Abstract
OBJECTIVE
We examined effects of race/ethnicity and neighborhood, a proxy of socioeconomic status, on cancer incidence in New York City neighborhoods: East Harlem (EH), Central Harlem (CH), and Upper East Side (UES).
METHODS
In this ecological study, Community Health Survey (CHS) data (2002–2006) and New York State Cancer Registry incidence data (2007–2011) were stratified by gender, age, race/ethnicity, and neighborhood. Logistic regression models were fitted to each cancer incidence rate with race/ethnicity, neighborhood, and CHS-derived risk factors as predictor variables.
RESULTS
Neighborhood was significantly associated with all cancers and 14 out of 25 major cancers. EH and CH residence conferred higher risk of all cancers compared to UES (OR=1.34; CI: 1.07, 1.68 and OR=1.39; CI: 1.12, 1.72, respectively). Prevalence of diabetes and tobacco smoking were the largest contributors to high cancer rates.
CONCLUSIONS
Despite juxtaposition and similar proximity to medical centers, cancer incidence disparities persist among EH, CH, and UES neighborhoods. Targeted, neighborhood-specific outreach may aid in reducing cancer incidence rates.
Keywords: neoplasms, incidence, New York City, healthcare disparities, socioeconomic factors
INTRODUCTION
New York City (NYC) is a highly populated, geographically concentrated metropolitan area with large racial/ethnic and socioeconomic status (SES) diversity, making it suitable for investigating racial/ethnic and SES disparities in cancer incidence and determinants. However, few studies have attempted to systematically disentangle the effect of SES and race/ethnicity on cancer risk (Islami et al. 2013, Klein Rosenthal, Kinney, and Metzger 2014).
Most prior studies on cancer disparities in NYC as well as other locations have focused on race/ethnicity (Whitman et al. 2011, Hirschman, Whitman, and Ansell 2007, Jandorf et al. 2008, Mayberry et al. 1995, Cruz et al. 2007). For studies that attempted to disentangle effects of SES disparities as well as race/ethnicity, only single cancer sites have been investigated to date (Richards et al. 2011, McCarthy et al. 2010, Whitman et al. 2011). Different population studies observed that living in SES-deprived neighborhoods was independently associated with lifestyle health risks, such as excess body weight (Janssen et al. 2006, Mobley et al. 2006), tobacco smoking (Hanibuchi et al. 2014), lower physical activity (van Lenthe, Brug, and Mackenbach 2005), increased stress (Cheng et al. 2014), and lower fruit and vegetable consumption (Giskes et al. 2006, Dubowitz et al. 2008). Cultural and social differences influence collective behaviors as well as individual psychological and physiological states. Systemic inflammation and tobacco smoking were significantly associated with low SES populations compared to nearby high SES populations (Hostinar et al. 2014, Levin et al. 2014).
Using a range of state and local data sources, we investigated the association between both race/ethnicity and neighborhood of residence in NYC—as proxy for SES— and incidence of all cancers combined as well as major specific cancers. Analysis was restricted to three neighborhoods: Upper East Side (UES), Central Harlem (CH), and East Harlem (EH). Although these neighborhoods are in similar proximity to medical centers, they are characterized by extreme differences in ethnic/racial composition, SES (Buchholz N. 2012), and cancer incidence rates.
METHODS
The study was conducted in three neighborhoods of NYC: UES, CH and EH (Figure S1). Selected socio-demographic characteristics of the three neighborhoods are listed in Table 2.
Table 2.
Descriptive characteristics of Community Health Survey respondents by United Health Fund (UHF) New York City neighborhoods: East Harlem, Central Harlem, and Upper East Side, 2002–2006
| Category | Missing n (% of total surveyed) | Characteristic | Category total | Upper East Side | East Harlem | Central Harlem | P-value |
|---|---|---|---|---|---|---|---|
| Neighborhood | 0 | Number of participants (%) | 2958 | 1181 (40) | 761 (26) | 1016 (34) | |
| Age | 154 (5.2) | Age, years (mean, sd) | 2804 | 1109/1181 58.7±15 |
736/761 54.7±14 |
959/1016 55.3±15 |
0.06 |
| 154 (5.2) | Age groups | <0.001 | |||||
| 35–44 years (%) | 750(27) | 244 (22) | 215 (29) | 291(30) | |||
| 45–54 years (%) | 659(24) | 222 (23) | 196 (27) | 241 (23) | |||
| 55–64 years (%) | 543(19) | 230 (21) | 132 (18) | 181(19) | |||
| 65–74 years (%) | 426(15) | 177 (16) | 111 (15) | 138 (14) | |||
| 75+ years (%) | 426(15) | 217 (20) | 82 (11) | 127 (13) | |||
| Gender | 0 | Females (%) | 1829 (62) | 691 (59) | 488 (64) | 650 (64) | 0.01 |
| Males (%) | 1129 (38) | 490 (41) | 273 (36) | 366 (36) | |||
| Race/ethnicity | 0 | Black, Non, Hispanic (%) | 993 (34) | 34 (3) | 232 (31) | 727 (72) | <0.001 |
| 0 | Hispanic, all races (%) | 682 (23) | 71 (6) | 453 (60) | 158 (16) | ||
| 0 | White, Non, Hispanic | 1283 (4) | 1076 (91) | 76 (10) | 131 (13) | ||
| Household income | 656 (22) | Poverty level ≥30% based on zipcode (%) | 839 (36) | 32 (4) | 408 (64) | 399 (50) | <0.001 |
| Education | 32 (0.1) | College degree (%) | 1340 (46) | 870 (74) | 145 (19) | 325 (32) | <0.001 |
| Self, assessed health | 22 (0.7) | General health rated as “fair/poor” (%) | 733 (25) | 151 (13) | 277 (37) | 305 (30) | <0.001 |
| 7 (0.2) | US born (%) | 2397 (81) | 961 (82) | 596 (78) | 840 (83) | 0.05 | |
| Sexual identity | 190 (8.0) | Homosexual and bisexual (%) | 118 (5) | 63 (7) | 15 (3) | 40 (5) | 0.02 |
| Neighborhood safety | 14 (2.4) | Feel “extremely safe” in neighborhood (%) | 95 (17) | 68 (33) | 12 (7) | 15 (8) | <0.001 |
| Body Mass Index | 208 (7.0) | Body mass index, (kg/m2) mean, sd | 2750 | 1105/1181 24.6±4.4 |
702/761 28.5±7.0 |
943/1016 27.8±5.7 |
<0.001 |
| Co-morbities | 9 (0.4) | Diabetes mellitus (%) | 273 (12) | 51 (6) | 102 (17) | 120 (16) | <0.001 |
| 17 (2.9) | Hypercholester olemia (%) | 191 (34) | 83 (40) | 49 (30) | 59 (31) | 0.08 | |
| 522 (29) | Hypertension (%) | 705 (38) | 219 (29) | 181 (41) | 305 (45) | <0.001 | |
| 14 (0.6) | Current asthma (%) | 136 (6) | 35 (4) | 50 (8) | 51 (7) | <0.001 | |
| Health Screening | 971 (41) | Ever colonoscopy (%) | 893 (64) | 448 (71) | 182 (55) | 263 (59) | <0.001 |
| 250 (17) | Mammogram in past 2 years (%) | 1002 (80) | 393 (79) | 258 (82) | 351 (79) | 0.43 | |
| 70 (4.6) | PAP smear in past 3 years (%) | 1233 (86) | 477 (86) | 313 (85) | 443 (86) | 0.93 | |
| 105 (5.8) | HIV test in past 12 months (%) | 373 (22) | 77 (11) | 114 (27) | 182 (30) | <0.001 | |
| 11 (0.4) | Presence of primary care physician (%) | 2471 (84) | 1044 (89) | 584 (77) | 843 (83) | 0.001 | |
| Access to Health care | 43 (1.5) | Uninsured (%) | 46 (8) | 61 (5) | 91 (12) | 94 (9) | <0.001 |
| Lifestyle risk factors | |||||||
| Tobacco smoking | 29 (1.0) | Current smokers (%) | 613 (21) | 172 (15) | 191 (25) | 250 (25) | <0.001 |
| Past smokers (%) | 892 (30) | 467 (40) | 165 (19) | 260 (29) | <0.001 | ||
| Never smokers (%) | 1424 (49) | 532 (45) | 396 (53) | 496 (49) | <0.001 | ||
| 6 (2.6) | Exercise in past 30 days (%) | 1676 (73) | 739 (85) | 405 (64) | 532 (67) | <0.001 | |
| 50 (2.2) | Binge drinking (%) | 245 (11) | 92 (11) | 75 (3.3) | 78 (10) | 0.53 | |
| 46 (4.0) | Fruit and vegetable servings/day ≥ 5 (%) | 117 (11) | 75 (18) | 18 (6) | 24 (6) | <0.001 | |
| 582 (20) | ≥ 3 sex partners (%) | 148 (6) | 70 (8) | 31 (5) | 47 (6) | 0.18 |
Neighborhood boundaries
United Hospital Fund (UHF) neighborhoods differentiated NYC neighborhoods. UHF neighborhoods are administrative boundaries defined by several adjoining zip code areas with similar characteristics, designated to approximate NYC Community Planning Districts ((NYCDOHMH) 2011). The places and populations contained within UHF areas often share common histories, built environments, and SES characteristics, and their use in ecological analysis is a scale of spatial disaggregation commonly used by each of the institutions from which data were obtained: the New York State Cancer Registry (NYSCR), the NYC Department of Mental Health and Hygiene, and previous NYC-based ecological studies (Klein Rosenthal, Kinney, and Metzger 2014).
Incidence data
Cancer incidence data for the three UHF neighborhoods were obtained from the NYSCR. Incidence counts for each cancer were aggregated for 2007–2011 by neighborhood, age group, race (non-Hispanic white, non-Hispanic black, and Hispanic of all races), and gender. A total of 26 major cancers defined according to the International Classification of Diseases for Oncology (ICD-O-3) categories (SEER 2012) were included: all invasive malignant tumors, oral cavity and pharynx, esophageal, stomach, colorectal, colon, rectum and rectosigmoid, liver, pancreas, larynx, lung, melanoma of the skin, female breast, cervix uteri, corpus uterus and uterus NOS, ovary, prostate, testis, urinary bladder including in situ, kidney and renal pelvis, brain and other nervous system, thyroid, Hodgkin lymphoma, non-Hodgkin lymphoma, myeloma, and leukemia. To maintain stable cancer incidence rates across aggregated strata, two age groups were included: 35–64 years and greater or equal to 65 years except for all malignant neoplasms as well as lung, breast, prostate, and colorectal cancers, for which we used 5 age groups: 35–44, 45–54, 55–64, 65–74, and 75+.
The age-specific incidence rates were calculated by dividing the number of new cases by the NYC Census population for 5 years (2007–2011), obtained from the NYC Department of Mental Health and Hygiene Office of Vital Statistics, for each gender-age group, neighborhood and race/ethnicity stratum. Cancer incidence rates were age-standardized using the 2000 US population standard.
Risk and protective factor data
Individual data on exposures which included demographic, health, and lifestyle cancer risk factors from 2002–2006 were obtained from the NYC Community Health Survey (CHS). The CHS is an annual, cross-sectional telephone survey using a stratified random-digit dialing sample design to ensure adequate sample per UHF area conducted by the Department of Health and Mental Hygiene in all NYC neighborhoods (Table S1). CHS data and cancer incidence rates were stratified by gender, UHF neighborhood, age group, and race/ethnicity. A total of 2974 individuals with survey responses from 2002–2006 comprised the CHS dataset, of whom 170 were excluded due to missing age data and most other variables. Each stratum count was converted to prevalence data for dichotomous and categorical variables as well as means for continuous variables.
Categorical variables of cancer risk factors that were obtained from CHS included: presence of a primary care physician, tobacco smoking status (current, former, never), exercise in the last 30 days, presence of co-morbidities (receiving diagnosis by a health care provider of diabetes, high blood pressure, or high cholesterol, respectively), binge drinking (five or more alcoholic drinks on one occasion in the past 30 days), asthma attack in the past 12 months, foreign born, screening history (receiving mammogram in past 2 years, PAP smear in past 3 years, and ever receiving colonoscopy), recreational needle use, ever receiving HIV test, and HIV test in the past 12 months.
Means were calculated by stratum for continuous variables including body-mass index (BMI) based on self-reported height and weight, household poverty level based on zip code poverty percentage (<10%, 10–20%, 20–30%, >30%), fruit and vegetable intake (none, 1–4 servings/day, 5+ servings/day), self-assessed health (excellent, very good, good, fair, poor), and number of sexual partners in the past 12 months (none, one, two, three or more). Means for categorical responses were obtained after ordinal re-labeling: fruit and vegetable servings per day as 0=none, 2.5 for 1–4, and 7.5 for > 5; general health as 1=poor, 2=fair, 3=good, 4=very good, and 5=excellent; number of sex partners as 1=one partner, 2=two partners, and 4.5 for ≥3 partners.
Statistical analysis
Specific cancer incidence (2007–2011) was stratified by UHF neighborhood, age category (35–44, 45–54, 55–64, 65–74, and ≥75 years), gender, and race/ethnicity (white, black, and Hispanic). Basic models included cancer incidence as the outcome variable and the aforementioned four fixed independent predictor covariates. Logistic regression analysis determined effect estimates for sex, age group, race, and neighborhood covariates for all cancers and specific cancer incidence rates. Odds ratios (ORs) and 95% confidence intervals (CIs) were obtained using the youngest age group and males as reference categories.
Significant differences in CHS risk factor means and proportions between the three neighborhoods were measured using ANOVA and chi-square tests, respectively. An alternative set of logistic regression models were fitted and included the four covariates from the basic models plus CHS-obtained risk and protective factors as modifiable predictors. Stepwise logistic regression techniques were used to fit CHS-derived factors in the model, using a P=0.10 threshold.
The log-likelihood ratio differences between basic models and fitted alternative models were calculated for all cancers and specific cancers. Alternative models were retained if log-likelihood ratio differences had a P-value of 0.05. Incidence rates were age-standardized for ≥35 years old using the 2000 US population standard for the purposes of this paper. All statistical analyses were conducted using SAS® software, Version 9.4.
RESULTS
A total of 12, 251 cancer cases were included in this study. The distribution and incidence rates of all cancers combined and the commonest cancers for the UES, EH, and CH are shown in Table 1.
Table 1.
Distributions of cancer counts and age-standardized cancer incidence rates per 100,000 of all cancers combined and the four commonest cancers in New York City United Hospital Fund (UHF) neighborhoods: Upper East Side, East Harlem, and Central Harlem for ≥35 years, 2007–2011*
| Cancer Site | Upper East Side | East Harlem | Central Harlem | |||
|---|---|---|---|---|---|---|
|
| ||||||
| n (%) | Incidence rate (Men/Women) | n (%) | Incidence rate (Men/Women) | n (%) | Incidence rate (Men/Women) | |
| All malignant neoplasms | 6459 (53) | 1010/874 | 2243 (18) | 1029/857 | 3549 (29) | 1066/897 |
| Lung | 676 (46) | 102/90 | 301 (21) | 160/104 | 485 (33) | 130/102 |
| Colorectal | 390 (41) | 64/89 | 208 (22) | 97/76 | 346 (37) | 103/87 |
| Female breast | 1148 (56) | - /286 | 345 (17) | - /258 | 545 (27) | - /280 |
| Prostate | 903 (49) | 283/ - | 330 (18) | 293/ - | 607 (33) | 354/ - |
Source: New York State Cancer Registry
Risk and Protective Factors
Selected risk and protective factor differences between UES, CH, and EH are presented in Table 2. Smoking status and cigarettes per day were the most frequently significant CHS risk factors in the fitted logistic regression models and were positively associated with 10 out of 26 cancers, including all cancers (OR=2.33; 95% CI: 1.88, 2.89). Diabetes prevalence (OR=1.45; 95% CI: 1.19, 1.78), number of sexual partners (OR=1.24; 95% CI: 1.13, 1.36), neighborhood poverty (OR=0.83; 95% CI: 0.75, 0.93), and college graduation (OR=0.78; 95% CI: 0.63, 0.98), were associated with incidence of all cancers combined.
Additional common CHS variables that were significantly associated with cancer outcomes include fruit and vegetable consumption (breast, oral, liver, kidney cancers, as well as leukemia), BMI (colorectal, liver, uterine, ovarian, and lung cancers), graduating college (colon cancer, myeloma), and neighborhood poverty level (brain and laryngeal cancers, as well as leukemia).
Cancer Incidence, 2007 to 2011
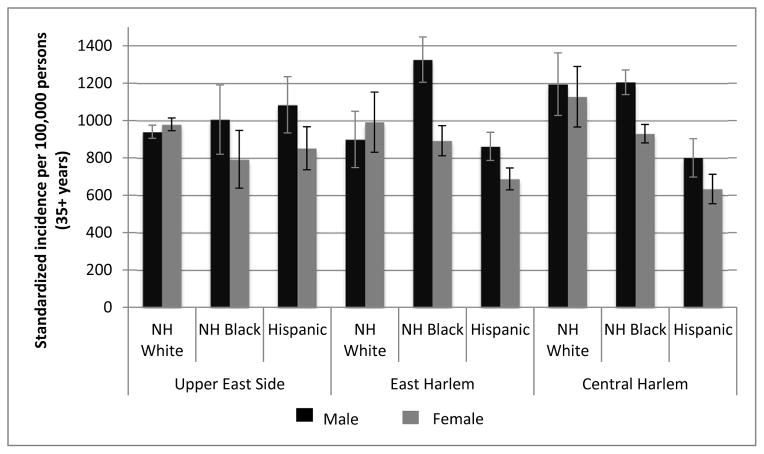
EH black men have the highest incidence rates (aged ≥35 years) for all cancers combined (Figure 1). Compared to UES, incidence rates for all cancers combined are highest for CH blacks and whites for both genders. For blacks, cancer incidence is higher in EH and CH compared to UES. Conversely, for Hispanics, cancer incidence is higher in UES versus EH and CH. UES Hispanic men have higher cancer incidence than UES white men, although confidence intervals between these two subgroups overlap. Women have higher all combined cancer incidence than males in UES and EH white subgroups. This is due to the higher breast cancer incidence in UES white women of 336 (95% CI: 260, 356) while prostate cancer in UES white men is comparatively lower at 280 (95% CI: 261, 300). Inequalities among the same cancers are also seen for EH whites: 299 (95% CI: 98, 388) for breast cancer among women and 186 (95% CI: 117, 255) for prostate cancer among men. Standardized incidence rates for all ages and by specific major cancer sites among UHF neighborhoods are displayed in Figure S1.
Figure 1.
Age-standardized cancer incidence rates per 100,000 for all sites by United Health Fund New York City neighborhood and race/ethnicity, 2007 to 2011. Vertical bars indicate 95% confidence intervals. NH = non-Hispanic
After risk factors adjustment, EH or CH residency were significantly associated with 34% and 39% higher odds incidence for all cancers combined, respectively, compared to UES. White race was associated with significantly higher incidence of all cancers compared to both other racial/ethnic groups (Table 3).
Table 3.
Associations of neighborhood and race with overall cancer incidence and incidence for the most common four sites, 2007–2011
| Cancer | Model | Race | UHF neighborhood | Community Health Survey (CHS) variables and association direction | Likelihood ratio test | ||
|---|---|---|---|---|---|---|---|
| Black vs. White | Hispanic vs. White | East Harlem vs. Upper East Side | Central Harlem vs. Upper East Side | P-value | |||
| All Cancers | Model 1a | 1.08 (1.02, 1.16) | 0.83 (0.78, 0.89) | 0.99 (0.93, 1.07) | 0.99 (0.93, 1.07) | ||
| Model 2b | 0.84 (0.74, 0.95) | 0.64 (0.56, 0.74) | 1.34 (1.07, 1.68) | 1.39 (1.12, 1.72) | Poverty level − Number of sex partners + Diabetes + Current smoking + College graduate − |
<0.001 | |
| Female breast | Model 1 | 0.74 (0.63, 0.88) | 0.88 (0.75, 1.04) | 0.88 (0.74, 1.04) | 0.92 (0.78, 1.08) | ||
| Model 2 | 1.13 (0.91, 1.42) | 1.03 (0.75, 1.35) | 0.84 (0.70, 1.02) | 0.95 (0.79, 1.14) | Fruit and vegetable consumption − Health insurance possession − Heavy smoker (>10 cigarettes +/day) Mammogram in past 2 years + Physical exercise in past 30 days + |
<0.001 | |
| Prostate | Model 1 | 1.44 (1.22, 1.69) | 0.83 (0.70, 1.00) | 1.05 (0.88, 1.25) | 1.13 (0.96, 1.33) | ||
| Model 2 | 1.41 (1.14, 1.75) | 0.76 (0.61, 1.01) | 0.85 (0.65, 1.11) | 0.95 (0.74, 1.21) | Felt “extremely safe” in neighborhood − Heterosexual orientation + |
<0.001 | |
| Lung | Model 1 | 1.33 (1.10, 1.60) | 0.78 (0.64, 0.96) | 1.25 (1.02, 1.51) | 1.21 (1.00, 1.47) | ||
| Model 2 | 1.50 (1.21, 1.87) | 0.82 (0.67, 1.01) | 1.27 (1.04, 1.55) | 1.17 (0.96, 1.42) | Sex and age interaction Body Mass Index (BMI) − Current smoking + |
<0.0001 | |
| Colorectal | Model 1 | 1.43 (1.13, 1.80) | 1.12 (0.88, 1.43) | 1.22 (0.96, 1.56) | 1.34 (1.06, 1.70) | ||
| Model 2 | 1.09 (0.80, 1.47) | 0.71 (0.49, 1.03) | 1.21 (0.95, 1.54) | 1.38 (1.09, 1.76) | Sex and age interaction Asthma + BMI + Has primary care physician − |
0.002 | |
Model 1 includes terms for age category, gender, race/ethnicity, and United Health Fund neighborhood.
Model 2 includes terms for the four categories in Model 1 in addition to statistically significant Community Health Survey (CHS) variables.
Two of the commonest cancers, lung and colorectal cancer, were significantly influenced by neighborhood with 27% higher risk of lung cancer incidence in EH and 38% higher risk of colorectal cancer in CH after risk factor adjustment.
Other neighborhood-influenced cancers were esophageal, kidney, brain, and non-Hodgkin lymphoma, which were higher in the UES than CH and EH. Cancers significantly associated with both neighborhood and race/ethnicity were oral, colon, liver, laryngeal, cervix, and bladder cancer in addition to melanoma and leukemia. Except for bladder cancer, black race was also associated with higher incidence. Incidence rates for melanoma and leukemia were also lower in blacks than whites. UES residence compared to CH and EH was associated with a higher incidence for melanoma, leukemia, and laryngeal cancer. EH and/or CH residency were significantly associated with a higher incidence of oral and colon cancers. Odds of cervical cancer were 2.3-times higher for EH residents compared to UES while the odds of liver cancer were 2.9-times higher in CH compared to UES.
Cancers of the prostate, stomach, pancreas, uterus, testis, myelomas, and thyroid were significantly influenced by race/ethnicity, but not neighborhood. Blacks had > 2-times the risk for cancers of the oral cavity, stomach, colon, liver, larynx, myeloma, cervix, and endometrium. Hispanic ethnicity was associated with a higher incidence of myeloma and stomach cancer. Prostate, lung, and pancreatic cancer had a higher incidence in blacks compared to whites. While whites had a 35% significantly higher risk for breast cancer incidence compared to blacks prior to risk and protective variable adjustment, the association became statistically insignificant after adjustment for diet, insurance status, tobacco smoking, mammography, and exercise.
No significant associations with either race/ethnicity or neighborhood were observed for cancers of the rectum, ovary as well as Hodgkin’s lymphoma after adjustments for risk and protective factors.
DISCUSSION
Cancer incidence disparities remains among three neighborhoods, EH, CH, and UES, in Upper Manhattan despite contiguity. Neighborhood was a statistically significant predictor of incidence for all cancers combined, as well as 14 out of 25 specific cancers in this study. In 10 out of these 14 cancers, including all cancers combined, the association of neighborhood did not become significant until after risk factors were included in the models, underscoring the pivotal role of modifiable risk factors in cancer development.
Lung cancer incidence rates were reflective of tobacco smoking prevalence among neighborhoods; more tobacco smokers resided in EH and CH from 2002–2006 and lung cancer incidence is concurrently higher in these neighborhoods. Likewise, breast cancer associations did not change after risk factor adjustments, coinciding with the fact that a similar proportion of women had mammograms in the past 2 years across all three neighborhoods (P=0.43) and three races (P=0.44). Similarly, for colorectal cancer, the significant positive association with black race was diminished after adjustment for presence of a primary care physician. Although only 41% of CHS-takers responded as to whether they had ever received colonoscopy, the CHS proportion of those who reported who affirmatively self-reported ever having had a colonoscopy is similar to what has been found in a previous New York City study in which this data was independently collected (69% in this study vs. 64% as reported by the Community Health Survey) (Crookes et al. 2014). Because the consistency of colonoscopy screening every 10 years had not been inquired as part of CHSs, the presence of a primary care physician may have served as a proxy for colorectal cancer screening (Crawford MJ 2004) and follow-up after screening, which was negatively associated with cancer incidence in our study.
Despite adjustment for neighborhood and risk factors, significant racial/ethnic association persisted for certain cancer types. Black men have higher incidence of prostate cancer, although contributions of specific dietary and modifiable factors associated with SES levels have been inconsistent (Ahn et al. 2008, Tuohimaa et al. 2004, Ahonen et al. 2000) and unable to account for wide racial/ethnic disparities (Cheng et al. 2009, Hankey et al. 1999). Stomach cancer (Siegel, Naishadham, and Jemal 2012, Siegel et al. 2014), pancreatic cancer (Howlader N, Enewold et al. 2014), and myeloma (Howlader N, Gebregziabher et al. 2006) have been shown to be higher in non-white populations despite SES adjustments, although low SES indicators such as lower education and income levels are also associated with myeloma (Baris et al. 2000) and pancreatic cancer incidence (Standop et al. 2012). Although this study found significant associations between increased pancreatic cancer and increased number of sex partners, the latter variable is correlated to other factors which have been demonstrated to be associated with pancreatic cancer such as current smoking (Lynch et al. 2009) (ρ=0.35; P=0.04) and alcohol binge drinking (Michaud et al. 2010, Lucenteforte et al. 2012)(ρ=0.74; P<0.001), suggesting potential residual confounding. Despite this, the adjustment was inadequate in explaining the positive association between black race/ethnicity and pancreatic cancer incidence. Similarly, higher incidence of thyroid and testicular cancer in whites compared to blacks is consistent with previous studies (Howlader N, Gajendran, Nguyen, and Ellison 2005). Although whites are genetically predisposed to thyroid cancer (Reitzel et al. 2014, Zhuang et al. 2014), higher rates among whites are also a consequence of over-diagnosis since thyroid cancer is associated with rising SES among blacks and Hispanics (Reitzel et al. 2014).
A limitation of the study is that true risk and protective factor adjustments may not have been complete due to a lack of adequate variable measurements in the CHS. The CHS survey did not gather information on exposure to environmental carcinogens (e.g., radiation, occupational agents, air pollution), reproductive factors, family cancer history, other genetic predisposition factors, HIV status, and infectious disease (e.g., Human papillomavirus).
CHS data were not fully representative of the underlying population since they excluded households without a landline telephone service as well as adults living in institutional group housing (Hygiene 2013). Since the primary aim of this study was to analyze the effects of NYC neighborhoods, data on other race/ethnicities, including those who identify with more than one race/ethnicity, were excluded from the analysis due to small numbers. Additionally, since CHS data for co-morbidities were self-reported, they may have a low sensitivity for assessing health conditions. Self-reported diabetes excludes undiagnosed cases, unreported diabetes, as well as information on glycemic control or cardiovascular health (Thorpe et al. 2009). Lastly, due to small cancer rates for less common specific cancers, age could not be adequately adjusted for.
A fallacy common to ecological studies is the lack of ability to control for confounding and effect modifying effects without misclassification. However, this study allowed an opportunity to understand the relationships of cancer incidence rates in different neighborhood populations with a variety of lifestyle and risk factors of interest obtained over the course of five years. This allowed an examination of a contextual cultural framework for cancer risk and exposure with a five-year latency period.
Multiple aspects of social environments have indeed been demonstrated to be linked to modifiable cancer risk factors, such as obesity (Thorpe et al. 2009), diabetes (Smalls et al. 2014), and smoking ((CDC) 2007). Many modifiable disparities have been observed between NYC neighborhoods (Shareck, Kestens, and Frohlich 2014, Frieden et al. 2008), with EH, CH, and the UES serving as a microcosm for inequalities in cancer screening, co-morbidities, and modifiable lifestyle risks. Primary strategies for reducing cancer risk in the CH and EH neighborhoods include diabetes prevention and awareness, increased academic education, and reducing tobacco smoking. Although further study in NYC has identified disparities in the diabetes care continuum (Thorpe et al. 2009), dietary lifestyle, and tobacco smoking (Frieden et al. 2008), programs that focus on social and demographic neighborhood differences are also necessary to narrow the disparity gap.
Conclusions
Both race/ethnicity and neighborhood residency are important in determining cancer risk. Cancer incidence health burdens may result from preventable causes, in part due to risk factor vulnerability borne from social community ties at the neighborhood level, but also due to race/ethnicity. Due to unique and contrasting neighborhood characteristics, community-based outreach programs aimed at preventive measures may be beneficial in reducing cancer rates. Key domains for cancer risk reduction include interventions in diabetes and tobacco smoking.
Supplementary Material
Figure S1. Geographic location of three neighborhoods and zip codes of Upper Manhattan: Upper East Side (10021, 10028, 10044, 10128), Central Harlem (10026, 10027, 10030, 10037, 10039), and East Harlem (10029, 10035)
Figure S2. Age-standardized cancer incidence rates for specific cancers by United Health Fund New York City neighborhood and sex, 2007 to 2011. Vertical bars indicate 95% confidence intervals.
Acknowledgments
The authors would like to thank the New York State Cancer Registry and the New York Department of Health and Mental Hygiene for providing data sources. The project was partially supported by the Tisch Cancer Institute and by NIEHS P30 grant # ES023515 (The Mount Sinai Transdisciplinary Center on Early Environmental Exposures).
Footnotes
The authors declare that they have no conflict of interest.
References
- (CDC), Centers for Disease Control. Decline in smoking prevalence--New York City, 2002–2006. MMWR Morb Mortal Wkly Rep. 2007;56(24):604–8. [PubMed] [Google Scholar]
- (NYCDOHMH), Bureau of Environmental Surveillance and Policy. New York City Neighborhood Definitions Used by the Environmental Public Health Tracking Portal to Categorize Poverty Level. 2014 Dec 19; 2011 [cited October 15, 2014 2014]. Available from http://www.nyc.gov/html/doh/downloads/pdf/tracking/povertymapdocument.pdf.
- Ahn J, Peters U, Albanes D, Purdue MP, Abnet CC, Chatterjee N, Horst RL, Hollis BW, Huang WY, Shikany JM, Hayes RB. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;100(11):796–804. doi: 10.1093/jnci/djn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland) Cancer Causes Control. 2000;11(9):847–52. doi: 10.1023/a:1008923802001. [DOI] [PubMed] [Google Scholar]
- Baris D, Brown LM, Silverman DT, Hayes R, Hoover RN, Swanson GM, Dosemeci M, Schwartz AG, Liff JM, Schoenberg JB, Pottern LM, Lubin J, Greenberg RS, Fraumeni JF., Jr Socioeconomic status and multiple myeloma among US blacks and whites. Am J Public Health. 2000;90(8):1277–81. doi: 10.2105/ajph.90.8.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz N, Resnick S, Konty K. The New York City Community Health Survey Atlas. The New York City Department of Health and Mental Hygiene. 2012 2010. [Google Scholar]
- Cheng I, Witte JS, McClure LA, Shema SJ, Cockburn MG, John EM, Clarke CA. Socioeconomic status and prostate cancer incidence and mortality rates among the diverse population of California. Cancer Causes Control. 2009;20(8):1431–40. doi: 10.1007/s10552-009-9369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Li X, Lou C, Sonenstein FL, Kalamar A, Jejeebhoy S, Delany-Moretlwe S, Brahmbhatt H, Olumide AO, Ojengbede O. The association between social support and mental health among vulnerable adolescents in five cities: findings from the study of the well-being of adolescents in vulnerable environments. J Adolesc Health. 2014;55(6 Suppl):S31–8. doi: 10.1016/j.jadohealth.2014.08.020. [DOI] [PubMed] [Google Scholar]
- Crawford MJ, Kerker B, Kaye K, Mostashari F. New Yorkers Without Health Care Coverage Are Not Getting the Care They Need. NYC Vital Signs. 2004;3(1):1–4. [Google Scholar]
- Crookes DM, Njoku O, Rodriguez MC, Mendez EI, Jandorf L. Promoting colorectal cancer screening through group education in community-based settings. J Cancer Educ. 2014;29(2):296–303. doi: 10.1007/s13187-013-0599-1. [DOI] [PubMed] [Google Scholar]
- Cruz GD, Shulman LC, Kumar JV, Salazar CR. The cultural and social context of oral and pharyngeal cancer risk and control among Hispanics in New York. J Health Care Poor Underserved. 2007;18(4):833–46. doi: 10.1353/hpu.2007.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowitz T, Heron M, Bird CE, Lurie N, Finch BK, Basurto-Davila R, Hale L, Escarce JJ. Neighborhood socioeconomic status and fruit and vegetable intake among whites, blacks, and Mexican Americans in the United States. Am J Clin Nutr. 2008;87(6):1883–91. doi: 10.1093/ajcn/87.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enewold L, Harlan LC, Tucker T, McKenzie S. Pancreatic Cancer in the USA: Persistence of Undertreatment and Poor Outcome. J Gastrointest Cancer. 2014 doi: 10.1007/s12029-014-9668-x. [DOI] [PubMed] [Google Scholar]
- Frieden TR, Bassett MT, Thorpe LE, Farley TA. Public health in New York City, 2002–2007: confronting epidemics of the modern era. Int J Epidemiol. 2008;37(5):966–77. doi: 10.1093/ije/dyn108. [DOI] [PubMed] [Google Scholar]
- Gajendran VK, Nguyen M, Ellison LM. Testicular cancer patterns in African-American men. Urology. 2005;66(3):602–5. doi: 10.1016/j.urology.2005.03.071. [DOI] [PubMed] [Google Scholar]
- Gebregziabher M, Bernstein L, Wang Y, Cozen W. Risk patterns of multiple myeloma in Los Angeles County, 1972–1999 (United States) Cancer Causes Control. 2006;17(7):931–8. doi: 10.1007/s10552-006-0030-x. [DOI] [PubMed] [Google Scholar]
- Giskes K, Turrell G, van Lenthe FJ, Brug J, Mackenbach JP. A multilevel study of socio-economic inequalities in food choice behaviour and dietary intake among the Dutch population: the GLOBE study. Public Health Nutr. 2006;9(1):75–83. doi: 10.1079/phn2005758. [DOI] [PubMed] [Google Scholar]
- Hanibuchi T, Nakaya T, Honjo K, Ikeda A, Iso H, Inoue M, Sawada N, Tsugane S. Neighborhood contextual factors for smoking among middle-aged Japanese: A multilevel analysis. Health Place. 2014;31c:17–23. doi: 10.1016/j.healthplace.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Hankey BF, Feuer EJ, Clegg LX, Hayes RB, Legler JM, Prorok PC, Ries LA, Merrill RM, Kaplan RS. Cancer surveillance series: interpreting trends in prostate cancer--part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999;91(12):1017–24. doi: 10.1093/jnci/91.12.1017. [DOI] [PubMed] [Google Scholar]
- Hirschman J, Whitman S, Ansell D. The black:white disparity in breast cancer mortality: the example of Chicago. Cancer Causes Control. 2007;18(3):323–33. doi: 10.1007/s10552-006-0102-y. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Ross KM, Chen E, Miller GE. Modeling the Association Between Lifecourse Socioeconomic Disadvantage and Systemic Inflammation in Healthy Adults: The Role of Self-Control. Health Psychol. 2014 doi: 10.1037/hea0000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975–2011. http://seer.cancer.gov/csr/1975_2011/ [Google Scholar]
- Hygiene, The New York City Department of Health and Mental. Community Health Survey: Methodology 2013. 2014 [cited January 7 2014]. Available from http://www.nyc.gov/html/doh/html/data/chs-methods.shtml.
- Islami F, Kahn AR, Bickell NA, Schymura MJ, Boffetta P. Disentangling the effects of race/ethnicity and socioeconomic status of neighborhood in cancer stage distribution in New York City. Cancer Causes Control. 2013;24(6):1069–78. doi: 10.1007/s10552-013-0184-2. [DOI] [PubMed] [Google Scholar]
- Jandorf L, Bursac Z, Pulley L, Trevino M, Castillo A, Erwin DO. Breast and cervical cancer screening among Latinas attending culturally specific educational programs. Prog Community Health Partnersh. 2008;2(3):195–204. doi: 10.1353/cpr.0.0034. [DOI] [PubMed] [Google Scholar]
- Janssen I, Boyce WF, Simpson K, Pickett W. Influence of individual- and area-level measures of socioeconomic status on obesity, unhealthy eating, and physical inactivity in Canadian adolescents. Am J Clin Nutr. 2006;83(1):139–45. doi: 10.1093/ajcn/83.1.139. [DOI] [PubMed] [Google Scholar]
- Klein Rosenthal J, Kinney PL, Metzger KB. Intra-urban vulnerability to heat-related mortality in New York City, 1997–2006. Health Place. 2014;30:45–60. doi: 10.1016/j.healthplace.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin KA, Dundas R, Miller M, McCartney G. Socioeconomic and geographic inequalities in adolescent smoking: a multilevel cross-sectional study of 15 year olds in Scotland. Soc Sci Med. 2014;107:162–70. doi: 10.1016/j.socscimed.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucenteforte E, La Vecchia C, Silverman D, Petersen GM, Bracci PM, Ji BT, Bosetti C, Li D, Gallinger S, Miller AB, Bueno-de-Mesquita HB, Talamini R, Polesel J, Ghadirian P, Baghurst PA, Zatonski W, Fontham E, Bamlet WR, Holly EA, Gao YT, Negri E, Hassan M, Cotterchio M, Su J, Maisonneuve P, Boffetta P, Duell EJ. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4) Ann Oncol. 2012;23(2):374–82. doi: 10.1093/annonc/mdr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch SM, Vrieling A, Lubin JH, Kraft P, Mendelsohn JB, Hartge P, Canzian F, Steplowski E, Arslan AA, Gross M, Helzlsouer K, Jacobs EJ, LaCroix A, Petersen G, Zheng W, Albanes D, Amundadottir L, Bingham SA, Boffetta P, Boutron-Ruault MC, Chanock SJ, Clipp S, Hoover RN, Jacobs K, Johnson KC, Kooperberg C, Luo J, Messina C, Palli D, Patel AV, Riboli E, Shu XO, Rodriguez Suarez L, Thomas G, Tjonneland A, Tobias GS, Tong E, Trichopoulos D, Virtamo J, Ye W, Yu K, Zeleniuch-Jacquette A, Bueno-de-Mesquita HB, Stolzenberg-Solomon RZ. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol. 2009;170(4):403–13. doi: 10.1093/aje/kwp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberry RM, Coates RJ, Hill HA, Click LA, Chen VW, Austin DF, Redmond CK, Fenoglio-Preiser CM, Hunter CP, Haynes MA, et al. Determinants of black/white differences in colon cancer survival. J Natl Cancer Inst. 1995;87(22):1686–93. doi: 10.1093/jnci/87.22.1686. [DOI] [PubMed] [Google Scholar]
- McCarthy AM, Dumanovsky T, Visvanathan K, Kahn AR, Schymura MJ. Racial/ethnic and socioeconomic disparities in mortality among women diagnosed with cervical cancer in New York City, 1995–2006. Cancer Causes Control. 2010;21(10):1645–55. doi: 10.1007/s10552-010-9593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud DS, Vrieling A, Jiao L, Mendelsohn JB, Steplowski E, Lynch SM, Wactawski-Wende J, Arslan AA, Bas Bueno-de-Mesquita H, Fuchs CS, Gross M, Helzlsouer K, Jacobs EJ, Lacroix A, Petersen G, Zheng W, Allen N, Ammundadottir L, Bergmann MM, Boffetta P, Buring JE, Canzian F, Chanock SJ, Clavel-Chapelon F, Clipp S, Freiberg MS, Michael Gaziano J, Giovannucci EL, Hankinson S, Hartge P, Hoover RN, Allan Hubbell F, Hunter DJ, Hutchinson A, Jacobs K, Kooperberg C, Kraft P, Manjer J, Navarro C, Peeters PH, Shu XO, Stevens V, Thomas G, Tjonneland A, Tobias GS, Trichopoulos D, Tumino R, Vineis P, Virtamo J, Wallace R, Wolpin BM, Yu K, Zeleniuch-Jacquotte A, Stolzenberg-Solomon RZ. Alcohol intake and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium (PanScan) Cancer Causes Control. 2010;21(8):1213–25. doi: 10.1007/s10552-010-9548-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley LR, Root ED, Finkelstein EA, Khavjou O, Farris RP, Will JC. Environment, obesity, and cardiovascular disease risk in low-income women. Am J Prev Med. 2006;30(4):327–332. doi: 10.1016/j.amepre.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Reitzel LR, Nguyen N, Li N, Xu L, Regan SD, Sturgis EM. Trends in thyroid cancer incidence in Texas from 1995 to 2008 by socioeconomic status and race/ethnicity. Thyroid. 2014;24(3):556–67. doi: 10.1089/thy.2013.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CA, Kerker BD, Thorpe L, Olson C, Krauskopf MS, Silver LS, Weber TK, Winawer SJ. Increased screening colonoscopy rates and reduced racial disparities in the New York Citywide campaign: an urban model. Am J Gastroenterol. 2011;106(11):1880–6. doi: 10.1038/ajg.2011.191. [DOI] [PubMed] [Google Scholar]
- Shareck M, Kestens Y, Frohlich KL. Moving beyond the residential neighborhood to explore social inequalities in exposure to area-level disadvantage: Results from the Interdisciplinary Study on Inequalities in Smoking. Soc Sci Med. 2014;108:106–14. doi: 10.1016/j.socscimed.2014.02.044. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin. 2012;62(5):283–98. doi: 10.3322/caac.21153. [DOI] [PubMed] [Google Scholar]
- Smalls BL, Gregory CM, Zoller JS, Egede LE. Direct and indirect effects of neighborhood factors and self-care on glycemic control in adults with type 2 diabetes. J Diabetes Complications. 2014 doi: 10.1016/j.jdiacomp.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Standop J, Kuhn Y, Glowka TR, Schaefer N, Overhaus M, Schmitz V, Hirner A, Kalff JC. Association of socio-economic status and stage of pancreatic cancer at time of surgery in a German setting. Hepatogastroenterology. 2012;59(120):2614–7. doi: 10.5754/hge10334. [DOI] [PubMed] [Google Scholar]
- Surveillance, Epidemiology, and End Results Program. ICD-0-3 SEER site/histology valifation list. 2012 [cited January 1, 2015. Available from http://www.seer.cancer.gov/icd-o-3/sitetype.icdo3.d20121205.pdf.
- Thorpe LE, Upadhyay UD, Chamany S, Garg R, Mandel-Ricci J, Kellerman S, Berger DK, Frieden TR, Gwynn C. Prevalence and control of diabetes and impaired fasting glucose in New York City. Diabetes Care. 2009;32(1):57–62. doi: 10.2337/dc08-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuohimaa P, Tenkanen L, Ahonen M, Lumme S, Jellum E, Hallmans G, Stattin P, Harvei S, Hakulinen T, Luostarinen T, Dillner J, Lehtinen M, Hakama M. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries. Int J Cancer. 2004;108(1):104–8. doi: 10.1002/ijc.11375. [DOI] [PubMed] [Google Scholar]
- van Lenthe FJ, Brug J, Mackenbach JP. Neighbourhood inequalities in physical inactivity: the role of neighbourhood attractiveness, proximity to local facilities and safety in the Netherlands. Soc Sci Med. 2005;60(4):763–75. doi: 10.1016/j.socscimed.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Whitman S, Ansell D, Orsi J, Francois T. The racial disparity in breast cancer mortality. J Community Health. 2011;36(4):588–96. doi: 10.1007/s10900-010-9346-2. [DOI] [PubMed] [Google Scholar]
- Zhuang Y, Wu W, Liu H, Shen W. Common genetic variants on FOXE1 contributes to thyroid cancer susceptibility: evidence based on 16 studies. Tumour Biol. 2014;35(6):6159–66. doi: 10.1007/s13277-014-1896-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Geographic location of three neighborhoods and zip codes of Upper Manhattan: Upper East Side (10021, 10028, 10044, 10128), Central Harlem (10026, 10027, 10030, 10037, 10039), and East Harlem (10029, 10035)
Figure S2. Age-standardized cancer incidence rates for specific cancers by United Health Fund New York City neighborhood and sex, 2007 to 2011. Vertical bars indicate 95% confidence intervals.