Abstract
Alcohol use and sexual risk behaviors are multidimensional phenomena involving many genetic and environmental factors. 5-HT transporter linked promoter region (5-HTTLPR) polymorphism constitutes an important factor affecting alcohol use problems and risky sexual behaviors. This paper narratively reviews studies on 5-HTTLPR polymorphism and its associations with alcohol use problems and sexual risk behaviors. We searched the electronic databases, PubMed, Ovid, and Google Scholar for articles using MeSH terms. Relevant articles were reviewed and eligible articles were selected for the study. Many studies have reported a significant but moderate association between 5-HTTLPR polymorphism and alcohol use problems. These studies have implicated the presence of at least one S allele to be associated with significant increases in alcohol use problems. Similarly, some studies associate the S allele with increased sexual risk behaviors. Effective alcohol cessation initiatives and STI/HIV prevention programs should be modified to account for 5-HTTLPR polymorphism before planning interventions; genetic effects could moderate the intervention effect.
Keywords: 5-HTTLPR polymorphism, Alcohol use problems, Risky sexual behaviors, Alcohol cessation programs, STI/HIV prevention programs
Introduction
Alcohol use and sexual risk-taking are complex and multifactorial behaviors involving an interplay between genetic, environmental, and social factors (Edenberg et al. 1998; McHale et al. 2009). Alcohol use is associated with uninhibited behaviors and impaired judgment. It has been implicated in high-risk sexual behaviors like failure to use condoms and having multiple sexual partners. Though the threshold for alcohol use precipitating risky sexual behaviors differ between individuals, the degree of intoxication and history of alcoholism have been associated with increased risky behaviors in both risk-prone and risk-averse individuals (Bryant 2006; Organista and Kubo 2005). Alcohol use also tends to compromise information processing, emotional regulation, and executive functioning, which could be important in adhering to safe sex practices and other preventive behaviors. Accordingly, many studies have shown alcohol use to be a major risk factor for sexually transmitted infections (STIs) (Kalichman et al. 2007; Rees et al. 2001).
Several studies have demonstrated an association between genetic variables and alcohol use problems. One of the implicated genes is the serotonin transporter gene (SLC6A4) (Feinn et al. 2005). As early as 1987, Cloninger (1987) showed that deficits in central serotoninergic neurotransmission was associated with early onset alcoholism as well as addictive behaviors. Serotonin is a monoamine neurotransmitter involved in many psychological and physiological processes like mood, behavior, sleep regulation, vascular tone, food intake, pain, motor activity, and platelet functions (Coccaro et al. 1990; Glennon and Dukat 2002; Hoyer et al. 2002). Serotonin is also involved in complex novelty-seeking behaviors (Vormfelde et al. 2006). Serotonin is synthesized by the cell bodies of raphe nuclei and is carried to the cerebral cortex, subcortical regions, and hippocampus, thereby regulating many complex reward circuitry pathways within the central nervous system. Serotonin released into the synaptic cleft acts on serotonin receptors on post-synaptic neuronal membranes. Some of the serotonin released into the synaptic cleft undergoes a process of reuptake through serotonin transporters (5-HT transporter, 5-HTT) located on the pre-synaptic neuronal membranes and metabolized by monoamine oxidase enzymes (Heils et al. 1995). This is the mechanism for synaptic regulation of serotonin levels, which in turn, controls the magnitude and duration of post-synaptic serotoninergic neuronal responses. Alterations in these mechanisms could be responsible for several behavioral and neurocognitive dysfunctions.
The serotonin transporter gene is located on chromosome 17q12. The promoter and the 14 exons of the serotonin transporter gene span 31 Kbs on chromosome 17 (Lesch et al. 1994). A polymorphic region of the serotonin transporter gene is situated in a guanine-cytosine (GC-rich) region made up of 20–23 bp repeats. This region is identified by a 43-bp insertion-deletion at the promoter region (5-HT transporter linked promoter region, 5-HTTLPR) (Heils et al. 1996). This variation in the promoter is associated with changes in the transcriptional activity of the gene. The two alleles that constitute this variation in the promoter region are termed the long (L) and the short (S) alleles and exhibit different phenotypes (Nakamura et al. 2000). The effects of SS and SL genotypes varies significantly from LL genotype. Cells with a homozygous L allele (LL), in comparison to those containing S allele (SL and SS), synthesize 1.4–1.7 times more 5-HTT messenger RNA (mRNA) (Lesch et al. 1996). The LL variant has a threefold higher basal activity for 5-HTT than the SS and SL variants. In addition, cells with the LL variant are capable of serotonin reuptake from the synaptic cleft at a rate 1.9–2.2 times than that of SS and SL variants (Lesch et al. 1996).
Since the first study by Sander et al. (1998), several studies have been conducted on alcohol-dependent populations to evaluate the role of allelic variations of the 5-HTT transporter genes. Most of the studies demonstrate an association between 5-HTTLPR polymorphism and alcohol use problems, most commonly, alcohol dependence. According to the DSM V, “alcohol dependence is maladaptive pattern of alcohol use, leading to clinically significant impairment or distress” (American Psychological Association 2013). The role of 5-HTTLPR on alcohol use problems has been controversial. Similarly, although not conclusive, some recent studies have shown an association between 5-HTTLPR polymorphism and sexual risk behaviors (Sales et al. 2014). In the wake of recent studies on the associations between 5-HTTLPR polymorphism, alcohol use problems, and sexual risk behaviors, it is important to review all these factors jointly for the development of effective interventions for alcohol use problems and STIs. The main objective of this paper is to review and analyze serotonin transporter promoter polymorphism and its associations with alcohol use problems and risky sexual behaviors.
Search strategy and selection criteria
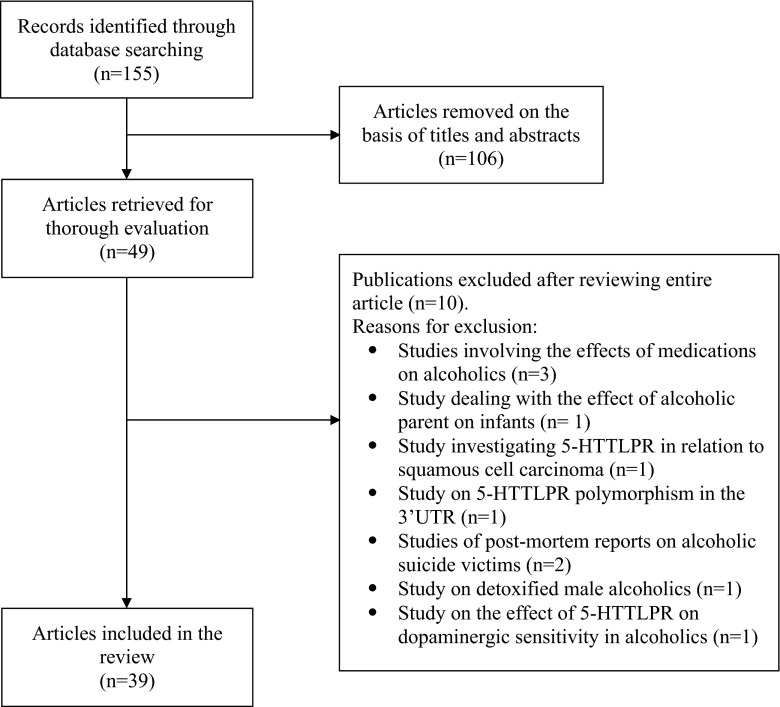
We searched the electronic databases, PubMed, Ovid, and Google Scholar for articles published between January 1995 and December 2014 (20 years) by combining the following search terms: “serotonin transporter polymorphism,” “serotonin transporter promoter polymorphism,” “5HTTLPR,” “5HTT linked polymorphic region,” “SCL6A4” and “alcohol,” “drinking,” “substance abuse,” “risky sexual behaviors,” and “sexual risk.” A total of 39 studies that met the review criteria were included in this study (Fig. 1). We followed a narrative review method to summarize studies on the associations between 5-HTTLPR polymorphism and two risk factors: alcohol use problems and sexual risk behaviors.
Fig. 1.
Flow diagram indicating the selection of included articles
5-HTTLPR polymorphism and alcohol use problems
Several studies point to greater strength in the S allele to predict alcohol dependence. A meta-analysis of 22 studies (n = 8050) showed that there was significant association between the S allele and alcohol dependence. This association was stronger for SS and SL genotypes when compared to the LL genotype. This meta-analysis also reported a 15 % greater likelihood of having at least one S allele in patients diagnosed with alcohol dependence (McHugh et al. 2010). Another meta-analysis of 17 studies by Feinn et al. (2005), reported similar associations between the S allele and alcohol dependence. This study reported an odds ratio of 1.18 (95 % confidence intervals (CI) = 1.03–1.33) for the S allele in alcohol-dependent subjects; hence an 18 % higher risk for developing alcohol dependence.
The association between the S allele and alcohol dependence has also been strong in research across genders and ethnicities. In a study involving 118 subjects of Han Chinese origin, the S allele was associated with increased risk for alcohol dependence and subjects with L alleles were found to be less susceptible (Wang et al. 2011). In a study done by Enoch et al. (2011), among 360 treatment-seeking African-American male patients, the frequency of the S allele was found to be significantly associated with alcohol dependence. A study by Lin et al. (2007) demonstrated that the SS genotype was significantly associated with alcohol consumption and dependence in a group of male Han Chinese population from Taiwan. In a longitudinal study of 583 adolescents, Merenäkk et al. (2011) showed that the SS genotype was associated with increased alcohol consumption. Similarly, van der Zwaluw et al. (2010) reported associations between the S allele and higher incidence of alcohol consumption in a group of 428 Dutch adolescents.
Research has also found the S allele to be related to variations in drinking patterns. Herman et al. (2005) also reported that the S allele was associated with a higher risk for binge drinking among women. Hammoumi et al. (1999) showed that Caucasian subjects with the SS and SL genotypes had increased frequency of binge drinking, compared to the LL genotype. The S alleles were also associated with early onset of binge drinking when compared to LL variants. A study among college students at George Washington University showed that the S allele was associated with increased risk of binge drinking (Herman et al. 2005). Matsushita et al. (2001) reported a higher frequency of the SS genotype among a sub-group of Japanese male adult alcoholics with binge drinking behaviors. Herman et al. reported an association between the S allele and alcoholism in young Caucasian women (Herman et al. 2005). Hallikainen et al. (1999) found that the S allele was associated with early onset alcoholism in a Finnish study. In a Japanese study, subjects with late-onset alcoholism showed increased frequencies of the S allele (Ishiguro et al. 1999). Hallikainen et al. (1999) also reported that the S allele was significantly associated with type 2 alcoholism (onset of alcoholism before 25 years of age) in the Finnish study.
In contrast to those findings pointing to the power of the S allele in the development of alcohol use disorders, a Korean study found that participants with L alleles showed higher risk for alcohol dependence (Kweon et al. 2005). This study also showed a gene-dose effect which suggested that people with the LL genotype had a higher risk for alcoholism when compared to the LS and SS variants. Additionally, increased frequency of the L allele was seen in individuals with a positive family history of alcohol dependence. In a study involving 97 Portuguese alcohol-dependent subjects, Pombo et al. (2008) showed that most (30.7 %) had the LL genotype, with 19.8 % displaying the SS genotype and the remaining had the SL genotype. Studies found that the L allele predicted alcohol dependence across ages, genders, and ethnicities. Gokturk et al. (2008) found an association between LL genotype and severe alcoholism in women. In a study of 305 German adolescent subjects, the LL genotype was associated with increased alcohol consumption in adolescent female participants (Skowronek et al. 2006). Schuckit et al. (1999) reported an association of LL genotype with higher incidence of alcoholism in a population of male subjects.
The L variants were also associated with early onset of alcohol use disorders in several studies. Daws et al. (2009) analyzed the genotypes of adolescents from mixed races (mostly Caucasian and Hispanics) and found that the LL genotype was associated with early onset of alcoholism when compared to SS and SL genotypes. They also found an association between the LL genotype and longer duration of alcohol abuse. Participants with the LL genotype also showed an increased number of 5-HTT indicated by indirect measures like platelet binding profiles of selective serotonin reuptake inhibitor (SSRI) drugs. This indicates faster depletion of serotoninergic activity and increased risk for alcoholism. Matsushita et al. (2001) showed that individuals with LL and LS genotypes had earlier onset of alcohol use when compared to subjects with SS genotype. In a study of factors affecting age of onset of alcoholism, it was observed that the LL genotype was relatively more susceptible to early onset alcoholism because of inadequate levels of serotonin functioning (Johnson 2000; Johnson and Ait-Daoud 2000).
There are other studies that do not show a relationship between 5-HTTLPR polymorphism and alcohol dependence. For example, a meta-analysis of 25 case control studies (n = 8885) found no link between 5-HTTLPR polymorphism and alcoholism (Villalba et al. 2015). In a German study conducted among 250 institutionalized alcoholics and 94 healthy controls, no significant differences were observed with respect to S and L allele genotypes between the two groups (Kohnke et al. 2006). Similarly, in a Spanish study of 165 alcohol-dependent patients, 113 heroine-dependent patients and 420 healthy controls, Saiz et al. (2009) showed that there were no associations between 5-HTTLPR polymorphism and alcohol dependence. Rasmussen et al. (2009) studied an elderly population of women with a history of severe alcoholism and also found no association between 5-HTTLPR polymorphism and alcohol dependence. Similarly, in a family based study on adult males, Stoltenberg et al. (2002) did not find any association between the 5-HTTLPR polymorphism and diagnosis of alcoholism or levels of alcoholism. Table 1 shows the summary of studies examining associations between HTTLPR polymorphism and alcohol use problems.
Table 1.
Summary of literature references of allelic association of 5-HTT promoter with risk of alcohol use problems
| Reference | Population | Co-occurring clinical feature | Mean age (years) | Male (%) | Sample size | Risk allele | Type of study |
|---|---|---|---|---|---|---|---|
| Gelernter et al. (1997)) | European ancestry | – | – | 77.8 | 274 | – | Population based |
| Edenberg et al. (1998) | European ancestry | – | – | – | 131 | No bias | Family based |
| Sander et al. (1998) | German | High severity alcoholism | 39.04 | – | 531 | SS | Population based |
| Hallikainen et al. (1999) | Finnish | Type II alcoholism | 43.91 | 100 | 105 | S | Gender based |
| Hammoumi et al. (1999) | French | 43.61 | 71.6 | 140 | – | – | |
| Schuckit et al. (1999) | European Ancestry | 100 | 41 | LL | Gender based | ||
| Ishiguro et al. (1999) | Japanese | Early onset | 52.24 | 92.2 | 166 | L | Population based |
| Gorwood et al. (2000) | French | Suicidality | 43.61 | – | 171 | S allele unrelated to alcoholism | Population based, alcohol dependent |
| Thompson et al. (2000) | European ancestry | Tourette syndrome | – | – | 256 | No bias | Population based |
| Lichtermann et al. (2000) | German, Hungarian | – | – | 82.6 | 102 | S | Family based study |
| Matsushita et al. (2001) | Japanese | – | 50.5 | 100 | 962 | SS | |
| Preuss et al. (2000) | German, Hungarian | Suicidality | 41.02 | 80.4 | 280 | S | Population based |
| Pastorelli et al. (2001) | Italian | Liver cirrhosis | – | 60 | No bias | Population based | |
| Kranzler et al. (2002) | European ancestry | Early onset | – | 74.9 | 555 | No bias | Population based |
| Kranzler et al. (2002) | African-American | Early onset | – | 75.0 | 151 | No bias | Population based |
| Stoltenberg et al. (2002) | Caucasian | Antisocial | – | 100 | 44 | No bias | Family based association |
| Johann et al. (2003) | German | ADHD | 43.1 | 83.4 | 534 | – | – |
| Nellissery et al. (2003) | European ancestry | Depression | 41.05 | 59.5 | 556 | – | – |
| Nellissery et al. (2003) | African-American | Depression | 38.85 | 43.8 | 59 | – | – |
| Konishi et al. (2004) | Mexican-American | 38.24 | 100 | 451 | – | – | |
| Mannelli et al. (2005) | African-American | Cocaine abuse | 36.29 | 70 | 1411 | S | Population based |
| Herman et al. (2005) | Caucasian | – | – | 0 | 412 | S | Gender based |
| Olsson et al. (2005) | Australian | – | Range (14–24) | – | – | L | Population based |
| Kweon et al. (2005) | Korean | – | – | – | – | L | Population based |
| Skowronek et al. (2006) | German | – | 15 | 48 | 305 | LL | Population and gender effects |
| Kohnke et al. (2006) | German | – | – | – | 215 | No association | Population based |
| Gokturk et al. (2008) | Caucasian | Anxiety /depression | Range (18–75) | 0 | 110 | LL | Gender based |
| Pombo et al. (2008) | Portuguese | – | – | – | 97 | LL | Population based |
| Pinto et al. (2008) | – | – | – | – | 48 | S | |
| Lin et al. (2007) | Han Chinese | Depression | 40 | 100 | 133 | SS | Population based |
| Saiz et al. (2009) | Caucasian/Spanish | – | 47.8 | 84.8 | 165 | NS | Population based |
| Dawes et al. (2009) | Mixed races (Whites, Hispanics, Biracial, American Indians) | – | 18.7 | 62 | 21 | LL | – |
| Rasmussen et al. (2009) | – | – | 69.2 | 0 | 1365 | No Significance | Gender based |
| van der Zwaluw et al. (2010) | Dutch Caucasian | – | 13.4 | 48 | 428 | S | Population based |
| Merenakk et al. (2011) | Estonian | – | Range (9–18) | – | 583 | SS | Longitudinal study |
| Wang et al. (2011) | Yunnan Han Chinese | – | – | – | 118 | S | Population based |
| Enoch et al. (2011) | African-American | – | 34 | 100 | 360 | S | Population based |
5-HTTLPR polymorphism and sexual risk behaviors
Research has shown that 5-HTTLPR polymorphism can adversely affect neuropsychological factors like information processing, executive functioning, memory, anxiety, depression, suicidality, and other specific behaviors and temperaments (Cysique et al. 2004; McArthur et al. 1989). There are several neurobiological mechanisms associated with 5-HTTLPR polymorphism which include neuroticism, reward dependence, delayed gratification of tasks, harm avoidance, novelty seeking, etc. (Carver and Miller 2006). Sexual risk behaviors such as unprotected sex, risky sexual encounters, and multiple sex partners are examples of novelty-seeking behaviors (Zuckerman 1979) and have been associated with 5-HTTLPR polymorphism (Wood and Nizam 2014).
There are very few studies on the associations between 5-HTTLPR polymorphism and risky sexual behaviors. In a study done by Wood et al. (Wood and Nizam 2014), among 284 undergraduate students, it was found that participants with the LL genotype had better cognition about risky sexual behaviors as assessed by three measures: the Cloninger Temperament and Character Inventory (TCI), the Physical Risk Frequency Inventory (PRFI), and the Physical Risk Assessment Inventory (PRAI). Hamer et al. (2002) reported that participants with SS and SL genotypes had more frequent sexual encounters than those with the LL genotype. In a study conducted by Sales et al. (2014), it was recommended that 5-HTTLPR polymorphism should be ascertained before enrolment into STI/HIV prevention interventions because participants with the S allele were more resistant to risk reduction strategies when compared to those with L alleles. This study also reported increased levels of anxiety and lack of assertiveness in sexual decisions in participants with S alleles when compared to those with L alleles, predisposing them to greater risk for sexually transmitted infections and HIV.
Paaver et al. (2007) studied the effects of 5-HTTLPR polymorphism among adolescents who participated in the Estonian Children Personality Behavior and Health Study and concluded that participants with the S allele reported higher impulsivity and error rates measured by visual comparison task (VCT) assessments. In another study of the same population, Paaver et al. (2008) also showed that participants with the SS genotype were more susceptible to stressful familial and environmental triggers and showed increased disinhibition and impulsivity. Table 2 shows the summary of studies that showed associations between 5-HTTLPR polymorphism and sexual risk behaviors.
Table 2.
Summary of literature references of allelic association of 5-HTT promoter with sexual risk behavior
| Reference | Population | Co-occurring clinical feature | Mean age (years) | Male (%) | Sample size | Risk allele | Type of study |
|---|---|---|---|---|---|---|---|
| Hamer (2002) | – | – | – | 100 | – | S | – |
| Kogan et al. (2010) | African-American adolescents | – | Wave 1–13.96 Wave 2–16.04 |
– | 185 | S | Longitudinal, prospective design |
| Wood et al., (2014) | – | – | – | 38.3 | 284 | S | – |
| Sales et al. (2014) | African-American adolescent | Depression | 18.1 | 0 | 254 | S | Randomized trial |
Studies linking serotoninergic activity with alcoholism and other substance use as well as high-risk sexual behaviors
There are very few studies that consider serotoninergic activity, alcohol use problems, and high-risk sexual behaviors jointly. The studies on differential responses to interventions for alcohol cessation and associated neurobiological factors showed that there were strong associations between serotonin deficiencies and heavy alcohol use and risky sexual behaviors due to impulsivity (Bowirrat and Oscar-Berman 2005; Trobst et al. 2002). Kogan et al. (2010) has further identified a relationship between early onset substance use (including alcohol) and significant high-risk sexual behaviors with 5-HTTLPR polymorphism, specifically associated with S allele. In their study among rural African-American youth, early onset substance use measured at age 14 was significantly related to high-risk sexual behaviors measured in a follow-up visit at age 16.
Discussion
The associations between alcohol use problems, sexual risk behaviors, and 5-HTTLPR polymorphism are highly variable. Currently there are many views on the actual mechanism impacting alcohol dependence and addictive behaviors in the presence of 5-HTTLPR polymorphism. Heinz et al. (2001) found that LL genotypes had decreased levels of serotonin in the synaptic cleft and this could be responsible for higher levels of impulsivity. Further, Johnson and Ait-Daoud (2000) suggested that decreased serotonin levels in the synaptic cleft could be responsible for persons with LL genotypes having early onset problem drinking. Both the authors hypothesized that LL homozygotes have an increased amount of 5-HTT in pre-synaptic membranes, thereby leading to faster cessation of serotonin activity in the synapse. This functional deficiency of serotonin has been implicated as a factor for many addiction-related variables like early onset problem drinking, heavy drinking patterns, and impulsivity. The unanimity of the authors’ conclusions about the LL genotype showing greater propensity for addictive behaviors and problem drinking, as well as the reversal of these symptoms with SSRI medications, suggests a broader hypothesis that decreased serotonin levels could be responsible for addictive behaviors (Dawes et al. 2009).
Another view is that the S allele is associated with increased alcohol dependence, addictive behaviors, and personality traits like neuroticism (Feinn et al. 2005; Herman et al. 2003; Munafò et al. 2006). The homeostatic effects of serotoninergic systems in genesis, differentiation, and maturation of neurons are thought to be responsible for these effects. Serotonin is an important mitogenetic and morphogenetic factor for brain development (Gaspar et al. 2003). The S allele has decreased serotonin transporters and increased serotoninergic activity in the synapse. Through unknown mechanisms, serotonin affects the formation and plasticity of both neocortical and subcortical brain matter (Lesch and Gutknecht 2005). This hypothesis has been confirmed in knockout animals where serotonin transporter genes were completely removed from the genome of the experimental animals. Distinctive brain and behavioral irregularities were observed in these animals, which were consistent with those observed in SS and SL genotypes (Lesch and Gutknecht 2005). These studies suggest that excess of serotonin in S alleles produces irreversible changes in brain development and thereby potentially increased likelihood for addictive and high-risk sexual behaviors.
Although considerable research has focused on S and L alleles of 5-HTTLPR polymorphism, many other alleles have also been discovered. Based on the number of repeats, 5-HTTLPR has been classified into the S allele with 14 repeats and L allele with 16 repeats. However, other alleles with 15, 19, 20, and 22 repeats have also been described (Delbrück et al. 1997; Gelernter et al. 1997; Kunugi et al. 1996; Michaelovsky et al. 1999). Because of the relative rarity of these alleles, not many researchers have focused on this topic. This is, indeed, a limitation because it restricts our understanding about the overarching 5-HTTLPR polymorphism phenomena. In addition, there are two subtypes of L alleles: LA and LG, identified as early as 2005 (Hu et al. 2005). Studies show differences between LA and LG subtypes in relation to alcoholism (Enoch et al. 2011; Kranzler et al. 2011; Philibert et al. 2008; Thompson et al. 2010; Wang et al. 2012). Comparative transcriptional activity between the LG, LA, and the S alleles showed that the LA allele is associated with increased transcriptional activity of 5-HTT, compared to LG, which is closer to the S allele (Hu et al. 2005). Therefore, inconsistencies in associations between S or L alleles and alcohol use problems and risky sexual behaviors could be due to these LG and LA subtypes. Novel investigations based on the LA and LG variants could help in explaining some of the previously reported inconsistencies.
The mechanisms linking 5-HTTLPR polymorphism, alcohol use problems, and risky sexual behaviors are not fully understood. There could be gene × environment interactions influencing this relationship. Many studies have researched and established such interactions influencing 5-HTTLPR polymorphism and alcoholism. Environmental factors include many complex associations (e.g., stressful events, childhood abuse, peer pressure, unstable family environments, delinquency, etc.) play significant role in later development of alcohol use problems. In a study done by Vaht et al. (2014) among 1075 participants, it was observed that the effects of 5-HTTLPR on alcohol use was affected by a significant genotype × gender × cohort interaction (p < 0.001). Females with an SS genotype in the older cohort (15 years old) reported significantly delayed first alcohol consumption when compared to females with SS genotype in the younger cohort (9 years old). This study suggested that such effects could be due to the environmental differences between the two cohorts. In a study done by Nilsson et al. (2005), 5-HTT genotype and family relations interacted with one another and marginally predicted alcohol consumption in a group of 200 adolescents (p = 0.05). Similar results were observed in a study by Kim et al. (2015) among 5091 adolescents where a significant gene × environment interaction influenced alcohol misuse in these participants. This study showed that low-activity alleles of 5-HTTLPR polymorphisms (S and LG alleles), when compounded with family conflicts, resulted in significant increase in alcohol misuse. Studies have also suggested that serotonin neurotransmission is a plastic phenomenon which changes in response to stressors and life experiences (Nilsson et al. 2005; Uher and McGuffin 2008). Thus, the effect of genetic susceptibility can be enhanced or decreased depending upon life circumstances and environmental factors such as parental influences, delinquent friends, unstable family structure, low socioeconomic status, substance abuse among peers, gender discrimination, STI/HIV status, sex education, sexual behavior, norms, etc. Genetic factors may contribute an inherent susceptibility for alcohol abuse and sexual risk behaviors, whereas environmental factors are likely to act as moderators and induce expressions of these traits. For example, Kogan et al. (2010) study reported high-risk sexual behaviors at age 16 if the subjects had learned high-risk behaviors at age 14 only if they had S allele. Through similar mechanisms, genetic propensity to alcohol use and sexual risk behaviors could be influenced by environmental factors leading to more dramatic expression of these behaviors.
The biological mechanisms of 5-HTTLPR polymorphism and alcohol use problems and sexual risk behaviors are still hazy. At this point, only associations have been made, but exact mechanisms have not been established. This opens a broad scope for genetic and environmental studies to understand the mechanisms and develop models for such associations.
Conclusion
Considering the existence of 5-HTTLPR polymorphism and its behavioral outcomes discussed above, alcohol use problems and high-risk sexual behaviors cannot be considered entirely as acts of volition. The effects of this polymorphism on alcohol use problems and risky sexual behaviors should be considered when developing HIV/STI and alcohol prevention programs. Future research should focus on larger and robust studies with enhanced external validity that explore 5-HTTLPR polymorphism, alcohol use problems, and sexual risk behaviors. This would help to establish protocols for effective STI/HIV prevention programs and alcohol cessation programs, possibly including pharmacotherapy (e.g., SSRI treatment) where deficiencies are noted. Finally, we recommend that a model linking factors associated with 5-HTTLPR polymorphism, alcohol use problems, and sexual risk behaviors be developed to further understand and explore these complex relationships.
Acknowledgments
Conflict of interest
The authors declare that they have no competing interests.
Compliance with ethical standards
This study does not involve human participants and/or animals. This study does not involve participant recruitment or enrollment and consequently no informed consent procedures
References
- American Psychological Association . The diagnostic and statistical manual of mental disorders: DSM 5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and reward deficiency syndrome. Am J Med Genet B Neuropsychiatr Genet. 2005;132B(1):29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Bryant KJ. Expanding research on the role of alcohol consumption and related risks in the prevention and treatment of HIV/AIDS. Substance Use Misuse. 2006;41(10–12):1465–1507. doi: 10.1080/10826080600846250. [DOI] [PubMed] [Google Scholar]
- Carver CS, Miller CJ. Relations of serotonin function to personality: current views and a key methodological issue. Psychiatry Res. 2006;144(1):1–15. doi: 10.1016/j.psychres.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236(4800):410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Siever LJ, Owen KR, Davis KL. Serotonin in mood and personality disorders. Washington, DC: American Psychiatric Association; 1990. [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10(6):350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- Dawes MA, et al. Drinking histories in alcohol-use-disordered youth: preliminary findings on relationships to platelet serotonin transporter expression with genotypes of the serotonin transporter. J Stud Alcohol Drugs. 2009;70(6):899–907. doi: 10.15288/jsad.2009.70.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbrück S, et al. A novel allelic variant of the human serotonin transporter gene regulatory polymorphism. Cytogenet Genome Res. 1997;79(3–4):214–220. doi: 10.1159/000134726. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, et al. A family-based analysis of whether the functional promoter alleles of the serotonin transporter gene HTT affect the risk for alcohol dependence. Alcohol Clin Exp Res. 1998;22(5):1080–1085. doi: 10.1111/j.1530-0277.1998.tb03704.x. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Gorodetsky E, Hodgkinson C, Roy A, Goldman D. Functional genetic variants that increase synaptic serotonin and 5-HT3 receptor sensitivity predict alcohol and drug dependence. Mol Psychiatry. 2011;16(11):1139–1146. doi: 10.1038/mp.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinn R, Nellissery M, Kranzler HR. Meta‐analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2005;133B(1):79–84. doi: 10.1002/ajmg.b.30132. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4(12):1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African-and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet. 1997;101(2):243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Dukat M. Serotonin receptors and drugs affecting serotonergic neurotransmission. In: Lemke TL, Williams DA, editors. Foye’s principles of medicinal chemistry. Baltimore: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- Gokturk C, Schultze S, Nilsson KW, von Knorring L, Oreland L, Hallman J. Serotonin transporter (5-HTTLPR) and monoamine oxidase (MAOA) promoter polymorphisms in women with severe alcoholism. Arch Women’s Mental Health. 2008;11(5–6):347–355. doi: 10.1007/s00737-008-0033-6. [DOI] [PubMed] [Google Scholar]
- Gorwood P, Batel P, Ades J, Hamon M, Boni C. Serotonin transporter gene polymorphisms, alcoholism, and suicidal behavior. Biol Psychiatry. 2000;48(4):259–264. doi: 10.1016/S0006-3223(00)00840-4. [DOI] [PubMed] [Google Scholar]
- Hallikainen T, et al. Association between low activity serotonin transporter promoter genotype and early onset alcoholism with habitual impulsive violent behavior. Mol Psychiatry. 1999;4(4):385–388. doi: 10.1038/sj.mp.4000526. [DOI] [PubMed] [Google Scholar]
- Hamer D. Genetics of sexual behavior. molecular genetics and the human personality. In: Benjamin J, Ebstein RP, Belmaker RH, editors. Molecular genetics and the human personality. Washington, DC: American Psychiatric Publishing; 2002. [Google Scholar]
- Hammoumi S, et al. Does the short variant of the serotonin transporter linked polymorphic region constitute a marker of alcohol dependence? Alcohol. 1999;17(2):107–112. doi: 10.1016/S0741-8329(98)00040-8. [DOI] [PubMed] [Google Scholar]
- Heils A, et al. Functional promoter and polyadenylation site mapping of the human serotonin (5-HT) transporter gene. J Neural Trans/Gen Sect JNT. 1995;102(3):247–254. doi: 10.1007/BF01281159. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66(6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Mann K, Weinberger DR, Goldman D. Serotonergic dysfunction, negative mood states, and response to alcohol. Alcohol Clin Exp Res. 2001;25(4):487–495. doi: 10.1111/j.1530-0277.2001.tb02240.x. [DOI] [PubMed] [Google Scholar]
- Herman AI, Philbeck JW, Vasilopoulos NL, Depetrillo PB. Serotonin transporter promoter polymorphism and differences in alcohol consumption behaviour in a college student population. Alcohol Alcohol. 2003;38(5):446–449. doi: 10.1093/alcalc/agg110. [DOI] [PubMed] [Google Scholar]
- Herman AI, Kaiss KM, Ma R, Philbeck JW, Hasan A, Dasti H, Depetrillo PB. Serotonin transporter promoter polymorphism and monoamine oxidase type A VNTR allelic variants together influence alcohol binge drinking risk in young women. Am J Med Genet B Neuropsychiatr Genet. 2005;133(1):74–78. doi: 10.1002/ajmg.b.30135. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71(4):533–554. doi: 10.1016/S0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29(1):8–16. doi: 10.1097/01.ALC.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, et al. Association between drinking-related antisocial behavior and a polymorphism in the serotonin transporter gene in a Japanese population. Alcohol Clin Experiment Res. 1999;23(7):1281–1284. doi: 10.1111/j.1530-0277.1999.tb04289.x. [DOI] [PubMed] [Google Scholar]
- Johann M, Bobbe G, Putzhammer A, Wodarz N. Comorbidity of alcohol dependence with attention‐deficit hyperactivity disorder: differences in phenotype with increased severity of the substance disorder, but not in genotype (Serotonin Transporter and 5‐Hydroxytryptamine‐2c Receptor) Alcohol Clin Exp Res. 2003;27(10):1527–1534. doi: 10.1097/01.ALC.0000090143.00703.07. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Serotonergic agents and alcoholism treatment: rebirth of the subtype concept and hypothesis. Alcohol Clin Exp Res. 2000;24(10):1597–1601. [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N. Neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Psychopharmacology. 2000;149(4):327–344. doi: 10.1007/s002130000371. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Simbayi LC, Kaufman M, Cain D, Jooste S. Alcohol use and sexual risks for HIV/AIDS in sub-Saharan Africa: systematic review of empirical findings. Prev Sci. 2007;8(2):141–151. doi: 10.1007/s11121-006-0061-2. [DOI] [PubMed] [Google Scholar]
- Kim J, et al. Interaction effects between the 5‐hydroxy tryptamine transporter‐linked polymorphic region (5‐HTTLPR) genotype and family conflict on adolescent alcohol use and misuse. Addiction. 2015;110(2):289–299. doi: 10.1111/add.12753. [DOI] [PubMed] [Google Scholar]
- Kogan SM, Beach SR, Philibert RA, Brody GH, Y-F C, Lei M-K. 5-HTTLPR status moderates the effect of early adolescent substance use on risky sexual behavior. Health Psychol. 2010;29(5):471–476. doi: 10.1037/a0020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnke MD, Kolb W, Lutz U, Maurer S, Batra A. The serotonin transporter promotor polymorphism 5-HTTLPR is not associated with alcoholism or severe forms of alcohol withdrawal in a German sample. Psychiatr Genet. 2006;16(6):227–228. doi: 10.1097/01.ypg.0000218629.11375.b0. [DOI] [PubMed] [Google Scholar]
- Konishi T, Luo HR, Calvillo M, Mayo MS, Lin KM, Wan YJ. ADH1B*1, ADH1C*2, DRD2 (−141C Ins), and 5-HTTLPR are associated with alcoholism in Mexican American men living in Los Angeles. Alcohol Clin Exp Res. 2004;28(8):1145–1152. doi: 10.1097/01.ALC.0000134231.48395.42. [DOI] [PubMed] [Google Scholar]
- Kranzler H, Lappalainen J, Nellissery M, Gelernter J. Association study of alcoholism subtypes with a functional promoter polymorphism in the serotonin transporter protein gene. Alcohol Clin Exp Res. 2002;26(8):1330–1335. doi: 10.1111/j.1530-0277.2002.tb02675.x. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, et al. A double-blind, randomized trial of sertraline for alcohol dependence: moderation by age of onset and 5-HTTLPR genotype. J Clin Psychopharmacol. 2011;31(2):22–30. doi: 10.1097/JCP.0b013e31820465fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunugi H, et al. Serotonin transporter gene polymorphisms: ethnic difference and possible association with bipolar affective disorder. Mol Psychiatry. 1996;2(6):457–462. doi: 10.1038/sj.mp.4000334. [DOI] [PubMed] [Google Scholar]
- Kweon YS, Lee HK, Lee CT, Lee KU, Pae CU. Association of the serotonin transporter gene polymorphism with Korean male alcoholics. J Psychiatr Res. 2005;39(4):371–376. doi: 10.1016/j.jpsychires.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Gutknecht L. Pharmacogenetics of the serotonin transporter. Prog Neuro-Psychopharmacol Biol Psychiatry. 2005;29(6):1062–1073. doi: 10.1016/j.pnpbp.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Balling U, Gross J, Strauss K, Wolozin BL, Murphy DL, Riederer P. Organization of the human serotonin transporter gene. J Neural Trans / Gen Sec JNT. 1994;95(2):157–162. doi: 10.1007/BF01276434. [DOI] [PubMed] [Google Scholar]
- Lesch KP, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lichtermann D, et al. Support for allelic association of a polymorphic site in the promoter region of the serotonin transporter gene with risk for alcohol dependence. Am J Psychiatry. 2000;157(12):2045–2047. doi: 10.1176/appi.ajp.157.12.2045. [DOI] [PubMed] [Google Scholar]
- Lin SC, Wu PL, Ko HC, Wu JY, Huang SY, Lin WW, Lu RB. Specific personality traits and dopamine, serotonin genes in anxiety-depressive alcoholism among Han Chinese in Taiwan. Prog Neuro-Psychopharmacol Biol Psychiatry. 2007;31(7):1526–1534. doi: 10.1016/j.pnpbp.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Mannelli P, Patkar AA, Murray HW, Certa K, Peindl K, Mattila-Evenden M, Berrettini WH. Polymorphism in the serotonin transporter gene and response to treatment in African American cocaine and alcohol-abusing individuals. Addict Biol. 2005;10(3):261–268. doi: 10.1080/13556210500235540. [DOI] [PubMed] [Google Scholar]
- Matsushita S, Yoshino A, Murayama M, Kimura M, Muramatsu T, Higuchi S. Association study of serotonin transporter gene regulatory region polymorphism and alcoholism. Am J Med Genet. 2001;105(5):446–450. doi: 10.1002/ajmg.1405. [DOI] [PubMed] [Google Scholar]
- McArthur JC, et al. Low prevalence of neurological and neuropsychological abnormalities in otherwise healthy HIV-1-infected individuals: results from the multicenter AIDS Cohort Study. Ann Neurol. 1989;26(5):601–611. doi: 10.1002/ana.410260504. [DOI] [PubMed] [Google Scholar]
- Mchale SM, Bissell J, Kim J-Y. Sibling relationship, family, and genetic factors in sibling similarity in sexual risk. J Fam Psychol. 2009;23(4):562–572. doi: 10.1037/a0014982. [DOI] [PubMed] [Google Scholar]
- Mchugh RK, Hofmann SG, Asnaani A, Sawyer AT, Otto MW. The serotonin transporter gene and risk for alcohol dependence: a meta-analytic review. Drug Alcohol Depend. 2010;108(1):1–6. doi: 10.1016/j.drugalcdep.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenakk L, Maestu J, Nordquist N, Parik J, Oreland L, Loit HM, Harro J. Effects of the serotonin transporter (5-HTTLPR) and α2A-adrenoceptor (C-1291G) genotypes on substance use in children and adolescents: a longitudinal study. Psychopharmacology. 2011;215(1):13–22. doi: 10.1007/s00213-010-2109-z. [DOI] [PubMed] [Google Scholar]
- Michaelovsky E, Frisch A, Rockah R, Peleg L, Magal N, Shohat M, Weizman R. A novel allele in the promoter region of the human serotonin transporter gene. Mol Psychiatry. 1999;4(1):97–99. doi: 10.1038/sj.mp.4000449. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Clark TG, Roberts KH, Johnstone EC. Neuroticism mediates the association of the serotonin transporter gene with lifetime major depression. Neuropsychobiology. 2006;53(1):1–8. doi: 10.1159/000089915. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry. 2000;5(1):32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- Nellissery M, et al. Alleles of a functional serotonin transporter promoter polymorphism are associated with major depression in alcoholics. Alcohol Clin Exp Res. 2003;27(9):1402–1408. doi: 10.1097/01.ALC.0000085588.11073.BB. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, et al. Role of the serotonin transporter gene and family function in adolescent alcohol consumption. Alcohol Clin Exp Res. 2005;29(4):564–570. doi: 10.1097/01.ALC.0000159112.98941.B0. [DOI] [PubMed] [Google Scholar]
- Olsson C, Byrnes G, Lotfi-Miri M, Collins V, Williamson R, Patton C, Anney R. Association between 5-HTTLPR genotypes and persisting patterns of anxiety and alcohol use: results from a 10-year longitudinal study of adolescent mental health. Mol Psychiatry. 2005;10(9):868–876. doi: 10.1038/sj.mp.4001677. [DOI] [PubMed] [Google Scholar]
- Organista KC, Kubo A. Pilot survey of HIV risk and contextual problems and issues in Mexican/Latino migrant day laborers. J Immigr Minor Health. 2005;7(4):269–281. doi: 10.1007/s10903-005-5124-0. [DOI] [PubMed] [Google Scholar]
- Paaver M, Nordquist N, Parik J, Harro M, Oreland L, Harro J. Platelet MAO activity and the 5-HTT gene promoter polymorphism are associated with impulsivity and cognitive style in visual information processing. Psychopharmacology. 2007;194(4):545–554. doi: 10.1007/s00213-007-0867-z. [DOI] [PubMed] [Google Scholar]
- Paaver M, Kurrikoff T, Nordquist N, Oreland L, Harro J. The effect of 5-HTT gene promoter polymorphism on impulsivity depends on family relations in girls. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32(5):1263–1268. doi: 10.1016/j.pnpbp.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Pastorelli R, et al. Genetic determinants of alcohol addiction and metabolism: a survey in Italy. Alcohol Clin Exp Res. 2001;25(2):221–227. doi: 10.1111/j.1530-0277.2001.tb02202.x. [DOI] [PubMed] [Google Scholar]
- Philibert RA, Sandhu H, Hollenbeck N, Gunter T, Adams W, Madan A. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. Am J Med Genet B Neuropsychiatr Genet. 2008;147(5):543–549. doi: 10.1002/ajmg.b.30657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto E, Reggers J, Gorwood P, Boni C, Scantamburlo G, Pitchot W, Ansseau M. The short allele of the serotonin transporter promoter polymorphism influences relapse in alcohol dependence. Alcohol Alcohol. 2008;43(4):398–400. doi: 10.1093/alcalc/agn015. [DOI] [PubMed] [Google Scholar]
- Pombo S, de Quinhones LP, Bicho M, Barbosa A, Ismail F, Cardoso N. Association of the functional serotonin transporter promoter polymorphism (5-HTTLPR) with externalizing and internalizing aggressivity and alcohol abuse. Acta Med Port. 2008;21(6):539–546. [PubMed] [Google Scholar]
- Preuss UW, et al. Serotonin transporter gene regulatory region polymorphism (5-HTTLPR), [3H]paroxetine binding in healthy control subjects and alcohol-dependent patients and their relationships to impulsivity. Psychiatry Res. 2000;96(1):51–61. doi: 10.1016/S0165-1781(00)00190-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen H, Bagger Y, Tanko LB, Christiansen C, Werge T. Lack of association of the serotonin transporter gene promoter region polymorphism, 5-HTTLPR, including rs25531 with cigarette smoking and alcohol consumption. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(5):575–580. doi: 10.1002/ajmg.b.30880. [DOI] [PubMed] [Google Scholar]
- Rees V, Saitz R, Horton NJ, Samet J. Association of alcohol consumption with HIV sex- and drug-risk behaviors among drug users. J Subst Abus Treat. 2001;21(3):129–134. doi: 10.1016/S0740-5472(01)00190-8. [DOI] [PubMed] [Google Scholar]
- Saiz PA, et al. Differential role of serotonergic polymorphisms in alcohol and heroin dependence. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33(4):695–700. doi: 10.1016/j.pnpbp.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Sales JM, Diclemente RJ, Brody GH, Philibert RA, Rose E. Interaction between 5-HTTLPR polymorphism and abuse history on adolescent African-American females’ condom use behavior following participation in an HIV prevention intervention. Prev Sci. 2014;15(3):257–267. doi: 10.1007/s11121-013-0378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander T, et al. Serotonin transporter gene variants in alcohol-dependent subjects with dissocial personality disorder. Biol Psychiatry. 1998;43(12):908–912. doi: 10.1016/S0006-3223(97)00356-9. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Mazzanti C, Smith TL, Ahmed U, Radel M, Iwata N, Goldman D. Selective genotyping for the role of 5-HT2A, 5-HT2C, and GABA alpha 6 receptors and the serotonin transporter in the level of response to alcohol: a pilot study. Biol Psychiatry. 1999;45(5):647–651. doi: 10.1016/S0006-3223(98)00248-0. [DOI] [PubMed] [Google Scholar]
- Skowronek MH, Laucht M, Hohm E, Becker K, Schmidt MH. Interaction between the dopamine D4 receptor and the serotonin transporter promoter polymorphisms in alcohol and tobacco use among 15-year-olds. Neurogenetics. 2006;7(4):239–246. doi: 10.1007/s10048-006-0050-4. [DOI] [PubMed] [Google Scholar]
- Stoltenberg SF, Twitchell GR, Hanna GL, Cook EH, Fitzgerald HE, Zucker RA, Little KY. Serotonin transporter promoter polymorphism, peripheral indexes of serotonin function, and personality measures in families with alcoholism. Am J Med Genet. 2002;114(2):230–234. doi: 10.1002/ajmg.10187. [DOI] [PubMed] [Google Scholar]
- Thompson MD, Gonzalez N, Nguyen T, Comings DE, George SR, O’Dowd BF. Serotonin transporter gene polymorphisms in alcohol dependence. Alcohol. 2000;22(2):61–67. doi: 10.1016/S0741-8329(00)00105-1. [DOI] [PubMed] [Google Scholar]
- Thompson RD, Heffner JL, Strong JA, Blom TJ, Anthenelli RM. Relationship between the serotonin transporter polymorphism and obsessive–compulsive alcohol craving in alcohol-dependent adults: a pilot study. Alcohol. 2010;44(5):401–406. doi: 10.1016/j.alcohol.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobst KK, Herbst JH, Masters HL, Costa PT. Personality pathways to unsafe sex: personality, condom use, and HIV risk behaviors. J Res Pers. 2002;36(2):117–133. doi: 10.1006/jrpe.2001.2334. [DOI] [Google Scholar]
- Uher R, Mcguffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13(2):131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Vaht M, Merenäkk L, Mäestu J, Veidebaum T, Harro J. Serotonin transporter gene promoter polymorphism (5-HTTLPR) and alcohol use in general population: interaction effect with birth cohort. Psychopharmacology. 2014;231(13):2587–2594. doi: 10.1007/s00213-013-3427-8. [DOI] [PubMed] [Google Scholar]
- van der Zwaluw CS, et al. A serotonin transporter polymorphism (5-HTTLPR) predicts the development of adolescent alcohol use. Drug Alcohol Depend. 2010;112(1):134–139. doi: 10.1016/j.drugalcdep.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Villalba K, Attonito J, Mendy A, Devieux JG, Gasana J, Dorak TM. A meta-analysis of the associations between the SLC6A4 promoter polymorphism (5HTTLPR) and the risk for alcohol dependence. Psychiatr Genet. 2015;25(2):47–58. doi: 10.1097/YPG.0000000000000078. [DOI] [PubMed] [Google Scholar]
- Vormfelde SV, Hoell I, Tzvetkov M, Jamrozinski K, Sehrt D, Brockmöller J, Leibing E. Anxiety-and novelty seeking-related personality traits and serotonin transporter gene polymorphisms. J Psychiatr Res. 2006;40(6):568–576. doi: 10.1016/j.jpsychires.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Zhong SR, Bao JJ, Dou SJ, Wu WY, Jing Q (2011) Association of polymorphism in the serotonin transporter gene promote with the susceptibility to alcohol dependence in Yunnan Han population Yi Chuan 33(1):48–53 [DOI] [PubMed]
- Wang T-Y, et al. Interaction between serotonin transporter and serotonin receptor 1 B genes polymorphisms may be associated with antisocial alcoholism. Behav Brain Funct. 2012;8:18. doi: 10.1186/1744-9081-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D, Nizam H The role of a serotonin transporter gene polymorphism in relation to novelty seeking and risky sexual behavior. In: National Conference on Undergraduate Research April 3-April 5, 2014), 2014. University of Kentucky
- Zuckerman M (1979) Sensation seeking. Corsini Encyclopedia Psychol 1–4