Abstract
The purpose of the current study was to examine changes in frequency of discussion about melanoma preventive behaviors among adults who received melanoma genetic test reporting and counseling and their children and grandchildren, correspondence of frequency of discussion with intentions, and content of discussions. Participants received CDKN2A/p16 testing and counseling (N = 24, 46 % p16-positive). Discussions about preventive behaviors were assessed before testing and 1 and 6 months post-testing. Intentions to discuss preventive behaviors and perceived preparedness to discuss risk were assessed post-testing. Open-ended questions assessed content of reported discussions. Discussion of preventive behaviors declined following test reporting, with more rapid decline reported by noncarriers. There was a large gap between the percentage of participants who intended to discuss preventive behaviors and who then reported discussions 1 and 6 months after counseling. Participants felt prepared to discuss melanoma risk but also suggested resources to facilitate discussions. Genetic test reporting and counseling alone did not sustain discussions about preventive behaviors for a hereditary cancer with children and grandchildren. The gap between intentions to have discussions and reported discussions has implications for augmentation of counseling to support at-risk families’ discussions about preventive behaviors.
Keywords: Melanoma, CDKN2A/p16, Genetic testing, Family communication, Prevention
Introduction
Of all melanomas, 5 to 10 % are hereditary, and of those, 20 to 40 % are associated with a pathogenic mutation in CDKN2A/p16 (Florell et al. 2005; Goldstein et al. 2007). Genetic testing and counseling provide an opportunity to inform individuals and their family of their risk for melanoma and to provide education on preventive behaviors to mitigate genetic risk (Leachman et al. 2009). Individuals at elevated risk for melanoma should engage in daily photoprotective practices (e.g., use of sunscreen, physical barriers to block ultraviolet radiation and prevent sunburns), perform screening practices (e.g., implement skin self-examinations, obtain total body skin examinations by a health care provider), and minimize risk behaviors that increase exposure to ultraviolet radiation (UVR; e.g., use of tanning booths, intentional tanning outdoors) (Kefford et al. 2002; Niendorf and Tsao 2006). Adherence to preventive behaviors is often suboptimal, particularly prior to receipt of genetic test reports and counseling (Aspinwall et al. 2008, 2009; Azzarello et al. 2006; Geller et al. 2003). However, provision of p16 testing results alongside recommendations for preventive behaviors can improve adherence (Aspinwall et al. 2008, 2009, 2013b, 2014b; Glanz et al. 2013).
Studies have begun to explore communication within families about melanoma risk and prevention (Hay et al. 2005, 2008). This work included melanoma survivors and unaffected first-degree relatives and did not involve genetic testing. Most melanoma survivors (50–94 %) engage in discussions with family about skin cancer risk, frequently with children (Harris et al. 2010; Hay et al. 2005). Topics of discussion often include photoprotection and physician-led skin examinations but less often, skin self-examinations (Hay et al. 2005). Other findings indicate that while melanoma survivors are concerned about their family member’s risk for the disease, they often do not discuss this concern (Oliveria et al. 2013). The literature on familial disclosure of genetic test results suggests that there may be barriers to discussing hereditary disease risk: concerns about the effect of disclosure (Barsevick et al. 2008; Hallowell et al. 2005; Hamilton et al. 2005), lack of knowledge about genetics (Barsevick et al. 2008; Costalas et al. 2003), and logistical challenges such as finding a time or place for disclosure (Daly et al. 2001; Hallowell et al. 2005). While yet unstudied in genetic test reporting, one might expect based on the theory of planned behavior (Ajzen 1991) that increased intentions to discuss hereditary risk or preventive behaviors would predict discussion occurrence. Even if an individual intends to hold these discussions, barriers may make these discussions challenging.
Little is known about family communication about melanoma preventive behaviors following genetic test reporting and counseling. Thus far, hereditary cancer studies have focused on disclosure of genetic test results and whether family members pursue testing, and less on communication about preventive behaviors (Fehniger et al. 2013; Gaff et al. 2007; Lafreniere et al. 2013). Genetic test results could surpass barriers to discussion by providing added motivation or serving as a tool to facilitate communication about preventive behaviors. There has been no research, to our knowledge, that examines individuals’ discussions about melanoma preventive behaviors with children and grandchildren after melanoma genetic testing. Younger family members should receive information on melanoma preventive behaviors because implementing them early in life decreases risk (Oliveria et al. 2006; Pustisek et al. 2010). To address these gaps, the current study examined the extent to which individuals intended to discuss melanoma preventive behaviors with children and grandchildren following p16 genetic test reporting and counseling. Next, this study investigated whether individuals followed through on their intentions by examining reported frequency of discussions among melanoma preventive behaviors before and after testing. We hypothesized that individuals, particularly those receiving a positive test result, would report increased frequency of discussing melanoma preventive behaviors with offspring following receipt of test results.
Materials and methods
Study sample and procedures
Participants were part of a larger study on the impact of melanoma genetic testing on behavioral and psychological outcomes (the “test reporting study”) (Aspinwall et al. 2008, 2009, 2013a, b, 2014a, b). Participants in this larger study were adults from two large melanoma pedigrees with identified p16 mutations (V126D or 5’UTR-34G>T) that had contributed DNA samples for research (Cannon-Albright et al. 1992; Kamb et al. 1994). Adult participants from these families were contacted and offered the opportunity to receive clinically confirmed, personal genetic test results. Although all participants had received extensive prior counseling regarding their elevated melanoma risk based on family history via research participation, none were aware of their p16 genetic status or the presence of a cancer-causing genetic mutation in the family. Participants who agreed to participate in the test reporting study received p16 test results and one session of genetic counseling free of charge.
Participants attended an individualized counseling session covering pre-test disclosure information, their result, and tailored risk and management information (Aspinwall et al. 2008). Sessions were conducted by a licensed genetic counselor using a standardized counseling protocol (see supplement to Aspinwall et al. 2008). Participants were informed that a p16 mutation increased risk of melanoma 35- to 70-fold above the general population risk to 50 % by age 50 and 76 % by age 80. After providing written informed consent, participants were offered and all accepted the opportunity to receive their test result. Those receiving a p16-negative result were informed that they still had up to a 1.7-fold residual risk due to other familial risks (Hansen et al. 2004). All participants were advised to manage their risk by preventing sunburns, avoiding the sun between 10 AM and 4 PM, seeking shade, using sunblock with sun protection factor 30+ with reapplication every 2 h, and wearing protective clothing. Melanoma screening recommendations (e.g., annual total body skin exams, monthly skin self-exams) were also provided (American Academy of Dermatology 2015; American Cancer Society 2015). Participants who received p16-positive results were informed that their children would be at 50 % risk for inheriting the mutation and that screening is recommended for at-risk children beginning at age 10 (Champine et al. 2013; Kefford et al. 1999). All participants received a letter 1 month after test reporting reiterating their test results, the associated risks for melanoma, and preventive recommendations.
Participants in the test reporting study were asked to complete study questionnaires at baseline (before test reporting) and immediately post-test reporting (May–November, 2005). Participants were invited to enroll in a follow-up study of the long-term behavioral and psychological responses to p16 test reporting. Individuals who agreed to participate in the follow-up study were asked to complete questionnaires 1 and 6 months after test reporting. All procedures were approved by the relevant Institutional Review Board.
Eligibility for current analysis
Out of the 61 participants who completed a baseline assessment, 44 reported having one or more children or grandchildren. There were no significant differences between those reporting that they had at least one child/grandchild and those who did not on demographic characteristics (age, sex, income, education), p16 test result, personal melanoma history, or baseline engagement in photoprotective, screening, and risk behaviors. Of the 44, there was 5 % attrition from baseline to post-test reporting, 21 % attrition from post-test reporting to 1 month, and 19 % attrition from 1 to 6 months (Fig. 1). There were no significant differences between participants who provided only baseline data and who provided baseline, 1 and 6 months data, with respect to age, p16 test result, personal melanoma history, or baseline levels of photoprotective, screening, and risk behaviors. A significantly higher proportion of females completed all assessments (74 % females vs 40 % males, χ2(1) = 4.9, p = 0.03).
Fig. 1.
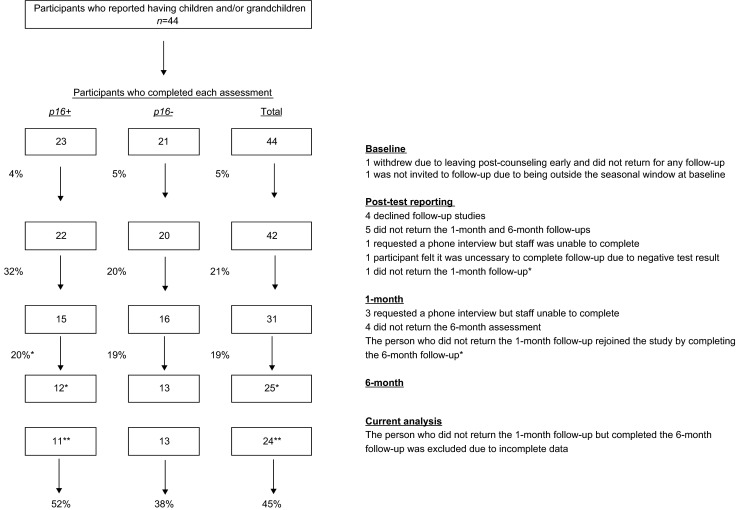
Participation across time points for individuals with children or grandchildren. Note: Percentages between boxes represent attrition rates between study time points. Percentages at the bottom of the figure are overall attrition rates from baseline to 6 months for participants included in the current analysis. *Six-month data reflect reinclusion of the participant who did not return for the 1-month follow-up but did return for the 6-month follow-up. **Current analysis does not include the participant that rejoined the study at 6 months due to incomplete data
Measures
Demographic characteristics
Participants completed a demographic questionnaire at baseline with questions about age, gender, ethnicity, marital status, education, household income, and how many children and grandchildren they had. Melanoma history was confirmed through the Utah Cancer Registry, the Utah Population Database (2014), and pathology reports.
Genetic test
Genetic testing was performed in a Clinical Laboratory Improvement Act certified laboratory, Myriad Genetics Laboratory Salt Lake City, UT. Participants had DNA sequencing for the specific mutation (V126D or 5′UTR-34G>T) identified in their family.
Family discussion
At 1 and 6 months, participants were asked how frequently they discussed their genetic test result with their children or grandchildren in the past month on a five-point Likert scale from 1 = “not at all” to 5 = “a lot.” At baseline, 1 and 6 months post-test reporting, study questionnaires included questions on frequency of discussion of the following melanoma preventive behaviors with children and grandchildren (same Likert scale as above): (1) photoprotective behaviors (using sunscreen, wearing protective clothing, avoiding UVR during peak hours), (2) screening behaviors (skin self-examinations, skin exams by medical professionals), and (3) risk behaviors (sunbathing, using tanning booths). The questions at baseline asked how often participants discussed each preventive behavior, and at 1 and 6 months asked for frequency of discussion in the last month. At 1 and 6 months, participants were asked open-ended questions about the content of discussions with children and grandchildren about preventive behaviors.
Intention to discuss, preparedness for discussing risk
Immediately after test reporting, participants completed questions concerning intention to discuss preventive behaviors with offspring and how prepared they felt for these discussions. The questions mirrored those administered at baseline and 1 month except that they asked how often participants intended to discuss preventive behaviors in the future with children and grandchildren. Responses were on a five-point Likert scale ranging from “1 = much less than I have been doing” to “5 = much more than I have been doing.” Participants were asked one question regarding how prepared they felt to discuss the family’s genetic risk for melanoma with their children and grandchildren on a five-point Likert scale ranging from “1 = not at all” to “5 = very much.” They were asked open-ended questions on their preparedness for discussions and their perceptions of how the risk counseling session could have better prepared them for discussions.
Analytic approach
Twenty-four participants reported having at least one child or grandchild at baseline and completed assessments at baseline, immediately post-test reporting, and 1 and 6 months. Because there were few unaffected carriers (n = 3), we combined participants who had a personal history of melanoma with those who did not. There were no demographic differences between participants who had a history of melanoma and those who did not. Participants’ initial visits were clustered deliberately into a compressed timeframe to reduce the effects of season. There were no significant differences in reported communication frequency based on season.
We first calculated average intentions to discuss preventive behaviors with children and grandchildren. We conducted repeated-measures analyses of variance (ANOVAs) to examine whether reported frequency of discussion of photoprotective, screening, and risk behaviors increased after test reporting (baseline to 1 and 6 months) and differed between p16 carriers and noncarriers. We conducted correlational analyses to examine whether higher intentions to discuss preventive behaviors were linked with more frequent reported discussions. To compare participants who reported at least some discussion of preventive behaviors with those that did not based on intentions to have these discussions, we dichotomized intentions to discuss preventive behaviors into “intentions to have the same or less frequent discussions” and “intentions to have more frequent discussions,” and 1 and 6 months reported frequency of discussions (i.e., “not at all” and “a little” vs “some,” “moderately,” or “a lot”). A criterion of p <0.05 was used for statistical significance.
We coded open-ended responses using a grounded theory approach (Hsieh and Shannon 2005; Strauss and Corbin 1990). We coded responses about the content of discussions, preparedness to discuss melanoma risk, and information participants could have received that would facilitate these conversations. All responses were coded by two individuals. Inter-rater reliability was calculated as the percent of codes on which the two individuals agreed. Inter-rater reliability of qualitative coding was good, with initial coding yielding 87–100 % agreement across the categories coded.
Results
Participants’ (n = 24) demographics at baseline are in Table 1. Approximately half received a positive p16 test result (n = 11, 46 %). Eight participants had a personal history of melanoma (33 %), all of whom received p16-positive test results. On average, each participant had 4.3 children (SD = 1.8) and 5 grandchildren (SD = 7.5).
Table 1.
Participant demographic characteristics
|
p16-positive n = 11 |
p16-negative n = 13 |
Overall N = 24 |
|||||
| n | % | n | % | n | % | ||
| Gender | Male | 5 | 45 | 5 | 38 | 10 | 42 |
| Female | 6 | 55 | 8 | 62 | 14 | 58 | |
| Marital status | Married | 11 | 100 | 13 | 100 | 24 | 100 |
| Personal history of melanoma | 8 | 72 | 0 | 0 | 8 | 33 | |
| Median | Median | Median | |||||
| Education level | Some college | Bachelor’s degree | Bachelor’s degree | ||||
| Income | $70–79,999 | $50,000–59,999 | $50–59,999 | ||||
| Age (years) | 52.0 | 36.0 | 42.0 | ||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Number of prior melanoma diagnosesa | 3.0 | 2.7 | 0.0 | 0.0 | 3.0 | 2.7 | |
| Age (years)b | 51.1 | 14.6 | 41.1 | 13.7 | 45.8 | 14.8 | |
| Number of children ≤18b | 1.5 | 1.8 | 2.5 | 1.7 | 1.9 | 1.8 | |
| Number of children >18b | 3.5 | 3.2 | 1.4 | 2.4 | 2.4 | 3.0 | |
| Number of grandchildren ≤18b | 7.5 | 9.2 | 2.7 | 4.9 | 5.0 | 7.5 | |
| Number of grandchildren >18b | 0.09 | 0.3 | 0.0 | 0.0 | 0.04 | 0.2 | |
aCalculated for the eight participants who had a personal history of melanoma. All were p16-positive
bCarriers (p16-positive) did not differ significantly from noncarriers (p16-negative) on their age, number of children under age 18, number of children over age 18, number of grandchildren under age 18, and number of grandchildren over age 18 (all ps > 0.05)
Table 2 contains descriptives (means, standard deviations) for participant demographic characteristics (melanoma history, p16 carrier status) and primary study variables (discussion of preventive behaviors, intentions to discuss preventive behaviors). The table also displays all possible correlations between demographic variables and reported discussion of preventive behaviors (baseline, 1 month, 6 months) and post-counseling intentions to discuss preventive behaviors. Participants with a personal history of melanoma (affected) did not differ from those without a personal history of melanoma (unaffected) on frequency of discussion of preventive behaviors at baseline and 1 month. At 6 months, affected individuals reported more frequent discussions than unaffected individuals (photoprotection: t(22) = 2.4, p = 0.026, screening: t(22) = 3.0, p = 0.006, risk: t(22) = 2.6, p = 0.016).
Table 2.
Descriptive statistics and correlations between participant characteristics, reported baseline discussions, post-counseling discussion intentions and preparedness, and reported discussions of photoprotection, screening, and risk behaviors with children and grandchildren 1 and 6 months after genetic test reporting and counseling
| SD | Mean | Melanoma History | p16 status | Discussions at Baseline | Post-counseling Intentions | Discussions at 1 month | Discussions at 6 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Photo | Screen | Risk | Photo | Screen | Risk | Prep | Photo | Screen | Risk | Photo | Screen | Risk | ||||
| Melanoma history (n) | n = 8 personal history | 1 | ||||||||||||||
| p16 status (n) | n = 11 p16+, n = 13 p16- | 0.77*** | 1 | |||||||||||||
| Baseline discussion frequency, mean (SD) | Photo 3.80 (1.18) | 0.13 | 0.02 | 1 | ||||||||||||
| Screen 2.80 (1.22) | 0.35 | 0.30 | 0.61** | 1 | ||||||||||||
| Risk 2.54 (1.61) | 0.37 | 0.33 | 0.57** | 0.93*** | 1 | |||||||||||
| Post-counseling intentions to discuss melanoma preventive behaviorsa, mean (SD) | Photo 3.71 (.69) | 0.04 | 0.40 | 0.08 | 0.08 | 0.13 | 1 | |||||||||
| Screen 3.83 (.64) | 0.05 | 0.38 | 0.18 | 0.01 | 0.12 | 0.87*** | 1 | |||||||||
| Risk 3.79 (.72) | 0.33 | 0.51* | 0.31 | 0.25 | 0.40 | 0.75*** | 0.68*** | 1 | ||||||||
| Prepared 4.36 (.65) | 0.00 | −0.02 | 0.16 | −0.01 | −0.01 | −0.04 | 0.05 | 0.18 | 1 | |||||||
| One-month discussion frequency, mean (SD) | Photo 2.92 (1.25) | 0.27 | 0.41 | 0.52** | 0.48* | 0.58** | 0.32 | 0.37 | 0.46* | −0.01 | 1 | |||||
| Screen 2.33 (1.31) | 0.37 | 0.54** | 0.39 | 0.65** | 0.66*** | 0.35 | 0.33 | 0.45* | −0.10 | 0.76*** | 1 | |||||
| Risk 1.67 (.87) | 0.26 | 0.37 | 0.43* | 0.57** | 0.62** | 0.23 | 0.22 | 0.44* | 0.13 | 0.76*** | 0.82*** | 1 | ||||
| Six-month discussion frequency, mean (SD) | Photo 1.92 (.93) | 0.46* | 0.27 | 0.42* | 0.41* | 0.46* | −0.11 | −0.02 | 0.23 | 0.13 | −0.04 | −0.01 | −0.03 | 1 | ||
| Screen 1.58 (.72) | 0.55** | 0.43* | 0.36 | 0.64** | 0.64** | −0.08 | −0.06 | 0.08 | −0.02 | 0.30 | 0.53** | 0.28 | 0.34 | 1 | ||
| Risk 3.71 (.69) | 0.49* | 0.36 | 0.40 | 0.63** | 0.62** | 0.12 | −0.03 | 0.44* | 0.16 | 0.26 | 0.45* | 0.57** | 0.40 | 0.47 | 1 | |
Photo photoprotection, Screen screening, Prep prepared
*p < 0.05; **p < 0.01; ***p < 0.001; significant correlations are bolded
aParticipants were asked about their intentions to discuss preventive behaviors “in the future”
Gap between intentions to discuss preventive behaviors and reported discussions
We examined the degree to which participants reported following through on their intentions to discuss preventive behaviors with offspring (Fig. 2). On average, participants reported that they intended to discuss preventive behaviors “somewhat more than they have been doing already” (Table 2). Higher intentions to discuss risk behaviors were significantly correlated with reported discussions (1 month: r = 0.44, p = 0.03; 6 months: r = 0.44, p = 0.03), but intentions were uncorrelated with reported frequency of discussion for photoprotection and screening. There were substantial proportions of participants (1 month: 21–59 %, 6 months: 60–88 %) who reported intentions of increasing discussion of preventive behaviors with offspring but who reported discussing preventive behaviors infrequently at 1 and 6 months. Further, among participants who intended to have the same or less frequent discussions, it was relatively rare for them to discuss preventive behaviors more than intended. Although participants reported feeling moderately well prepared to discuss genetic risk with children/grandchildren (mean = 4.4, SD = 0.7), feelings of preparedness were unrelated to reported frequency of discussions at 1 and 6 months (Table 2).
Fig. 2.
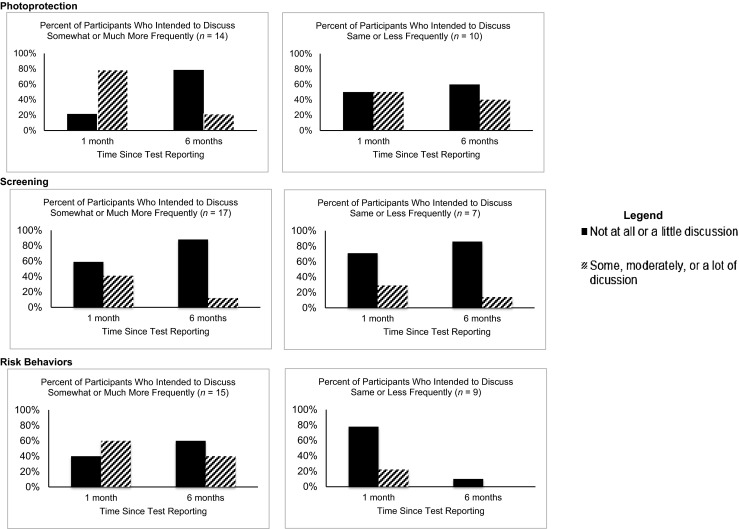
Discussion intentions immediately after test reporting and reported discussion 1 and 6 months later, stratified by participants who intended to discuss more frequently versus less frequently. Note: The dark bars on the left panels illustrate the participants who at post-counseling indicated intentions to have more frequent discussions with children and grandchildren but who at 1 and 6 months reported having had little or no discussion
Themes for preparedness to discuss preventive behaviors and informational needs
After test reporting, 88 % of participants provided open-ended responses about preparedness to discuss the family’s genetic risk for melanoma with their children and grandchildren, and suggestions for information that would be helpful for understanding their risk or facilitating discussions with offspring. Participants reported that research participation helped them feel prepared (19 %) and that they had the knowledge needed for discussions (10 %). They also felt prepared due to personal or family history of melanoma (10 %) and because they already discussed melanoma risk with children (5 %). Participants described the need to discuss melanoma risk and prevention so that children and grandchildren can “take control” by implementing preventive behaviors (19 %) and so that children and grandchildren are aware of the risk (10 %). Fourteen percent expressed concern about their ability to communicate information about genetic risk to offspring, and 43 % provided ideas for resources that would be helpful. Examples included written and pictorial information (e.g., pamphlets containing charts that describe one’s risk, internet-based resources) and information about UVR exposure (e.g., differences in protection afforded by sunscreen versus clothing) and other risk factors for melanoma.
Changes in reported frequency of discussions about melanoma prevention with children and grandchildren
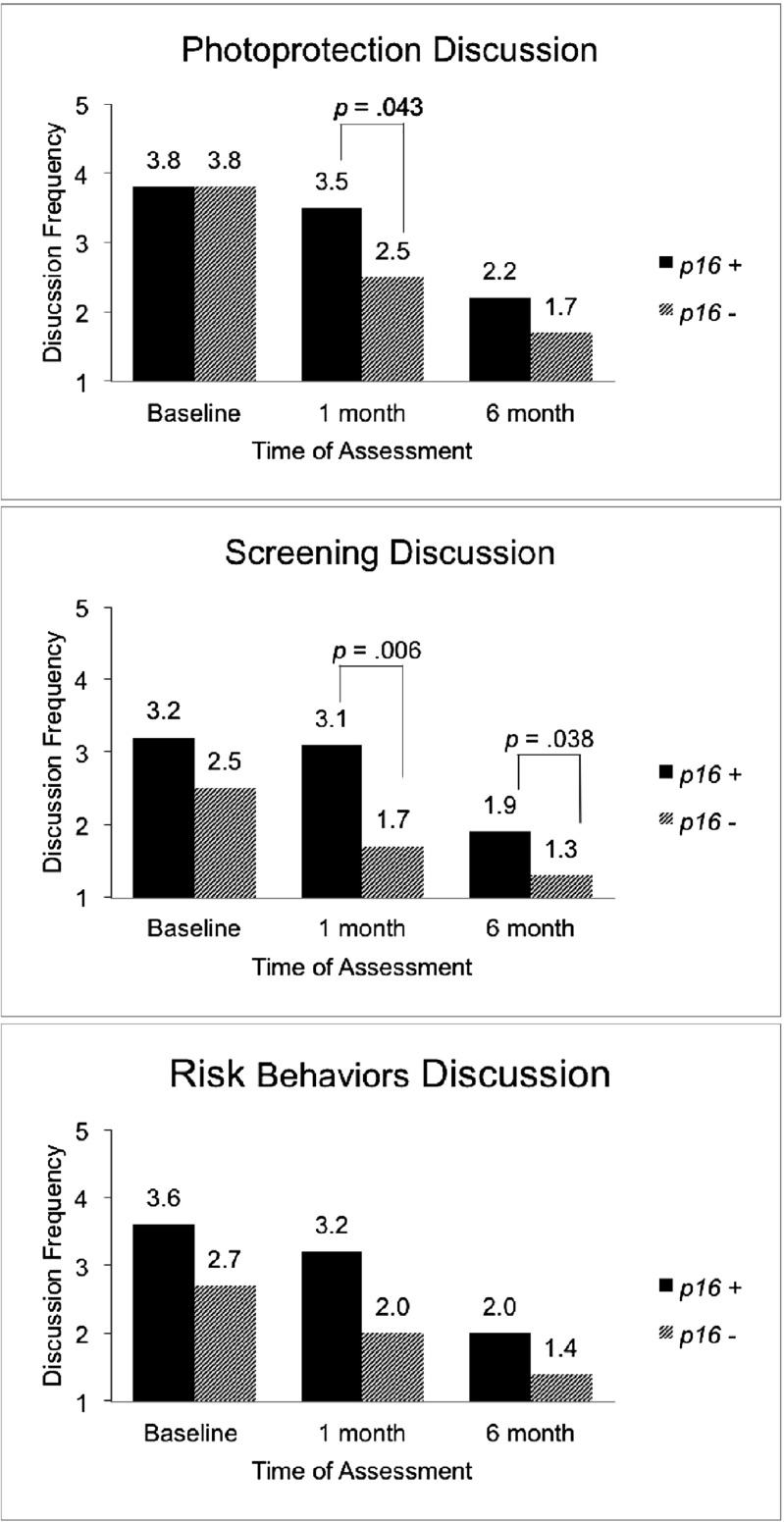
The repeated-measures ANOVAs indicated there was a significant main effect for time for discussion of each preventive behavior (photoprotection: Wilks’ λ = 0.23, F(2,21) = 35.53, p < 0.001; screening: Wilks’ λ = 0.36, F(2,21) = 18.76, p < 0.001; risk: Wilks’ λ = 0.36, F(2,21) = 18.91, p < 0.001) (Fig. 3). Carriers reported more frequent overall discussion of screening (F(1,22) = 7.09, p = 0.014) and risk behaviors (F(1,22) = 4.47, p = 0.046) than noncarriers. Contrary to hypotheses, the frequency of discussion of preventive behaviors decreased significantly in both groups from baseline to 6 months (Fig. 3). The decrease in reported frequency of discussion occurred between baseline and 1 month for noncarriers for photoprotective and screening behaviors, remaining stable and low from 1 to 6 months. There was no change between all three time points for discussion of risk behaviors among noncarriers. In contrast, among carriers, baseline levels of discussion frequency were sustained for all three behaviors at 1 month, but dropped significantly between 1 and 6 months. Although there was no significant carrier status by time interaction (photoprotection: Wilks’ λ = 0.81, F(2,21) = 2.44, p = 0.11; screening: Wilks’ λ = 0.85, F(2,21) = 1.82, p = 0.19; risk: Wilks’ λ = 0.95, F(2,21) = 0.56, p = 0.58), indicating that there were no differences between carriers and noncarriers in changes in their level of discussion about preventive behaviors over time, we note that the carrier status groups seemed to differ with respect to the time at which discussion dropped from baseline, with noncarriers reporting decreased discussion frequency at 1 month and carriers reporting decreases at 6 months.
Fig. 3.
Mean frequency of discussion of preventive behaviors with children and grandchildren by p16 carrier status before (baseline) and after (1 and 6 months) test reporting and counseling. Note: Significant differences between p16 carrier groups within time point are noted by brackets
Role of personal melanoma history and genetic test result disclosure
Exploratory analyses using repeated-measures ANOVAs to examine discussion of preventive behaviors by personal melanoma history indicated a trend such that carriers with a personal history of melanoma (n = 8) more frequently discussed photoprotective and screening behaviors at 6 months than carriers without a personal history of melanoma (n = 3; p = 0.057, p = 0.077, respectively). There were strong correlations, within time point, between discussion of the genetic test result and discussion about the three preventive behaviors (1 month, rs = 0.87 to 0.91, ps < 0.001; 6 months, rs = 0.49, ps < 0.05). At 1 month, 71 % of participants, and at 6 months, 33 % reported discussing their genetic test result with children/grandchildren.
Content of reported discussions
At 1 month, 17 participants (71 %) indicated that they had discussions photoprotective behaviors, 15 (63 %) about screening behaviors, and 14 (58 %) about risk behaviors. For photoprotection, the most frequent topic discussed was sunscreen use (82 %), followed by use of physical barriers to decrease UVR exposure (29 %), and decreasing time outside during peak exposure (29 %). Additional topics included healthy eating and recommendations to protect one’s skin anytime there is UVR exposure (18 %). For screening, participants’ conversations included implementing skin self-exams (47 %), types of dermatological changes to look for (47 %), and the importance of obtaining a total body skin exam (40 %). When discussing risk behaviors, participants reported advising offspring not to use tanning beds or engage in other intentional tanning (57 %), to use precautions such as sunscreen, physical barriers, and decreasing time outside (50 %), and discussing norms for tanness (e.g., being tan is “not beautiful,” 7 %). Additional topics included statements about the dangers of risky behaviors (21 %). At 6 months, 9 participants (38 %) provided responses about “whether anything important came up” in discussions with offspring about preventive behaviors. Participants reported discussing sunscreen use (56 %), decreasing time spent outdoors (33 %), using physical barriers to protect from UVR (22 %), implementing skin self-exams (22 %), and avoiding tanning (11 %). An additional topic was the extent to which melanoma risk due to the p16 mutation differs depending on skin type (11 %).
Discussion
The current study examined an understudied consequence of genetic test reporting related to cancer prevention among families. Our results indicate that genetic test reporting and individualized genetic counseling alone do not appear to sustain individuals’ discussions about preventive behaviors for a hereditary cancer with their offspring who are at risk for the illness. Noncarriers reported a decrease in frequency of discussion. This is not unexpected, as their negative test result meant their offspring were no longer at risk for the genetic mutation. Carriers discussed screening and risk behaviors more frequently than noncarriers. However, their frequency of discussions about preventive behaviors also declined over time. This is notable given that the majority (73 %) of carriers had a personal history of melanoma. The decline in frequency of discussion for carriers generally occurred later (between 1 and 6 months) than for noncarriers (between test reporting and 1 month). Not only did the frequency of discussions decline over time, but these declines occurred despite participants reporting intentions to discuss preventive behaviors more frequently. Consistent with topics identified in prior studies (Hay et al. 2005), participants reported discussing recommended preventive behaviors, particularly sunscreen use. Physical UVR barriers, avoidance of peak exposure times, and risk behaviors were mentioned by fewer participants.
Our findings suggest that there may be barriers to discussions about melanoma preventive behaviors with children and grandchildren. Based on the results, one barrier could be a perceived need for additional resources (e.g., information sheets or websites) to be used in discussions with offspring. Participants provided initial ideas on how genetic counseling could be augmented to facilitate discussions about preventive behaviors with younger family members. Individuals who receive genetic testing and counseling may benefit from receiving information about how to approach preventive behavior conversations with children and grandchildren (Montgomery et al. 2013). This could include guidance from genetic counselors and other health professionals on how to make these discussions informative, constructive, and relevant to the needs and activities of their family, and how to continue these discussions over time. Supplemental written, pictorial, or internet-based resources could help facilitate these discussions. It may also be useful to provide education on the rationale for implementation of melanoma preventive behaviors early in life. Among the melanoma preventive behaviors, additional education on the effectiveness of physical barriers for UVR exposure, sunscreen reapplication (Aspinwall et al. 2014b) and use of shade when outdoors is warranted. Outside of the context of genetic counseling, future work could explore which provider(s) would be best suited to providing information on melanoma preventive behaviors and the most effective ways of communicating this information to facilitate individuals’ comprehension.
The findings should be interpreted with limitations and alternative explanations in mind. At baseline, participants may have reported more frequently discussing melanoma preventive behaviors due to having received prior counseling about their risk for melanoma or their plans to participate in the current study. Our study design precluded us from ruling out the possibility that decreased frequency in communication about photoprotection was partially attributable to changing seasons (however, this concern does not apply to findings about screening or use of tanning booths). Nevertheless, for high-risk families, especially those living at high altitude, photoprotection is an important behavior year-round. Our analysis was limited to the subsample of participants who completed questionnaires at all time points. Among participants in the test reporting study who completed baseline and 1-month assessments (n = 31), we detected similar decreases in discussion frequency over time. We therefore included the 6-month time point in order to expand the time frame in which participants could have reported preventive behavior discussions. The current study was limited by the relatively small sample size; however, to our knowledge, this is the largest available study on reported discussion of preventive behaviors with children and grandchildren after melanoma genetic test reporting. The demographic characteristics of our sample, including relatively high number of offspring, are representative of the local geographic region (United States Census Bureau 2013). Our findings await replication with larger, multi-site samples. However, it remains striking that even in the local culture, where there is a strong orientation toward family, genealogy, large family size, and personal agency for health (Leaf et al. 2010) participants did not report frequently having discussions about preventive behaviors with their offspring. The current study relied on self-reported frequency of preventive behavior discussions. While it is impossible to rule out inaccurate reporting, the declines in discussion frequency suggest that participants did not provide socially desirable answers (i.e., more frequent discussion of preventive behaviors).
Future work to identify barriers to and facilitators of communication about melanoma preventive behaviors could examine potential concern about alarming offspring, negative reactions from family, existing conflict around preventive behaviors, and reasons for not discussing risk and preventive behaviors with family (Gaff et al. 2007; Hay et al. 2005). Additional factors that could be examined include predictors of ongoing discussions with children and grandchildren about preventive behaviors (Harris et al. 2010), family structure (e.g., how closely or distantly family members are related), communication style, perceived control over health conditions with a genetic basis, involvement in monitoring and supporting adherence to preventive behaviors (Dimatteo 2004; Harris et al. 2010; McCann et al. 2009; Modi et al. 2012), and children’s/grandchildren’s perceptions (e.g., helpfulness of discussions). Depending on children’s and grandchildren’s adherence to preventive behaviors, it may be necessary to provide targeted risk communication or adherence intervention to these younger members of melanoma-prone families. Variability in discussions about preventive behaviors may be due to families’ changing participation in seasonal outdoor activities and could be examined. Future studies could also assess how discussions vary with offspring of different ages and relationship (e.g., children vs. grandchildren) and the relationship between discussion of preventive behaviors and disclosure of genetic test results.
In summary, our findings indicate that while participants who received melanoma genetic testing reported considerable intentions to discuss preventive behaviors with offspring and reported feeling prepared for these discussions, discussion occurred at lower levels and represented a significant decline from baseline. Individuals who receive melanoma genetic testing could benefit from additional support to have ongoing discussions with offspring about melanoma preventive measures, particularly given literature indicating the importance of effective family communication in promoting adherence to medical recommendations (Dimatteo 2004). Given the suspected etiology of early life sunburns in the development of melanoma, it will be important to improve communication about preventive behaviors with family members who are at risk for the disease at an opportune time to promote prevention behaviors and the establishment of lifelong habits (Balk 2011).
Acknowledgments
The authors acknowledge the generous participation of study participants; Marybeth Hart, Erin Dola, and Lisa Wadge for contributions to study development; and Amber Kostial, Emily Bullough, Michelle Welch, Hoda Wali, Candace Larson, and Taylor Haskell for service as study/clinic coordinators. This work was supported by a Funding Incentive Seed Grant, University of Utah; Huntsman Cancer Foundation; Tom C. Mathews, Jr. Familial Melanoma Research Clinic endowment; Pedigree and Population Resource of Huntsman Cancer Institute (HCI); Utah Population Database; Utah Cancer Registry funded by N01-PC-35141 from the National Cancer Institute (NCI) SEER Program and the Utah State Department of Health and University of Utah. The authors acknowledge the use of core facilities supported by the NCI Cancer Center Support Grant 5P30CA420-14 awarded to HCI and the genetic counseling core supported by Huntsman Cancer Foundation. Investigators (LGA, SAL, TKS) were supported by NCI (R01 CA158322-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of NCI or the National Institutes of Health.
Compliance with ethics guidelines
Yelena Wu, Lisa G. Aspinwall, Timothy Michaelis, Tammy Stump, and Wendy Kohlmann declare that they have no conflict of interest.
Sancy Leachman serves on a Medical and Scientific Advisory Board for Myriad Genetics, for which she has received an honorarium. She collaborated with Myriad to validate an assay that is unrelated to research reported here.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5).
Informed consent was obtained from all patients for being included in the study.
References
- Ajzen I (1991) The theory of planned behavior. Organ Behav Hum Decis Process 50:179–211. doi:10.1016/0749-5978(91)90020-T
- American Academy of Dermatology (2015) Melanoma: tips for finding and preventing. www.aad.org. Accessed 15 April 2015
- American Cancer Society (2015) Can melanoma skin cancer be prevented? www.cancer.org. Accessed 15 April 2015
- Aspinwall LG, Leaf SL, Dola ER, Kohlmann W, Leachman SA. CDKN2A/p16 genetic test reporting improves early detection intentions and practices in high-risk melanoma families. Cancer Epidemiol Biomarkers Prev. 2008;17:1510–1519. doi: 10.1158/1055-9965.EPI-08-0010. [DOI] [PubMed] [Google Scholar]
- Aspinwall LG, Leaf SL, Kohlmann W, Dola ER, Leachman SA. Patterns of photoprotection following CDKN2A/p16 genetic test reporting and counseling. J Am Acad Dermatol. 2009;60:745–757. doi: 10.1016/j.jaad.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Aspinwall LG, Taber JM, Leaf SL, Kohlmann W, Leachman SA. Genetic testing for hereditary melanoma and pancreatic cancer: a longitudinal study of psychological outcome. Psychooncology. 2013;22:276–289. doi: 10.1002/pon.2080. [DOI] [PubMed] [Google Scholar]
- Aspinwall LG, Taber JM, Leaf SL, Kohlmann W, Leachman SA. Melanoma genetic counseling and test reporting improve screening adherence among unaffected carriers 2 years later. Cancer Epidemiol Biomarkers Prev. 2013;22:1687–1697. doi: 10.1158/1055-9965.EPI-13-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinwall LG, Taber JM, Kohlmann W, Leaf SL, Leachman SA. Perceived risk following melanoma genetic testing: a 2-year prospective study distinguishing subjective estimates from recall. J Genet Couns. 2014;23:421–437. doi: 10.1007/s10897-013-9676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinwall LG, Taber JM, Kohlmann W, Leaf SL, Leachman SA. Unaffected family members report improvements in daily routine sun protection 2 years following melanoma genetic testing. Genet Med. 2014 doi: 10.1038/gim.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzarello LM, Dessureault S, Jacobsen PB. Sun-protective behavior among individuals with a family history of melanoma. Cancer Epidemiol Biomarkers Prev. 2006;15:142–145. doi: 10.1158/1055-9965.EPI-05-0478. [DOI] [PubMed] [Google Scholar]
- Balk SJ. Ultraviolet radiation: a hazard to children and adolescents. Pediatrics. 2011;127:588–597. doi: 10.1542/peds.2010-3502. [DOI] [PubMed] [Google Scholar]
- Barsevick AM, et al. Intention to communicate BRCA1/BRCA2 genetic test results to the family. J Fam Psychol. 2008;22:303. doi: 10.1037/0893-3200.22.2.303. [DOI] [PubMed] [Google Scholar]
- Cannon-Albright LA, et al. Assignment of a locus for familial melanoma, MLM, to chromosome 9p13-p22. Science. 1992;258:1148–1152. doi: 10.1126/science.1439824. [DOI] [PubMed] [Google Scholar]
- Champine M, Kohlmann W, Leachman SA. Genetic counseling and testing for hereditary melanoma: an updated guide for dermatologists. Hered Genet. 2013;S2:004. [Google Scholar]
- Costalas JW, Itzen M, Malick J, Babb JS, Bove B, Godwin AK, Daly MB (2003) Communication of BRCA1 and BRCA2 results to at-risk relatives: a cancer risk assessment program's experience. In: American journal of medical genetics part C: seminars in medical genetics, vol 1. Wiley Online Library, pp 11-18 [DOI] [PubMed]
- Daly MB, Barsevick A, Miller SM, Buckman R, Costalas J, Montgomery S, Bingler R. Communicating genetic test results to the family: a six-step, skills-building strategy. Fam Community Health. 2001;24:13–26. doi: 10.1097/00003727-200110000-00004. [DOI] [PubMed] [Google Scholar]
- Dimatteo MR. The role of effective communication with children and their families in fostering adherence to pediatric regimens. Patient Educ Couns. 2004;55:339–344. doi: 10.1016/j.pec.2003.04.003. [DOI] [PubMed] [Google Scholar]
- Fehniger J, Lin F, Beattie MS, Joseph G, Kaplan C. Family communication of BRCA1/2 results and family uptake of BRCA1/2 testing in a diverse population of BRCA1/2 carriers. J Genet Couns. 2013;22:603–612. doi: 10.1007/s10897-013-9592-4. [DOI] [PubMed] [Google Scholar]
- Florell SR, et al. Population-based analysis of prognostic factors and survival in familial melanoma. J Clin Oncol. 2005;23:7168–7177. doi: 10.1200/JCO.2005.11.999. [DOI] [PubMed] [Google Scholar]
- Gaff CL, et al. Process and outcome in communication of genetic information within families: a systematic review. Eur J Hum Genet. 2007;15:999–1011. doi: 10.1038/sj.ejhg.5201883. [DOI] [PubMed] [Google Scholar]
- Geller AC, et al. Skin cancer prevention and detection practices among siblings of patients with melanoma. J Am Acad Dermatol. 2003;49:631–638. doi: 10.1067/S0190-9622(03)02126-1. [DOI] [PubMed] [Google Scholar]
- Glanz K, et al. Melanoma genetic testing, counseling, and adherence to skin cancer prevention and detection behaviors. Cancer Epidemiol Biomarkers Prev. 2013;22:607–614. doi: 10.1158/1055-9965.EPI-12-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AM, et al. Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents. J Med Genet. 2007;44:99–106. doi: 10.1136/jmg.2006.043802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallowell N, Ardern‐Jones A, Eeles R, Foster C, Lucassen A, Moynihan C, Watson M. Communication about genetic testing in families of male BRCA1/2 carriers and non-carriers: patterns, priorities and problems. Clin Genet. 2005;67:492–502. doi: 10.1111/j.1399-0004.2005.00443.x. [DOI] [PubMed] [Google Scholar]
- Hamilton RJ, Bowers BJ, Williams JK. Disclosing genetic test results to family members. J Nurs Scholarsh. 2005;37:18–24. doi: 10.1111/j.1547-5069.2005.00007.x. [DOI] [PubMed] [Google Scholar]
- Hansen CB, Wadge LM, Lowstuter K, Boucher K, Leachman SA. Clinical germline testing for melanoma. Lancet Oncol. 2004;5:314–419. doi: 10.1016/S1470-2045(04)01469-X. [DOI] [PubMed] [Google Scholar]
- Harris JN, Hay J, Kuniyuki A, Asgari MM, Press N, Bowen DJ. Using a family systems approach to investigate cancer risk communication within melanoma families. Psycho-Oncology. 2010;19:1102–1111. doi: 10.1002/pon.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J, Ostroff J, Martin A, Serle N, Soma S, Mujumdar U, Berwick M. Skin cancer risk discussions in melanoma-affected families. J Cancer Educ. 2005;20:240–246. doi: 10.1207/s15430154jce2004_13. [DOI] [PubMed] [Google Scholar]
- Hay J, Shuk E, Brady MS, Berwick M, Ostroff J, Halpern A. Family communication after melanoma diagnosis. Arch Dermatol. 2008;144:553–554. doi: 10.1001/archderm.144.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- Kamb A, et al. Analysis of the p16 gene (CDKN2) as a candidate for the chromosome 9p melanoma susceptibility locus. Nat Genet. 1994;8:23–26. doi: 10.1038/ng0994-22. [DOI] [PubMed] [Google Scholar]
- Kefford R, et al. Counseling and DNA testing for individuals perceived to be genetically predisposed to melanoma: a consensus statement of the melanoma genetics consortium. J Clin Oncol. 1999;17:3245–3251. doi: 10.1200/JCO.1999.17.10.3245. [DOI] [PubMed] [Google Scholar]
- Kefford R, et al. Genetic testing for melanoma. Lancet Oncol. 2002;3:653–654. doi: 10.1016/S1470-2045(02)00894-X. [DOI] [PubMed] [Google Scholar]
- Lafreniere D, Bouchard K, Godard B, Simard J, Dorval M. Family communication following BRCA1/2 genetic testing: a close look at the process. J Genet Couns. 2013;22:323–335. doi: 10.1007/s10897-012-9559-x. [DOI] [PubMed] [Google Scholar]
- Leachman SA et al (2009) Selection criteria for genetic assessment of patients with familial melanoma. J Am Acad Dermatol 61:677.e671-677. doi:10.1016/j.jaad.2009.03.016 [DOI] [PMC free article] [PubMed]
- Leaf SL, Aspinwall LG, Leachman SA. God and agency in the era of molecular medicine: religious beliefs predict sun-protection behaviors following melanoma genetic testing reporting. Arch Psychol Relig. 2010;32:87–112. doi: 10.1163/008467210X12626615185784. [DOI] [Google Scholar]
- McCann S, MacAuley D, Barnett Y, Bunting B, Bradley A, Jeffers L, Morrison PJ. Family communication, genetic testing and colonoscopy screening in hereditary non-polyposis colon cancer: a qualitative study. Psychooncology. 2009;18:1208–1215. doi: 10.1002/pon.1487. [DOI] [PubMed] [Google Scholar]
- Modi AC, et al. Pediatric self-management: a framework for research, practice, and policy. Pediatrics. 2012;129:e473–485. doi: 10.1542/peds.2011-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SV, et al. Preparing individuals to communicate genetic test results to their relatives: report of a randomized control trial. Fam Cancer. 2013;12:537–546. doi: 10.1007/s10689-013-9609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendorf KB, Tsao H. Cutaneous melanoma: family screening and genetic testing. Dermatol Ther. 2006;19:1–8. doi: 10.1111/j.1529-8019.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- Oliveria SA, Saraiya M, Geller AC, Heneghan MK, Jorgensen C. Sun exposure and risk of melanoma. Arch Dis Child. 2006;91:131–138. doi: 10.1136/adc.2005.086918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria SA, et al. Melanoma survivors: health behaviors, surveillance, psychosocial factors, and family concerns. Psychooncology. 2013;22:106–116. doi: 10.1002/pon.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustisek N, Sikanic-Dugic N, Hirsl-Hecej V, Domljan ML. Acute skin sun damage in children and its consequences in adults. Coll Anthropol. 2010;34:233–237. [PubMed] [Google Scholar]
- Strauss A, Corbin JM. Basics of qualitative research: grounded theory procedures and techniques. Thousand Oaks: Sage Publications; 1990. [Google Scholar]
- United States Census Bureau (2013) American community survey. http://www.census.gov/acs/www/. Accessed 13 Apr 2015
- University of Utah (2014) Utah population database. http://healthcare.utah.edu/huntsmancancerinstitute/research/updb/. Accessed 10 Nov 2014