Abstract
Asthma exacerbations are important components of asthma morbidity. The Inner City Asthma Consortium was established in the early 1990’s to identify risk factors for and to evaluate treatments to reduce asthma symptoms and exacerbations. Early studies identified atopy and inadequate treatment as important drivers of asthma morbidity. Later studies demonstrated that good adherence to guidelines based asthma care could virtually eliminate symptoms and reduce but not eliminate exacerbations. Looking at exacerbations by season, risk factors were found to vary across the different seasons. Of the seven factors identified, allergic status and pulmonary functions were found to be important for exacerbations in all seasons but allergy had its strongest effect in the fall season. Therefore, additional therapy directed at reducing the role of allergy was evaluated and found to significantly reduce exacerbations even in participants with good symptom control when receiving guidelines based therapy. Despite this aggressive therapy, exacerbations remain albeit at a lower level and with less seasonal variation. An ongoing trial evaluating two different approaches to pre-seasonal interventions directed at individuals at high risk for exacerbations during the subsequent season may offer an effective alternative to minimize exacerbations and to limit the amount of therapy necessary.
Keywords: Asthma, Exacerbations, Children, Inner City Asthma Consortium, Control
INTRODUCTION
Exacerbations are a distinct and, perhaps, the most important component driving asthma morbidity. This distinction is highlighted by The Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma which identifies two domains of disease control: impairment, which focuses on symptoms and functional impairment, and risk which focuses on risk of asthma exacerbations, progressive loss of pulmonary function, and side effects.1 Exacerbations are an important cause of asthma morbidity and cost. Data from the 2008–10 Medical Expenditures Panel survey, a nationally representative study of medical costs in the U.S., found that approximately 50% of self-reported patients with asthma reported having an exacerbation in the previous year.2 Patients with exacerbations have significantly higher medical expenses than those without exacerbations.3 While individuals with severe asthma have higher rates of exacerbations, patients with mild asthma still account for a large percentage of exacerbations.4 Finally, disparities exist in risks for exacerbations with the young, minorities, and the poor having higher rates.5 These disparities have not decreased in recent years.6
Inception of the Inner City Asthma Consortium
Asthma disparities are not a recent phenomenon. In 1987, a review of asthma morbidity and mortality using data on the U.S population found asthma morbidity and mortality increasing with higher rates in poor and minority populations.7 Other studies reported that geographic areas with high concentrations of poor and minorities residents also had high levels of asthma morbidity and mortality.8,9 The Inner City Asthma Consortium was established in 1991 to identify the causes of the disparities in asthma morbidity and to develop strategies to reduce them. Over the past 24 years the National Institute of Allergy and Infectious Diseases has funded five iterations of the Inner City Asthma Consortium, which have performed a series of epidemiologic studies and clinical trials that have expanded our understanding of exacerbations.10 This article will review what has been learned from this effort about asthma exacerbations in inner-city populations.
The original NIAID Inner-City Asthma Program, The National Cooperative Inner City Asthma Study (NCICAS), 1991–1995, was a two-phase study. The first phase was an epidemiologic study of 4 to 9 year old children with asthma and living in selected U.S. inner cities. The children were carefully followed over 12 months to document their asthma symptoms and exacerbations with extensive measurement of the home environment and psychosocial outcomes. NCICAS Phase 1 documented a high level of symptoms and exacerbations in this population.11 Not unexpectedly, most of the children had high rates of allergic sensitization with multiple positive allergen skin test responses. Unexpectedly, however, house dust mite was not an important allergen and other allergens such as cockroach12 and mouse13 represented a significant percentage of allergic sensitization and were present in large quantities in inner city homes. The combination of exposure and sensitization to cockroach was found to be a very strong determinate of asthma exacerbations but less important in the frequency of symptoms.12 Many other factors were found to be driving asthma morbidity among the NCICAS children including inadequate treatment, lack of access to treatment, poor adherence to treatment, childhood behavioral problems, caretaker mental health problems and allergic sensitization.11,12,14,15
The Role of Environmental Allergens and Exacerbations
The first phase of the NCICAS suggested that given these many inter-related medical and psychosocial determinant of disease a simple disease education program would be insufficient to ameliorate the high level of morbidity. Therefore during NCICAS Phase 2, an intervention was developed and applied in a randomized design to 5 to 11 years in an attempt to help the family deal with the competing issues in their lives so they could better manage their child’s asthma. Families in the intervention were assigned an Asthma Counselor who was a trained social worker familiar with the social welfare resources available in the local area and also trained in asthma education. At the beginning of the study, families were evaluated to determine which risk factors were relevant to them; the intervention was then tailored by the Asthma Counselor to the identified risk factors. In addition to asthma education, the Asthma Counselor provided the caretakers with referrals to appropriate community resources for education on smoking cessation, as well as on psychological and social issues. No direct medical care was given to the families, but the caretaker was trained to discuss their concerns about their child’s asthma with their primary care provider and to obtain an asthma action plan. To lower the burden of environmental allergens, the families with children who were skin test positive to cockroach and were randomized to the intervention were instructed on environmental cleanup and professional pest control companies visited the homes twice for cockroach elimination. The control group received only general asthma education.
The intervention was found effective in lowering the level of symptoms, but a significant level of symptoms still remained.16 The intervention had a lesser effect on asthma exacerbations. The use of commercial pest companies and home cleanup efforts had only a small impact on home cockroach levels and the decrease in allergen levels only lasted a short time.17
One assumption for the relatively small effect of the intervention was the high level of environmental allergens, which likely played a significant role in maintaining the high level of disease activity. It was postulated that this continuing allergen stimulus reduced the effectiveness of the asthma treatment the children were receiving. It was also obvious from the results of the limited NCICAS cockroach intervention that the use of professional pest companies and minimal cleanup efforts would not be sufficient.
The next effort therefore was to reduce the environmental burden of allergens with the hope that this would make the child’s asthma easier to control and reduce the level of medication needed to achieve that control. As a consequence, the Inner City Asthma Study (ICAS), a randomized trial conducted between 1996 and 2001, enrolled children with asthma and a sensitization to at least one indoor allergen, ages 5 to 11 years. The intervention group received comprehensive home cleanup which included HEPA air filters, mattress and pillow covers, and a HEPA vacuum cleaner and the cleanup was supervised by research assistants who visited the home between 5 to 7 times during the course of the 12-month intervention. The families of children sensitized to cockroach also received visits from professional exterminators for cockroach elimination. The intervention reduced the level of allergens in the home and this decrease in allergen levels was associated with the improvement in asthma symptoms and a lesser improvement in asthma attacks.18
Treatment Interventions to Prevent Exacerbations
The results of the NCICAS and ICAS interventions demonstrated that inner-city children with asthma whose family understands the disease, feels empowered to discuss treatment issues with the child’s physician, and is given the opportunity to reduce indoor allergens, can have improved symptoms and, to a lesser degree, reduced asthma exacerbations. However, these efforts are not sufficient to bring the child’s asthma (measured by either symptoms or exacerbations) under optimal control.
Because, in general, participants in previous inner-city trials were not receiving sufficient therapy from their primary care providers, the Asthma Control Evaluation (ACE) trial was developed and implemented by the Inner City Asthma Consortium, between 2002 and 2008. The ACE trial sought to determine if asthma treatment, based on EPR-3 guidelines, was sufficient to eliminate exacerbations among children 12 to 20 years old who had moderate to severe asthma. The ACE study was also designed to determine whether therapy guided by a defined algorithm based on symptoms and pulmonary function would be as effective as a more aggressive approach in which treatment could be increased over the level selected based on symptoms and pulmonary functions based on elevations of exhaled nitric oxide (FeNO). Both the control and FeNO -based treatment groups received asthma treatment in accordance to EPR-3 guidelines. A computer algorithm was developed to enable treating physicians at the 10 research sites to select and change treatment levels in a standardized fashion.
In both the FeNO -based treatment and the control groups, the greatest improvement in symptoms occurred during a 3-week run-in period when the participants were transitioned into guidelines based care. During this time asthma symptoms decreased by more than 60%. Adherence to medication was high in both the control and intervention group. Over the course of the 46-week treatment period, between 71%–78% of the participants were judged to be in good control, based on symptoms and pulmonary function. The FeNO guided treatment resulted in approximately 120 micrograms higher dose of daily fluticasone compared to the control group. Despite this increase in ICS, the level of symptoms did not differ between the two groups. However, 42% of the participants in the control group versus 32.1% (p<0.01) in the FeNO-guided treatment group developed asthma exacerbations requiring a burst of prednisone during the course of the study. The study concluded that the level of therapy recommended by EPR-3 guidelines resulted in excellent overall asthma control.19 Although the findings indicated that higher doses of ICS resulting from the FeNO-guided treatment had the potential to reduce exacerbations, almost 1/3 of the children experienced an exacerbation during their 48-week study participation.
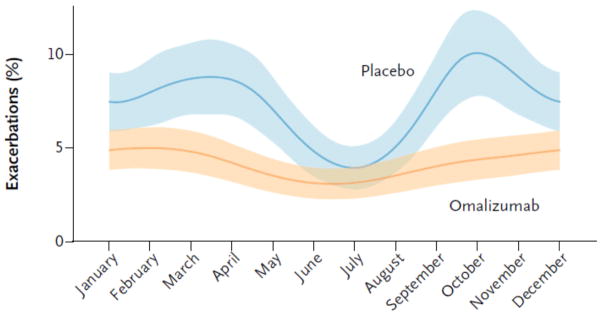
Based on the NCICAS observation that allergic sensitization is a driving force for asthma exacerbations in inner-city children and the ACE observation that, while aggressive ICS therapy lowers the rate of exacerbations, exacerbations still remained a frequent event, the Inner-City Anti-IgE Therapy for Asthma (ICATA) trial was designed. The ICATA trial evaluated whether suppression of the allergic signal with the use of anti-IgE (omalizumab), would reduce exacerbations even in the presence of “optimal”, EPR-3 directed therapy. Inner-city children ages 6 to 20 years of age with moderate-to-severe asthma, who qualified for omalizumab based on their total IgE and weight, were randomized to EPR-3 guidelines-based therapy with added omalizumab versus placebo for a one-year treatment period. Therapy was standardized using a defined algorithm, modified from previous ICAC studies, with a recent exacerbation history added to symptoms and pulmonary function as the outcomes determining the need for stepping therapy up or down during the course of the intervention. Omalizumab significantly lowered the exacerbation rate over that of the placebo arm-an approximate 38% decrease. This decrease in exacerbations was larger than the approximately 23% decrease seen in the ACE study in the FeNO group and was achieved though the dose of inhaled corticosteroid treatment was decreased during the ICATA study in the omalizumab treated participants. The expected fall and the lesser spring peak in exacerbations were clearly seen in the control group but were absent in the omalizumab group (figure 1). The ICATA study clearly showed that allergy plays an important role in the seasonal variation in asthma exacerbations, a role that could not be eliminated by EPR3-based treatment alone. Interestingly, despite the elimination of seasonal peaks, the average monthly rate of exacerbations continued to be around 5% in the omalizumab group, showing that in addition to allergy, some other factors may determine a portion of asthma exacerbations.20
Figure 1.
Seasonal variation in exacerbations among children 6 to 20 years of age receiving guidelines based therapy with or without omalizumab: Inner-City Anti-IgE Therapy for Asthma (ICATA)
From: Busse WW et al Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med 2011;364:1005–15.
Exacerbation Risk Factors
The findings of the ACE and ICATA studies emphasized the need to identify risk factors associated with asthma exacerbations among children receiving guidelines based therapy in order to develop new approaches to further reduce the rate of exacerbations. As respiratory viruses, particular rhinovirus infections, are linked with exacerbations,21 it would seem logical to direct efforts in this direction. However, a viral infection is not sufficient to cause an asthma exacerbation. Rhinovirus infections are nearly universal among children with asthma during the fall and winter seasons, but in most cases they do not result in exacerbations.22 Other factors obviously contribute to the asthma exacerbation process.
In an initial attempt to better understand which non-viral risk factors were associated with exacerbations versus symptoms, data from the ACE study were evaluated more extensively. Twelve variables, which were collected at the ACE baseline visit, were included in the analyses. Baseline asthma symptoms and previous exacerbations were the principal determinants of both future symptoms and exacerbations but pulmonary function, specifically bronchial reactivity, was a significant predictor of exacerbations but not symptoms. Neither set of predictors explained much of the future variance in symptoms (11.4%) or exacerbations (12.6%).23
Seasonal Variations
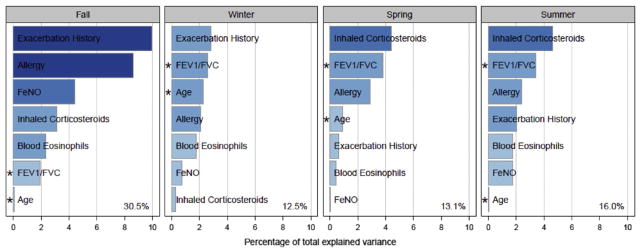
Descriptive analyses of the temporal variation in exacerbations and risk factors from various ICAC studies pointed to a different approach to understanding exacerbations. These analyses showed that both asthma exacerbations among inner city children followed a similar seasonal pattern of fall peaks and summer lows seen in children with asthma around the world24 and many of the risk factors for exacerbations, such as pulmonary functions, also have season variation in their levels. A study was undertaken to incorporate the season variation in risk factors in order to obtain better insight into predictors of exacerbations. We evaluated data from 400 participants from the ACE and the ICATA studies who received standardized, guidelines-based treatment for approximately 1 year. Exacerbations were stratified by season of occurrence (spring, summer, winter, fall). Certain variables, such as skin test results and IgE were derived, from baseline data while other predictors (previous exacerbations, ICS treatment requirements, FEV1/FVC and FENO) were drawn from measurements taken in the previous season. For example, FEV1/FVC and FENO measured during the summer were used to predict fall exacerbations.
A number of informative and novel findings arose from this analytic approach. First, the factors which predicted exacerbations varied by the season. Exacerbations in the previous season were the most important predictor for fall and winter exacerbations, but were much less important for spring and summer exacerbations. While a higher requirement for ICS was the most important predictor for spring and summer exacerbations, it was a minor predictor for fall and winter exacerbations. Pulmonary function, e.g. FEV1/FVC, was an important predictor for all seasons but was least predictive for fall exacerbations. Finally, allergic status was a predictor for all seasons but was most strongly associated with exacerbations in the fall (Figure 2). Using multivariate modeling, 30.5% of the variance in fall exacerbations could be explained by our model; for the other seasons the amount of variance explained ranged from 12.5% to 16%.25
Figure 2.
Risk factors for exacerbation by season among children ages 6 to 20 years
From: Teach SJ, et al J Allergy Clin Immunol. 2015 Jun;135(6):1465–1473
Even though a number of the risk factors were measured before the start of the season, timing of when the risk factors were measured may still have influenced which risk factors were selected. For example a very high FeNO may be predictive of an exacerbation in the short term (same season exacerbation), but not an exacerbation in several months.
Given the seasonal nature of exacerbations with the highest rates in the fall and varying risk factors for each season, the next intervention strategy evaluated by the ICAC was a seasonal-based treatment approach to reduce exacerbations. The ICAC investigators specifically targeted fall exacerbations by beginning the intervention shortly before the start of school and continuing it through the fall season. Two approaches were evaluated, the use of short term omalizumab based on the results of the ICATA study or a boost in inhaled ICS above the dose needed to control symptoms based on the findings from the ACE study. This study, the Preventative Omalizumab or Step-up Therapy for Severe Fall Exacerbations (PROSE) was designed and implemented by the Inner City Asthma Consortium between 2009 and 2013. Inner-city children between the ages of 6 and 16 were included. The results of this trial are pending.
SUMMARY
The findings from the various studies of the Inner City Asthma consortium over the last several decades have given us a greater understanding and appreciation of the causes and treatment of asthma exacerbations among poor and minority children living in the inner city. For example, the risk factors for exacerbations are not the same for every season; the months following an exacerbation are an especially vulnerable time for future exacerbations; allergy is a major driving force for exacerbations and symptoms among this population; finally, the combination of allergic sensitization and exposure to certain allergens, such as cockroach, identifies individuals who are at increased risk for exacerbations.12 We have also learned that removing allergens from the environment is efficacious but, unfortunately, not easily accomplished17,18 and even if allergen levels are reduced in the home, other locations such as schools and day care are important sources for continuing exposure.26,27 Adherence to medication did not appear to be a risk factor in the inner-city studies due to the high level of adherence achieved in these closely followed cohorts. However, in other situations where adherence is not high, efforts to increase adherence have proven beneficial. For example, increasing use of medication in the summer months has been shown to be associated with reduced fall exacerbations.28
EPR-3 directed therapy can achieve excellent control of symptoms and reduce exacerbations in the majority of children with asthma in inner-city populations. However, many challenges, such as access to a level of care needed for treatment, obtaining the medications, and understanding their use are barriers that must be overcome. Despite excellent control of symptoms, exacerbations still remain a significant problem that cannot be controlled with guidelines-based conventional treatment (ICS or ICS/LABA combination) in many patients. The use of anti-IgE has reduced exacerbations by almost 38%, even in the setting of apparent excellent disease control. Other biologics targeting various components of Th2 inflammation may have similar results, but have not been tested in this population. While current biologics are too expensive to make this approach cost effective, it is hoped that future technological developments in the field will render biologics available at a reduced cost. A seasonal approach or focused therapy during periods of high risk may help limit the cost by further reducing the use of expensive treatments.
Despite the use of omalizumab, a 5% average monthly rate of exacerbation remained and was constant, throughout the year. The factors which drive these remaining exacerbations are still unknown. Better understanding of these factors will be necessary to further reduce or eliminate asthma exacerbations in children.
Abbreviations
- ICAC
Inner City Asthma Consortium
- NCICAS
National Cooperative Inner City Asthma Study
- ICAS
Inner City Asthma Study
- ACE
Asthma Control Evaluation
- ICATA
Inner-City Anti-IgE Therapy for Asthma
- PROSE
Preventative Omalizumab or Step-up Therapy for Severe Fall Exacerbations
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Heart, Lung and Blood Institute. National Asthma Education and Prevention Program Expeert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 2007. NIH Publication 07-4051. [Google Scholar]
- 2.Slejko JF, Ghushchyan VH, Sucher B, et al. Asthma control in the United States, 2008–2010: Indicators of poor asthma control. J Allergy Clin Immunol. 2014;133:1579–87. doi: 10.1016/j.jaci.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129:1229–35. doi: 10.1016/j.jaci.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 4.Carroll CL, Schramm CM, Zucker AR. Severe exacerbations in children with mild asthma: characterizing a pediatric phenotype. J Asthma. 2008;45:513–7. doi: 10.1080/02770900802017751. [DOI] [PubMed] [Google Scholar]
- 5.Schiller JS, Ward BW, Freeman G. Early release of selected estimates based on data from the 2013 National Health Interview Survey. National Center for Health Statistics; Jun, 2014. [Accessed April 30, 2015]. Available from: http://www.cdc.gov/nchs/nhis.htm. [Google Scholar]
- 6.Akinbami LJ, Moorman JE, Simon AE, Schoendorf KC. Trends in racial disparities for asthma outcomes among children 0 to 17 years, 2001–2010. J Allergy Clin Immunol. 2014;134:547–53. doi: 10.1016/j.jaci.2014.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans R, 3rd, Mullally DI, Wilson RW, et al. National trends in the morbidity and mortality of asthma in the US. Prevalence, hospitalization and death from asthma over two decades: 1965–1984. Chest. 1987;91:65S–74S. [PubMed] [Google Scholar]
- 8.Carr W, Zeitel L, Weiss K. Variations in asthma hospitalizations and deaths in New York City. Am J Public Health. 1992;82:59–65. doi: 10.2105/ajph.82.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottlieb DJ, Beiser AS, O’Connor GT. Poverty, race, and medication use are correlates of asthma hospitalization rates. A small area analysis in Boston. Chest. 1995;108:28–35. doi: 10.1378/chest.108.1.28. [DOI] [PubMed] [Google Scholar]
- 10.Togias A, Fenton MJ, Gergen PJ, Rotrosen D, Fauci AS. Asthma in the inner city: the perspective of the National Institute of Allergy and Infectious Diseases. J Allergy Clin Immunol. 2010;125:540–4. doi: 10.1016/j.jaci.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 11.Kattan M, Mitchell H, Eggleston P, et al. Characteristics of inner-city children with asthma: the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997;24:253–62. doi: 10.1002/(sici)1099-0496(199710)24:4<253::aid-ppul4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 13.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. I. The prevalence of mouse allergen in inner-city homes. The National Cooperative Inner-City Asthma Study. J Allergy Clin Immunol. 2000;106:1070–4. doi: 10.1067/mai.2000.110796. [DOI] [PubMed] [Google Scholar]
- 14.Bauman LJ, Wright E, Leickly FE, et al. Relationship of adherence to pediatric asthma morbidity among inner-city children. Pediatrics. 2002;110:e6. doi: 10.1542/peds.110.1.e6. [DOI] [PubMed] [Google Scholar]
- 15.Weil CM, Wade SL, Bauman LJ, Lynn H, Mitchell H, Lavigne J. The relationship between psychosocial factors and asthma morbidity in inner-city children with asthma. Pediatrics. 1999;104:1274–80. doi: 10.1542/peds.104.6.1274. [DOI] [PubMed] [Google Scholar]
- 16.Evans R, 3rd, Gergen PJ, Mitchell H, et al. A randomized clinical trial to reduce asthma morbidity among inner-city children: results of the National Cooperative Inner-City Asthma Study. J Pediatr. 1999;135:332–8. doi: 10.1016/s0022-3476(99)70130-7. [DOI] [PubMed] [Google Scholar]
- 17.Gergen PJ, Mortimer KM, Eggleston PA, et al. Results of the national cooperative inner-city asthma study (NCICAS) environmental intervention to reduce cockroach allergen exposure in inner-city homes. J Allergy Clin Immunol. 1999;103:501–6. doi: 10.1016/s0091-6749(99)70477-x. [DOI] [PubMed] [Google Scholar]
- 18.Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–80. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 19.Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065–72. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–34. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olenec JP, Kim WK, Lee WM, et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125:1001–6. e1. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruchalla RS, Sampson HA, Matsui E, et al. Asthma morbidity among inner-city adolescents receiving guidelines-based therapy: role of predictors in the setting of high adherence. J Allergy Clin Immunol. 2009;124:213–21. 21 e1. doi: 10.1016/j.jaci.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gergen PJ, Mitchell H, Lynn H. Understanding the seasonal pattern of childhood asthma: results from the National Cooperative Inner-City Asthma Study (NCICAS) J Pediatr. 2002;141:631–6. doi: 10.1067/mpd.2002.127510. [DOI] [PubMed] [Google Scholar]
- 25.Teach SJ, Gergen PJ, Szefler SJ, et al. Seasonal risk factors for asthma exacerbations among inner-city children. J Allergy Clin Immunol. 2015;135:1465–73. doi: 10.1016/j.jaci.2014.12.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanchongkittiphon W, Sheehan WJ, Friedlander J, et al. Allergens on desktop surfaces in preschools and elementary schools of urban children with asthma. Allergy. 2014;69:960–3. doi: 10.1111/all.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tranter DC, Wobbema AT, Norlien K, Dorschner DF. Indoor allergens in Minnesota schools and child care centers. J Occup Environ Hyg. 2009;6:582–91. doi: 10.1080/15459620903103454. [DOI] [PubMed] [Google Scholar]
- 28.Spahn J, Sheth K, Yeh WS, Stempel DA, Stanford RH. Dispensing of fluticasone propionate/salmeterol combination in the summer and asthma-related outcomes in the fall. J Allergy Clin Immunol. 2009;124:1197–203. doi: 10.1016/j.jaci.2009.08.042. [DOI] [PubMed] [Google Scholar]