Abstract
Spreading depolarizations (SD) are coordinated waves of synchronous depolarization, involving large numbers of neurons and astrocytes as they spread slowly through brain tissue. The recent identification of SDs as likely contributors to pathophysiology in human subjects has led to a significant increase in interest in SD mechanisms, and possible approaches to limit the numbers of SDs or their deleterious consequences in injured brain. Astrocytes regulate many events associated with SD. SD initiation and propagation is dependent on extracellular accumulation of K+ and glutamate, both of which involve astrocytic clearance. SDs are extremely metabolically demanding events, and signaling through astrocyte networks is likely central to the dramatic increase in regional blood flow that accompanies SD in otherwise healthy tissues. Astrocytes may provide metabolic support to neurons following SD, and may provide a source of adenosine that inhibits neuronal activity following SD. It is also possible that astrocytes contribute to the pathophysiology of SD, as a consequence of excessive glutamate release, facilitation of NMDA receptor activation, brain edema due to astrocyte swelling, or disrupted coupling to appropriate vascular responses after SD. Direct or indirect evidence has accumulated implicating astrocytes in many of these responses, but much remains unknown about their specific contributions, especially in the context of injury. Conversion of astrocytes to a reactive phenotype is a prominent feature of injured brain, and recent work suggests that the different functional properties of reactive astrocytes could be targeted to limit SDs in pathophysiological conditions.
Keywords: Spreading depression, migraine, stroke, traumatic brain injury, reactive astrocyte, Ca2+ waves, adenosine, glutamate, neurovascular coupling
2. Spreading depolarization (SD) and implications for brain pathology
Spreading depolarizations (SD) are unusual events that involve the sustained depolarization of regions of brain tissue, and are observed to propagate slowly in a feed-forward manner across the cortical surface. The first clear documentation of the consequences of SD was made by Aristides Leao, who showed that strong stimulation of a region of the exposed cortex could result in a propagating abolition of spontaneous electrical activity, a phenomenon he termed “spreading depression” (Leao 1944). Since Leao’s initial observations, there has been a large body of work focused on characterizing the underlying ionic shifts involved in SD, the mediator(s) responsible for SD propagation, and the implications of these events for a range of pathophysiological disorders. SD involves near-complete depolarization of both neurons and astrocytes, and associated intracellular Na+ and Ca2+ loading. Extracellular K+ accumulation is a hallmark of SD and, depending on the specific experimental conditions, K+ and glutamate together appear responsible for the depolarization of neighboring tissue, to continue the feed-forward propagation of SD events (Pietrobon and Moskowitz 2014; Somjen 2001). Large amounts of energy are required to reestablish ionic gradients after SD, and these demands are substantially higher than for other pathophysiological events in brain, including seizures (Dreier et al. 2013). Effective vascular supply is therefore required following SD, for provision of metabolic substrates to challenged tissues. Based on these characteristics, a number of consequences of SD can be used to track the progression of SD events in experimental and clinical settings: 1) suppression of electrocorticographic activity, 2) extracellular potential changes (DC shifts), 3) intracellular or extracellular ionic shifts, and accompanying cellular swelling, and 4) regional blood flow dynamics.
Several lines of evidence have converged to strongly suggest that SD underlies important aspects of migraine, including aura (Charles and Baca 2013; Eikermann-Haerter and Ayata 2010; Lauritzen 1985; Milner 1958). Changes in regional cerebral blood flow (rCBF) during migraine aura in humans match flow changes associated with SD observed in animal models (Hadjikhani et al. 2001; Lauritzen and Olesen 1984). Meningeal nociceptor activation occurs following SD induction, and is thought to underlie the initiation of the headache (Zhang et al. 2010). Recent work suggests that SD induction of headache involves pannexin 1 signaling in neurons and communication with astrocytes to mediate trigeminal nerve activation around pial vessels (Karatas et al. 2013). These studies and others, suggest that SD may in fact trigger the onset of migraine headaches in some patients (Ayata 2010).
SD may also be one of the primary contributors to the progression of acute brain injuries. Leao described a SD-like event following carotid arterial occlusion in the rabbit (Leao 1947), and subsequent work in rodent models made a strong case for the involvement of SD in the enlargement of ischemic infarction (Hartings et al. 2003; Hossmann 1996; Mies et al. 1993; Nedergaard and Astrup 1986). The metabolic requirements for recovery from SD likely exceed the metabolic capacity of injured or poorly-perfused brain tissue, and lead to a progressive failure of ionic homeostasis. More recently, SDs have been recorded from human subjects in the intensive care unit, including in populations of patients with large ischemic strokes, subarachnoid hemorrhage, and traumatic brain injury. The accumulating evidence from these studies implies that SDs are common in the injured human brain, including at late time points following the onset of injury. These data also suggest that SDs which occur in clusters in severely compromised tissue (as assessed by suppressed electrocorticographic activity) contribute to poor patient outcomes (Dreier 2011; Hartings et al. 2011; Lauritzen et al. 2011). Thus SDs are being considered as a potential target for therapeutic intervention, and understanding mechanisms involved in SD regulation and resupply of metabolic substrates after SD should be valuable in developing interventions that can be provided at relatively late time points after the onset of brain injury.
3. Scope of review
In the 7 decades since Leao’s original descriptions of spreading depression, a majority of studies of SD mechanisms have concentrated on neuronal activity, but the responses of astrocytes in SD have certainly not been neglected. Relatively early recordings made from astrocytes during SD argued that depolarization of astrocyte networks likely makes a large contribution to extracellular voltage shifts recorded during SD (Reviewed in (Charles and Brennan 2009; Somjen 2001)). As will be discussed below, SD also results in large amounts of K+ uptake into astrocytes, astrocyte swelling, and is also accompanied by striking Ca2+ waves that spread through astrocyte networks together with SD. Much effort has centered on the question of whether astrocytes or neurons are primarily responsible for driving SD, and led to the general conclusion that many obvious astrocyte responses appear to follow (rather than lead) SD. However, the fact that astrocytes may not be the main drivers of SD does not mean that astrocyte function may not be a key determinant of the SD severity or outcome. In fact, the rapid advances in astrocyte biology in the last decade suggest that these cells likely play central roles in many of the features that may be targetable, to mitigate deleterious consequences of SD in injured brain. The current review concentrates on functions of astrocytes in SD (Figure 1), especially those that might be considered to reduce the incidence of SD, and/or deleterious consequences of SD in injured brain. Since astrocytes become reactive in injured brain, we will also address the yet understudied issue related to the potential impact of reactive astrocytes on SD and functional outcomes. Current views of astrocytic roles in normal synaptic, neurovascular, and metabolic functions are not covered here in detail, but can be found in a number of comprehensive reviews (Attwell et al. 2010; Brown and Ransom 2007; Gordon et al. 2007; Nedergaard and Verkhratsky 2012; Orellana and Stehberg 2014; Pellerin et al. 2007; Scemes and Giaume 2006).
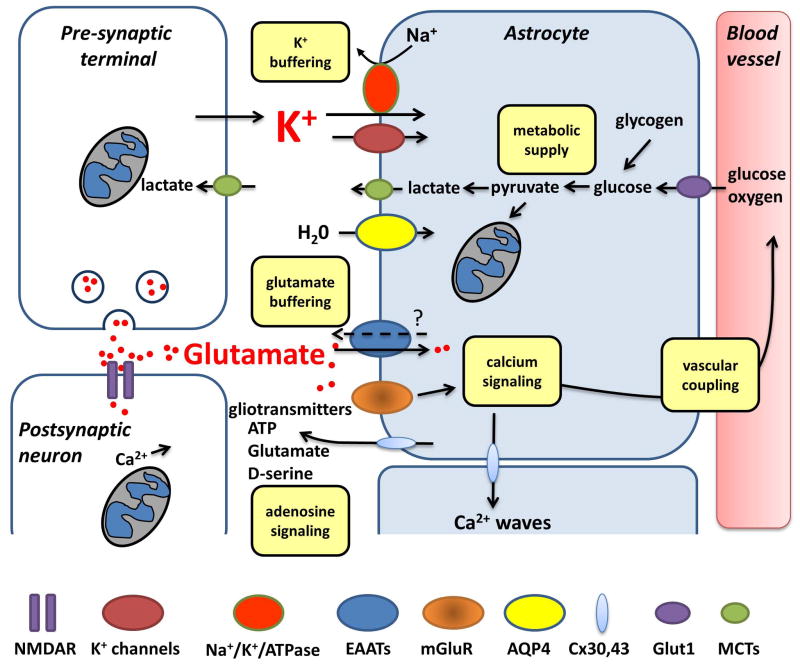
Figure 1. Major mechanisms by which astrocytes can participate in spreading depolarization (SD).
Waves of SD involve large extracellular K+ and glutamate increases, as well as severe demands on ATP production. Astrocyte uptake of K+ (via channels and the activity of Na+/K+/ATPase) can delay the onset or progression of SD, and participate in recovery of tissues after SD. Glutamate uptake by astrocytes could also limit the spread of SD, and prevent excessive accumulation of glutamate and excitotoxic NMDA receptor activation. It is not yet known to what degree reverse operation of excitatory amino acid transporters (EAATs) or vesicular release of glutamate from astrocytes may contribute SD or its consequences. Astrocyte metabolism is critical for transport activities, and it is also possible that lactate from astrocytes could provide an energy source for neurons during recovery from SD. Astrocytes could serve as a significant source of extracellular adenosine, which is enhanced in the extracellular space following SD. Adenosine could be derived as a consequence of intracellular depletion of ATP and transport of adenosine via equilibrative transporters, or ATP release and degradation by ecto-ATPase activity. Astrocyte Ca2+ waves are prominent during SD, and are expected to modulate the dramatic vascular dynamics that are associated with SD, and could also regulate availability of the co-agonist required for NMDAR activation (D-serine). Gap junctions composed of connexins (Cx30 and Cx43) can mediate release of ATP and glutamate, and also allow intercellular passage of mediators such as IP3. The importance of glial-derived ATP and glutamate for the propagation of astrocyte Ca2+ waves is not yet clear for SD. Astrocytes are well established to swell substantially following SD, involving water flux through aquaporin 4 channels (AQP4). However, it is important to keep in mind that the ionic fluxes and metabolic demands of SD are much greater than those observed during regulated synaptic transmission, implying that astrocyte roles described under other recording conditions may not directly apply to the unusual circumstances of SD.
4. Metabolic support
As noted above, the metabolic demands of SD are likely the largest experienced by a volume of brain tissue, far exceeding the requirements of normal synaptic transmission. Even pathological events such as seizure activity involve orders of magnitude lower demands on glucose and O2 consumption (Dreier et al. 2013). Animal studies have demonstrated large increases in brain lactate, and decreased glucose and extracellular pH during SD (Cruz et al. 1999; Csiba et al. 1985; Hashemi et al. 2008). These findings have recently been extended to recordings in clinical settings associated with SDs (Feuerstein et al. 2010; Sakowitz et al. 2013), where it was found that the decrease in glucose only occurred as tissue started repolarizing, as indicated by the coincidence of the onset of the glucose drop with the onset of the recovery of extracellular K+ concentrations. This large metabolic demand is not met by the increased local supply, and glucose concentrations fall (Rogers et al. 2013). Increased glycolysis in both neurons and astrocytes could contribute to increased lactate production, but the fact that SD rapidly depletes astrocyte glycogen stores (Bures 1956; Krivanek 1958; Krivanek 1961), implies that astrocytic metabolism contributes, at least in part, to the greatly accelerated glycolysis during the recovery phase of SD. The demonstration of pH decreases within astrocytes during SD (Chesler and Kraig 1987) is consistent with accelerated astrocytic glycolysis, although other routes of H+ accumulation are possible. Some work has been done to show that supplementing astrocyte glycogen stores can delay the onset or propagation rate of SD in brain slices (Seidel and Shuttleworth 2011), but it is not yet known whether selectively increasing astrocyte glycogen content in vivo can limit metabolic depletion following SD in brain injury models.
While the glycolytic capacity of astrocytes is often emphasized, astrocyte oxidative metabolism also appears to significantly increase during SD and contribute to preservation of tissue viability. Studies of oxidative metabolism also have the advantage that pharmacological tools are available that can help distinguish between astrocytic and neuronal sources, by exploiting preferential uptake of substrates through membrane transporters. Thus increased astrocytic oxidative metabolism has been suggested from [14C]-butyrate studies made during SD in rats (Dienel et al. 2001). Similarly, selective inhibition of astrocyte TCA cycle activity can be accomplished with fluorocitrate (FC) or its precursor fluoroacetate (FAc), where selectivity for astrocytes over neurons is due to selective expression of monocarboxylate transporters (Clarke et al. 1970; Fonnum et al., 1997). In brain slices, exposure to FAc alone is sufficient to generate SD, implying that failure of astrocyte metabolism could be sufficient to trigger neuronal depolarization (Canals et al. 2008). In addition, the rate of depolarization in vivo is significantly increased by focal injection or dialysis of either FC or FAc (Largo et al. 1996; Largo et al. 1997; Lian and Stringer 2004a), a result which could be due to impairment in the ability of astrocytes to take up glutamate and/or K+ at the advancing SD wavefront (see below).
The α2 isoform of the Na+/K+-ATPase is expressed predominately by astrocytes in the adult brain (Cameron et al. 1994). Mutations in the gene encoding the α2 isoform (ATP1A2) underlie a form of familial hemiplegic migraine (FHM2) (De Fusco et al. 2003; de Vries et al. 2009), and FHM2 mutant mice show both increased propagation rate and reduced electrical threshold for SD in vivo (Leo et al. 2011). This increase in SD susceptibility could be the result of reduced astrocytic K+ clearance and/or enhanced glutamate accumulation.
Astrocyte metabolism also mitigates deleterious consequences of SD, under certain experimental conditions. Lian and Stringer (2004b) examined FC administration in anesthetized rats, with or without the additional challenge of SD. They demonstrated that SD alone was not sufficient to cause damage in healthy brain, consistent with many previous reports (e.g. (Nedergaard and Hansen 1988), but when brain tissue was pre-exposed to FC, experimentally-induced SD was followed by severe energy depletion and injury (Lian and Stringer 2004b).
Whether or not astrocytes also directly provide metabolic fuel for neurons during SD is an interesting question, with relevance for therapeutic approaches to limit SD damage. The concept of astrocyte-neuron lactate shuttling (Pellerin and Magistretti 2012) has been most extensively investigated under conditions more relevant to physiological neuronal activity, with less known of the relative contributions of metabolic substrate transfer under extreme conditions such as SD. In [14C]-glucose studies of SD in rat, it was concluded that SD results in significant efflux of lactate into venous blood, as well as diffusion throughout other brain regions (Cruz et al. 1999). It is possible that lactate production is disposed of as a byproduct of glycolytic metabolism during the extreme conditions of SD, but it remains to be determined whether astrocytic lactate production could be utilized by neurons, in different phases of SD recovery. In this context, the capacity of neurons to utilize oxidative fuels after SD may be an important limiting factor that may prevent utilization in injured tissues where oxygen supply is reduced due to inappropriate neurovascular coupling after SD (see below). In addition, imaging studies have used NADH autofluorescence as a marker of oxygen availability in brain following SD, and revealed that even in apparently “healthy” brain, oxygen availability (and hence oxidative metabolic capacity) may be quite heterogenous after SD (Takano et al. 2007).
5. Adenosine signaling
Adenosine accumulates in and around most cells as a consequence of extreme energy consumption, as ATP is progressively depleted in the intracellular space to the lower energy purine, and moves from its source to the extracellular space via equilibrative transporters. Adenosine accumulation is well established during brain ischemia, and usually is considered to be neuroprotective, acting by limiting brain activity in metabolically compromised tissues (Dunwiddie and Masino 2001). It was recently confirmed that SD can generate significant adenosine accumulation following SD in brain slices, even without metabolic substrate removal. The accumulated adenosine appeared to underlie the suppression of evoked synaptic activity that persists following SD, via A1 receptor activation (Lindquist and Shuttleworth 2012). When SD occurs on the backdrop of metabolic inhibition, adenosine accumulation is significantly enhanced, both in vitro and in vivo (Lindquist and Shuttleworth 2014). Neurons may be thought of as a more likely source of adenosine derived as a consequence of metabolic depletion, since neurons are more vulnerable to substrate removal (see above), (Parkinson et al. 2002), due to different metabolic demands of the two cell types. Although it was suggested that astrocytes could be a source of adenosine during mild hypoxia (Martin et al. 2007), subsequent work suggests that this may be unlikely, and that neurons probably are a better candidate (Fujita et al. 2012). However, the level of metabolic demand seen during SD, even in otherwise “healthy” brain tissue, may lead to much larger adenosine accumulations than observed during hypoxia alone, and the assumption that neurons rather than astrocytes are likely the major source of adenosine during metabolic compromise has not yet been tested in the case of SD under these different conditions.
Another source of extracellular adenosine is ATP released into the extracellular space, followed by breakdown by extracellular ATPases. Recent work has emphasized the possibility of vesicular ATP release from astrocytes, and based on results from animals deficient in regulated release from astrocytes, significant roles for adenosine accumulation have been proposed in a range of physiological processes (Blutstein and Haydon 2013; Halassa et al. 2009). Adenosine derived from astrocytic ATP release has been implicated in src kinase-dependent regulation of NMDA trafficking in neurons (Deng et al. 2011), and could potentially contribute to reduced ischemic lesion volumes in dnSNARE mice that lack vesicular release from astrocytes (Hines and Haydon 2013). However, this issue is controversial, as it has been argued that there is unlikely to be a significant contribution from ATP released by astrocytes, based on evidence that deleting extracellular conversion of AMP to adenosine does not reduce A1-receptor–dependent suppression of evoked synaptic activity (Lovatt et al. 2012). In the same study, it was shown that neuronal depolarization can be sufficient to generate A1 effects and deletion of the enzyme identified as responsible for AMP adenosine conversion (Nt5e) did not increase seizure expansion (Lovatt et al. 2012). While this suggests that neuronal adenosine accumulation may predominate during seizures or other evoked synaptic activity, it remains possible that after SD, adenosine release from astrocytes might also contribute. Under these conditions, accumulation as a consequence of metabolic depletion in astrocytes after SD may be the more likely source of adenosine.
Two reports suggest that adenosine accumulation protects brain tissue from deleterious consequences of SD. In one study FAc exposures led to release of adenosine from astrocyte cultures and suppressed synaptic activity in brain slices in an A1-receptor dependent manner (Canals et al. 2008). In addition, A1 receptor antagonism increased the number of spontaneous SDs generated by FAc, and impaired functional recovery of synaptic transmission. Together these findings suggest a protective role of adenosine, released as a consequence of severe compromise of astrocyte metabolism (Canals et al. 2008). More recently it was shown that short-term SD-preconditioning was mediated by A1 receptor activation. In neocortical slices, repetitive SDs (induced every 15 min by elevating extracellular K+) led to run-down in the membrane potential shifts and amplitude of neuronal Ca2+ transients associated with SD. In addition, anoxic depolarization induced by oxygen and glucose deprivation was significantly delayed following a single SD (Gniel and Martin 2013). An A1 receptor antagonist clearly reduced these effects, and block of equilibrative nucleoside transporters or ecto-5′-nucleotidase activity were both partially effective, suggesting that multiple sources of adenosine could be involved (Gniel and Martin 2013).
6. Astrocyte Ca2+ waves
Initial studies of Ca2+ waves in astrocyte cultures showed propagation rates similar to rates of SD in vivo, and astrocyte Ca2+ waves have been demonstrated during SD in brain slice and in vivo. Suggestions for a causative role in SD propagation were based on the effectiveness of gap junction blockers and the fact that astrocyte Ca2+ waves had been seen to precede the advancing SD wavefront (Basarsky et al. 1998; Kunkler and Kraig 1998; Nedergaard et al. 1995). However, subsequent studies showed that low concentrations of the same gap junction blockers resulted in an increased SD velocity, while higher concentrations (similar to those used in the initial studies (Nedergaard et al. 1995)) resulted in an immediate decline in the velocity and even complete block of SD (Martins-Ferreira and Ribeiro 1995). These observations suggested that, at high concentrations, these gap junction blockers were likely no longer acting solely on astrocyte-specific hemichannels (Martins-Ferreira and Ribeiro 1995). Additional studies have shown that SD can occur even when astrocyte Ca2+ waves are completely abolished, and astrocytic Ca2+ waves occur before changes in light transmittance (IOS) associated with SD (Basarsky et al. 1998) and spread further than both IOS signals and measurable DC-potential shifts (Peters et al. 2003). The observations that 1) the gap junction inhibitor carbenoxolone slowed the progression of astrocyte Ca2+ waves without affecting SD, and 2) that NMDA antagonism blocked SD, but astrocyte Ca2+waves persisted, albeit more slowly (Peters et al. 2003), further implies that astrocyte Ca2+ waves are not required for the progression of SD in brain slices. A pharmacological analysis of SDs generated by endothelin-1 application in vivo supports this view (Kleeberg et al. 2004), as do studies with KCl stimulation, showing that inhibition of astrocytic Ca2+ waves did not depress SD, and that the waves appeared in astrocytes after neuronal Ca2+ elevations (Chuquet et al. 2007). These findings appear consistent with conclusions that while prominent astrocyte Ca2+ waves are associated with SD, they are not responsible for generating the propagating depolarization under most conditions (Somjen 2001; Zhou et al. 2010).
The discussion above implies that astrocyte Ca2+ waves do not drive SD, and suggests that targeting these astrocytic events will not be useful to outright prevent SDs. However, the evidence accumulated from these studies provides strong support for robust Ca2+ signaling through astrocyte networks associated with SD, and implies that these waves could make substantial contributions to processes associated with tissue injury or survival following SD. It is also noted that a recent report showed that anesthesia suppressed Ca2+ signaling through astrocyte networks (Thrane et al. 2012), suggesting that in vivo effects of astrocyte Ca2+ waves on SD may be greatly underappreciated.
7. Neurovascular Coupling
Under normal (non-ischemic) conditions, the cerebral blood flow (CBF) changes in mouse associated with experimentally-induced SD usually occur in three phases: 1) a brief initial vasoconstriction (~10–20 seconds) coincident with the tissue depolarization, 2) a longer-lasting hyperemia (~1–2 min) which begins at the end of the tissue depolarization (marked by recovery of the negative DC shift) and peaks after repolarization, and 3) a slow-developing oligemia which can last for more than one hour following SD onset ((Ayata et al. 2004a; Busija et al. 2008; Lauritzen 1994; Piper et al. 1991) for review). The initial vasoconstrition is much less pronounced, and sometimes entirely absent, in other species including rat and cat (Ayata et al. 2004b; Piper et al. 1991). The long oligemia phase is present in all species and is associated with an impairment of the cerebrovascular reactivity to dilating stimuli such as hypercapnia, K+, autoregulation and somatosensory stimulation (Florence et al. 1994; Lacombe et al. 1992; Wahl et al. 1987). It is of note that in animals undergoing repetitive SDs, the perfusion response is attenuated, despite similar cortical depolarization (Brennan et al. 2007).
When visualized with methods that permit tracking of the two-dimensional spread of flow changes (e.g. laser speckle contrast imaging), SD is usually easily visualized as propagating waves of blood flow increases, that closely follow the spread of tissue depolarization (Ayata et al. 2004a; Dunn et al. 2001). Such increases in blood flow provide metabolic substrates needed to fuel recovery of ionic homeostasis, but even with effective vascular coupling, tissues may be close to the limit of their ability to recover following SD. Thus the long-lasting oligemia following SD is accompanied by increased CMRO2 and desaturation of cortical haemoglobin (Chang et al. 2010), leading to tissue hypoxia (Piilgaard and Lauritzen 2009). Indeed, when visualized at higher resolution, signs of hypoxia can be observed in tissues more distant to penetrating arterioles following SD, in otherwise healthy brain (Takano et al. 2007).
In the context of ischemic injuries, CBF responses to SD can be transformed, such that the initial vasoconstrictive phase becomes prolonged and the hyperemic phase is diminished (Dreier et al. 1998; Hoffmann et al. 2012). The resulting “spreading ischemia” that can be seen under these conditions is obviously a dangerous set of conditions, as it occurs just when brain tissue is experiencing unusually severe metabolic demands. The mechanism(s) underlying this conversion of CBF responses in injured tissue are not fully identified, but it is noted that reduced baseline perfusion further exacerbates hypoperfusion responses and limits hyperemia (Feuerstein et al. 2014; Hoffmann et al. 2012), and elevated extracellular K+ also leads to a more pronounced and prolonged CBF decrease (Dreier et al. 1998). In addition, reduction in the availability of the endogenous vasodilator nitric oxide (NO) can transform the normal hyperemic response to a pronounced hypoperfusion (Dreier et al. 1998). It has been suggested that scavenging of NO by hemoglobin may contribute to deficits following subarachnoid hemorrhage, as a result of spreading ischemia (Dreier et al., 1998). Exogenous application of adenosine can attenuate the hypoperfusion phase of SD recorded in the presence of nitric oxide synthase inhibition (Dreier et al. 2004), and may suggest approaches to target spreading ischemia.
Although experimental evidence is currently limited, it is reasonable to presume that astrocyte signaling contributes significantly to vascular responses following SD, and that astrocyte dysfunction could contribute to impaired neurovascular coupling in injured brain. Previous works have described in detail the evidence that astrocyte processes surround multiple synapses, and are connected either directly, or via astrocyte networks to smooth muscle cells (SMC) of intracerebral arterioles and endothelial cells of capillaries (Kacem et al. 1998; Mathiisen et al. 2010). In addition, astrocyte endfeet are enriched with aquaporin-4, connexin 43, purinergic receptors and K+ channels that confer on astrocytes the ability to link neuronal activity to regional cerebral blood flow, and contribute to the flow of signaling molecules to and from blood vessels (Petzold and Murthy 2011). From work in experimental systems without SD, it has been established that selectively increasing astrocytic intracellular Ca2+ induces Ca2+ waves that spread through astrocytic network leads to vasoconstriction only if Ca2+ increases reached astrocyte endfeet (Mulligan and MacVicar 2004). The polarity of vascular responses after astrocyte Ca2+ increases was subsequently shown to depend on the metabolic state of brain slices, with oxygen depletion leading to a PGE2-mediated vasodilation, rather than a vasoconstriction (Gordon et al. 2008).
As discussed above, SD is closely associated with prominent waves of intracellular Ca2+ increase through astrocyte networks (Chuquet et al. 2007; Peters et al. 2003), and initial vasoconstrictive responses during SD can be abolished with inhibition of internal Ca2+ release in astrocytes (Chuquet et al. 2007). Vasoconstriction is mediated by release of arachidonic acid (AA) from astrocytes, which enters SMC and is converted to 20-hydroxyeicosatetraenoic acid (20-HETE), while there is evidence that vasodilation is induced by activation of astrocytic metabotropic glutamate receptors and either cyclooxygenase products or combined activation of K+ channels on astrocytes and SMC (Mulligan and MacVicar 2004; Petzold and Murthy 2011). A recent report shows that 20-HETE is generated by SD, with a time course that approximates the long-lasting oligemia following SD. Furthermore, an inhibitor of 20-HETE synthesis reduced CBF decreases following SD, suggesting the utility of such agents to improve blood flow, particularly following SDs in injured brain (Fordsmann et al. 2013). It will be of interest to determine whether preventing astrocyte Ca2+ waves influences 20-HETE production associated with SD.
K+ release from astrocyte networks via inward rectifying K+ (Kir) channels has also been discussed as a potential mechanism for neurovascular coupling (Kofuji and Newman 2004), although following synaptic stimulation is appears that “K+ siphoning” – redistribution of K+ from areas of neuronal activity through astrocyte networks to the vasculature - does not contribute significantly to local vasodilation (Attwell et al. 2010). However, it remains to be determined whether K+ siphoning through astrocytes might play a more significant role in the special situation of SD, where large volumes of tissue are coordinately depolarized and K+ fluxes are much larger (60–70 mM) than in the case of focal electrical or reflex stimulation (5–10 mM). In addition to the possible involvement of Kir channels, an alternative mechanism linked to the opening of large-conductance Ca2+-activated K+ (BK) channels on astrocyte endfeet, releasing K+ onto vessels (Filosa et al. 2006) could also participate.
A recent report suggests a link between enhanced astrocyte Ca2+ signaling and enhanced vasodilation in a form of familial migraine linked to mutation in casein kinase Iδ (Brennan et al. 2013). In mice carrying the mutation, SD threshold is reduced, and arterial dilation is enhanced. Interestingly, astrocytes from these mice show enhanced spontaneous Ca2+ oscillations waves, possibly contributing to decreased SD threshold and increased vasodilation (Brennan et al. 2013).
8. Extracellular K+ and swelling
Large extracellular K+ elevations are a cardinal sign of SD, and peak extracellular concentrations in the range of 60–70 mM are reliably recorded during SD in vivo (Somjen 2001). When tested in anesthetized rats, selective inhibition of astrocyte metabolism with FAc or FC significantly slowed recovery of extracellular K+ after SD (Lian and Stringer 2004a). The lack of effect of Kir channel antagonist barium, together with the size of the effects seen with selective inhibition of astrocyte metabolism, was taken to suggest Na+/K+-ATPase activity as the predominant form of extracellular K+ clearance following SD (Lian and Stringer 2004a). As noted above, the α2 isoform of the Na+/K+-ATPase is expressed predominately by astrocytes in the adult brain (Cameron et al. 1994). This isoform has a higher Km than that expressed on neurons, and is saturated at extracellular K+ levels (see (Brown and Ransom 2014) review and original article (Verkhratsky and Butt 2007)) that are very similar to the threshold K+ concentrations required to initiate SD (10–15 mM, see Somjen 2001).
Passive uptake through K+ channels expressed on astrocytes may also be important for setting the threshold for SD initiation (Hoffmann 1992; Walz 2000). The relative contribution of these mechanisms under the special circumstances of SD is an interesting topic, with implications for targeting approaches to limit the onset of SD in pathophysiological conditions. While much study of astrocytic K+ buffering has been done with synaptic stimulation (Amedee et al. 1997; Kofuji and Newman 2004), the situation with SD may be quite different, in part because a large volume of tissue becomes simultaneously involved. This may limit the ability of mechanisms such as spatial buffering to meaningfully modify extracellular K+, either during or after the passage of SD. It has been pointed out that astrocytic K+ buffering/siphoning is not currently an attractive target for prevention of post-ischemic SD waves, in part because of the lack of astrocyte-specific targets (Kimelberg and Nedergaard 2010) and additional work on this topic could be very helpful.
Cellular swelling was recognized in the initial work of Leao (1944), and contributes to optical changes that permit the propagation of SD to be quite easily visualized, even by eye in some preparations (Somjen 2001). Neurons swell rapidly at the onset of SD (Takano et al. 2007; Zhou et al. 2010), and astrocytes also swell following SD, due mainly to K+ uptake and the water movement that follows Cl− uptake. Expression of AQP4 gives astrocytes the ability to readily swell in response to hypoosmotic challenges (Andrew et al. 2007; Risher et al. 2009), and also appears to contribute to K+ uptake during SD. Studies using a K+-sensing fluorophore found that deletion of AQP4 led to impaired K+ uptake following SD, suggesting a role for these channels in passive K+ uptake (Padmawar et al. 2005). AQP4 knockouts were demonstrated to show significant protection against ischemia (Manley et al. 2000), and it will be interesting to determine whether this is directly related to decreased astrocyte swelling with peri-infarct SDs.
As noted above, two-photon imaging of astrocyte volume reveal that astrocytes swell transiently following SD (Zhou et al. 2010), and under normoxic conditions astrocytes recover their normal volume relatively quickly. In contrast, in a rodent stroke model it has been shown that the astrocyte swelling can long-outlast neuronal swelling responses (Risher et al. 2012). Interestingly, long-lasting swelling was associated with prolonged DC potential shifts that indicate metabolically compromised tissues, and in addition a stepwise increase in astrocyte swelling was discovered during clusters of SDs, which may be relevant to the deleterious consequences of SD clusters in injured brain (Risher et al. 2012). A similar phenomenon of sustained astrocyte swelling was also described recently with a rodent TBI model (Sword et al. 2013). There are a number of mechanisms by which astrocyte swelling could contribute to injury, including vascular compression and subsequent ischemia. Indeed, persistent astrocyte swelling has been suggested to contribute to demonstrable lack of capillary flow even in apparently uninjured brain (Tomita et al. 2003), and with stepwise increases in astrocyte volume following SD clusters in injured brain, vascular compression could significantly contribute to the mismatch in supply versus demand. Activation of Ca2+ signaling or release of neurotoxic factors from astrocyte networks have also been considered as links between persistent astrocyte swelling and neuronal injury (Risher et al. 2012). Persistent astrocytic swelling will also effectively reduce the volume of the extracellular space which will likely increase tissue susceptibility to SD since threshold concentrations of both K+ and glutamate will be more readily reached.
9. Extracellular glutamate levels and toxicity
It is well known that extracellular glutamate levels increase substantially during SD, and NMDA-type glutamate receptor antagonists can be effective at limiting SD initiation or propagation, especially under normoxic conditions (Pietrobon and Moskowitz 2014; Somjen 2001). Beneficial effects of NMDA antagonism in stroke models may be by limiting spread of SDs into less hypoxic areas, more distant from initial ischemic cores, and/or by preventing deleterious consequences of glutamate release onto metabolically compromised neurons. Ketamine, an NMDA antagonist was recently shown to effectively inhibit SD in swine cortex (Sanchez-Porras et al. 2014). In clinical studies, ketamine has been suggested to reduce SD incidence (Sakowitz et al. 2009), and a retrospective case review showed reduced numbers of SDs in patients administered ketamine in the intensive care unit (Hertle et al. 2012).
Astrocytes could serve as a significant source of glutamate during SD (see (Malarkey and Parpura 2008) for review), and glutamate release from astrocytes could conceivably contribute significantly to the progression, or deleterious consequences of SD in injury conditions. Volume-activated Cl− channels are one potential route of glutamate release from astrocytes during SD (Basarsky et al. 1999). As discussed above, astrocytes swell during SD and this appears to be sufficient to activate these channels. Thus extracellular glutamate increases during SD in brain slices were recorded in the absence of neuronal synaptic release, and prevented by a blocker of volume-activated Cl− channels (see discussion in (Kimelberg et al. 2006)). Blockers of volume-activated Cl− channels could be particularly interesting to test in injury models where astrocyte swelling is prominent during SD. Vesicular release is a second possible route of astrocytic glutamate release during SD, although it is noted that the mechanism(s) of astrocytic glutamate release is a debated issue (Araque et al. 2014; Nedergaard and Verkhratsky 2012). Evidence using animals with an astrocyte-specific dominant-negative SNARE (dnSNARE) that limits vesicular release of glutamate (and potentially ATP) shows ischemic injuries were reduced and behavioral recovery was enhanced (Hines and Haydon 2013). However, a recent report has challenged the astrocytic specificity of dnSNARE in these transgenic mice (Fujita et al. 2014), therefore a definitive conclusion about the contribution of gliotransmitters in SD it still awaited.
Reversal of astrocytic glutamate transporters could also contribute to glutamate accumulation, and targeting of these transporters could be considered as an approach to limit glutamate excitotoxicity associated with SD. The importance of astrocyte function for removal of synaptically-released glutamate is well established, and involves GLAST (EAAT1) and GLT1 (EAAT2) (Anderson and Swanson 2000; Danbolt 2001; Swanson et al. 2004). Transporter-mediated glutamate uptake is driven by Na+ co-transport, which in turn requires maintenance of astrocytic metabolism for Na+/K+-ATPase activity to maintain Na+ gradients. It is not yet clear to what extent astrocytic (and neuronal) glutamate transport may reverse during SD, when intracellular Na+ loading is severe and coupled with metabolic depletion (see also section 11).
Agents that increase expression of astrocytic glutamate transporters have been identified (e.g. ceftriaxone), and shown to be effective at reducing infarct volumes following ischemic challenges (Rothstein et al. 2005). In brain slice studies, ceftriaxone exposures resulted in increased glutamate transport activity, and provided some protection against the onset of SD triggered by ischemic-type stimuli (Lipski et al. 2007), although the mechanism for increased transport was not clear, and possibly not due to increased surface expression of the transporter.
Inhibition of astrocyte glutamate uptake has been demonstrated by exposures to Zn2+ (Suh et al. 2007), which is of interest because significant Zn2+ release has been demonstrated during SD (Carter et al. 2011). It is not yet known how much Zn2+ accumulation occurs in astrocytes following SD, and whether this may be sufficient to compromise normal glutamate uptake in vivo. However such an action on astrocyte function could potentially contribute to beneficial effects of Zn2+ chelation following stroke (Koh et al. 1996), in addition to the many other deleterious actions that Zn2+ accumulation could have following SD (Shuttleworth and Weiss 2011).
It is also possible that astrocytes may facilitate NMDAR activation during the late phase of SD, which from brain slice studies appears to be a major contributor to neuronal vulnerability in metabolically-compromised tissues (Aiba and Shuttleworth 2012), as a consequence of D-serine released by astrocyte Ca2+ waves. D-serine serves as a co-agonist with glutamate, and is required for activation of NMDA receptors (Mothet et al. 2005). D-serine was originally thought to be produced and secreted only by astrocytes because of the selective expression of the enzyme serine racemate (Wolosker et al. 1999). However recent data suggest that neuronal-derived D-serine could also contribute to the activity of NMDA receptors (Benneyworth et al. 2012; Rosenberg et al. 2013) see (Martineau et al. 2014) for review). It seems possible that both cell types contribute to D-serine increases that permit NMDA-dependent toxicity following SD in injurious conditions.
10. Changes in Astrocyte Phenotype
In the setting of acute brain injury, SDs may persist over several hours and even days (Dreier 2011; Lauritzen et al. 2011). During this time frame, astrocytes may undergo profound transcriptional, morphological and functional alterations that change them into “reactive astrocytes”. These phenotypic changes could occur as a consequence of multiple components of ischemia or neuronal injury cascades, or perhaps also as a consequence of the occurrence of SDs themselves. Regardless of their etiology, reactive astrocyte phenotypes are generally characterized by hypertrophy of processes and overexpression of intermediate filaments such as GFAP and vimentin (Burda and Sofroniew 2014). What is less appreciated is the fact that the functions of reactive astrocytes are also modified. It has been long debated whether reactive astrocytes are harmful or beneficial for disease outcomes since both types of effects have been observed in a context-dependent manner (Escartin and Bonvento 2008). Indeed, astrocyte reactivity is a highly heterogeneous state that depends on the type of inducing injury. A recent transcriptomic analysis reveals that reactive astrocytes undergo extensive, but transient, alterations in gene expression (>1000 genes) that depends on the injury models (Zamanian et al. 2012).
SD by itself can trigger astrocyte reactivity. SD induces an upregulation in GFAP mRNA and protein levels in vivo in the absence of tissue injury (Bonthius et al. 1995; Kraig et al. 1991). The spatial distribution of GFAP up-regulation matches the observed spatial pattern of SD and signs of reactivity were not observed in animals where SD propagation was prevented by the NMDAR antagonist MK801. SD triggered by a single application of 3M KCl for 10 min in rats also increases the expression of nestin, an intermediate filament protein expressed in the CNS and a sensitive marker for astrocyte reactivity (Holmin et al. 2001). Single, daily SDs induced for 1 or 2 weeks in mouse frontal cortex also led to marked increases in GFAP expression without neuronal injury throughout the conditioned cortex (Sukhotinsky et al. 2011).
What are the molecular triggers of the astrocyte reactivity during SD? Many different types of signaling molecules are able to initiate or to regulate specific aspects of reactive astrogliosis (Kang and Hebert 2011; Sofroniew and Vinters 2010). In organotypic slices (Kunkler et al. 2004) and in the rat brain (Jander et al. 2001), SD has been shown to induce the release of multiple pro-inflammatory cytokines such as interleukin 1alpha (IL-1α), interleukin 1beta (IL-1β) and tumor necrosis factor alpha (TNFα), that are all potent triggers of astrocyte reactivity (Kang and Hebert 2011). Karatas et al. recently described a molecular pathway whereby SD could lead to astrocyte reactivity (Karatas et al. 2013). SD induces Pannexin 1 megachannel opening in neurons, inducing the release of IL-1β and high-mobility group box 1 (HMGB1), two pro-inflammatory proteins that induce nuclear factor kappa B (NF-KB) translocation in neighboring astrocytes after 30 minutes (Karatas et al. 2013). The NF-KB pathway is a well-known trigger of astrocyte reactivity (Brambilla et al. 2009), and indeed, those astrocytes overexpress inducible NO synthase and cyclooxygenase 2, also indexes of reactivity. Additional signaling pathways could be involved in astrocyte reactivity following chronic SD such as signal transducer and activator of transcription-3 (STAT3) and mitogen-activated protein kinase (MAPK) (Kang and Hebert, 2011). Finally, excessive release of ATP and glutamate during SD may also activate purinergic and metabotropic glutamate receptors in astrocytes and transduce an activation cascade (Sofroniew and Vinters 2010). It will be very important to identify the molecules responsible for astrocyte reactivity during SD, as the functional changes they trigger may be different depending on the signaling pathway involved.
11. Functional consequences of astrocyte reactivity during chronic repetitive SD
Astrocytes participate in almost all of the events associated with SD (see previous sections). However, it is not yet known how these cells, once they become reactive, impact the incidence of SD or its deleterious consequences in injured brain. Swelling after SD was slower in GFAP−/− astrocytes than in wild-type, suggesting a role for these intermediate filaments in the swelling response during SD (Anderova et al. 2001), but it is not yet known whether increased GFAP expression in reactive astrocytes modifies SD-induced swelling. Interestingly, transcriptomic analysis of genes induced by ischemia strongly suggests that ischemic reactive astrocytes may be protective since they overexpress high levels of neurotrophic factors and cytokines, including cardiotrophin-like cytokine factor 1 (CLCF1), leukemia inhibitory factor (LIF), and interleukin 6 (IL6), and thrombospondins that may help repair and rebuild lost synapses (Zamanian et al. 2012). We will review specific and important functions regulated by astrocytes that are changed when they become reactive, which could directly influence the outcomes of repetitive SDs. It has been shown in a variety of models that reactive astrocytes display changes in glutamate uptake capacities. For example, the expression of glutamate transporters is decreased in reactive astrocytes after repetitive SD (Douen et al. 2000). This alteration in astrocyte glutamate uptake could exacerbate excitotoxic processes occurring during chronic SD and contribute to neurodegeneration. However, glutamate transporters have been suggested to operate in a reverse mode under severe pathological conditions such as ischemia, when astrocyte membrane potential and ionic homeostasis are disrupted (Rossi et al. 2000). In this case, decreased expression of glutamate transporters may actually reduce glutamate efflux during subsequent ischemia, and reduce SD propagation and neuronal damage as suggested by Douen and colleagues (Douen et al. 2000).
Importantly, glutamate uptake efficacy is not only regulated by transcription of astrocyte transporters but also by post-translational mechanisms. Astrocytes chronically activated with the cytokine ciliary neurotrophic factor (CNTF) in the rat striatum display no change in the total expression level of glutamate transporters, but rather a hyper-glycosylation and enrichment of transporters in functional raft domains (Escartin et al. 2006) where glutamate transport is known to be more efficient (Butchbach et al. 2004). These post-translational changes are associated with an enhanced buffering of excitotoxic concentrations of glutamate (Escartin et al. 2006) and produce significant neuroprotective effects (Beurrier et al. 2010). Changes in astrocyte morphology may also indirectly influence glutamate uptake. Indeed, altering the distance of glutamate transporters from synaptic sites of glutamate release influences the efficacy of glutamate removal, as shown in the hypothalamus of lactating rats (Oliet et al. 2001) and in the hippocampus of knock-out mice for the astrocyte protein connexin 30 (Pannasch et al. 2014). These morphological changes may occur acutely during SD-induced swelling (Risher et al. 2009; Risher et al. 2012) or more chronically when astrocytes become reactive. Such chronic changes involve up-regulation of intermediate filaments, enlargement of proximal processes and a complex reorganization of processes (Oberheim et al. 2008; Wilhelmsson et al. 2006) while the astrocytic domain (i.e. the 3D maximal volume contacted by astrocytes) remains grossly unaffected, at least in models of axotomy or electrical lesions (Wilhelmsson et al. 2006). Therefore, the fine morphological organization of the tripartite synapse could be profoundly changed when astrocytes are reactive, but any direct demonstration that such changes influence glutamate buffering and transmission during chronic SD is currently lacking.
The network of astrocytes connected by gap junction channels plays a central role in K+ and glutamate buffering capacity (Pannasch et al. 2011; Steinhauser et al. 2012). How could the reactive status of astrocytes affect network properties and influence the late phases of chronic SD? Both increased and decreased levels of expression and permeability of connexins, the molecular components of the gap junction channels, have been described in inflammatory and pathological conditions involving reactive astrocytes (Giaume et al. 2010). It is therefore difficult to predict how networks of reactive astrocytes function during chronic SD. Most importantly, in addition to its role in K+ and glutamate buffering, astrocyte networks participate in the distribution of metabolic substrates from capillaries to distal active neurons (Rouach et al. 2008). This activity-dependent trafficking of energy metabolites sustains active neurotransmission and could be changed when astrocytes are reactive (Escartin and Rouach 2013). It would be important to test this hypothesis using dye or glucose-analogue coupling experiments in situ. Metabolic plasticity of reactive astrocytes represents an understudied field of research. Data from in vitro models of reactive astrocytes induced by pro-inflammatory cytokines show that they display a different metabolic profile (Belanger et al. 2011). In an in vivo model, reactive astrocytes were shown to become more oxidative with a reduction in the rate of glycolysis at the expense of the oxidation of fatty acids or ketone bodies (Escartin et al. 2007). However, it remains to be determined whether such metabolic plasticity occurs during SD. In particular, it is not yet known whether glycogen content and use or lactate release could be altered during chronic SD. Similarly, the extensive literature implying that astrocytes are prominent actors regulating neurovascular coupling (Petzold and Murthy 2011) does not address the issue as to whether acutely or chronically reactive astrocytes perform differently to regulate blood flow.
We have recently provided evidence that reactive astrocytes may have beneficial effects against SD. Activation of astrocytes with chronic exposure to CNTF in vivo led to substantial increases in SD threshold, when tested subsequently in brain slice preparations (Seidel et al. 2014). Increased K+ clearance by CNTF-activated astrocytes was implicated as a possible mechanism, and it remains to be determined whether this or other consequences of astrocyte reactivity may contribute to increased SD thresholds following chronic SD in vivo. Furthermore, these findings raise the possibility that enhancing specific components of astrocyte reactivity could be useful to limit or even prevent the incidence of SD associated with brain disorders and injury.
12. Perspectives
The SD field has expanded significantly in recent years, in part due to the demonstration that SD is a general phenomenon associated with multiple disease conditions in patients. Of particular significance is the fact that SDs can occur for many days following a stroke or traumatic brain injury, opening up a wide time window of opportunity for interventions that can effectively limit the numbers of SD, or their consequences. There are a handful of clinical reports suggesting that agents that target neuronal excitability or receptors (e.g. ketamine) could be a promising therapy, but evidence for efficacy is very limited. Selective targeting of astrocyte function in this extended time window could be valuable in limiting the initiation of SD (by increasing removal of K+ and/or glutamate), preventing astrocytic swelling, or by limiting possible contributions of astrocytes to the progression of NMDA-dependent neuronal injury.
There is a large (and growing) body of literature describing roles of astrocytes in regulated synaptic transmission, and there is a temptation to extrapolate from this work to deduce likely roles of astrocytes in regulating SD. However this should be done with significant caution. The total durations of depolarization associated with SD are extraordinarily long when compared with action-potential dependent synaptic events, including burst firing during seizures. The ionic loading and metabolic burden of SD is extreme and involves large volumes of tissue, and this will likely result in reversal of transporters, recruitment of alternative metabolic pathways and changes in distribution of ions and other signaling molecules through astrocyte networks. Thus further identification of specific contributions of astrocytes to SD will benefit from the application of new approaches to selectively modify aspects of astrocyte function in SD models.
The morphological changes associated with conversion of astrocytes to a reactive phenotype are widely appreciated, but there is less understanding of the functional changes that occur in parallel. Some of these functional changes could contribute to significant alterations in the ability to generate SD. There is a significant need to characterize the functions of reactive astrocytes in situ, including detailed analysis of changes in astrocytic regulation of cerebral blood flow, K+ homeostasis, and edema. Ideally, such studies should compare different experimental approaches for astrocyte activation, since each may have specific consequences on astrocytic function. Such work is expected to lead to insights into specific signaling pathways that could be used to selectively enhance protective functions of astrocytes against SD, ideally in a way that is reversible and could be applied transiently after the onset of an acute injury.
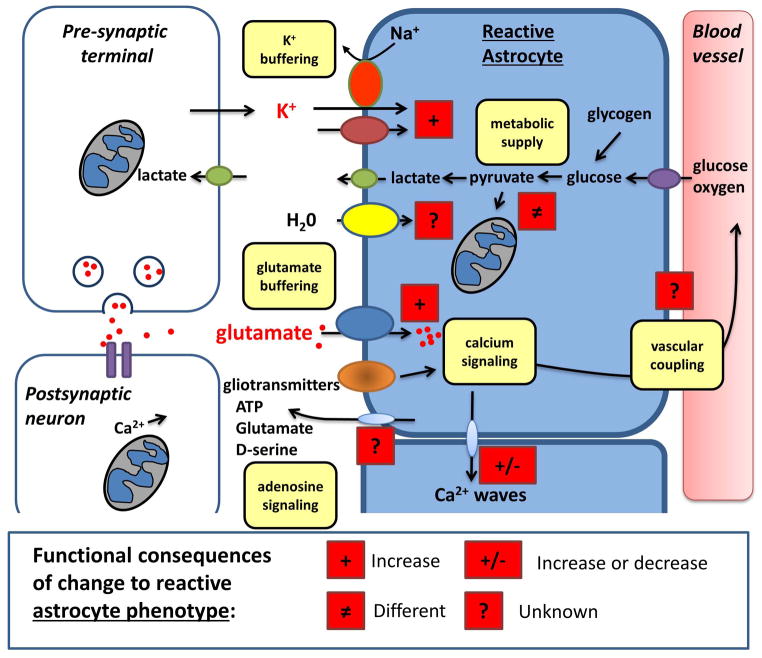
Figure 2. Modifications of function in reactive astrocytes.
Following conversion to a reactive phenotype, the functional properties of astrocytes can be significantly modified in ways that are relevant to SD and its deleterious consequences. The symbols used in this figure follow the key provided in Figure 1, and possible differences from functions in Figure 1 are highlighted with red boxes. Buffering of extracellular K+ may be increased sufficiently to raise the threshold for SD initiation or propagation. Likewise, increased accumulation of glutamate from the extracellular space may limit the spread of SDs, as well as reducing the likelihood of NMDAR-dependent toxicity. Reactive astrocytes undergo modifications in energy metabolism, which could render them more resistant to the metabolic challenges of SD in the context of various brain injuries. There is currently a notable lack of information on how changing to a reactive phenotype modifies other key astrocyte functions related to SD. For example, it is not known whether astrocyte Ca2+ waves are modified, and whether this could be significant for the critical issue of disrupted neurovascular coupling that accompanies SD in injured brain tissue. There is also a lack of information on the influence on cell swelling mechanisms, ionic homeostasis, and possible release of gliotransmitters. Some of these modifications seen in reactive astrocytes could be targeted to reduce the number of SDs and/or reduce their deleterious consequences in injured brain.
Acknowledgments
Supported by NIH grant NS051288 (C.W.S) & Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA; http://www.cea.fr/) and Centre National de la Recherche Scientifique (CNRS; http://www.cnrs.fr/) (G.B.).
References
- Aiba I, Shuttleworth CW. Sustained NMDA receptor activation by spreading depolarizations can initiate excitotoxic injury in metabolically compromised neurons. J Physiol. 2012;590(Pt 22):5877–93. doi: 10.1113/jphysiol.2012.234476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amedee T, Robert A, Coles JA. Potassium homeostasis and glial energy metabolism. Glia. 1997;21(1):46–55. doi: 10.1002/(sici)1098-1136(199709)21:1<46::aid-glia5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Anderova M, Kubinova S, Mazel T, Chvatal A, Eliasson C, Pekny M, Sykova E. Effect of elevated K(+), hypotonic stress, and cortical spreading depression on astrocyte swelling in GFAP-deficient mice. Glia. 2001;35(3):189–203. doi: 10.1002/glia.1084. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32(1):1–14. [PubMed] [Google Scholar]
- Andrew RD, Labron MW, Boehnke SE, Carnduff L, Kirov SA. Physiological evidence that pyramidal neurons lack functional water channels. Cereb Cortex. 2007;17(4):787–802. doi: 10.1093/cercor/bhk032. [DOI] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81(4):728–39. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468(7321):232–43. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayata C. Cortical spreading depression triggers migraine attack: pro. Headache. 2010;50(4):725–30. doi: 10.1111/j.1526-4610.2010.01647.x. [DOI] [PubMed] [Google Scholar]
- Ayata C, Dunn AK, Gursoy OY, Huang Z, Boas DA, Moskowitz MA. Laser speckle flowmetry for the study of cerebrovascular physiology in normal and ischemic mouse cortex. J Cereb Blood Flow Metab. 2004a;24(7):744–55. doi: 10.1097/01.WCB.0000122745.72175.D5. [DOI] [PubMed] [Google Scholar]
- Ayata C, Shin HK, Salomone S, Ozdemir-Gursoy Y, Boas DA, Dunn AK, Moskowitz MA. Pronounced hypoperfusion during spreading depression in mouse cortex. J Cereb Blood Flow Metab. 2004b;24(10):1172–82. doi: 10.1097/01.WCB.0000137057.92786.F3. [DOI] [PubMed] [Google Scholar]
- Basarsky TA, Duffy SN, Andrew RD, MacVicar BA. Imaging spreading depression and associated intracellular calcium waves in brain slices. J Neurosci. 1998;18(18):7189–99. doi: 10.1523/JNEUROSCI.18-18-07189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basarsky TA, Feighan D, MacVicar BA. Glutamate release through volume-activated channels during spreading depression. J Neurosci. 1999;19(15):6439–45. doi: 10.1523/JNEUROSCI.19-15-06439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger M, Allaman I, Magistretti PJ. Differential effects of pro- and anti-inflammatory cytokines alone or in combinations on the metabolic profile of astrocytes. J Neurochem. 2011;116(4):564–76. doi: 10.1111/j.1471-4159.2010.07135.x. [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Li Y, Basu AC, Bolshakov VY, Coyle JT. Cell selective conditional null mutations of serine racemase demonstrate a predominate localization in cortical glutamatergic neurons. Cell Mol Neurobiol. 2012;32(4):613–24. doi: 10.1007/s10571-012-9808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurrier C, Faideau M, Bennouar KE, Escartin C, Kerkerian-Le Goff L, Bonvento G, Gubellini P. Ciliary neurotrophic factor protects striatal neurons against excitotoxicity by enhancing glial glutamate uptake. PLoS One. 2010;5(1):e8550. doi: 10.1371/journal.pone.0008550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blutstein T, Haydon PG. The Importance of astrocyte-derived purines in the modulation of sleep. Glia. 2013;61(2):129–39. doi: 10.1002/glia.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius DJ, Lothman EW, Steward O. The role of extracellular ionic changes in upregulating the mRNA for glial fibrillary acidic protein following spreading depression. Brain Res. 1995;674(2):314–28. doi: 10.1016/0006-8993(95)00035-o. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G, Ivanov D, Nathanson L, Barnum SR, Bethea JR. Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol. 2009;182(5):2628–40. doi: 10.4049/jimmunol.0802954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan KC, Bates EA, Shapiro RE, Zyuzin J, Hallows WC, Huang Y, Lee HY, Jones CR, Fu YH, Charles AC, et al. Casein kinase idelta mutations in familial migraine and advanced sleep phase. Science translational medicine. 2013;5(183):183ra56, 1–11. doi: 10.1126/scitranslmed.3005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan KC, Beltran-Parrazal L, Lopez-Valdes HE, Theriot J, Toga AW, Charles AC. Distinct vascular conduction with cortical spreading depression. J Neurophysiol. 2007;97(6):4143–51. doi: 10.1152/jn.00028.2007. [DOI] [PubMed] [Google Scholar]
- Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55(12):1263–71. doi: 10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- Brown AM, Ransom BR. Astrocyte glycogen as an emergency fuel under conditions of glucose deprivation or intense neural activity. Metab Brain Dis. 2014 doi: 10.1007/s11011-014-9588-2. [DOI] [PubMed] [Google Scholar]
- Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81(2):229–48. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bures J. Some metabolic aspects of Leao’s spreading depression. J Neurochem. 1956;1(2):153–8. doi: 10.1111/j.1471-4159.1956.tb12067.x. [DOI] [PubMed] [Google Scholar]
- Busija DW, Bari F, Domoki F, Horiguchi T, Shimizu K. Mechanisms involved in the cerebrovascular dilator effects of cortical spreading depression. Prog Neurobiol. 2008;86(4):379–95. doi: 10.1016/j.pneurobio.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchbach ME, Tian G, Guo H, Lin CL. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: importance for excitatory amino acid transporter localization and function. J Biol Chem. 2004;279(33):34388–96. doi: 10.1074/jbc.M403938200. [DOI] [PubMed] [Google Scholar]
- Cameron R, Klein L, Shyjan AW, Rakic P, Levenson R. Neurons and astroglia express distinct subsets of Na, K-ATPase alpha and beta subunits. Brain Res Mol Brain Res. 1994;21(3–4):333–43. doi: 10.1016/0169-328x(94)90264-x. [DOI] [PubMed] [Google Scholar]
- Canals S, Larrosa B, Pintor J, Mena MA, Herreras O. Metabolic challenge to glia activates an adenosine-mediated safety mechanism that promotes neuronal survival by delaying the onset of spreading depression waves. J Cereb Blood Flow Metab. 2008 doi: 10.1038/jcbfm.2008.71. [DOI] [PubMed] [Google Scholar]
- Carter RE, Aiba I, Dietz RM, Sheline CT, Shuttleworth CW. Spreading depression and related events are significant sources of neuronal Zn2+ release and accumulation. J Cereb Blood Flow Metab. 2011;31(4):1073–84. doi: 10.1038/jcbfm.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JC, Shook LL, Biag J, Nguyen EN, Toga AW, Charles AC, Brennan KC. Biphasic direct current shift, haemoglobin desaturation and neurovascular uncoupling in cortical spreading depression. Brain. 2010;133(Pt 4):996–1012. doi: 10.1093/brain/awp338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles A, Brennan K. Cortical spreading depression-new insights and persistent questions. Cephalalgia. 2009;29(10):1115–24. doi: 10.1111/j.1468-2982.2009.01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AC, Baca SM. Cortical spreading depression and migraine. Nature reviews Neurology. 2013;9(11):637–44. doi: 10.1038/nrneurol.2013.192. [DOI] [PubMed] [Google Scholar]
- Chesler M, Kraig RP. Intracellular pH of astrocytes increases rapidly with cortical stimulation. Am J Physiol. 1987;253(4 Pt 2):R666–70. doi: 10.1152/ajpregu.1987.253.4.R666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuquet J, Hollender L, Nimchinsky EA. High-resolution in vivo imaging of the neurovascular unit during spreading depression. J Neurosci. 2007;27(15):4036–44. doi: 10.1523/JNEUROSCI.0721-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DD, Nicklas WJ, Berl S. Tricarboxylic acid-cycle metabolism in brain. Effect of fluoroacetate and fluorocitrate on the labelling of glutamate, aspartate, glutamine and gamma-aminobutyrate. Biochem J. 1970;120(2):345–51. doi: 10.1042/bj1200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz NF, Adachi K, Dienel GA. Rapid efflux of lactate from cerebral cortex during K+-induced spreading cortical depression. J Cereb Blood Flow Metab. 1999;19(4):380–92. doi: 10.1097/00004647-199904000-00004. [DOI] [PubMed] [Google Scholar]
- Csiba L, Paschen W, Mies G. Regional changes in tissue pH and glucose content during cortical spreading depression in rat brain. Brain Res. 1985;336(1):167–70. doi: 10.1016/0006-8993(85)90430-5. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- De Fusco M, Marconi R, Silvestri L, Atorino L, Rampoldi L, Morgante L, Ballabio A, Aridon P, Casari G. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat Genet. 2003;33(2):192–6. doi: 10.1038/ng1081. [DOI] [PubMed] [Google Scholar]
- de Vries B, Frants RR, Ferrari MD, van den Maagdenberg AM. Molecular genetics of migraine. Human genetics. 2009;126(1):115–32. doi: 10.1007/s00439-009-0684-z. [DOI] [PubMed] [Google Scholar]
- Deng Q, Terunuma M, Fellin T, Moss SJ, Haydon PG. Astrocytic activation of A1 receptors regulates the surface expression of NMDA receptors through a Src kinase dependent pathway. Glia. 2011;59(7):1084–93. doi: 10.1002/glia.21181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Liu K, Cruz NF. Local uptake of (14)C-labeled acetate and butyrate in rat brain in vivo during spreading cortical depression. J Neurosci Res. 2001;66(5):812–20. doi: 10.1002/jnr.10063. [DOI] [PubMed] [Google Scholar]
- Douen AG, Akiyama K, Hogan MJ, Wang F, Dong L, Chow AK, Hakim A. Preconditioning with cortical spreading depression decreases intraischemic cerebral glutamate levels and down-regulates excitatory amino acid transporters EAAT1 and EAAT2 from rat cerebal cortex plasma membranes. J Neurochem. 2000;75(2):812–8. doi: 10.1046/j.1471-4159.2000.0750812.x. [DOI] [PubMed] [Google Scholar]
- Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17(4):439–47. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Isele T, Reiffurth C, Offenhauser N, Kirov SA, Dahlem MA, Herreras O. Is spreading depolarization characterized by an abrupt, massive release of gibbs free energy from the human brain cortex? Neuroscientist. 2013;19(1):25–42. doi: 10.1177/1073858412453340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Korner K, Ebert N, Gorner A, Rubin I, Back T, Lindauer U, Wolf T, Villringer A, Einhaupl KM, et al. Nitric oxide scavenging by hemoglobin or nitric oxide synthase inhibition by N-nitro-L-arginine induces cortical spreading ischemia when K+ is increased in the subarachnoid space. J Cereb Blood Flow Metab. 1998;18(9):978–90. doi: 10.1097/00004647-199809000-00007. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Tille K, Dirnagl U. Partial antagonistic effect of adenosine on inverse coupling between spreading neuronal activation and cerebral blood flow in rats. Neurocrit Care. 2004;1(1):85–94. doi: 10.1385/NCC:1:1:85. [DOI] [PubMed] [Google Scholar]
- Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab. 2001;21(3):195–201. doi: 10.1097/00004647-200103000-00002. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Eikermann-Haerter K, Ayata C. Cortical spreading depression and migraine. Curr Neurol Neurosci Rep. 2010;10(3):167–73. doi: 10.1007/s11910-010-0099-1. [DOI] [PubMed] [Google Scholar]
- Escartin C, Bonvento G. Targeted activation of astrocytes: a potential neuroprotective strategy. Mol Neurobiol. 2008;38(3):231–41. doi: 10.1007/s12035-008-8043-y. [DOI] [PubMed] [Google Scholar]
- Escartin C, Brouillet E, Gubellini P, Trioulier Y, Jacquard C, Smadja C, Knott GW, Kerkerian-Le Goff L, Deglon N, Hantraye P, et al. Ciliary neurotrophic factor activates astrocytes, redistributes their glutamate transporters GLAST and GLT-1 to raft microdomains, and improves glutamate handling in vivo. J Neurosci. 2006;26(22):5978–89. doi: 10.1523/JNEUROSCI.0302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Pierre K, Colin A, Brouillet E, Delzescaux T, Guillermier M, Dhenain M, Deglon N, Hantraye P, Pellerin L, et al. Activation of astrocytes by CNTF induces metabolic plasticity and increases resistance to metabolic insults. J Neurosci. 2007;27(27):7094–104. doi: 10.1523/JNEUROSCI.0174-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Rouach N. Astroglial networking contributes to neurometabolic coupling. Front Neuroenergetics. 2013;5:4. doi: 10.3389/fnene.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein D, Manning A, Hashemi P, Bhatia R, Fabricius M, Tolias C, Pahl C, Ervine M, Strong AJ, Boutelle MG. Dynamic metabolic response to multiple spreading depolarizations in patients with acute brain injury: an online microdialysis study. J Cereb Blood Flow Metab. 2010;30(7):1343–55. doi: 10.1038/jcbfm.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein D, Takagaki M, Gramer M, Manning A, Endepols H, Vollmar S, Yoshimine T, Strong AJ, Graf R, Backes H. Detecting tissue deterioration after brain injury: regional blood flow level versus capacity to raise blood flow. J Cereb Blood Flow Metab. 2014;34(7):1117–27. doi: 10.1038/jcbfm.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9(11):1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Florence G, Bonvento G, Charbonne R, Seylaz J. Spreading depression reversibly impairs autoregulation of cortical blood flow. Am J Physiol. 1994;266(4 Pt 2):R1136–40. doi: 10.1152/ajpregu.1994.266.4.R1136. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Johnsen A, Hassel B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia. 1997;21(1):106–13. [PubMed] [Google Scholar]
- Fordsmann JC, Ko RW, Choi HB, Thomsen K, Witgen BM, Mathiesen C, Lonstrup M, Piilgaard H, MacVicar BA, Lauritzen M. Increased 20-HETE synthesis explains reduced cerebral blood flow but not impaired neurovascular coupling after cortical spreading depression in rat cerebral cortex. J Neurosci. 2013;33(6):2562–70. doi: 10.1523/JNEUROSCI.2308-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Chen MJ, Li B, Smith NA, Peng W, Sun W, Toner MJ, Kress BT, Wang L, Benraiss A, et al. Neuronal Transgene Expression in Dominant-Negative SNARE Mice. J Neurosci. 2014;34(50):16594–604. doi: 10.1523/JNEUROSCI.2585-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Williams EK, Jensen TK, Smith NA, Takano T, Tieu K, Nedergaard M. Cultured astrocytes do not release adenosine during hypoxic conditions. J Cereb Blood Flow Metab. 2012;32(1):e1–7. doi: 10.1038/jcbfm.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11(2):87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- Gniel HM, Martin RL. Cortical Spreading Depression-induced Preconditioning in Mouse Neocortex is Laminar Specific. J Neurophysiol. 2013 doi: 10.1152/jn.00855.2011. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456(7223):745–9. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Mulligan SJ, MacVicar BA. Astrocyte control of the cerebrovasculature. Glia. 2007;55(12):1214–21. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, Kwong KK, Cutrer FM, Rosen BR, Tootell RB, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98(8):4687–92. doi: 10.1073/pnas.071582498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology. 2009;57(4):343–6. doi: 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartings JA, Bullock MR, Okonkwo DO, Murray LS, Murray GD, Fabricius M, Maas AI, Woitzik J, Sakowitz O, Mathern B, et al. Spreading depolarisations and outcome after traumatic brain injury: a prospective observational study. Lancet Neurol. 2011;10(12):1058–64. doi: 10.1016/S1474-4422(11)70243-5. [DOI] [PubMed] [Google Scholar]
- Hartings JA, Rolli ML, Lu XC, Tortella FC. Delayed secondary phase of peri-infarct depolarizations after focal cerebral ischemia: relation to infarct growth and neuroprotection. J Neurosci. 2003;23(37):11602–10. doi: 10.1523/JNEUROSCI.23-37-11602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi P, Bhatia R, Nakamura H, Dreier JP, Graf R, Strong AJ, Boutelle MG. Persisting depletion of brain glucose following cortical spreading depression, despite apparent hyperaemia: evidence for risk of an adverse effect of Leao’s spreading depression. J Cereb Blood Flow Metab. 2008 doi: 10.1038/jcbfm.2008.108. [DOI] [PubMed] [Google Scholar]
- Hertle DN, Dreier JP, Woitzik J, Hartings JA, Bullock R, Okonkwo DO, Shutter LA, Vidgeon S, Strong AJ, Kowoll C, et al. Effect of analgesics and sedatives on the occurrence of spreading depolarizations accompanying acute brain injury. Brain. 2012;135(Pt 8):2390–8. doi: 10.1093/brain/aws152. [DOI] [PubMed] [Google Scholar]
- Hines DJ, Haydon PG. Inhibition of a SNARE-sensitive pathway in astrocytes attenuates damage following stroke. J Neurosci. 2013;33(10):4234–40. doi: 10.1523/JNEUROSCI.5495-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann EK. Cell swelling and volume regulation. Can J Physiol Pharmacol. 1992;70(Suppl):S310–3. doi: 10.1139/y92-277. [DOI] [PubMed] [Google Scholar]
- Hoffmann U, Sukhotinsky I, Atalay YB, Eikermann-Haerter K, Ayata C. Increased glucose availability does not restore prolonged spreading depression durations in hypotensive rats without brain injury. Exp Neurol. 2012;238(2):130–2. doi: 10.1016/j.expneurol.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmin S, von Gertten C, Sandberg-Nordqvist AC, Lendahl U, Mathiesen T. Induction of astrocytic nestin expression by depolarization in rats. Neurosci Lett. 2001;314(3):151–5. doi: 10.1016/s0304-3940(01)02292-3. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Periinfarct depolarizations. Cerebrovasc Brain Metab Rev. 1996;8(3):195–208. [PubMed] [Google Scholar]
- Jander S, Schroeter M, Peters O, Witte OW, Stoll G. Cortical spreading depression induces proinflammatory cytokine gene expression in the rat brain. J Cereb Blood Flow Metab. 2001;21(3):218–25. doi: 10.1097/00004647-200103000-00005. [DOI] [PubMed] [Google Scholar]
- Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23(1):1–10. [PubMed] [Google Scholar]
- Kang W, Hebert JM. Signaling pathways in reactive astrocytes, a genetic perspective. Mol Neurobiol. 2011;43(3):147–54. doi: 10.1007/s12035-011-8163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Kocak E, Sen ZD, Dalkara T. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013;339(6123):1092–5. doi: 10.1126/science.1231897. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Macvicar BA, Sontheimer H. Anion channels in astrocytes: biophysics, pharmacology, and function. Glia. 2006;54(7):747–57. doi: 10.1002/glia.20423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 2010;7(4):338–53. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeberg J, Petzold GC, Major S, Dirnagl U, Dreier JP. ET-1 induces cortical spreading depression via activation of the ETA receptor/phospholipase C pathway in vivo. Am J Physiol Heart Circ Physiol. 2004;286(4):H1339–46. doi: 10.1152/ajpheart.00227.2003. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience. 2004;129(4):1045–56. doi: 10.1016/j.neuroscience.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272(5264):1013–6. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Kraig RP, Dong LM, Thisted R, Jaeger CB. Spreading depression increases immunohistochemical staining of glial fibrillary acidic protein. J Neurosci. 1991;11(7):2187–98. doi: 10.1523/JNEUROSCI.11-07-02187.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivanek J. Changes of brain glycogen in the spreading EEG-depression of Leao. J Neurochem. 1958;2(4):337–43. doi: 10.1111/j.1471-4159.1958.tb12383.x. [DOI] [PubMed] [Google Scholar]
- Krivanek J. Some metabolic changes accompanying Leao’s spreading cortical depression in the rat. J Neurochem. 1961;6:183–9. doi: 10.1111/j.1471-4159.1961.tb13463.x. [DOI] [PubMed] [Google Scholar]
- Kunkler PE, Hulse RE, Kraig RP. Multiplexed cytokine protein expression profiles from spreading depression in hippocampal organotypic cultures. J Cereb Blood Flow Metab. 2004;24(8):829–39. doi: 10.1097/01.WCB.0000126566.34753.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Kraig RP. Calcium waves precede electrophysiological changes of spreading depression in hippocampal organ cultures. J Neurosci. 1998;18(9):3416–25. doi: 10.1523/JNEUROSCI.18-09-03416.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe P, Sercombe R, Correze JL, Springhetti V, Seylaz J. Spreading depression induces prolonged reduction of cortical blood flow reactivity in the rat. Exp Neurol. 1992;117(3):278–86. doi: 10.1016/0014-4886(92)90137-f. [DOI] [PubMed] [Google Scholar]
- Largo C, Cuevas P, Somjen GG, Martin del Rio R, Herreras O. The effect of depressing glial function in rat brain in situ on ion homeostasis, synaptic transmission, and neuron survival. J Neurosci. 1996;16(3):1219–29. doi: 10.1523/JNEUROSCI.16-03-01219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largo C, Ibarz JM, Herreras O. Effects of the gliotoxin fluorocitrate on spreading depression and glial membrane potential in rat brain in situ. J Neurophysiol. 1997;78(1):295–307. doi: 10.1152/jn.1997.78.1.295. [DOI] [PubMed] [Google Scholar]
- Lauritzen M. On the possible relation of spreading cortical depression to classical migraine. Cephalalgia. 1985;5(Suppl 2):47–51. doi: 10.1177/03331024850050S208. [DOI] [PubMed] [Google Scholar]
- Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain. 1994;117 (Pt 1):199–210. doi: 10.1093/brain/117.1.199. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31(1):17–35. doi: 10.1038/jcbfm.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen M, Olesen J. Regional cerebral blood flow during migraine attacks by Xenon-133 inhalation and emission tomography. Brain. 1984;107 (Pt 2):447–61. doi: 10.1093/brain/107.2.447. [DOI] [PubMed] [Google Scholar]
- Leao A. Spreading depression of activity in the cerebral cortex. Journal of Neurophysiology. 1944;7:359–390. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- Leao A. Further observations on the spreading depression of activity in the cerebral cortex. J Neurophysiology. 1947;10:409–414. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- Leo L, Gherardini L, Barone V, De Fusco M, Pietrobon D, Pizzorusso T, Casari G. Increased susceptibility to cortical spreading depression in the mouse model of familial hemiplegic migraine type 2. PLoS Genet. 2011;7(6):e1002129. doi: 10.1371/journal.pgen.1002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian XY, Stringer JL. Astrocytes contribute to regulation of extracellular calcium and potassium in the rat cerebral cortex during spreading depression. Brain Res. 2004a;1012(1–2):177–84. doi: 10.1016/j.brainres.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Lian XY, Stringer JL. Energy failure in astrocytes increases the vulnerability of neurons to spreading depression. Eur J Neurosci. 2004b;19(9):2446–54. doi: 10.1111/j.0953-816X.2004.03289.x. [DOI] [PubMed] [Google Scholar]
- Lindquist BE, Shuttleworth CW. Adenosine receptor activation is responsible for prolonged depression of synaptic transmission after spreading depolarization in brain slices. Neuroscience. 2012;223:365–76. doi: 10.1016/j.neuroscience.2012.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist BE, Shuttleworth CW. Spreading depolarization-induced adenosine accumulation reflects metabolic status in vitro and in vivo. J Cereb Blood Flow Metab. 2014 doi: 10.1038/jcbfm.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Wan CK, Bai JZ, Pi R, Li D, Donnelly D. Neuroprotective potential of ceftriaxone in in vitro models of stroke. Neuroscience. 2007;146(2):617–29. doi: 10.1016/j.neuroscience.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Lovatt D, Xu Q, Liu W, Takano T, Smith NA, Schnermann J, Tieu K, Nedergaard M. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci U S A. 2012;109(16):6265–70. doi: 10.1073/pnas.1120997109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Mechanisms of glutamate release from astrocytes. Neurochem Int. 2008;52(1–2):142–54. doi: 10.1016/j.neuint.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6(2):159–63. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Martin ED, Fernandez M, Perea G, Pascual O, Haydon PG, Araque A, Cena V. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55(1):36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- Martineau M, Parpura V, Mothet JP. Cell-type specific mechanisms of D-serine uptake and release in the brain. Front Synaptic Neurosci. 2014;6:12. doi: 10.3389/fnsyn.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Ferreira H, Ribeiro LJ. Biphasic effects of gap junctional uncoupling agents on the propagation of retinal spreading depression. Braz J Med Biol Res. 1995;28(9):991–4. [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58(9):1094–103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- Mies G, Iijima T, Hossmann KA. Correlation between peri-infarct DC shifts and ischaemic neuronal damage in rat. Neuroreport. 1993;4(6):709–11. doi: 10.1097/00001756-199306000-00027. [DOI] [PubMed] [Google Scholar]
- Milner PM. Note on a possible correspondence between the scotomas of migraine and spreading depression of Leao. Electroencephalogr Clin Neurophysiol. 1958;10(4):705. doi: 10.1016/0013-4694(58)90073-7. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci U S A. 2005;102(15):5606–11. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431(7005):195–9. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Astrup J. Infarct rim: effect of hyperglycemia on direct current potential and [14C]2-deoxyglucose phosphorylation. J Cereb Blood Flow Metab. 1986;6(5):607–15. doi: 10.1038/jcbfm.1986.108. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Cooper AJ, Goldman SA. Gap junctions are required for the propagation of spreading depression. J Neurobiol. 1995;28(4):433–44. doi: 10.1002/neu.480280404. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Hansen AJ. Spreading depression is not associated with neuronal injury in the normal brain. Brain Res. 1988;449(1–2):395–8. doi: 10.1016/0006-8993(88)91062-1. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Verkhratsky A. Artifact versus reality--how astrocytes contribute to synaptic events. Glia. 2012;60(7):1013–23. doi: 10.1002/glia.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B, Nedergaard M. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008;28(13):3264–76. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292(5518):923–6. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- Orellana JA, Stehberg J. Hemichannels: new roles in astroglial function. Front Physiol. 2014;5:193. doi: 10.3389/fphys.2014.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmawar P, Yao X, Bloch O, Manley GT, Verkman AS. K+ waves in brain cortex visualized using a long-wavelength K+-sensing fluorescent indicator. Nat Methods. 2005;2(11):825–7. doi: 10.1038/nmeth801. [DOI] [PubMed] [Google Scholar]
- Pannasch U, Freche D, Dallerac G, Ghezali G, Escartin C, Ezan P, Cohen-Salmon M, Benchenane K, Abudara V, Dufour A, et al. Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci. 2014;17(4):549–58. doi: 10.1038/nn.3662. [DOI] [PubMed] [Google Scholar]
- Pannasch U, Vargova L, Reingruber J, Ezan P, Holcman D, Giaume C, Sykova E, Rouach N. Astroglial networks scale synaptic activity and plasticity. Proc Natl Acad Sci U S A. 2011;108(20):8467–72. doi: 10.1073/pnas.1016650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson FE, Sinclair CJ, Othman T, Haughey NJ, Geiger JD. Differences between rat primary cortical neurons and astrocytes in purine release evoked by ischemic conditions. Neuropharmacology. 2002;43(5):836–46. doi: 10.1016/s0028-3908(02)00083-7. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55(12):1251–62. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32(7):1152–66. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters O, Schipke CG, Hashimoto Y, Kettenmann H. Different mechanisms promote astrocyte Ca2+ waves and spreading depression in the mouse neocortex. J Neurosci. 2003;23(30):9888–96. doi: 10.1523/JNEUROSCI.23-30-09888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71(5):782–97. doi: 10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Pietrobon D, Moskowitz MA. Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nature reviews Neuroscience. 2014;15(6):379–93. doi: 10.1038/nrn3770. [DOI] [PubMed] [Google Scholar]
- Piilgaard H, Lauritzen M. Persistent increase in oxygen consumption and impaired neurovascular coupling after spreading depression in rat neocortex. J Cereb Blood Flow Metab. 2009;29(9):1517–27. doi: 10.1038/jcbfm.2009.73. [DOI] [PubMed] [Google Scholar]
- Piper RD, Lambert GA, Duckworth JW. Cortical blood flow changes during spreading depression in cats. Am J Physiol. 1991;261(1 Pt 2):H96–102. doi: 10.1152/ajpheart.1991.261.1.H96. [DOI] [PubMed] [Google Scholar]
- Risher WC, Andrew RD, Kirov SA. Real-time passive volume responses of astrocytes to acute osmotic and ischemic stress in cortical slices and in vivo revealed by two-photon microscopy. Glia. 2009;57(2):207–21. doi: 10.1002/glia.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher WC, Croom D, Kirov SA. Persistent astroglial swelling accompanies rapid reversible dendritic injury during stroke-induced spreading depolarizations. Glia. 2012;60(11):1709–20. doi: 10.1002/glia.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ML, Feuerstein D, Leong CL, Takagaki M, Niu X, Graf R, Boutelle MG. Continuous online microdialysis using microfluidic sensors: dynamic neuro-metabolic changes during spreading depolarisation. ACS Chem Neurosci. 2013 doi: 10.1021/cn400047x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg D, Artoul S, Segal AC, Kolodney G, Radzishevsky I, Dikopoltsev E, Foltyn VN, Inoue R, Mori H, Billard JM, et al. Neuronal D-serine and glycine release via the Asc-1 transporter regulates NMDA receptor-dependent synaptic activity. J Neurosci. 2013;33(8):3533–44. doi: 10.1523/JNEUROSCI.3836-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403(6767):316–21. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322(5907):1551–5. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Sakowitz OW, Kiening KL, Krajewski KL, Sarrafzadeh AS, Fabricius M, Strong AJ, Unterberg AW, Dreier JP. Preliminary evidence that ketamine inhibits spreading depolarizations in acute human brain injury. Stroke. 2009;40(8):e519–22. doi: 10.1161/STROKEAHA.109.549303. [DOI] [PubMed] [Google Scholar]
- Sakowitz OW, Santos E, Nagel A, Krajewski KL, Hertle DN, Vajkoczy P, Dreier JP, Unterberg AW, Sarrafzadeh AS. Clusters of spreading depolarizations are associated with disturbed cerebral metabolism in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2013;44(1):220–3. doi: 10.1161/STROKEAHA.112.672352. [DOI] [PubMed] [Google Scholar]
- Sanchez-Porras R, Santos E, Scholl M, Stock C, Zheng Z, Schiebel P, Orakcioglu B, Unterberg AW, Sakowitz OW. The effect of ketamine on optical and electrical characteristics of spreading depolarizations in gyrencephalic swine cortex. Neuropharmacology. 2014;84:52–61. doi: 10.1016/j.neuropharm.2014.04.018. [DOI] [PubMed] [Google Scholar]
- Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54 (7):716–25. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel JL, Faideau M, Aiba I, Pannasch U, Escartin C, Rouach N, Bonvento G, Shuttleworth CW. Ciliary neurotrophic factor (CNTF) activation of astrocytes decreases spreading depolarization susceptibility and increases potassium clearance. Glia. 2015;63(1):91–103. doi: 10.1002/glia.22735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel JL, Shuttleworth CW. Contribution of astrocyte glycogen stores to progression of spreading depression and related events in hippocampal slices. Neuroscience. 2011;192:295–303. doi: 10.1016/j.neuroscience.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth CW, Weiss JH. Zinc: new clues to diverse roles in brain ischemia. Trends Pharmacol Sci. 2011;32(8):480–6. doi: 10.1016/j.tips.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001;81(3):1065–96. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- Steinhauser C, Seifert G, Bedner P. Astrocyte dysfunction in temporal lobe epilepsy: K+ channels and gap junction coupling. Glia. 2012;60(8):1192–202. doi: 10.1002/glia.22313. [DOI] [PubMed] [Google Scholar]
- Suh SW, Bergher JP, Anderson CM, Treadway JL, Fosgerau K, Swanson RA. Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316,819 ([R-R*, S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmet hyl)propyl]-1H-indole-2-carboxamide) J Pharmacol Exp Ther. 2007;321(1):45–50. doi: 10.1124/jpet.106.115550. [DOI] [PubMed] [Google Scholar]
- Sukhotinsky I, Dilekoz E, Wang Y, Qin T, Eikermann-Haerter K, Waeber C, Ayata C. Chronic daily cortical spreading depressions suppress spreading depression susceptibility. Cephalalgia. 2011 doi: 10.1177/0333102411425865. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Curr Mol Med. 2004;4(2):193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- Sword J, Masuda T, Croom D, Kirov SA. Evolution of neuronal and astroglial disruption in the peri-contusional cortex of mice revealed by in vivo two-photon imaging. Brain. 2013;136(Pt 5):1446–61. doi: 10.1093/brain/awt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Lovatt D, Hansen AJ, Kasischke KA, Nedergaard M. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10(6):754–62. doi: 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]
- Thrane AS, Rangroo Thrane V, Zeppenfeld D, Lou N, Xu Q, Nagelhus EA, Nedergaard M. General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. Proc Natl Acad Sci U S A. 2012;109(46):18974–9. doi: 10.1073/pnas.1209448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M, Tanahashi N, Takeda H, Takao M, Tomita Y, Amano T, Fukuuchi Y. Astroglial swelling in the neuronal depolarization ensemble. Acta Neurochir Suppl. 2003;86:219–22. doi: 10.1007/978-3-7091-0651-8_47. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Butt A. Glia neurobiology. Wiley and Sons; 2007. [Google Scholar]
- Wahl M, Lauritzen M, Schilling L. Change of cerebrovascular reactivity after cortical spreading depression in cats and rats. Brain Res. 1987;411(1):72–80. doi: 10.1016/0006-8993(87)90682-2. [DOI] [PubMed] [Google Scholar]
- Walz W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem Int. 2000;36(4–5):291–300. doi: 10.1016/s0197-0186(99)00137-0. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A. 2006;103(46):17513–8. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci U S A. 1999;96(23):13409–14. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32(18):6391–410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci. 2010;30(26):8807–14. doi: 10.1523/JNEUROSCI.0511-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Gordon GR, Feighan D, MacVicar BA. Transient swelling, acidification, and mitochondrial depolarization occurs in neurons but not astrocytes during spreading depression. Cereb Cortex. 2010;20(11):2614–24. doi: 10.1093/cercor/bhq018. [DOI] [PubMed] [Google Scholar]