Abstract
Ovarian cancer is the fifth most deadly cancer in women in the United States and despite advances in surgical and chemotherapeutic treatments survival rates have not significantly improved in decades. The poor prognosis for ovarian cancer patients is largely due to the extremely high (80%) recurrence rate of ovarian cancer and because the recurrent tumors are often resistant to the widely utilized platinum-based chemotherapeutic drugs. In this study, expression of Rad6, an E2 ubiquitin-conjugating enzyme, was found to strongly correlate with ovarian cancer progression. Furthermore, in ovarian cancer cells Rad6 was found to stabilize β-catenin promoting stem cell-related characteristics, including expression of stem cell markers and anchorage-independent growth. Cancer stem cells can promote chemoresistance, tumor recurrence and metastasis, all of which are limiting factors in treating ovarian cancer. Thus it is significant that Rad6 overexpression led to increased resistance to the chemotherapeutic drug carboplatin and correlated with tumor cell invasion. These findings show the importance of Rad6 in ovarian cancer and emphasize the need for further studies of Rad6 as a potential target for the treatment of ovarian cancer.
Keywords: Rad6, stem cell, platinum resistance, ovarian cancer
1. Introduction
Ovarian cancer (OC) is a serious problem worldwide and is the most deadly gynecological malignancy in women in the USA [1]. OC is typically asymptomatic during early development and thus is frequently detected at late stages where prognosis is poor. Currently, the five year survival rate is only 45.6%, a number which has changed little in decades [2]. This highlights the need to find new treatment options for patients with ovarian cancer.
Rad6 is an E2 ubiquitin-conjugating enzyme originally identified in yeast where it is required for DNA repair, induced mutagenesis and proliferation [3]. Humans have two homologs, Rad6A and Rad6B, which were able to complement mutant Saccharomyces cerevisiae Rad6 in DNA repair [4]. In humans, Rad6 has been shown to regulate gene transcription through modulation of chromatin conformation by histone modification and degradation [5–7]. Furthermore, Rad6 plays a pivotal role in choosing which DNA repair pathway is used [8,9]. Loss of Rad6 sensitizes cells to DNA damage and chemotherapeutic drugs [7]. Conversely, overexpression of Rad6 corresponds with mitotic abnormalities and can lead to transformation [10]. High levels of Rad6 correlate with melanoma development and progression [11]. Elevated Rad6 was also found in breast cancer, where it promotes malignant progression through stimulation of the Wnt/β-catenin signaling pathway [10,12].
In this report we examined the expression of Rad6 in ovarian cancer patient tissue and found that its expression correlated with tumor stage. In OC-derived cell lines increased Rad6 expression led to increased expression of stem cell markers and components signaling pathways that promote stemness. Cells expressing higher levels of Rad6 were also more capable of anchorage-independent growth, a key property of cancer stem cells (CSCs). Furthermore, ectopic overexpression of Rad6 increased resistance to carboplatin in ovarian cancer cells. Therefore, Rad6 is important for the progression of ovarian cancer and promotes stem cell characteristics that can provide the tumor with increased capacity for chemoresistance, proliferation and metastasis.
2. Methods and materials
2.1. Cell Lines and reagents
Fallopian tube epithelial cells (FTSEC or FTEC) were used as normal ovarian cells (generously provided by Dr. Amir Jazaeri) [13]. OV90 and SKOV3 cells were purchased from ATCC, isogenic A2780 (cisplatin-sensitive) and A2780/CP70 (cisplatin-resistant) cells were previously described [14]. OV90 and SKOV3 cells were cultured in 1:1 DMEM/F12 (Mediatech). FTSEC, A2780 and A2780/CP70 cells were cultured in RPMI [14]. All media were supplemented with 10% FBS and 1x Penicillin/Streptomycin. Carboplatin was from Sigma and Rad6 expression vector was obtained from Addgene [15]. The siRNAs used in this study were purchased from Dharmacon and the transfections were done using Lipofectamine 2000 (Invitrogen) following the manufacturer’s protocol. Antibodies specific to the following proteins were used: Gli1 (Cell Signaling Technology); GAPDH, ALDA1H1, BMI1, Nanog, OCT4, Myc, and β-Catenin (Santa Cruz Biotechnology); Rad6 (Bethyl Laboratories); H2B, H3K79me3 and SOX2 (Abcam); and Ub-H2B (Millipore).
2.2. Clonogenic survival assays
For clonogenic survival assays, 500 cells were plated in 6-well culture dishes in triplicate [16]. Cells were allowed to attach overnight and treated with indicated concentrations of carboplatin or vehicle control (DMSO) overnight. After the drug treatment cells were washed three times with PBS and two times with growth medium and allowed to form colonies in complete growth medium. After 8 to 12 days colonies were fixed in methanol, stained with crystal violet (0.5% w/v) and counted as described [16]. Only colonies containing more than 25 cells were counted.
2.3. Western blotting
For Western blot analysis cell lysates were prepared after washing three times with ice-cold PBS. Cells were lysed in ice-cold cytoskeletal (CSK) buffer freshly supplemented with protease and phosphatase inhibitors (Roche) as described [17]. After quantitation of the protein concentrations, samples were normalized and prepared in 5x Laemmli buffer and heated to 100°C for 15 min. Denatured samples were resolved by SDS-PAGE gel followed by immunoblotting as described [17].
2.4. Sphere formation assays
The adherent ovarian cancer cells growing in log phase were harvested by trypsinization, counted and seeded in ultra-low attachment 6 well dishes at 1×104 to 1000 cells/well. The cells were allowed to grow and form spheres as detailed elsewhere [18]. In brief, the counted cells were carefully dispersed as single cells, cultured in stem cell-specific serum free media (2 mL) in an ultra-low attachment six well plates for 10–12 days. This defined media (1:1 DMEM/F-12) was supplemented with 1% penicillin-streptomycin, B27 and N2 supplements (all from Gibco), and growth factors [recombinant human epidermal growth factor (EGF) and fibroblast growth factor (FGF), both from Invitrogen]. These conditions support stem cell growth, and with time, cells proliferate to form floating single cell cloned spheres, known as OC spheres. After seeding the cells were observed daily to ensure that spheres were forming as a result of multiplication from a single cell and not due to cell adherence. Fresh media containing growth supplements EGF (20 ng/ml) and FGF (20 ng/ml) was added every 72 h. The spheres containing ≥ 50 cells were scored as large (true stem cell spheres), while spheres <50 but >15 cells were considered to be small spheres.
2.5. Generation of carboplatin-resistant cells
The carboplatin-resistant SKOV3 cell lines were generated by intermittently exposing the original SKOV3 cell line to sequentially increased concentrations of carboplatin. The SKOV3 cells were exposed to different concentrations of carboplatin for 72 hours and then allowed to recover in the drug free medium. This process was repeated to get resistant cell lines at a range of concentrations of carboplatin (1 to 20 μM). Resistance was confirmed by clonogenic survival assay. The cell lines were named according to level of carboplatin resistance. The cell line widely used here is SKOV3/CP20 which is resistant to 20 μM carboplatin.
2.6. Immunohistochemistry
Normal ovarian tissue and ovarian cancer tumor samples were purchased from US BIOMAX and expression of Rad6 protein was measured by immunohistochemistry. Tissue sections were incubated with anti-Rad6 antibody, followed by a specific biotinylated secondary antibody (1:250 dilution), and then conjugated HRP streptavidin and DAB chromogen and tissues were counterstained with hematoxylin. Stained sections were analyzed by Zeiss Axioscope 2 microscope and images captured by AxioCam camera at 400x magnifications were analyzed by NIS Element AR software (Nikon). This process was carried out without any knowledge of the identity of each tissue sample to prevent bias in scoring the samples.
2.7. 3-D culture invasion assay
OC cell spheres generated as described above were harvested and immobilized in Matrigel according to manufacturer’s instructions (Cultrex). Briefly, 4-chambered glass slides were coated with thin layer of Matrigel (80 μL/well). Approximately 100 spheres in PBS were mixed (1:1 ratio) with Matrigel and 500 μl was layered on top of the solidified basement Matrigel-coated chamber slide. The slide was incubated at 37°C for 30 min. After Matrigel had solidified complete growth media supplemented with EGF (20 ng/ml) was added. The spheres were allowed to grow in this 3-D culture media for 72 hr. The spheres were monitored and imaged by Zeiss Axioscope 2 microscope and images were captured for every 24 hr by AxioCam camera at 100x magnification.
2.8. Statistical analysis
Clonogenic survival data and sphere formation data presented are averages of three independent experiments. The experiments performed in triplicate each time. Error bars represents the ± SEM. Data were analyzed either by GraphPad Prism 6 or Excel 2010 (Microsoft).
3. Results
3.1. Rad6 expression correlates with ovarian cancer stage
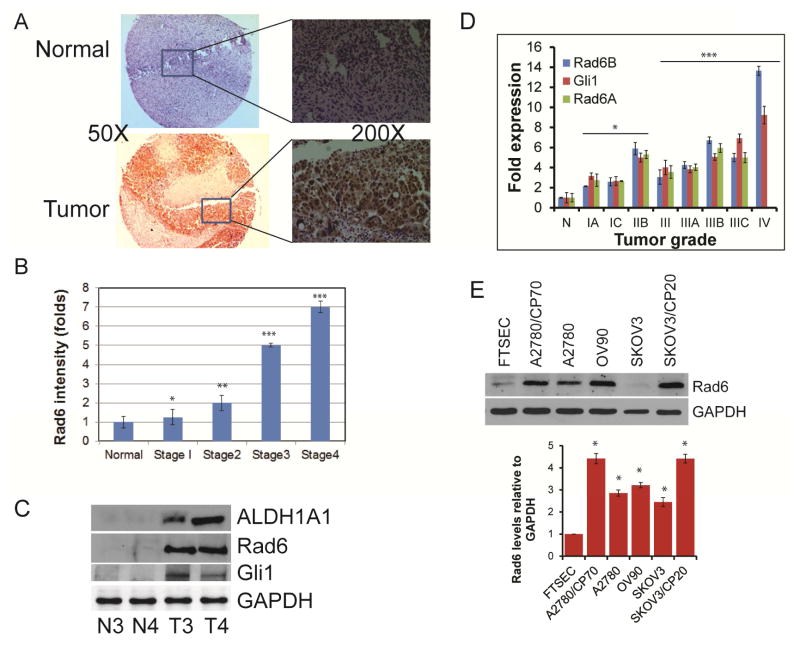
Rad6 levels correlate with severity of melanoma and breast cancer [10,11]. To determine if Rad6 is also associated with disease progression in OC, normal and staged OC tissue samples were stained for Rad6 (Fig. 1A). The identities of the samples were concealed to limit any bias in scoring for Rad6 signal intensity. Consistent with the findings in aforementioned cancers, Rad6 levels correlated well with disease progression (Fig. 1B). Aberrant Hedgehog (Hh)/Gli signaling is commonly associated with tumor growth, metastasis, and resistance to chemotherapy [16,19] and we previously demonstrated that ALDH1A1 regulates DNA repair and checkpoint progression to enhance resistance to platinum drugs [14]. Therefore, we examined stage 3 and 4 OC and adjacent normal tissue for expression of these proteins in addition to Rad6. All three proteins were highly expressed in the tumor samples compared to normal tissue (Fig. 1C). In order to determine if the high levels of these proteins is due to enhanced transcription of their genes in the OC tumors, mRNA levels of each were quantitated. Gli1 and Rad6 message correlated with OC stage similarly to protein, with Gli1 mRNA being 4 to almost 10-fold higher in grade 3 and nearly 10-fold higher in stage 4 and Rad6 3 to 7-fold more in stage 3 and 13-fold greater in stage 4 tissue compared to normal ovarian tissues (Fig. 1D).
Fig. 1.
Rad6 expression correlates with ovarian cancer progression. A. Ovarian cancer tissue microarray containing both normal and all four stages of serous ovarian carcinoma from US Biomax was used for immunohistochemical analysis. Tissues were incubated with primary Rad6 antibody and DAB chromogen and then counterstained with hematoxylin. Shown are representative normal (top panel) and stage 3 tumor tissue (bottom panel) and selected areas are also shown at high magnification. B. Rad6 stained normal (n=16) and staged ovarian tumor (n=60) samples were scored for Rad6 intensity by double blind analysis and average values plotted. C. Protein was harvested from paired normal (N) and stage 3 and 4 ovarian tumor (T3 & T4, respectively) samples, and levels of Rad6, ALDH1A1 and Gli1 were measured by Western blot. D. Quantitative RT-PCR of Rad6 and Gli1 mRNA levels in normal ovarian (n=6) and graded ovarian tumors (n=42) of the HORT104 ovarian cDNA array (OriGene). Message levels were normalized to GAPDH mRNA and quantified using the ΔΔCt method. E. Total protein was isolated form normal, immortalized FTEC and ovarian cancer cell lines and Rad6 levels were quantitated by Western blot. Shown are a representative blot and the average values obtained from 3 independent experiments with each band adjusted to the corresponding GAPDH control. Statistical analysis: * indicates P<0.05, **P<0.01, ***P<0.001.
To further establish the correlation between Rad6 and OC we analyzed a normal, immortalized cell line derived from fallopian tube epithelial cells, a proposed OC precursor [13], and several OC cell lines, including 2 isogenic pairs of platinum-sensitive (A2780 and SKOV3) and platinum-resistant (A2780/CP70 and SKOV3/CP20) lines. As expected the OC cell lines had higher Rad6 expression than normal cells (Fig. 1E). Interestingly, Rad6 levels were also significantly higher in the cell lines with platinum drug resistance.
3.2. Rad6 levels correlate with platinum drug resistance and stem-like characteristics
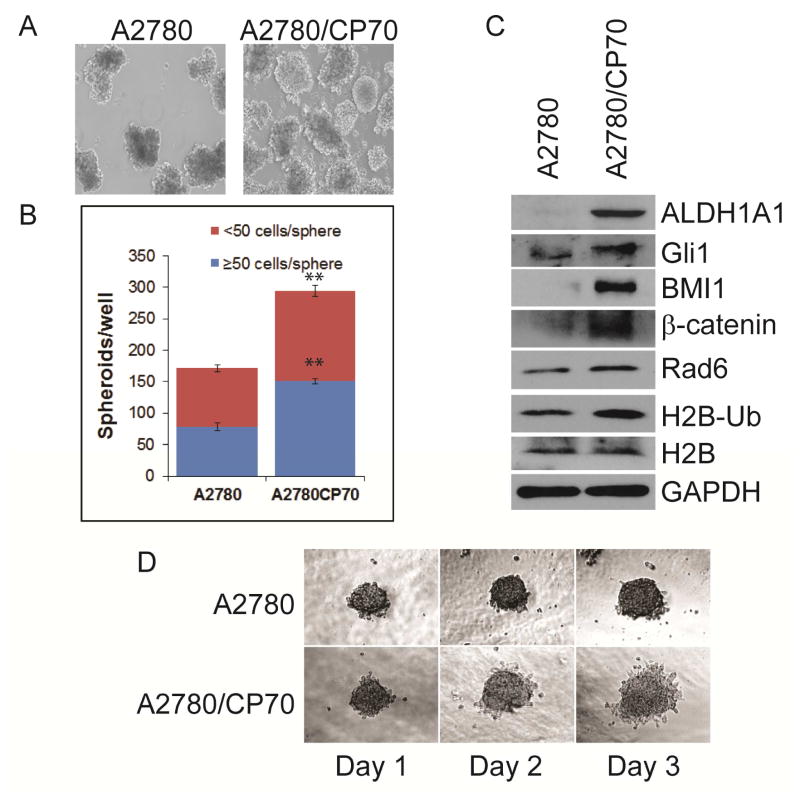
Carboplatin-resistant A2780/CP70 cells exhibited greater Rad6 expression than its isogenic carboplatin-sensitive counterpart (Fig. 1E). SKOV3/CP20 cells were generated by sequential exposure to increasing concentrations of carboplatin and analyzed for Rad6 expression. Rad6 levels increased with the level of platinum resistance in these cells (Fig. S1). Together these results show that Rad6 correlates with platinum resistance in ovarian cancer cells; a finding that is consistent with earlier studies reporting that Rad6 can promote chemoresistance [7]. Rad6 is also known to stabilize β-catenin and stimulate Wnt signaling [12], a pathway associated with stem cell properties [20]. Thus, we examined the capability of the isogenic platinum-sensitive and –resistant A2780 cells for anchorage-independent growth as spheres, an indicator of stem cell-like properties. The resistant A2780/CP70 cell line generated substantially more spheres in culture (Fig. 2A & B). To further confirm the correlation between Rad6, stemness and platinum resistance we examined the expression levels of several stem cell markers (ALDH1A1, BMI1, SOX2, Nanog, OCT4) and signaling proteins (Gli1 and β-catenin) and found that all were upregulated in the platinum-resistant cells (Fig. 2C and not shown). An increase in histone 2B (H2B) ubiquitination and formation of histone H3K79me3 were also seen in these cells. Alterations in H2B ubiquitination have previously been shown to enhance transcription in cancer cells [21] and Rad6 has been implicated in H2B ubiquitination [5]. Histone H3K79 trimethylation is dependent upon H2B monoubiquitantion by Rad6 and is a marker for an open transcriptionally active region [22,23]. Additionally, Matrigel invasion assays showed that the more stem cell-like, platinum resistant A2780/CP70 cells were also more invasive than their platinum-sensitive counterpart (Fig. 2D).
Fig. 2.
Rad6 expression correlates with stemness and platinum resistance in OC cells. A. Isogenic platinum-sensitive (A2780) platinum-resistant (A2780/CP70) ovarian cancer cells were tested for capacity for anchorage-independent growth (as described in Methods & Materials). B. The large (>50 cells) and small (<50 cells) OC spheres formed by each cell line were counted and average value presented. C. Anchorage-independent growth (represented by OC sphere formation) is characteristic of CSCs; therefore, protein was harvested from these cells and analyzed for the Rad6 levels, the presence of stem cell markers (ALDH1A1 & BMI1), expression of signaling molecules that promote stemness (Gli1 & β-catenin), and ubiquitination of histone 2B by Rad6 and related downstream histone H3 trimethylation H3K79me3. D. The isogenic A2780 and A2780/CP70 cell lines were analyzed by Matrigel invasion assay to determine invasive potential of these cells. Statistical analysis: * indicates P<0.05, **P<0.01.
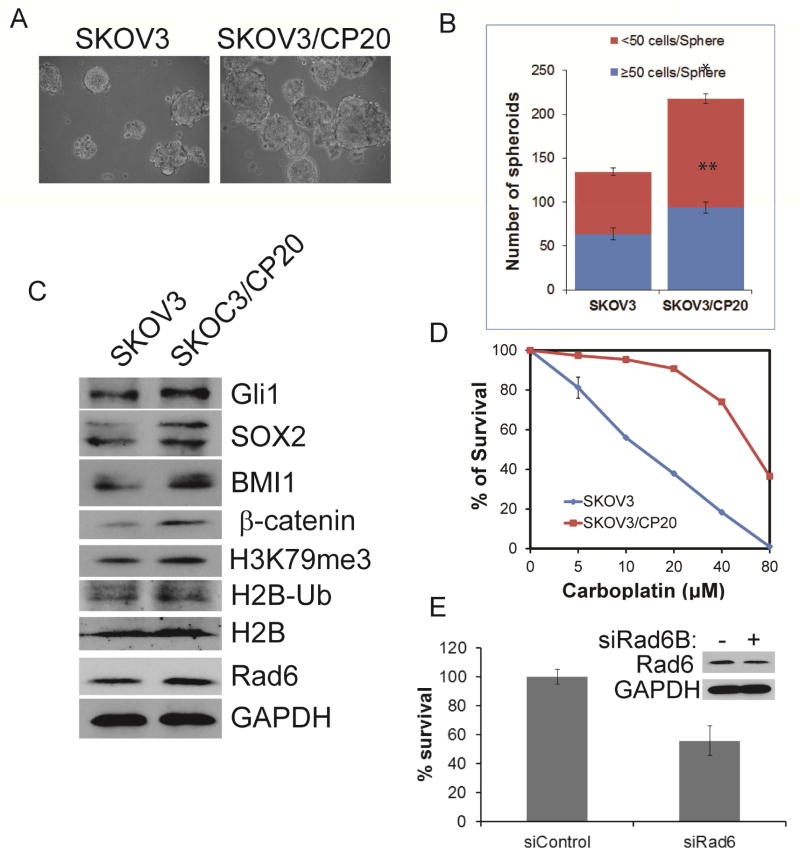
At least 80% of OC cases in the US involve serous tumors [24]; therefore, it is important to confirm the correlation between stemness, platinum resistance and Rad6 expression using cell lines derived from serous tumor (SKOV3). As seen with the isogenic A2780 cell lines, the platinum-resistant SKOV3/CP20 cells were more proficient at anchorage-independent growth (Fig. 3A & B), and expressed higher levels of Rad6, stem cell markers, and Wnt and Hh signaling molecules (β-catenin and Gli1) than the isogenic platinum-sensitive SKOV3 (Fig. 3C). The SKOV3/CP20 generated for this work were confirmed to be resistant to the platinum chemotherapy drug carboplatin (to 20 μM, hence the name CP20 - Fig. 3D). Importantly, knocking down Rad6B using siRNAs attenuated SKOV3 cells’ clonogenic potential (Fig. 3E). This shows that the effects are not cell line specific but are found in cells derived from several types of ovarian cancers.
Fig. 3.
Rad6 expression correlates with stemness in serous OC cells. A. Isogenic platinum-sensitive (SKOV3) and platinum-resistant (SKOV3/CP20) serous OC cells were assayed for capability of anchorage-independent growth as spheres. B. Average values of numbers of small (<50 cells) and large (>50 cells) spheres formed by SKOV3 and SKOV3/CP20 cells from 3 independent experiments. C. Total protein was examined for expression of stemness markers (SOX2 & BMI1) as well as pro-stem cell signaling proteins (Gli1 & β-catenin) and ubiquitination of histone H2B by Rad6 and the subsequent trimethylation of histone 3 (H3K79me3). D. Clonogenic survival assay for analyzing carboplatinum resistance in SKOV3 and SKOV3/CP20 cells. Clonogenic survival was assessed in control and Rad6 siRNAs transfected SKOV3 cells. E, and the representing Rad6 blot (inset) showing knockdown level of about 50%. Statistical analysis: * indicates P<0.05, **P<0.01.
3.3. Ectopic overexpression of Rad6 induces carboplatin-resistance and stem-like properties
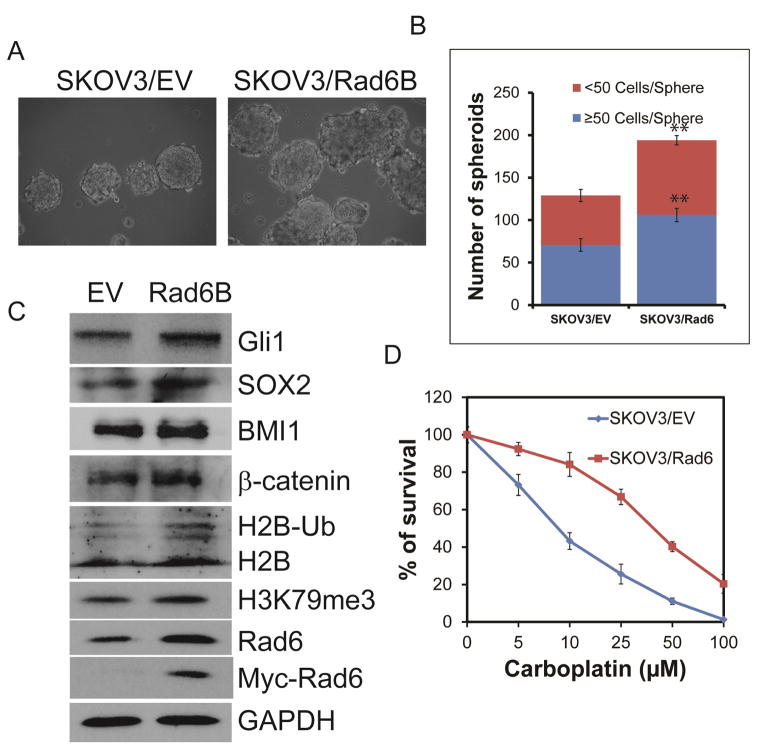
Rad6 expression correlates with OC severity (Fig. 1A–D), resistance to platinum-based chemotherapeutic drugs (Fig. 1E & S1) and with anchorage-independent growth as spheres and expression of stem cell markers and the signaling molecules β-catenin and Gli1 in OC cell lines (Fig 2 & 3). In order to determine if Rad6 is responsible for the enhanced platinum resistance and the development of stem cell properties, it was overexpressed in SKOV3 cells. Increasing Rad6 expression in these cells was accompanied by increased sphere formation, which is indicative of stem cell characteristics (Fig 4A & B). This was accompanied by increased expression of stem cell markers (SOX2, BMI1, ALDH1A1, Nanog, and OCT4) and ubiquitinated histone 2B (Fig. 4C and not shown) in the SKOV3/Rad6 cells. Rad6 overexpression in these normally platinum-sensitive cells significantly increased resistance to carboplatin in clonogenic survival assays (Fig. 4D). Taken together this data shows that overexpression of Rad6 in ovarian cancer cells can lead to increased resistance to platinum-based chemotherapeutic drugs and enhanced stem cell characteristics potentially leading to more aggressive tumor growth and metastasis. This is significant in light of evidence showing that chemotherapy drug-resistant CSCs can lead to tumor recurrence [25].
Fig. 4.
Ectopic expression of Rad6 promotes platinum drug resistance and stemness in OC cells. SKOV3 cells were transfected with a plasmid to express Rad6B. A. The ability of parental and Rad6-overexpressing SKOV3 cells for anchorage-independent growth as spheres was determined. B. The average number of OC spheres produced by parental and Rad6-overexpressing cells from three independent experiments. C. Levels of stem cell markers (SOX2 & BMI1) signaling molecules that promote stemness (Gli1 & β-catenin), and ubiquitination of histone 2B by Rad6 and trimethylation of histone 3 (H3K79me3) were measured by Western blot. D. The parental and Rad6-expressing cells were analyzed for resistance to the chemotherapy drug carboplatin by clonogenic survival assay. Statistical analysis: * indicates P<0.05, **P<0.01.
4. Discussion
Ovarian cancer remains a serious health issue worldwide and in the United States alone there are more than 22,000 new cases annually. Because of its largely asymptomatic progression OC typically goes undetected until reaching an advanced stage with widespread intra-abdominal involvement and/or metastatic spread. While survival rates of many cancers have improved with current detection and treatment methods, OC outcomes have changed little in decades [2]. Therefore, there is a need for better understanding of the nature and pathology of OC in order to develop better treatment options. Thus in this study we have explored the role of Rad6 in ovarian cancer. Expression of the E2 ubiquitin-conjugating enzyme Rad6 correlates well with progression of OC tumors (Fig. 1A–D). This finding correlates with previous studies of Rad6 expression in melanoma and breast cancer [10,11]. This is important because Rad6 has been implicated in regulating proliferation, DNA repair and resistance to chemotherapeutic drugs [3,7–9].
The expression of Rad6 in laboratory cell lines derived from OC was also high and correlated with resistance to platinum-based drugs (carboplatin and cisplatin – Fig. 1D). When an OC cell line (SKOV3) was made resistant to increasing levels of the chemotherapy drug carboplatin, Rad6 expression increased with resistance (Fig. S1), suggesting Rad6 expression is important for developing drug resistance. This was confirmed by measuring carboplatin resistance in OC cells overexpressing Rad6. These cells showed significantly greater resistance to carboplatin than vector control (Fig. 4D). This confirms previous studies correlating Rad6 expression with drug resistance [7].
Rad6 has previously been shown to stabilize β-catenin a member of the Wnt signaling pathway [12]. Wnt signaling through β-catenin promotes stemness and self-renewal [20]. There is a wealth of evidence that cancer stem cells (CSCs) promote tumor initiation, cellular heterogeneity, avoidance or resistance to treatment, recurrence and metastasis [26]. Chemotherapy resistance, recurrence and metastasis are major limitations in the current treatment of OC that need to be addressed; therefore, OC cells were analyzed for expression of stem cell markers and capacity for anchorage-independent growth (stem cell characteristic). Analysis of two sets of isogenic OC cell lines showed that the platinum-resistant cell lines that express higher levels of Rad6 (A2780/CP70 & SKOV3/CP20) were enriched in protein markers of stemness compared to platinum-sensitive counterparts (A2780 & SKOV3) which express lower levels of Rad6 (Fig. 2C & 3C). Furthermore, downregulation of Rad6B attenuated clongenic potential of OC cells (Fig 3E). On the other hand, when OC cells were transfected with a plasmid expressing Rad6B, these cells exhibited increased levels of stem cell markers (Fig. 4C) and Rad6 levels correlated with histone modifications suggesting open, actively transcribed chromatin. Each of these cell lines expressing higher levels of Rad6 and stem cell markers were more capable of anchorage-independent growth as OC spheres than their isogenic counterparts that had less Rad6 (Fig. 2A & 2B). Rad6 overexpressing SKOV3 cells formed more spheres than the vector control cells (Fig. 4A & B). Together these findings suggest that Rad6 promotes stemness in OC cells by stabilizing β-catenin. A model representation of these findings is presented in the Supplementary material (Fig. S2). Increased Rad6 expression in OC cells means there is more Rad6-mediated ubiquitin signaling leading to β-catenin stabilization (increased Wnt signaling) and enhanced Gli1 expression (increased Hh signaling). Enhanced activity of these signaling pathways combined with Rad6-directed chromatin remodeling leads to increased expression of proteins that promote stem cell-like properties leading to chemoresistance. Rad6 also partners with Rad18, an E3 ubiquitin ligase to regulate multiple DNA repair pathways including translesion synthesis and the Fanconi anemia/BRCA pathway [27,28].
Since most ovarian cancer cases are diagnosed at later stages and Rad6 expression correlates with progression of ovarian cancer, many patients will already have high levels of Rad6 expressing tumor cells. Rad6 promotes chemoresistance and stem cell characteristics, which could lead to enhanced disease recurrence and metastasis. These findings suggest that development of new therapies targeting Rad6 may be warranted in the treatment of ovarian cancer.
Supplementary Material
Highlights.
Rad6 expression positively correlates with progression of ovarian cancer.
Increased Rad6 expression lead to resistance to chemotherapeutic platinum drugs.
Rad6 induces stem cell-like properties in ovarian cancer cells.
Acknowledgments
We would like to thank Joel Andrews, Ph.D. for help with microscopy and Dr. Amir Jazaeri, M.D. Anderson Cancer Center for generously providing FTECs.
Funding: This work is supported by NIH grant R01GM098956, and Abraham Mitchell Cancer Research Scholar Endowment grant (K.P.).
Abbreviations
- OC
ovarian cancer
- Hh
hedgehog
- CSC
cancer stem cell
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.SEER Cancer Statistics Review, 1975–2012. n.d. [Google Scholar]
- 3.Haynes R, Kunz B. The Molecular Biology of the Yeast Saccharomyces cerevisiae: Life Cycle and Inheritance. Cold Spring Harbor Laboratory; n.d. [Google Scholar]
- 4.Koken MH, Reynolds P, Jaspers-Dekker I, Prakash L, Prakash S, Bootsma D, et al. Structural and functional conservation of two human homologs of the yeast DNA repair gene RAD6. Proc Natl Acad Sci U S A. 1991;88:8865–8869. doi: 10.1073/pnas.88.20.8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robzyk K, Recht J, Osley MA. Rad6-dependent ubiquitination of histone H2B in yeast. Science. 2000;287:501–504. doi: 10.1126/science.287.5452.501. [DOI] [PubMed] [Google Scholar]
- 6.Kao CF, Hillyer C, Tsukuda T, Henry K, Berger S, Osley MA. Rad6 plays a role in transcriptional activation through ubiquitylation of histone H2B. Genes Dev. 2004;18:184–195. doi: 10.1101/gad.1149604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyakhovich A, Shekhar MPV. RAD6B overexpression confers chemoresistance: RAD6 expression during cell cycle and its redistribution to chromatin during DNA damage-induced response. Oncogene. 2004;23:3097–3106. doi: 10.1038/sj.onc.1207449. [DOI] [PubMed] [Google Scholar]
- 8.Bailly V, Lamb J, Sung P, Prakash S, Prakash L. Specific complex formation between yeast RAD6 and RAD18 proteins: a potential mechanism for targeting RAD6 ubiquitin-conjugating activity to DNA damage sites. Genes Dev. 1994;8:811–820. doi: 10.1101/gad.8.7.811. [DOI] [PubMed] [Google Scholar]
- 9.Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 10.Shekhar MPV, Lyakhovich A, Visscher DW, Heng H, Kondrat N. Rad6 overexpression induces multinucleation, centrosome amplification, abnormal mitosis, aneuploidy, and transformation. Cancer Res. 2002;62:2115–2124. [PubMed] [Google Scholar]
- 11.Rosner K, Mehregan DR, Kirou E, Abrams J, Kim S, Campbell M, et al. Melanoma Development and Progression Are Associated with Rad6 Upregulation and β-Catenin Relocation to the Cell Membrane. J Skin Cancer. 2014;2014:439205. doi: 10.1155/2014/439205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shekhar MPV, Gerard B, Pauley RJ, Williams BO, Tait L. Rad6B is a positive regulator of beta-catenin stabilization. Cancer Res. 2008;68:1741–1750. doi: 10.1158/0008-5472.CAN-07-2111. [DOI] [PubMed] [Google Scholar]
- 13.Jazaeri AA, Bryant JL, Park H, Li H, Dahiya N, Stoler MH, et al. Molecular requirements for transformation of fallopian tube epithelial cells into serous carcinoma. Neoplasia N Y N. 2011;13:899–911. doi: 10.1593/neo.11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng E, Mitra A, Tripathi K, Finan MA, Scalici J, McClellan S, et al. ALDH1A1 maintains ovarian cancer stem cell-like properties by altered regulation of cell cycle checkpoint and DNA repair network signaling. PloS One. 2014;9:e107142. doi: 10.1371/journal.pone.0107142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen DE, Brzovic PS, Klevit RE. E2-BRCA1 RING interactions dictate synthesis of mono- or specific polyubiquitin chain linkages. Nat Struct Mol Biol. 2007;14:941–948. doi: 10.1038/nsmb1295. [DOI] [PubMed] [Google Scholar]
- 16.Tripathi K, Mani C, Barnett R, Nalluri S, Bachaboina L, Rocconi RP, et al. Gli1 protein regulates the S-phase checkpoint in tumor cells via Bid protein, and its inhibition sensitizes to DNA topoisomerase 1 inhibitors. J Biol Chem. 2014;289:31513–31525. doi: 10.1074/jbc.M114.606483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark DW, Tripathi K, Dorsman JC, Palle K. FANCJ protein is important for the stability of FANCD2/FANCI proteins and protects them from proteasome and caspase-3 dependent degradation. Oncotarget. 2015 doi: 10.18632/oncotarget.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S, Raina K, Agarwal C, Agarwal R. Silibinin strongly inhibits the growth kinetics of colon cancer stem cell-enriched spheroids by modulating interleukin 4/6-mediated survival signals. Oncotarget. 2014;5:4972–4989. doi: 10.18632/oncotarget.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpenter RL, Lo H-W. Hedgehog Pathway and GLI1 Isoforms in Human Cancer. Discov Med. 2012;13:105–113. [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhl SJ, Kuhl M. On the role of Wnt/β-catenin signaling in stem cells. Biochim Biophys Acta BBA - Gen Subj. 2013;1830:2297–2306. doi: 10.1016/j.bbagen.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Espinosa JM. Histone H2B ubiquitination: the cancer connection. Genes Dev. 2008;22:2743–2749. doi: 10.1101/gad.1732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steger DJ, Lefterova MI, Ying L, Stonestrom AJ, Schupp M, Zhuo D, et al. DOT1L/KMT4 Recruitment and H3K79 Methylation Are Ubiquitously Coupled with Gene Transcription in Mammalian Cells. Mol Cell Biol. 2008;28:2825–2839. doi: 10.1128/MCB.02076-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulze JM, Hentrich T, Nakanishi S, Gupta A, Emberly E, Shilatifard A, et al. Splitting the task: Ubp8 and Ubp10 deubiquitinate different cellular pools of H2BK123. Genes Dev. 2011;25:2242–2247. doi: 10.1101/gad.177220.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2013;24(Suppl 6):vi24–32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 25.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. discussion 1895–1896. [DOI] [PubMed] [Google Scholar]
- 26.Ajani JA, Song S, Hochster HS, Steinberg IB. Cancer Stem Cells: The Promise and the Potential. Semin Oncol. 2015;42(Supplement 1):S3–S17. doi: 10.1053/j.seminoncol.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Park HK, Wang H, Zhang J, Datta S, Fei P. Convergence of Rad6/Rad18 and Fanconi Anemia Tumor Suppressor Pathways upon DNA Damage. PLoS ONE. 2010;5:e13313. doi: 10.1371/journal.pone.0013313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palle K, Vaziri C. Rad18 E3 ubiquitin ligase activity mediates Fanconi anemia pathway activation and cell survival following DNA Topoisomerase 1 inhibition. Cell Cycle Georget Tex. 2011;10:1625–1638. doi: 10.4161/cc.10.10.15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.