Abstract
Over the past decade, considerable progress has been made with respect to the analytical methods for analysis of glycans from biological sources. Regardless of the specific methods that are used, glycan analysis includes isolation, identification, and quantitation. Derivatization is indispensable to increase their identification. Derivatization of glycans can be performed by permethylation or carbodiimide coupling / esterification. By introducing a fluorophore or chromophore at their reducing end, glycans can be separated by electrophoresis or chromatography. The fluorogenically labeled glycans can be quantitated using fluorescent detection. The recently developed approaches using solid-phase such as glycoprotein immobilization for glycan extraction and on-tissue glycan mass spectrometry imaging demonstrate advantages over methods performed in solution. Derivatization of sialic acids is favorably implemented on the solid support using carbodiimide coupling, and the released glycans can be further modified at the reducing end or permethylated for quantitative analysis. In this review, methods for glycan isolation, identification, and quantitation are discussed.
Keywords: Glycomics, Glycoproteomics, GIG, Isobaric Labeling, Mass Spectrometry, Solid-Phase, Porous Graphitized Carbon, Permethylation
1 Introduction
Protein glycosylation is one of the most common protein modifications that affect the biological activities of all living organisms. Protein glycosylation is involved in many biological pathways including cell-cell signaling, protein stability, and solubility [1]. Aberrant protein glycosylation is associated with several pathological states such as hereditary inclusion body myopathy [2], cancer [3], immune responses [4,5], human immunodeficiency virus [6], and heart diseases [7,8]. The disease-associated alterations in glycosylation can be exploited for diagnosis or targeted treatment of diseases [9]. The structural elucidation of glycans is crucial for therapeutic glycoproteins such as antibodies because protein glycosylation can impact the efficiency and safety of glycoprotein-based drugs [10,11].
The modification of proteins through enzymatic glycosylation is so complicated that the complexity of the glycome has surpassed that of the genome, namely because glycosylation is regulated by a variety of factors that are quite heterogeneous among cell types and species [12]. Due to the complex nature of glycans and their non-template-driven biosynthesis, the elucidation of the glycome has lagged far behind the elucidation of the genome and proteome [13]. As a result of such complexity, studies on the biological roles of glycosylation are inaccessible to most biomedical researchers. Together with the presence of isomers and other modifications, glycan analysis has become imperative [14].
A variety of methods have been developed for the isolation, identification, and quantitation of glycans. N-glycans are generally cleaved from glycoproteins using endoglycosidases [15]; O-glycans are released by O-glycosidase or chemical reactions such as β-elimination [16] and hydrazinolysis [17]. Glycans often require derivatization to increase their ionization efficiency for mass spectrometry (MS) [18,19], or they bear fluorogenic tags for fluorescent sensitivity [20,21]. The labeled glycans are quantitatively analyzed by HPLC in combination with a database [22]. With the advancement of MS instrumentation, the structures of derivatized glycans can be directly identified by matrix-assisted laser desorption/ionization (MALDI)- [23] or electrospray ionization (ESI)-MS/MS [24]. Through the use of isotopic labeling, glycans are quantified by MS1 via heavy-light tags on their reducing end [25,26] or via permethylation [27,28]. Recently, isobaric mass tags have become an attractive tool for glycan quantitation [29,30].
An overview of the methods for N-glycan isolation, identification, and quantitation is discussed, including in-solution isolation, solid-phase extraction, tissue imaging, MS identification, separation, and isotopic/isobaric quantitation. The solid-phase techniques are described in detail. A systematic method is deliberately illustrated for glycan extraction, derivatization, profiling, and quantitation. O-glycan can be analyzed using similar strategies described in this work, even though O-glycan is largely isolated by β-elimination [31]. In this review, we focus on protein N-glycosylations and their N-glycans.
2. N-glycan isolation methods
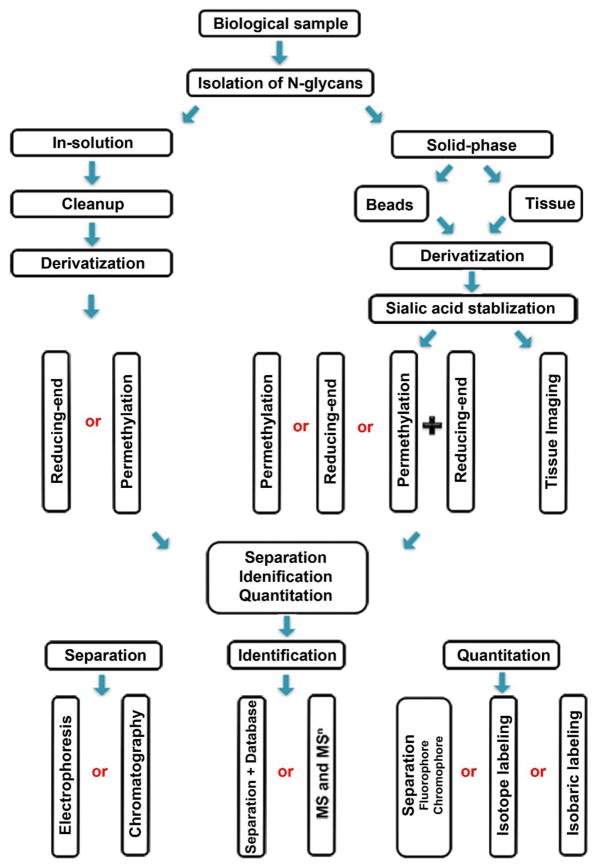
N-glycan isolation is a critical step in sample preparation (Figure 1). It can be performed by either direct digestion of glycoproteins in solution or on a solid-phase using enzymes [32,33]. Release of N-glycans using in-solution extraction requires the isolation of glycans from proteins or peptides, followed by desalting [34]. N-glycans can be imaged on tissue sections using MS [35,36].
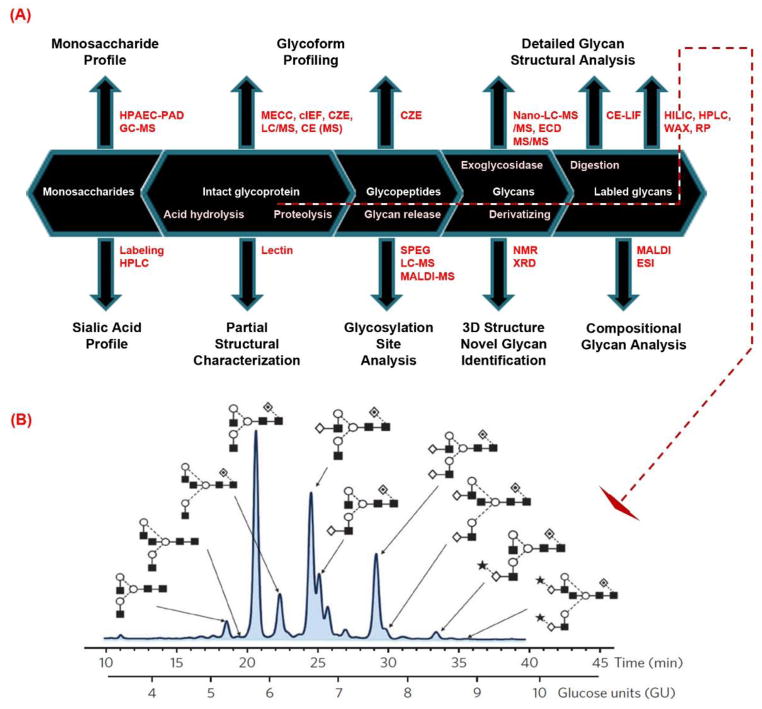
Figure 1. Strategies for isolation, identification, and quantitation of N-glycans from biological specimens.
Proteins extracted from biological samples are digested with enzymes for glycan extraction in solution or on resin. Glycans from in-solution digestion require chromatographic cleanup prior to derivatization by reducing end labeling or permethylation; while the immobilized glycoproteins can be derivatized directly on the solid support. Sialic acids can be stabilized before glycans are released for derivatization. Glycans are further quantitatively analyzed through different separation techniques, or in combination with fluorescent/stable isotopic labeling and mass spectrometry.
2.1. In-solution extraction
In-solution extraction has been well-established and widely used for the analysis of N-glycans. The procedure is relatively straightforward and it is summarized by the direct isolation of N-glycans from their glycoproteins by enzymatic digestion. Isolation of N-glycans can be performed on a chromatographic column [37], filter [38,39], or cotton HILIC SPE micro-tips [40]. For example, the denatured glycoproteins are deglycosylated using PNGase F and the flow-through is purified using a two-step SPE procedure (C18 and PGC) [37,41,42]. N-glycans can be released from glycoproteins by hydrazine hydrolysis (e.g., 65°C) [43], while O-glycans are separately cleaved at a lower temperature (e.g., 60°C) [44]. The glycans released from hydrazinolysis must be re-acetylated to produce a reducing end. The hydrophilic glycans can bind to HILIC absorbents. This procedure has been used for the analysis of N-glycans in a variety of biological specimens [41,45] and is beneficial to study neutral N-glycans that do not contain fragile sialic acids. However, many samples such as sera or plasma consist of many sialylated glycans, so it would be problematic to study native N-glycans or N-glycopeptides from in-solution digestion without the derivatization of sialic acid residues [31]. This may be due to labile nature of sialic acid residue and difficult ionization of sialic acids in ESI [46]. Although sialylated glycans can be stabilized by permethylation [47] or methyl esterification in solution [48], it engenders some impediments such as sample loss, cleanup, and throughput.
2.2. Solid-phase extraction
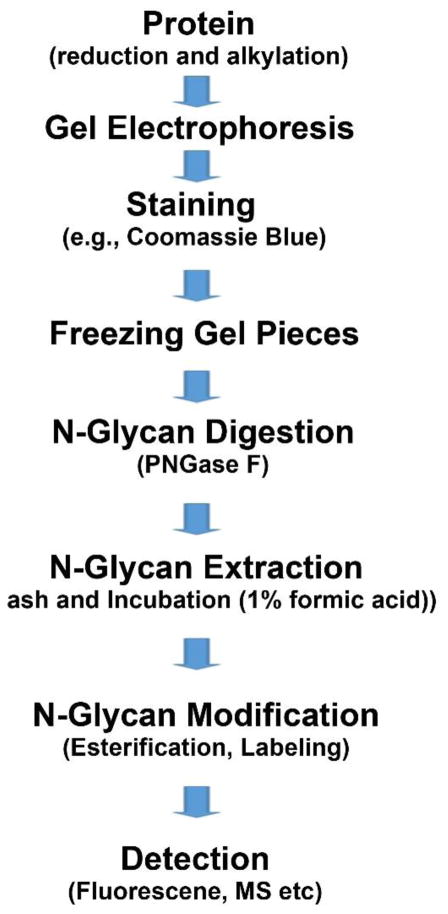
Solid-phase extraction (SPE) is a newly emerging approach for glycan analysis. The immobilization step can be carried out using a physical barrier or chemical reaction. The physical immobilization utilizes different physical properties of proteins, such as size, ionic bond, and hydrophobic and polar interactions between proteins and the surface. SPE has been utilized for the extraction of N-glycans using a gel based matrix such as 1-D gel bands [49], 2D-PAGE gel spots [50,51], or gel immobilization [22]. Glycoproteins are denatured through reduction and alkylation before being separated through 1-D or 2-D gel electrophoresis [52]. The protocol developed by Royle et al. [53] is depicted in Figure 2.
Figure 2. Schematic flowchart of in-gel digestion of glycoproteins for N-glycan extraction.
The method is based on a protocol described in the literature [53]. Proteins are first chemically treated by reduction and alkylation. Proteins are stained with Coomassie blue and directly digested in-gel to release N-glycans. N-glycans are modified through different methods, e.g., esterification for sialic acid or labeling for reducing ends/permethylation. Quantitative analysis is carried out by fluorescence detection or mass spectrometry.
Glycoproteins are immobilized in the cross-linked gel or membrane (e.g., PVDF) [54], while the other remaining molecules are extensively washed away. The glycoproteins are visualized by staining and they are excised for N-glycan release [53]. Glycoproteins can be covalently immobilized on solid-phase for N-glycan processing. Different from the filter approach, glycoproteins are chemically conjugated to the functional groups on solid support, such as amine, epoxy, aldehyde, or EHS ester [55]. The protein N-terminus is often used for immobilization of global proteins. For example, N-termini have primary amines that actively react with NHS ester [56,57], aldehyde [33,58,59], epoxy [55,60], and carboxylic acid [61]. In addition to N-termini of proteins, amino acids containing functional groups including –OH, -SH, and -COOH can also be conjugated to the solid support. For example, threonine (T) and tyrosine (Y) bear –OH, cysteine (C) –SH, aspartic acid (D) and glutamic acid (E) –COOH, and lysine (K) –NH2. These amino acid groups can react with a variety of functional groups as described in Table 1. Glycoproteins can be selectively enriched on the solid support by other amino acids. Different from lysine or N-terminus, cysteine-based immobilization specifically captures those proteins that contain cysteine. Chemicals, e.g., maleimide [62–65], pyridyil disulfide [66,67] and vinyl sulfone [68], can be applied for cysteine conjugation. Conversely, aspartic acid and glutamic acid have carboxylic acid that reacts with primary amines [69]. The hydroxyl group on serine (Ser) or threonine (Thr) can also react with epoxy for rapid immobilization [70].
Table 1.
List of reactive amino acid side groups in proteins for solid-phase immobilization. Amino acid side groups, lysine, cysteine, aspartic acid, and serine, are potentially used as active sites for immobilization.
| Amino acid side groups | Amino acids | Functional groups on solid-phase | Pros and Cons | Applications |
|---|---|---|---|---|
| -NH2 | Lysine (Lys) N-termini | -COOH (Carboxylic acid) Aldehyde | Fast; non-selective Fast, high yield; non-selective | Two-step process of diimide-activated amidation [61]. Dynamic immobilization of proteins [58]; Glycan extraction [33]; Glycan on-line extraction and separation [59]. |
| NHS (active ester) | Fast; inactive if exposed to moister in air | Protein on self-assembled monolayer [56]; Protein on gold surface for biospecific interaction [57]. | ||
| Epoxy | Fast; non-selective | Protein immobilization on epoxy-activated thin polymer film [60]; Thiol-sulfide exchange followed by covalent immobilization [55]. | ||
| -SH | Cysteine (Cys) | Maleimide | Quick reaction; slow hydrolysis under aqueous conditions | Bis(maleimidohexane) (BMH) for polyclonal anti-human IgG [62]; N-maleimidocaproyl succinimide (EMCS) for fibronectin [63]; sulfosuccinimidyl 4[p-maleimidopheny] butyrate (sulfo-SMPB) for DNA chip [64]; Maleimide monolayer for glycan microarray [65]. |
| Pyridyil disulfide | Reversible; poor solubility | Pyrodyl disulfide-functionalized nanoparticles [66];Peptide-oligonucleotide conjugation [67]. | ||
| Vinyl sulfone | Stable, high yield; non-selective | Vinyl sulfone silica [68] | ||
| -COOH | Aspartic acid (Asp) Glutamic acid (Glu) C-termini | -NH2 (Amine) | Mildly acidic condition (pH = 5–6); Non-specific modification (sialic acid), solubility. | Enzyme on beads [69] |
| -OH | Serine (Ser) Threonine (Thr) | Epoxy | Easy protocols, neutral pH, strong bond; slow reaction. | Bifunctional epoxy/thiol-reactive support for quick immobilization [70] |
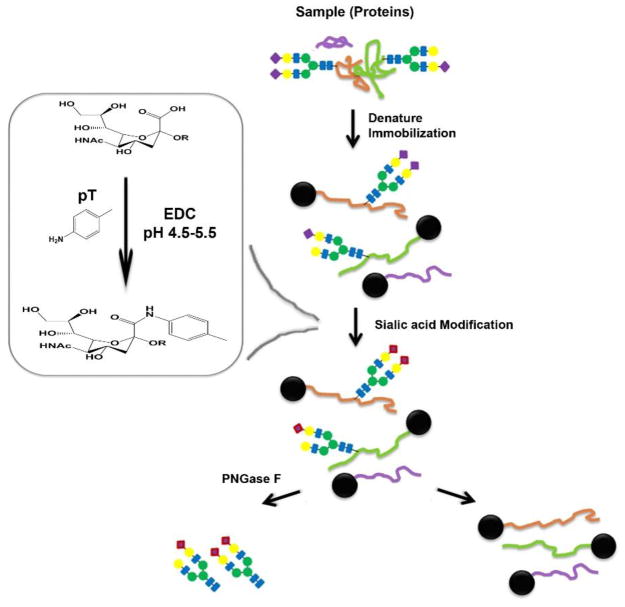
Glycoprotein immobilization can be employed for glycan extraction. Conjugation of glycoproteins only occurs on amino acids while carbohydrate moieties remain intact. One method utilizes the N-termini or lysines for chemical immobilization, allowing derivatization of glycans on solid-phase. Such a strategy has been successfully demonstrated on a platform termed ‘Glycoprotein Immobilization for Glycan Extraction (GIG)’ [33,59,71]. The schematic workflow of GIG is shown in Figure 3, and it features several key steps such as protein extraction, denaturation and conjugation, sialic acid stabilization, N-glycan release, and protein digestion. By adjusting the pH of a binding buffer, one could selectively conjugate N-termini and/or lysines. An advantage of GIG includes the derivatization of glycans on solid-phase with diverse chemical or enzymatic treatments [72]. For example, sialic acid protection is required prior to MS analysis. If a reaction is performed in solution, proteins presumably precipitate in mildly acidic condition (pH 5.0). When peptides instead of proteins are used for the carbodiimide coupling of sialic acids, it is not feasible to purify the derivatized glycans from the coupling reagents. Not only has an excess amount of reagents been used for complete labeling, but their similar chromatographic properties also make isolation difficult. Sialic acid carbodiimide coupling can be easily performed during GIG while unnecessary reagents are washed off. N-glycans are thus procured using enzymes such as PNGases.
Figure 3. N-glycan enrichment and extraction using glycoprotein immobilization for glycan extraction (GIG).
GIG consists of five steps, including protein extraction, protein immobilization, sialic acid stabilization, N-glycan release, and protein digestion. Proteins are immobilized on the solid support via reductive amination of protein amines. Stabilization of sialic acids increases identification. After release of N-glycans, proteins can additionally be digested with trypsin for global proteomics.
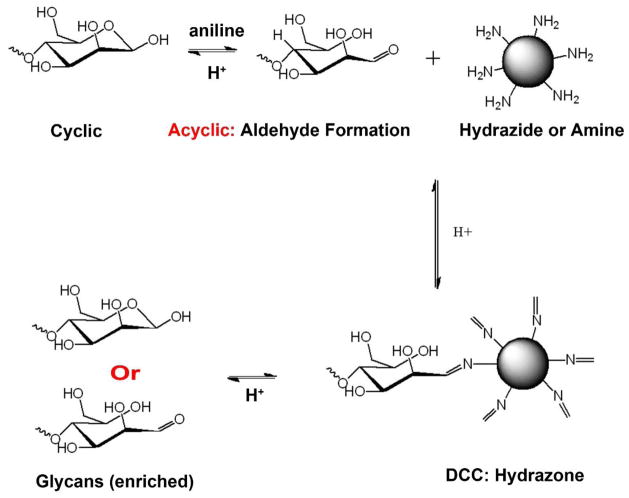
Dynamic covalent chemistry (DCC) can be also utilized for the direct enrichment of glycans. DCC consists of reversible chemical reactions at thermodynamic equilibrium [73]. For example, native N-glycans have a reducing-end that can form an acyclic configuration (i.e., aldose) by nucleophilic attack [34,74]. As illustrated in Figure 4, the acyclic form generates one active aldehyde that can conjugate to hydrazide [34,75], amine [76] or aminooxy groups [77]. These covalent bonds are stable at a higher pH, e.g., in PBS (pH 7.4) or NH4HCO3 (pH 8.0). Our previous studies indicated that hydrazone bonds are effective for the enrichment of N-glycans [34,74]; the release of N-glycans is performed in acid conditions via hydrolysis (0.1% TFA).
Figure 4. N-glycan enrichment and purification using reversible hydrazide chemistry.
N-glycans released by PNGase F are conjugated to beads functionalized with hydrazide groups. The hydrazides in acetic acid perform a nucleophilic attack on the carbonyl carbon of the acyclic reducing terminal residue of N-glycans to form a stable Schiff’s base conjugation (pH > 5.0). Aniline is added as a catalyst to quicken the reaction. After washing unbound molecules, N-glycans are released from beads via acidic hydrolysis (10% formic acid). This method allows for the enrichment and purification of N-glycans from biological samples. (Reprint with permission)
2.3. On-target extraction for N-glycan imaging
The primary goal of N-glycan imaging on tissue sections is to reveal the spatial distribution of glycans for identification of histology-dependent changes in protein glycosylation, which could lead to identification of glycan biomarkers, treatment targets and the improvement of systematic models of glycosylation. Affinity-based histostaining using lectins, which are naturally occurring glycan-binding proteins, is widely used to image certain glycan epitopes on tissues. Despite their effectiveness and ease of use, lectins are limited in several ways. First, lectins lack the specificity to distinguish glycan structures that share similar epitopes. Second, lectin staining provides a qualitative means for tissue analysis, however it lacks quantitative accuracy. Lastly, due to steric hindrance and binding competition between lectins, it is not suitable for high-throughput glycan analysis. Mass spectrometry imaging (MSI) is a powerful technique that overcomes some of the limitations of histostaining by providing a highly specific, semi-quantitative method for high-throughput profiling of N-glycans directly from tissue sections immobilized on glass slides. The application of MSI for N-glycan imaging has been demonstrated on frozen [78] and formalin-fixed paraffin embedded human samples and animal models [35,36]. Depending on the way that the tissue was prepared, it is first treated with wash solutions and antigen retrieval buffers so that the proteins regain their reactivity. The tissue is then treated with the PNGase F enzyme, which can be applied to the tissue using either a sprayer or an array printer. Following incubation at 37 °C, DHB matrix, which facilitates the deglycosylation, is sprayed over the sample. MALDI-MS/MS analysis is used to image the glycans that have been released and detached from the slide through enzymatic treatment. Using this technique, N-glycans can be simultaneously identified and imaged from a single tissue section in one experiment.
In addition to ex vivo glycan imaging, metabolic labeling via click chemistry followed by tagging with fluorescence probes has been used for in vivo imaging of zebra fish development [79] and Caenorhabditis elegans [80].
3. Glycan separation and identification
Identification of N-glycans is a crucial step in understanding their roles in the regulation of biological activities. N-glycan consists of a core Man3GlcNAc2Asn, where Man stands for mannose, GlcNAc for N-acetylglucosamine, and Asn for asparagine [81]. In general, N-glycans can be identified through MS analysis or separation of fluorescently labeled glycans alone (Figure 1).
3.1. Identification by chromatographic separation
Glycosylation analysis is summarized in Figure 5, and it includes the characterization of N-glycans in intact glycoproteins, glycopeptides, and structural analysis of N-glycans [82]. In this review, we focus on the analysis of N-glycans after chemical or enzymatic release. NMR (nuclear magnetic resonance) or XRD (X-ray diffraction) is capable of determining 3D structure of molecules, but it often fails to elucidate complex N-glycans and requires a large amount of sample. MALDI or ESI alone can be used for the compositional analysis of N-glycans, but these approaches could prove inefficient in the analysis of complex N-glycans without separation. Since the hydrophobicity of N-glycans increases with their size, it is routine to separate N-glycans by their hydrophobicity prior to MS analysis. N-glycans may be labeled with fluorescent tags, further increasing their hydrophobicity. The fluorescent labeling only occurs at the reducing end of N-glycans, thus ensuring each N-glycan will react with a single fluorescent tag, making this approach suitable for quantitative analysis [20].
Figure 5. Strategies for analysis of protein glycosylation for characterization of N-glycans in intact glycoproteins, glycopeptides, and structural analysis of N-glycans.
(A) Protein glycosylation can be analyzed using a variety of approaches. Partial intact glycoprotein characterization can be achieved by lectins, e.g., ConA, AAL, SNA etc.; their glycoforms can be profiled using different separation techniques such as MECC, cIEF, CZE,CE or LC/MS. Glycoproteins can also be analyzed through bottom-up approaches by proteolysis, whereas glycosites can be identified using SPEG. Glycans are further digested from glycopeptides or glycoproteins; the labeled glycans are analyzed for their composition and structures by MALDI- or ESI-MS/MS, or their structures are identified by different separation methods, for instance, CE-LIF, HILIC, HPLC, or RPLC. (B) N-glycan profiling of human serum IgG by using the approach described in (A). Glycans are determined by glucose units (GU) while their relative abundance is based on fluorescent detection [82]. (Reprint with permission)
Liquid chromatography (LC) is an effective technique for the separation of fluorescently labeled N-glycans, including HILIC, anion-exchange, RP, or CE. For example, HILIC utilizes amide-bonded stationary phases, thus the larger N-glycans have a longer retention time than the smaller ones. The number of monomers in linear oligomers of glucose is often expressed as glucose units (GU) [83]. The dependency of the logarithm of GU on the retention time is described by a polynomial equation [84]. The calibration curve can be acquired by the analysis of a mixture of linear glucose oligomers that are prepared by partial hydrolysis of dextran (a.k.a. dextran ladder). N-glycan structures are then assigned based on HILIC elution positions [85]. Since the GU value is glycan-specific, it can be used as a library for the structural assignment of unknown glycans without directly relying on mass spectrometry analysis. Using these approaches, 10 N-glycans from heavy chain human serum IgG were identified by HPLC-HILIC (Figure 5B). The same methodology has been applied for the detection of serum glycan biomarkers in lung cancer [86], stomach cancer [87], and ovarian tumors [88]. Results indicated that an increase in sialylation was observed on glycoproteins in the cancerous state, while an increased level of core fucosylated biantennary N-glycans and a decreased expression of mono-galactosylated/core fucosylated biantennary N-glycans were present with increasing disease progression [89].
3.2. Identification by mass spectrometry
Mass spectrometry has become a popular tool for molecule analysis. Advancements in both the speed and sensitivity of MS instrumentation have greatly benefited the glycomic field. The most common ionization techniques include MALDI, ESI and APCI for glycan analysis. APCI utilizes gas-phase ion-molecule reactions at atmospheric pressure, and it is mainly used with polar and relatively nonpolar compounds with a molecular weight less than 2000 Da [90]. MALDI is a fast analytical approach to obtain a snapshot of glycan profiles, while ESI can detect glycans with a wide range of molecular weights. To elucidate the composition and structure of glycans, several fragmentation techniques have been developed, i.e., CID (collision induced dissociation), HCD (higher-energy collisional dissociation), and ETD (electron transfer dissociation). CID is a MS technique to induce fragmentation of molecular ions in the gas phase and it can be used to partially or completely determine the structure of a molecule. Known as higher-energy C-trap dissociation, HCD fragmentation takes place prior to trapping the ions in the mass spectrometer’s ion trap. HCD provides beam type CID tandem MS with detection of fragmented ions at a higher resolution in the Orbitrap mass analyzer [91]. CID/HCD is a powerful tool for the identification of glycan structure and peptide backbone because it enables the elucidation of glycosylation sites by maintaining the glycan-peptide linkage [92]. The generated MS and MS/MS spectra can be used for automatic annotation of N-glycan structure, such as GlycoWorkbench [93], SimGlycan [94], GRITS (www.grits-toolbox.org).
MALDI-MS has been widely used for glycan identification and quantitation. Serum is most commonly used for the analysis of potential glycan markers. By profiling glycans, Kirmiz et al. found that the glycosylation patterns of patients with breast cancer were strikingly different from those without breast cancer [45]. Serum N-glycans were surveyed from ovarian cancer patients for the discovery of potential N-glycan biomarkers [42]. Size-selective enrichment of N-glycans was achieved using highly ordered mesoporous carbon material, which greatly improved the identification of N-glycans. Qin et al. used this approach for the analysis of N-glycans and they identified several core-fucosylated N-glycans that could distinguish liver cancer patients from healthy patients [95]. To stabilize sialylated N-glycans, Miura et al. developed a rapid and simple solid-phase esterification on sialic acid residues for quantitation of sialic acid by MALDI-TOF-MS [96]. In addition, the formation of on-target aniline Schiff-based modification facilitates the laser energy absorption and ultimately improves detection of glycans by MALDI-MS, and it is particularly useful for sialylated species [33,97]. However, yields of N-glycan identification using this technique are low without separation since over 350 structures are estimated to be present in the selected group of serum proteins [41].
3.3. Separation by liquid chromatography or electrophoresis
A variety of separation techniques have been applied for N-glycan enrichment. The structural heterogeneity of glycans presents a formidable analytical challenge, but it offers the feasibility to separate the glycans using different separation methods. Native N-glycans have abundant polar groups (OH) or charged groups (sialic acids), allowing them to be separated using LC or electrophoresis. HPLC is a method that has been widely applied for separating glycan mixtures and analyzing oligosaccharide profiles [83,98]. Recently, a microfluidics device has been implemented as a new platform to use in tandem with HPLC. For example, a microchip has been developed in which porous graphitized carbon particles are packed in micro-channels for the profiling of native N-glycans [99]. This technique was employed to analyze sera from two groups of prostate cancer patients with different diagnoses. More than 300 N-glycan species were identified and differentially expressed in different groups [100]. Glycoproteins can also be immobilized on the solid-phase for direct profiling with graphitized carbon particles in a 2D device, resulting in the faster analysis of isomers and profiling of N-glycans [59].
Capillary electrophoresis (CE) is another method of choice for analysis of glycan heterogeneity and their structural moieties. Native glycoproteins can be separated using high-performance CE to study glycan-mediated heterogeneity and the characterization of glycoprotein-derived N-glycans [32]. CE may have better selectivity for separation of isomeric structures on fluorescently labeled glycans [101,102]. It is complicated to interface CE with MS for automated separation and identification of glycans, even though a few groups have successfully coupled CE with MS [103,104].
4. Glycan quantitation
Quantitation of glycans can be accomplished using a variety of methods, such as fluorescent labeling, UV detection, isotope labeling, and isobaric tag.
4.1. Labeling tags for fluorescent or UV detection
Presence of a unique reducing end on N-glycan core GlcNAc (N-acetylglucosamine) in addition to the high sensitivity of fluorescence or UV detection allows for application of fluorescent or UV-activated labeling of N-glycans for quantitation. Most N-glycan labels are aromatic amines that are conjugated to glycans by reductive amination. Several types of tags have been used in N-glycan labeling and quantitation by fluorescent detection, e.g., 2-AA, 2-AB, APTS, and AA-Ac. These tags have been used in different ways and applications: 2-AA is suitable for applications such as profiling complex N-glycan patterns by HILIC [105] and rapid analysis by gel electrophoresis [20]; 2-AB is favorable when used for profiling complex N-glycan patterns by anion exchange and reverse phase HPLC; APTS is commonly used for CE. AA-Ac is a relatively new tag for rapid and sensitive profiling and structural analysis of glycans by HPLC and LC/MS. Other tags have been developed to increase sensitivity in UV detection, such as 1-(2-naphthyl)-3-methyl-5-pyrazolone (NMP), 1-pheyl-3-methyl-5-pyrazolone (PMP) [106]. Separation using these tags has been summarized by Ruhaak et al. [107].
4.2. MS1-mass shift isotope tags
Quantitation of glycans has been successfully performed using mass spectrometry. As shown in Figure 1, N-glycans can be labeled with stable isotope coded tags on their reducing end or permethylated by replacing –OH with –OCH3. Ideally, the stable isotope labeling methods should have minimal effect on the physical properties of glycans. For example, when a glycan reducing end is derivatized by a stable isotopic tag, 13C (heavy) is often used to replace 12C (light) as opposed to deuterium for 1H to ensure that the derivatized glycans have very similar chromatographic properties [108]. The heavy or light isotopic tags can be incorporated into a pair of samples by either metabolic treatment or chemical synthesis [109,110]. The relative abundance of the glycan is determined from the ratio of the light- and heavy-labeled molecules. Recently, Orlando et al. utilized metabolic incorporation of 15N-Glutamine into the glycans of cultured cells taking advantage of the fact that the amide chain of glutamine is the sole source of nitrogen for the biosynthesis of GlcNAc [111]. Walker et al. were able to synthesize stable-isotope tags using hydrophobic hydrazide for chemical derivatization of glycans via their reducing ends [112]. The tags allow for quantitation and are also capable of enhancing glycan hydrophobicity, so that the routine LC-MS for peptides can be applied for glycan quantitation and identification. However, this mass-shift approach is usually limited to pair-wise measurement in addition to the spectrum complexity for quantitation of a large number of glycans from biological samples.
4.3. Tandem mass isobaric tags
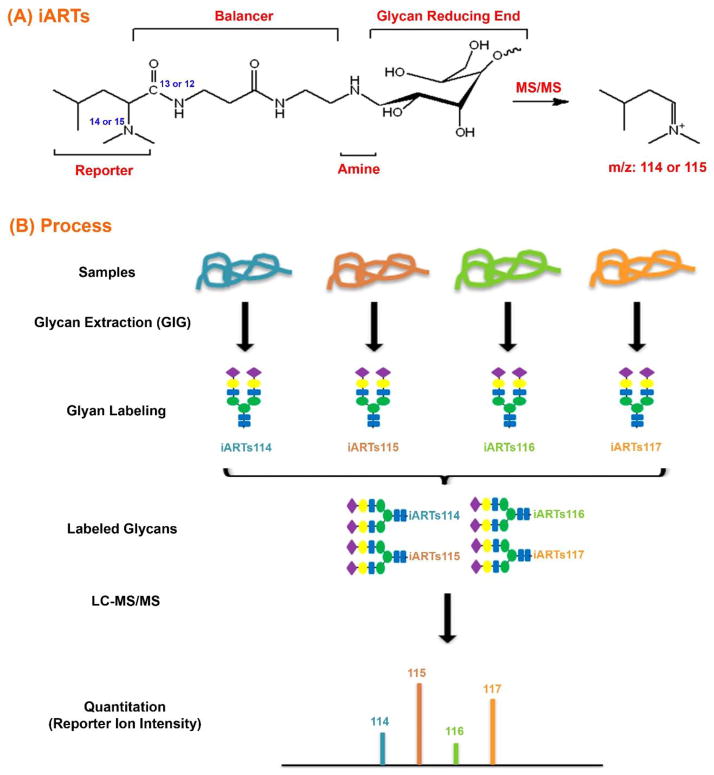
Glycan quantitation can also be carried out through fragmented reporter ions using MS/MS (Figure 6). Tandem mass tag consists of a reporter, a balancer, and a primary amine (Figure 6A). Stable isotope coded tags are chemically conjugated to glycans, typically on their reducing end. Different samples are labeled with a set of isobaric tags. The labeled glycans are pooled together to form a single peak where MS1 spectra show the same m/z for individual glycan precursors from different samples; upon fragmentation, the reporter ions depict the relative abundance of glycans in different samples (Figure 6B). Bowman and Zaia synthesized tags that produce a 4-dalton mass shift in the tandem MS, allowing for the direct comparison of three samples or triplicate measurements within one mass spectral analysis [113]. Due to distinct moieties of the tags, the labeled glycans may have varied retention times in LC-MS/MS, causing variation in the identification of precursor ions for MS/MS fragmentation. Hahne et al. reported an aminoxyTMT (tandem mass tag) tag for labeling the carbonyl group of N-glycans with limited success [29]. Our group successfully designed a novel carbonyl-reactive tag using primary amine for conjugation of the reducing end of N-glycans [30]. The reaction is achieved by Schiff-base chemistry similar to that of 2-AA or 2-AB fluorescent labeling; thus, similar protocols can easily be developed that include our isobaric tag labeling strategy.
Figure 6. Schematic diagram of isobaric tag and its labeling process for glycan quantitative analysis.
(A) An isobaric tag, iARTs, consists of a reporter, a balancer, and an amine. Under acidic solution, the reducing end of a glycan forms an aldose that reacts to the amine. Upon MS/MS fragmentation, the reporter ion is generated for quantitation. (B) Isobaric tag labeling process incudes several key steps such as glycan extraction (GIG), labeling, and LC-MS/MS.
5. Systematic platform for analysis of protein glycosylation
Systematic approaches have been pursued to study protein glycosylation, including glycans and protein glycosites. A rapid glycoprotein sample preparation platform, GlykoPrep™, has been reported by ProZyme, for streamlined N-glycan preparation including release, purification and labeling [114]. The platform relies on antibodies to target and enrich glycoproteins for the isolation of glycans. It dramatically increases throughput and can process up to 192 samples within 3 h.
It is ideal to analyze both glycans and protein glycosites in an integrated platform. As shown in Figure 7A, iGIG enables one to analyze glycans, glycosites, and intact glycopeptides simultaneously. Many researchers have studied site-specific N-glycosylation by analysis of N-glycans (Figure 7A.1) and direct digestion of intact glycoproteins (Figure 7A.2), followed by enrichment of intact glycopeptides using HILIC [115,116]. To reduce false discovery, it would be more precise to search intact glycopeptides using database comprised of identified peptides from MS/MS instead of using an entire database. For example, glycopeptides are first deglycosylated by PNGase F to release both deglycosylated peptides (Figure 7A.1) and N-glycans (Figure 7A.2). The deglycosylated peptides are searched against the entire database (e.g., Homo sapiens), whereas N-glycans are determined by MS or fluorescent detection. Furthermore, intact glycopeptides can be analyzed separately by LC-MS/MS after HILIC enrichment (Figure 7A.3). The MS and MS/MS spectra can be searched using databases from A.1 and A.2.
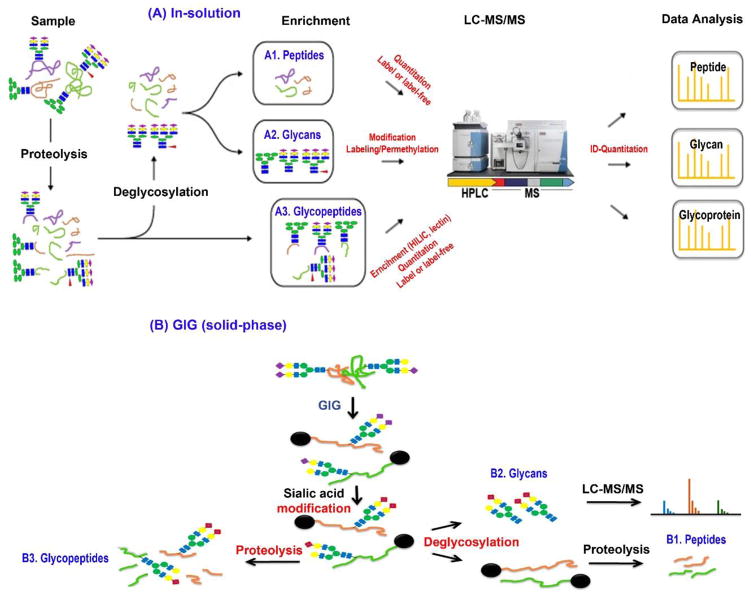
Figure 7. Systematic approaches for analysis of protein N-glycosylation.
Glycans, deglycopeptides and intact glycopeptides are systematically analyzed using (A) In-solution and (B) GIG. Intact glycopeptides are searched using databases from N-glycans and global peptides. In solution, peptides (A.1) are quantitatively analyzed by LC-MS/MS while N-glycans can be determined by labeling or permethylation (A.2); intact glycopeptides are enriched by chromatography (HILIC) or lectin (A.3). On GIG, glycoproteins are immobilized on resin for derivatization of glycan; sialic acids are stabilized (B.2) before tryptic digestion for glycopeptide identification (B3); deglycosylated peptides are quantitatively analyzed by LC-MS/MS (B.1).
The workflow in Figure 7A may cause partial or complete loss of the fragile sialic acids during sample processing and MS ionization/detection. The iGIG platform can be used for analysis of intact glycopeptides and especially sialylated glycopeptides (Figure 7B) [117]. Compared with the in-solution approach, glycoproteins are first immobilized to the amine-reactive aldehydes on resin by reductive amination. Sialylated glycoproteins are then derivatized by carbodiimide coupling, e.g., p-Toluidine and EDC in pH 4.5–5.5 [33,72]. The derivatized sialic acids are stable in an acidic solution (e.g., 0.1% TFA) and during MS ionization (e.g., MALDI or ESI). Glycoproteins are deglycosylated using PNGase F to identify peptides and N-glycans (Figure 7B.1&2). Meanwhile glycoproteins are directly digested from the resin with trypsin (Figure 7B.3). These steps are incorporated in the workflow illustrated in Figure 7A. To improve specificity, glycoproteins can also be enriched using specific lectins before GIG immobilization. However, we only focused on the identification of N-glycans in this review.
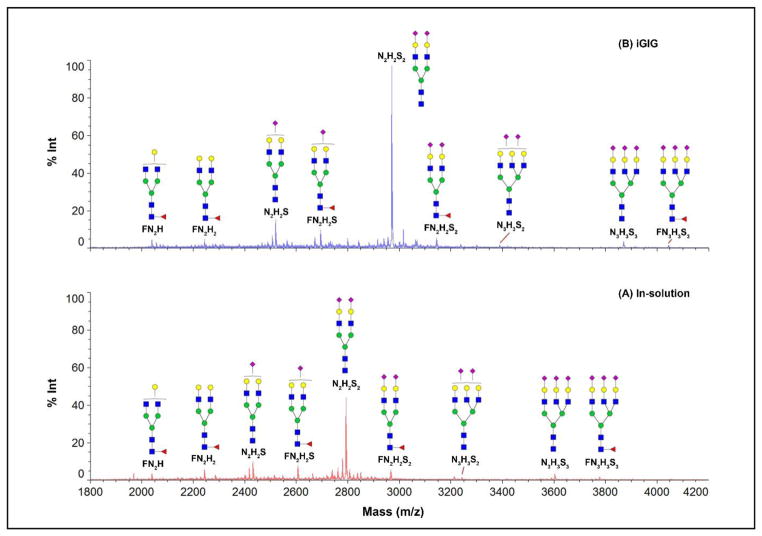
Use of iGIG for preparation of N-glycans shows similar results in comparison to the solution approach. As shown in Figure 8, N-glycans from healthy human sera are prepared by an in-solution method (A) and GIG (B). The solution method follows procedures described by Morelle & Michalski [31]. The N-glycan profiles have similar pattern, featuring the most abundant peak for N2H2S2 followed by N2H2S. In iGIG, a similar N-glycan pattern is observed (Figure 8B). The intensity of N-glycans from solution and GIG can be compared by spiking internal standards (e.g., Maltoheptaose (DP7), 1 μL @ 25 μM). For instance, the intensity of N-glycan (N2H2S2) from iGIG is ~2-fold greater than that from the solution method. It is expected that low abundant N-glycans can be identified through enhanced ionization and improved ion competition by iGIG. Additionally, the relative abundance of non-sialylated N-glycans should be the same regardless of the solution or iGIG method. N-glycans, such as FN2H or FN2H2, exhibit similar intensities since they are not affected by carbodiimide coupling. Overall, iGIG not only can protect sialic acids, but also enhance the identification of sialylated glycans. This feature will benefit the identification of intact sialylated glycopeptides as well.
Figure 8. MALDI-MS spectra of permethylated N-glycans from human serum.
N-glycans are prepared in solution (A) and on solid-phase (B) using 20 μL serum. In the in-solution method, serum is directly digested using PNGase F after protein denaturation; the released N-glycans are then permethylated. On solid-phase, glycoproteins are immobilized on beads for glycan modification, e.g., sialic acid stabilization before permethylation. The top nine N-glycans are listed, in which iGIG yields more N-glycans based on their respective intensities. The same m/z is observed on glycans that do not contain sialic acids. A higher intensity is observed on sialylated glycans due to their protection.
N-glycans extracted from the iGIG platform can be also labeled on their reducing end for fluorescent detection. Although fluorescent detection does not elucidate glycan structures, it offers higher sensitivity for glycan analysis. Fluorescent labeling is commonly performed in acidic conditions, e.g., 15–30% acetic acid or citric acid, at 37–90°C from 1 h to overnight incubation. Typically, 2-AA or 2-AB labeling is performed at 65°C for 3 h for complete reaction [53]. Under these conditions, the labile sialic acids may be lost to some extent due to acidic hydrolysis [118,119]. Therefore, stabilization of sialic acids prior to 2-AA or 2-AB labeling could largely minimize loss of the sialic acid residues. iGIG enables the derivatization of sialic acid residues in pH > 5.0, and the modified N-glycans are very stable in low pH and/or high temperature (< 80°C). N-glycans, after fluorescent labeling, can be quantitatively analyzed by HILIC or HPLC separation and fluorescent detection. The combination of exact mass and retention time, together with glycan fragments, provides a powerful means to elucidate glycan structure and its effect in the disease progression. With the advancement of software development [120], it will be possible to explore glycan biomarkers from body fluids or tissue specimens in the near future.
6. Summary
Accurate analysis of protein glycosylation requires a robust platform for sample preparation. Solid-phase is a newly emerging technique for the analysis of protein glycosylation. The rapid immobilization of proteins on the solid support makes it an ideal method for rapid sample processing and manipulation. The in-solution method requires several sample cleanup steps and it is often impossible to isolate glycans from other reagents based on their chromatographic properties. Cleanup on resin is achieved by extensive washing. Sample loss is effectively reduced because a permanent bond is formed by the chemical immobilization of proteins.
iGIG has been developed to meet some of these sample preparation challenges that are faced by many researchers. iGIG can be particularly useful for detailed structural analysis of N-glycans by exoglycosidase enzymes. In solution, glycans need to be purified after enzymatic digestion, compared to glycans that are still linked to glycoproteins on resin which do not require purification. Exoglycosidase enzymes can remove individual monosaccharides in series with intermediate washing steps. These advantages not only quicken the analysis, but also mitigate sample loss and facilitate the removal of chemical reagents from bound glycoproteins.
iGIG can be employed for the analysis of intact glycopeptides. The additional challenges faced by modifications of sialic acid in solution can be reconciled using solid-phase. With enrichment of glycoproteins by lectins, the number of identified glycosites and glycoforms is expected to increase. The modified glycans can also be permethylated for detailed structural analysis.
Acknowledgments
We are grateful to Dr. Stefani Thomas for assistance in manuscript preparation. This work was supported by National Institutes of Health, National Heart Lung and Blood Institute, Johns Hopkins Proteomics Center (N01-HV-00240) and Programs of Excellence in Glycosicences (PEG, P01HL107153), and National Institutes of Health, National Cancer Institute, the Early Detection Research Network (EDRN, U01CA152813), and the Clinical Proteomic Tumor Analysis Consortium (CPTAC, U24CA160036).
Abbreviations
- GIG
glycoprotein Immobilization for glycan extraction
- iGIG
integrated glycoprotein immobilization for glycoprotein and glycan analysis
- SPE
solid-phase extraction
- SPEG
solid-phase extraction of glycosite-containing peptides
- DCC
dynamic covalent chemistry
- 2-AA
2-aminobenzoic acid
- 2-AB
2-aminobenzamide
- 2-AP
2-aminopyridine
- AA-Ac
3-(acetylamino)-6-aminoacridine
- APTS
1-aminopyrene-3,6,8-trisulfonic acid
- AAL
aleuria aurantia lectin
- SNA
sambucus nigra lectin
- ConA
concanavalin A
- DHB
2,5-Dihydoxybenzoic acid
- EDC
N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide
- TFA
trifluoroacetic acid
- HILIC
hydrophilic interaction liquid chromatography
- HPLC
high performance liquid chromatography
- RPLC
reverse-phase liquid chromatography
- bRPLC
basic reverse-phase liquid chromatography
- MECC
micellar electro kinetic capillary chromatography
- cIEF
capillary isoelectric focusing
- CZE
capillary zone electrophoresis
- APCI
atmospheric pressure chemical ionization
- ESI
electrospray ionization
- MALDI
matrix-assisted laser desorption/ionization
Footnotes
The authors declared no conflict of interest.
References
- 1.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 2.Galeano B, Klootwijk R, Manoli I, Sun M, et al. Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J Clin Invest. 2007;117:1585–1594. doi: 10.1172/JCI30954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peracaula R, Barrabés S, Sarrats A, Rudd PM, de Llorens R. Altered glycosylation in tumours focused to cancer diagnosis. Dis Markers. 2008;25:207–218. doi: 10.1155/2008/797629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 5.Lowe JB. Glycosylation, immunity, and autoimmunity. Cell. 2001;104:809–812. doi: 10.1016/s0092-8674(01)00277-x. [DOI] [PubMed] [Google Scholar]
- 6.Kwong PD, Doyle ML, Casper DJ, Cicala C, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 7.Martos R, Baugh J, Ledwidge M, O'Loughlin CC, et al. Diastolic heart failure Evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115:888–895. doi: 10.1161/CIRCULATIONAHA.106.638569. [DOI] [PubMed] [Google Scholar]
- 8.Yang S, Chen L, Sun S, Shah P, et al. Glycoproteins identified from heart failure and treatment models. Proteomics. 2015;15:567–579. doi: 10.1002/pmic.201400151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reis CA, Osorio H, Silva L, Gomes C, David L. Alterations in glycosylation as biomarkers for cancer detection. J Clin Pathol. 2010;63:322–329. doi: 10.1136/jcp.2009.071035. [DOI] [PubMed] [Google Scholar]
- 10.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8:226–234. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 11.Dwek RA, Butters TD, Platt FM, Zitzmann N. Targeting glycosylation as a therapeutic approach. Nat Rev Drug Discov. 2002;1:65–75. doi: 10.1038/nrd708. [DOI] [PubMed] [Google Scholar]
- 12.Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. doi: 10.1093/glycob/12.4.43r. [DOI] [PubMed] [Google Scholar]
- 13.Raman R, Raguram S, Venkataraman G, Paulson JC, Sasisekharan R. Glycomics: an integrated systems approach to structure-function relationships of glycans. Nat Methods. 2005;2:817–824. doi: 10.1038/nmeth807. [DOI] [PubMed] [Google Scholar]
- 14.Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 15.Tarentino AL, Gomez CM, Plummer TH., Jr Deglycosylation of asparagine-linked glycans by peptide: N-glycosidase F. Biochemistry. 1985;24:4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- 16.Carlson DM. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968;243:616–626. [PubMed] [Google Scholar]
- 17.Takasaki S, Kobata A. [4] Microdetermination of sugar composition by radioisotope labeling. Methods Enzymol. 1978;50:50–54. doi: 10.1016/0076-6879(78)50006-2. [DOI] [PubMed] [Google Scholar]
- 18.Snovida SI, Chen VC, Perreault H. Use of a 2, 5-dihydroxybenzoic acid/aniline MALDI matrix for improved detection and on-target derivatization of glycans: a preliminary report. Anal Chem. 2006;78:8561–8568. doi: 10.1021/ac061375r. [DOI] [PubMed] [Google Scholar]
- 19.Walker SH, Lilley LM, Enamorado MF, Comins DL, Muddiman DC. Hydrophobic derivatization of N-linked glycans for increased ion abundance in electrospray ionization mass spectrometry. J Am Soc Mass Spectrom. 2011;22:1309–1317. doi: 10.1007/s13361-011-0140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bigge J, Patel T, Bruce J, Goulding P, et al. Nonselective and efficient fluorescent labeling of glycans using 2-amino benzamide and anthranilic acid. Anal Biochem. 1995;230:229–238. doi: 10.1006/abio.1995.1468. [DOI] [PubMed] [Google Scholar]
- 21.Xia B, Kawar ZS, Ju T, Alvarez RA, et al. Versatile fluorescent derivatization of glycans for glycomic analysis. Nat Methods. 2005;2:845–850. doi: 10.1038/nmeth808. [DOI] [PubMed] [Google Scholar]
- 22.Royle L, Campbell MP, Radcliffe CM, White DM, et al. HPLC-based analysis of serum N-glycans on a 96-well plate platform with dedicated database software. Anal Biochem. 2008;376:1–12. doi: 10.1016/j.ab.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg D, Sutton-Smith M, Paulson J, Dell A. Automatic annotation of matrix-assisted laser desorption/ionization N-glycan spectra. Proteomics. 2005;5:865–875. doi: 10.1002/pmic.200401071. [DOI] [PubMed] [Google Scholar]
- 24.Jensen PH, Karlsson NG, Kolarich D, Packer NH. Structural analysis of N-and O-glycans released from glycoproteins. Nat Protoc. 2012;7:1299–1310. doi: 10.1038/nprot.2012.063. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence R, Olson SK, Steele RE, Wang L, et al. Evolutionary differences in glycosaminoglycan fine structure detected by quantitative glycan reductive isotope labeling. J Biol Chem. 2008;283:33674–33684. doi: 10.1074/jbc.M804288200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia B, Feasley CL, Sachdev GP, Smith DF, Cummings RD. Glycan reductive isotope labeling for quantitative glycomics. Anal Biochem. 2009;387:162–170. doi: 10.1016/j.ab.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang P, Mechref Y, Kyselova Z, Goetz JA, Novotny MV. Comparative glycomic mapping through quantitative permethylation and stable-isotope labeling. Anal Chem. 2007;79:6064–6073. doi: 10.1021/ac062098r. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez-Manilla G, Warren NL, Abney T, Atwood J, et al. Tools for glycomics: relative quantitation of glycans by isotopic permethylation using 13CH3I. Glycobiology. 2007;17:677–687. doi: 10.1093/glycob/cwm033. [DOI] [PubMed] [Google Scholar]
- 29.Hahne H, Neubert P, Kuhn K, Etienne C, et al. Carbonyl-reactive tandem mass tags for the proteome-wide quantification of N-linked glycans. Anal Chem. 2012;84:3716–3724. doi: 10.1021/ac300197c. [DOI] [PubMed] [Google Scholar]
- 30.Yang S, Yuan W, Yang W, Zhou J, et al. Glycan Analysis by Isobaric Aldehyde Reactive Tags and Mass Spectrometry. Anal Chem. 2013;85:8188–8195. doi: 10.1021/ac401226d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morelle W, Michalski JC. Analysis of protein glycosylation by mass spectrometry. Nat Protoc. 2007;2:1585–1602. doi: 10.1038/nprot.2007.227. [DOI] [PubMed] [Google Scholar]
- 32.Taverna M, Baillet A, Biou D, Schlüter M, et al. Analysis of carbohydrate-mediated heterogeneity and characterization of N-linked oligosaccharides of glycoproteins by high performance capillary electrophoresis. Electrophoresis. 1992;13:359–366. doi: 10.1002/elps.1150130174. [DOI] [PubMed] [Google Scholar]
- 33.Yang S, Li Y, Shah P, Zhang H. Glycomic analysis using glycoprotein immobilization for glycan extraction. Anal Chem. 2013;85:5555–5561. doi: 10.1021/ac400761e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang S, Zhang H. Glycan analysis by reversible reaction to hydrazide beads and mass spectrometry. Anal Chem. 2012;84:2232–2238. doi: 10.1021/ac202769k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toghi Eshghi S, Yang S, Wang X, Shah P, et al. Imaging of N-linked glycans from formalin-fixed paraffin-embedded tissue sections using MALDI mass spectrometry. ACS Chem Biol. 2014;9:2149–2156. doi: 10.1021/cb500405h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powers TW, Neely BA, Shao Y, Tang H, et al. MALDI imaging mass spectrometry profiling of N-glycans in formalin-fixed paraffin embedded clinical tissue blocks and tissue microarrays. PLoS One. 2014;9:12. doi: 10.1371/journal.pone.0106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu YQ, Gilar M, Kaska J, Gebler JC. A rapid sample preparation method for mass spectrometric characterization of N-linked glycans. Rapid Commun Mass Spectrom. 2005;19:2331–2336. doi: 10.1002/rcm.2067. [DOI] [PubMed] [Google Scholar]
- 38.Zhou H, Froehlich JW, Briscoe AC, Lee RS. The GlycoFilter: a simple and comprehensive sample preparation platform for proteomics, N-glycomics and glycosylation site assignment. Mol Cell Proteomics. 2013;12:2981–2991. doi: 10.1074/mcp.M113.027953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 40.Selman MH, Hemayatkar M, Deelder AM, Wuhrer M. Cotton HILIC SPE microtips for microscale purification and enrichment of glycans and glycopeptides. Anal Chem. 2011;83:2492–2499. doi: 10.1021/ac1027116. [DOI] [PubMed] [Google Scholar]
- 41.Aldredge D, An HJ, Tang N, Waddell K, Lebrilla CB. Annotation of a serum N-glycan library for rapid identification of structures. J Proteome Res. 2012;11:1958–1968. doi: 10.1021/pr2011439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.An HJ, Miyamoto S, Lancaster KS, Kirmiz C, et al. Profiling of glycans in serum for the discovery of potential biomarkers for ovarian cancer. J Proteome Res. 2006;5:1626–1635. doi: 10.1021/pr060010k. [DOI] [PubMed] [Google Scholar]
- 43.Chen YJ, Wing DR, Guile GR, Dwek RA, et al. Neutral N-glycans in adult rat brain tissue. Euro J Biochem. 1998;251:691–703. doi: 10.1046/j.1432-1327.1998.2510691.x. [DOI] [PubMed] [Google Scholar]
- 44.Patel T, Bruce J, Merry A, Bigge C, et al. Use of hydrazine to release in intact and unreduced form both N-and O-linked oligosaccharides from glycoproteins. Biochemistry. 1993;32:679–693. doi: 10.1021/bi00053a037. [DOI] [PubMed] [Google Scholar]
- 45.Kirmiz C, Li B, An HJ, Clowers BH, et al. A serum glycomics approach to breast cancer biomarkers. Mol Cell Proteomics. 2007;6:43–55. doi: 10.1074/mcp.M600171-MCP200. [DOI] [PubMed] [Google Scholar]
- 46.Harvey DJ. Structural determination of N-linked glycans by matrix-assisted laser desorption/ionization and electrospray ionization mass spectrometry. Proteomics. 2005;5:1774–1786. doi: 10.1002/pmic.200401248. [DOI] [PubMed] [Google Scholar]
- 47.Kang P, Mechref Y, Klouckova I, Novotny MV. Solid-phase permethylation of glycans for mass spectrometric analysis. Rapid Commun Mass Spectrom. 2005;19:3421–3428. doi: 10.1002/rcm.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powell AK, Harvey DJ. Stabilization of sialic acids in N-linked oligosaccharides and gangliosides for analysis by positive ion matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun Mass Spectrom. 1996;10:1027–1032. doi: 10.1002/(SICI)1097-0231(19960715)10:9<1027::AID-RCM634>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 49.Šagi D, Kienz P, Denecke J, Marquardt T, Peter-Katalinić J. Glycoproteomics of N-glycosylation by in-gel deglycosylation and matrix-assisted laser desorption/ionisation-time of flight mass spectrometry mapping: Application to congenital disorders of glycosylation. Proteomics. 2005;5:2689–2701. doi: 10.1002/pmic.200401312. [DOI] [PubMed] [Google Scholar]
- 50.Kleinert P, Kuster T, Arnold D, Jaeken J, et al. Effect of glycosylation on the protein pattern in 2-D-gel electrophoresis. Proteomics. 2007;7:15–22. doi: 10.1002/pmic.200600297. [DOI] [PubMed] [Google Scholar]
- 51.Hamid UMA, Royle L, Saldova R, Radcliffe CM, et al. A strategy to reveal potential glycan markers from serum glycoproteins associated with breast cancer progression. Glycobiology. 2008;18:1105–1118. doi: 10.1093/glycob/cwn095. [DOI] [PubMed] [Google Scholar]
- 52.Küster B, Wheeler SF, Hunter AP, Dwek RA, Harvey DJ. Sequencing of N-linked oligosaccharides directly from protein gels: in-gel deglycosylation followed by matrix-assisted laser desorption/ionization mass spectrometry and normal-phase high-performance liquid chromatography. Anal Biochem. 1997;250:82–101. doi: 10.1006/abio.1997.2199. [DOI] [PubMed] [Google Scholar]
- 53.Royle L, Radcliffe CM, Dwek RA, Rudd PM. Glycobiology Protocols. Springer; 2007. pp. 125–143. [Google Scholar]
- 54.Wilson NL, Schulz BL, Karlsson NG, Packer NH. Sequential analysis of N-and O-linked glycosylation of 2D-PAGE separated glycoproteins. J Proteome Res. 2002;1:521–529. doi: 10.1021/pr025538d. [DOI] [PubMed] [Google Scholar]
- 55.Rusmini F, Zhong Z, Feijen J. Protein immobilization strategies for protein biochips. Biomacromolecules. 2007;8:1775–1789. doi: 10.1021/bm061197b. [DOI] [PubMed] [Google Scholar]
- 56.Patel N, Davies MC, Hartshorne M, Heaton RJ, et al. Immobilization of protein molecules onto homogeneous and mixed carboxylate-terminated self-assembled monolayers. Langmuir. 1997;13:6485–6490. [Google Scholar]
- 57.Johnsson B, Löfås S, Lindquist G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal Biochem. 1991;198:268–277. doi: 10.1016/0003-2697(91)90424-r. [DOI] [PubMed] [Google Scholar]
- 58.Ruzicka J, Carroll AD, Lähdesmäki I. Immobilization of proteins on agarose beads, monitored in real time by bead injection spectroscopy. Analyst. 2006;131:799–808. doi: 10.1039/b603768b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang S, Toghi Eshighi S, Chiu H, DeVoe D, Zhang H. Glycomic analysis by glycoprotein immoblization for glycan extraction and liquid chromatography on mcirofluidic chip. Anal Chem. 2013;85:10117–10125. doi: 10.1021/ac4013013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen B, Pernodet N, Rafailovich MH, Bakhtina A, Gross RA. Protein immobilization on epoxy-activated thin polymer films: effect of surface wettability and enzyme loading. Langmuir. 2008;24:13457–13464. doi: 10.1021/la8019952. [DOI] [PubMed] [Google Scholar]
- 61.Jiang K, Schadler LS, Siegel RW, Zhang X, et al. Protein immobilization on carbon nanotubes via a two-step process of diimide-activated amidation. J Mater Chem. 2004;14:37–39. [Google Scholar]
- 62.Viitala T, Vikholm I, Peltonen J. Protein immobilization to a partially cross-linked organic monolayer. Langmuir. 2000;16:4953–4961. [Google Scholar]
- 63.Gauvreau V, Chevallier P, Vallières K, Petitclerc É, et al. Engineering surfaces for bioconjugation: developing strategies and quantifying the extent of the reactions. Bioconjugate Chem. 2004;15:1146–1156. doi: 10.1021/bc049858u. [DOI] [PubMed] [Google Scholar]
- 64.Jongsma MA, Litjens RH. Self-assembling protein arrays on DNA chips by auto-labeling fusion proteins with a single DNA address. Proteomics. 2006;6:2650–2655. doi: 10.1002/pmic.200500654. [DOI] [PubMed] [Google Scholar]
- 65.Houseman BT, Gawalt ES, Mrksich M. Maleimide-functionalized self-assembled monolayers for the preparation of peptide and carbohydrate biochips. Langmuir. 2003;19:1522–1531. [Google Scholar]
- 66.van der Vlies AJ, O’Neil CP, Hasegawa U, Hammond N, Hubbell JA. Synthesis of pyridyl disulfide-functionalized nanoparticles for conjugating thiol-containing small molecules, peptides, and proteins. Bioconjugate Chem. 2010;21:653–662. doi: 10.1021/bc9004443. [DOI] [PubMed] [Google Scholar]
- 67.Corey DR. 48000-fold acceleration of hybridization by chemically modified oligonucleotides. J Am Chem Soc. 1995;117:9373–9374. [Google Scholar]
- 68.Morales-Sanfrutos J, Lopez-Jaramillo J, Ortega-Muñoz M, Megia-Fernandez A, et al. Vinyl sulfone: a versatile function for simple bioconjugation and immobilization. Org Biomol Chem. 2010;8:667–675. doi: 10.1039/b920576d. [DOI] [PubMed] [Google Scholar]
- 69.Fernandez-Lafuente R, Rosell C, Rodriguez V, Santana C, et al. Preparation of activated supports containing low pK amino groups. A new tool for protein immobilization via the carboxyl coupling method. Enzyme Microb Tech. 1993;15:546–550. doi: 10.1016/0141-0229(93)90016-u. [DOI] [PubMed] [Google Scholar]
- 70.Grazú V, Abian O, Mateo C, Batista-Viera F, et al. Novel bifunctional epoxy/thiol-reactive support to immobilize thiol containing proteins by the epoxy chemistry. Biomacromolecules. 2003;4:1495–1501. doi: 10.1021/bm034262f. [DOI] [PubMed] [Google Scholar]
- 71.Yang S, Zhang H. Glycomic Analysis of Glycans Released from Glycoproteins Using Chemical Immobilization and Mass Spectrometry. Curr Protoc Chem Biol. 2015:191–208. doi: 10.1002/9780470559277.ch140085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shah P, Yang S, Sun S, Aiyetan P, et al. Mass spectrometric analysis of sialylated glycans with use of solid-phase labeling of sialic acids. Anal Chem. 2013;85:3606–3613. doi: 10.1021/ac3033867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rowan SJ, Cantrill SJ, Cousins GR, Sanders JK, Stoddart JF. Dynamic covalent chemistry. Angew Chem Int Ed. 2002;41:898–952. doi: 10.1002/1521-3773(20020315)41:6<898::aid-anie898>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 74.Yang S, Zhang H. Solid-phase glycan isolation for glycomics analysis. Protoemics Clin Appl. 2012;6:596–608. doi: 10.1002/prca.201200045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang H, Li X-j, Martin DB, Aebersold R. Identification and quantification of N-linked glycoproteins using hydrazide chemistry, stable isotope labeling and mass spectrometry. Nat Biotechnol. 2003;21:660–666. doi: 10.1038/nbt827. [DOI] [PubMed] [Google Scholar]
- 76.del Amo V, Slawin AM, Philp D. Manipulating Replication Processes within a Dynamic Covalent Framework. Org Lett. 2008;10:4589–4592. doi: 10.1021/ol8018665. [DOI] [PubMed] [Google Scholar]
- 77.SetháHorne W. Promoting peptide α-helix formation with dynamic covalent oxime side-chain cross-links. Chem Commun. 2011;47:10915–10917. doi: 10.1039/c1cc12010g. [DOI] [PubMed] [Google Scholar]
- 78.Powers TW, Jones EE, Betesh LR, Romano PR, et al. Matrix assisted laser desorption ionization imaging mass spectrometry workflow for spatial profiling analysis of N-linked glycan expression in tissues. Anal Chem. 2013;85:9799–9806. doi: 10.1021/ac402108x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. In vivo imaging of membrane-associated glycans in developing zebrafish. Science. 2008;320:664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laughlin ST, Bertozzi CR. In vivo imaging of Caenorhabditis elegans glycans. ACS Chem Biol. 2009;4:1068–1072. doi: 10.1021/cb900254y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Varki A. In: N-glycans. Varki A, Cummings RD, Esko JD, Freeze HH, et al., editors. Cold Spring Harbor Laboratory Press; New York: 2009. pp. 633–648. [PubMed] [Google Scholar]
- 82.Mariño K, Bones J, Kattla JJ, Rudd PM. A systematic approach to protein glycosylation analysis: a path through the maze. Nat Chem Biol. 2010;6:713–723. doi: 10.1038/nchembio.437. [DOI] [PubMed] [Google Scholar]
- 83.Guile GR, Rudd PM, Wing DR, Prime SB, Dwek RA. A rapid high-resolution high-performance liquid chromatographic method for separating glycan mixtures and analyzing oligosaccharide profiles. Anal Biochem. 1996;240:210–226. doi: 10.1006/abio.1996.0351. [DOI] [PubMed] [Google Scholar]
- 84.Berthod A, Chang SS, Kullman JP, Armstrong DW. Practice and mechanism of HPLC oligosaccharide separation with a cyclodextrin bonded phase. Talanta. 1998;47:1001–1012. doi: 10.1016/s0039-9140(98)00179-9. [DOI] [PubMed] [Google Scholar]
- 85.Rudd PM, Endo T, Colominas C, Groth D, et al. Glycosylation differences between the normal and pathogenic prion protein isoforms. Proc Natl Acad Sci. 1999;96:13044–13049. doi: 10.1073/pnas.96.23.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arnold JN, Saldova R, Galligan MC, Murphy TB, et al. Novel glycan biomarkers for the detection of lung cancer. J Proteome Res. 2011;10:1755–1764. doi: 10.1021/pr101034t. [DOI] [PubMed] [Google Scholar]
- 87.Bones J, Byrne JC, O’Donoghue N, McManus C, et al. Glycomic and glycoproteomic analysis of serum from patients with stomach cancer reveals potential markers arising from host defense response mechanisms. J Proteome Res. 2010;10:1246–1265. doi: 10.1021/pr101036b. [DOI] [PubMed] [Google Scholar]
- 88.Saldova R, Piccard H, Pérez-Garay M, Harvey DJ, et al. Increase in sialylation and branching in the mouse serum N-glycome correlates with inflammation and ovarian tumour progression. PLOS ONE. 2013;8:e71159. doi: 10.1371/journal.pone.0071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bones J, Mittermayr S, O’Donoghue N, Guttman As, Rudd PM. Ultra performance liquid chromatographic profiling of serum N-glycans for fast and efficient identification of cancer associated alterations in glycosylation. Anal Chem. 2010;82:10208–10215. doi: 10.1021/ac102860w. [DOI] [PubMed] [Google Scholar]
- 90.Klaus U, Pfeifer T, Spiteller M. APCI-MS/MS: A powerful tool for the analysis of bound residues resulting from the interaction of pesticides with DOM and humic substances. Environ Sci Technol. 2000;34:3514–3520. [Google Scholar]
- 91.Jedrychowski MP, Huttlin EL, Haas W, Sowa ME, et al. Evaluation of HCD-and CID-type fragmentation within their respective detection platforms for murine phosphoproteomics. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M111.009910. M111.009910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scott NE, Parker BL, Connolly AM, Paulech J, et al. Simultaneous glycan-peptide characterization using hydrophilic interaction chromatography and parallel fragmentation by CID, higher energy collisional dissociation, and electron transfer dissociation MS applied to the N-linked glycoproteome of Campylobacter jejuni. Mol Cell Proteomics. 2011;10:M000031-MCP000201. doi: 10.1074/mcp.M000031-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ceroni A, Maass K, Geyer H, Geyer R, et al. GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans†. J Proteome Res. 2008;7:1650–1659. doi: 10.1021/pr7008252. [DOI] [PubMed] [Google Scholar]
- 94.Albanese J, Glueckmann M, Lenz C. SimGlycan Software*: a new predictive carbohydrate analysis tool for MS/MS data. Appl Biosystems. 2010:p1–p7. [Google Scholar]
- 95.Qin H, Zhao L, Li R, Wu Ra, Zou H. Size-selective enrichment of N-linked glycans using highly ordered Mesoporous carbon material and detection by MALDI-TOF MS. Anal Chem. 2011;83:7721–7728. doi: 10.1021/ac201198q. [DOI] [PubMed] [Google Scholar]
- 96.Miura Y, Shinohara Y, Furukawa J, Nagahori N, Nishimura SI. Rapid and Simple Solid-Phase Esterification of Sialic Acid Residues for Quantitative Glycomics by Mass Spectrometry. Chem Eur J. 2007;13:4797–4804. doi: 10.1002/chem.200601872. [DOI] [PubMed] [Google Scholar]
- 97.Snovida SI, Rak-Banville JM, Perreault H. On the use of DHB/aniline and DHB/N, N-dimethylaniline matrices for improved detection of carbohydrates: automated identification of oligosaccharides and quantitative analysis of sialylated glycans by MALDI-TOF mass spectrometry. J Am Soc Mass Spectrom. 2008;19:1138–1146. doi: 10.1016/j.jasms.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 98.Harz H, Burgdorf K, Höltje JV. Isolation and separation of the glycan strands from murein of Escherichia coli by reversed-phase high-performance liquid chromatography. Anal Biochem. 1990;190:120–128. doi: 10.1016/0003-2697(90)90144-x. [DOI] [PubMed] [Google Scholar]
- 99.Chu CS, Niñonuevo MR, Clowers BH, Perkins PD, et al. Profile of native N-linked glycan structures from human serum using high performance liquid chromatography on a microfluidic chip and time-of-flight mass spectrometry. Proteomics. 2009;9:1939–1951. doi: 10.1002/pmic.200800249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hua S, An HJ, Ozcan S, Ro GS, et al. Comprehensive native glycan profiling with isomer separation and quantitation for the discovery of cancer biomarkers. Analyst. 2011;136:3663–3671. doi: 10.1039/c1an15093f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kamoda S, Ishikawa R, Kakehi K. Capillary electrophoresis with laser-induced fluorescence detection for detailed studies on N-linked oligosaccharide profile of therapeutic recombinant monoclonal antibodies. J Chromatogr A. 2006;1133:332–339. doi: 10.1016/j.chroma.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 102.Pabst M, Altmann F. Glycan analysis by modern instrumental methods. Proteomics. 2011;11:631–643. doi: 10.1002/pmic.201000517. [DOI] [PubMed] [Google Scholar]
- 103.Nakano M, Higo D, Arai E, Nakagawa T, et al. Capillary electrophoresis-electrospray ionization mass spectrometry for rapid and sensitive N-glycan analysis of glycoproteins as 9-fluorenylmethyl derivatives. Glycobiology. 2009;19:135–143. doi: 10.1093/glycob/cwn115. [DOI] [PubMed] [Google Scholar]
- 104.Szabo Z, Guttman A, Rejtar T, Karger BL. Improved sample preparation method for glycan analysis of glycoproteins by CE-LIF and CE-MS. Electrophoresis. 2010;31:1389–1395. doi: 10.1002/elps.201000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ruhaak LR, Steenvoorden E, Koeleman CA, Deelder AM, Wuhrer M. 2-picoline-borane: A non-toxic reducing agent for oligosaccharide labeling by reductive amination. Proteomics. 2010;10:2330–2336. doi: 10.1002/pmic.200900804. [DOI] [PubMed] [Google Scholar]
- 106.You J, Sheng X, Ding C, Sun Z, et al. Detection of carbohydrates using new labeling reagent 1-(2-naphthyl)-3-methyl-5-pyrazolone by capillary zone electrophoresis with absorbance (UV) Anal Chim Acta. 2008;609:66–75. doi: 10.1016/j.aca.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 107.Ruhaak L, Zauner G, Huhn C, Bruggink C, et al. Glycan labeling strategies and their use in identification and quantification. Anal Bioanal Chem. 2010;397:3457–3481. doi: 10.1007/s00216-010-3532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Julka S, Regnier F. Quantification in proteomics through stable isotope coding: a review. J Proteome Res. 2004;3:350–363. doi: 10.1021/pr0340734. [DOI] [PubMed] [Google Scholar]
- 109.Goshe MB, Smith RD. Stable isotope-coded proteomic mass spectrometry. Curr Opin Biotechnol. 2003;14:101–109. doi: 10.1016/s0958-1669(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 110.Tao WA, Aebersold R. Advances in quantitative proteomics via stable isotope tagging and mass spectrometry. Curr Opin Biotechnol. 2003;14:110–118. doi: 10.1016/s0958-1669(02)00018-6. [DOI] [PubMed] [Google Scholar]
- 111.Orlando R, Lim JM, Atwood JA, III, Angel PM, et al. IDAWG: Metabolic incorporation of stable isotope labels for quantitative glycomics of cultured cells. J Proteome Res. 2009;8:3816–3823. doi: 10.1021/pr8010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Walker SH, Budhathoki-Uprety J, Novak BM, Muddiman DC. Stable-isotope labeled hydrophobic hydrazide reagents for the relative quantification of N-linked glycans by electrospray ionization mass spectrometry. Anal Chem. 2011;83:6738–6745. doi: 10.1021/ac201376q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bowman MJ, Zaia J. Tags for the stable isotopic labeling of carbohydrates and quantitative analysis by mass spectrometry. Anal Chem. 2007;79:5777–5784. doi: 10.1021/ac070581b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Szabo Z, Guttman A, Bones J, Shand RL, et al. Ultrasensitive capillary electrophoretic analysis of potentially immunogenic carbohydrate residues in biologics: Galactose-α-1, 3-galactose containing oligosaccharides. Mol Pharm. 2012;9:1612–1619. doi: 10.1021/mp200612n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Parker BL, Thaysen-Andersen M, Solis N, Scott NE, et al. Site-specific glycan-peptide analysis for determination of N-glycoproteome heterogeneity. J Proteome Res. 2013;12:5791–5800. doi: 10.1021/pr400783j. [DOI] [PubMed] [Google Scholar]
- 116.An HJ, Froehlich JW, Lebrilla CB. Determination of glycosylation sites and site-specific heterogeneity in glycoproteins. Curr Opin Chem Biol. 2009;13:421–426. doi: 10.1016/j.cbpa.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang S, Mishra S, Chen L, Zhou J-y, et al. An Integrated Glycoprotein Immobilization Method for Glycopeptide and Glycan Analysis of Cardiac Hypertrophy. Anal Chem. 2015 doi: 10.1021/acs.analchem.5b01663. Revision-submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lamari FN, Kuhn R, Karamanos NK. Derivatization of carbohydrates for chromatographic, electrophoretic and mass spectrometric structure analysis. J Chromatogr B. 2003;793:15–36. doi: 10.1016/s1570-0232(03)00362-3. [DOI] [PubMed] [Google Scholar]
- 119.Landberg E, Pahlsson P, Lundblad A, Arnetorp A, Jeppsson JO. Carbohydrate composition of serum transferrin isoforms from patients with high alcohol consumption. Biochem Biophys Res Commun. 1995;210:267–274. doi: 10.1006/bbrc.1995.1656. [DOI] [PubMed] [Google Scholar]
- 120.Campbell MP, Royle L, Radcliffe CM, Dwek RA, Rudd PM. GlycoBase and autoGU: tools for HPLC-based glycan analysis. Bioinformatics. 2008;24:1214–1216. doi: 10.1093/bioinformatics/btn090. [DOI] [PubMed] [Google Scholar]