (Figure 4B), or plate-bound anti-DR5 mAb (Figure 4C)] was increased in the presence of triptolide. Moreover, the inclusion of triptolide reduced the mTRAIL-2PK3 effector cell: Renca target cell ratio needed for 50% specific lysis from 13.5 to 3.2 (Figure 4A; right panel). We also determined this combination synergistically killed Renca using triptolide and plate-bound anti-DR5 mAb (Figure 4D). Consistent with the effects of triptolide on ACHN cells, Renca cells showed decreased HSP70 protein expression after in vitro treatment with triptolide (Figure 4E). Collectively, these data suggest triptolide-treated Renca cells demonstrate increased sensitivity to TRAIL-induced apoptosis and decreased HSP70 expression, similar to their human RCC counterparts.
Figure 4. Triptolide sensitizes Renca cells to TRAIL receptor agonistic-induced death and decreases HSP70 expression.
A-B. 51Cr-labeled Renca cells (104/well) were cultured with (A) 2PK3 cells or mTRAIL-2PK3 cells, or (B) P815 cells or MD5-1-coated P815 cells with or without triptolide at the indicated E:T ratios. % specific lysis was determined after 16 h. The mTRAIL-2PK3 effector cell: Renca target cell ratio needed to induce 50% specific target cell lysis (as indicated by the dashed line) was determined by nonlinear regression analysis using GraphPad Prism. C. Renca cells were cultured with the indicated concentrations of triptolide in microtiter wells coated with anti-DR5 mAb. % anti-DR5 mAb-induced cell death was determined using MTT after 24 h. D. Isobologram analysis of the combination of triptolide and anti-DR5 mAb (MD5-1) demonstrates a synergistic interaction on Renca cells (The effective doses of both drugs that result in cell death that is 90% (•) and 75% (⦿) of untreated cells). The lines of additivity are shown. The boxed and unboxed numbers in the lower left quadrant are the ED90 and ED75 combination indices, respectively, calculated using the CalcuSyn software package. E. HSP70 expression decreases in Renca cells after triptolide treatment. Renca cells were cultured for 24 h with increasing doses of triptolide for 24 h. HSP70 expression was then determined by flow cytometry. Isotype mAb staining is shown for reference. Data presented (average + SE) are representative of at least 2 independent experiments consisting of at least 3 replicates in each group.
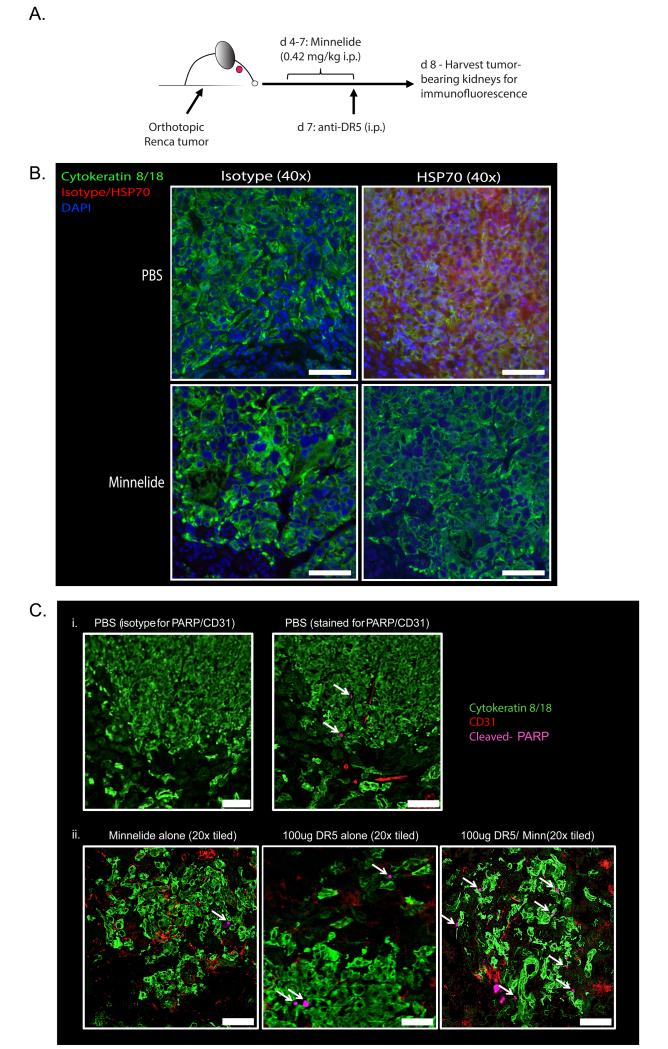
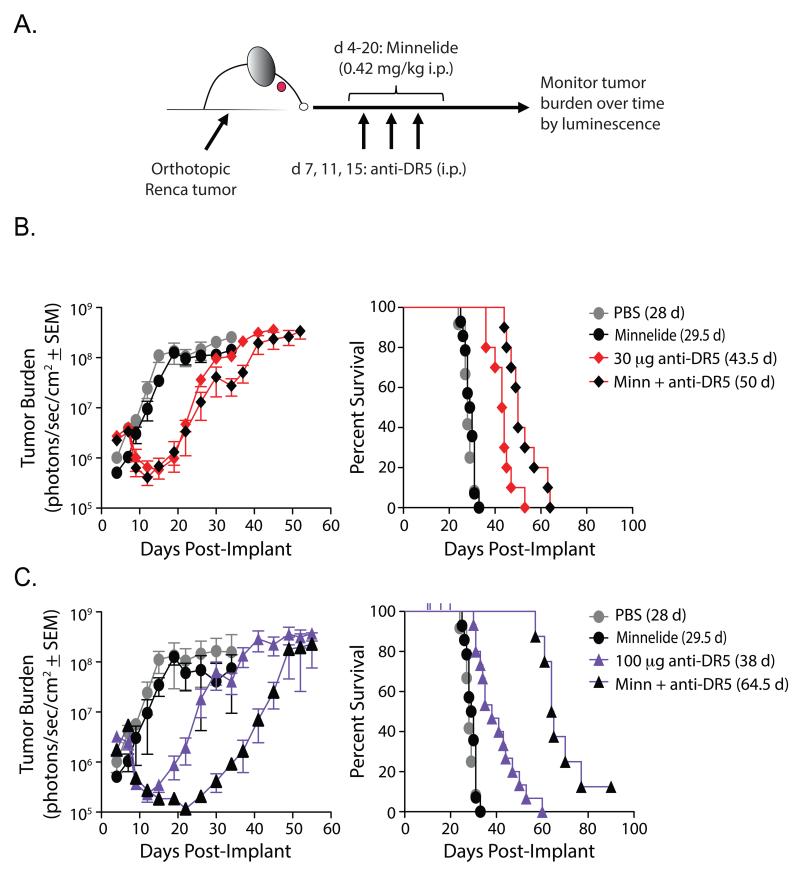
Combination Minnelide and anti-DR5 mAb therapy of Renca-bearing mice significantly reduces tumor burden and prolongs survival
Triptolide holds promise as an antitumor therapeutic, but solubility issues initially limited its potential clinical use [16]. Consequently, the water-soluble analog Minnelide was developed [17], and preclinical studies with Minnelide have demonstrated similar effects on tumor cells compared to triptolide. For example, treatment of Renca-bearing mice with Minnelide reduced HSP70 expression within the tumor (Figure 5A-B). We saw very little, if any, HSP70 staining in areas of the tumor-bearing kidney sections free of tumor (i.e., cytokeratin 8/18 negative areas). We went on to test the efficacy of Minnelide, anti-DR5 mAb therapy, or the combination as treatment against established, orthotopic Renca tumors in BALB/c mice [25]. Starting on day 4 post-tumor implantation, mice were given Minnelide [0.42 mg/kg QD, i.p.] for 17 days; mice only given anti-DR5 mAb were given saline during the same period. Mice were treated with anti-DR5 mAb (30 or 100 μg/dose) i.p. on day 7 for day 8 harvests (Figure 5A) and on days 7, 11, and 15 for longitudinal studies (Figure 6A). To assess tumor cell death in situ following Minnelide and/or anti-DR5 mAb administration, tumors were harvested and stained for cleaved poly-ADP ribose polymerase (PARP). While PBS (Figure 5Ci) and Minnelide (Figure 5Cii) alone showed some tumor cell death, the Minnelide/anti-DR5 mAb combination showed increased tumor cell apoptosis (Figure 5Cii). Next, tumor burden was measured at various times by bioluminescent imaging following Minnelide, anti-DR5 mAb, or combination treatment (Figure 6B-C). Despite showing antitumor activity in other models [18, 19], administration of Minnelide alone provided no antitumor effect in Renca-bearing mice compared to PBS treatment. In contrast, there was a significant reduction in total tumor burden in mice receiving either anti-DR5 mAb alone or in combination with Minnelide (Figure 6B-C, left), with combination therapy of Minnelide and three 100 μg doses of anti-DR5 mAb significantly delaying tumor outgrowth compared to anti-DR5 mAb alone (Figure 6B, left). With regard to enhancing survival, Minnelide treatment offered no advantage over PBS alone (Figure 6B-C, right). Both low-dose and high-dose anti-DR5 mAb therapy provided a survival advantage over mice treated with either Minnelide alone or PBS, which is consistent with previous reports [45, 47, 52]. Of note, both the low-dose and high-dose combination therapy improved survival when compared with their respective stand-alone anti-DR5 therapy (Figure 6B-C, right). Further, the high-dose combination therapy significantly improved survival compared with the low-dose combination therapy. Of clinical importance, however, was the observation that high-dose combination therapy resulted in nontumor-related deaths (censored from percentages, but indicated by ticks on the survival curve in Figure 6C, right). Together, these data demonstrate immunotherapy using Minnelide in combination with anti-DR5 mAb can provide a significant survival advantage when used to treat established, orthotopic renal cell tumors.
Figure 5. Minnelide modulates HSP70 expression in vivo in established orthotopic RCC tumors and sensitizes tumor cells to anti-DR5 mAb-induced apoptosis.
A. Experimental design for immunofluorescence imaging of protein expression within tumors. BALB/c mice were challenged with luciferase-expressing Renca cells in the kidney. On d 4-7, mice received daily i.p. injections of Minnelide (0.42 mg/kg). Mice received anti-DR5 mAb (MD5-1; 100 μg i.p.) on d 7. Tumor-bearing kidneys were harvested on d 8 and prepared for immunofluorescence imaging to measure (B) HSP70 and (C) cleaved-PARP expression. Slides were also stained for cytokeratin 8 & 18 and CD31 to visualize tumor cells and vasculature, respectively, and DAPI. Images in B are at 40× (scale bar = 50 μm), while individual 20× images (scale bar = 100 μm) were taken and tiled together to visualize tumor nodules from each mouse in C (Data in B-C are representative of 3 mice/group).
Figure 6. Minnelide enhances the activity of anti-DR5 mAb therapy in vivo against established orthotopic RCC tumors.
A. Experimental design to monitor tumor burden and survival. Starting on d 4, mice received daily i.p. injections of Minnelide (0.42 mg/kg) through d 20. On d 7, 11, 14 mice received anti-DR5 mAb (MD5-1; 30 μg or 100 μg i.p.). Tumor burden was monitored via bioluminescence starting on d 4. Mean +/− SEM tumor burden and survival for (B) low (30 μg) dose and (C) high (100 μg) dose anti-DR5 are shown. Data are cumulative from 3 independent experiments; n = ≥ 5 mice/group/experiment). For clarity, data for anti-DR5 mAb- and Minnelide/anti-DR5 mAb-treated mice are separated based on dosage of anti-DR5 mAb used. PBS and Minnelide only groups are the same in the upper and lower panels.
Discussion
The excitement generated over TRAIL receptor agonists as cancer treatment options began shortly after the initial description of TRAIL [6, 8], primarily because of the unique ability of these reagents to induce apoptosis specifically in tumor cells while having minimal toxicity on normal cells and tissues. Despite being tested clinically in patients with a variety of types of cancer, recombinant human TRAIL (Dulanermin) did not achieve significant clinical efficacy. A number of hypotheses have been posited to explain this lack of efficacy, including short distribution and elimination half-lives and the ability to engage all four membrane-bound TRAIL receptors. As an alternative to recombinant soluble TRAIL, TRAIL-R1 and –R2-specific agonistic mAb have also tested therapeutically. mAb have a longer in vivo half-life compared to soluble TRAIL, and will only bind to one TRAIL receptor. The agonistic anti-mouse DR5-specific mAb, MD5-1, has exhibited strong antitumor activity in some mouse tumor models [53]. The MD5-1 mAb (by itself), however, has had limited therapeutic efficacy in orthotopic models of renal cell carcinoma, requiring it to be used in combination with other agents to optimize efficacy [45, 54]. This lack of efficacy reflects a common challenge for therapies based on TRAIL receptor agonists – tumor cells can have multiple mechanisms of resistance [55, 56], including altered TRAIL receptor expression or signaling [57-61], overexpression of cFLIP [33, 62], and overexpression or constitutive activation of a variety of pro-survival molecules [37, 63-65]. The goal of the present study was to examine the mechanism(s) of action whereby triptolide/Minnelide treatment increased the responsiveness of human and mouse RCC cells to TRAIL receptor agonist-induced death. Our studies revealed triptolide sensitized human and mouse RCC cells to TRAIL receptor agonists, which is consistent with data examining other tumor types [13, 35, 36, 66-68]. The main aspect distinguishing the present study from previous ones, however, is this is the first report to include an in vivo assessment of Minnelide and agonistic anti-DR5 mAb in an orthotopic tumor model in immunocompetent mice. We feel the data presented are an important first step toward future clinical testing of these agents.
Identification of a single mechanism governing tumor cell sensitivity to TRAIL has been elusive. Initially, it was believed expression of TRAIL-R3 and –R4 was sufficient to protect a cell from TRAIL, based on data where TRAIL-R3 or –R4 overexpression protected cells from TRAIL-induced death [69-72]. Similarly, a variety of drugs have been identified to increase TRAIL death receptor expression on tumor cells. Triptolide was initially identified through a small molecule screen for compounds that inhibit human heat shock response [32]. Heat shock proteins have diverse regulatory capacity in a variety of aspects of cell growth and death, and HSP70 is needed within cells to ensure the proper folding of newly synthesized proteins, help in the translocation of these new proteins across intracellular membranes, and movement of proteins in and out of the nucleus [73]. Overexpression of HSP70 also increases tumorigenic potential and resistance to apoptotic stimuli [74]. Cell death following death receptor signaling can occur without (Type I) and with (Type II) the help of the intrinsic (mitochondrial) pathway [75]. Triptolide will increase TRAIL-R1 and/or –R2 expression on tumor cells [24, 35], but selective HSP70 suppression with siRNA will upregulate TRAIL-R1 and –R2 and downregulate cFLIP [76]. Interestingly, we also saw a dramatic increase in TRAIL-R3 and –R4 expression on the human RCC cells after incubation with triptolide, suggesting expression of the TRAIL decoy receptors likely does not play a protective role on these cells. HSP70 also inhibits apoptosis downstream of mitochondrial events that lead to the release of cytochrome c, formation of the Apaf-1 apoptosome, and activation of ‘executioner’ caspases (e.g., caspase-3) [77-79]. While TRAIL receptor modulation can alter the sensitivity of a tumor cell to TRAIL death receptor agonists, it is becoming apparent that concomitant or independent modulation of intracellular pro- and anti-apoptotic proteins will also tip the balance to favor increased TRAIL sensitivity. Thus, it would appear that HSP70 has the ability to regulate extrinsic and intrinsic components critical for TRAIL-mediated apoptosis.
By including studies that used an orthotopic mouse model of RCC, we found that combination therapy consisting of Minnelide and agonistic anti-DR5 mAb significantly reduced tumor burden in mice and prolonged survival compared to either agent alone (Figure 5). However, the Minnelide/anti-DR5 mAb therapy did not provide lasting protection from tumor outgrowth. It can be speculated apoptotic tumor cell death is a sub-optimal stimulus to initiate an anti-tumor immune response that provides lasting protection against tumor outgrowth [80], so pairing Minnelide/anti-DR5 mAb with an immunostimulatory signal could have significant translational implications. When anti-DR5 mAb is used in tandem with costimulatory signals – e.g., anti-CD40 mAb to stimulate DC activation, anti-4-1BB mAb to stimulate T cells, and cytokine (IL-2) administration – tumors can be eradicated through the induction of an effective antitumor immune response [45, 81]. Thus, adding IL-2 to the Minnelide/anti-DR5 mAb combination therapy could prove effective for eradicating established metastatic disease by stimulating the death of tumors (though Minnelide/anti-DR5 mAb) while activating an immune response against the dying tumor (through IL-2). The use of high-dose IL-2 or interferon alpha as an immunotherapy against metastatic RCC is toxic in many patients [5], but their use can lead to primary and metastatic tumor eradication along with providing robust, lasting protection in a subset of patients [82]. By pairing Minnelide/anti-DR5 mAb with IL-2 treatments, it is tempting to speculate that the IL-2 dose necessary for optimal response could be decreased—thereby eliminating, or at least decreasing, IL-2 associated toxicity. Clearly, extending the present study to include a modulator/activator of immune responses would be clinically relevant and could provide important data for the advancement of current immunotherapies.
The timing and duration of the Minnelide/anti-DR5 mAb combination therapy used herein was based on historical precedent [17, 52, 83], and it proved effective in reducing tumor burden and prolonging survival (Figure 5). However, future studies aimed at optimizing therapy duration would be enlightening. Of note in Figure 5E-F, tumor outgrowth after therapy did not occur until after Minnelide/anti-DR5 mAb therapy had been completed (i.e., starting after the lowest tumor burden measurement at day 21 post-implant, which corresponded with the end of Minnelide treatments). Future investigation extending the duration of Minnelide treatments, increasing the Minnelide dosage, and/or increasing the number of anti-DR5 mAb treatments could improve efficacy of this combination therapy. Such optimization could aid the translation of these preclinical results into therapies for RCC patients. We were surprised by the lack of an in vivo antitumor effect when Minnelide was used as a monotherapy against orthotopic Renca tumors, based on previous reports. The lack of in vivo therapeutic efficacy of Minnelide alone might be due to the dose used and/or the time frame over which it was administered. In studies of the in vivo use of Minnelide as a monotherapy against osteosarcoma and pancreatic cancer, it was administered over a longer timeframe [17, 18], and future studies to examine the efficacy of long-term Minnelide treatment as a monotherapy for RCC should be considered. Another important factor that can contribute to the extent of therapy responsiveness is the anatomical location where the experimental tumors are growing, as recent data indicate that orthotopic tumors are less responsive to immunotherapy compared to the same tumor types located subcutaneously [54]. Moreover, additional in vivo studies are needed to compare the therapeutic benefits of agents that specifically inhibit HSP70 (e.g., HSP70 siRNA) versus those that are more “broad-spectrum” HSP70 inhibitors in combination with TRAIL receptor agonists [84]. Triptolide inhibits the induction of the heat shock response by blocking heat shock transcription factor 1 (HSF1) activation and production of several heat shock proteins [32].
In recent studies in which Minnelide was used to treat neuroblastoma and pancreatic cancer, death of tumor cells was induced through both apoptotic and autophagic mechanisms [12, 15]. While the induction of apoptosis is of obvious interest and importance anti-tumor therapies, recent clinical reports have demonstrated that the induction of autophagy in RCC could increase their sensitivity to radiation therapy (which is minimally effective against RCC in most cases) [85]. Further, the induction of autophagy could also contribute to the effectiveness of novel chemotherapeutics [86, 87]. With this in mind, examining the induction of autophagy in triptolide-treated RCC cells and the degree to which the autophagy pathway contributes to the efficacy of Minnelide/anti-DR5 mAb combination therapy would enhance our mechanistic understanding and could offer insights for how to improve treatments for RCC. In addition to increasing sensitivity to cell death, triptolide can affect other aspects of tumor cells and the immune system. Triptolide inhibits IFN-γ-induced PD-L1 expression on tumor cells [88], a key pathway that can limit antitumor immunity. Triptolide can also inhibit T cell and DC activation [89, 90], which would be detrimental to cancer therapies designed to stimulate a T cell response. It is unknown how triptolide affects other immune suppressive factors induced by tumors (such as MDSC), which can be inhibited by other drugs currently used to treat RCC (5-FU) [91]. Additionally, there are little data describing the “normal cell” effects of drugs that sensitize tumor cells to TRAIL receptor agonists.
In conclusion, we have shown that triptolide/Minnelide sensitizes RCC cells to TRAIL receptor agonist-induced apoptosis in in vitro and in vivo models. We demonstrate that combination therapy of Minnelide with anti-DR5 mAb is effective in decreasing tumor burden and increasing the survival of tumor-bearing mice in an orthotopic model of RCC. Our data suggest that Minnelide used in combination with anti-DR5 mAb treatment could offer a novel therapeutic against metastatic RCC that could replace or supplement current therapies with sub-optimal efficacy.
Materials and methods
Animals
Female BALB/c mice (7-8 wk old) were purchased from the National Cancer Institute (Frederick, MD). All mice were housed under pathogen-free conditions at the AALAC-accredited University of Minnesota Animal Care Facility. All animal procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee.
Cell lines
The human RCC cell lines ACHN, A498, Caki-1, 769-P, and 786-O were obtained from the American Type Culture Collection (Rockville, MD). A498 and ACHN were cultured in Eagle’s MEM supplemented with 10% FCS, 1% nonessential amino acids, 1 mM sodium pyruvate, and 1% streptomycin/penicillin solution. Caki-1, 769-P, and 786-O were cultured in RPMI 1640 supplemented as above. A variant of the murine renal adenocarcinoma cell line, Renca, that stably expresses firefly Luciferase and green fluorescent protein (GFP; Renca-GLE), was obtained from Dr. Andrew Wilber (Southern Illinois University School of Medicine, Springfield, IL). Renca-GLE cells were maintained in complete RPMI supplemented with 0.3 μg/ml puromycin and 300 μg/ml zeocin.
In vitro killing
Tumor cell sensitivity to triptolide and/or recombinant human TRAIL (rTRAIL) was determined as follows. Human RCC cells were added to 96-well flat-bottom plates (2 × 104 cells/well) and allowed to attach overnight. Titrated doses of triptolide and/or rTRAIL (Peprotech, Rocky Hill, NJ)were then added. In some cases, cells were treated with the HSP70 inhibitor VER-155008 (Sigma, St. Louis, MO) alone or in combination with rTRAIL. Cell death was determined 24 h later by crystal violet staining [34], with results presented as percent cell death: [1 − (O.D. cells treated per O.D. cells not treated) × 100]. To assess tumor cell death induced by anti-DR5 mAb, protein A coated microtiter plates (Thermo Fisher Scientific, Rockford, IL) were coated with increasing concentrations of the agonistic anti-DR5 mAb MD5-1 (eBioscience, San Diego, CA) overnight. The plates were washed with PBS, and Renca-GLE cells were added (2 × 104 cells/well) in the presence or absence of triptolide. Cell death was determined 24 h later using MTT. For analysis of apoptosis, tumor cells were incubated with triptolide and/or rTRAIL for 8 h and apoptotic cell death was measured by flow cytometry using FITC-conjugated annexin V (R&D Systems, Minneapolis, MN) and propidium iodide (Sigma, St. Louis, MO) as described [92, 93]. After staining, cells were analyzed on a BD LSR II (BD Biosciences, San Diego, CA) and FlowJo software (TreeStar Inc, Ashland, OR).
DEVDase activity
DEVDase activation was assessed as previously described [58]. Briefly, cells (5 × 105/well in a 24-well plate) were treated with triptolide and/or rTRAIL at the indicated concentrations for 8 h. The cells were harvested and DEVDase activity was measured using the carboxyfluorescein-labeled aspartic acid-glutamic acid-valine-aspartic acid (DEVD) fluoromethyl ketone peptide caspase inhibitor, according to the manufacturer’s protocol (Immunochemistry Technologies, Bloomington, MN). Cells were incubated with the 1× FLICA solution for 1 hour at 37°C before analysis.
Flow cytometry
Untreated and triptolide-treated cells were blocked using a cocktail of anti-CD16/32 and normal mouse serum in PBS containing 2 mg/ml bovine serum albumin and 0.02% NaN3 (FACS buffer) prior to surface staining with the PE-conjugated DJR1 (eBioscience), DJR2-4 (eBioscience), 90906 (R&D Systems), 104918 (R&D Systems) specific for TRAIL-R1, -R2, -R3, or -R4, respectively, or a PE-conjugated mouse IgG1 isotype control mAb (eBioscience) at 4°C for 30 min. Cells were analyzed immediately or fixed in 2% paraformaldehyde for 30 min at 4°C, washed with FACS buffer, and stored until analysis. For intracellular staining of HSP70, cells were permeablized in FACS buffer containing 0.5% saponin at 4°C for 30 min, and incubated with PE-conjugated anti-HSP70 (clone W27; Santa Cruz Biotechnology, Dallas, TX) or mouse IgG2a isotype control (BioLegend, San Diego, CA) at 4°C for 30 min. After staining, cells were analyzed on a BD LSR II and FlowJo software.
Quantitative RT-PCR
Total RNA was isolated with TRIzol reagent (Life Technologies, Carlsbad, CA) and 1 mg was reverse-transcribed using Superscript III (Life Technologies). The resulting cDNA was used as a template for real-time PCR for HSPA1A, HSPA1B, and 18S rRNA was performed using TaqMan primer/probe sets. The TaqMan primer/probe sets for HSPA1A (Hs00359163_s1), HSPA1B (Hs00271244_s1), pan HSPA1A & HSPA1B (Hs00271229_s1), and 18S rRNA were purchased from Life Technologies.
siRNA transfection
For transfection, 5 × 104 ACHN cells in 0.5 ml RPMI were incubated overnight in 24-well plates. Lipofectamine RNAiMAX (2 μl; Invitrogen) was added to 50 μl Opti-MEM I medium. HSPA1A/HSPA1B-specific or scramble (ON-TARGETplus Non-targeting pool; 20 μM; both siRNA were purchased from GE Dharmacon, Lafayette, CO) siRNA was added to 50 μl Opti-MEM I medium. Diluted RNAiMAX was added to diluted HSP70 or scramble siRNA, and incubated at room temperature for 10 min. The transfection reagents/siRNA complexes were added to the cells containing 0.5 ml RPMI. Cells were collected after 48 h for mRNA isolation/qRT-PCR or assessment of TRAIL sensitivity.
Western blotting
Tumor cells were added to 24-well plates (5 × 105 cells/well) and allowed to adhere overnight before adding triptolide. After 24 h, cells were lysed in PBS containing 1% Nonidet P-40 and Complete Mini protease inhibitors (1 tablet/10 ml solution, Roche), and the protein concentrations of the lysates were determined by the colorimetric bicinchoninic acid analysis (Thermo Fisher Scientific). Equal amounts of protein were separated by SDS-PAGE, transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA), and blocked with 5% nonfat dry milk in PBS-Tween-20 (0.05% v/v) overnight. The membrane was incubated with the mouse anti-human HSP70 mAb (Enzo Life Sciences, Farmingdale, NY; diluted 1:1000) or goat anti-human actin pAb (Santa Cruz Biotechnology, Santa Cruz, CA) overnight with gentle agitation at 4°C. After washing, the membrane was incubated with an anti-mouse-HRP or anti-goat-HRP (diluted 1:5000, Jackson ImmunoResearch, West Grove, PA) for 1 h. Following several washes, the blots were developed by chemiluminescence according to the manufacturer’s protocol (SuperSignal West Pico Chemiluminescent Substrate, Thermo Fisher Scientific) and imaged on a FluorChem M Digital Darkroom (Protein Simple, Santa Clara, CA).
Cell-mediated killing of murine tumor cells
Renca-GLE cells were labeled with 100 μCi of 51Cr for 1 h at 37°C, washed three times, and resuspended in complete medium. The 51Cr-labeled tumor cells (104/well) were incubated with varying numbers of 2PK3 or mTRAIL-2PK3 effector cells [94, 95] or anti-DR5 mAb (MD5-1)-coated or uncoated P815 cells [52] for 16 h in the presence or absence of triptolide. Assays were performed in round-bottom 96-well plates and the percent specific lysis was calculated as: 100 × (experimental c.p.m. - spontaneous c.p.m.)/(total c.p.m. - spontaneous c.p.m.). Spontaneous and total 51Cr releases were determined in the presence of either medium alone or 1% NP-40, respectively.
Isobologram analysis
Calculations of synergistic cytotoxicity following treatment with triptolide and anti-mouse DR5 mAb (MD5-1) were determined by isobologram analysis [96]. Interaction indices were calculated using the CalcuSyn software package (Biosoft). Combination index (CI) points falling below the line of additivity (<1.0) represent synergism.
Tumor challenge and therapy
Orthotopic (intrarenal) tumor challenge was done as described previously [25, 97, 98]. Briefly, mice were anesthetized, a skin incision was made on the left flank, and 2 × 105 Renca-GLE cells were injected through the intact peritoneum into the left kidney in a 0.1 ml volume of HBSS. Animals were treated with Minnelide (0.42 mg/kg body weight) i.p. [18, 19] from days 4-20 post-implant. Control animals received equal volume of carrier (saline). Animals were treated with MD5-1 mAb (30 μg/dose or 100 μg/dose delivered i.p.) given on days 7, 11, and 15 post-tumor implant.
Immunofluorescence imaging of tumors
Immunofluorescent imaging was performed as previously described [99]. Briefly, tumor-bearing and contralateral kidneys were harvested on day 8 of the tumor challenge/therapy scheme described above (1 day post 100μg MD5-1 mAb treatment). Tissues were snap frozen in OCT, cut to 7 μm thickness, and fixed in acetone for 10 min at −20°C. To assess tumor cell death in vivo, sections were stained using unconjugated rabbit anti-cytokeratin 8 (NB100-91850) and 18 (NBP1-67610; Novus Biologics; Littleton, CO), AF488-conjugated donkey anti-rabbit secondary Ab (Jackson ImmunoResearch Laboratories; West Grove, PA), PE-conjugated anti-CD31 (MEC13.3; eBioscience), and AlexaFluor 647-conjugated anti-human/mouse cleaved PARP (Asp214, clone F21-852; BD Pharmingen). To assess HSP70 expression in vivo after Minnelide treatment, sections were stained using unconjugated rabbit anti-cytokeratin 8 (NB100-91850) and 18 (NBP1-67610; Novus Biologics; Littleton, CO), AF488-conjugated donkey anti-rabbit secondary Ab (Jackson ImmunoResearch Laboratories; West Grove, PA), and PE-conjugated anti-HSP70 (clone W27; Santa Cruz Biotechnology) or mouse IgG2a isotype control (BioLegend). Cover slides were applied using ProLong Gold® Antifade Mountant with DAPI (Life Technologies) and imaged with a Leica DM5500 B microscope.
Bioluminescent imaging of tumor growth
Bioluminescent imaging was done using an IVIS 100 (Caliper Life Sciences, Hopkinton, MA) as described previously [25, 97, 98]. Briefly, 10 min prior to imaging, mice were injected i.p. with 0.1 ml of a 15 mg/ml solution of D-Luciferin (GoldBio.com, St. Louis, MO) and then anesthetized via inhalation of oxygenated isoflurane. Live mice were imaged for 10 sec. Renca-GLE generated photon flux (photons/second) was calculated within a defined region of interest using Living Image software (version 2.5; Perkin Elmer, Waltham, MA).
Statistical Analysis
Data were analyzed using GraphPad Prism® (La Jolla, CA). Specific tests to determine statistical significance are indicated in the figure legends. Statistical signficance is indicated as follows: **** p < 0.001, *** p < 0.005, ** p < 0.01, * p < 0.05, and ns, no significance. Data scatter plots are presented as mean values ± SEM, and data shown as bar graphs are presented as mean + SEM.
Acknowledgements
We thank Deb Lins (University of Minnesota Center for Immunology) for assistance with the 51Cr release experiments, Dr. Susan K. Rathe for assistance with the isobologram analyses, and Elizabeth M. Steinert for immunofluorescence advice. This work was supported by the American Cancer Society 2013 Williston ND Postdoctoral Fellowship (ELB), a Kidney Cancer Association Research Scholarship administered by the Urology Care Foundation (ELB), a University of Minnesota Doctoral Dissertation Fellowship (BRJ), an American Associations of Immunologists Careers in Immunology Fellowship (TSG and BRJ), and the National Institutes of Health Grants CA132827 (KLS), CA170946 (AS), and CA109446 (TSG).
Footnotes
Author contributions
ELB planned and performed experiments, analyzed data, and wrote the paper. TAK planned and performed experiments, and analyzed data. BRJ planned and performed experiments, analyzed data, and wrote the paper. KAM performed experiments and analyzed data. KLS performed experiments and analyzed data. VS planned experiments and provided key reagents. SB planned experiments and provided key reagents. AKS contributed to the conception of the study and provided key reagents. TSG planned experiments and wrote the paper. All authors read and approved the final manuscript.
Disclosures
EL Brincks, BR James, KA Murphy, TA Kucaba, KL Schwertfeger, V Sangwan, and TS Griffith declare that they have no conflict of interest. The University of Minnesota has filed a patent for Minnelide, which has been licensed to Minneamrita Therapeutics, LLC. AK Saluja is the co-founder and the Chief Scientific Officer of this company; S Banerjee is a consultant with this company. This relationship has been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policies.
References
- 1.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–32. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 2.Tan HJ, Norton EC, Ye Z, Hafez KS, Gore JL, Miller DC. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307:1629–35. doi: 10.1001/jama.2012.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, Borkowski A, Colombel M, Klotz L, Skinner E, Keane T, Marreaud S, Collette S, Sylvester R. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59:543–52. doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Patil S, Ishill N, Deluca J, Motzer RJ. Stage migration and increasing proportion of favorable-prognosis metastatic renal cell carcinoma patients: implications for clinical trial design and interpretation. Cancer. 2010;116:347–54. doi: 10.1002/cncr.24713. [DOI] [PubMed] [Google Scholar]
- 5.Ather MH, Masood N, Siddiqui T. Current management of advanced and metastatic renal cell carcinoma. Urology J. 2010;7:1–9. [PubMed] [Google Scholar]
- 6.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–90. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 7.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–63. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 8.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–82. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 9.Micheau O, Shirley S, Dufour F. Death receptors as targets in cancer. Br J Pharmacol. 2013;169:1723–44. doi: 10.1111/bph.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todaro M, Lombardo Y, Francipane MG, Alea MP, Cammareri P, Iovino F, Di Stefano AB, Di Bernardo C, Agrusa A, Condorelli G, Walczak H, Stassi G. Apoptosis resistance in epithelial tumors is mediated by tumor-cell-derived interleukin-4. Cell Death Differ. 2008;15:762–72. doi: 10.1038/sj.cdd.4402305. [DOI] [PubMed] [Google Scholar]
- 11.Antonoff MB, Chugh R, Borja-Cacho D, Dudeja V, Clawson KA, Skube SJ, Sorenson BS, Saltzman DA, Vickers SM, Saluja AK. Triptolide therapy for neuroblastoma decreases cell viability in vitro and inhibits tumor growth in vivo. Surgery. 2009;146:282–90. doi: 10.1016/j.surg.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Krosch TC, Sangwan V, Banerjee S, Mujumdar N, Dudeja V, Saluja AK, Vickers SM. Triptolide-mediated cell death in neuroblastoma occurs by both apoptosis and autophagy pathways and results in inhibition of nuclear factor-kappa B activity. Am J Surg. 2013;205:387–96. doi: 10.1016/j.amjsurg.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clawson KA, Borja-Cacho D, Antonoff MB, Saluja AK, Vickers SM. Triptolide and TRAIL combination enhances apoptosis in cholangiocarcinoma. J Surg Res. 2010;163:244–9. doi: 10.1016/j.jss.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudeja V, Mujumdar N, Phillips P, Chugh R, Borja-Cacho D, Dawra RK, Vickers SM, Saluja AK. Heat shock protein 70 inhibits apoptosis in cancer cells through simultaneous and independent mechanisms. Gastroenterology. 2009;136:1772–82. doi: 10.1053/j.gastro.2009.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mujumdar N, Mackenzie TN, Dudeja V, Chugh R, Antonoff MB, Borja-Cacho D, Sangwan V, Dawra R, Vickers SM, Saluja AK. Triptolide induces cell death in pancreatic cancer cells by apoptotic and autophagic pathways. Gastroenterology. 2010;139:598–608. doi: 10.1053/j.gastro.2010.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, Vickers SM, Saluja AK. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67:9407–16. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- 17.Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S, Schumacher RJ, Blazar BR, Georg GI, Vickers SM, Saluja AK. A preclinical evaluation of Minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med. 2012;4:156ra139. doi: 10.1126/scitranslmed.3004334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee S, Thayanithy V, Sangwan V, Mackenzie TN, Saluja AK, Subramanian S. Minnelide reduces tumor burden in preclinical models of osteosarcoma. Cancer Lett. 2013;335:412–20. doi: 10.1016/j.canlet.2013.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rousalova I, Banerjee S, Sangwan V, Evenson K, McCauley JA, Kratzke R, Vickers SM, Saluja A, D’Cunha J. Minnelide: a novel therapeutic that promotes apoptosis in non-small cell lung carcinoma in vivo. PLoS One. 2013;8:e77411. doi: 10.1371/journal.pone.0077411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Chen F, Wang S, Guo X, Shi P, Wang W, Xu B. Low-dose triptolide in combination with idarubicin induces apoptosis in AML leukemic stem-like KG1a cell line by modulation of the intrinsic and extrinsic factors. Cell Death Dis. 2013;4:e948. doi: 10.1038/cddis.2013.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li CJ, Chu CY, Huang LH, Wang MH, Sheu LF, Yeh JI, Hsu HY. Synergistic anticancer activity of triptolide combined with cisplatin enhances apoptosis in gastric cancer in vitro and in vivo. Cancer Lett. 2012;319:203–13. doi: 10.1016/j.canlet.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Yang S, Su Y, Xiao Z, Wang C, Li X, Lin L, Fenton BM, Paoni SF, Ding I, Keng P, Okunieff P, Zhang L. Enhanced antitumor effect of combined triptolide and ionizing radiation. Clin Cancer Res. 2007;13:4891–9. doi: 10.1158/1078-0432.CCR-07-0416. [DOI] [PubMed] [Google Scholar]
- 23.Chen YW, Lin GJ, Hueng DY, Huang SH, Chia WT, Shieh YS, Ma KH, Sytwu HK. Enhanced anti-tumor activity of triptolide in combination with irradiation for the treatment of oral cancer. Planta Med. 2014;80:255–61. doi: 10.1055/s-0033-1360315. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Sangwan V, Banerjee S, Chugh R, Dudeja V, Vickers SM, Saluja AK. Triptolide sensitizes pancreatic cancer cells to TRAIL-induced activation of the death receptor pathway. Cancer Lett. 2014;348:156–66. doi: 10.1016/j.canlet.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norian LA, Kresowik TP, Rosevear HM, James BR, Rosean TR, Lightfoot AJ, Kucaba TA, Schwarz C, Weydert CJ, Henry MD, Griffith TS. Eradication of metastatic renal cell carcinoma after adenovirus-encoded TNF-related apoptosis-inducing ligand (TRAIL)/CpG immunotherapy. PLoS One. 2012;7:e31085. doi: 10.1371/journal.pone.0031085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsubara H, Mizutani Y, Hongo F, Nakanishi H, Kimura Y, Ushijima S, Kawauchi A, Tamura T, Sakata T, Miki T. Gene therapy with TRAIL against renal cell carcinoma. Mol Cancer Ther. 2006;5:2165–71. doi: 10.1158/1535-7163.MCT-05-0522. [DOI] [PubMed] [Google Scholar]
- 27.Macher-Goeppinger S, Aulmann S, Tagscherer KE, Wagener N, Haferkamp A, Penzel R, Brauckhoff A, Hohenfellner M, Sykora J, Walczak H, Teh BT, Autschbach F, Herpel E, Schirmacher P, Roth W. Prognostic value of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors in renal cell cancer. Clin Cancer Res. 2009;15:650–9. doi: 10.1158/1078-0432.CCR-08-0284. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Zhu W, Leng T, Shu M, Huang Y, Xu D, Qiu P, Su X, Yan G. Triptolide-induced cell cycle arrest and apoptosis in human renal cell carcinoma cells. Oncol Reports. 2011;25:979–87. doi: 10.3892/or.2011.1158. [DOI] [PubMed] [Google Scholar]
- 29.Ozoren N, El-Deiry W. Heat shock protects HCT116 and H460 cells from TRAIL-induced apoptosis. Exp Cell Res. 2002;281:175–81. doi: 10.1006/excr.2002.5660. [DOI] [PubMed] [Google Scholar]
- 30.Atkins D, Lichtenfels R, Seliger B. Heat shock proteins in renal cell carcinomas. Contrib Nephrol. 2005;148:35–56. doi: 10.1159/000086042. [DOI] [PubMed] [Google Scholar]
- 31.Santarosa M, Favaro D, Quaia M, Galligioni E. Expression of heat shock protein 72 in renal cell carcinoma: possible role and prognostic implications in cancer patients. Eur J Cancer. 1997;33:873–7. doi: 10.1016/s0959-8049(97)00002-6. [DOI] [PubMed] [Google Scholar]
- 32.Westerheide SD, Kawahara TL, Orton K, Morimoto RI. Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J Biol Chem. 2006;281:9616–22. doi: 10.1074/jbc.M512044200. [DOI] [PubMed] [Google Scholar]
- 33.Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol. 1998;161:2833–40. [PubMed] [Google Scholar]
- 34.Flick DA, Gifford GE. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984;68:167–75. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- 35.Carter BZ, Mak DH, Schober WD, Dietrich MF, Pinilla C, Vassilev LT, Reed JC, Andreeff M. Triptolide sensitizes AML cells to TRAIL-induced apoptosis via decrease of XIAP and p53-mediated increase of DR5. Blood. 2008;111:3742–50. doi: 10.1182/blood-2007-05-091504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiaowen H, Yi S. Triptolide sensitizes TRAIL-induced apoptosis in prostate cancer cells via p53-mediated DR5 up-regulation. Mol Biol Rep. 2012;39:8763–70. doi: 10.1007/s11033-012-1737-2. [DOI] [PubMed] [Google Scholar]
- 37.Griffith TS, Fialkov JM, Scott DL, Azuhata T, Williams RD, Wall NR, Altieri DC, Sandler AD. Induction and regulation of tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand-mediated apoptosis in renal cell carcinoma. Cancer Res. 2002;62:3093–9. [PubMed] [Google Scholar]
- 38.Antonoff MB, Chugh R, Skube SJ, Dudeja V, Borja-Cacho D, Clawson KA, Vickers SM, Saluja AK. Role of Hsp-70 in triptolide-mediated cell death of neuroblastoma. J Surg Res. 2010;163:72–8. doi: 10.1016/j.jss.2010.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–11. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aghdassi A, Phillips P, Dudeja V, Dhaulakhandi D, Sharif R, Dawra R, Lerch MM, Saluja A. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007;67:616–25. doi: 10.1158/0008-5472.CAN-06-1567. [DOI] [PubMed] [Google Scholar]
- 41.Schlecht R, Scholz SR, Dahmen H, Wegener A, Sirrenberg C, Musil D, Bomke J, Eggenweiler HM, Mayer MP, Bukau B. Functional analysis of Hsp70 inhibitors. PLoS One. 2013;8:e78443. doi: 10.1371/journal.pone.0078443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li D, Duncan RF. Transient acquired thermotolerance in Drosophila, correlated with rapid degradation of Hsp70 during recovery. Eur J Biochem. 1995;231:454–65. doi: 10.1111/j.1432-1033.1995.tb20719.x. [DOI] [PubMed] [Google Scholar]
- 43.Mao RF, Rubio V, Chen H, Bai L, Mansour OC, Shi ZZ. OLA1 protects cells in heat shock by stabilizing HSP70. Cell Death Dis. 2013;4:e491. doi: 10.1038/cddis.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffith TS, Rauch CT, Smolak PJ, Waugh JY, Boiani N, Lynch DH, Smith CA, Goodwin RG, Kubin MZ. Functional analysis of TRAIL receptors using monoclonal antibodies. J Immunol. 1999;162:2597–605. [PubMed] [Google Scholar]
- 45.Westwood JA, Darcy PK, Guru PM, Sharkey J, Pegram HJ, Amos SM, Smyth MJ, Kershaw MH. Three agonist antibodies in combination with high-dose IL-2 eradicate orthotopic kidney cancer in mice. J Transl Med. 2010;8:42. doi: 10.1186/1479-5876-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosevear HM, Lightfoot AJ, Griffith TS. Conatumumab, a fully human mAb against death receptor 5 for the treatment of cancer. Curr Opin Investig Drugs. 2010;11:688–98. [PubMed] [Google Scholar]
- 47.Stagg J, Sharkey J, Pommey S, Young R, Takeda K, Yagita H, Johnstone RW, Smyth MJ. Antibodies targeted to TRAIL receptor-2 and ErbB-2 synergize in vivo and induce an antitumor immune response. Proc Natl Acad Sci U S A. 2008;105:16254–9. doi: 10.1073/pnas.0806849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiezorek J, Holland P, Graves J. Death receptor agonists as a targeted therapy for cancer. Clin Cancer Res. 2010;16:1701–8. doi: 10.1158/1078-0432.CCR-09-1692. [DOI] [PubMed] [Google Scholar]
- 49.Kindler HL, Richards DA, Garbo LE, Garon EB, Stephenson JJ, Jr., Rocha-Lima CM, Safran H, Chan D, Kocs DM, Galimi F, McGreivy J, Bray SL, Hei Y, Feigal EG, Loh E, Fuchs CS. A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann Oncol. 2012;23:2834–42. doi: 10.1093/annonc/mds142. [DOI] [PubMed] [Google Scholar]
- 50.Paz-Ares L, Balint B, de Boer RH, van Meerbeeck JP, Wierzbicki R, De Souza P, Galimi F, Haddad V, Sabin T, Hei YJ, Pan Y, Cottrell S, Hsu CP, RamLau R. A randomized phase 2 study of paclitaxel and carboplatin with or without conatumumab for first-line treatment of advanced non-small-cell lung cancer. J Thorac Oncol. 2013;8:329–37. doi: 10.1097/JTO.0b013e31827ce554. [DOI] [PubMed] [Google Scholar]
- 51.Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R, Barrett M, Judson I, Kaye S, Fox NL, Halpern W, Corey A, Calvert H, de Bono J. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–94. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 52.Haynes NM, Hawkins ED, Li M, McLaughlin NM, Hammerling GJ, Schwendener R, Winoto A, Wensky A, Yagita H, Takeda K, Kershaw MH, Darcy PK, Smyth MJ. CD11c+ dendritic cells and B cells contribute to the tumoricidal activity of anti-DR5 antibody therapy in established tumors. J Immunol. 2010;185:532–41. doi: 10.4049/jimmunol.0903624. [DOI] [PubMed] [Google Scholar]
- 53.Takeda K, Yamaguchi N, Akiba H, Kojima Y, Hayakawa Y, Tanner JE, Sayers TJ, Seki N, Okumura K, Yagita H, Smyth MJ. Induction of tumor-specific T cell immunity by anti-DR5 antibody therapy. J Exp Med. 2004;199:437–48. doi: 10.1084/jem.20031457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devaud C, Westwood JA, John LB, Flynn JK, Paquet-Fifield S, Duong CP, Yong CS, Pegram HJ, Stacker SA, Achen MG, Stewart TJ, Snyder LA, Teng MW, Smyth MJ, Darcy PK, Kershaw MH. Tissues in different anatomical sites can sculpt and vary the tumor microenvironment to affect responses to therapy. Mol Ther. 2014;22:18–27. doi: 10.1038/mt.2013.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–37. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 56.Aggarwal BB, Bhardwaj U, Takada Y. Regulation of TRAIL-induced apoptosis by ectopic expression of antiapoptotic factors. Vitam Horm. 2004;67:453–83. doi: 10.1016/S0083-6729(04)67023-3. [DOI] [PubMed] [Google Scholar]
- 57.Shin MS, Kim HS, Lee SH, Park WS, Kim SY, Park JY, Lee JH, Lee SK, Lee SN, Jung SS, Han JY, Kim H, Lee JY, Yoo NJ. Mutations of tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1) and receptor 2 (TRAIL-R2) genes in metastatic breast cancers. Cancer Res. 2001;61:4942–6. [PubMed] [Google Scholar]
- 58.VanOosten RL, Moore JM, Karacay B, Griffith TS. Histone deacetylase inhibitors modulate renal cell carcinoma sensitivity to TRAIL/Apo-2L-induced apoptosis by enhancing TRAIL-R2 expression. Cancer Biol Ther. 2005;4:1104–1112. doi: 10.4161/cbt.4.10.2022. [DOI] [PubMed] [Google Scholar]
- 59.VanOosten RL, Moore JM, Ludwig AT, Griffith TS. Depsipeptide (FR901228) enhances the cytotoxic activity of TRAIL by redistributing TRAIL receptor to membrane lipid rafts. Mol Ther. 2005;11:542–552. doi: 10.1016/j.ymthe.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 60.Zhang XD, Franco A, Myers K, Gray C, Nguyen T, Hersey P. Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE-inhibitory protein expression to TRAIL-induced apoptosis of melanoma. Cancer Res. 1999;59:2747–53. [PubMed] [Google Scholar]
- 61.Lee SH, Shin MS, Kim HS, Lee HK, Park WS, Kim SY, Lee JH, Han SY, Park JY, Oh RR, Kang CS, Kim KM, Jang JJ, Nam SW, Lee JY, Yoo NJ. Somatic mutations of TRAIL-receptor 1 and TRAIL-receptor 2 genes in non-Hodgkin’s lymphoma. Oncogene. 2001;20:399–403. doi: 10.1038/sj.onc.1204103. [DOI] [PubMed] [Google Scholar]
- 62.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer JL, Schroter M, Scaffidi C, Krammer PH, Peter ME, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–21. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 63.Griffith TS, Kucaba TA, O’Donnell MA, Burns J, Benetatos C, McKinlay MA, Condon S, Chunduru S. Sensitization of human bladder tumor cells to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis with a small molecule IAP antagonist. Apoptosis. 2011;16:13–26. doi: 10.1007/s10495-010-0535-3. [DOI] [PubMed] [Google Scholar]
- 64.Fulda S, Meyer E, Debatin KM. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene. 2002;21:2283–94. doi: 10.1038/sj.onc.1205258. [DOI] [PubMed] [Google Scholar]
- 65.Lee TJ, Lee JT, Park JW, Kwon TK. Acquired TRAIL resistance in human breast cancer cells are caused by the sustained cFLIP(L) and XIAP protein levels and ERK activation. Biochem Biophys Res Commun. 2006;351:1024–30. doi: 10.1016/j.bbrc.2006.10.163. [DOI] [PubMed] [Google Scholar]
- 66.Borja-Cacho D, Yokoyama Y, Chugh RK, Mujumdar NR, Dudeja V, Clawson KA, Dawra RK, Saluja AK, Vickers SM. TRAIL and triptolide: an effective combination that induces apoptosis in pancreatic cancer cells. J Gastrointest Surg. 2010;14:252–60. doi: 10.1007/s11605-009-1065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee KY, Park JS, Jee YK, Rosen GD. Triptolide sensitizes lung cancer cells to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by inhibition of NF-kappaB activation. Exp Mol Med. 2002;34:462–8. doi: 10.1038/emm.2002.64. [DOI] [PubMed] [Google Scholar]
- 68.Panichakul T, Intachote P, Wongkajorsilp A, Sripa B, Sirisinha S. Triptolide sensitizes resistant cholangiocarcinoma cells to TRAIL-induced apoptosis. Anticancer Res. 2006;26:259–65. [PubMed] [Google Scholar]
- 69.Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–20. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- 70.Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang C-P, DuBose RF, Goodwin RG, Smith CA. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med. 1997;186:1165–1170. doi: 10.1084/jem.186.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–8. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 72.Pan G, Ni J, Yu G, Wei YF, Dixit VM. TRUNDD, a new member of the TRAIL receptor family that antagonizes TRAIL signalling. FEBS Lett. 1998;424:41–5. doi: 10.1016/s0014-5793(98)00135-5. [DOI] [PubMed] [Google Scholar]
- 73.Mosser DD, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004;23:2907–18. doi: 10.1038/sj.onc.1207529. [DOI] [PubMed] [Google Scholar]
- 74.Samali A, Orrenius S. Heat shock proteins: regulators of stress response and apoptosis. Cell Stress Chaperones. 1998;3:228–36. doi: 10.1379/1466-1268(1998)003<0228:hspros>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ozoren N, El-Deiry WS. Defining characteristics of Types I and II apoptotic cells in response to TRAIL. Neoplasia. 2002;4:551–7. doi: 10.1038/sj.neo.7900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhuang H, Jiang W, Zhang X, Qiu F, Gan Z, Cheng W, Zhang J, Guan S, Tang B, Huang Q, Wu X, Huang X, Jiang W, Hu Q, Lu M, Hua ZC. Suppression of HSP70 expression sensitizes NSCLC cell lines to TRAIL-induced apoptosis by upregulating DR4 and DR5 and downregulating c-FLIP-L expressions. J Mol Med. 2013;91:219–35. doi: 10.1007/s00109-012-0947-3. [DOI] [PubMed] [Google Scholar]
- 77.Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–75. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 78.Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem. 2000;275:25665–71. doi: 10.1074/jbc.M906383199. [DOI] [PubMed] [Google Scholar]
- 79.Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476–83. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- 80.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–63. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Teng MW, Westwood JA, Darcy PK, Sharkey J, Tsuji M, Franck RW, Porcelli SA, Besra GS, Takeda K, Yagita H, Kershaw MH, Smyth MJ. Combined natural killer T-cell based immunotherapy eradicates established tumors in mice. Cancer Res. 2007;67:7495–504. doi: 10.1158/0008-5472.CAN-07-0941. [DOI] [PubMed] [Google Scholar]
- 82.McDermott DF. Immunotherapy and targeted therapy combinations in renal cancer. Curr Clin Pharmacol. 2011;6:207–13. doi: 10.2174/157488411797189451. [DOI] [PubMed] [Google Scholar]
- 83.Shanker A, Brooks AD, Tristan CA, Wine JW, Elliott PJ, Yagita H, Takeda K, Smyth MJ, Murphy WJ, Sayers TJ. Treating metastatic solid tumors with bortezomib and a tumor necrosis factor-related apoptosis-inducing ligand receptor agonist antibody. J Natl Cancer Inst. 2008;100:649–62. doi: 10.1093/jnci/djn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang X, Chen M, Zhou J, Zhang X. HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy. Int J Oncol. 2014;45:18–30. doi: 10.3892/ijo.2014.2399. [DOI] [PubMed] [Google Scholar]
- 85.Anbalagan S, Pires IM, Blick C, Hill MA, Ferguson DJ, Chan DA, Hammond EM. Radiosensitization of renal cell carcinoma in vitro through the induction of autophagy. Radiother Oncol. 2012;103:388–93. doi: 10.1016/j.radonc.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 86.Turcotte S, Chan DA, Sutphin PD, Hay MP, Denny WA, Giaccia AJ. A molecule targeting VHL-deficient renal cell carcinoma that induces autophagy. Cancer Cell. 2008;14:90–102. doi: 10.1016/j.ccr.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turcotte S, Sutphin PD, Giaccia AJ. Targeted therapy for the loss of von Hippel-Lindau in renal cell carcinoma: a novel molecule that induces autophagic cell death. Autophagy. 2008;4:944–6. doi: 10.4161/auto.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang M, Fu J. Triptolide inhibits interferon-gamma-induced programmed death-1-ligand 1 surface expression in breast cancer cells. Cancer Lett. 2008;270:337–41. doi: 10.1016/j.canlet.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 89.Chen BJ. Triptolide, a novel immunosuppressive and anti-inflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. Leuk Lymphoma. 2001;42:253–65. doi: 10.3109/10428190109064582. [DOI] [PubMed] [Google Scholar]
- 90.Liu Y, Chen Y, Lamb JR, Tam PK. Triptolide, a component of Chinese herbal medicine, modulates the functional phenotype of dendritic cells. Transplantation. 2007;84:1517–26. doi: 10.1097/01.tp.0000289990.55668.0d. [DOI] [PubMed] [Google Scholar]
- 91.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 92.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–20. [PubMed] [Google Scholar]
- 93.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Kayagaki N, Yagita H, Okumura K. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med. 2002;195:161–9. doi: 10.1084/jem.20011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Yamaguchi N, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in NK cell-mediated and IFN-gamma-dependent suppression of subcutaneous tumor growth. Cellular immunology. 2001;214:194–200. doi: 10.1006/cimm.2001.1896. [DOI] [PubMed] [Google Scholar]
- 96.Berenbaum MC. Criteria for analyzing interactions between biologically active agents. Adv Cancer Res. 1981;35:269–335. doi: 10.1016/s0065-230x(08)60912-4. [DOI] [PubMed] [Google Scholar]
- 97.James BR, Brincks EL, Kucaba TA, Boon L, Griffith TS. Effective TRAIL-based immunotherapy requires both plasmacytoid and CD8a DC. Cancer Immunol Immunother. 2014;63:685–697. doi: 10.1007/s00262-014-1548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.James BR, Tomanek-Chalkley A, Askeland EJ, Kucaba T, Griffith TS, Norian LA. Diet-induced obesity alters dendritic cell function in the presence and absence of tumor growth. J Immunol. 2012;189:1311–1321. doi: 10.4049/jimmunol.1100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.James BR, Anderson KG, Brincks EL, Kucaba TA, Norian LA, Masopust D, Griffith TS. CpG-mediated modulation of MDSC contributes to the efficacy of Ad5-TRAIL therapy against renal cell carcinoma. Cancer Immunol Immunother. 2014;63:1213–1227. doi: 10.1007/s00262-014-1598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]