Abstract
Recent advances in genetic testing for heritable cardiac diseases have led to increasing involvement of the genetic counselor in cardiology practice. We present a series of cases collected from a nationwide query of genetics professionals regarding issues related to cost and utilization of genetic testing. Three themes emerged across cases: (1) choosing the most appropriate genetic test, (2) choosing the best person to test, and (3) interpreting results accurately. These cases demonstrate that involvement of a genetic counselor throughout the evaluation, diagnosis, and continuing management of individuals and families with inherited cardiovascular conditions helps to promote the efficient use of health care dollars.
Keywords: Genetic counselor, genetic testing, cardiology, cardiomyopathy, long QT syndrome
INTRODUCTION
In the past decade, cardiovascular genetic services have rapidly emerged at the forefront of what is now considered state of the art cardiology care. Improvements in the understanding of inherited cardiac conditions have been followed by a boom in the availability of cardiovascular genetic tests leading to changes in practice in both the cardiology and genetics fields. Cardiology and genetics sub-specialists ordering genetic testing have been called upon to make decisions about patient and test selection and to incorporate genetic information into care plans as needed. It has become evident over recent years that the financial impact of genetic tests both on the patient and the healthcare system is a relevant consideration when incorporating these services into patient care.
The need for integration of genetic medicine (often via a genetic counselor) into cardiology practice has been increasingly recognized as a benefit for patients and their families.1–10 Genetic counselors help patients and their providing physicians understand the implications of complex genetic information for the care of the patient and, in some cases, their family1–4. The value of the genetic counseling process has been documented previously, often with regard to hereditary cancer syndromes10–21. One conclusion from many of these publications is that the medical genetics and psychosocial counseling expertise of the genetic counselor in the application of genetic testing in clinical care positively impacts the use of healthcare dollars and adds value to patient care.
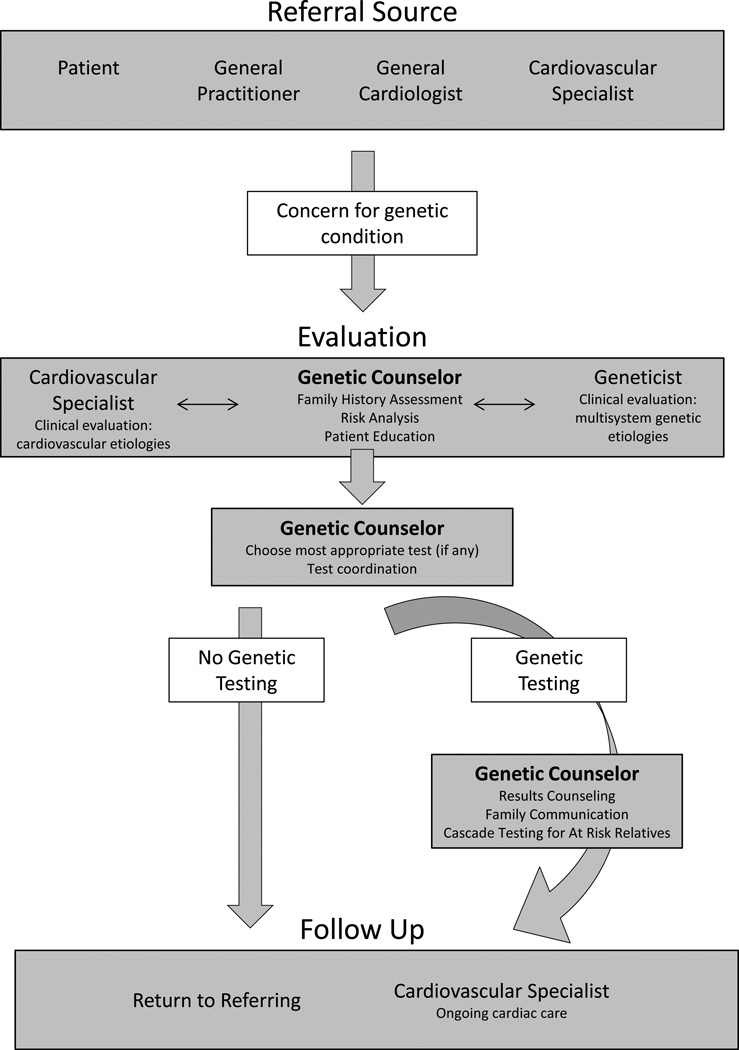
We present a series of cases that reveal specific issues related to appropriate genetic test utilization. These issues can be avoided or diminished by inclusion of a genetic counselor in the care of patients undergoing genetic risk evaluation and testing for hereditary cardiac diseases. In particular, these cases illustrate opportunities for health care savings through collaboration with a genetic counselor whose skill set facilitates the integration of the most appropriate genetic testing options (Table 1). The patient process is diagramed in Figure 1 which highlights specific points in patient care at which a genetic counselor can impact genetic testing decisions and utilization. Three themes emerge in these cases: (1) choosing the most appropriate genetic test, (2) choosing the best person to test, and (3) interpreting results accurately.
Table 1.
Genetic Counselor Roles in Clinical Care
| Risk Assessment |
|
| Education |
|
| Genetic testing |
|
| Result Interpretation |
|
| Result disclosure |
|
| Client-centered counseling |
|
Figure 1.
Integrating a genetic counselor into clinical care. Flow chart depicts referral through genetic counseling and evaluation, determination of testing (or not), result disclosure, to communication of information to family members and referring providers. White boxes indicate decision points related to genetic testing. The genetic counselor may coordinate testing for family members once a positive mutation is identified.
Genetic Evaluations for Inherited Cardiovascular Conditions
Examples of common indications for referral for genetic counseling and genetic testing are listed in Table 2. There are some generalities that can be made about inherited risks for cardiovascular disease that are important for understanding the impact of the cases described. Specific pathology, diagnosis, genetic etiology, and management have been extensively reviewed by others22–30 and will not be reviewed in detail.
Inherited cardiovascular diseases are associated with an increased risk for sudden cardiac death.
Identification of inherited monogenic cardiovascular disease in a patient typically confers a 50% risk for immediate relatives to be predisposed to the same disease.
Variable expressivity, in which clinical signs and symptoms vary among family members, and incomplete penetrance, in which some mutation-positive individuals may never develop disease, frequently complicate risk prediction for family members.
Life-long periodic cardiac evaluations are typically recommended for at-risk family members.
The likelihood of identifying a mutation varies across conditions and is dependent on which family member undergoes genetic testing.
If a mutation has been identified in a family, genetic testing can frequently determine which family members are predisposed to the condition and which are not.
Genetic testing can often be inconclusive because testing may identify rare genetic variants that may not be related to the inherited disease in the family.
Table 2.
Indications for Referral to a Cardiovascular Genetic Counselor
| Condition suspected in patient or family history: |
Genetic testing available |
Guidelines (references) for: |
Genetic testing yield |
|
|---|---|---|---|---|
| Genetic Counseling |
Genetic Testing |
|||
| ◦ Unexplained sudden death, sudden infant death syndrome | + /−a | 1, 8 | 1, 8 | ~5% (SIDS) – ~35% (SUD) |
| ◦ Sudden cardiac arrest, and/or idiopathic ventricular fibrillation | ||||
| Inherited Arrhythmias: | 1, 8 | 1, 8 | ||
| ◦ Long QT syndrome | + | 70–80% | ||
| ◦ Brugada syndrome | + | 20–30% | ||
| ◦ Catecholaminergic polymorphic ventricular tachycardia | + | 60 –70% | ||
| ◦ Short QT syndrome | + | unknown | ||
| ◦ Progressive cardiac conduction disease | +/− | unknown | ||
| Cardiomyopathies: | 1, 23 | 1, 23 | ||
| ◦ Hypertrophic cardiomyopathy | + | 30–50% | ||
| ◦ Dilated cardiomyopathy (idiopathic or familial) | + | 30–40% | ||
| ◦ Arrhythmogenic right ventricular dysplasia/cardiomyopathy | + | 30–50% | ||
| ◦ Restrictive cardiomyopathy | + | unknown | ||
| ◦ Left ventricular noncompaction cardiomyopathy | + | ~20% | ||
| Conditions affecting the aorta and other blood vessels: | 24 | 24 | ||
| ◦ Familial or early onset thoracic aortic aneurysm and dissection | + | 4–15% | ||
| ◦ Marfan syndrome | + | 75–90% | ||
| ◦ Loeys-Dietz syndrome | + | ~85% | ||
| ◦ Ehlers-Danlos syndrome (vascular and classic types) | + | vascular 95%/classic 50% | ||
| ◦ Arterial tortuosity syndrome | + | |||
| ◦ Bicuspid aortic valve | +/− | unknown | ||
| Congenital heart disease | +/−a | Varies | ||
| Coronary artery disease, early-onset and/or familial | − | |||
| ◦ Familial hypercholesterolemia | + | 41 | 60–80% | |
| Pulmonary arterial hypertension, idiopathic or familial | + | 42 | 42 | 25% simplex 75% familial |
| Known familial mutation for cardiovascular condition | + | 1, 8, 23, 24 | 1, 8, 23, 24 | |
+ = available; +/− = available for limited or specific indications; − = not clinically available;
availability of testing depends on risk assessment and evaluation
METHODS
Cases were solicited through a nationwide query of genetic counselors involved in clinical cardiovascular genetics practice. Cases were then selected by group consensus. The seven cases presented in this series are representative of recurrent themes where steps taken by the genetic counselor reduced excessive and inefficient testing choices, resulting in significant health care savings. The monetary values listed in this document are general figures based on the listed cost of laboratory testing at the time the manuscript was written.
CASES
Choosing the Most Appropriate Genetic Test
Choosing the most appropriate genetic testing strategy results in both the efficient use of healthcare dollars and the ability to answer the clinical question at hand. The process of selecting a genetic test is complicated by the number of genes associated with inherited cardiovascular conditions, increased number of labs offering testing, and the emergence of gene panels that include testing for many genes at once. Most inherited cardiovascular conditions exhibit genetic heterogeneity, whereby mutations in different genes, or different mutations in the same gene, can lead to the same disease. Mutations within a given gene may also confer differing phenotypes (i.e. dilated vs. hypertrophic cardiomyopathy).
Case 1
A 30-year-old man with a family history of Brugada Syndrome (BrS) sought genetic testing to determine whether he inherited the predisposition to BrS. A mutation in SCN5A had previously been identified in the patient’s brother who has BrS. In such cases, a genetic counselor would typically offer the at-risk relative genetic testing for the familial SCN5A mutation, typically at a cost of $350–$900, as well as help identify other at-risk family members who are candidates for genetic counseling and consideration of genetic testing. In fact, in the absence of the involvement of a genetic counselor, the patient underwent genetic testing through two multi-gene panels: one for BrS, and one for LQTS. Both panels include the SCN5A gene and were able to assess that the patient had in fact inherited his brother’s mutation. However, the patient was also tested for many genes that were not clinically relevant. The two panel testing strategy cost over $10,000 incurring over $9,000 in unnecessary costs.
Case 2
A 14-year-old male experienced a cardiac arrest of unknown etiology. During hospitalization prior to the patient’s death, genetic testing was ordered to assess possible genetic etiologies. The tests ordered included three separate multi-gene panels: Long QT syndrome (LQTS), catecholaminergic polymorphic ventricular tachycardia (CPVT), and arrhythmogenic right ventricular cardiomyopathy (ARVC), which together cover more than 20 genes for a total cost of over $15,000. In spite of this extensive and expensive testing, a specific genetic cause for the boy’s cardiac arrest was not identified. The family was later referred to a genetic counselor who was able to help the family understand the implications of the patient’s unexplained arrest in the setting of the family history and negative genetic test results, and provide supportive counseling. In reviewing the patient’s testing history, the genetic counselor noted that a single multi-condition panel could have been ordered for approximately $5,000, saving $10,000 from the prior order; this test would have included all of the genes ordered on the three separate panels, plus several dozen other genes associated with cardiac arrest.
Case 3
A 67-year-old woman with a history of a type A aortic dissection presented for follow up with her managing physician. She had previously been evaluated by a genetic counselor and a geneticist as her family history was significant for a 6’8” son who died of a type A dissection at the age 39 years, and a father who had been diagnosed with a subclavian aneurysm at age 75 years. She had findings suggestive of a hereditary connective tissue disorder and had normal sequencing of FBN1 (Marfan syndrome), as well as TGFBR1 and TGFBR2 (Loeys Dietz syndrome). At the time of a re-evaluation by the genetics team, additional testing for a newly described genetic cause of familial aneurysms and dissections, SMAD3 (Aneurysm Osteoarthritis syndrome), was recommended by the genetic counselor. Although the referring provider originally misinterpreted the recommendation for SMAD3 testing and repeated the FBN1, TGFBR1 and TGFBR2 analysis, the genetic counselor identified and canceled the unnecessary duplicate testing and coordinated the SMAD3 testing. The SMAD3 testing identified a mutation responsible for the family’s vascular presentation. The involvement of the genetic counselor allowed for recognition of and testing for this newly described condition, as well as prevented over $2000 worth of duplicate and unnecessary testing. With the identification of a specific mutation, the genetic diagnosis was established and genetic testing was subsequently available for family members.
KEY POINT.
Involvement of a genetic counselor can help reduce unnecessary healthcare costs by making sure the most appropriate testing is ordered.
Choosing the Best Person to Test
Collecting and analyzing an in-depth, multi-generational family history can impact genetic testing decisions and help identify the most pertinent person to test. This process will maximize the odds of an informative genetic test result and thereby improve post-test screening options and interventions for family members. Identifying this starting point requires careful consideration and must be tailored to each family. Ideally, the initial person tested should be the most significantly affected family member, the youngest affected family member, or the individual with additional extra-cardiac findings in the case of syndromic heart disease. Sometimes healthy individuals present to a cardiovascular genetics clinic due to a family history of sudden death. In these situations, the most appropriate individual to initiate genetic testing is often deceased, and post-mortem testing can only be done if a specimen appropriate for genetic testing is available.
In families where previous genetic testing identified a causal mutation, implementing testing in a cascade manner will be most efficient. Cascade testing involves testing family members one-at-a-time or in small groups based on their relational proximity to the affected proband, in order to minimize unnecessary tests. When an individual is genotype negative for a familial mutation, this also means their children and descendants are not at risk for the familial mutation.
Case 4
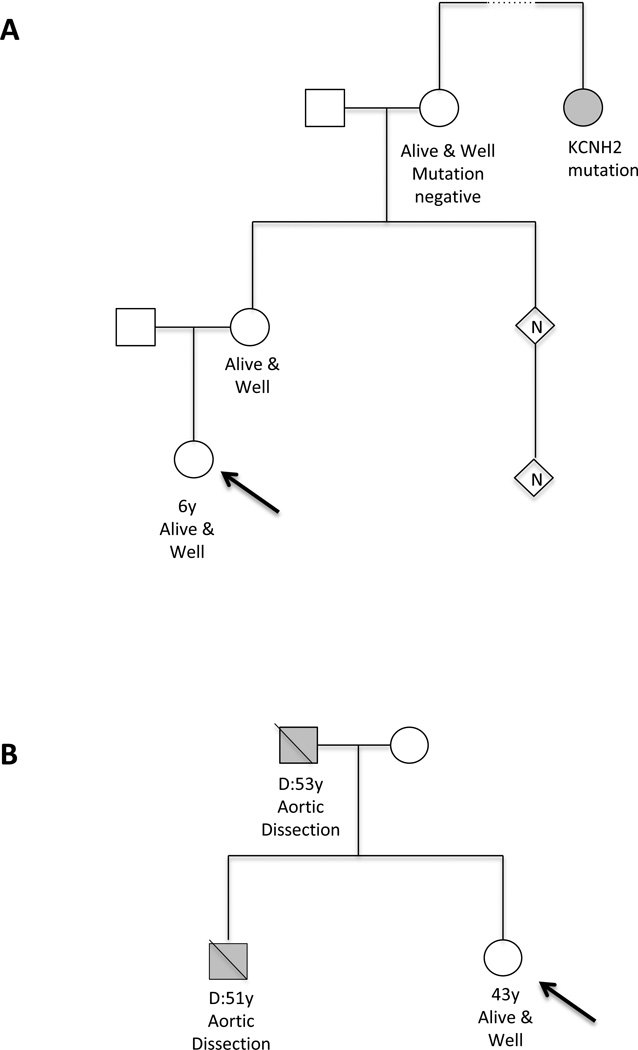
A 6-year-old girl presented to genetics clinic due to a family history of LQTS. A familial LQT2-causative mutation in KCNH2 had been identified in a distant maternal cousin (Figure 2). The patient and numerous family members had been undergoing cardiology evaluations annually since birth due to the family history. The patient and her mother had normal cardiology evaluations. Referral to the genetic counselor allowed for refinement of who within the family actually needed screening. The closest shared relative, the patient’s maternal grandmother, was living and not known to have LQTS. The genetic counselor advised the family that an optimal testing strategy would be to offer familial genetic testing to the patient’s grandmother. The cost of a single test for a known mutation is $350–900. The grandmother underwent genetic testing for the KCNH2 mutation; results revealed that the grandmother did not carry the familial mutation. Therefore, her eight children and grandchildren were not at increased risk for LQTS and would not need to continue to undergo related cardiology evaluations nor would they need genetic testing. Performing genetic testing on the grandmother as opposed to testing her eight children saved $2800–7200. Significant additional cost savings were realized from discontinuation of cardiovascular screening in the patient and other family members who were no longer considered at risk for the condition.
Figure 2.
Pedigree examples for case 4 (A), and case 5 (B). Arrow indicates individual presenting for genetic counseling (proband). Standard pedigree notation using circles for females and squares for males. Filled symbol represents affected individual. Diagonal line indicates individual is deceased.
Case 5
A healthy 43-year-old female was referred to a cardiovascular genetics clinic for evaluation following her brother’s sudden death due to aortic dissection at age 51 (Figure 2). Her father also died suddenly of aortic dissection at age 53. No living family member, including the patient, had any aortic abnormalities detectable on imaging. The genetic counselor discussed with the family why it would be most informative for them to begin testing on a post-mortem specimen, however, no specimen suitable for genetic testing was available. Due to significant limitations in genetic testing for this clinical indication, testing an unaffected family member would most likely yield an uninformative result that would not change recommendations for ongoing aortic imaging in the patient and other at-risk relatives. Instead of genetic testing, appropriate clinical screening recommendations were made to the family, promoting a more efficient use of healthcare dollars and avoiding the cost of uninformative genetic testing. Genetic tests for familial aortic disease range in cost from around $1800 to more than $5000, depending on the laboratory and number of genes included.
KEY POINT.
Through pedigree analysis, a genetic counselor can help reduce costs and strengthen utility of genetic testing in a family by identifying the most appropriate individuals to test first.
Interpreting Results Accurately
In addition to the multifaceted logistics of ordering genetic tests, the interpretation of genetic test results can be complex. While a positive result (identification of a pathogenic mutation) can confirm a diagnosis and allow for familial genetic testing, a negative test result can be a challenge with regard to determining the next steps for the patient and family. Furthermore, variants of unknown significance (VUS) may be identified for which there is limited or insufficient evidence to draw conclusions about pathogenicity. While some VUS may eventually be reclassified as pathogenic mutations, many VUS may represent rare benign variations that are not the cause of familial disease. Since there is not enough information to make an accurate interpretation, a VUS should not be used to confirm or rule out an inherited condition.
Case 6
Following a young sudden death, the asymptomatic brother of the deceased underwent genetic testing for LQTS, which identified a variant that was reported as a “probable disease causing mutation” in an LQTS related gene. Clinical guidelines discourage offering unaffected relatives clinical testing for gene variants of uncertain significance for the purpose of medical management. However, in this case, it was assumed that the cause of the sudden death was LQTS and that the gene variant was pathogenic. Clinical evaluations and genetic testing for the presumed disease causing mutation were performed on over 20 additional relatives, many of whom were subsequently given LQTS diagnoses. Several family members then presented to a specialized multidisciplinary LQTS clinic that included a genetic counselor and were re-evaluated. A careful evaluation of clinical data (i.e. EKGs) and genetic testing results did not reveal a clinical correlation with the presumed disease causing mutation. Namely, individuals who carried this variant did not demonstrate a prolonged QT, casting doubt on the clinical significance of this variant. Reviewing the genetic test results in more detail, the genetic counselor noted that while the laboratory report indicated that the variant had been previously published in three unrelated probands with LQTS, the three published reports were in fact describing the same individual. Thus, the evidence for pathogenicity was weak and the variant was more appropriately classified as a VUS. Finally, a copy of the autopsy report from the deceased individual was obtained and reviewed. There were clear structural cardiac abnormalities that may well have contributed to the cause of death in the proband and were not consistent with LQTS. Genetic testing for the 20 relatives was an avoidable cost of ~$350–900 each for a total of $7000–$18000. Some individuals also had implantable cardioverter defibrillators (ICDs) implanted based on the presence of the VUS. Implantation of one ICD may cost on the order of $20,000–35,00031, 32{Abriel, 2013 #1}. Additional potential costs associated with an ICD include absence from work, risks associated with surgical complications, as well as psychological stressors related to ICDs33, 34. This case highlights the importance of careful interpretation of genetic test results, the value of clinical correlation with mutation status, and the dangers of genetically testing asymptomatic family members for a VUS.
Case 7
A healthy 65-year-old woman presented to a genetic counselor with a family history of hypertrophic cardiomyopathy (HCM). A VUS in the MYBPC3 gene had been identified in her affected sister. Analysis of this large family’s history revealed a very strong family history of sudden death and unspecified heart problems. The patient had been undergoing screening for HCM for several years and did not have findings of the disease. She came to the genetic counseling session wishing to be tested for the sister’s VUS. The genetic counselor advised her that presymptomatic testing (using testing to assess risk of disease in healthy individuals) for a VUS is generally not recommended, since a positive or negative result would not be informative, and she would need to continue screening for HCM regardless of the genetic test result. At the end of the session, the patient planned to continue HCM screening every 3 to 5 years and genetic testing was not pursued.
Two years later, the genetic counselor identified literature that suggested the VUS detected in the sister was indeed a disease-causing mutation. The genetic counselor contacted the laboratory who had reported the sister’s VUS, and the laboratory re-classified the variant from VUS to pathogenic, allowing for informative presymptomatic testing in the family. The patient underwent genetic testing for the familial mutation and tested negative, eliminating the need for ongoing HCM screening for herself and confirming that her twelve children and fifteen grandchildren were not at risk for this mutation. Involvement of the genetic counselor in this case was critical for appropriate interpretation and use of a genetic test result over the span of several years. In this family, thousands of healthcare dollars have been saved by allowing presymptomatic testing to eliminate mutation-negative family members from the HCM screening protocol.
KEY POINT.
Continued involvement of a genetic counselor facilitates up-to-date interpretation of genetic test results and can help to avoid unnecessary downstream costs.
DISCUSSION
Genetic testing is increasingly being incorporated into clinical care for individuals with inherited cardiovascular conditions. The inclusion of a genetic counselor in the care of patients with hereditary cardiovascular disease has been recommended in a number of consensus statements and practice guidelines1, 9, 23, 24, 35. Similarly, insurance companies are increasingly recognizing the value of a genetic counselor for appropriate utilization of genetic tests. Many insurers have adopted policies to help guide coverage decisions for genetic testing. These policies often recommend, or in some cases require, genetic counseling provided by a genetic counselor for patients undergoing genetic testing for specific hereditary conditions, including Long QT syndrome. Cigna Medical Coverage Policy 0193 was one of the earliest, and it is reasonable to anticipate an increase in adoption of these policies by more insurance companies over time.
Several studies have demonstrated the cost-effectiveness of including genetic testing in cascade family screening for hypertrophic cardiomyopathy, inherited arrhythmia conditions, and familial hypercholesterolemia5, 6, 36–39. These analyses are dependent on the cost of the genetic test and the likelihood of identifying a mutation in the proband. Scenarios where the cost of testing is high, or testing is done in an individual with a low likelihood of identifying a mutation, would be less cost effective. However, when a familial mutation is defined, familial screening is possible and genetic testing becomes a cost-effective screening tool. This suggests that a careful approach to genetic testing is called for in order to maximize the cost-effectiveness of genetic testing in clinical practice.
Early involvement of a genetic counselor facilitates familial screening by helping to determine an etiology for disease. Appropriate pedigree analysis and risk assessment provide the basis for decisions about genetic testing. Case 3 highlights how knowledge of a newly available testing option provides a key opportunity to arrive at a specific genetic diagnosis. Once a familial mutation is known, other family members can undergo presymptomatic genetic testing to determine their risk of disease. Presymptomatic testing can determine which family members require ongoing cardiovascular surveillance and which ones do not. On average, half of at-risk family members will not have the familial mutation and thus will not require screening.
Expertise in choosing the most appropriate tests helps to prevent excessive costs related to genetic testing. For example, it is important to know when familial testing has already identified a specific gene mutation in a family so targeted testing can be performed and significant cost savings can be realized (illustrated in cases 1 and 4). In other situations, although genetic testing may be available, testing the person being seen in clinic that day may not be informative, and is not indicated, as in case 5 and 6. When it is determined that testing first in another family member would be more appropriate, a genetic counselor is a resource to help facilitate testing in that family member. Even in an urgent setting such as case 2, the genetic testing strategy can be streamlined to minimize costs and obtain the most useful information. In cases in which a person’s prognosis is dire and a genetic cause has not been identified, genetic counselors can also discuss DNA banking as an option to guarantee a sample is available for any new tests that may be developed in the future40.
Technological advances in molecular analysis are driving rapid expansion of cardiovascular genetic testing options. Large multi-gene test panels and whole exome sequencing are increasingly available for inherited cardiac conditions and may offer an economical approach. It is important to note, however, that the composition of panels, methods used, costs, and turn-around-time will vary. It is also important to recognize that the inclusion of more genes on a panel increases the likelihood of identifying a VUS, which is an inconclusive result and can be troubling for patients and providers. Genetic counselors are aware of the most current test availability, as well as each test’s benefits and limitations, which is key to providing the most relevant genetic information to patients and their physicians.
It is important for clinical and molecular information to be interpreted together to provide the best care for the patient and their family. For example, the presence of a VUS in an unaffected individual should be considered with caution and must be correlated with clinical evaluations as illustrated by cases 6 and 7. Additional information gained from family history and evaluation of family members can help to clarify whether or not a VUS might be reclassified and considered useful for family testing on a clinical or research basis. Continued involvement of a genetic counselor, as in case 7, can help facilitate the ongoing process of providing the most current update of genetic information.
The presented cases demonstrate the importance of appropriate test selection and accurate result interpretation in the care of patients with inherited cardiovascular conditions. The inclusion of a genetic counselor as part of the multidisciplinary team throughout the evaluation, diagnosis, and continuing care of individuals who have an inherited cardiovascular condition results in high quality care and appropriate utilization of genetic testing that meets the clinical needs while optimizing use of healthcare dollars. The genetic counselor is the medical professional most able to navigate the testing process for maximum cost effectiveness.
ACKNOWLEDGEMENTS
The project described was supported in part by the Indiana University Health – Indiana University School of Medicine Strategic Research Initiative.
Source of Funding:
Michael J. Ackerman, MD, PhD, is a consultant for Boston Scientific, Gilead Sciences, Metronic, St. Jude Medical. MJA and Mayo Clinic receive royalties from Transgenomic with respect to their FAMILION-LQTS and FAMILION-CPVT genetic tests. Katie Spoonamore, MS, CGC, is supported by the Indiana University Health – Indiana University School of Medicine Strategic Research Initiative.
Footnotes
Conflicts of Interest:
For the remaining authors none were declared.
REFERENCES
- 1. Ackerman MJ, Priori SG, Willems S, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) Heart Rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020. Guidelines for genetic testing for channelopathies and cardiomyopathies.
- 2. Caleshu C, Day S, Rehm HL, et al. Use and interpretation of genetic tests in cardiovascular genetics. Heart. 2010;96:1669–1675. doi: 10.1136/hrt.2009.190090. Case-based discussion of principles and approaches to best use of genetic testing in cardiovascular genetics.
- 3. Dunn KE, Caleshu C, Cirino AL, et al. A clinical approach to inherited hypertrophy: the use of family history in diagnosis, risk assessment, and management. Circ Cardiovasc Genet. 2013;6:118–131. doi: 10.1161/CIRCGENETICS.110.959387. Assessment and description of family history assessment as dynamic, ongoing process relevant to cardiovascular genetics care.
- 4. Ingles J, Yeates L, Semsarian C. The emerging role of the cardiac genetic counselor. Heart Rhythm. 2011;8:1958–1962. doi: 10.1016/j.hrthm.2011.07.017. Extensive description of development of, current role, and ideal role of genetic counselor in cardiovascular genetics.
- 5. Ingles J, McGaughran J, Scuffham PA, et al. A cost-effectiveness model of genetic testing for the evaluation of families with hypertrophic cardiomyopathy. Heart. 2012;98:625–630. doi: 10.1136/heartjnl-2011-300368. Assesses cost-effectiveness of including genetic screening for family members in comparison to clinical screening alone.
- 6. Perez MV, Kumarasamy NA, Owens DK, et al. Cost-effectiveness of genetic testing in family members of patients with long-QT syndrome. Circ Cardiovasc Qual Outcomes. 2011;4:76–84. doi: 10.1161/CIRCOUTCOMES.110.957365. Assesses cost-effectiveness of three strategies regarding a first degree relative of LQTS patient.
- 7. Sturm AC, Hershberger RE. Genetic testing in cardiovascular medicine: current landscape and future horizons. Curr Opin Cardiol. 2013;28:317–325. doi: 10.1097/HCO.0b013e32835fb728. Review of current genetic testing options and guidance on incorporating genetics in to cardiovascular medicine.
- 8. Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10:e85–e108. doi: 10.1016/j.hrthm.2013.07.021. International consensus statement on diagnosis and management of patients with inherited primary arrhythmias.
- 9. Hershberger RE, Lindenfeld J, Mestroni L, et al. Genetic evaluation of cardiomyopathy--a Heart Failure Society of America practice guideline. J Card Fail. 2009;15:83–97. doi: 10.1016/j.cardfail.2009.01.006. Heart Failure Society guidelines on use of genetics for various cardiomyopathy evaluations.
- 10. Judge DP. Use of genetics in the clinical evaluation of cardiomyopathy. JAMA. 2009;302:2471–2476. doi: 10.1001/jama.2009.1787. Demonstrates benefits of genetics in individual and family evaluation for cardiomyopathies.
- 11. Aktan-Collan K, Mecklin JP, de la Chapelle A, et al. Evaluation of a counselling protocol for predictive genetic testing for hereditary non-polyposis colorectal cancer. J Med Genet. 2000;37:108–113. doi: 10.1136/jmg.37.2.108. Concludes that inclusion of genetic counseling is considered useful and supportive by patients.
- 12. Balmana J, Sanz J, Bonfill X, et al. Genetic counseling program in familial breast cancer: analysis of its effectiveness, cost and cost-effectiveness ratio. Int J Cancer. 2004;112:647–652. doi: 10.1002/ijc.20458. Suggests that genetic testing and screening is cost-effect for preventive medicine approach to inherited cancer conditions
- 13. Brierley KL, Campfield D, Ducaine W, et al. Errors in delivery of cancer genetics services: implications for practice. Conn Med. 2010;74:413–423. Discussion of errors leading to assessment of expectation that clinicians cover all genetic concepts and testing, suggests role for genetic counselors in this area.
- 14. Brierley KL, Blouch E, Cogswell W, et al. Adverse events in cancer genetic testing: medical, ethical, legal, and financial implications. Cancer J. 2012;18:303–309. doi: 10.1097/PPO.0b013e3182609490. Case series demonstrating impact of errors in genetic testing on cost effectiveness, liabilty, and patients and families.
- 15. Christie J, Quinn GP, Malo T, et al. Cognitive and psychological impact of BRCA genetic counseling in before and after definitive surgery breast cancer patients. Ann Surg Oncol. 2012;19:4003–4011. doi: 10.1245/s10434-012-2460-x. Pre-test counseling improves understanding and decision-making in patients.
- 16. Griffith GL, Edwards RT, Gray J. Cancer genetics services: a systematic review of the economic evidence and issues. Br J Cancer. 2004;90:1697–1703. doi: 10.1038/sj.bjc.6601792. Systematic review of economics of cancer genetic services. Shows cost-effectiveness of genetic testing.
- 17. Forrest LE, Burke J, Bacic S, et al. Increased genetic counseling support improves communication of genetic information in families. Genet Med. 2008;10:167–172. doi: 10.1097/GIM.0b013e318164540b. Concludes that genetic counseling for proband increases likelihood of at-risk family member contact for genetic services.
- 18. Meiser B, Halliday JL. What is the impact of genetic counselling in women at increased risk of developing hereditary breast cancer? A meta-analytic review. Soc Sci Med. 2002;54:1463–1470. doi: 10.1016/s0277-9536(01)00133-2. Concludes that genetic counseling decreases patient anxiety and improves understanding of individual risk.
- 19. Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med. 2012;79:560–568. doi: 10.3949/ccjm.79a.11091. Explains process of genetic counseling, and describes value of genetic counseling in caring for patients and families with inherited conditions.
- 20. Miller CE, Krautscheid P, Baldwin EE, et al. Genetic counselor review of genetic test orders in a reference laboratory reduces unnecessary testing. Am J Med Genet A. 2014;164A:1094–1101. doi: 10.1002/ajmg.a.36453. Describes how the utilization of genetic counselors in the laboratory reduces healthcare costs to multiple stakeholders.
- 21. Plon SE, Cooper HP, Parks B, et al. Genetic testing and cancer risk management recommendations by physicians for at-risk relatives. Genet Med. 2011;13:148–154. doi: 10.1097/GIM.0b013e318207f564. Impact of genetic testing results on decision-making. Utilization of genetics professionals increases efficacy.
- 22. Abriel H, Zaklyazminskaya EV. Cardiac channelopathies: genetic and molecular mechanisms. Gene. 2013;517:1–11. doi: 10.1016/j.gene.2012.12.061. Channelopathy review.
- 23. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e783–e831. doi: 10.1161/CIR.0b013e318223e2bd. Guidelines for treatment of HCM including genetics.
- 24. Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. doi: 10.1161/CIR.0b013e3181d4739e. Guidelines for treatment of thoracic aortic disease including genetics.
- 25.Jacoby D, McKenna WJ. Genetics of inherited cardiomyopathy. Eur Heart J. 2012;33:296–304. doi: 10.1093/eurheartj/ehr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381:242–255. doi: 10.1016/S0140-6736(12)60397-3. [DOI] [PubMed] [Google Scholar]
- 27.Murray B. Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C): a review of molecular and clinical literature. J Genet Couns. 2012;21:494–504. doi: 10.1007/s10897-012-9497-7. [DOI] [PubMed] [Google Scholar]
- 28. Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. Consensus statement on diagnosis and management of inherited arrhythmias, including genetics.
- 29. Schwartz PJ, Ackerman MJ, George AL, Jr, et al. Impact of genetics on the clinical management of channelopathies. J Am Coll Cardiol. 2013;62:169–180. doi: 10.1016/j.jacc.2013.04.044. Discussion of best use of genetics in care for patients with cardiac channelopathies.
- 30. Teekakirikul P, Kelly MA, Rehm HL, et al. Inherited cardiomyopathies: molecular genetics and clinical genetic testing in the postgenomic era. J Mol Diagn. 2013;15:158–170. doi: 10.1016/j.jmoldx.2012.09.002. Overview of genetics and diagnosis of inherited cardiomyopathies.
- 31. Groeneveld PW, Matta MA, Suh JJ, et al. Costs and quality-of-life effects of implantable cardioverter-defibrillators. Am J Cardiol. 2006;98:1409–1415. doi: 10.1016/j.amjcard.2006.06.041. Systematic review of ICD and Quality of Life outcomes.
- 32. Hlatky MA, Mark DB. The high cost of implantable defibrillators. Eur Heart J. 2007;28:388–391. doi: 10.1093/eurheartj/ehl311. Commetary on the cost of ICDs and improving cost-effectiveness.
- 33. Kirkfeldt RE, Johansen JB, Nohr EA, et al. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014;35:1186–1194. doi: 10.1093/eurheartj/eht511. Assessment of ICD risks and their frequency.
- 34. Schwartz PJ, Spazzolini C, Priori SG, et al. Who are the long-QT syndrome patients who receive an implantable cardioverter-defibrillator and what happens to them?: data from the European Long-QT Syndrome Implantable Cardioverter-Defibrillator (LQTS ICD) Registry. Circulation. 2010;122:1272–1282. doi: 10.1161/CIRCULATIONAHA.110.950147. Description of cohort of patients with LQTS receiving ICDs.
- 35. Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385–e484. doi: 10.1161/CIRCULATIONAHA.106.178233. ACC/AHA guidelines regarding management of ventricular arrhythmias. Includes genetics.
- 36. Bai R, Napolitano C, Bloise R, et al. Yield of genetic screening in inherited cardiac channelopathies: how to prioritize access to genetic testing. Circ Arrhythm Electrophysiol. 2009;2:6–15. doi: 10.1161/CIRCEP.108.782888. Assesses utility and cost-effectiveness of genetic testing for patients with inherited channelopathies.
- 37. Wordsworth S, Leal J, Blair E, et al. DNA testing for hypertrophic cardiomyopathy: a cost-effectiveness model. Eur Heart J. 2010;31:926–935. doi: 10.1093/eurheartj/ehq067. Assesses cost-effectivness of using genetic testing in diagnoses and management of HCM.
- 38. Nherera L, Marks D, Minhas R, et al. Probabilistic cost-effectiveness analysis of cascade screening for familial hypercholesterolaemia using alternative diagnostic and identification strategies. Heart. 2011;97:1175–1181. doi: 10.1136/hrt.2010.213975. Demonstrates that family genetic screening is cost effective in FH.
- 39. Wonderling D, Umans-Eckenhausen MA, Marks D, et al. Cost-effectiveness analysis of the genetic screening program for familial hypercholesterolemia in The Netherlands. Semin Vasc Med. 2004;4:97–104. doi: 10.1055/s-2004-822992. Systematic genetic screening of FH families is cost-effective.
- 40. MacLeod HDE, Honeywell C, Rutberg J. Genetic Counselors: An Important Resource for Families Following a Young Sudden Cardiac Death. Academic Forensic Pathology. 2013;3:183–190. Describes role of genetic counselor in cases of sudden cardiac death in a young person.
- 41. Goldberg AC, Hopkins PN, Toth PP, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S1–S8. doi: 10.1016/j.jacl.2011.04.003. National Lipid Association guidance includes discussion of genetic testing.
- 42. McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:14S–34S. doi: 10.1378/chest.126.1_suppl.14S. Guidelines for pulmonary arterial hypertension, including discussion of genetic testing and genetic counseling.