Abstract
Pycnogenol (PYC) is a patented mix of bioflavonoids with potent anti-oxidant and anti-inflammatory properties. Previously, we showed that PYC administration to rats within hours after a controlled cortical impact (CCI) injury significantly protects against the loss of several synaptic proteins in the hippocampus. Here, we investigated the effects of PYC on CA3-CA1 synaptic function following CCI. Adult Sprague Dawley rats received an ipsilateral CCI injury followed 15 min later by intravenous injection of saline vehicle or PYC (10mg/kg). Hippocampal slices from the injured (ipsilateral) and uninjured (contralateral) hemispheres were prepared at seven and fourteen days post-CCI for electrophysiological analyses of CA3-CA1 synaptic function and induction of long-term depression (LTD). Basal synaptic strength was impaired in slices from the ipsilateral, relative to the contralateral, hemisphere at seven days post-CCI and susceptibility to LTD was enhanced in the ipsilateral hemisphere at both post-injury timepoints. No interhemispheric differences in basal synaptic strength or LTD induction were observed in rats treated with PYC. The results show that PYC preserves synaptic function after CCI and provides further rationale for investigating the use of PYC as a therapeutic in humans suffering from neurotrauma.
Keywords: traumatic brain injury, hippocampus, synaptic transmission, oxidative stress, Pycnogenol®
Introduction
Cognitive loss following traumatic brain injury (TBI) is closely associated with synaptic loss and/or dysfunction (Scheff et al., 2005; Wakade et al., 2010). In experimental models of TBI, synaptic deficits have been especially well-characterized in the hippocampus, a brain region that is critical to the acquisition of contextual and spatial learning. Many commonly reported hippocampal deficits include the loss of synaptic proteins and contacts (Ansari et al., 2008b, 2013; Gao et al., 2011; Kharlamov et al., 2011; Kumar et al., 2002; Scheff et al., 2005; Wakade et al., 2010). posttranslational modification and/or trafficking of glutamate receptors (Bell et al., 2009; Park et al., 2013; Schumann et al., 2008), reduced basal synaptic strength, and/or altered synaptic plasticity (Albensi et al., 2000; D’Ambrosio et al., 1998; Miyazaki et al., 1992; Norris and Scheff, 2009; Sanders et al., 2000; Schwarzbach et al., 2006; Sick et al., 1998). These deficits are not always permanent and can be offset by endogenous reparative mechanisms, leading to the recovery of at least some cognitive functions. Work from our lab, and others, has shown that the loss of synaptic contacts and synaptic strength following TBI is followed by extensive, if not complete, recovery within weeks following injury (Norris and Scheff, 2009; Scheff et al., 2005). These observations have led to extensive investigations into the injury-related mechanisms responsible for suppressing synaptic function and/or delaying synaptic re-wiring.
Oxidative stress and neuroinflammation are two of the most pervasive and destructive processes in central nervous system (CNS) disease and injury (Lin and Beal, 2006). Synapses are particularly vulnerable to both processes (Mattson and Liu, 2002; Sama and Norris, 2013), showing reduced function and/or poorer recovery after acute injury when levels of reactive oxygen/nitrogen species and cytokines are elevated (Ansari et al., 2008b). Oxidative stress and neuroinflammation arise within hours following TBI (Ansari et al., 2008b, 2014) and may persist indefinitely (Loane et al., 2014) leading to an inhospitable environment for establishing new synaptic circuits. Several anti-oxidant/anti-inflammatory compounds, including flavones and flavonoids, have shown promise as an intervention for normalizing brain redox state and promoting neural recovery following TBI (Ansari et al., 2013; Chen et al., 2012; Li et al., 2014; Scheff et al., 2013; Theadom et al., 2013; Wang et al., 2014). Pycnogenol® (PYC) is a patented French maritime pine bark extract containing a complex mixture of bioflavonoids (Packer et al., 1999). PYC has been shown to neutralize reactive oxygen and nitrogen species, reduce pro-inflammatory cytokine levels, and prevent degeneration and apoptosis in a variety of cell types exposed to injurious stimuli (Ansari et al., 2008a; Kobayashi et al., 2000; Liu et al., 2000; Peng et al., 2002; Rong et al., 1994; Scheff et al., 2013). We previously showed that peripheral administration of pycnogenol after controlled cortical impact (CCI) injury ameliorates the loss of both presynaptic and postsynaptic proteins in the cortex and hippocampus (Ansari et al., 2013; Scheff et al., 2013). The synapto-protective effects of PYC were obtained even when therapy was delayed up to four hours following injury (Ansari et al., 2013). Here, we extend those findings to show that PYC also hastens the recovery of CA1 synaptic function following TBI and reduces the susceptibility to long-term synaptic depression (LTD). The results provide further support for the consideration of PYC as a possible treatment for neurotrauma and possibly other CNS disorders.
Materials and methods
Adult male Sprague-Dawley rats (250–275g) (Harlan Laboratories, Indianapolis, IN) were used in this study. Animal protocol and procedures were approved by the Institutional Animal Care and Use Committee of the University of Kentucky. Animals were housed two/cage on a 12 h light/dark cycle and provided access to food and water ad libitum. Rats were subjected to a moderate unilateral controlled cortical impact (CCI) utilizing an electronic controlled pneumatic impact device (ECPI) (TBI0310, Precision System & Instrumentation, Fairfax Station, VA) with a hard stop Bimba cylinder (Bimba Manufacturing, Monee, IL). Each animal was anesthetized with 2% isofluorane and placed in a Kopf stereotaxic frame (Tujunga, CA) with the incisor bar set at −5. Body temperature for each rat was monitored and maintained at 36°C with a Space Gel heating pad (Braintree Scientific, Braintree, MA). Following a midline incision and retraction of the skin, a 6-mm-diameter craniotomy was made lateral to midline and approximately midway between bregma and lamda with a Michele hand trephine (Miltex, NY). The skull disk was removed without disturbing the dura. The exposed brain was injured with the ECPI device using a 5-mm-diameter beveled tip that compressed the cortex to a depth of 2.0 mm at a velocity of 3.5 m/s. Following the injury, the craniotomy site was sealed with MASCOT adhesive (EMS, Hatfield, PA) and an 8mm disc formed from clear polyester (0.5 mm) (Midwest Products, Hobart, IN). The surgical procedure was completed in 15–20 min.
At 15 min post injury, rats received a single intravenous (i.v.) injection of either vehicle (saline) or PYC (10mg/kg) (gift from Horphag Research Inc., USA) and allowed to recover in their home cage. The dosage was based upon therapeutic effects of PYC as previously described (Ansari et al., 2013).
Hippocampal slice preparation
Methods for preparing hippocampal slices from rats are similar to those used in our previous work.(Mathis et al., 2011; Norris et al., 2010; Norris and Scheff, 2009; Sama et al., 2012) At either 7 or 14 days after CCI injury, rats were euthanized under CO2 anesthesia. Brains were then rapidly removed and stored briefly in ice-cold, oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (ACSF) containing (in mM) 124 NaCl, 2 KCl, 1.25 KH2PO4, 2 MgSO4, 0.5 CaCl2, 26 NaHCO3, and 10 dextrose (pH 7.4). Coronal hippocampal slices (450-μm thick) from both hemispheres were cut parallel to the alvear fibers using a McIlwain Tissue Chopper (Stoelting Co., Wood Dale, IL) and transferred to netting in a custom holding chamber.(Mathis et al., 2011) Slices were bathed in oxygenated ACSF (containing 2mM CaCl) at an interface with warm (32°C), humidified air and permitted to equilibrate for at least 1.5h before transfer to an RC-22 recording chamber (Warner Instruments, Hamden, CT) for electrophysiological analysis.
Field potential recordings
Slices were submerged in oxygenated ACSF (32°C) and perfused at a rate of 1–2mL/min. Schaffer collaterals were activated with a bipolar platinum/iridium electrode located in stratum radiatum of CA3-CA1 border. Stimulus intensity was controlled by a constant current stimulus isolation unit (World Precision Instruments, Sarasota, FL), and stimulus timing was controlled by Clampex 9.2 software (Molecular Devices, Sunnyvale, CA). Field potentials were recorded in CA1 stratum radiatum using a glass micropipette (1–6MΩ), filled with ACSF and containing a Ag/AgCl wire. Physiologic activity was amplified 100×, Bessel-filtered at 1kHz, and digitized at 10kHz using a Multiclamp 700B amplifier and a Digidata 1320 digitizer (Molecular Devices).
Fiber excitability and synaptic strength measures
Methods for assessing presynaptic fiber excitability and basal synaptic strength were similar to those used in our previous work.(Furman et al., 2012; Norris and Scheff, 2009; Sama et al., 2012) For each slice, twin stimulus pulses (S1 and S2), separated by 50ms, were delivered at nine different intensity levels (range 30–500μA) at a rate of 0.1Hz. Five field potentials at each stimulus level were averaged, and measurements of fiber volley (FV) amplitude (in mV) and excitatory postsynaptic potential (EPSP) slope (mV/ms) were performed offline using Clampfit software (Molecular Devices). FV amplitudes were plotted against stimulation intensity to estimate the relative level of presynaptic excitability, and EPSP slope measures were plotted against their corresponding FV amplitudes to estimate the relative strength of existing CA3-CA1 synaptic contacts. Fiber excitability and synaptic strength curves for each hemisphere were fit (SigmaPlot 12, Systat Software Inc. San Jose, CA) with a sigmoidal equation of the form:
where a equals the maximal amplitude of the distribution, b equals the distribution slope, x equals the stimulation intensity (or FV amplitude), and x0 equals the stimulation intensity (or FV amplitude) required for half-maximal response amplitude. Maximal synaptic strength for each slice was also estimated by taking the maximal EPSP slope amplitude during the input/output curve and dividing by the corresponding FV amplitude. Paired-pulse facilitation (PPF) was calculated by dividing the S2 EPSP slope by the S1 EPSP and multiplying by 100. To estimate population spike (PS) threshold, the EPSP slope amplitude at which a population spike first appeared in the ascending phase of the field potential was calculated and averaged across five successive trials at the spike threshold stimulation level.
Induction and measurement of long-term depression (LTD)
Methods for inducing and measuring LTD are similar to those used in our previous work (Norris et al., 1996; Sama et al., 2012). Following input/output curves, single stimulus pulses were delivered at a rate of 0.033 Hz to establish a baseline. While the ½ maximal stimulus intensity from the input/output curve is often used for setting the baseline and LTD stimulation levels, we instead used the stimulus intensity necessary to elicit an approximately 1 mV EPSP. This procedure was implemented to minimize possible hemispheric differences in the level of postsynaptic depolarization achieved during LTD induction (see discussion in (Mathis et al., 2011; Norris and Scheff, 2009). It is well-established that the induction of both LTD and long-term potentiation (LTP) are strongly dependent on postsynaptic depolarization levels (Malinow and Miller, 1986; Mulkey and Malenka, 1992; Wigstrom and Gustafsson, 1986). We have previously reported that, relative to the contralateral hemisphere, slices from the ipsilateral hippocampus following TBI typically show reduced EPSP amplitudes (less depolarization) in response to the same level of presynaptic stimulation at 7 days post-CCI (Norris and Scheff, 2009). Using the ½ maximal stimulus intensity in the CCI model would therefore necessarily lead to the elicitation of smaller EPSP amplitudes during LTD stimulation for ipsilateral relative to contralateral slices, which may negatively affect the extent to which postsynaptic plasticity mechanisms are engaged.
After a stable baseline period of no less than 20 min, slices received a 15 minute train of 1 Hz stimulation at baseline stimulation intensity, followed by another 60 min baseline period (stimulation rate = 0.033 Hz). Within each hemisphere, EPSP measures from the last 10 min of the post-1 Hz stimulation baseline were averaged across slices and expressed as a percentage of the pre-1 Hz stimulation baseline level.
Statistics
All statistical analyses were conducted using Prism V6 (GraphPad Software, San Diego, CA). For each animal, electrophysiological measures, including synaptic strength parameters, were averaged across all slices within each hemisphere (one to four slices) and the sample size (n) used for all statistical comparisons reflects the number of animals used per post-injury time point. During LTD experiments, some slices showed excessive upward or downward drift (>20% over the pre or post-1 Hz baseline) and were excluded from statistical analyses without regard to treatment/injury group. Effects of injury and PYC treatment were determined by two-way repeated measures analysis of variance (ANOVA). Where appropriate, the Protected Fisher’s Least Significant Difference test was used for post hoc comparisons. Significance for all statistical comparisons was set at p < 0.05.
RESULTS
PYC treatment facilitates the recovery of synaptic strength following CCI
At seven and fourteen days after CCI, hippocampal slices from both the contra- and ipsilateral hemispheres were prepared from rats receiving a single IV injection of PYC (n = 14) or vehicle (n = 15). Thirty-two rats were used in all (7 day vehicle, n = 8; 7 day PYC, n = 9; 14 day vehicle, n = 7; 14 day PYC, n = 8). In each slice, measures of FV amplitude, EPSP slope amplitude, PPF, and population spike threshold were collected in CA1 stratum radiatum across nine stimulus intensities. These parameters were compared across slices from the contra-(uninjured) and ipsilateral (injured) hemispheres within the same animals. While the contralateral hemisphere may be affected in a variety of ways by a CCI injury (Hall et al., 2005), our previous work (Norris and Scheff, 2009) investigating multiple CA1 electrophysiological parameters (including all of the parameters investigated in the present study), found no differences between contralateral slices from CCI-injured animals and slices (contra- or ipsi-) from sham-operated animals.
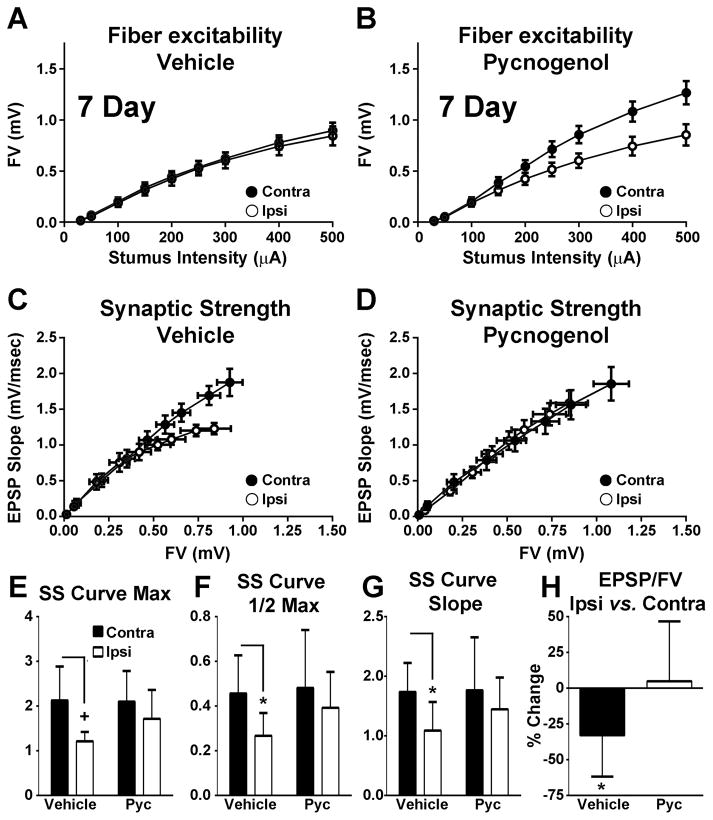
Figure 1 shows FV and synaptic strength curves for rats at 7 day post-CCI. Curves were fitted using 3 parameter Sigmoidal equations to assess curve amplitudes, slopes, and half-maximal amplitude levels (see methods). Though FV curves for slices from the contra- and ipsilateral hemispheres of vehicle-treated rats were very similar (Figure 1A), the curves for PYC-treated rats (Figure 1B) were not. A significant injury effect [F(1, 15) = 7.178; p < 0.05] was found for the FV distribution amplitude (data not shown) attributable in large part to an increase (p < 0.01) in the FV amplitude in the contra- vs. ipsilateral hemisphere of PYC-treated rats. This indicates that PYC may affect FV amplitude in naïve rats. No differences for any FV curve parameters were found for vehicle-treated rats.
Figure 1. Effects of PYC on CA3 axon excitability and CA3-CA1 basal synaptic strength at 7 days post CCI.
(A–B) Input/output curves for mean ± SEM FV amplitudes in CA1 stratum radiatum of hippocampal slices from contralateral (Contra) and ipsilateral (Ipsi) hemispheres of rats treated with vehicle (A, n = 8 rats/group) or PYC (B, n = 9 rats). (C–D) Mean ± SEM EPSP slope measures plotted against FV measures from the same rats shown in A and B. (E–G) Mean ± SD values for synaptic strength (SS) curve parameters shown in C and D: maximum distribution amplitude (E, Max), half maximum activation level (F, 1/2 Max), and distribution slope (G, Slope). (H) Mean ± SD maximum EPSP/FV ratio. +p < 0.01, * p < 0.05 determined using repeated measures ANOVA and post hoc analyses.
In addition to changes in afferent excitability, we also observed qualitative and quantitative differences in synaptic strength across treatment conditions (Figure 1C–H). The average synaptic strength curve for ipsilateral hippocampal slices exhibited a downward shift relative to the contralateral hemisphere in the vehicle group (Figure 1C), but not in the PYC group (Figure 1D). Significant effects of injury were found for all three synaptic strength curve parameters [Figure 1E–G: max amplitude F(1, 14) = 9.94, p < 0.01; half maximal amplitude F(1, 14) = 7.51, p < 0.05; slope F(1, 14) = 8.87, p < 0.01). Post hoc tests showed that curve amplitude, half max amplitude, and slope levels (Figure 1E) were significantly reduced in the ipsilateral hemisphere of vehicle-treated, but not in PYC-treated rats (amplitude p < 0.01; half max p < 0.05; slope p < 0.05).
Though these results are consistent with a protective effect of PYC on synaptic strength, the results may be attributable, instead, to a PYC-mediated increase in afferent excitability (see Figure 1B). To test this possibility, we calculated the maximal EPSP-to-FV ratio for each slice (see Methods). A significant injury X drug interaction [F(1, 15) = 5.617, p < 0.05) showed that the EPSP-to-FV ratio was significantly reduced in the ipsilateral, relative to the contralateral, hemisphere in vehicle-treated (p < 0.05), but not in pycnogenol-treated rats (Figure 1H). These results demonstrate that PYC helps protect against synaptic strength deficits in the ipsilateral hippocampus at 7 days post-CCI, regardless of its effects on afferent excitability.
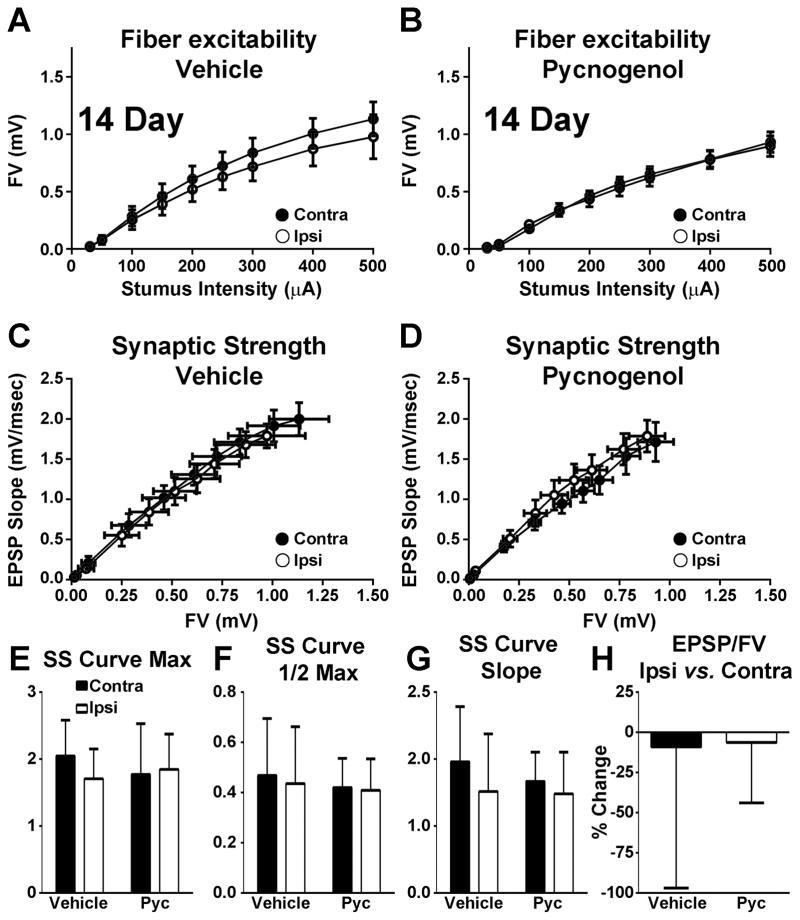
In contrast to the 7 day post-CCI time point, neither injury nor drug treatment appeared to affect afferent excitability and synaptic strength when assessed at 14 days post-CCI (Figure 2). Both FV and synaptic strength curves (Figure 2A–D) were qualitatively similar across hemispheres regardless of drug treatment. Moreover, no differences in any synaptic strength curve parameters were found across injury/drug treatment conditions (Figure 2E–H).
Figure 2. Effects of PYC on CA3 axon excitability and CA3-CA1 basal synaptic strength at 7 days post CCI.
(A–B) Input/output curves for mean ± SEM FV amplitudes in CA1 stratum radiatum of hippocampal slices from contralateral (Contra) and ipsilateral (Ipsi) hemispheres of rats treated with vehicle (A, n = 7 rats/group) or PYC (B, n = 8 rats/group). (C–D) Mean ± SEM EPSP slope measures plotted against FV measures from the same rats shown in A and B. (E–G) Mean ± SD values for synaptic strength (SS) curve parameters shown in C and D: maximum distribution amplitude (E, Max), half maximum activation level (F, 1/2 Max), and distribution slope (G, Slope). (H) Mean ± SD maximum EPSP/FV ratio.
Finally, there were no injury or drug effects observed for PPF or population spike threshold at either post-injury time point (data not shown). These parameters are illustrated in Table 1 and will not be discussed further.
Table 1.
Values for paired pulse facilitation (PPF) and population spike (PS) threshold in hippocampal slices from the contralateral (Contra) and Ipsilateral (Ipsi) hemispheres.
| 7 Days Post-CCI | PPF | PS Threshold |
|---|---|---|
| Vehicle | Contra. 129.9 ± 24.3 Ipsi. 123.1 ± 20.2 |
Contra. 0.49 ± 0.1 Ipsi. 0.36 ± 0.1 |
| PYC | Contra. 124.6 ± 15.2 Inj. 122.9 ± 14.5 |
Contra. 0.60 ± 0.2 Inj. 0.52 ± 0.2 |
| 14 Days Post-CCI | PPF | PS Threshold |
|---|---|---|
| Vehicle | Contra. 134.9 ± 14.3 Ipsi. 133.1 ± 22.7 |
Contra. 0.47 ± 0.3 Ipsi. 0.48 ± 0..2 |
| PYC | Contra. 133.9 ± 16.5 Ipsi. 129.6 ± 18.3 |
Contra. 0.60 ± 0.2 Ipsi. 0.58 ± 0.3 |
All values expressed as mean ± SD.
PYC reduces susceptibility to LTD following TBI
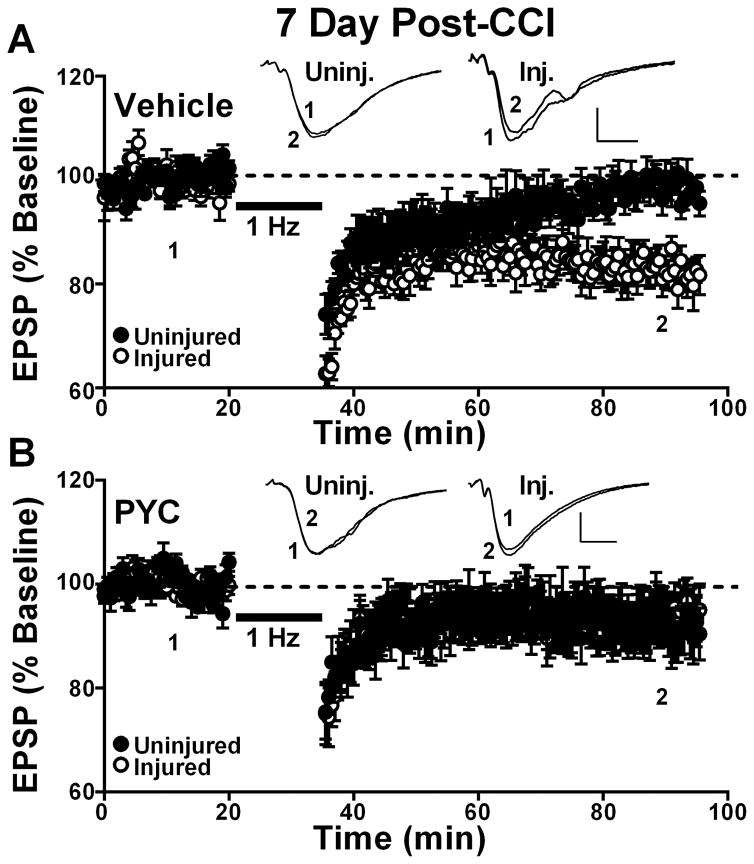
After input/output curves were recorded, the stimulus intensity for some slices was adjusted to yield an EPSP amplitude of approximately 1mV. CA3-CA1 field potentials were then elicited every 30 seconds for a baseline period of no less than 20 minutes, followed by a 15 minute train of 1Hz stimulation (to induce LTD), and another 60 minute baseline period as described previously.(Sama et al., 2012). At the 7 day post-CCI time point, a significant effect of injury [F(1,13) = 4.6, p ≤ 0.05)], as well as an injury x drug treatment interaction [F(1,13) = 4.9, p ≤ 0.05)], were observed. In vehicle-treated rats (Figure 3A), the EPSP slope in slices from the ipsilateral hemisphere was significantly depressed to 82.9 ± 7.4% (mean ± SD) of the initial baseline level (p < 0.05) when measured at 60 min after delivery of 1 Hz stimulation, indicative of LTD (Figure 3A). In contrast, EPSP slope measures in slices from the contralateral hemisphere were nearly identical before and 60 minutes after 1 Hz stimulation (98.5 ± 5.1%, mean ± SD), indicative of no LTD (Figure 3A). Post hoc analysis confirmed that LTD was significantly greater in the ipsilateral relative to the contralateral hemisphere of vehicle-treated rats (p < 0.05). For the PYC group (Figure 3B), no significant LTD was found in either the ipsilateral or contralateral hemisphere following 1 Hz stimulation (ipsilateral 94.2 ± 14.7%; contralateral 92.9 ± 11.9%, mean ± SD).
Figure 3. Effects of PYC on LTD induction in hippocampal CA1 at 7 days post CCI.
Time plots showing mean ± SEM EPSP slope measures collected in CA1 stratum radiatum of uninjured and injured hemispheres before (1) and 60 min after (2) the delivery of a 900 pulse train (1 Hz, bar) to the Schaffer collaterals. Responses are normalized to the pre-1 Hz baseline. Vehicle treated rats (n = 8) are shown in (A) and rats treated with PYC (n = 8) are shown in (B). Insets: representative field potentials averaged over 10 min before (1) and at 60 min after (2) the delivery of 1Hz stimulation. Calibration bars 0.5 mV/5 ms.
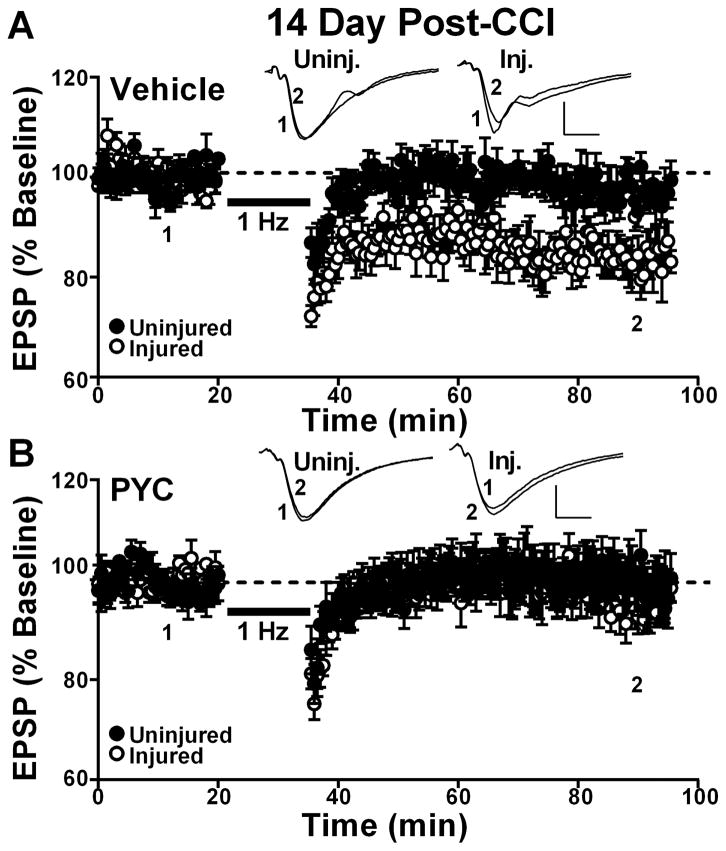
Similar results were observed at the 14 day post-CCI timepoint (Figure 4). ANOVA revealed a significant effect of drug treatment [F(1,9) = 6.2, p ≤ 0.05)] on LTD induction. Post hoc analyses showed that vehicle-treated rats exhibited significant LTD in the ipsilateral (84.4 ± 7.9%) relative to the contralateral hemisphere (97.4 ± 5.1%, mean ± SD) (p < 0.05), while PYC-treated rats showed no differences in LTD across hemispheres (injured 98 ± 9.2%; uninjured 99.3 ± 11.9%, mean ± SD, Figure 4B and 4C). Together, the results demonstrate that PYC reduces susceptibility to LTD within the first two weeks following TBI.
Figure 4. Effects of PYC on LTD induction in hippocampal CA1 at 14 days post CCI.
Time plots showing mean ± SEM EPSP slope measures collected in CA1 stratum radiatum of uninjured and injured hemispheres before (1) and 60 min after (2) the delivery of a 900 pulse train (1 Hz, bar) to the Schaffer collaterals. Responses are normalized to the pre-1 Hz baseline. Vehicle treated rats (n = 7) are shown in (A) and rats treated with PYC (n = 6) are shown in (B). Insets: representative field potentials averaged over 10 min before (1) and at 60 min after (2) the delivery of 1Hz stimulation. Calibration bars 0.5 mV/5 ms.
Discussion
Loss of functional synapses in the hippocampus may account for many of the cognitive deficits exhibited after TBI. We previously showed that following a moderate CCI injury, there is a loss of several key synaptic proteins occurring subsequent to the elevation of oxidative stress markers.(Ansari et al., 2013; Scheff et al., 2013) Post-injury treatment with PYC prevented the loss of synaptic proteins and significantly negated elevation of oxidative stress, suggesting that synapse loss and/or lack of synapse restoration is driven in part by oxidative stress mechanisms. The present findings are in general agreement with these reports and provide the first evidence that PYC not only preserves synaptic protein levels after TBI, but also protects synaptic function.
In addition to damaging neocortical tissue, CCI injury causes the extensive loss of CA3 neurons in the hippocampus (Baldwin et al., 1997) leading to reduced CA3-CA1 synaptic density in CA1 stratum radiatum (Scheff et al., 2005). Consistent with these observations, several groups have reported on the loss and/or the posttranslational modification of glutamatergic receptors (Bell et al., 2009; Park et al., 2013; Schumann et al., 2008), neurotransmitter release machinery (Agrawal et al., 2015; Ansari et al., 2013; Darwish et al., 2012; Griesbach et al., 2008; Scheff et al., 2013; Wu et al., 2006), membrane anchoring proteins (Ansari et al., 2013; Campbell et al., 2012; Scheff et al., 2013; Wakade et al., 2010), and second messenger cascades involved in synaptic plasticity (Bales et al., 2010; Campbell et al., 2012; Schwarzbach et al., 2006; Yan et al., 2014). Functionally, these changes appear as deficits in CA3 fiber volleys (Norris and Scheff, 2009), reduced CA1 synaptic strength, and/or impaired long-term synaptic potentiation (LTP) (Albensi et al., 2000; D’Ambrosio et al., 1998; Miyazaki et al., 1992; Norris and Scheff, 2009; Schwarzbach et al., 2006; Sick et al., 1998). Synaptic alterations at the anatomical, biochemical, and physiological levels are typically most severe within the first few days following a CCI injury, before exhibiting a remarkable degree of recovery. For instance, loss of CA3 axon excitability and LTP deficits observed at two days post-TBI is mostly recovered by 7 days post-CCI (Norris and Scheff, 2009). CA1 synaptic strength also recovers, albeit more slowly, and perhaps less completely with slight deficits still apparent at 7 days (Norris and Scheff, 2009).
Relative to investigations of LTP, fewer studies (Albensi et al., 2000; D’Ambrosio et al., 1998) have investigated alterations in LTD following TBI. Historically, LTD induction using the 1 Hz/900 pulse stimulus protocol has been difficult to achieve in hippocampal slices from healthy adult (> three weeks-old) animals, particularly when the extracellular Ca2+/Mg2+ ratio is at, or is close to one (Dumas, 2012; Foster and Kumar, 2007; Foster and Norris, 1997; Fujii et al., 1991; Norris et al., 1996; O’Dell and Kandel, 1994; Shankar et al., 2008; Vouimba et al., 2000; Wexler and Stanton, 1993). Generally, LTD-induction in slices from healthy adult slices can be greatly enhanced using modifications that presumably increase postsynaptic Ca2+ influx during 1 Hz stimulation, such as higher bath Ca2+ or lower bath Mg2+ levels (Norris et al., 1996), GABA receptor blockers (Wagner and Alger, 1995), or Ca2+ channel activators (Coussens et al., 1997). In some studies where a relatively high bath Ca2+/Mg2+ ratio was used, induction of LTD in slices from adult animals was associated with improved neurologic function, including better cognitive scores (Lee et al., 2005; Liu et al., 2014; Mills et al., 2014). However, other studies using a 1:1 bath Ca2+/Mg2+ ratio have shown that LTD is more closely associated with impaired neurologic function, Ca2+ dysregulation, advanced age, and/or elevated amyloid levels (Chen et al., 2013; Foster and Kumar, 2007; Foy et al., 2008; Norris et al., 1998; Norris et al., 1996b; Shankar et al., 2008; Vouimba et al., 2000). In the present study, we used a 1:1 bath Ca2+/Mg2+ ratio and observed little-to-no LTD in the contralateral uninjured hemisphere of adult rats at either seven or fourteen days post-CCI. In contrast, slices from the injured ipsilateral hemisphere showed significant depression in responspe to 1 Hz stimulation at both post-injury time points (see Figures 3 and 4). These observations suggest that CCI injury increases the susceptibility to LTD induction in the ipsilateral hippocampus of adult rats, perhaps through the disruption of Ca2+ homeostasis and/or the hyperactivation of LTD-related mechanisms, including the protein phosphatase calcineurin (Mulkey et al., 1994). Indeed, several studies have shown that calcineurin is converted to a high-activity proteolytic fragment in neurons following acute nervous system injury (Huang et al., 2005; Mohmmad Abdul et al., 2011; Shioda et al., 2007; Shioda et al., 2006; Wu et al., 2004). That LTD was found in the ipsilateral hippocampus at 14 days post-CCI, even though synaptic strength deficits were largely recovered at this time point, suggests that susceptibility to LTD may persist long after injury and contribute to lingering cognitive deficits. A full post-injury time course for LTD changes will be addressed in future studies.
Like many other neuronal compartments, synapses are highly sensitive to redox state. Oxidative stress has been shown to disrupt synaptic structure, function, and plasticity and may be a primary mechanism for synaptic deficits found in animal models of aging, injury, and disease.(Mattson and Liu, 2002) These deficits may arise from the direct oxidation of key synaptic proteins, or indirectly from damage to mitochondrial function and/or to damage-induced elevations in harmful neuroinflammatory mediators. The secondary injury cascade associated with TBI involves an increase in the levels of numerous free radical species as well as a reduction in the levels of several key anti-oxidants (Ansari et al., 2008b). Reduction of oxidative stress levels using a variety of different flavonoids has been shown to reduce neuronal damage and neuroinflammation and improve cognitive function (Spencer, 2008, 2009; Williams and Spencer, 2012). The appeal of flavonoids as therapeutic agents for neurodegenerative conditions is based largely on their relative safety, their ability to traverse the blood-brain barrier, and their combinatorial impact on multiple molecular targets.
PYC is a patented blend of multiple bioflavonoids including polymeric catechin and epicatechin, caffeic acid, ferulic acid, and taxifolin, which independently have been reported to impart neuroprotection (Rohdewald, 2002). Similar to other flavonoids, PYC exhibits powerful free radical scavenging properties (Nelson et al., 1998) and favorably affects the viability of neuronal and vascular endothelial cells (Ansari et al., 2008a; Liu et al., 2000; Rezzani et al., 2010). In our previous work (Ansari et al., 2013; Scheff et al., 2013), PYC administration (i.p. or i.v) to rats following CCI was associated with elevated levels of antioxidants (e.g. glutathione and glutathione peroxidase) shown by others to improve synaptic function/plasticity (Furling et al., 2011; Robillard et al., 2011), while concurrently reducing pro-inflammatory cytokines (e.g. IL-6 and TNFα) linked to synaptic impairments (Oda et al., 2000; Tancredi et al., 2000; Pickering and O’Connor, 2007; Sama et al., 2012). Consistent with these findings, we have shown that PYC largely prevents the loss of pre- and post-synaptic proteins (Ansari et al., 2013; Scheff et al., 2013), prevents deficits in synaptic strength (Figure 1), and reduces the susceptibility to synaptic depression (Figure 3,4) in ipsilateral hippocampus following CCI injury. Together, our present and previous work suggests that PYC improves and/or protects the structure and functional integrity of synapses by suppressing the harmful actions of free radicals and inflammatory cytokines. Of course, the role(s) of oxidative stress and neuroinflammation in synaptic function is highly complex (Watson et al., 2006; del Rey et al., 2013) and the molecular targets of PYC are many and diverse (Maimoona et al., 2011). Pinpointing the exact mechanisms coupling PYC to synapto-protection will therefore be a major challenge requiring extensive additional research.
While the specific mechanisms of action remain unsettled, the present results provide further support for the translational potential of PYC. Perhaps most remarkably, we observed beneficial effects of PYC after only a single peripheral administration, even when given up to four hours post-CCI (Ansari et al., 2013). But, before PYC is ready for testing in the clinic, additional studies will be necessary to work-out key treatment parameters including the plasma/CSF levels of PYC and the duration of its effects following a single administration. In addition, it will be important to determine if multiple injections, given at “critical” periods following injury, are of greater benefit than a single dose. Regardless, the present experimental results offer proof of concept that post-injury treatment with PYC is a feasible approach to promoting functional recovery. Together with previous placebo-controlled trials showing that PYC is well-tolerated and exerts beneficial effects on lipid profiles, oxidative stress markers, and cognition (Ryan et al., 2008), our work provides important precedence for investigating the effects of PYC in humans suffering from neurotrauma.
Highlights.
Effects of Pycnogenol® on synaptic function in brain injured rats were examined
Pycnogenol® protected synaptic strength and prevented synaptic depression after TBI
Pycnogenol® treatment in humans may help promote recovery from CNS injuries
Acknowledgments
Work supported by grants from the Kentucky Spinal Cord and Head Injury Research Trust Fund (12-16A to SWS and 12-10A to CMN). Special thanks to Erin Abner, Richard J. Kryscio, and Frederick Schmitt of the Sanders-Brown Center on Aging for help on statistical analyses.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- CCI
controlled cortical impact
- CNS
central nervous system
- LTD
long-term depression
- PYC
pycnogenol
- TBI
traumatic brain injury
- FV
fiber volley
- EPSP
excitatory postsynaptic potential
- PPF
paired-pulse facilitation
- PS
population spike
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal R, Noble E, Tyagi E, Zhuang Y, Ying Z, Gomez-Pinilla F. Flavonoid derivative 7,8-DHF attenuates TBI pathology via TrkB activation. Biochim Biophys Acta. 2015;1852:862–872. doi: 10.1016/j.bbadis.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albensi BC, Sullivan PG, Thompson MB, Scheff SW, Mattson MP. Cyclosporin ameliorates traumatic brain-injury-induced alterations of hippocampal synaptic plasticity. Exp Neurol. 2000;162:385–389. doi: 10.1006/exnr.1999.7338. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Keller JN, Scheff SW. Protective effect of Pycnogenol in human neuroblastoma SH-SY5Y cells following acrolein-induced cytotoxicity. Free Radic Biol Med. 2008a;45:1510–1519. doi: 10.1016/j.freeradbiomed.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med. 2008b;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MA, Roberts KN, Scheff SW. Dose- and Time-Dependent Neuroprotective Effects of Pycnogenol (R) following Traumatic Brain Injury. J Neurotrauma. 2013;30:1542–1549. doi: 10.1089/neu.2013.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MA, Roberts KN, Scheff SW. A time course of NADPH-oxidase up- regulation and endothelial nitric oxide synthase activation in the hippocampus following neurotrauma. Free Radic Biol Med. 2014;77:21–29. doi: 10.1016/j.freeradbiomed.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin SA, Gibson T, Callihan CT, Sullivan PG, Palmer E, Scheff SW. Neuronal cell loss in the CA3 subfield of the hippocampus following cortical contusion utilizing the optical disector method for cell counting. J Neurotrauma. 1997;14:385–398. doi: 10.1089/neu.1997.14.385. [DOI] [PubMed] [Google Scholar]
- Bales JW, Ma X, Yan HQ, Jenkins LW, Dixon CE. Expression of protein phosphatase 2B (calcineurin) subunit A isoforms in rat hippocampus after traumatic brain injury. J Neurotrauma. 2010;27:109–120. doi: 10.1089/neu.2009.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JD, Park E, Ai J, Baker AJ. PICK1-mediated GluR2 endocytosis contributes to cellular injury after neuronal trauma. Cell Death Differ. 2009;16:1665–1680. doi: 10.1038/cdd.2009.106. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Low B, Kurz JE, Patel SS, Young MT, Churn SB. Mechanisms of dendritic spine remodeling in a rat model of traumatic brain injury. J Neurotrauma. 2012;29:218–234. doi: 10.1089/neu.2011.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Hung TH, Wang YH, Lin CW, Wang PY, Lee CY, Chen SF. Wogonin improves histological and functional outcomes, and reduces activation of TLR4/NF-kappaB signaling after experimental traumatic brain injury. PLoS One. 2012;7:e30294. doi: 10.1371/journal.pone.0030294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Lin R, Chang L, Xu S, Wei X, Zhang J, Wang C, Anwyl R, Wang Q. Enhancement of long-term depression by soluble amyloid beta protein in rat hippocampus is mediated by metabotropic glutamate receptor and involves activation of p38MAPK, STEP and caspase-3. Neuroscience. 2013;253:435–443. doi: 10.1016/j.neuroscience.2013.08.054. [DOI] [PubMed] [Google Scholar]
- Coussens CM, Kerr DS, Abraham WC. Glucocorticoid receptor activation lowers the threshold for NMDA-receptor-dependent homosynaptic long-term depression in the hippocampus through activation of voltage-dependent calcium channels. J Neurophysiol. 1997;78:1–9. doi: 10.1152/jn.1997.78.1.1. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Selective loss of hippocampal long-term potentiation, but not depression, following fluid percussion injury. Brain Res. 1998;786:64–79. doi: 10.1016/s0006-8993(97)01412-1. [DOI] [PubMed] [Google Scholar]
- Darwish H, Mahmood A, Schallert T, Chopp M, Therrien B. Mild traumatic brain injury (MTBI) leads to spatial learning deficits. Brain Inj. 2012;26:151–165. doi: 10.3109/02699052.2011.635362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas TC. Postnatal alterations in induction threshold and expression magnitude of long-term potentiation and long-term depression at hippocampal synapses. Hippocampus. 2012;22:188–199. doi: 10.1002/hipo.20881. [DOI] [PubMed] [Google Scholar]
- Foster TC, Kumar A. Susceptibility to induction of long-term depression is associated with impaired memory in aged Fischer 344 rats. Neurobiol Learn Mem. 2007;87:522–535. doi: 10.1016/j.nlm.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Norris CM. Age-associated changes in Ca(2+)-dependent processes: relation to hippocampal synaptic plasticity. Hippocampus. 1997;7:602–612. doi: 10.1002/(SICI)1098-1063(1997)7:6<602::AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Foy MR, Baudry M, Foy JG, Thompson RF. 17beta-estradiol modifies stress-induced and age-related changes in hippocampal synaptic plasticity. Behav Neurosci. 2008;122:301–309. doi: 10.1037/0735-7044.122.2.301. [DOI] [PubMed] [Google Scholar]
- Fujii S, Saito K, Miyakawa H, Ito K, Kato H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Res. 1991;555:112–122. doi: 10.1016/0006-8993(91)90867-u. [DOI] [PubMed] [Google Scholar]
- Furling D, Ghribi O, Lahsaini A, Mirault ME, Massicotte G. Impairment of synaptic transmission by transient hypoxia in hippocampal slices: improved recovery in glutathione peroxidase transgenic mice. Proc Natl Acad Sci U S A. 2000;97:4351–4356. doi: 10.1073/pnas.060574597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD, Van Eldik LJ, Norris CM. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J Neurosci. 2012;32:16129–16140. doi: 10.1523/JNEUROSCI.2323-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Deng P, Xu ZC, Chen J. Moderate traumatic brain injury causes acute dendritic and synaptic degeneration in the hippocampal dentate gyrus. PLoS One. 2011;6:e24566. doi: 10.1371/journal.pone.0024566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Gomez-Pinilla F, Sutton RL. Voluntary exercise or amphetamine treatment, but not the combination, increases hippocampal brain-derived neurotrophic factor and synapsin I following cortical contusion injury in rats. Neuroscience. 2008;154:530–540. doi: 10.1016/j.neuroscience.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, Scheff SW. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Huang W, Fileta JB, Dobberfuhl A, Filippopolous T, Guo Y, Kwon G, Grosskreutz CL. Calcineurin cleavage is triggered by elevated intraocular pressure, and calcineurin inhibition blocks retinal ganglion cell death in experimental glaucoma. Proc Natl Acad Sci U S A. 2005;102:12242–12247. doi: 10.1073/pnas.0505138102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharlamov EA, Lepsveridze E, Meparishvili M, Solomonia RO, Lu B, Miller ER, Kelly KM, Mtchedlishvili Z. Alterations of GABA(A) and glutamate receptor subunits and heat shock protein in rat hippocampus following traumatic brain injury and in posttraumatic epilepsy. Epilepsy Res. 2011;95:20–34. doi: 10.1016/j.eplepsyres.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Kobayashi MS, Han D, Packer L. Antioxidants and herbal extracts protect HT-4 neuronal cells against glutamate-induced cytotoxicity. Free Radic Res. 2000;32:115–124. doi: 10.1080/10715760000300121. [DOI] [PubMed] [Google Scholar]
- Kumar A, Zou L, Yuan X, Long Y, Yang K. N-methyl-D-aspartate receptors: transient loss of NR1/NR2A/NR2B subunits after traumatic brain injury in a rodent model. J Neurosci Res. 2002;67:781–786. doi: 10.1002/jnr.10181. [DOI] [PubMed] [Google Scholar]
- Lee HK, Min SS, Gallagher M, Kirkwood A. NMDA receptor-independent long-term depression correlates with successful aging in rats. Nat Neurosci. 2005;8:1657–1659. doi: 10.1038/nn1586. [DOI] [PubMed] [Google Scholar]
- Li Z, Dong X, Zhang J, Zeng G, Zhao H, Liu Y, Qiu R, Mo L, Ye Y. Formononetin protects TBI rats against neurological lesions and the underlying mechanism. J Neurol Sci. 2014;338:112–117. doi: 10.1016/j.jns.2013.12.027. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu F, Lau BH, Peng Q, Shah V. Pycnogenol protects vascular endothelial cells from beta-amyloid-induced injury. Biol Pharm Bull. 2000;23:735–737. doi: 10.1248/bpb.23.735. [DOI] [PubMed] [Google Scholar]
- Liu X, Gu QH, Duan K, Li Z. NMDA receptor-dependent LTD is required for consolidation but not acquisition of fear memory. J Neurosci. 2014;34:8741–8748. doi: 10.1523/JNEUROSCI.2752-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Kumar A, Stoica BA, Cabatbat R, Faden AI. Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J Neuropathol Exp Neurol. 2014;73:14–29. doi: 10.1097/NEN.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Miller JP. Postsynaptic hyperpolarization during conditioning reversibly blocks induction of long-term potentiation. Nature. 1986;320:529–530. doi: 10.1038/320529a0. [DOI] [PubMed] [Google Scholar]
- Mathis DM, Furman JL, Norris CM. Preparation of Acute Hippocampal Slices from Rats and Transgenic Mice for the Study of Synaptic Alterations during Aging and Amyloid Pathology. J Vis Exp. 2011 doi: 10.3791/2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Liu D. Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromolecular Med. 2002;2:215–231. doi: 10.1385/NMM:2:2:215. [DOI] [PubMed] [Google Scholar]
- Mills F, Bartlett TE, Dissing-Olesen L, Wisniewska MB, Kuznicki J, Macvicar BA, Wang YT, Bamji SX. Cognitive flexibility and long-term depression (LTD) are impaired following beta-catenin stabilization in vivo. Proc Natl Acad Sci U S A. 2014;111:8631–8636. doi: 10.1073/pnas.1404670111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S, Katayama Y, Lyeth BG, Jenkins LW, DeWitt DS, Goldberg SJ, Newlon PG, Hayes RL. Enduring suppression of hippocampal long- term potentiation following traumatic brain injury in rat. Brain Res. 1992;585:335–339. doi: 10.1016/0006-8993(92)91232-4. [DOI] [PubMed] [Google Scholar]
- Mohmmad Abdul H, Baig I, Levine H, 3rd, Guttmann RP, Norris CM. Proteolysis of calcineurin is increased in human hippocampus during mild cognitive impairment and is stimulated by oligomeric Abeta in primary cell culture. Aging Cell. 2011;10:103–113. doi: 10.1111/j.1474-9726.2010.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Lau BH, Ide N, Rong Y. Pycnogenol inhibits macrophage oxidative burst, lipoprotein oxidation, and hydroxyl radical-induced DNA damage. Drug Dev Ind Pharm. 1998;24:139–144. doi: 10.3109/03639049809085598. [DOI] [PubMed] [Google Scholar]
- Norris CM, Blalock EM, Chen KC, Porter NM, Thibault O, Kraner SD, Landfield PW. Hippocampal ‘zipper’ slice studies reveal a necessary role for calcineurin in the increased activity of L-type Ca(2+) channels with aging. Neurobiol Aging. 2010;31:328–338. doi: 10.1016/j.neurobiolaging.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J Neurosci. 1998;18:3171–3179. doi: 10.1523/JNEUROSCI.18-09-03171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long- term potentiation reversal during aging. J Neurosci. 1996;16:5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Scheff SW. Recovery of afferent function and synaptic strength in hippocampal CA1 following traumatic brain injury. J Neurotrauma. 2009;26:2269–2278. doi: 10.1089/neu.2009.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda S, Hori T, Oomura Y. Interleukin-6 inhibits long-term potentiation in rat hippocampal slices. Li AJ, Katafuchi T, Brain Res. 2000;748:30–38. doi: 10.1016/s0006-8993(96)01283-8. [DOI] [PubMed] [Google Scholar]
- O’Dell TJ, Kandel ER. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learn Mem. 1994;1:129–139. [PubMed] [Google Scholar]
- Packer L, Rimbach G, Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, pycnogenol. Free Radic Biol Med. 1999;27:704–724. doi: 10.1016/s0891-5849(99)00090-8. [DOI] [PubMed] [Google Scholar]
- Park Y, Luo T, Zhang F, Liu C, Bramlett HM, Dietrich WD, Hu B. Downregulation of Src-kinase and glutamate-receptor phosphorylation after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1642–1649. doi: 10.1038/jcbfm.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng QL, Buz’Zard AR, Lau BH. Pycnogenol protects neurons from amyloid-beta peptide-induced apoptosis. Brain Res Mol Brain Res. 2002;104:55–65. doi: 10.1016/s0169-328x(02)00263-2. [DOI] [PubMed] [Google Scholar]
- Pickering M, O’Connor JJ. Pro-inflammatory cytokines and their effects in the dentate gyrus. Prog Brain Res. 2007;163:339–354. doi: 10.1016/S0079-6123(07)63020-9. [DOI] [PubMed] [Google Scholar]
- Rezzani R, Porteri E, De Ciuceis C, Bonomini F, Rodella LF, Paiardi S, Boari GE, Platto C, Pilu A, Avanzi D, Rizzoni D, Agabiti Rosei E. Effects of melatonin and Pycnogenol on small artery structure and function in spontaneously hypertensive rats. Hypertension. 2010;55:1373–1380. doi: 10.1161/HYPERTENSIONAHA.109.148254. [DOI] [PubMed] [Google Scholar]
- Robillard JM, Gordon GR, Choi HB, Christie BR, MacVicar BA. Glutathione restores the mechanism of synaptic plasticity in aged mice to that of the adult. PLoS One. 2011;6:e20676. doi: 10.1371/journal.pone.0020676. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. 2002;40:158–168. doi: 10.5414/cpp40158. [DOI] [PubMed] [Google Scholar]
- Rong Y, Li L, Shah V, Lau BH. Pycnogenol protects vascular endothelial cells from t-butyl hydroperoxide induced oxidant injury. Biotechnol Ther. 1994;5:117–126. [PubMed] [Google Scholar]
- Ryan J, Croft K, Mori T, Wesnes K, Spong J, Downey L, Kure C, Lloyd J, Stough C. An examination of the effects of the antioxidant Pycnogenol on cognitive performance, serum lipid profile, endocrinological and oxidative stress biomarkers in an elderly population. J Psychopharmacol. 2008;22:553–562. doi: 10.1177/0269881108091584. [DOI] [PubMed] [Google Scholar]
- Sama DM, Mohmmad Abdul H, Furman JL, Artiushin IA, Szymkowski DE, Scheff SW, Norris CM. Inhibition of soluble tumor necrosis factor ameliorates synaptic alterations and Ca2+ dysregulation in aged rats. PLoS ONE. 2012;7:e38170. doi: 10.1371/journal.pone.0038170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sama DM, Norris CM. Calcium dysregulation and neuroinflammation: discrete and integrated mechanisms for age-related synaptic dysfunction. Ageing Res Rev. 2013;12:982–995. doi: 10.1016/j.arr.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Sick TJ, Perez-Pinzon MA, Dietrich WD, Green EJ. Chronic failure in the maintenance of long-term potentiation following fluid percussion injury in the rat. Brain Res. 2000;861:69–76. doi: 10.1016/s0006-8993(00)01986-7. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Ansari MA, Roberts KN. Neuroprotective effect of Pycnogenol(R) following traumatic brain injury. Exp Neurol. 2013;239:183–191. doi: 10.1016/j.expneurol.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Hicks RR, Baldwin SA, Robinson S, Brackney C. Synaptogenesis in the hippocampal CA1 field following traumatic brain injury. J Neurotrauma. 2005;22:719–732. doi: 10.1089/neu.2005.22.719. [DOI] [PubMed] [Google Scholar]
- Schumann J, Alexandrovich GA, Biegon A, Yaka R. Inhibition of NR2B phosphorylation restores alterations in NMDA receptor expression and improves functional recovery following traumatic brain injury in mice. J Neurotrauma. 2008;25:945–957. doi: 10.1089/neu.2008.0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbach E, Bonislawski DP, Xiong G, Cohen AS. Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus. 2006;16:541–550. doi: 10.1002/hipo.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda N, Han F, Moriguchi S, Fukunaga K. Constitutively active calcineurin mediates delayed neuronal death through Fas-ligand expression via activation of NFAT and FKHR transcriptional activities in mouse brain ischemia. J Neurochem. 2007;102:1506–1517. doi: 10.1111/j.1471-4159.2007.04600.x. [DOI] [PubMed] [Google Scholar]
- Shioda N, Moriguchi S, Shirasaki Y, Fukunaga K. Generation of constitutively active calcineurin by calpain contributes to delayed neuronal death following mouse brain ischemia. J Neurochem. 2006;98:310–320. doi: 10.1111/j.1471-4159.2006.03874.x. [DOI] [PubMed] [Google Scholar]
- Sick TJ, Perez-Pinzon MA, Feng ZZ. Impaired expression of long-term potentiation in hippocampal slices 4 and 48 h following mild fluid-percussion brain injury in vivo. Brain Res. 1998;785:287–292. doi: 10.1016/s0006-8993(97)01418-2. [DOI] [PubMed] [Google Scholar]
- Spencer JP. Flavonoids: modulators of brain function? Br J Nutr. 2008;99(E Suppl 1):ES60–77. doi: 10.1017/S0007114508965776. [DOI] [PubMed] [Google Scholar]
- Spencer JP. The impact of flavonoids on memory: physiological and molecular considerations. Chem Soc Rev. 2009;38:1152–1161. doi: 10.1039/b800422f. [DOI] [PubMed] [Google Scholar]
- Tancredi V, D’Antuono M, Cafè C, Giovedì S, Buè MC, D’Arcangelo G, Onofri F, Benfenati F. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. J Neurochem. 2000;75:634–643. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- Theadom A, Mahon S, Barker-Collo S, McPherson K, Rush E, Vandal AC, Feigin VL. Enzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCT. Eur J Neurol. 2013;20:1135–1144. doi: 10.1111/ene.12099. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Foy MR, Foy JG, Thompson RF. 17beta-estradiol suppresses expression of long-term depression in aged rats. Brain Res Bull. 2000;53:783–787. doi: 10.1016/s0361-9230(00)00377-4. [DOI] [PubMed] [Google Scholar]
- Wagner JJ, Alger BE. GABAergic and developmental influences on homosynaptic LTD and depotentiation in rat hippocampus. J Neurosci. 1995;15:1577–1586. doi: 10.1523/JNEUROSCI.15-02-01577.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade C, Sukumari-Ramesh S, Laird MD, Dhandapani KM, Vender JR. Delayed reduction in hippocampal postsynaptic density protein-95 expression temporally correlates with cognitive dysfunction following controlled cortical impact in mice. J Neurosurg. 2010;113:1195–1201. doi: 10.3171/2010.3.JNS091212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Wang HD, Cong ZX, Zhou XM, Xu JG, Jia Y, Ding Y. Puerarin ameliorates oxidative stress in a rodent model of traumatic brain injury. J Surg Res. 2014;186:328–337. doi: 10.1016/j.jss.2013.08.027. [DOI] [PubMed] [Google Scholar]
- Wexler EM, Stanton PK. Priming of homosynaptic long-term depression in hippocampus by previous synaptic activity. Neuroreport. 1993;4:591–594. doi: 10.1097/00001756-199305000-00034. [DOI] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. Postsynaptic control of hippocampal long-term potentiation. J Physiol (Paris) 1986;81:228–236. [PubMed] [Google Scholar]
- Williams RJ, Spencer JP. Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic Biol Med. 2012;52:35–45. doi: 10.1016/j.freeradbiomed.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Wu HY, Tomizawa K, Oda Y, Wei FY, Lu YF, Matsushita M, Li ST, Moriwaki A, Matsui H. Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration. J Biol Chem. 2004;279:4929–4940. doi: 10.1074/jbc.M309767200. [DOI] [PubMed] [Google Scholar]
- Yan HQ, Shin SS, Ma X, Li Y, Dixon CE. Differential effect of traumatic brain injury on the nuclear factor of activated T Cells C3 and C4 isoforms in the rat hippocampus. Brain Res. 2014;1548:63–72. doi: 10.1016/j.brainres.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]