Abstract
Currently, there is no consensus regarding services required to help families with consanguineous marriages manage their increased genetic reproductive risk. Genetic services for communities with a preference for consanguineous marriage in the UK remain patchy, often poor. Receiving two disparate explanations of the cause of recessive disorders (cousin marriage and recessive inheritance) leads to confusion among families. Further, the realisation that couples in non-consanguineous relationships have affected children leads to mistrust of professional advice. British Pakistani families at-risk for recessive disorders lack an understanding of recessive disorders and their inheritance. Such an understanding is empowering and can be shared within the extended family to enable informed choice. In a three-site qualitative study of British Pakistanis, we explored family and health professional perspectives on recessively inherited conditions. Our findings suggest, firstly, that family networks hold strong potential for cascading genetic information, making the adoption of a family-centred approach an efficient strategy for this community. However, this is dependent on provision of high-quality and timely information from health care providers. Secondly, families’ experience was of ill-coordinated and time-starved services, with few having access to specialist provision from Regional Genetics Services; these perspectives were consistent with health professionals’ views of services. Thirdly, we confirm previous findings that genetic information is difficult to communicate and comprehend, further complicated by the need to communicate the relationship between cousin marriage and recessive disorders. A communication tool we developed and piloted is described and offered as a useful resource for communicating complex genetic information.
Electronic supplementary material
The online version of this article (doi:10.1007/s12687-015-0252-2) contains supplementary material, which is available to authorized users.
Keywords: Family-centred approach, Consanguinity, British Pakistanis, Recessive disorders, Genetic communication
Introduction
Over one billion people live in societies where consanguineous marriages are common (Bittles 2012) and, across the world, 15 % of all newborns have consanguineous parents. Consanguineous marriage is defined as a union in which the couple are related as second cousins or closer. Parental consanguinity is associated with an increased birth prevalence of children with severe recessively inherited disorders.1 One feature of a global decline in infant mortality is that more affected children survive. This unmasks the extent of congenital disorders, and tackling consanguinity-related disorders have emerged as a global public health challenge (Bittles 1990; Alwan and Modell 1997).
Recessive disorders are transmitted by parents who carry one copy of a gene that can cause a disorder. When both parents carry the same gene for the disorder, every child they have has a 25 % risk of suffering from that recessive disorder. The chance that both parents will be carriers of the same disorder producing gene is influenced by the extent to which the gene is endemic in a community or in particular families. Consequently, recessive disorders manifest differently in communities with different modes of partner choice with implications for policy in devising genetic services.
In populations of Northern European origin, where parental consanguinity is uncommon, an overall 2–3 % risk of a congenital disorder includes a 0.17 % risk of a recessive disorder (4–5 % of the total) (Baird et al. 1988), manifestation of recessive disorders is sporadic and thinly scattered throughout the population. In contrast, consanguineous communities have a higher risk of congenital disorders and recessive gene variants tend to cluster in extended family groupings: this increases the chance that couples will both carry the same recessive variant, with a corresponding increase in the birth prevalence of recessive disorders (Modell and Darr 2002). For example, in some groups of Middle Eastern or Pakistani origin, consanguinity-associated disorders may almost double the total birth prevalence of congenital disorders (Bittles 1990; Bundey and Alam 1993). A recent prospective birth cohort study of 13,776 babies and their families recruited between 2007 and 2011, the Born in Bradford study (Wright et al. 2012), identified that 1922 (37 %) of 5127 babies of Pakistani origin had parents in first-cousin unions. In this study, consanguinity was also associated with a doubling of risk for congenital anomaly; 6 % of the offspring of first-cousin unions and 5 % of those more distantly related but still consanguineous parents had an anomaly (Sheridan et al. 2013).
In Western Europe and North America, transnational migration has resulted in a mix of communities in which consanguineous marriage is uncommon, and those in which it is customary (Darr 2009; Bittles 2012). In the UK, it is common for groups of Pakistani, Bangladeshi and Middle Eastern origin, some groups of Indian origin, Irish travellers and some refugee groups (Modell and Darr 2002). In the Born in Bradford study, 59 % of pregnant Pakistani-origin women (n 5127) reported being in consanguineous marriages (Bhopal et al. 2013)—the highest incidence of such marriages in these groups. We estimate that these groups contribute three quarters of consanguineous marriages in the UK, with consequent marked variations in the birth prevalence of recessive disorders by ethnic origin.
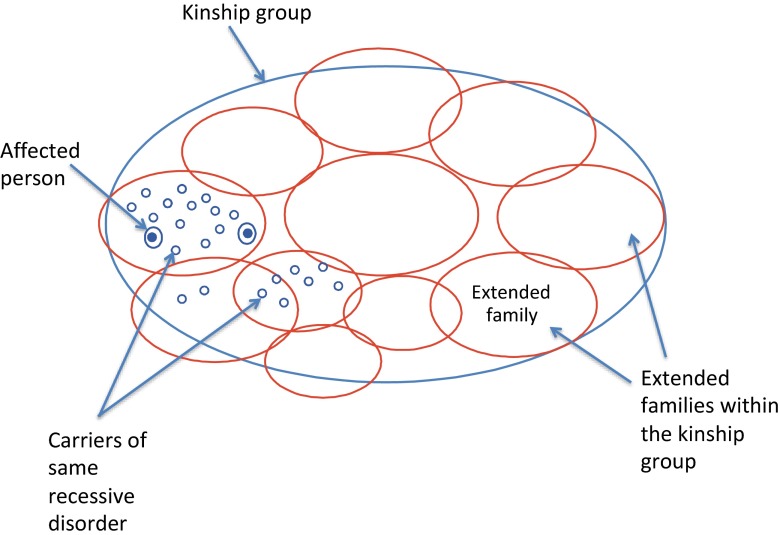
The Eastern Mediterranean Regional Office of the World Health Organisation has recognised that “consanguineous marriage is an integral part of cultural and social life in many areas and that attempts to discourage it at the population level are undesirable and inappropriate”. It proposed a family-centred approach for identifying extended families at increased risk, and for providing genetic counselling and cascade genetic testing when feasible (Alwan and Modell 1997; Modell and Darr 2002). In a family-centred approach, the diagnosis of an affected child leads to identification of an extended family at increased genetic risk. It proposes that familial links are both genetic links and potential channels for information and support (Darr 1997; Ahmed et al. 2002; Darr et al. 2012). Thus, the clustered concentration of potentially at-risk individuals within extended family groups (Fig. 1) provides a social structure that could lend itself to the effective transmission of genetic information via these kinship ties. The acceptability and potential effectiveness of such an approach have been confirmed in Pakistan using thalassaemia as an example (Ahmed et al. 2002).
Fig. 1.
Clustering of recessive genes in a consanguineous kinship group. In communities with customary consanguineous marriage, the population make-up consists largely of several or more kinship groups (referred to, for example, as tribes, clans, biraderis). Each kinship group is made up of a large number of extended families. Figure 1 shows the clustered concentration of individuals potentially at-risk for the same recessive disorder in extended families of one such kinship group. Other recessive disorders may also be clustered in this or other extended families within the same kinship group. This is in contrast to the sporadic manifestation of recessive disorders in populations with random partner choice
An alternative strategy aiming to reduce impairment2 by discouraging cousin marriage through promoting awareness of the associated genetic risk has been tried in the Middle East using media campaigns and teaching of health professionals (Samavat and Modell 2004), and in the UK using a media campaign, leaflets, a video and schools roadshow (Haslam 2001). In both situations, this isolated policy of raising awareness had no detectable impact on marriage choices but prompted negative community reaction (Director, Heart of Birmingham PCT 2008, personal communication). This strategy has two major flaws: firstly, the assumption that communication of overall population risk provides sufficient motivation for individuals to adjust partner choice to reduce genetic risk; secondly, the health message presented the cause of impairments as cousin marriage rather than both partners being carriers of the same recessive disorder. This inaccuracy is evident to community members who see cousin couples with healthy children and non-cousin couples with children with impairments (Darr et al. 2012).
Thus, the attempt at public engagement by over-simplification proved confusing and counter-productive. It did, however, demonstrate the pivotal role of accurate information in genetics. Table 1 details the information that needs to be understood by families in order to make informed choices.
Table 1.
Sequences of information required by different family members
| Level 1: Information required by all family members | 1. Genes for a recessive disorder may be inherited from parents and ancestors |
| Level 2: Information required by carrier couples to make informed choice about future children | 1. Both parents carry one gene for a recessive disorder |
| 2. One-in-four risk in every pregnancy of having an affected child | |
| 3. Prenatal diagnosis may be available to detect if child is affected/unaffected | |
| Level 3: Information required by carrier parents to pass onto their children to ensure they are able to make informed choices about genetic testing, partner choice and future children | 1. Carrier parents may pass on their gene for the recessive disorder to their children |
| 2. Unaffected children may carry the same recessive gene | |
| 3. Genetic test may be available to detect carrier status of unaffected persons | |
| 4. Unaffected persons who are carriers could have affected children if their partner also carries the same recessive gene | |
| 5. A blood relative is more likely to carry the same recessive gene than an unrelated person | |
| Level 4: Information required by carriers to pass onto siblings and other extended family members to ensure they are able to make informed choices about genetic testing, partner choice and future children | 1. The recessive gene has been transmitted through family ancestors |
| 2. Siblings of a carrier, and their children, may also carry a gene for the same disorder | |
| 3. Genetic test may be available to detect carrier status of unaffected relatives | |
| 4. A person who is a blood relative is more likely to carry the same recessive gene than an unrelated person |
There is a strong theoretical case for integrating a family-centred approach for communities with a consanguineous kinship pattern into existing genetics services (Modell and Darr 2002). Such integration requires the co-existence of three key components: (1) active kinship networks and a willingness of at-risk families to share genetic information; (2) health professionals with adequate training and resources to deliver information and support services; and (3) the availability of information tools to facilitate effective communication between professionals and families and within families.
This qualitative study explored community, family and health professional perspectives in a Pakistani origin community in the UK. A previous paper (Darr et al. 2012) based on focus group discussions with community members demonstrated their willingness to engage with debates in genetics. Here, we present the perspectives of parents and extended families that include a member with a recessive disorder, and health professionals involved in the care of people with recessive disorders. Early in the study, it became clear that interviewees had inaccurate or limited knowledge of recessive inheritance. A communication tool for health professionals and families was therefore developed and piloted. The rationale for the tool, its development and utility is described.
Methods
Ethical approval for this Department of Health funded study was gained from the local NHS Research Ethics Committee. The fieldwork (June 2007–September 2008) was conducted in three cities in the North, North-West and Midlands regions of the UK, selected because (1) they include a large Pakistani-origin community; (2) impairments due to recessive disorders were recognised as important local health issues; and (3) the research team had existing contacts with clinicians, necessary to gain access to respondents. Working in three different sites minimised potential geographical bias related to community or service factors. Qualitative interviews, using topic guides, were undertaken with affected families (face to face) and with relevant health workers (by telephone). We explored attitudes and experience, and the communication of genetic risk information.
Family interviews
Criteria for family selection were as follows: (1) a child with a recessively inherited disorder and (2) carrier testing and prenatal diagnosis available for the condition. The study included 16 extended families with 13 recessively inherited disorders covering a range of severity (Table 2). In total, 54 interviews were conducted, 24 with primary interviewees (all parents of the person with a recessive disorder) and 30 with secondary interviewees (29 extended family members and 1 sibling) (Table 3).
Table 2.
Range of conditions: 16 families with 13 diagnoses
| Condition | No. of families | Classed as | |
|---|---|---|---|
| 1 | Congenital goitrous hypothyroidism (inherited) | 1 | Less severe, manageable |
| 2 | 3M syndrome | 1 | Severe, manageable |
| 3 | Glanzmann’s thrombasthenia | 1 | Severe, manageable |
| 4 | Thalassaemia major | 3 | Severe, manageable |
| 5 | 4-Hydroxybutyric aciduria (succinic semialdehyde dehydrogenase deficiency) | 1 | Severe |
| 6 | Cohen syndrome | 1 | Severe |
| 7 | Fabry’s disease (aspartylglucosaminuria (AGU)) | 1 | Severe |
| 8 | Morquio disease (mucopolysaccharidosis IVB) | 1 | Severe |
| 9 | Muscular dystrophy and cleft palate | 1 | Severe |
| 10 | Fraser syndrome | 1 | Very severe |
| 11 | I-cell disease (mucolipidosis II) | 1 | Very severe |
| 12 | Multiple sulphatase deficiency | 1 | Very severe |
| 13 | Sanfilippo A (mucopolysaccharidosis 3A) | 2 | Very severe |
Classification of conditions:
Less severe: Relatively simple treatment gives effectively normal length and quality of life.
Severe: potential physical, intellectual or functional impairment. Treatment burdensome, survival into adult life
Very severe: physical, intellectual or functional impairment. Treatment relatively ineffective, death in childhood/early adult life
Table 3.
Details of family interviewees in 16 extended families (showing interviewees’ relationship to person with the disorder)
| Relationship to patient | Male | Female | Total |
|---|---|---|---|
| Parent | 8 | 16 | 24 |
| Sibling | 1 | 1 | |
| Grandparents | 1 | 4 | 5 |
| Uncle/aunt | 5 | 6 | 11 |
| Spouse of uncle/aunt | 1 | 3 | 4 |
| Cousin | 3 | 6 | 9 |
| Total | 18 | 36 | 54 |
Fifteen families were recruited by local paediatricians and/or their bilingual Specialist Health Visitor and an additional family through a patient support group. Parents were approached by the recruiting health professional during a clinic appointment, or by phone. Each was given an information pack containing a covering letter, bilingual information sheet (Urdu and English), and for those not literate in English or Urdu, an audiocassette version of the information sheet in Urdu (the main written language in Pakistan). The initial (primary) interviewee was the parent suggested by the recruiting health professional. The primary interviewee was then given bilingual information sheets for use in recruiting secondary interviewees within the extended family. Guidance on the selection of these secondary interviewees included taking account of personal circumstance, perceived willingness to be interviewed and availability for interview.
Written consent was obtained using a bilingual consent form prior to each interview. All but one participant agreed for the interviews to be recorded. All but one of the interviews were in English, Punjabi or Urdu, and often bilingual. All were conducted by the lead author (fluent in English, Urdu and Punjabi). One was conducted in Pushto with the help of a Pushto-speaking genetic health professional. Punjabi and Pushto are regional languages of Pakistan. A rudimentary family tree was taken at the beginning of each interview. Interviews took 1–5 hours depending on family size, the issues raised, delays due to interviewees’ commitments to children or other relatives and partaking in hospitality offered.
Health professional interviews
Fifteen telephone interviews, lasting 20–40 min, were conducted in the three fieldwork sites (Table 4). Interviews were recorded.
Table 4.
Health professionals interviewed and their role
| Role | No. of interviewed | |
|---|---|---|
| Secondary care | Consultant Geneticist | 1 |
| Genetic Counsellor | 2 | |
| Consultant Paediatrician | 3 | |
| Primary care | GP | 2 |
| Health Visitor | 2 | |
| Midwife | 2 | |
| Specialist Health Visitor | 1 | |
| Voluntary sector | Family Welfare Advisors | 2 |
| Total | 15 |
Development of communication tool
It became apparent at an early stage that parents and extended family interviewees had limited or inaccurate understanding of the condition and its inheritance, the relevance of cousin marriage and implications for other family members. The absence of a tool to facilitate communication about recessive inheritance by professionals and within families also became apparent. To aid communication, we developed a communication tool designed to explain genetic risk associated with recessive inheritance and its relationship to cousin marriage, in continuous consultation with the families. The tool was designed after the first ten interviews, developed in collaboration with the next four interviewees, and piloted at the end of the initial interview with the remaining 40 interviewees. An open-ended questionnaire was used to elicit responses to the tool. The interviewees retained the tool after the interview.
The communication tool draws on a prototype web-based genetic information resource for families and health professionals using haemoglobin disorders as an example (the Accessible Publishing of Genetic Information system (APoGI), http://www.chime.ucl.ac.uk/APoGI/). The outstanding lesson learnt from this prototype was the central importance of using simple, uncluttered images. Transmission of genetic information characteristically is not a one-off event but a long-term process. The tool is therefore designed as a resource for both health professionals and families, providing consistent information that can be utilised over a prolonged time period. It is intended for use by a health professional with a family member in the first instance. The family member is then given an exact copy for their own use and to assist them in communicating with the extended family. Box 1 summarises the information the tool is designed to convey. Pictorial representation is used to explain recessive inheritance and the impact of consanguineous marriage, accompanied by minimal text, thereby promoting visual impact, reducing translation costs for multilingual application and reducing reliance on literacy.
Box 1: Information that tool is capable of conveying
| 1. What is a carrier (how did it happen?) |
| 2. Both parents have to be carriers to be at risk of having an affected child |
| 3. In principle affected person can have healthy children (if clinically possible) |
| 4. How carriers are distributed in the immediate family, in extended family and wider society |
| 5. How marriage within the extended family can potentially increase the chance of having an affected child |
| 6. You have to be a carrier and not a cousin to be at risk |
| 7. Most people are carriers of one or more gene variants for a recessive disorder; to have a problem both parents must carry the same gene variant |
| The tool directs individuals to further sources of information and help. |
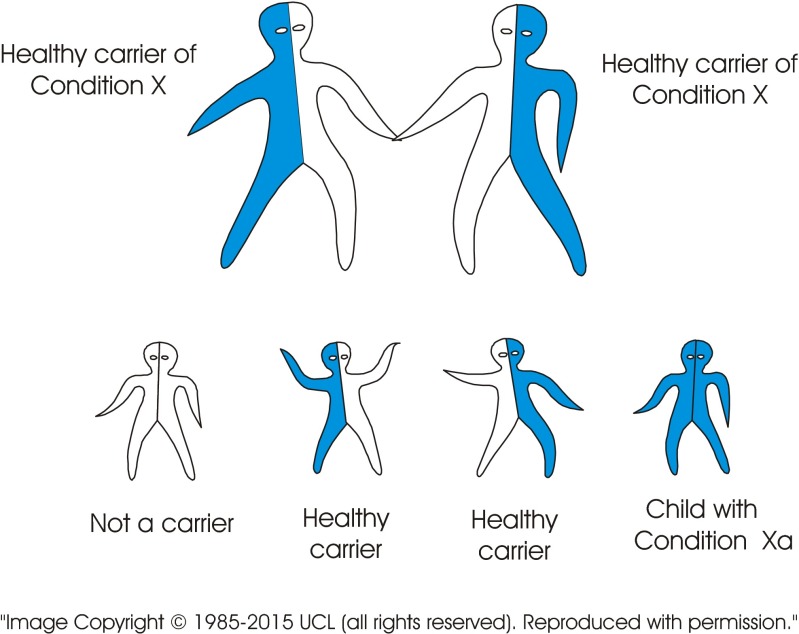
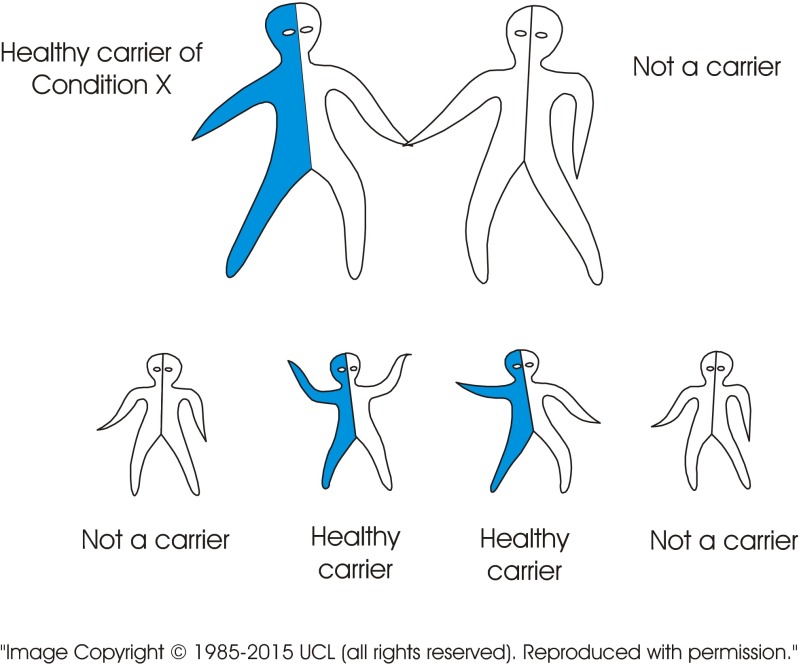
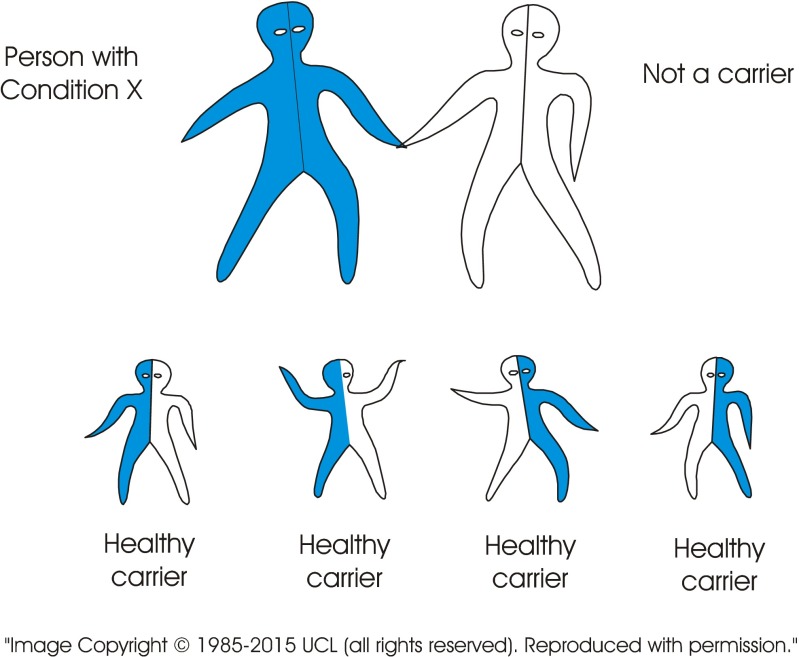
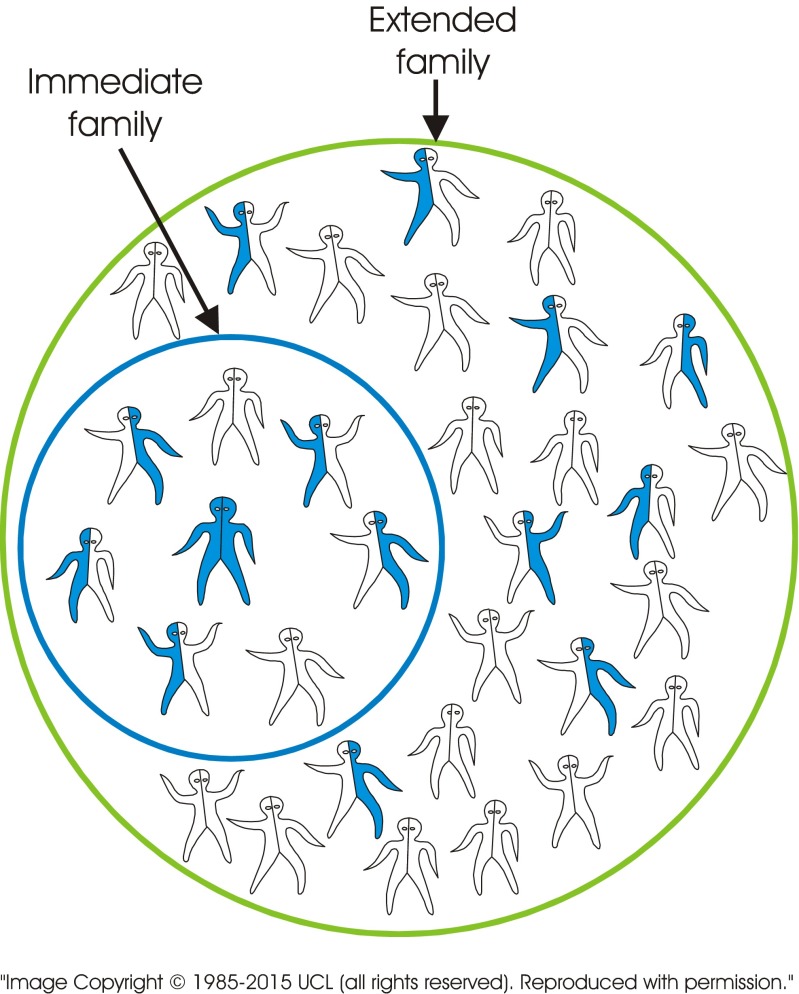
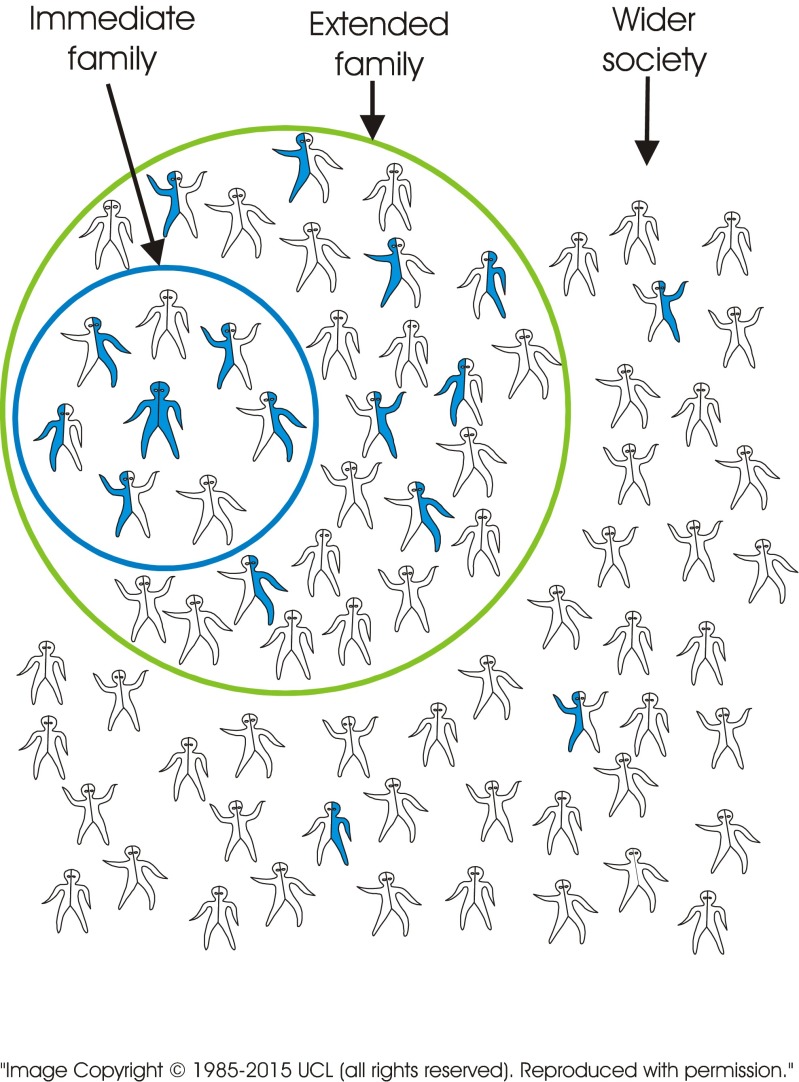
The standard visual image currently used to explain recessive inheritance is of a single image of a carrier couple. In contrast, the new tool consists of a series of images including several combinations of genotypes. The images may be presented in different sequences depending on the target audience. Figure 2 (carrier × carrier) shows a couple who both carry the same recessive disorder and the risks for their children. It emphasises (a) the fundamental characteristic of recessive inheritance—i.e. for a couple to have an affected child, both must carry a gene for the same recessive disorder, and (b) that unaffected children may or may not be healthy carriers. Figure 3 (carrier × non-carrier) shows (a) that carriers can transmit the variant gene to their children, and (b) how recessive disorders can be transmitted through generations without any evidence that they are “in the family”. Figure 4 (patient × non-carrier) shows that an affected person can have unaffected children providing their partner does not carry the same disorder. Figure 5 shows the likely prevalence of the gene variant in the nuclear and extended family. Figure 6 sets this in the context of the whole community and so shows how consanguineous marriage increases the risk of a recessive disorder if the recessive gene is endemic in the extended family. Finally, Fig. 7 shows that almost everyone carries at least one recessive disorder so (a) it is not exceptional to be a carrier, but (b) it is exceptional to know the specific disorder you may carry and that this knowledge can help minimise associated risks.
Fig. 2.
A couple can only have a child with a recessive disorder if both are carriers of the same recessive disorder
Fig. 3.
When only one of a couple is a carrier of a recessive disorder, the children may be healthy carriers. None of the children can suffer from the disorder
Fig. 4.
Adults with a disorder who are able to have children, can have healthy children if their partner is not a carrier of the same disorder. All children will be healthy carriers. None of the children will suffer from the disorder
Fig. 5.
There are many carriers in the family of a person with a recessive disorder
Fig. 6.
Carriers of the same disorder are less frequent among people who are not related by blood
Fig. 7.
Most people carry at least one gene for a recessive disorder
Alongside the use of several combinations of genotypes, the particularly distinguishing feature of this tool is that it extends explanation of recessive inheritance beyond the nuclear family to include extended family members and the community.
The tool can be produced in booklet format but also has the potential, as part of APoGI, to be web-based with an electronic delivery system compatible with existing health service structures. It could be deployed throughout the health service, in any language or languages for any population group regardless of linguistic or cultural background and hence can also be an international resource. A print version of the tool is currently being used as an educational and communication tool by AD for training health professionals and Community Health Development Workers, commissioned by Public Health Departments within the National Health service (NHS).
Analysis
All interviews were transcribed, translated when required, and analysed using the Framework Approach (Ritchie and Spencer 1994). Emerging themes and sub-themes were identified and organised for analysis both manually and using an Excel spreadsheet. Two project team members (AD and BM) regularly discussed coding and results, with input from other team members.
Results
Families
Kinship networks
The majority of family members interviewed reported having contact with grandparents, parents, siblings, sibling’s children, in-laws and other extended family members, maintaining greater contact with some than others. Most extended families occupied houses close together, with regular visiting. Interviewees spoke of families settled in the UK for several generations having grown in size, with gradually loosening ties with Pakistan. Only one interviewee, a divorcee, was relatively isolated with no contact with her in-laws and most of her natal family in Pakistan. People spoke of a variety of marriage forms, with marriages between close relatives continuing alongside marriages emerging from friendship and social networks.
Diagnosis and information giving
The majority of parents (22/24) received information about the diagnosis and cause of the condition in a clinical setting as a couple: two absentee parents received it from the parent who was present. Hospital doctors gave most parents (18) the diagnoses with relatively few receiving it from the Regional Genetics Centres (RGC) or General Practitioners (GPs). Thus most initial conversations about cause took place with professionals other than RGC staff. RGCs have a specialist remit to transmit information about genetic disorders and support families in managing their genetic risk, but only eight of the 16 families had had contact with an RGC.
Every parent interviewed except one, a recent arrival from Pakistan, said they had heard the health message they interpreted as “cousin marriage causes disabilities in children”. The majority of parents said they had received this information from a health professional whilst a few said it was common community knowledge. The same was true for extended family members. The majority of interviewees either rejected and/or were confused by the message as they were surrounded by cousin couples with healthy children and aware of non-cousin couples with children with impairments. A typical comment was that “If it is cousins then every child of a cousin couple should be disabled”.
Over half of the parents (fourteen), including the two seen at RGCs, recalled initially being given two disparate explanations of cause simultaneously: (1) being married to a cousin and (2) genetic inheritance. Some (four) recalled only being told that the condition was caused by cousin marriage. Others (six) recalled being told that it was an inherited disorder without mention of cousin marriage, though all were aware of this as background community knowledge. Several parents recalled health professionals attempting to explain a medical cause by using drawings they said they found too complex to understand, let alone retain and discuss with others:
The doctor did explain it. He really tried but it was all lines and circles…like he drew it out, this, about there are 1 in 4 or something. Then I came home and with everything going on, you know, I couldn’t remember half of it, still can’t, didn’t know the words and how to explain it. Mother of child with severe disorder
Table 5 shows that health professionals are the primary source of information for parents, who then become the primary source of information for other family members. Information was circulating within these families to the point where it was becoming family knowledge, demonstrating that kinship is utilised as a channel for information about genetic risk.
Table 5.
Who parents and extended family members had received initial information from
| Relationship to patient | Health professional | Spouse | Parent of affected child | Family knowledge | Cousin | Total |
|---|---|---|---|---|---|---|
| Parent | 22 | 2 | 24 | |||
| Extended family member | 0 | 1 | 26 | 2 | 1 | 30 |
| Total | 22 | 3 | 26 | 2 | 1 | 54 |
Adequacy of information, consequences for families and reaction to tool
Parents were only able to transmit information they had received, understood and retained, which for the majority was patchy, confusing and inadequate. The families fell into three groups: (1) families that were well-informed (two), (2) families with variable degrees of understanding (two), and (3) families with little if any understanding (twelve).
Families that were well informed: In two families. the interviewed parents had a sufficient grasp of the information required to make informed choices about having more children and raise awareness of genetic risk and genetic testing within the extended family, i.e. recessive inheritance, the impact of marrying close blood relatives (box 2, steps 1–5) and preventative options. Both had children with thalassaemia major, a recessive disorder for which there is an established, national community-based screening and counselling service (Anionwu and Atkin 2001) and an active national family support group. From the outset, these families had received a consistent explanation of cause (recessive inheritance) that they conveyed to other family members with the support of a bilingual thalassaemia counsellor. This remained the dominant discourse in both families, although both couples and their extended families had community knowledge of the link made between cousin marriage and impairments. Another common feature was that both families had had long-term contact with a variety of health professionals. One had a second child with a very rare severe disorder for which carrier testing is not available, and the other’s child had undergone a bone marrow transplant, thus providing opportunities for repetition, questioning and sifting of information. In both cases, extended family members were using carrier testing to make marriage choices and in the family with the rare recessive disorder two family members had chosen to marry outside the family to reduce their genetic risk.
Box 2: Explanation of the impact of marrying within the family in the context of recessive inheritance
| 1. You and your partner carry a gene that is inherited as a recessive |
| 2. When both partners are carriers there is a 1 in 4 chance in each pregnancy that a child can be born with the disorder |
| 3. You have inherited this gene from one of your parents |
| 4. Some other people in your family will also have inherited the same gene |
| 5. If you marry a close blood relative there is a greater chance that he/she may also have inherited the same gene for a recessive disorder |
Families with variable degrees of understanding: In these two families, the parents had received competing explanations about cause with consequent confusion and diminished faith in professional advice. Both sets of parents had understood recessive inheritance and preventative options, over time, but the entanglement with cousin marriage created confusion from the outset:
But when I went to the (condition name) party and I saw that there were so many [white people]. Then I went back to Dr (hospital doctor) and I said, you said it was because me and my husband are first cousins. But I’ve been to a party and they’re all white [presumably not married to first cousins]. What’s going on here? And he goes it’s just one of those things. And I said it’s one of those things with me and my husband. It’s not because we’re first cousins. Mother of a 12-year old with a severe recessive disorder
And
I asked the doctor and the genetic counsellor at the Regional Genetic Centre why they say it’s because we’re cousins when it’s because we’re both carriers. How is it because we’re cousins, I said, and they tried to explain but it still wasn’t a proper answer. I still don’t understand why they go on about cousins. I don’t know, confuses everything, when it’s about carriers. Mother of a 9-year old with a severe recessive disorder
The parents had shared information with other family members but interviews with extended family members showed that, sparked by the initial conversations with the parents, the dominant discourse continued to be confused and about cousin marriage. For most, knowledge of their own genetic risk was either non-existent or patchy and there was a lack of reliance on health professionals for advice. For example, both families included a couple awaiting the results of carrier testing, but neither were able to articulate the implications if one or both partners were found to be carriers. Both were anxious about the results but neither thought to seek professional support. Although they were aware that they could be at risk of having a child with a disorder and hence were undergoing carrier testing they did not have a grasp of the full spectrum of information required to instil confidence in the choices they were making or could make in the future. One of these interviewees had been given a leaflet by the genetic counsellor but found it difficult to understand—one of only two instances of a family member receiving written material. Only one of the ten interviewed extended family members in these two families had a grasp of recessive inheritance, this gained through self-study.
Seven members of these two families participated in the communication tool pilot. All found it immensely useful, and it alleviated anxiety for both the interviewees above. For one father of an affected child, the images allowed him to understand the medical explanation and also how marrying a close blood relative can increase the chance of two carriers coming together. Given the rare recessive gene in his family, he said, this now posed questions about who his children might marry and that needed to be discussed, whereas before he had regarded the association of the disorder with cousin marriage as nonsensical and had ignored it. The tool had reconciled the disparate explanations and resolved the confusion. His teenaged son commented:
They should have told us all this before.
One aunt through marriage said she had never thought her immediate family could be at risk as she and her husband were not cousins. She now wanted to discuss genetic testing with her husband, for him and in the future for their young children, using the tool:
I didn’t know all this, that it could affect my children too. I want to discuss this with my husband.
Similarly, another aunt had not considered her immediate family to be at risk and wanted to have discussions, using the tool, with her husband and children who were of marriageable age. Feeling empowered with the information, she felt able to take on the task of explaining the facts to her family:
No I’d prefer it to be that [the tool, rather than a health professional coming to the home]…because, you know, explains it all to you. I want to show this to my husband and daughter.
Families with little if any understanding: In the remaining 12 families, knowledge of recessive inheritance was patchy and/or inaccurate among both parents and extended family members. The dominant discourse about cause in all but one of these families focused on cousin marriage. The latter, though the exception, illustrates the pervasive, anxiety-generating impact of the message. This interviewed mother of a child with a less severe manageable condition had an inaccurate grasp of recessive inheritance, was unaware of carrier testing but understood there was a risk in every pregnancy and about prenatal diagnosis. Though a health professional had never mentioned cousin marriage to her, awareness of the message as background community knowledge resulted in latent anxiety about future children:
My son’s got a (name of medicine for condition) problem; do you think that if I have another child it’ll have any other disability? Because em, we’re related and…do you think…..the blood and…well I’ve heard that they can be disabled or blind, anything…so…
Anxiety characterised all these family situations as did lack of faith in professional advice. Without a rational explanation of cause, some chose to believe that affected births were a chance event beyond their control and therefore the will of God or just luck.
I don’t know but the lady doctor said ‘you know it maybe because you married your first cousin.’ And that’s what I thought as well, I just thought because we got married with my first cousin, that’s why it happened. Which now, I think no, it didn’t. It happened because God wanted it to happen but a lot of white people have a lot of disabled children, but they don’t marry first cousins. It’s just pure luck …I’ve got an abnormal gene. Mother of an 8-year old with severe disorder
This could not be equated with fatalism and abdication of personal responsibility; instead, it was an attempt at making sense of events when medical information was inconsistent with observed reality. In the first part of the interview, this mother spoke of genetic risk, albeit inaccurate, in terms of her own nuclear family.
If one of them (her children) has an abnormal gene and one normal gene and they get married into the relatives, they have a three in four chance of having a child with (condition in family). I’ve got a one in four and they’ve got a three in four.
However, on engaging with the communication tool, her understanding of recessive inheritance had clarified sufficiently not only to understand the genetic risk of her nuclear family, but also that of extended family members. Her reaction after seeing Fig. 5:
That could be any one of my sisters though, and any one of (husband’s name) sisters. I should go through this with my husband because this is like, it hits you in the face, you know. Because this could be my nieces and nephews, more of a chance of my nieces and nephews. It’s actually scared me quite a bit actually because his sister’s just got her kid married to her sister’s daughter and there’s a chance that they could have a child with (name of condition), if they’ve both inherited an abnormal gene.
Unmet needs characterised most of these family situations. Locating cause in the marriage choice of parents had the effect of disempowering parents from proactively engaging with the implications of genetic risk and services, for themselves and their families, that arises from an understanding of recessive inheritance. Instead, risk remains stagnant within the marriage choice with little choice for management other than a sense of helplessness, as one father stated:
It does make us, you know, it does make us wish that we weren’t married because we’re cousins but it’s too late now.
Families were therefore at the mercy of the knowledge, communication skills and limited time of the professional responsible for the care of the affected person. For example, one mother had just given birth to a child affected by a severe disorder. This was the second such birth in the family, the mother’s 15-year-old brother also being affected. During these 15 years, the only advice given was not to marry within the family—advice that was ignored. The mother is British born, fluent in English and feels devastated by her child’s condition. Though the hospital doctor is highly praised for his caring manner, the family had received no information about recessive inheritance or prenatal diagnosis and had no involvement of the RGC or GP for their care. Working through the communication tool, when prenatal diagnosis was also discussed, she said she would have used it to avoid an affected birth, had she known about it.
In eight families the parents continued to have further children after the initial diagnosis. In these families, there were 27 births, of which 7 (in four families) were affected—one family had three, one had two and two had one subsequent, affected birth.
The tool was piloted with 40 interviewees. Of those, 39 said it had improved their understanding of the cause of their child’s condition. Many said they were relieved to receive information that made sense. One grandmother, a matriarch within her family, was silent and refused to respond when asked if she had understood the information in the tool. The researcher’s impression was that she had understood the information and had become aware that marrying close blood relatives in her family now needed careful consideration. See box 3 for further reactions to the tool.
Box 3: Reactions to information in communication tool
|
“I’m getting myself tested, like tomorrow. You should think about your grandkids and your great grandkids as well. Okay carry on, I get it now.”
18-year old first cousin of person affected by a severe disorder |
|
“First person I’d discuss it with is my husband, explain to him because he wanted to know the answers and I couldn’t explain before. I’d explain to my mum, I’d explain to my khala [maternal aunt] who’s pregnant, obviously tell her that its not something you have to have a child like that, but this is how it works. Yeah, so I’d explain to anybody that probably would be pregnant and probably would be close. In my family or somebody who wants to know, friend or someone I could explain to them now.”
First cousin of mother of child with a recessive disorder |
|
“Well, usually writing is a lot more boring than pictures, and when you see a lot you don’t want to read it. The shorter the better…..When I saw these pictures I knew straight away that that was a couple and that was the kid and the shaded areas that they were the carriers, you can tell straight away and it helps a lot and I’ve learnt a lot. I didn’t know what these genes were and what… and I didn’t want to know. But now that I know I wanna do more and I wanna have that test and I want to see the outcome. These diagrams are very useful as well. They do explain it. Like if I showed that to my mum, if I was explaining them out I would know…I could really explain it properly…She’d understand. Yeah…you know so I would explain it to them now, and I would explain it to my sisters, to my mum…….so I would be able to explain this to her now and she would be more comfortable about her[sister affected by genetic disorder].”
Older sister of child with a recessive disorder |
|
With this, it’s here and I could show the pictures. Now I understand, I can show my mum, my family, anyone really.
Mother of child with severe disorder |
|
“……because it’s sort of the pictures, it would be more easy for her [mother] to understand than to just do the lines and for her to understand the crosses. She would actually understand with explanation what happens here……I think the carrier status will be useful and I think this kind of thing would be useful for people to understand because I don’t think they understand the concept of healthy carriers.”
Cousin of child with recessive disorder |
|
I used to think it was me, because I’ve never been very well, that I had something to do with her getting sick…..I understand it now, these, these pictures. I want to send these pictures to my brother in Pakistan. He will be able to understand it, as well.
Mother of child with recessive disorder, not literate in English or mother tongue |
Health professionals
Range of professionals providing information
Currently, only RGC staff has a specialist remit and related training to communicate information about genetic risk. However, a range of other professionals, including GPs, health visitors, paediatricians, midwives and voluntary sector advisers encountered situations where they were required to discuss genetic risk with at-risk family members:
We do discuss cause but it’s only done briefly. You have to explain about the cause so that you can get them to go see the genetics people. Consultant Paediatrician
The interviewed RGC staff members were clear that explanations about cause should be centred on recessive inheritance and not cousin marriage:
I never talk about cousin marriage with reference to the parents. That’s an issue for their brothers and sisters. RGC staff
Professional training about genetics for all interviewees had been mainly scientific with only one professional having encountered a lecture about communicating genetic risk in consanguineous communities as part of professional development. A number mentioned that their dealing with service delivery issues was a result of “learning on the job” rather than any formal training.
Lack of training and effective tools to aid communication was evident in the experiences of Primary, Secondary and Voluntary sector staff:
We get stuff in for the parents off the internet, the consultants, to try and explain it, but that’s difficult in itself because it’s very medical jargoned. The families just think ‘well, what on earth is all this about’, but no, I wouldn’t say we have access to appropriate things really. Health Visitor
I would definitely like clarity about cousin marriage, because at the moment, I’ve got to be honest, I wouldn’t always feel comfortable debating with somebody in a family home about cousin marriages. Voluntary sector, Disability Advisor
Lack of co-ordination between service sectors
There were several examples of individual professional commitment to optimising family care; for example, hospital doctors forging close links with local voluntary disability organisations providing long-term support to families; specialist posts to support minority ethnic families; and involvement in local research to improve service delivery. These initiatives, however, were individual and ad hoc; current links between the various agencies responsible for diagnosis, genetic counselling, clinical care and on-going support lack formal co-ordination with loopholes that compromise patient care. One GP’s experience is illustrative:
We’re just not in the loop. We should be involved at all stages. We could do so much. I have mothers coming to me and the families are distraught and we just haven’t been involved. I’ve been going through some of the notes of some of our families and there’s no mention of the genetic service. GP
Time constraints
Lack of time due to the workload associated with the increasing numbers of children with recessive disorders and complex needs is a major issue for clinical staff and compromises optimal care, with insufficient multi-disciplinary provision:
We just don’t have time for joint clinics. Consultant Paediatrician
Limited time is also a factor for RGC staff who consequently do few home visits that would bring them into contact with other family members. Further, to maintain confidentiality, their remit is to work with the presenting individual/family rather than proactively with their extended family. The onus for communicating genetic risk information, therefore, lies with the presenting family members, who as noted above, often have poor personal understanding and no tools with which to communicate information to others. Support staff such as Health Visitors and Disability/Advocacy Workers emerged as people better able to devote time to supporting families, including extended family members, as their remit was to provide long-term support to the whole family.
Challenges of working in a multi-ethnic society
All professionals recognised that they were working in a professional environment in which the informal professional culture equated consanguinity with impairments in children:
It’s in the air and… It’s definitely out there.. and yeah, it’s difficult to deal with, and it’s insensitively dealt with by lots of people. RGC staff
It’s certainly not perceived as a good thing. .but I’m not going by what people have said.. it’s just a vibe you pick up.. and then actual needs of this community. I don’t feel they’ve been picked up before I came into post. Specialist Health Visitor
Some professionals feared that because such perceptions were known to service users, that this tainted views of their service:
We don’t have a [positive] history with the community and I think we are perceived as a service, where people feel that cousin marriage might be criticised. RGC staff
Further, RGC staff from the white ethnic group perceived that lack of a common language and cultural understandings with some of the families they saw limited their ability to communicate effectively. One ensured that in such situations he worked with a genetic counsellor of an appropriate linguistic and cultural background. But, it was noted that not all RGC staff have access to such support.
Of all those interviewed, only RGC staff had immediate access to a tool to explain recessive inheritance. That tool included two images: carrier × carrier and carrier × non-carrier. All interviewees responded positively to the suggestion that they may be helped to communicate more effectively with a tool that pictorially explained recessive inheritance and the impact of marrying close blood relatives.
Discussion
Of the three basic components for the implementation of the family-centred approach—active kinship networks and a willingness of families to share genetic information; appropriately trained professionals with adequate resources; and the availability of communication tools—the first is being met. Health professionals are struggling to address family need and this leaves most families to struggle unsupported. In developing a new communication tool, in consultation with families in the study, we have addressed the third basic component.
In North European populations, where partners are not “arranged” by families, with marital choices introducing a degree of genetic randomness, genetic counselling and extended family studies are usually offered for dominant or X-linked disorders, because relatives have a high chance of being asymptomatic carriers, and may use this knowledge to reduce their personal and reproductive risks (Samavat and Modell 2004). Relatives of carriers of recessive genes also have a high chance of being carriers, but extended family studies are rarely offered because (a) the risk that their partner will carry the same disorder is usually low, and (b) at present, it is rarely possible to detect all DNA variants that can cause a given disorder. Hence, cascade screening is less cost-effective for most recessive than for dominantly inherited disorders (Krawczak et al. 2001). However, as Table 6 shows, in communities where consanguineous marriage is common, families with recessive disorders move into a risk category comparable with that of families with dominant or X- linked disorders because (a) a carrier who marries within their extended family has a high (around 30 %) risk that their partner is also a carrier and (b) carriers are highly likely to carry the DNA variant found in the presenting affected relative. Thus, when a precise (usually DNA-based) diagnosis is possible for an affected person, carrier diagnosis, prenatal diagnosis and genetic counselling are usually all possible for the extended family. It would seem logical then, to adopt a policy of offering comparable genetic counselling services to consanguineous families at risk for recessive disorders, as recommended by the WHO (Alwan and Modell 1997). Our study suggests that British Pakistani families are willing to and do share genetic information. Where they are appropriately informed and supported by a well-coordinated health service, the cascade of information is effective in informing and enabling informed choice.
Table 6.
Comparison of characteristics of recessive disorders in populations with random partner choice and populations with customary consanguineous marriage
| Random partner choice | Customary consanguineous marriage |
|---|---|
| Occur unpredictably and sporadically | Often occur in identifiable extended families |
| Diagnosis of first affected identifies an at-risk couple | Diagnosis of first affected identifies a large family grouping at increased risk. |
| ∼30 % of extended family are carriersa | ∼30 % of extended family are carriers |
| Carriers usually at low risk (usually <2 %) of marrying another carrier | Carriers marrying within the family have ∼30 % risk of marrying another carrier |
| Usually caused by two different variants of the same gene (dependent on relative frequency of variants) | Usually caused by two identical variants of the same gene |
| Marriages with unrelated people blend extended families into the wider community. | Frequent consanguineous marriages maintain extended family structures, contacts and communication. |
aAhmed S, Saleem M, Modell B, Petrou M (Ahmed et al. 2002) Screening extended families for genetic counselling for genetic haemoglobin disorders in Pakistan. N Engl J Med 347(15):1162–1168
In a study of British Pakistani attitudes to sharing genetic information, Shaw and Hurst (2009) erroneously cite Krawczak et al.’s (2001)) analysis of cystic fibrosis in North European populations to state that cascade screening is not efficacious in the context of genetic services for British Pakistanis. That analysis, however, did not include consideration of the clustering of recessives in consanguineous populations. Based on interviews with British Pakistani adults referred to a genetics clinic, the authors also discount the particular potential of genetic information sharing within these family clusters, stating that information was largely withheld from family members. However, their data and interpretation relies heavily on families’ social and cultural context with little reference to participants’ understanding of the cause of the disorder, and the relevance and timeliness of information to families’ needs. By introducing and monitoring an intervention we have demonstrated the importance of accurate information on families’ ability to make sense of their genetic risk and the impact of its absence. Many factors, including social and cultural context, can be important determinants of attitude and behaviour (Allford et al. 2014; Wertz et al. 1990), but the first and essential step towards informed choice and risk reduction is an understanding of recessive inheritance itself. If this is not understood the cause of not sharing genetic risk information and continuing births of affected children can be wrongly located within the culture of the families. Service deficiencies being masked by an emphasis on cultural context has a long documented history (Darr 2009; Ahmad and Bradby 2007; Ahmad et al. 2000; Atkin et al. 1998, Darr 1991). Our study, alongside others (Darr 1991, Khan et al. 2010, Ahmed et al. 2002), shows that families are prepared to pass on and use genetic information. That they do not do this consistently reflects a failure on the part of services. In two families in our study, there was a strong and sophisticated understanding of the conditions, nature of their genetic inheritance, and a shared understanding of implications for the extended family. Communication with professionals over an extended period of time and strong and supportive relations with health care providers were central to these families’ understanding. That others did not possess a meaningful understanding does not reflect the irrelevance of cascade genetic screening, but of the poor and ill-coordinated nature of genetic service provision, leaving families ill-informed and poorly resourced. A significant component of that service failure is that families do not have a ready tool for understanding and communicating about complex genetic risk information.
The study also shows that initial conversations about cause were taking place in Secondary Care; that the majority of these conversations included discussion of cousin marriage and that professionals in this sector were ill equipped to empower families to understand their genetic risk. They had insufficient time to adequately counsel families, had limited training beyond the technical aspects of recessive inheritance, no useful communication tools to aid them (relying mainly on hand drawn diagrams and oral transmission of information) and expected counselling to be provided by specialists at the RGCs. But, only half of the families were seen by RGC staff and of those who were, none said that they received an explanation of the impact of cousin marriage that they understood. Our findings add to previous literature that illustrate health professionals’ need for training, resources and support in meeting the growing need to deliver genetic services to a diverse population (Darr 2009; Dyson 2007; Kai et al.; 2007; Atkin et al. 1998).
Study findings also reveal a lack of understanding of the nuances of the link between recessive inheritance and cousin marriage. Effective communication about the impact of having children with close blood relatives entails following a strictly ordered sequence (see box 2, steps 1–5). Discussion of the possible consequences of having children with close blood relatives (step 5) without first ensuring a complete grasp of recessive inheritance (steps 1–4) is likely not only to be futile but potentially counterproductive as the campaigns in Iran and Birmingham demonstrated. As the risk of having an affected child is the same for carrier couples whether they are related or not, marrying close blood kin is not the main, but an additional risk factor for understanding a consanguineous couple’s own genetic risk and the future risk for other blood-related extended family members. The continued emphasis on consanguinity instead of a focus on the nature of recessive inheritance remains a key component of the experience of these parents; something consistently shown to be damaging to family engagement with genetic risk information that also prohibits engagement with services (Darr 2009; Ahmad et al. 2007; Ahmad et al. 2000; Atkin et al. 1998, Darr 1991).
In the future, prospective screening is likely to become available for carriers of a wide range of recessive disorders. When a carrier is detected in a “consanguineous family”, this shows that that particular gene is present in the extended family and indicates risk, in the same way as the diagnosis of an affected child. Carriers therefore need the same help with informing family members of possible risk—the tool is very suitable for this purpose.
Conclusion
Adaptation of existing services to accommodate genomic advances is recommended by the Human Genetics Strategy Group (Department of Health 2012), alongside efficiency and equity of access to services, as guiding principles. The clustering of recessive disorders and sharing of genetic information in British Pakistani extended families, if supported by trained and appropriately resourced professionals, suggests that these criteria could be met by integration of the family centred approach within existing NHS structures. Further research is needed among other groups with consanguineous marriages.
Genetic infrastructure development is in its infancy. The growing literature on genetic communication and development of guidelines for professionals (Gaff et al. 2007; Forrest et al. 2007) is encouraging. This provides background knowledge against which enquiry and action now need to progress towards the crucial development of communication tools for professionals and families, with rigorous standards, to accompany the communication process (Modell et al., in preparation). Communication is a central activity in genetics and accurate information a major therapeutic intervention, without which the whole process of understanding personal and familial genetic risk, genetic testing, counselling and access to services is jeopardised.
The UK Department of Health has acknowledged the need for local commissioning groups and service providers to address the health service needs of consanguineous communities within the overall framework of integrating genomic medicine into future health services (Department of Health 2010, Department of Health 2012). A first step would be to form multidisciplinary groups in areas where consanguinity related recessives are a health concern, to develop local frameworks with the co-operation of representatives of a range of relevant professionals from the Tertiary, Secondary, Primary and Voluntary sectors as well as the lay public.
Electronic supplementary material
(DOC 43 kb)
(DOC 27 kb)
(DOC 35 kb)
Acknowledgments
We wish to thank the study participants and members of the project advisory committee. We also thank the two anonymous reviewers for their useful comments and suggestions.
Compliance with Ethical Standards
All study participants gave written consent before taking part in focus group discussions and interviews.
This research was funded by a Department of Health research grant (Health Services Research Programme).
Footnotes
This paper deals with autosomal recessive disorders. For brevity, we refer to these as recessive disorders throughout the article.
Impairment is increasingly used in place of disability in social science/social care contexts. For example, the Union of the Physically Impaired Segregation (1976 as quoted in Winter 2003) defined physical impairment as the condition of a person “lacking part or all of a limb, organ or mechanism of a person” and disability as the “disadvantage or restriction of activity caused by a contemporary social organization which excludes people with physical impairments from participation in the mainstream of social activities.”
References
- Ahmad WIU, Atkin K, Chamba R. “Causing havoc to their children”: parental and professional perspectives on consanguinity and childhood disability. In: Ahmad WIU, editor. Ethnicity. Disability and Chronic Illness, Buckingham: Open University Press; 2000. [Google Scholar]
- Ahmad W IU, Bradby H (2007) Locating ethnicity and health: exploring concepts and contexts. Sociol Health Ill 29(6):795–810, ISSN 0141-9889 [DOI] [PubMed]
- Ahmed S, Saleem M, Modell B, Petrou M. Screening extended families for genetic counselling for genetic haemoglobin disorders in Pakistan. N Engl J Med. 2002;347(15):1162–1168. doi: 10.1056/NEJMsa013234. [DOI] [PubMed] [Google Scholar]
- Allford A, Qureshi N, Barwell J, Lewis C, Kai J. What hinders minority ethnic access to cancer genetic services and what may help? Eur J Hum Genet. 2014;22(7):866–874. doi: 10.1038/ejhg.2013.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwan A, Modell B (1997) Community control of genetic and con- genital disorders. EMRO technical publication series 24. WHO Regional Office for the Eastern Mediterranean Region, Cairo
- Atkin K, Ahmad WIU, Anionwu EN. Screening and counselling for sickle cell disorders and thalassaemia: the experience of parents and health professionals. Soc Sci Med. 1998;47:1639–51. doi: 10.1016/S0277-9536(98)00261-5. [DOI] [PubMed] [Google Scholar]
- Anionwu EN, Atkin K (2001) The Politics of Sickle Cell and Thalassaemia. Buckingham, Open University Press
- Baird PA, Anderson TW, Newcombe HB, Lowry RB. Genetic disorders in children and young adults: a population study. Am J Hum Genet. 1988;42:677–693. [PMC free article] [PubMed] [Google Scholar]
- Bhopal R, Petherick E, Wright J, Small N. Potential social, economic and general health benefits of consanguineous marriage: results from the Born in Bradford cohort study. Eur J Pub Health. 2013 doi: 10.1093/eurpub/ckt166. [DOI] [PubMed] [Google Scholar]
- Bittles AH (1990) Consanguineous marriage: current global incidence and its relevance to demographic research. Research report no. 90-186, Population Studies Center, University of Michigan. Data available at http://www.consang.net
- Bittles AH. Consanguinity in Context. New York, NY: Cambridge University Press; 2012. [Google Scholar]
- Bundey S, Alam H. A five-year prospective study of the health of children in different ethnic groups with particular reference to the effect of inbreeding. Eur J Hum Genet. 1993;1:206–219. doi: 10.1159/000472414. [DOI] [PubMed] [Google Scholar]
- Darr A (1991). The Social Implications of Thalassaemia among Muslims of Pakistani Origin in England — Family Experience and Service Delivery.Ph.D. thesis, Univ. London
- Darr A (1997) Consanguineous marriage and genetics: a positive relationship. In Clarke A and Parsons E (eds) Culture, Kinship and Genes: towards cross cultural genetics. Basingstoke, Macmillan
- Darr A. Cousin marriage, culture blaming and equity in service delivery. Divers Equality Health Care. 2009;6:7–9. [Google Scholar]
- Darr A, Small N, Ahmad WIU, Atkin A, Corry P, Benson J, Morton R. Examining the family-centred approach to genetic testing and counselling among UK Pakistanis: a community perspective. J Community Genet. 2012;4(1):49–57. doi: 10.1007/s12687-012-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health (2010) Tackling health inequalities in infant and maternal health outcomes. Report of the Infant Mortality National Support Team. Dec 2012
- Department of Health (2012) Building on our inheritance: genomic technology in healthcare. Human Genomics Strategy Group, Department of Health http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_ 132369
- Dyson S. Knowledge of sickle cell in a screened population. Health and Soc Care Community. 2007;5(2):84–93. doi: 10.1111/j.1365-2524.1997.tb00103.x. [DOI] [Google Scholar]
- Forrest LE, Delatycki MB, Skene L, Aitken MA. Communicating genetic information in families—a review of guidelines and position papers. Eur J Hum Genet. 2007 doi: 10.1038/sj.ejhg.5201822. [DOI] [PubMed] [Google Scholar]
- Gaff C, Clarke A, Atkinson P, Sivell S, Elwyn G, Iredale R, Thornton H, Dundon J, Shaw C, Edwards A. Process and outcome in communication of genetic information within families: a systematic review. Eur J Hum Genet. 2007;15:999–1011. doi: 10.1038/sj.ejhg.5201883. [DOI] [PubMed] [Google Scholar]
- Haslam J (2001) Harsh troth. The Guardian http://www.guardian. co.uk/society/2001/mar/14/guardiansocietysupplement6
- Kai J, Beavan J, Faull C, Dodson L, Gill P, Beighton A (2007) Professional uncertainty and disempowerment responding to ethnic diversity in health care: a qualitative study. PLoS medicine 4(11):e323, http://www.plosmedicine.org/article/ info%3Adoi%2F10.1371%2Fjournal.pmed.0040323 [DOI] [PMC free article] [PubMed]
- Khan N, Benson J, MacLeod R, Kingston H (2010) Developing and evaluating a culturally appropriate genetic service for consanguineous South Asian families. J Community Genet, June 1(2):73-81. doi: 10:007/s12687-010-0012-2 [DOI] [PMC free article] [PubMed]
- Krawczak M, Cooper DN, Schmidtke J (2001) Estimating the efficacy and efficiency of cascade genetic screening. Am J Hum Genet 2:361–370 [DOI] [PMC free article] [PubMed]
- Modell B, Darr A. Genetic counselling and customary consanguineous marriage. Nat Rev Genet. 2002;3:225–229. doi: 10.1038/nrg754. [DOI] [PubMed] [Google Scholar]
- Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Bryman A, Burgess R, editors. Analysing qualitative data. London: Routledge; 1994. pp. 173–194. [Google Scholar]
- Samavat A, Modell B. Iranian national thalassaemia screening programme. BMJ. 2004;329:1134–1137. doi: 10.1136/bmj.329.7475.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A, Hurst A. “I don’t see any point in telling them”: attitudes to sharing information in the family and carrier testing of relatives among British Pakistani adults. Ethn Health. 2009;14(2):205–24. doi: 10.1080/13557850802071140. [DOI] [PubMed] [Google Scholar]
- Sheridan E, Wright J, Small N, Corry P, Oddie S, Whibley C, Petherick E, Malik T, Pawson N, McKinney P, Parslow R 2013 Risk factors for congenital anomaly in a multiethnic birth cohort: an analysis of the Born in Bradford study. The Lancet published online July 4th http://dx.doi.org/10.1016/50140-6736(13)61132-0. [DOI] [PubMed]
- Wertz D, Fletcher JC, Mulvihill JJ. Medical geneticists confront ethical dilemmas: cross cultural comparisons among 18 nations. Am J Hum Genet. 1990;46:1200–13. [PMC free article] [PubMed] [Google Scholar]
- Winter JA. The development of the Disability Rights Movement as a social problem solver. Disability Studies Quarterly. 2003;23(1):33–61. [Google Scholar]
- Wright J, Small N, Raynor P, on behalf of the Born in Bradford Scientific Collaborators Group et al. Cohort profile: the Born in Bradford multi-ethnic family cohort study. Int J Epidemiol. 2012 doi: 10.1093/ije/dys112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 43 kb)
(DOC 27 kb)
(DOC 35 kb)