Abstract
Introduction
There is no consensus chemotherapy regimen with concurrent radiation therapy (RT) for inoperable Stage IIIA/B NSCLC. This trial evaluated pemetrexed with carboplatin (PCb) or cisplatin (PC) with concurrent RT followed by consolidation pemetrexed.
Methods
In this open-label, non-comparative Phase II trial, patients with inoperable Stage IIIA/B NSCLC (initially all histologies, later restricted to nonsquamous) were randomized (1:1) to PCb or PC with concurrent RT (64–68 Gy over Days 1–45). Consolidation pemetrexed monotherapy was administered every 21 days for 3 cycles. Primary endpoint was 2-year overall survival (OS) rate.
Results
From June 2007 to November 2009, 98 patients were enrolled (PCb: 46; PC: 52). The 2-year OS rate was PCb: 45.4% (95% confidence interval [CI], 29.5%–60.0%); PC: 58.4% (95% CI, 42.6%–71.3%) and in nonsquamous patients was PCb: 48.0% (95% CI, 29.0%–64.8%); PC: 55.8% (95% CI, 38.0%–70.3%). Median time to disease progression was PCb: 8.8 months (95% CI, 6.0–12.6 months); PC: 13.1 months (95% CI, 8.3–not evaluable (NE). Median OS (months) was PCb: 18.7 (95% CI, 12.9-NE); PC: 27.0 (95% CI, 23.2-NE).The objective response rates were PCb: 52.2%; PC: 46.2%. Grade 4 treatment-related toxicities (% PCb/% PC) were: anemia, 0/1.9; neutropenia, 6.5/3.8; thrombocytopenia, 4.3/1.9; and esophagitis, 0/1.9. Most patients completed scheduled chemotherapy and RT during induction and consolidation phases. No drug-related deaths were reported during chemoradiotherapy.
Conclusions
Due to study design, efficacy comparisons cannot be made. However, both combinations with concurrent RT were active and well tolerated.
Keywords: NSCLC, pemetrexed, cisplatin, chemoradiotherapy, stage III
INTRODUCTION
Approximately 30% of patients with non-small cell lung cancer (NSCLC) have unresectable Stage IIIA/B disease at diagnosis.1 The standard of care for patients with good performance status is chemoradiotherapy.1,2 Surgical resection following chemoradiotherapy does not improve overall survival (OS).3 Because the 5-year survival rate for unresectable stage IIIA/B disease is only 25%,4 improvements are needed in chemoradiotherapy regimens.
Pemetrexed is a multitargeted antifolate.5 It has radiosensitizing activity in cultured cells and xenografts6–9 and has been tested in combination with radiotherapy (RT) and platinum in clinical trials.10–17 These trials have shown the feasibility of combining pemetrexed/platinum with RT in patients with locally advanced NSCLC. The safety profile of pemetrexed, its approved use with cisplatin in first-line nonsquamous NSCLC,18,19 and its utility in the maintenance setting20 suggest that pemetrexed may be used not only with chemoradiation but may also be safe and effective as consolidation therapy after concurrent chemoradiation.
On the basis of this rationale, a Phase I–II trial was designed to test pemetrexed/carboplatin (PCb) or pemetrexed/cisplatin (PC) with concurrent RT followed by pemetrexed consolidation in patients with favorable-prognosis inoperable Stage IIIA/B NSCLC. The Phase I portion of this trial established the maximum tolerated and Phase II doses of pemetrexed with either carboplatin or cisplatin given during RT, followed by pemetrexed consolidation.21 The results of the randomized Phase II portion of the trial are reported here. Because this trial was initiated prior to the observation that pemetrexed offers a survival advantage in patients with nonsquamous histology relative to squamous histology,22,23 this trial initially enrolled patients with all histologies. Following these reports, a protocol amendment subsequently excluded patients with squamous histology.
PATIENTS AND METHODS
Patient Eligibility
Men and woman (≥18 years old) with measurable Stage IIIA or IIIB (without pleural effusion) NSCLC24 were eligible. Initially, all histologies were allowed, but based on subset analyses from clinical trials of pemetrexed as first- and second-line therapy for Stage IV NSCLC19,22,23,25 the protocol was amended in October 2008 to exclude patients with squamous histology. Other key eligibility criteria were Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, weight loss <10% in the 3 months prior to diagnosis, a forced expiratory volume in 1 second >1000 mL, and written informed consent.
Key exclusion criteria were prior tumor resection; prior chemotherapy for this cancer; prior RT for any thoracic or neck condition; symptomatic heart disease; myocardial infarction within the past 6 months; any pleural effusion on chest x-ray or computed tomography (CT) scan; planning target volume >3 liters; lung volume receiving more than 20 Gy >40%; inability to discontinue nonsteroidal anti-inflammatory drugs for 2 days before, the day of, and 2 days after pemetrexed; and unwillingness or inability to take folic acid, vitamin B12, or dexamethasone as required by protocol.
This study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. The protocol was approved by local Human Institutional Review Boards and in accord with an assurance filed with and approved by the Department of Health and Human Services. Written informed consent was obtained.
Study Design and Treatment
This was an open-label, non-comparative randomized trial. Per protocol, the two study treatment arms were analyzed independently. Patients were randomized (1:1) to Arm A: pemetrexed 500 mg/m2 intravenously on Day 1 followed by carboplatin area under the curve (AUC) 5 intravenously on Day 1, or to Arm B: pemetrexed 500 mg/m2 intravenously on Day 1 followed by cisplatin 75 mg/m2 on Day 1. Both arms received chemotherapy every 3 weeks for 3 cycles with concurrent RT at a dose of 2 Gy per day administered 5 days a week to a total dose of 64–68 Gy. Randomization was performed without stratification factors.
Patients received consolidation pemetrexed 500 mg/m2 every 3 weeks for 3 cycles after RT completion. Consolidation chemotherapy did not begin until the absolute neutrophil count (ANC) was ≥1500/mm3 and platelets were ≥100,000/mm3. Subsequent cycles of therapy did not begin until ANC was ≥1000/mm3 and platelets were ≥75,000/mm3. For hematologic toxicities, therapy could be held up to 28 days from Day 1 of the previous cycle.
Patients received daily folic acid beginning at least 1 week prior to the first dose of pemetrexed, and continuing daily thereafter until 3 weeks after the last pemetrexed dose. Intramuscular vitamin B12 began at least 1 week prior to the first dose of pemetrexed and continued approximately every 9 weeks until 3 weeks after the last pemetrexed dose. Patients were premedicated with dexamethasone and received full supportive care. Prophylactic use of colony stimulating factors was not permitted, but granulocyte-colony stimulating factor (G-CSF) and erythropoietin were allowed, if clinically indicated. G-CSF was not administered on radiation days. Treatment continued until protocol completion, disease progression, unacceptable toxicity, or discontinuation for another reason. The ClinicalTrials.gov identifier is NCT00482014.
Radiotherapy
Chest irradiation began on chemotherapy Day 1. Radiotherapy was administered after chemotherapy. The total RT dose, which was calculated using algorithms accounting for tissue inhomogeneity, was 64 to 68 Gy. Treated volumes included the gross tumor volume plus margins, without elective nodal irradiation. A volumetric treatment planning CT study defined the gross tumor volume, clinical target volume, and planned target volume. Radiotherapy was administered in 2.0-Gy fractions, 5 days per week. The suggested beam energy was 6 to 10 MV, but investigators were permitted to use up to 18 MV.
If therapy interruption ≤1 week was necessary, irradiation was resumed and completed to the prescribed dose. If a longer interruption in therapy was required, treatment resumption was at the radiation oncologist’s discretion.
Chemotherapy Dose Adjustments
For Grade 3 and Grade 4 hematologic toxicities, the protocol mandated holding chemotherapy until recovery and then resuming at 75% or 50% dose as specified. Radiotherapy was held week to week until recovery. If these events recurred after dose reduction, the patient was discontinued from study treatment. If RT was held, chemotherapy was also held. For hemoglobin ≤8 g/dL the patient was transfused without treatment interruption.
For specified Grade ≥3 nonhematologic toxicities, chemotherapy was held up to 1 week until recovery to ≤Grade 1 and then resumed at the previous dose or a reduced dose depending on the toxicity. Radiotherapy was also held. Neurologic toxicities ≥Grade 2 required holding platinum until recovery to ≤Grade 1.
Patient Evaluations
Tumors could be measured by computed tomography (CT) or magnetic resonance imaging (MRI). The same assessment method was used throughout the study. During the study, tumor measurements were performed at the investigator’s discretion during chemoradiation, within 7 to 10 days before the first dose of consolidation therapy and after Cycle 3 of consolidation. Tumor measurements were also performed at the 30-day follow-up assessment. All patients achieving an objective response had a 4-week confirmation scan.
Assessments, such as physical examinations, performance status, hematology, blood chemistry, CTCAE Version 3.0 grading, and concomitant medication recording, were performed at prespecified intervals throughout the study. A late radiation morbidity assessment using the Radiation Therapy Oncology Group (RTOG) Late Morbidity Scoring Schema was performed approximately 90 days after the start of RT.
Upon completion of all study therapy, patients had a 30-day follow-up assessment including a tumor measurement. Thereafter, patients were followed monthly for 2 months, then every 2 months for 6 months, and then every 3 months until progression or for 24 months from initiation of study therapy, whichever came first. After progression, patients were followed every 3 months for survival.
Statistical Considerations
The primary objective was 2-year OS rate using Kaplan-Meier analyses.26 Based on SWOG 9504 yielding a 54% 2-year survival rate,27 the threshold of interest for pursuing a Phase III trial was 50%, and a 2-year survival of 30% or lower was of no interest. Assuming that patients would be accrued within 18 months and observed an additional 24 months, 46 patients per arm provided an approximate 82% power to detect a 2-year survival rate of ≥50%, with a 1-sided significance level of 0.05 in each arm. To allow for a 10% ineligibility rate, 51 patients per arm were planned.
Secondary objectives were median OS, objective response rates (ORR), time to disease progression, and toxicity and feasibility of these 2 regimens in this patient population. Time-to-event endpoints were analyzed using the Kaplan-Meier method.26 Response was measured according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.0.28 Toxicities were graded using the CTCAE Version 3.0.29
The intent-to-treat population included all enrolled (randomized) patients regardless of whether the patient received treatment. The safety population was all patients receiving ≥1 dose of pemetrexed plus carboplatin or cisplatin and 1 dose of radiotherapy.
RESULTS
Patient Characteristics and Treatment
From June 2007 through November 2009, 98 patients were enrolled (46 PCb; 52 PC) in 17 centers located in the United States and India. Patients were followed until October 2011. Figure 1 shows the CONSORT diagram. Table 1 shows baseline demographics. Notably, 13 patients (28.3%) in the PCb arm and 11 patients (21.2%) in the PC arm had squamous histology.
Figure 1. CONSORT diagram.
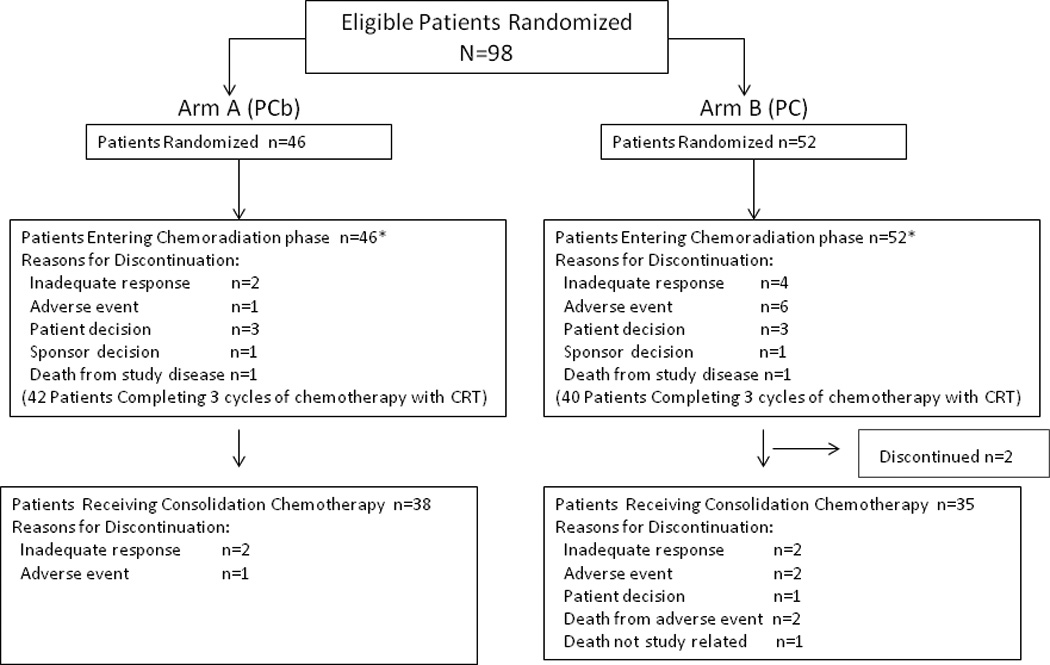
Patient disposition is shown. The reasons for discontinuation of the 2 patients prior to receiving consolidation therapy in the PC arm were not reported. Abbreviations: PC=pemetrexed-cisplatin; PCb=pemetrexed-carboplatin
Table 1.
Baseline Demographics (ITT Population)
| Parameter | PCb (N=46) |
PC (N=52) |
|---|---|---|
| Gender (n [%]) | ||
| Female | 16 (34.8) | 21 (40.4) |
| Male | 30 (65.2) | 31 (59.6) |
| Age (years) | ||
| Median (range) | 62.8 (43.7, 82.4) | 64.3 (45.8, 85.2) |
| Race (n [%]) | ||
| African | 5 (10.9) | 5 (9.6) |
| Caucasian | 36 (78.3) | 37 (71.2) |
| Hispanic | 1 (2.2) | 0 (0.0) |
| Asian | 4 (8.7) | 10 (19.2) |
| Primary tumor diagnosis (n [%]) | ||
| Adenocarcinoma | 19 (41.3) | 18 (34.6) |
| Large cell | 3 (6.5) | 3 (5.8) |
| Mixed histology | 0 (0.0) | 2 (3.8) |
| Poorly differentiated NSCLC | 9 (19.6) | 13 (25.0) |
| Squamous cell | 13 (28.3) | 11 (21.2) |
| Other | 2 (4.3) | 5 (9.6) |
| Disease stage | ||
| Stage IIIA | 20 (43.5) | 27 (51.9) |
| Stage IIIB | 26 (56.5) | 25 (48.1) |
Abbreviations: ITT = intent to treat; N = total population size; n = number of patients; PC=pemetrexed/cisplatin; PCb=pemetrexed/carboplatin.
Efficacy
In the intent-to-treat population, the 2-year OS rates were 45.4% (95% CI, 29.5–60.0) and 58.4% (95% CI, 42.6–71.3) with PCb and PC, respectively (Figure 2 and Table 2). The median OS was 18.7 months for PCb (95% CI, 12.9-not evaluable [NE]) and 27.0 months for PC (95% CI, 23.2-NE). In nonsquamous patients, median OS was not evaluable in either arm, whereas the 2-year survival rates were 48.0% (95% CI, 29.0–64.8) and 55.8% (95% CI, 38.0–70.3) with PCb and PC, respectively.
Figure 2. Kaplan-Meier plot of overall survival.
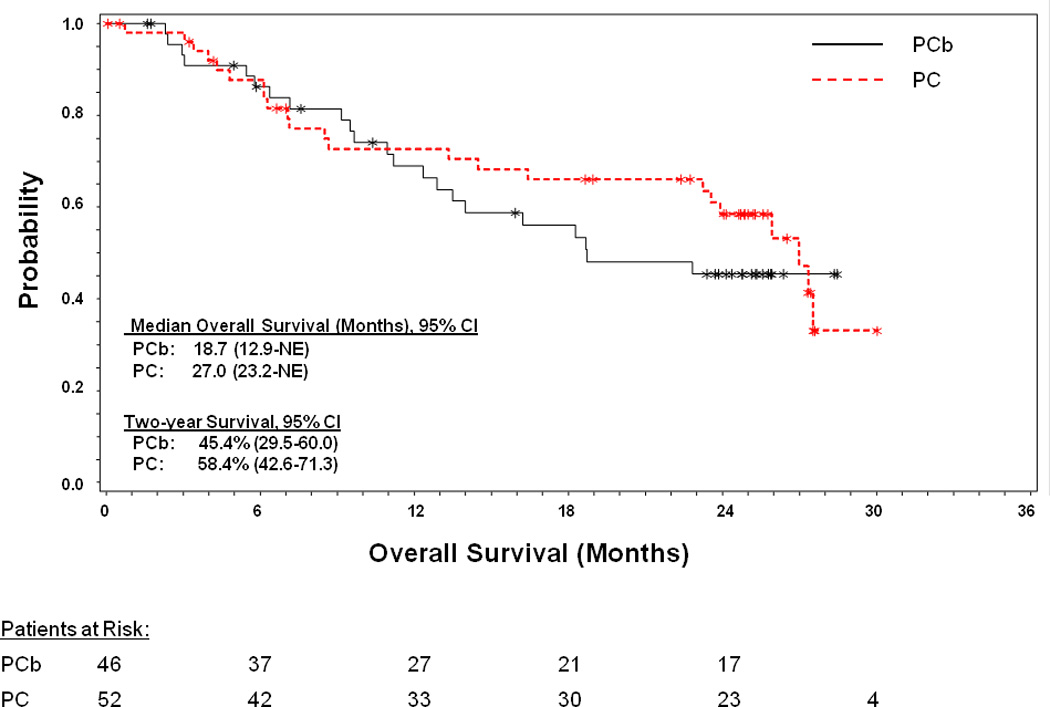
Overall survival was defined as the duration from date of randomization to date of death as the result of any cause. Censoring was done on the date the patient was last known to be alive, for patients who were still alive at the date of the last follow-up visit. Confidence intervals are based on the binomial distribution. Kaplan-Meier analyses were performed for the 2-year survival rate. Abbreviations: CI=confidence interval; NE=not evaluable; PC=pemetrexed-cisplatin; PCb=pemetrexed-carboplatin; * censured patients
Table 2.
Summary of Efficacy (ITT Population)
| Objective Tumor Responsea | ||||
|---|---|---|---|---|
| PCb | PC | |||
| Parameter | All N=46 |
Nonsquamous N=33 |
All N=52 |
Nonsquamous N=41 |
| Tumor response rate (n [%]) | 24 (52.2) | 18 (54.5) | 24 (46.2) | 16 (39.0) |
| Complete response (n [%]) | 3 (6.5) | 2 (6.1) | 2 (3.8) | 2 (4.9) |
| Partial response (n [%]) | 21 (45.7) | 16 (48.5) | 22 (42.3) | 14 (34.1) |
| Stable disease (n [%]) | 15 (32.6) | 10 (30.3) | 18 (34.6) | 17 (41.5) |
| Progressive disease (n [%]) | 1 (2.2) | 0 (0) | 6 (11.5) | 5 (12.2) |
| Unknown (n [%]) | 6 (13.0) | 5 (15.2) | 4 (7.7) | 3 (7.3) |
| Time to Event | ||||
| 2-year overall survival rate, % (95% CI) | 45.4 (29.5–60.0) | 48.0 (29.0–64.8) | 58.4 (42.6–71.3) | 55.8 (38.0–70.3) |
| Median overall survival, months (95% CI) | 18.7 (12.9-NE) | 22.8 (14.0-NE) | 27.0 (23.2-NE) | 25.9 (14.5-NE) |
| Median time-to-disease progression, months (95% CI) | 8.8 (6.0–12.6) | 9.0 (6.3–16.4) | 13.1 (8.3-NE) | 12.9 (7.8-NE). |
Abbreviations: CI=confidence interval; ITT=intent-to-treat; N=number in group; N=population size; NE=not evaluable; PC=pemetrexed/cisplatin; PCb=pemetrexed/carboplatin; RECIST=Response Evaluation in Solid Tumors.
Response was determined per RECIST criteria.
The ORR rates were 52.2% (complete response [CR], 6.5%; partial response [PR], 45.7%) with PCb and 46.2% (CR, 3.8%; PR, 42.3%) with PC (Table 2). In nonsquamous patients, the ORR was 54.5% with PCb and 39.0% with PC.
The median TTP was 8.8 months (95% CI, 6.0–12.6) with PCb and 13.1 months (95% CI, 8.3-NE) with PC (Figure 3 and Table 2).
Figure 3. Kaplan-Meier plot of time to progression.
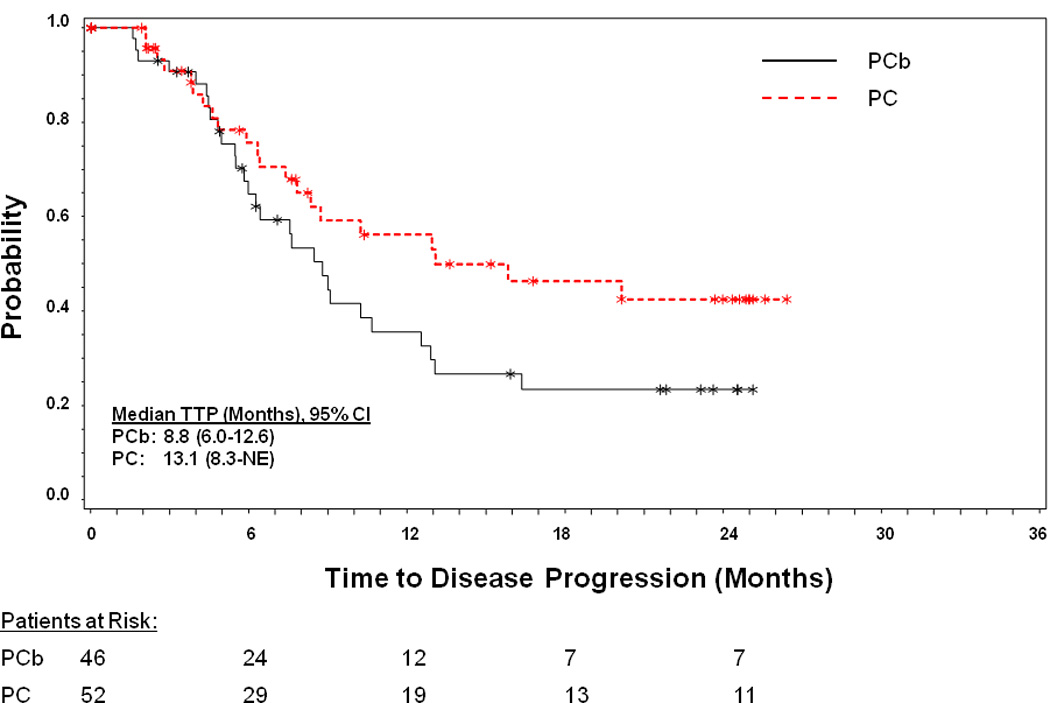
Time to progression was measured from randomization to the first observation of disease progression according to RECIST. Abbreviations: CI=confidence interval; NE=not evaluable; PC=pemetrexed-cisplatin; PCb=pemetrexed-carboplatin; RECIST; Response Evaluation in Solid Tumors; TTP=time to progression; *=censored patients.
Drug Exposure
All enrolled patients received ≥1 dose of PCb or PC. Forty-two PCb-treated (91.3%) and 40 PC-treated (76.9%) patients completed chemoradiotherapy. Thirty-eight PCb-treated (82.6%) and 35 PC-treated (67.3%) patients received consolidation therapy. Table 3 shows drug exposure during the chemoradiotherapy and consolidation periods.
Table 3.
Drug Exposure
| Chemoradiation | Consolidation | |||||
|---|---|---|---|---|---|---|
| PCb | PC | PCb | PC | |||
| P (N=46) | Cb (N=46) | P (N=52) | C (N=52) | PCb (N=38) | PC (N=35) | |
| Patients completing at least 1 cyclea | 46 (100) | 46 (100) | 52 (100) | 52 (100) | 38 (100) | 35 (100) |
| Patients completing at least 2 cyclesa | 46 (100) | 46 (100) | 46 (88.5) | 46 (88.5) | 38 (100) | 34 (97.1) |
| Patients completing at least 3 cyclesa | 42 (91.3) | 42 (91.3) | 42 (80.8) | 42 (80.8) | 35 (92.1) | 28 (80.0) |
| Mean number of cycles received (SD) | 2.9 (0.28) | 2.9 (0.28) | 2.7 (0.67) | 2.7 (0.67) | 2.9 (0.3) | 2.8 (0.5) |
| Patients with dose adjustments, n (%) | 5 (10.9) | 5 (10.9) | 7 (13.5) | 7 (13.5) | 5 (13.2) | 6 (17.1) |
| Dose omissions | 1 (2.2) | 1 (2.2) | 2 (3.8) | 1 (1.9) | 0 (0) | 2 (5.7) |
| Dose reductions | 4 (8.7) | 4 (8.7) | 6 (11.5) | 6 (11.5) | 5 (13.2) | 4 (11.4) |
| Actual mean dose (SD)b,c | 162.3 (11.5) | 1.6 (0.2) | 160.4 (14.9) | 24.1 (2.3) | 161.6 (15.5) | 157.7 (21.1) |
| Planned mean doseb,c | 166.7 | 1.7 | 166.7 | 25.0 | 166.7 | 166.7 |
| Dose intensityd | ||||||
| Mean (SD) | 97.4 (6.9) | 98.8 (13.4) | 96.2 (8.9) | 96.5 (9.2) | 97.0 (9.3) | 94.6 (12.6) |
| Median (range) | 100 (67.3–103.3) | 99.5 (58.5–125.0) | 100.0 (52.1–102.6) | 99.9 (52.1–111.5) | 100.0 (49.8–111.1) | 99.4 (50.3–101.7) |
Abbreviations: C=cisplatin; Cb=carboplatin; n=number in group; N=population size; P=pemetrexed; PC=pemetrexed/cisplatin; PCb=pemetrexed/carboplatin; SD=standard deviation.
Number of cycles is calculated as number of days on drug divided by 21 days and then rounded to a whole number.
For each patient, the actual mean dose is calculated as the dose received divided by number of weeks on treatment; the planned mean dose is same for all patients according to the protocol.
For pemetrexed and cisplatin, the unit is mg/m2/week; for carboplatin, the unit is area under the curve/week.
Dose intensity is calculated as mean dose received /mean dose planned × 100.
During the concurrent RT phase, the median (range) actual doses received were 66.0 (34.0–74.0) Gy and 66.0 (0–84.7) Gy in the PCb and PC arms, respectively. One patient in the PCb arm and 3 patients in the PC arm did not receive any RT. Interruptions of RT occurred in 39.1% of PCb-treated patients and 46.2% of PC-treated patients. Reasons for dose interruptions were adverse events (19.6% PCb; 13.5% PC), equipment failure (19.6% PCb; 7.7% PC), and patient decision (15.2% PCb; 26.9% PC). According to responses to a questionnaire administered to patients, 26 (56.5%) patients in the PCb arm and 25 (48.1%) patients in the PC arm completed RT with the total planned dose administered; approximately half of the patient population did not answer this questionnaire.
Safety
Most patients experienced ≥1 possibly drug-related treatment-emergent adverse event (TEAE; 89.1%, PCb; 73.1%, PC); approximately half of patients experienced ≥1 possibly drug-related Grade 3 or Grade 4 TEAE (50%, PCb; 42.3% PC). Possibly drug-related serious adverse events (SAEs) were experienced by 23.9% and 17.3% of patients in the PCb and PC arms, respectively. Drug-related SAEs occurring in ≥3% of patients in the PCb arm were: nausea (6.5%), vomiting (6.5%), dehydration (6.5%), anemia (4.3%), thrombocytopenia (4.3%), dysphagia (4.3%), and esophagitis (4.3%). Drug-related SAEs occurring in ≥ 3% of patients in the PC arm were: dehydration (7.7%), hypotension (5.8%), esophagitis (3.8%), and vomiting (3.8%).
Possibly drug-related AEs leading to discontinuation were experienced by 1 patient (2.2%) in the PCb arm (Grade 3 respiratory failure) and 4 patients (7.7%) in the PC arm (Grade 3 gastritis, Grade 2 reduced renal creatinine clearance, and 2 cases of Grade 3 renal failure). In addition, 1 patient in the PC arm discontinued due to a possibly drug-related SAE (Grade 5 myocardial infarction) during the consolidation phase.
Table 4 shows Grade 3 and Grade 4 AEs that were possibility related to study drug. A higher percentage of PCb-treated (19.6%) than PC-treated (7.7%) patients required transfusions. With the exception with of 1 patient in the PCb arm requiring platelets, all transfusions were of packed red blood cells. The arms had similar hospitalization rates (30.4%, PCb; 28.8%, PC).
Table 4.
Grade 3 and 4 Adverse Events, Possibly Related to Study Drug (Safety Population)
| PCb (N=46) |
PC (N=52) |
|||
|---|---|---|---|---|
| Grade 3 n (%) |
Grade 4 n (%) |
Grade 3 n (%) |
Grade 4 n (%) |
|
| Hematologic | ||||
| Anemia | 5 (10.9) | 0 (0.0) | 3 (5.8) | 1 (1.9) |
| Febrile neutropenia | 2 (4.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Leukopenia | 5 (10.9) | 0 (0) | 4 (7.7) | 0 (0) |
| Lymphopenia | 3 (6.5) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Neutropenia | 7 (15.2) | 3 (6.5) | 5 (9.6) | 2 (3.8) |
| Thrombocytopenia | 2 (4.3) | 2 (4.3) | 2 (3.8) | 1 (1.9) |
| Nonhematologic | ||||
| Alanine aminotransferase increased | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anorexia | 0 (0.0) | 0 (0.0) | 2 (3.8) | 0 (0.0) |
| Aspartate aminotransferase increased | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Asthenia | 0 (0.0) | 0 (0.0) | 2 (3.8) | 0 (0.0) |
| Bronchitis | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Decreased appetite | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Dehydration | 3 (6.5) | 0 (0.0) | 5 (9.6) | 0 (0.0) |
| Diarrhea | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Dysphagia | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dyspnea | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Esophagitis | 2 (4.3) | 0 (0.0) | 2 (3.8) | 1 (1.9) |
| Failure to thrive | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Fatigue | 3 (6.5) | 0 (0.0) | 2 (3.8) | 0 (0.0) |
| Gastritis | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Herpes esophagitis | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Hyperbilirubinemia | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hypokalemia | 3 (6.5) | 0 (0.0) | 2 (3.8) | 0 (0.0) |
| Hyponatremia | 1 (2.2) | 0 (0.0) | 2 (3.8) | 0 (0.0) |
| Hypotension | 2 (4.3) | 0 (0.0) | 4 (7.7) | 0 (0.0) |
| Localized infection | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mediastinitis | 1 (2.2) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Mucosal inflammation | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Muscular weakness | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Nausea | 2 (4.3) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Neuropathy peripheral | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Orthostatic hypotension | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Pneumonia | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Radiation esophagitis | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Radiation pneumonitis | 1 (2.2) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Renal failure | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Renal failure acute | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Respiratory failure | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Syncope | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vomiting | 2 (4.3) | 0 (0.0) | 3 (5.8) | 0 (0.0) |
| Weight decreased | 1 (2.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Abbreviations: n=number in group; N=population size; PC=pemetrexed/cisplatin; PCb=pemetrexed/carboplatin.
During study treatment, 1 (2.2%) patient in the PCb arm died from disease progression and 4 (7.7%) patients in the PC arm died: 1 (1.9%) patient from disease progression, 2 (3.8%) from AEs, and 1 (1.9%) from non–study related causes. The 2 deaths due to AEs in the PC arm occurred during the consolidation period: one due to dyspnea (not related to study drug), and one due to possibly drug-related myocardial infarction.
Post-Study Therapy
Both arms had similar rates of patients receiving any post-study therapy (PCb, 41.3%; PC, 42.3%). Post-study surgery (unspecified) was received by 6.5% of PCb-treated and 13.5% of PC-treated patients. Post-study chemotherapy was received by 21.7% of PCb-treated and 36.5% of PC-treated patients. The proportions of patients receiving post-study RT were similar (PCb 17.4%; PC 19.2%) in both arms.
DISCUSSION
Based on clinical trials showing that chemoradiotherapy was superior to thoracic RT,30–33 combined modality treatment has emerged as the standard of care for patients with unresectable Stage III NSCLC with adequate performance status.2,30 Currently, concurrent cisplatin/etoposide/RT or carboplatin/paclitaxel/RT are frequently used regimens.3,12,34–37 Despite improved survival with chemoradiotherapy relative to RT alone, outcomes are still poor, and modern chemoradiotherapy regimens are associated with significant toxicities. Therefore, there is a need for more efficacious regimens with improved toxicity profiles.
Here, we report the results of a randomized Phase II trial studying pemetrexed in combination with carboplatin or cisplatin and RT followed by pemetrexed consolidation. Pemetrexed has radiosensitizing activity in vitro and in xenografts, and it has been well tolerated when combined with platinum in patients with NSCLC.6–17 In this trial, the 2-year OS rates were 45.4% and 58.4% with PCb and PC, respectively. The PC arm exceeded the pre-specified criteria of a 2-year survival rate of 50% needed to proceed to a Phase III trial. Other Phase I–II trials having doses and schedules similar to this trial have tested pemetrexed in combination with platinum and RT10–17,21,38; among these, there has only been 1 randomized trial (pemetrexed, carboplatin, and RT with and without cetuximab).12 These prior trials have demonstrated the tolerability and efficacy of pemetrexed in combination with platinum and RT. Our trial is notable because it sought to examine dose delivery and safety of PC and PCb in the setting of uniform entry criteria and radiotherapy plan.
When this trial was planned, the preferential efficacy of pemetrexed in patients with nonsquamous histology22,23 was not known. A substantial percentage of patients with squamous histology (28.3% PCb; 21.2% PC) were enrolled prior to a protocol amendment excluding squamous patients. In the PC arm, the pre-specified 2-year survival rate of 50% was exceeded regardless of whether the intent-to-treat (ITT) (58.4%) or nonsquamous (55.8%) population was considered. In the PCb arm, the 2-year survival rate in patients with nonsquamous histology was 48% and was 45.4% in the ITT population. The results for both the ITT and nonsquamous populations of PC-treated patients are consistent with the 2-year survival rate of 54% obtained in the Phase 2 SWOG 9504 trial with cisplatin/etoposide/concurrent RT plus consolidation docetaxel27 and with the 2-year survival rate (57.5%) obtained in a small Phase 1 trial testing cisplatin/pemetrexed/concurrent RT plus consolidation pemetrexed.11
In patients with nonsquamous histology, median OS was not reached in either arm. In the ITT population, median OS rates were 18.7 months and 27.0 months with PCb and PC, respectively. Median OS in the PC arm was numerically superior to the updated median OS of 21.5 months reported for the entire study population of the LUN 01–24 trial (concurrent etoposide/cisplatin/RT with or without consolidation docetaxel).39
In the Phase III LUN 01–24 trial, which was terminated for futility due to lack of benefit, a regimen of cisplatin+etoposide+RT followed by docetaxel consolidation was associated with considerable Grades 3 through 5 toxicity (chemoradiotherapy/consolidation): 32.0%/24.7% Grade 3 or Grade 4 neutropenia, 9.9%/10.9% Grade 3 or Grade 4 febrile neutropenia, 17.2%/not determined esophagitis and not determined/9.6% Grades 3 through 5 pneumonitis.34 In contrast, the rates of drug-related Grade 3 and Grade 4 neutropenia (13.4%), Grade 3 and Grade 4 febrile neutropenia (0%), Grade 3 radiation pneumonitis (1.9%), Grade 3 and Grade 4 esophagitis (5.7%), and Grade 3 radiation esophagitis (1.9%) in the PC arm for the entire treatment period in this trial appear lower.
The data here also show that both PCb and PC are feasible. The mean dose intensities for the chemoradiotherapy and consolidation periods were >94% for each drug, allowing full dose delivery of planned systemic treatment in combination with therapeutic doses of radiotherapy. In this trial, 91.3% of PCb-treated and 76.9% of PC-treated patients completed chemoradiotherapy, whereas in SWOG 9504, 88% of patients completed chemoradiotherapy.27
This trial has a number of limitations. One limitation is that the enrollment criteria was changed mid study from patients with all histologies to those with nonsquamous tumors. Another limitation of this study is that it was not designed to test the role of consolidation therapy. This study is further limited by a small sample size and a statistical design that did not allow between-arm comparisons to be performed. As such, this study does not answer the carboplatin versus cisplatin question, nor does it allow comparison of the tested regimens to more established regiments such as cisplatin/etoposide or carboplatin/paclitaxel.
In conclusion, the 2-year OS rates were 45.4% (95% CI, 29.5–60.0) for PCb and 58.4% (95% CI, 42.6–71.3) for PC. Due to study design, no conclusions regarding the relative efficacy of these regimens can be made, although both combinations with concurrent RT were active and well tolerated.
ACKNOWLEDGMENTS
The authors wish to acknowledge the patients, their families, and the study personnel who participated in this clinical trial. The authors thank Anwar M. Hossain for reviewing the manuscript and providing statistical support. Medical writing support was provided by Lori Kornberg, who is a full-time employee of inVentiv Health Clinical.
Source of Funding: Dr. Choy’s institution received research funding from Eli Lilly and Company. Dr. Schwartzberg previously served as paid consultants for Eli Lilly and Company and its subsidiary, ImClone, and received payments for lectures from Eli Lilly and Company and Bristol Myers-Squibb. Dr. Garon’s institution received research funding and support to travel to meetings from Eli Lilly and Company, received funds for a consultancy from Boehringer Ingelheim, and received research funding from Pfizer, Genentech, and AstraZeneca. Dr. Govindan previously served as a paid consultant for Genentech, Astra Zeneca, GlaxoSmithKline, and Pfizer and serves as a paid consultant for Bristol-Myers Squibb, Merck, Boehringer-Ingelheim, and Covidien. Drs Treat and Obasaju and Messrs. Peng and Koustenis are employees of Eli Lilly and Company. Dr. Obasaju and Messrs. Peng and Koustenis own stock in Eli Lilly and Company.
This trial was funded by Eli Lilly and Company. This work was also funded in part by an NIH Cancer Center Support Grant (5P30 CA 142543-03) made to Dr. Choy’s institution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Drs Choksi, Dakhil, and Gerber have no relevant disclosures to report.
References
- 1.Lee CB, Stinchcombe TE, Rosenman JG, et al. Therapeutic advances in local-regional therapy for stage III non-small-cell lung cancer: evolving role of dose-escalated conformal (3-dimensional) radiation therapy. Clin Lung Cancer. 2006;8:195–202. doi: 10.3816/CLC.2006.n.047. [DOI] [PubMed] [Google Scholar]
- 2.Wagner TD, Yang GY. The role of chemotherapy and radiation in the treatment of locally advanced non-small cell lung cancer (NSCLC) Curr Drug Targets. 2010;11:67–73. doi: 10.2174/138945010790030956. [DOI] [PubMed] [Google Scholar]
- 3.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. [Accessed November 9, 2012];Surveillance Epidemiology and End Results Stat Fact Sheets: Lung and Bronchus [National Cancer Institute Web site] Available at: http://seer.cancer.gov/statfacts/html/lungb.html.
- 5.Goldman ID, Zhao R. Molecular, biochemical, and cellular pharmacology of pemetrexed. Semin Oncol. 2002;29:3–17. doi: 10.1053/sonc.2002.37461. [DOI] [PubMed] [Google Scholar]
- 6.Bischof M, Weber KJ, Blatter J, et al. Interaction of pemetrexed disodium (ALIMTA, multitargeted antifolate) and irradiation in vitro. Int J Radiat Oncol Biol Phys. 2002;52:1381–1388. doi: 10.1016/s0360-3016(01)02794-8. [DOI] [PubMed] [Google Scholar]
- 7.Bischof M, Huber P, Stoffregen C, et al. Radiosensitization by pemetrexed of human colon carcinoma cells in different cell cycle phases. Int J Radiat Oncol Biol Phys. 2003;57:289–292. doi: 10.1016/s0360-3016(03)00595-9. [DOI] [PubMed] [Google Scholar]
- 8.Mauceri HJ, Seetharam S, Salloum RM, et al. Treatment of head and neck and esophageal xenografts employing Alimta and concurrent ionizing radiation. Int J Oncol. 2001;19:833–835. doi: 10.3892/ijo.19.4.833. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida D, Ebara T, Sato Y, et al. Interaction of radiation and pemetrexed on a human malignant mesothelioma cell line in vitro. Anticancer Res. 2011;31:2847–2851. [PubMed] [Google Scholar]
- 10.Brade A, Bezjak A, MacRae R, et al. Phase I trial of radiation with concurrent and consolidation pemetrexed and cisplatin in patients with unresectable stage IIIA/B non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79:1395–1401. doi: 10.1016/j.ijrobp.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Cardenal F, Arnaiz MD, Morán T, et al. Phase I study of concurrent chemoradiation with pemetrexed and cisplatin followed by consolidation pemetrexed for patients with unresectable stage III non-small cell lung cancer. Lung Cancer. 2011;74:69–74. doi: 10.1016/j.lungcan.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Govindan R, Bogart J, Stinchcombe T, et al. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B trial 30407. J Clin Oncol. 2011;29:3120–3125. doi: 10.1200/JCO.2010.33.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li BS, Gong HY, Huang W, et al. Phase I study of pemetrexed, cisplatin, and concurrent radiotherapy in patients with locally advanced non-small cell lung cancer. Am J Clin Oncol. 2012;35:115–119. doi: 10.1097/COC.0b013e318209ab93. [DOI] [PubMed] [Google Scholar]
- 14.Surmont V, Smit EF, de Jonge M, et al. Pemetrexed and cisplatin with concurrent radiotherapy for locally advanced non-small cell and limited disease small cell lung cancer: results from 2 phase I studies. Lung Cancer. 2010;69:302–306. doi: 10.1016/j.lungcan.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Seiwert TY, Connell PP, Mauer AM, et al. A phase I study of pemetrexed, carboplatin, and concurrent radiotherapy in patients with locally advanced or metastatic non-small cell lung or esophageal cancer. Clin Cancer Res. 2007;13:515–522. doi: 10.1158/1078-0432.CCR-06-1058. [DOI] [PubMed] [Google Scholar]
- 16.Shen X, Denittis A, Werner-Wasik M, et al. Phase I study of 'dose-dense' pemetrexed plus carboplatin/radiotherapy for locally advanced non-small cell lung carcinoma. Radiat Oncol. 2011;6:17. doi: 10.1186/1748-717X-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y, Ma S, Ji Y, et al. Concomitant chemoradiotherapy using pemetrexed and carboplatin for unresectable stage III non-small cell lung cancer (NSCLC): preliminary results of a phase II study. Lung Cancer. 2011;72:327–332. doi: 10.1016/j.lungcan.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Alimta [package insert] Indianapolis, IN: Eli Lilly and Company; Nov, 2012. [Google Scholar]
- 19.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 20.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 21.Heinzerling JH, Choy H, Hughes R, et al. Toxicity and response of pemetrexed plus carboplatin or cisplatin with concurrent chest radiation therapy for patients with locally advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:1391–1396. [Google Scholar]
- 22.Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist. 2009;14:253–263. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 23.Scagliotti G, Brodowicz T, Shepherd FA, et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol. 2011;6:64–70. doi: 10.1097/JTO.0b013e3181f7c6d4. [DOI] [PubMed] [Google Scholar]
- 24.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer; 2002. 2012. [Google Scholar]
- 25.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Gandara DR, Chansky K, Albain KS, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003;21:2004–2010. doi: 10.1200/JCO.2003.04.197. [DOI] [PubMed] [Google Scholar]
- 28.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 29. [Accessed November 9, 2012];Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events, version 3.0 [National Cancer Institute Web site] Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 30.Stinchcombe TE, Fried D, Morris DE, et al. Combined modality therapy for stage III non-small cell lung cancer. Oncologist. 2006;11:809–823. doi: 10.1634/theoncologist.11-7-809. [DOI] [PubMed] [Google Scholar]
- 31.Jeremic B, Shibamoto Y, Acimovic L, et al. Randomized trial of hyperfractionated radiation therapy with or without concurrent chemotherapy for stage III non-small-cell lung cancer. J Clin Oncol. 1995;13:452–458. doi: 10.1200/JCO.1995.13.2.452. [DOI] [PubMed] [Google Scholar]
- 32.Jeremic B, Shibamoto Y, Acimovic L, et al. Hyperfractionated radiation therapy with or without concurrent low-dose daily carboplatin/etoposide for stage III non-small-cell lung cancer: a randomized study. J Clin Oncol. 1996;14:1065–1070. doi: 10.1200/JCO.1996.14.4.1065. [DOI] [PubMed] [Google Scholar]
- 33.Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med. 1992;326:524–530. doi: 10.1056/NEJM199202203260805. [DOI] [PubMed] [Google Scholar]
- 34.Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008;26:5755–5760. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 35.Vokes EE, Senan S, Treat JA, et al. PROCLAIM: A phase III study of pemetrexed, cisplatin, and radiation therapy followed by consolidation pemetrexed versus etoposide, cisplatin, and radiation therapy followed by consolidation cytotoxic chemotherapy of choice in locally advanced stage III non-small-cell lung cancer of other than predominantly squamous cell histology. Clin Lung Cancer. 2009;10:193–198. doi: 10.3816/CLC.2009.n.027. [DOI] [PubMed] [Google Scholar]
- 36.Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23:5883–5891. doi: 10.1200/JCO.2005.55.405. [DOI] [PubMed] [Google Scholar]
- 37.Vokes EE, Herndon JE, 2nd, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol. 2007;25:1698–1704. doi: 10.1200/JCO.2006.07.3569. [DOI] [PubMed] [Google Scholar]
- 38.Gadgeel SM, Ruckdeschel JC, Patel BB, et al. Phase II study of pemetrexed and cisplatin, with chest radiotherapy followed by docetaxel in patients with stage III non-small cell lung cancer. J Thorac Oncol. 2011;6:927–933. doi: 10.1097/JTO.0b013e3182156109. [DOI] [PubMed] [Google Scholar]
- 39.Jalal SI, Riggs HD, Melnyk A, et al. Updated survival and outcomes for older adults with inoperable stage III non-small-cell lung cancer treated with cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel: analysis of a phase III trial from the Hoosier Oncology Group (HOG) and US Oncology. Ann Oncol. 2012;23:1730–1738. doi: 10.1093/annonc/mdr565. [DOI] [PubMed] [Google Scholar]


