Abstract
Heterotopic pancreatic parenchyma recapitulates the normal pancreas in extra-pancreatic locations and on rare occasions can even give rise to pancreatic adenocarcinoma. The genetic signatures of pancreatic adenocarcinoma and its precursor lesions are well characterized. We explored the genetic alterations in precursor lesions (intraductal papillary mucinous neoplasms [IPMN], pancreatic intraepithelial neoplasia [PanIN]) in patients with pancreatic heterotopias but without concomitant pancreatic ductal adenocarcinomas. This allowed us to determine whether the stereotypical dysplasia—infiltrating carcinoma sequence also occurs in these extra-pancreatic foci. Seven cases of heterotopic pancreas with ductal precursor lesions were identified. These included two IPMNs with focal high grade dysplasia and five PanINs with low to moderate grade dysplasia (PanIN grades 1–2). Neoplastic epithelium was micro-dissected and genomic DNA was extracted. Sequencing of commonly mutated hotspots (KRAS, TP53, CDKN2A, SMAD4, BRAF, and GNAS) in pancreatic ductal adenocarcinoma and its precursor lesions was performed. Both IPMNs were found to have KRAS codon 12 mutations. The identification of KRAS mutations suggests a genetic pathway shared with IPMN of the pancreas. No mutations were identified in our heterotopic PanINs. One of the possible mechanisms for the development of dysplasia in these lesions is field effect. At the time of these resections there was no clinical or pathologic evidence of a prior or concomitant pancreatic lesion. However, a clinically undetectable lesion is theoretically possible. Therefore, while a field effect cannot be excluded, there was no evidence for it in this study.
Keywords: incipient IPMN, IPMN, PanIN, Meckel’s diverticulum, KRAS
1. Introduction
Invasive pancreatic ductal adenocarcinoma arises from a few distinct precursor lesions including intraductal papillary mucinous neoplasm (IPMN), pancreatic intraepithelial neoplasia (PanIN), mucinous cystic neoplasm, and intraductal tubulopapillary neoplasm (ITPN). The genetics of invasive pancreatic ductal adenocarcinoma are well described, with mutations correlating with different stages of disease and different morphologic entities [1]. Studies have supported a stepwise progression from precursor lesions to invasive pancreatic adenocarcinoma with an activating mutation in codon 12 of KRAS being a common and early event. The other most commonly mutated genes, which increase in number and prevalence (or frequency) with higher grades of dysplasia, are TP53, p16/CDKN2A, and SMAD4 [2, 3]. The IPMN precursor pathway overlaps with the PanIN precursor pathway with some significant differences. Whereas KRAS, GNAS, and RNF43 are frequently mutated in IPMN, GNAS is rarely mutated in PanIN lesions [4]. In addition, the prevalence of certain mutations in IPMN also associates with the different histologic subtypes. For example, GNAS mutations are more common in the intestinal subtype (almost 100%) than in the pancreaticobiliary (71%) and gastric subtypes (51%) of IPMNs [3, 5–8]. ITPN seems to follow yet a different molecular pathway, with PIK3CA targeted more commonly, and KRAS less frequently [9, 10].
Precursor lesions morphologically identical to IPMN and PanIN in the pancreas have occasionally been reported in heterotopic pancreas [11, 12]. Only one other study has examined the molecular changes associated with these lesions [11] and all of those lesions were identified in patients with concomitant pancreatic cancer. Our study is the first and largest study to examine these lesions in the absence of a known pancreatic cancer. We identified 7 cases of heterotopic pancreas with varying degrees of dysplasia in IPMN and PanIN lesions in our consultation files. Invasive carcinoma was absent in all cases. The purpose of this study was to examine these cases for mutations in a set of tumor suppressor genes and oncogenes commonly targeted in invasive pancreatic cancer, and to compare these changes to the well-characterized mutational profile of precursor lesions arising in the pancreas. In addition, the immunohistochemical expression of mucin was also evaluated in the IPMN cases.
2. Materials and Methods
2.1. Patient and Tissues
Seven cases of heterotopic pancreas with precursor lesions (ductal epithelial dysplasia) were identified from the consultation files of one of us (RHH). The study was conducted with the approval of the Johns Hopkins Medicine Institutional Review Board.
2.2. Histology
All of the cases were reviewed and the dysplasia was categorized and graded by an expert in pancreatic pathology (RHH) using the most recent World Health Organization guidelines [13]. Briefly, PanIN was defined as small, usually < 5mm, non-invasive, flat or papillary intraductal epithelial lesions. PanIN lesions were evaluated for the degree of cytologic and architecture atypia and were divided into 3 grades: low grade including PanIN1A and 1B: 1A is characterized by flat basally placed nuclei with abundant supranuclear cytoplasm, and PanIN 1B lesions have a papillary or micropapillary architecture with pseudostratified nuclei; intermediate-grade: PanIN 2 are predominantly papillary with accompanying nuclear abnormalities consisting of loss of polarity, nuclear crowding, enlarged nuclei, pseudostratification, and hyperchromasia; and high-grade lesions: PanIN 3 with cribriforming, micropapillary, and lumenal shedding or budding of small clusters of epithelial cells in association with nuclear changes consisting of dystrophic goblet cells, loss of nuclear polarity, mitoses, prominent nucleoli, and necrosis. IPMN was defined as a grossly visible intraductal mucin-producing cystic tumor forming papillary projections and measuring ≥ 1 cm in greatest dimension. IPMNs typically have longer papillae than PanINs. IPMN can also be classified into low-grade, intermediate-grade, and high-grade dysplasia. For similar appearing smaller lesions (0.5 to 1.0 cm), the term incipient IPMN has been used [14–16].
2.3. Sequencing
Dysplastic epithelium in each case was manually micro-dissected and genomic DNA was extracted as described [17]. DNA sequencing of the frequently mutated regions of 50 cancer-related genes using the AmpliSeq Cancer Hotspot panel v.2 on the Ion Torrent PGM (Life Technologies, Inc, San Diego, CA) was performed as previously described [18]. This panel includes genes commonly mutated in pancreatic ductal adenocarcinoma and precursor lesions (KRAS, TP53, CDKN2A, SMAD4, BRAF, and GNAS). RNF43 is not included in this panel.
3. Results
3.1. Clinicopathologic Parameters
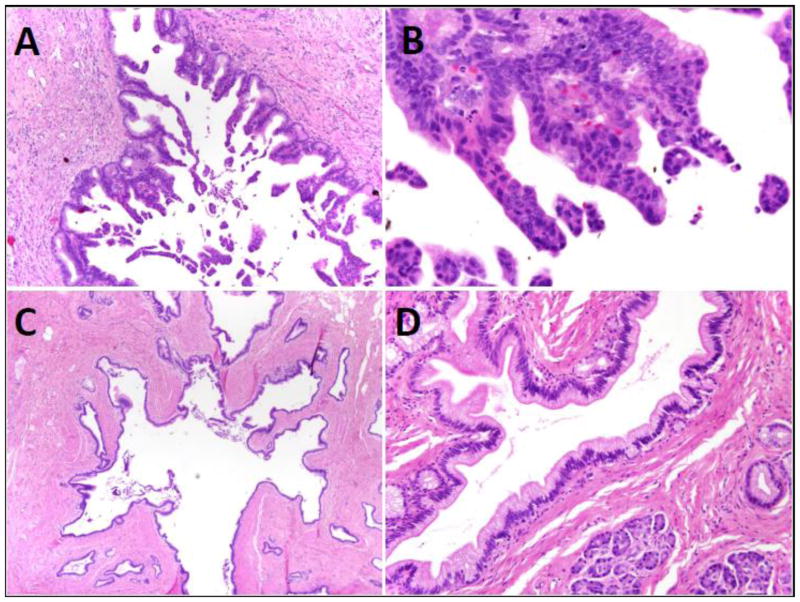
Detailed patient information is provided in Table 1. Precursor lesions in heterotopic foci were identified in 7 patients, 6 male and 1 female, with ages ranging from 15 to 82. Five of the lesions were incidentally identified following surgery for other lesions (Meckel’s diverticula in cases 1–3 and a gastrointestinal stromal tumor in case 6 and small bowel intussusception in case 7) and 2 were removed due to symptoms. In all of the cases both pancreatic acinar tissue and ducts were present. Islets were present in only one case (case 7). Both of the cases of IPMN showed minimal acute and chronic inflammation. Case 4 had a PanIN2 lesion with associated chronic inflammation involving the heterotopic pancreatic tissue. Inflammation was absent in all other cases with PanIN (cases 3, 5, 6, 7). Two of the cases of Meckel’s diverticula were found to have IPMNs with focal high-grade dysplasia arising in heterotopic pancreatic parenchyma. Both were of the gastric-foveolar type. One was <1 cm (0.9cm) and therefore classified as an incipient IPMN. Five cases of PanIN with grades ranging from 1 to 2 were identified, one in a Meckel’s diverticulum, one in the small bowel causing an intussusception, and 3 in the stomach (Figure 1). None of the patients had a history of co-existing pancreatic ductal adenocarcinoma.
Table 1.
Demographic, clinicopathologic, and genetic findings of 6 patients with ductal epithelial dysplasia in heterotopic pancreas
| Pt | Age/ Sex | Clinical presentation | Anatomic location | Size (cm) | Diagnosis | Mutation | Pancreas Imaging |
|---|---|---|---|---|---|---|---|
| 1 | 74/ M | Ileocecal fistula, ileal stricture | Meckel’s diverticulum | 0.9 | Incipient IPMN with focal HGD | KRAS p.G12D | Not available |
| 2 | 70/ M | Crohn’s disease, ileal stricture | Meckel’s diverticulum | 3.5 | IPMN with focal HGD | KRAS p.G12D | Multiple pancreatic cysts, largest 1.7 cm, stable |
| 3 | 15/ M | Bloody diarrhea due to Meckel’s diverticulum | Meckel’s diverticulum | 1.6 | PanIN-1 | Not available | |
| 4 | 82/F | Abdominal pain; imaging: gastric submucosal lesion | Stomach | 2.5 | PanIN-2 | None | Unremarkable |
| 5 | 53/ M | Epigastric pain; EGD/EUS: antral nonhealing ulcer and submucosal lesion | Stomach | 1.2 | PanIN-1 | None | Unremarkable |
| 6 | 56/ M | Gastrointestinal stromal tumor | Stomach | 1.3 | PanIN-2 | None | Unremarkable |
| 7 | 31/ M | Intussusception | Small Bowel | 1.0 | PanIN-1 | None | Unremarkable |
Abbreviations: Pt – patient, M – male, F – female, EGD: esophagogastroduodenoscopy; EUS – endoscopic ultrasound; IPMN – intraductal papillary mucinous neoplasm; PanIN – pancreatic intraepithelial neoplasia; HGD – high-grade dysplasia
Figure 1.
A) IPMN arising in Meckel’s diverticulum, H&E x40, B) High power view of high grade dysplasia in IPMN, H&E x400, C) PanIN-1 in stomach, H&E x40 D) High power view of low grade dysplasia in PanIN-1, H&E x200.
3.2. Mutational Analysis
Both cases of IPMN harbored activating mutations in KRAS at codon 12 (both p.G12D, the most common type of KRAS mutation in pancreatic neoplasia)[19]. GNAS mutations were not present in the IPMNs. No mutations were identified in any of the cases of PanIN. All lesions were wildtype for TP53, CDKN2A, SMAD4, BRAF, and GNAS.
4. Discussion
Heterotopic foci of pancreatic tissue may develop invasive ductal adenocarcinoma or its precursor lesions, histologically identical to those in the pancreas [20–22]. These foci provide an opportunity to study the development of these lesions in pancreatic tissue away from the confines and the microenvironment of the pancreas. Our analysis targeted mutational changes in precursor lesions in heterotopic pancreatic parenchyma. None of our lesions had an identifiable associated invasive ductal adenocarcinoma either in the heterotopic focus or in the pancreas, although the possibility of a clinically undetectable lesion cannot be excluded. The identification of clonal KRAS mutations in the IPMNs suggests a genetic pathway shared with IPMN of the pancreas. In addition, these IPMNs were of the gastric-foveolar subtype that, in the pancreas, most frequently carries mutations of KRAS and are less likely to harbor mutations in GNAS. GNAS mutations are more commonly found in the intestinal subtypes. We were unable to assay for RNF43, which has recently been recognized as a gene frequently mutated in IPMN [4].
No mutations were identified in any of our five PanIN lesions. In the only prior molecular study of PanIN occurring in heterotopic foci, three of the six cases harbored activating KRAS mutations at codon 12 [11]. All of those patients had concomitant invasive ductal adenocarcinoma of the pancreas bearing the same mutation. Although our case numbers were small, the lack of mutations in PanINs arising in heterotopic pancreatic parenchyma differs from similar lesions in orthotopic pancreatic parenchyma. In the pancreas essentially all PanIN lesions harbor a KRAS mutation, even in low grade lesions (PanIN-1) [23]. Higher grade lesions are associated with additional mutations such as CDKN2A inactivation [24], which was also not present in our cases. Interestingly, the control group in the Zhang study consisted of thirty-two cases of heterotopic pancreas of the upper gastrointestinal tract without associated PDAC, a group corresponding more closely to our cases [11]. Two out of the thirty-two of these control cases reportedly had PanIN-1A and both were KRAS wild type. No further details of mutational profiling were provided. Our five PanIN cases and the two controls with similar findings from the Zhang study raise the possibility that PanIN in heterotopic pancreas does not follow the same pathway as its analogue in the orthotopic pancreas. In concert with this, the Zhang cases with KRAS-mutated heterotopic PanIN and co-existing pancreatic ductal adenocarcinoma could be attributable to a widespread “field effect”.
The development of ductal adenocarcinoma in heterotopic pancreatic parenchyma is rare, with approximately 31 cases reported in the literature [25–27]. In only one case has molecular analysis been reported; a KRAS mutation at codon 12 was identified [28]. There was no mention of adenocarcinoma precursor lesions in that study. The finding of at least one instance of a KRAS mutated adenocarcinoma arising in heterotopic pancreatic tissue raises the possibility that at least a subset of these lesions may follow a pathway similar to that of pancreatic ductal adenocarcinomas.
A meta-analysis of studies performed mostly in the 1990’s pointed out that the incidence of KRAS mutation in PanIN1 depended heavily on the type of assay [29]. This was confirmed by the more recent study by Kanda et al. which demonstrated a higher rate of KRAS mutation (99%) using a combination of laser-capture microdissection with pyrosequencing and melt curve analysis [23]. Their reported limit of detection was approximately 5% mutant alleles. We believe our careful manual microdissection has a roughly equivalent ability to enrich for mutated tumor alleles, and our limit of detection is superior at 1% mutant alleles [18].
The rarity of these lesions is the major shortcoming of our study. Our numbers are small and statistical analysis is not possible. However, closer attention to these precursor lesions may provide information on the development of neoplasia in a setting distinct from that in the pancreas.
Acknowledgments
Funding: NIH Specialized Programs of Research Excellence (P30CA006973) to R.H.H.
Footnotes
Conflicts of interest for all authors: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cowan RW, Maitra A. Genetic progression of pancreatic cancer. Cancer J. 2014;20:80–4. doi: 10.1097/PPO.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 2.Hruban RH, Klimstra DS. Adenocarcinoma of the pancreas. Semin Diagn Pathol. 2014;31:443–51. doi: 10.1053/j.semdp.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reid MD, Saka B, Balci S, Goldblum AS, Adsay NV. Molecular genetics of pancreatic neoplasms and their morphologic correlates: an update on recent advances and potential diagnostic applications. Am J Clin Pathol. 2014;141:168–80. doi: 10.1309/AJCP0FKDP7ENVKEV. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa T, Kuboki Y, Tanji E, Yoshida S, Hatori T, Yamamoto M, Shibata N, Shimizu K, Kamatani N, Shiratori K. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep. 2011;1:161. doi: 10.1038/srep00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amato E, Molin MD, Mafficini A, Yu J, Malleo G, Rusev B, Fassan M, Antonello D, Sadakari Y, Castelli P, Zamboni G, Maitra A, Salvia R, Hruban RH, Bassi C, Capelli P, Lawlor RT, Goggins M, Scarpa A. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol. 2014;233:217–27. doi: 10.1002/path.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dal Molin M, Matthaei H, Wu J, Blackford A, Debeljak M, Rezaee N, Wolfgang CL, Butturini G, Salvia R, Bassi C, Goggins MG, Kinzler KW, Vogelstein B, Eshleman JR, Hruban RH, Maitra A. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg Oncol. 2013;20:3802–8. doi: 10.1245/s10434-013-3096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH, Wolfgang CL, Klein AP, Diaz LA, Jr, Allen PJ, Schmidt CM, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kloppel G, Adsay V, Konukiewitz B, Kleeff J, Schlitter AM, Esposito I. Precancerous lesions of the biliary tree. Best Pract Res Clin Gastroenterol. 2013;27:285–97. doi: 10.1016/j.bpg.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi H, Kuboki Y, Hatori T, Yamamoto M, Shimizu K, Shiratori K, Shibata N, Shimizu M, Furukawa T. The discrete nature and distinguishing molecular features of pancreatic intraductal tubulopapillary neoplasms and intraductal papillary mucinous neoplasms of the gastric type, pyloric gland variant. J Pathol. 2013;231:335–41. doi: 10.1002/path.4242. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi H, Kuboki Y, Hatori T, Yamamoto M, Shiratori K, Kawamura S, Kobayashi M, Shimizu M, Ban S, Koyama I, Higashi M, Shin N, Ishida K, Morikawa T, Motoi F, Unno M, Kanno A, Satoh K, Shimosegawa T, Orikasa H, Watanabe T, Nishimura K, Harada Y, Furukawa T. Somatic mutations in PIK3CA and activation of AKT in intraductal tubulopapillary neoplasms of the pancreas. Am J Surg Pathol. 2011;35:1812–7. doi: 10.1097/PAS.0b013e31822769a0. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Sanderson SO, Lloyd RV, Smyrk TC. Pancreatic intraepithelial neoplasia in heterotopic pancreas: evidence for the progression model of pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2007;31:1191–5. doi: 10.1097/PAS.0b013e31806841e1. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto H, Fujishima F, Ishida K, Tsuchida K, Shimizu T, Goto H, Sato A, Satomi S, Sasano H. Intraductal papillary mucinous neoplasm originating from a jejunal heterotopic pancreas: report of a case. Surg Today. 2014;44:349–53. doi: 10.1007/s00595-012-0486-0. [DOI] [PubMed] [Google Scholar]
- 13.Hruban R, Pitman M, Klimstra D. Atlas of Tumor Pathology. Washington DC: American Registry of Pathology and Armed Forces Institute of Pathology; 2007. Tumors of the Pancreas. Fourth Series. [Google Scholar]
- 14.Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, Biankin SA, Compton C, Fukushima N, Furukawa T, Goggins M, Kato Y, Kloppel G, Longnecker DS, Luttges J, Maitra A, Offerhaus GJ, Shimizu M, Yonezawa S. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–87. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 15.Matthaei H, Wu J, Dal Molin M, Shi C, Perner S, Kristiansen G, Lingohr P, Kalff JC, Wolfgang CL, Kinzler KW, Vogelstein B, Maitra A, Hruban RH. GNAS sequencing identifies IPMN-specific mutations in a subgroup of diminutive pancreatic cysts referred to as “incipient IPMNs”. Am J Surg Pathol. 2014;38:360–3. doi: 10.1097/PAS.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Kloppel G, Longnecker DS, Luttges J, Offerhaus GJ. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–86. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Tsiatis AC, Norris-Kirby A, Rich RG, Hafez MJ, Gocke CD, Eshleman JR, Murphy KM. Comparison of Sanger sequencing, pyrosequencing, and melting curve analysis for the detection of KRAS mutations: diagnostic and clinical implications. J Mol Diagn. 2010;12:425–32. doi: 10.2353/jmoldx.2010.090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin MT, Mosier SL, Thiess M, Beierl KF, Debeljak M, Tseng LH, Chen G, Yegnasubramanian S, Ho H, Cope L, Wheelan SJ, Gocke CD, Eshleman JR. Clinical validation of KRAS, BRAF, and EGFR mutation detection using next-generation sequencing. Am J Clin Pathol. 2014;141:856–66. doi: 10.1309/AJCPMWGWGO34EGOD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, Ding M, Bamford S, Cole C, Ward S, Kok CY, Jia M, De T, Teague JW, Stratton MR, McDermott U, Campbell PJ. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015:D805–11. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koh HC, Page B, Black C, Brown I, Ballantyne S, Galloway DJ. Ectopic pancreatic-type malignancy presenting in a Meckel’s diverticulum: a case report and review of the literature. World J Surg Oncol. 2009;7:54. doi: 10.1186/1477-7819-7-54. 7819-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MS, Cho BS, Park JS, Koo HC, Han HY, Kang DW. Premalignant lesion of heterotopic pancreas combined with gastritis cystica profunda in gastric fundus. J Gastrointestin Liver Dis. 2013;22:337–40. [PubMed] [Google Scholar]
- 22.Lemaire J, Delaunoit T, Molle G. Adenocarcinoma arising in gastric heterotopic pancreas. Case report and review of the literature. Acta Chir Belg. 2014;114:79–81. [PubMed] [Google Scholar]
- 23.Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B, Goggins M. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730, 733.e9. doi: 10.1053/j.gastro.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh M, Maitra A. Precursor lesions of pancreatic cancer: molecular pathology and clinical implications. Pancreatology. 2007;7:9–19. doi: 10.1159/000101873. [DOI] [PubMed] [Google Scholar]
- 25.Emerson L, Layfield LJ, Rohr LR, Dayton MT. Adenocarcinoma arising in association with gastric heterotopic pancreas: A case report and review of the literature. J Surg Oncol. 2004;87:53–7. doi: 10.1002/jso.20087. [DOI] [PubMed] [Google Scholar]
- 26.Ginori A, Vassallo L, Butorano MA, Bettarini F, Di Mare G, Marrelli D. Pancreatic adenocarcinoma in duodenal ectopic pancreas: a case report and review of the literature. Pathologica. 2013;105:56–8. [PubMed] [Google Scholar]
- 27.Goodarzi M, Rashid A, Maru D. Invasive ductal adenocarcinoma arising from pancreatic heterotopia in rectum: case report and review of literature. Hum Pathol. 2010;41:1809–13. doi: 10.1016/j.humpath.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Osanai M, Miyokawa N, Tamaki T, Yonekawa M, Kawamura A, Sawada N. Adenocarcinoma arising in gastric heterotopic pancreas: clinicopathological and immunohistochemical study with genetic analysis of a case. Pathol Int. 2001;51:549–54. doi: 10.1046/j.1440-1827.2001.01240.x. [DOI] [PubMed] [Google Scholar]
- 29.Lohr M, Kloppel G, Maisonneuve P, Lowenfels AB, Luttges J. Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: a meta-analysis. Neoplasia. 2005;7:17–23. doi: 10.1593/neo.04445. [DOI] [PMC free article] [PubMed] [Google Scholar]