Abstract
Insufficient regeneration of central nervous system (CNS) axons contributes to persisting neurological dysfunction after spinal cord injury (SCI). Peripheral nerve grafts (PNGs) support regeneration by thousands of injured intraspinal axons and help them bypass some of the extracellular barriers that form after SCI. However this number represents but a small portion of the total number of axons that are injured. Here we tested if rhythmic sensory stimulation during cycling exercise would boost the intrinsic regenerative state of neurons to enhance axon regeneration into PNGs after a lower thoracic (T12) spinal transection of adult rats. Using True Blue retrograde tracing, we show that 4 weeks of cycling improves regeneration into a PNG from lumbar interneurons but not by primary sensory neurons. The majority of neurons that regenerate their axon are within 5mm of the lesion and their number increased 70% with exercise. Importantly propriospinal neurons in more distant regions (5–20 mm from the lesion) that routinely exhibit very limited regeneration responded to exercise by increasing the number of regenerating neurons by 900%. There was no exercise-associated increase in regeneration from sensory neurons. Analyses using fluorescent in situ hybridization showed that this increase in regenerative response is associated with changes in levels of mRNAs encoding the regeneration associated genes (RAGs) GAP43, β-actin and Neuritin. While propriospinal neurons showed increased mRNA levels in response to SCI alone and then to grafting and exercise, sensory neurons did not respond to SCI, but there was a response to the presence of a PNG. Thus, exercise is a non-invasive approach to modulate gene expression in injured neurons leading to an increase in regeneration. This sets the stage for future studies to test whether exercise will promote axon outgrowth beyond the PNG and reconnection with spinal cord neurons, thereby demonstrating a potential clinical application of this combined therapeutic intervention.
Keywords: Spinal cord injury, Peripheral nerve grafts, Regeneration, Exercise, GAP43, β-actin, Neuritin
1 Introduction
Regeneration of severed CNS axon pathways is one of the major challenges in spinal cord injury (SCI) research. A combination of excess extrinsic growth inhibitory molecules and lack of robust intrinsic regenerative capacity in central nervous system (CNS) neurons greatly reduces the possibility of spontaneous structural and functional regeneration (Afshari et al., 2009; Ferguson and Son, 2011; Murray, 2014; Young, 2014). We and others have used a pre-degenerated peripheral nerve graft (PNG) to promote functional regeneration of damaged axons as they bypass the injured inhibitory CNS environment, yet the regenerative response is generally limited to a fraction of neurons axotomized by SCI (Bray et al., 1987; David and Aguayo, 1981; Houlé et al., 2006; Richardson et al., 1980; Tom et al., 2009). This supports the notion that successful CNS regeneration is limited by more than just the inhospitable environment presented by scar tissue in the injured spinal cord. Deletion of negative regulators of mammalian target of rapamycin (mTOR) such as phosphatase and tensin homolog (PTEN) or tuberous sclerosis protein 1 (TSC1) facilitates remarkable improvement in regeneration of optic nerve and corticospinal tract axons (Liu et al., 2010; Park et al., 2008). These studies highlight the potential for boosting the intrinsic regenerative potential of injured CNS neurons. We have shown that cycling exercise increases mTOR and decreases PTEN mRNA and protein levels in the injured spinal cord, with increased activation of the ribosomal protein S6 in intermediate grey (propriospinal) neurons (Liu et al., 2012). We also have reported that post injury exercise upregulates production of neurotrophic factors (BDNF, NT-3, NT-4 and GDNF), heat shock proteins and decreases caspase proteins in the spinal cord, suggesting a positive effect on neuron survival and regeneration (Côté et al., 2011; Keeler et al., 2012). Many groups, including ours, have demonstrated that providing exogenous neurotrophic factors rescues neurons after injury and can promote the regenerative effort when combined with a neural tissue transplant (Dolbeare and Houlé, 2003; Giehl and Tetzlaff, 1996; Tom et al., 2009; Xu et al., 1995). While exercise promotes axon regeneration in the PNS (Armada-da-Silva et al., 2013; Goulart et al., 2014; Molteni et al., 2004; Teodori et al., 2011), and elevates neurotrophins and other favorable factors in the spinal cord, the efficacy of exercise in promoting CNS axonal regeneration after SCI was unknown. In the present study, we tested whether exercise affected axon regeneration into a peripheral nerve graft (PNG) while measuring changes in the expression of regeneration associated genes (RAGs) in injured and regenerating neurons. We combined retrograde tracing using True Blue (TB) with fluorescence in situ hybridization (FISH) to analyze mRNA expression levels of growth associated protein 43 (GAP43), β-actin and Neuritin (candidate plasticity gene 15: cpg15) in two neuronal subpopulations that are major contributors of axons ascending in the spinal cord, propriospinal interneurons in the spinal cord intermediate grey and primary sensory neurons in dorsal root ganglia (DRGs). Here we demonstrate a positive influence of exercise on regeneration by CNS interneurons but not on the central process of sensory neurons, a result that is mirrored respectively by the presence or absence of an increase in RAGs.
Materials and methods
Surgical procedures and post-operative care were performed in compliance with protocols approved by Drexel University College of Medicine Institutional Animal Care and Use Committee and followed National Institutes of Health guidelines for the care and use of laboratory animals.
Surgical procedures and animal groups
Adult (225–250g) female Sprague Dawley (SD) rats (Charles River Laboratories International, Inc.) were used for this study (n=5 per group).
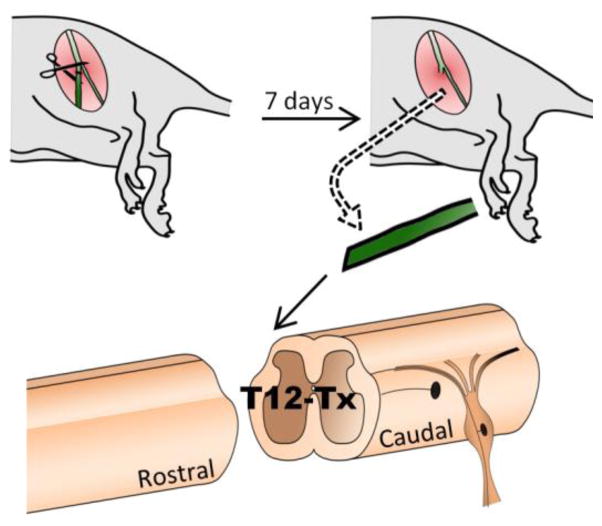
Briefly, rats were anesthetized using 5% Isoflurane in a plexiglass induction chamber and maintained on 1–3% Isoflurane throughout surgery. The T12 spinal cord was exposed by dorsal laminectomy. A 2–3 mm long segment of spinal cord was removed by vacuum aspiration using a glass micropipette. The complete transection (Tx) lesion cavity was filled with gel foam saturated with saline to achieve hemostasis. Pre-degenerated peripheral nerve (PN) segments were harvested from donor rats after cutting of the tibial nerve 7 days prior to grafting (Figure 1). At the time of spinal cord injury, donor rats were anesthetized with Ketamine (60 mg/kg, Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) and Xylazine (10 mg/kg, Anased, Lloyd Laboratories, Shenandoah, IA) and an 8–10 mm length of PN was harvested for use as a graft. One end of the PNG was apposed to the midline dorsal portion of the caudal lesion cavity wall to allow ingrowth of ascending axons. The distal end of the graft was left unapposed to spinal cord tissue, laying on top of the adjacent vertebral process (Figure 2). Donor rats were euthanized with Euthasol (390 mg/kg pentobarbital sodium and 50 mg/kg phenytoin sodium IP, Virbac, Fort Worth, TX) after harvesting peripheral nerve segments. Spinal cord injured rats received the antibiotic Cefazolin (25 mg/ml, Sandoz, Princeton, NJ) and the analgesic Sustained Release-Buprenorphine (1.0 mg/kg, Zoopharm, Laramie, WY) at the time of surgery. Urinary bladder was expressed twice a day throughout the survival period. Graft recipient rats received daily subcutaneous Cyclosporine A (10mg/kg, Teva Czech Industries, Sellersville, PA) for 2 weeks (wks) to prevent graft rejection, beginning 3 days prior to grafting before changing to oral administration (1mg/ml) continuing throughout the experiment.
Figure 1. Experimental approach for nerve grafting.
Tibial nerve is cut in donor rat and harvested 7 days later. Recipient rats receive T12 transection and an intraspinal graft of the harvested nerve segment apposing the caudal end of the lesion cavity.
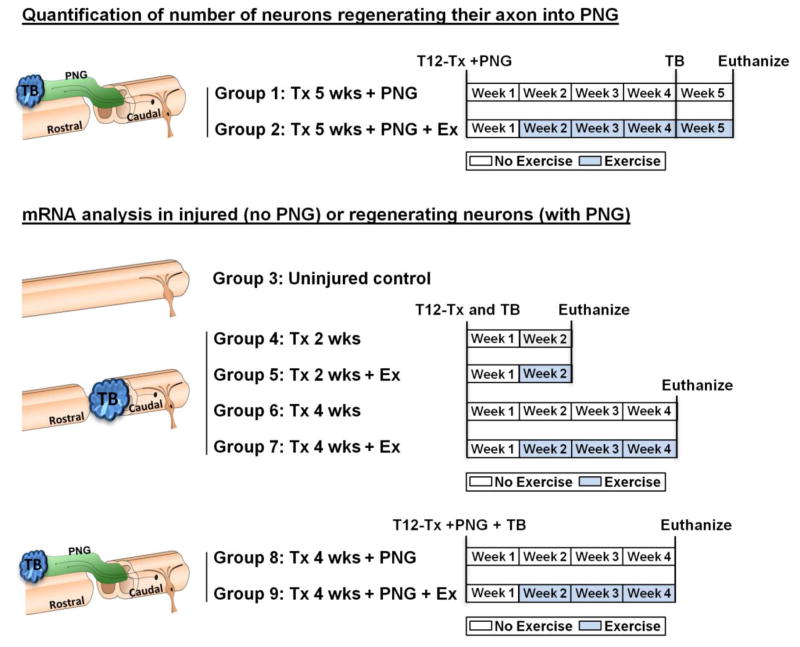
Figure 2. Animal groups and experimental timeline.
9 animal groups were divided into 2 categories. Groups 1 and 2 were used to quantify the number of regenerating neurons in the presence or absence of Ex. Groups 3–9 were used for mRNA analyses. Uninjured control rats were not exercised (Group 3). All other groups started exercise at the beginning of week 2 (5 days post injury) for 5 days/week and 30 min/day. The Tx rats were sacrificed at 2 wks post injury (Groups 4, 5) or 4 wks post injury (groups 6, 7). Tx + PNG rats were sacrificed 4 wks post injury (groups 8, 9). Tx= transection, Wks= weeks, Ex= exercise, TB= True Blue.
Quantification of number of neurons regenerating their axon into PNG
Figure 2 outlines the experimental groups used; Groups 1–2 were used to assess extent of regeneration into PNG ± EX. Group 1 (Tx 5 wks + PNG) rats sustained a complete transection injury and a PNG with no further experimentation for 4 weeks. Group 2 (Tx 5 wks + PNG + Ex) rats began cycling exercise 5 days post SCI and PN grafting (at the beginning of week 2 and continued till the end of week 5. For both groups, 4 weeks after injury, the distal end of the PNG was exposed, trimmed by 1 mm and exposed to gel foam soaked with TB (2% True Blue diaceturate salt, Sigma Aldrich, St. Louis, MO) to label by retrograde transport neurons that had extended their axon into the PNG. At the end of week 5, animals were euthanized with an overdose of Euthasol and perfused with 4% paraformaldehyde. We harvested T13-L5 spinal cord and T13 to L6 DRGs bilaterally. Tissue was stored in 30% sucrose at 4°C before preparing cryostat sections.
mRNA analysis in injured (no PNG) or regenerating neurons (with PNG)
Groups 3–9 were used to assess changes in mRNA expression after SCI and the intervention of exercise and PNG separately and in combination.
Group 3 rats served as uninjured controls and received no injury or PNG or Ex. Rats in Groups 4–9 received a T12 Tx and all groups were used to study exercise related changes in gene expression. Rats in Groups 8 and 9 received a PNG after Tx (see below). Rats in Groups 3–9 were euthanized at appropriate times with an overdose of Euthasol and perfused with 2% paraformaldehyde and stored in 30% sucrose at 4°C.
Groups 4 (Tx 2 wks) and 5 (Tx 2 wks + Ex) rats were used to measure acute effects of exercise in injured neurons. Ten rats received aT12 Tx and a piece of gel foam saturated in 2% TB was placed in the lesion cavity for 30 minutes to label axotomized neurons by retrograde transport of this fluorescent tracer. Group 4 rats were not exercised but cycling exercise began 5 days after injury (at the beginning of week 2) for Group 5 rats. All rats were euthanized 2 wks after SCI. A 2 wks post injury group was not prepared for the cell counting experiment because only a few axons are seen in the PNGs at that time point.
Groups 6 (Tx 4 wks) and 7 (Tx 4 wks + Ex) rats were used to measure sustained effects of exercise in injured neurons. Ten rats received Tx and TB labeling similar to Groups 4 and 5. Group 6 rats were not exercised while Group 7 rats received cycling exercise for 3 weeks. Both groups of rats were euthanized at 4 wks post injury.
Groups 8 (Tx 4 wks + PNG) and 9 (Tx 4 wks + PNG + Ex) rats were used to measure sustained effects of the presence of a PNG alone or the effects of exercise on regenerating neurons. Ten rats received Tx and acute placement of a PNG apposed as described above. Unlike Groups 1 and 2, the TB for these rats was placed at the distal end of the graft at the time of grafting. This allowed us to label regenerating neurons whose axons had reached the distal graft end without initiating changes in gene expression that would result from trimming of the distal graft end.
Cycling exercise
Rats were supported in a sling and hind limb paws dangling beneath them were secured to the pedals of a motorized cycling apparatus using surgical tape. Hind limbs were repetitively moved through a complete range of motion at 45 rpm for 30 minutes a day, 5 days per week as described previously (Côté et al., 2011; Houlé and Côté, 2013).
Tissue preparation and Staining
Counting the number of regenerating neurons
All neuron counting was done by personnel blind to the experimental condition. Blocks of spinal cord 0–5 mm from the graft-spinal cord interface were sectioned in a transverse plane at 30 μm on a cryostat. Sections were kept in serial order in phosphate buffer before every other section was serially mounted on a glass slide, air dried and cover slipped with Cytoseal 60 (Richard-Allan Scientific, Kalamazoo MI). TB labeled neurons were counted and binned in 1 mm segments from the block closest to the graft. Using fluorescence microscopy, TB positive neurons were identified as having a rounded to oval cell body of 20–30 μm diameter (see Figure 6 a1) with the proximal portion of processes filled with TB and the number of TB positive cells was counted manually in each section and the sum for each region of spinal cord was figured. Blocks of lumbar spinal cord (approximately 15 mm long) were sectioned longitudinally along the dorsal-ventral axis (horizontal plane) at 30 μm and mounted in serial order. Bilateral T13-L5 DRGs were sectioned at 30 μm and mounted in serial order. The number of TB positive neurons was figured for each pair of DRGs. Data are presented as mean ± SD.
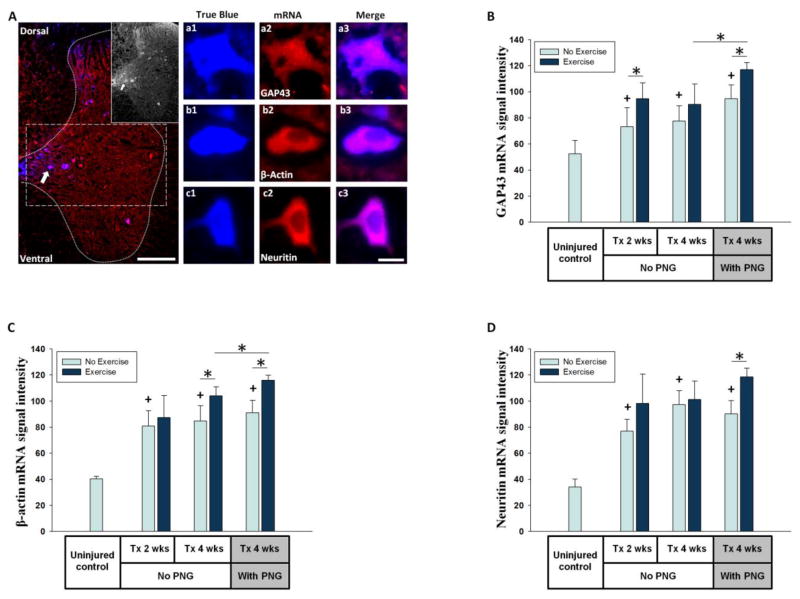
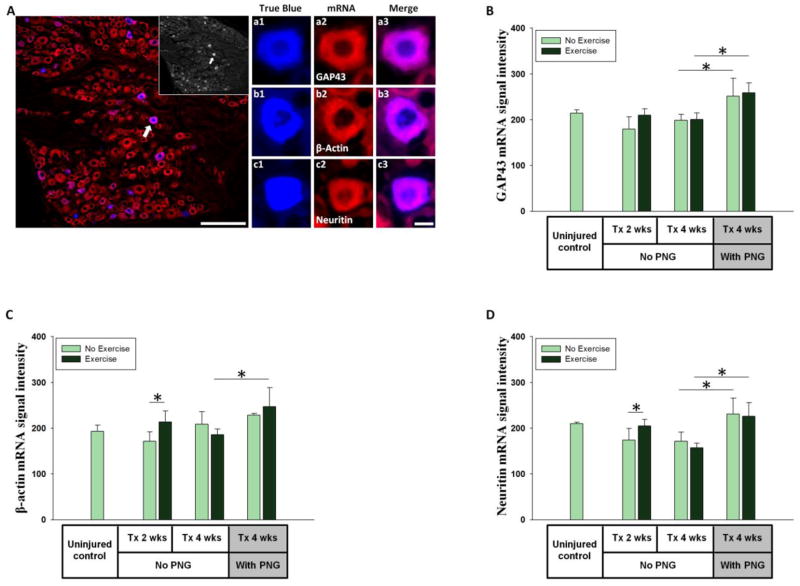
Figure 6. mRNA expression in propriospinal neurons.
(A) T13 spinal cord grey matter (dotted line) showing regenerating neurons labeled with TB placed at distal end of a PNG and stained for GAP43 mRNA (scale bar = 200μm). The inset shows TB positive neurons. Dash lined box is the region of interest for assessing propriospinal neurons in intermediate gray. a1 shows a higher magnification image of TB positive neuron stained for GAP43 mRNA (a2) and a3 shows a merged image. Similarly, b1-b3 and c1-c3 show individual TB positive neurons stained for βactin and Neuritin mRNAs respectively (scale bar = 10μm). (B, C, D) Y-axis shows average of FISH signal intensity per neuron for GAP43, β-actin, Neuritin mRNAs respectively. X-axis shows the animal groups. * indicates significant difference (p<0.05). + indicates significant difference (p<0.05) from uninjured control.
Gene expression in neurons
For fluorescent in situ hybridization (FISH), T13 spinal cord (between 1–2 mm caudal to the lesion) was sectioned in a transverse plane at 10 μm thickness and L1 DRGs were sectioned at 10 μm. Sections were mounted on Superfrost Plus slides ( isher rand, Pitts urgh, PA), air dried overnight and stored at −20°C. FISH reaction was performed as described previously using digoxygenin-tagged oligonucleotide probes for GAP43, β-actin and Neuritin mRNAs (Merianda et al., 2013). The probe sequences have been published (Willis et al., 2007). Scrambled probe was used as a negative control. Oligonucleotides were prepared with 3–5 “Int Amino Modifier C6 dT” modifications per 5 mer sequence (Integrated DNA Technologies, San Diego, CA). Probes were incubated overnight in digoxygenin (Digoxigenin-3-O-methylcarbonyl-ε-aminocaproic acid-N-hydroxysuccinimide ester, Roche Diagnostics, Indianapolis, IN) and N,N-Dimethylformamide (Sigma-Aldrich, St. Louis, MO) solution and purified using bench top column purification method (mini Quick Spin DNA Columns, Roche Diagnostics, Mannheim, Germany). The hybridized probes were identified by immunofluorescence using anti-digoxygenin antibody tagged with Cy3 (Jackson Immunoresearch, West Grove, PA). Five sections per animal through the L1 DRG and T13 spinal cord intermediate gray were analyzed for neurons expressing each mRNA. All TB positive neurons in 5 sections through each region were analyzed. This yielded at least 25 neurons per animal for analysis. Images were taken at 63X using a Leica epifluorescence microscope and all images were matched for exposure parameters. Intensity of the mRNA signal was quantified in individual TB labeled neurons using ImageJ and averaged between animals. Since tracer injection may affect gene expression, uninjured controls (Group 3) did not receive TB, so equal numbers of neurons of size and location comparable to injury only animals (Group 4) were randomly selected for analyses in corresponding regions of T13 spinal cord. It is possible that exercise has an effect on the mRNA levels of non-injured, non TB positive neurons, as suggested in Figure 6A, however, in the present study we focused specifically on injured/regenerating neurons labeled with TB. Therefore, except in the uninjured control group, only TB positive neurons were included in the analyses.
Statistical Analyses
Statistical analysis was performed using SigmaPlot 11.0 (Systat Software, Inc.). Results are expressed as mean ± standard deviation. Differences across groups in number of regenerating propriospinal neurons by distance caudal to the injury site (Figure 4b) or DRG level (Figure 5) were determined using a two-way ANOVA with repeated measures for group vs. location and a post-hoc Bonferroni’s multiple comparisons test. Significant differences across groups for remaining cell count analyses (Figures 4A and 4C) and for mRNA expression data ( igures 6 and 7) were determined y Student’s t-test. For all tests, the significance level was set to p < 0.05.
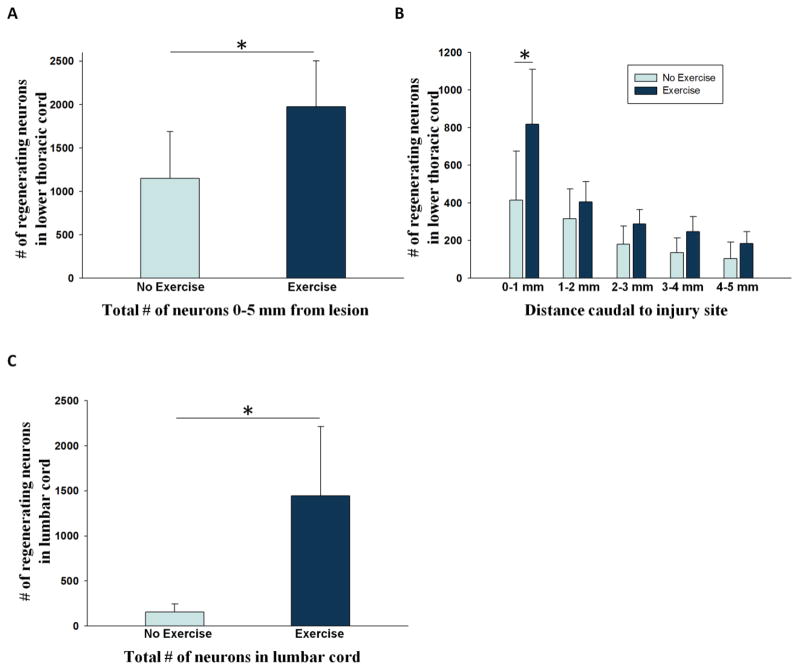
Figure 4. Exercise increases regeneration by spinal cord neurons.
(A) Total number of regenerating neurons within 5mm distance from injury site is significantly increased with exercise. (B) Number of regenerating neurons decreases as distance increases from the injury site. Post injury exercise increases the number of regenerating neurons within 1mm of the lesion. (C) Total number of regenerating neurons within lumbar spinal cord increases significantly with exercise. * indicates significant difference (p<0.05).
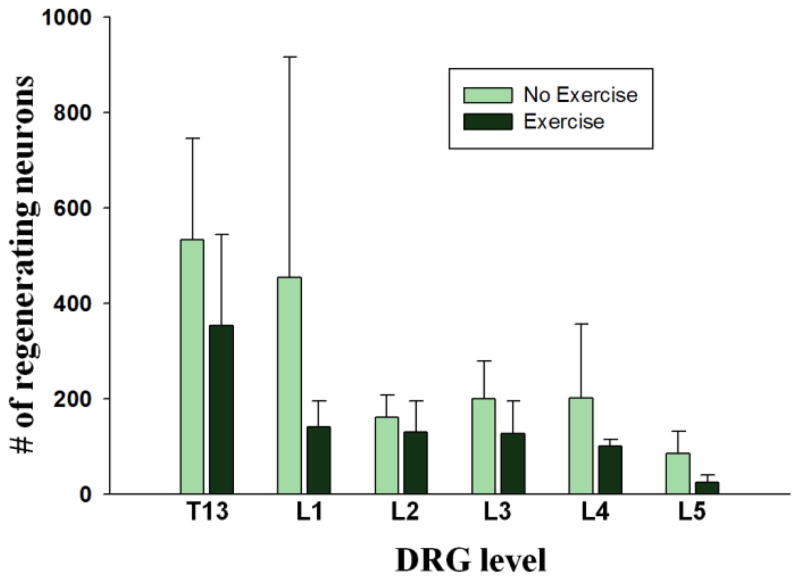
Figure 5. Exercise does not enhance axon regeneration from sensory neurons into PNGs.
The number of regenerating sensory neurons decreased as distance increased from the injury site. No significant change with exercise was observed in regenerating neurons from DRGs at any level.
Results
Exercise increases the number of regenerating spinal cord neurons
Neurons that regenerated their axons into the PNG were labeled by retrograde transport of TB from the distal end of the PNG and were visible under the fluorescent microscope using ultraviolet light. The majority of neurons that regenerated from the lower thoracic spinal cord were within the intermediate gray region, often in bilateral distribution around the central canal, consistent with the distribution of propriospinal neurons (Figure 3) (see Flynn et al., 2011). Very few TB labeled neurons were seen in either the dorsal or ventral horn.
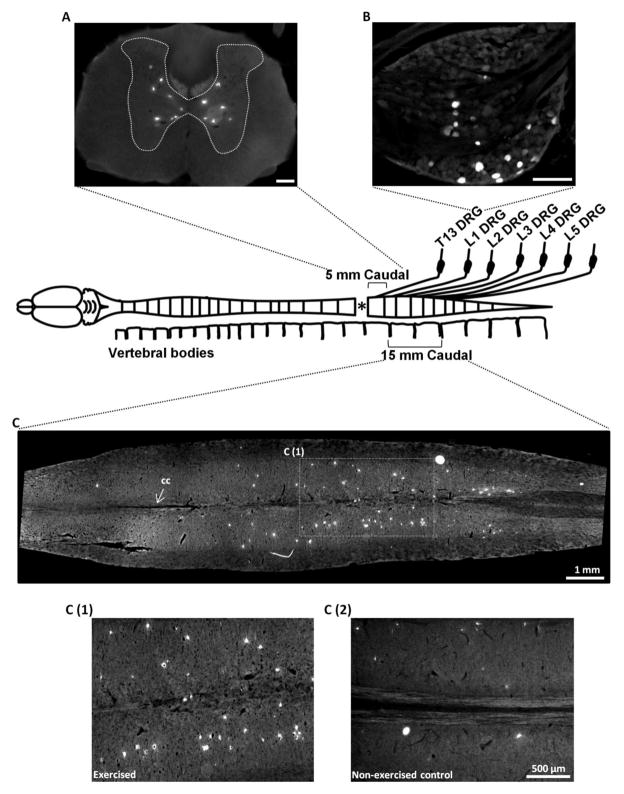
Figure 3. Identification of the number of regenerating spinal cord and sensory neurons caudal to injury.
Lower thoracic spinal cord was sectioned in transverse plane between 0–5 mm caudal to lesion (A, Scale bar = 200μm). Left and right DRGs were sectioned from T13 to L5 level (B, Scale bar = 200μm). C shows the Lumbar spinal cord from an exercised rat cut in horizontal plane. C (1) shows a high magnification image from the boxed region in C and C (2) shows a comparable region from a non-exercised control. TB positive neurons are defined as those regenerating their axon into the PNG. cc = central canal.
When considering the cross sections from the 5 mm block immediately caudal to the lesion (Figure 3B), exercise nearly doubled the number of regenerating neurons (1148 ± 541 in Tx 5 wks + PNG vs. 1973 ± 528 in Tx 5 wks + PNG + Ex condition, Figure 4A) with the highest number present within 1 mm of graft (414.2 ± 259.6 vs. 817.5 ± 292.8, Figure 4B). There was a gradual decline in the number of neurons as the distance from the graft/ lesion site increased. Individual regions (1–5 mm) more distant from the lesion site did not show an exercise related increase in the number of regenerating neurons (Figure 4B), but it is important to note that there was a significant increase in regenerating neurons within the 0–2 mm region as well.
The lumbar cord that was analyzed in a horizontal plane was approximately 15 mm in length. TB-labeled neurons were distributed uniformly throughout the rostral-caudal extent of this block with many in close proximity to the central canal (Figure 3C). Compared to Tx 5 wks + PNG group, exercised rats in Tx 5 wks + PNG + Ex group had a 9 fold increase in the number of regenerating neurons (156.4 ± 89.1 vs. 1445 ± 766.9 (Figures 3C1, 3C2 and 4C).
Exercise does not increase axon regeneration from sensory neurons
DRGs from both sides of the lower thoracic (T13) to lower lumbar (L5) level were sectioned at 30 μm and all TB positive neurons per ganglion were counted manually. Small and large (>30 μm diameter) DRG neurons were TB labeled in all ganglia (Figure 3B) and there was no significant difference in the number of regenerating neurons between Tx 5 wks + PNG and Tx 5 wks + PNG + Ex conditions based on neuron size. Similar to spinal cord neurons, most of the regenerating neurons in non-exercised animals were found in DRGs close to the graft/lesion site with a decrease as the distance from the lesion increased (Figure 5). Unlike the response of lumbar propriospinal neurons there was no exercise dependent increase in the number of regenerating DRG neurons at any of the spinal levels (Figure 5) or when totaled for all caudal ganglia that were examined (1639.25 ± 765.95 in Tx 5 wks + PNG vs. 875.25 ± 240.86 in Tx 5 wks + PNG + Ex condition, p= 0.106).
Exercise modulates the regeneration associated gene profile of axotomized and regenerating spinal cord neurons
We measured mRNA levels for three RAGs (GAP43, β-actin and Neuritin) in spinal cord neurons in response to axotomy, exercise, grafting and grafting plus exercise. In the injury and injury + Ex rats (Groups 4–7, Figure 2) application of TB tracer at the injury site labeled axotomized neurons, allowing for future selective identification of injured neurons in the spinal cord and DRGs. To analyze neurons that had regenerated their axon into a PNG, tracer was placed at the distal end of the PNG at the time of grafting so that only axons growing through to the distal end of the graft would be exposed to the tracer (Groups 8 and 9, Figure 2). TB positive neurons were analyzed in the intermediate gray (Figure 6A) between 1–2 mm caudal to the lesion. Since the effect of exercise on regeneration was significant at 0–2 mm from lesion (p=0.026) and neurons closer to the site of axotomy are more likely to regenerate their axon with or without exercise, we were able to sample a greater number of neurons per section when examining this region proximal to the injury. At 2 wks post injury the neurons are just beginning to extend their axons into the proximal end of PNG, while at 4 wks post injury most of the axons are approaching the distal end of the PNG (Amin et al., 2010), indicating that most neurons are in an active phase of regeneration at the 2 and 4 wks post-injury intervals analyzed here.
FISH signals for all 3 mRNAs were detected in many neurons in the intermediate gray of the uninjured control animals (Figure 6A). In Tx 2 wks rats, spinal cord neurons showed a significant upregulation of mRNA for all three RAGs. This injury response was maintained for at least 4 wks (Tx 4 wks) compared to uninjured control values (Figures 6 B, C, and D). Exercise had varying effects on propriospinal neurons. Short term (1 wk) exercise increased GAP43 mRNA FISH signals at 2 wks post injury but this was not maintained with continued exercise out to 4 wks post injury (Figure 6B). β-actin mRNA signals did not increase with acute exercise (Tx 2 wks + Ex) but did so with more prolonged daily exercise (Tx 4 wks + Ex; Figure 6C). Neuritin mRNA FISH signals were significantly increased with injury (Tx 2 wks or Tx 4 wks), yet no exercise related change in Neuritin mRNA was observed in Tx 2 wks + Ex or Tx 4 wks + Ex groups (Figure 6D).
In spinal cord neurons of non-exercised animals, the presence of a PNG did not result in a significant upregulation of any of the mRNAs above the response seen in the injury only Group 3 spinal cord. The combination of grafting and exercise (Tx 4 wks + PNG + Ex) caused a significant upregulation of all 3 mRNAs expression at 4 wks post grafting compared to the Tx 4 wks + PNG group, as well as to the Tx 4 wks + Ex group (Figures 6B, C, D).
Exercise has no significant effect on the regeneration associated gene profile of axotomized or regenerating DRG neurons
TB positive neurons in L1 DRGs (located approximately 25 mm from the lesion site), were analyzed for mRNA levels using FISH. As with the spinal cord neurons, FISH signals were detectable in the DRG neurons before injury. None of the RAG mRNAs were upregulated at Tx 2 wks or Tx 4 wks compared to uninjured controls in the L1 DRG neurons (Figure 7). βactin and Neuritin mRNA signals were significantly higher in injured neurons after 1 week of exercise but this response was not maintained with longer Ex contrary to what was observed for β-actin in spinal cord neurons (Tx 4 wks vs. Tx 4 wks + Ex; Figure 7C, D). No change was seen in GAP43 mRNA signals with exercise in injured DRG neurons at any timepoint (Tx 2 wks, Tx 2 wks + Ex, Tx 4 wks, Tx 4 wks + Ex, Figure 7B). Four weeks of exercise alone did not alter any of the mRNAs tested in sensory neurons (Tx 4 wks vs. Tx 4 wks + Ex). At 4 wks post injury, GAP43 and Neuritin mRNA FISH signals in the L1 DRGS were increased in the presence of PNGs without exercise (Tx 4 wks vs. Tx 4 wks + PNG) but not when Ex was combined with PNGs (Tx 4 wks + PNG vs. Tx 4 wks + PNG + Ex). This suggests that in these sensory neurons the major effect on mRNA levels is due to the presence of a PNG (Figure 7B, C and D).
Figure 7. mRNA expression in sensory neurons.
(A) A section from L1 DRG showing regenerating neurons labeled by TB placed at the distal end of a PNG and stained for GAP43 mRNA (scale bar = 200μm). The inset shows TB labeled neurons. a1 shows a magnified image of a TB positive neuron stained for GAP43 mRNA (a2) and a3 shows a merged image. Similarly, b1-b3 and c1-c3 show individual TB positive neurons stained for β-actin and Neuritin mRNAs respectively (scale bar = 10μm). (B, C, D) Y-axis shows average of FISH signal intensity per neuron for GAP43, β-actin, Neuritin mRNAs respectively. X-axis shows the animal groups. * indicates significant difference (p<0.05).
Discussion
In the present study, we used a cycling exercise that provides rhythmic sensory stimulation to the spinal cord and is known to increase intraspinal levels of neurotrophins and phosphorylation of the ribosomal protein S6 that is downstream of mTOR (Houlé and Côté, 2013). These local effects of exercise not only suggest a neuroprotective and pro-regenerative effect that could promote elongation of axons into a PNG but also the potential for enhanced synaptic activity and neuroplasticity that could increase axonal growth and reconnection with targets beyond the PNG. In support of the significant role that exercise appears to play in neuroplasticity we present evidence that the intrinsic regenerative effort of propriospinal neurons, but not primary sensory neurons is enhanced by cycling exercise and that this effort is correlated with upregulation of mRNA levels of the regeneration associated genes GAP43, βactin and Neuritin. An intraspinal PNG not only supports extension of axons but also provides a unique model for selectively studying the regenerative efforts of different populations of neurons and the application of possible therapeutic interventions to enhance regeneration.
Exercise increases regeneration from propriospinal neurons
The majority of axons that ascend into the PNG from spinal cord are from propriospinal neurons present in the intermediate gray region of spinal cord. These neurons greatly outnumber the motor neurons in the spinal cord and mediate a variety of functions while integrating and modulating both sensory and motor inputs (Chung et al., 1984; Conta and Stelzner 2009; Flynn et al., 2011). While short-axon propriospinal neurons in the lumbar enlargement modulate input to lower limb motor neuron pools (Gerasimenko et al., 2002; Jordan and Schmidt, 2002; Kostyuk and Maisky, 1972; Kostyuk et al., 1971), ascending and descending long-axon propriospinal neurons can mediate the crosstalk between lumbosacral and cervical regions (Miller, 1970; Miller et al., 1971, 1973). The number, location, elaborate projection patterns and functional capabilities of these neurons make them a suitable target for promoting functional recovery after SCI. An important study by Bareyre et al. (2004) showed that after dorsal spinal hemisection, axons of the corticospinal tract formed functional de novo spinal circuits with long descending propriospinal neurons to relay information to lumbar motor neuron pools. Findings by Courtine et al. (2008) convincingly demonstrated the ability of propriospinal neurons to restore functional locomotor recovery after a spinal cord injury that severed all supraspinal descending pathways using staggered lateral hemisections at T7 and T12 levels on opposite sides of the spinal cord. These studies highlight the remarkable plastic properties of propriospinal neurons and the observation that the circuitry for functional recovery could remain intact within the injured spinal cord and be potentiated for functional recovery using physical rehabilitation (Raineteau and Schwab, 2001).
The number of propriospinal neurons regenerating into a PNG is highest proximal to the lesion-graft interface and it decreases as the distance of neurons from the injury site increases. Exercise resulted in a significant increase in regeneration by neurons at both proximal (T13) and distal (L1-5) locations. Hind limb cycling may produce this effect by direct stimulation of the neurons via rhythmically activated primary afferents and/or via local upregulation of pro-regenerative neurotrophic factors within the spinal cord. Data supporting direct and indirect mechanisms suggests that both can contribute to an increase in regenerative effort. Unpublished data from our lab shows that exercise fails to upregulate brain derived neurotrophic factor (BDNF) after deafferentation of the spinal cord below the level of a transection injury indicating the critical need for afferent input to process and mediate some of the effects of cycling.
Exercise does not increase regeneration from DRGs
When provided with a PNG after SCI, primary sensory neurons showed a strong propensity for regeneration (~1700 DRG neurons vs. ~1300 propriospinal neurons). The majority of TB positive neurons were found in DRGs closer to the injury as the number of TB+ neurons per ganglion decreased as distance increased from the injury site. Given the effect of cycling on the regenerative effort of interneurons it is curious that the total number of regenerating DRG neurons did not change with exercise. This result was surprising since a study from the Twiss lab demonstrated an activity driven, neurotrophin dependent enhanced outgrowth of neurites from cultured adult DRG neurons and regeneration of peripheral axons after a nerve crush in vivo (Molteni et al., 2004). Sensory neurons in DRGs are pseudounipolar and have a central and a peripheral axonal process branching from each cell body. The regenerative response from the cell body shows branch specificity where the peripheral injury successfully mounts the growth associated response while injury to the central process fails to elicit a similar response (Chong et al., 1994; Schreyer and Skene, 1993). However, the regenerative capacity of the central DRG process can be potentiated by an injury (preconditioning lesion) to the peripheral branch (Neumann and Woolf, 1999; Richardson and Verge, 1987), which initiates the transport of retrograde signals to the cell body that may stimulate the regeneration associated gene program (Michaelevski et al., 2010; Rishal and Fainzilber, 2010; Tetzlaff et al., 1991). Epigenetic evidence of this distinct response comes from a study where a peripheral ut not central axon injury triggers PKCμ-dependent nuclear export of HDAC5 causing enhanced histone acetylation and pro-regenerative gene expression (Cho et al., 2013). Additionally, a more recent study has shown that histone acetyltransferase p300/CBP-associated factor (PCAF) modulates chromatin environment of essential RAGs to support regeneration after a peripheral but not central injury (Puttagunta et al., 2014). These studies strongly suggest that the intrinsic regenerative state of the injured neurons is a key determinant of their regenerative capacity (Rossi et al., 2007).
Neuronal subpopulations show unique changes in gene expression following injury, grafting and exercise
To address the disparity in regenerative response from sensory vs. propriospinal neurons, we examined the expression of RAGs in these neuronal subpopulations in regenerating/non-regenerating conditions (presence and absence of a PNG) and in exercise and no exercise conditions. Presence of TB tracer identified injured or regenerating neurons. The complete RAG program of injured neurons can consist of several genes and successful regeneration likely requires co-operative effort of multiple transcriptional events (Fagoe et al., 2014). We examined three key RAGs that have been shown to be strongly associated with the regenerative effort and are almost indispensable for a successful regeneration event. GAP43 mRNA levels are elevated during development (Benowitz and Routtenberg, 1997) and increase in successfully regenerating neurons but not in the injury conditions where no regeneration takes place (Mason et al., 2003; Skene and Willard, 1981). Abundance of GAP43 protein in the axonal and growth cone regions is consistent with its active involvement in axon elongation and synaptic plasticity (Skene, 1989). β-actin, being a cytoskeletal element, is not only involved in formation and extension of axons during regeneration, ut asymmetrical localization of β-actin in the growth cone facilitates growth cone turning in response to positive and negative stimuli (Leung et al., 2006; Yao et al., 2006). Neuritin (candidate plasticity gene 15 or cpg15) codes for a glycosylphosphatidylinositol-anchored axonal protein that is induced by extraneuronal cues such as neurotrophins and neuronal activity. Neuritin can promote neurite outgrowth, synapse formation and synapse stability and shape dendritic arbors of target neurons (Fujino et al., 2011; Naeve et al., 1997; Nedivi et al., 1996; Zhou and Zhou, 2014).
Responsiveness of these genes to extracellular cues and a strong association with the regenerative effort of neurons allowed us to investigate how different types of neurons respond to injury, grafting and exercise. An injury associated increase in RAG mRNAs is suggestive of a spontaneous regenerative drive in the propriospinal neurons which is further augmented by the presence of a growth promoting PNG and even more by the stimulating input from hindlimb exercise. However, the sensory neurons do not show injury associated changes in mRNAs for these RAGs. RAG mRNAs do upregulate in the presence of a PNG but are not further potentiated by exercise. This suggests that the positive extracellular cues from activated Schwann cells within the graft may stimulate the expression of RAGs. It should be noted that exercise did upregulate β-actin and Neuritin at 2 wks post injury, however, the elevation did not persist out to 4 wks post injury. Previous work in our laboratory has shown that while post-injury cycling exercise upregulates BDNF, GDNF and NT-4 mRNAs in spinal cord intermediate gray, DRG neurons remain unaffected by either injury or exercise (Keeler et al., 2012). However, in the same study, exercise reduced the injury-associated increase in Caspase-7 in both spinal cord and DRG samples. This indicates that our current exercise paradigm may provide for neuroprotection of injured DRGs but it is not sufficient to elicit a stronger regenerative response.
The absence of an effect of exercise on sensory axon growth may have some beneficial effect since aberrant sprouting of primary afferents can lead to development of neuropathic pain (Ondarza et al., 2003). Recent work from the Gallo lab has demonstrated that intracellular increase in actin could lead to axonal branch formation and increased sprouting from sensory axons (Spillane et al., 2011; Spillane et al., 2013). Although we did not test animals in the current study for SCI-associated neuropathic pain, a recent study from our lab has shown the beneficial effects of acute exercise in reducing afferent sprouting in the dorsal horn and preventing the onset of neuropathic pain in a contusion SCI model (Detloff et al., 2014). We have previously shown that neurons can regenerate into a PNG in a chronic injury situation (Houlé, 1991; Tom et al., 2009) and that chronically injured neurons are responsive to trophic factors applied at the injury site 4 or 8 weeks post injury (Houlé and Ye, 1999). Future studies will examine whether delayed exercise can have a growth promoting effect on chronically injured neurons. Another exciting possibility is to address whether exercise can potentiate the outgrowth of axons beyond the distal end of the PNG into the spinal cord to form synaptic contacts with neurons above the level of injury. Similar strategies could be employed to potentiate regeneration from descending neurons by apposing a PNG to the rostral end of the lesion cavity. Since both ascending and descending regenerative effort can be studied within the same animal, this strategy holds potential to promote both sensory and motor recovery in a spinalized animal. The current study brings forward an exciting possibility of using exercise driven regeneration to improve functional recovery after SCI.
Highlights.
Peripheral nerve grafts support CNS regeneration after SCI.
Post injury exercise increases regeneration into peripheral nerve grafts.
Regenerative effort is correlated with the levels of regeneration-associated mRNAs.
Propriospinal but not sensory neurons respond to exercise.
Data suggest exercise as a therapy to promote regeneration after SCI.
Acknowledgments
This work was supported by grants from the Craig H. Neilsen Foundation (224125 to JDH) and the National Institutes of Health (PO1-NS055976 to JDH and JLT). JLT is supported by the South Carolina SmartState Endowment Program (Center for Childhood Neurotherapeutics) through the University of South Carolina. Technical assistance by Scarlett Austin and Danielle Kulich is greatly appreciated.
Footnotes
Abbreviations: Ex= Exercise, FISH= Fluorescent in situ hybridization, PNG= Peripheral nerve graft, RAGs= Regeneration-associated genes, TB= True Blue, Tx= Transection.
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afshari FT, Kappagantula S, Fawcett JW. Extrinsic and intrinsic factors controlling axonal regeneration after spinal cord injury. Expert reviews in molecular medicine. 2009;11:e37. doi: 10.1017/S1462399409001288. [DOI] [PubMed] [Google Scholar]
- Amin AA, Houlé JD. The role of neurotrophic factors and their receptors in ascending and descending axon regeneration through intrapsinal peripheral nerve grafts (PNGs) Abstr Soc Neurosci. 2010;2010:588.28. [Google Scholar]
- Armada-da-Silva PA, Pereira C, Amado S, Veloso AP. Role of physical exercise for improving posttraumatic nerve regeneration. International review of neurobiology. 2013;109:125–149. doi: 10.1016/B978-0-12-420045-6.00006-7. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nature neuroscience. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends in neurosciences. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Bray GM, Villegas-Perez MP, Vidal-Sanz M, Aguayo AJ. The use of peripheral nerve grafts to enhance neuronal survival, promote growth and permit terminal reconnections in the central nervous system of adult rats. The Journal of experimental biology. 1987;132:5–19. doi: 10.1242/jeb.132.1.5. [DOI] [PubMed] [Google Scholar]
- Cho Y, Sloutsky R, Naegle KM, Cavalli V. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell. 2013;155:894–908. doi: 10.1016/j.cell.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong MS, Reynolds ML, Irwin N, Coggeshall RE, Emson PC, Benowitz LI, Woolf CJ. GAP-43 expression in primary sensory neurons following central axotomy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:4375–4384. doi: 10.1523/JNEUROSCI.14-07-04375.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K, Kevetter GA, Willis WD, Coggeshall RE. An estimate of the ratio of propriospinal to long tract neurons in the sacral spinal cord of the rat. Neuroscience letters. 1984;44:173–177. doi: 10.1016/0304-3940(84)90077-6. [DOI] [PubMed] [Google Scholar]
- Conta A, Stelzner D. The Propriospinal System. In: Watson C, Paxinos G, Kayalioglu G, editors. The Spinal Cord a Christopher and Dana Reeve Foundation Text and Atlas. New York: Academic Press; 2009. pp. 180–190. [Google Scholar]
- Côté MP, Azzam GA, Lemay MA, Zhukareva V, Houlé JD. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. Journal of neurotrauma. 2011;28:299–309. doi: 10.1089/neu.2010.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nature medicine. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Detloff MR, Smith EJ, Quiros Molina D, Ganzer PD, Houlé JD. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Experimental neurology. 2014;255:38–48. doi: 10.1016/j.expneurol.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolbeare D, Houlé JD. Restriction of axonal retraction and promotion of axonal regeneration by chronically injured neurons after intraspinal treatment with glial cell line-derived neurotrophic factor (GDNF) Journal of neurotrauma. 2003;20:1251–1261. doi: 10.1089/089771503770802916. [DOI] [PubMed] [Google Scholar]
- Fagoe ND, van Heest J, Verhaagen J. Spinal cord injury and the neuron-intrinsic regeneration-associated gene program. Neuromolecular medicine. 2014;16:799–813. doi: 10.1007/s12017-014-8329-3. [DOI] [PubMed] [Google Scholar]
- Ferguson TA, Son YJ. Extrinsic and intrinsic determinants of nerve regeneration. Journal of tissue engineering. 2011;2:2041731411418392. doi: 10.1177/2041731411418392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JR, Graham BA, Galea MP, Callister RJ. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology. 2011;60:809–822. doi: 10.1016/j.neuropharm.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Fujino T, Leslie JH, Eavri R, Chen JL, Lin WC, Flanders GH, Borok E, Horvath TL, Nedivi E. CPG15 regulates synapse stability in the developing and adult brain. Genes & development. 2011;25:2674–2685. doi: 10.1101/gad.176172.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko YP, Makarovskii AN, Nikitin OA. Control of locomotor activity in humans and animals in the absence of supraspinal influences. Neuroscience and behavioral physiology. 2002;32:417–423. doi: 10.1023/a:1015836428932. [DOI] [PubMed] [Google Scholar]
- Giehl KM, Tetzlaff W. BDNF and NT-3, but not NGF, prevent axotomy-induced death of rat corticospinal neurons in vivo. The European journal of neuroscience. 1996;8:1167–1175. doi: 10.1111/j.1460-9568.1996.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Goulart CO, Jurgensen S, Souto A, Oliveira JT, de Lima S, Tonda-Turo C, Marques SA, de Almeida FM, Martinez AM. A combination of Schwann-cell grafts and aerobic exercise enhances sciatic nerve regeneration. PloS one. 2014;9:e110090. doi: 10.1371/journal.pone.0110090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlé JD. Demonstration of the potential for chronically injured neurons to regenerate axons into intraspinal peripheral nerve grafts. Experimental neurology. 1991;113:1–9. doi: 10.1016/0014-4886(91)90139-4. [DOI] [PubMed] [Google Scholar]
- Houlé JD, Côté MP. Axon regeneration and exercise-dependent plasticity after spinal cord injury. Annals of the New York Academy of Sciences. 2013;1279:154–163. doi: 10.1111/nyas.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlé JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlé JD, Ye JH. Survival of chronically-injured neurons can be prolonged by treatment with neurotrophic factors. Neuroscience. 1999;94:929–936. doi: 10.1016/s0306-4522(99)00359-0. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Schmidt BJ. Propriospinal neurons involved in the control of locomotion: potential targets for repair strategies? Progress in brain research. 2002;137:125–139. doi: 10.1016/s0079-6123(02)37012-2. [DOI] [PubMed] [Google Scholar]
- Keeler BE, Liu G, Siegfried RN, Zhukareva V, Murray M, Houlé JD. Acute and prolonged hindlimb exercise elicits different gene expression in motoneurons than sensory neurons after spinal cord injury. Brain research. 2012;1438:8–21. doi: 10.1016/j.brainres.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk PG, Maisky VA. Propriospinal projections in the lumbar spinal cord of the cat. Brain research. 1972;39:530–535. doi: 10.1016/0006-8993(72)90459-3. [DOI] [PubMed] [Google Scholar]
- Kostyuk PG, Vasilenko DA, Lang E. Propriospinal pathways in the dorsolateral funiculus and their effects on lumbosacral motoneuronal pools. Brain research. 1971;28:233–249. doi: 10.1016/0006-8993(71)90657-3. [DOI] [PubMed] [Google Scholar]
- Leung KM, van Horck FP, Lin AC, Allison R, Standart N, Holt CE. Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nature neuroscience. 2006;9:1247–1256. doi: 10.1038/nn1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Detloff MR, Miller KN, Santi L, Houlé JD. Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury. Experimental neurology. 2012;233:447–456. doi: 10.1016/j.expneurol.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nature neuroscience. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MR, Lieberman AR, Anderson PN. Corticospinal neurons up-regulate a range of growth-associated genes following intracortical, but not spinal, axotomy. The European journal of neuroscience. 2003;18:789–802. doi: 10.1046/j.1460-9568.2003.02809.x. [DOI] [PubMed] [Google Scholar]
- Merianda TT, Vuppalanchi D, Yoo S, Blesch A, Twiss JL. Axonal transport of neural membrane protein 35 mRNA increases axon growth. Journal of cell science. 2013;126:90–102. doi: 10.1242/jcs.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelevski I, Segal-Ruder Y, Rozenbaum M, Medzihradszky KF, Shalem O, Coppola G, Horn-Saban S, Ben-Yaakov K, Dagan SY, Rishal I, Geschwind DH, Pilpel Y, Burlingame AL, Fainzilber M. Signaling to transcription networks in the neuronal retrograde injury response. Science signaling. 2010;3:ra53. doi: 10.1126/scisignal.2000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. Excitatory and inhibitory propriospinal pathways from lumbo-sacral to cervical segments in the cat. Acta physiologica Scandinavica. 1970;80:25A–26A. doi: 10.1111/j.1748-1716.1970.tb04849.x. [DOI] [PubMed] [Google Scholar]
- Miller S, Reitsma DJ, van der Meche FG. Excitatory ascending propriospinal actions between lumbosacral and cervical segments in the cat. The Journal of physiology. 1971;218(Suppl):76P–77P. [PubMed] [Google Scholar]
- Miller S, Reitsma DJ, van der Meche FG. Functional organization of long ascending propriospinal pathways linking lumbo-sacral and cervical segments in the cat. Brain research. 1973;62:169–188. doi: 10.1016/0006-8993(73)90626-4. [DOI] [PubMed] [Google Scholar]
- Molteni R, Zheng JQ, Ying Z, Gomez-Pinilla F, Twiss JL. Voluntary exercise increases axonal regeneration from sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8473–8478. doi: 10.1073/pnas.0401443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ. Axon regeneration: what needs to be overcome? Methods in molecular biology. 2014;1162:3–14. doi: 10.1007/978-1-4939-0777-9_1. [DOI] [PubMed] [Google Scholar]
- Naeve GS, Ramakrishnan M, Kramer R, Hevroni D, Citri Y, Theill LE. Neuritin: a gene induced by neural activity and neurotrophins that promotes neuritogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2648–2653. doi: 10.1073/pnas.94.6.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedivi E, Fieldust S, Theill LE, Hevron D. A set of genes expressed in response to light in the adult cerebral cortex and regulated during development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2048–2053. doi: 10.1073/pnas.93.5.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- Ondarza AB, Ye Z, Hulsebosch CE. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Experimental neurology. 2003;184:373–380. doi: 10.1016/j.expneurol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttagunta R, Tedeschi A, Soria MG, Hervera A, Lindner R, Rathore KI, Gaub P, Joshi Y, Nguyen T, Schmandke A, Laskowski CJ, Boutillier AL, Bradke F, Di Giovanni S. PCAF-dependent epigenetic changes promote axonal regeneration in the central nervous system. Nature communications. 2014;5:3527. doi: 10.1038/ncomms4527. [DOI] [PubMed] [Google Scholar]
- Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nature reviews. Neuroscience. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980;284:264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- Richardson PM, Verge VM. Axonal regeneration in dorsal spinal roots is accelerated by peripheral axonal transection. Brain research. 1987;411:406–408. doi: 10.1016/0006-8993(87)91096-1. [DOI] [PubMed] [Google Scholar]
- Rishal I, Fainzilber M. Retrograde signaling in axonal regeneration. Experimental neurology. 2010;223:5–10. doi: 10.1016/j.expneurol.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Rossi F, Gianola S, Corvetti L. Regulation of intrinsic neuronal properties for axon growth and regeneration. Progress in neurobiology. 2007;81:1–28. doi: 10.1016/j.pneurobio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Schreyer DJ, Skene JH. Injury-associated induction of GAP-43 expression displays axon branch specificity in rat dorsal root ganglion neurons. Journal of neurobiology. 1993;24:959–970. doi: 10.1002/neu.480240709. [DOI] [PubMed] [Google Scholar]
- Skene JH. Axonal growth-associated proteins. Annual review of neuroscience. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Skene JH, Willard M. Axonally transported proteins associated with axon growth in rabbit central and peripheral nervous systems. The Journal of cell biology. 1981;89:96–103. doi: 10.1083/jcb.89.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Jones SL, Korobova F, Marsick B, Lanier L, Svitkina T, Gallo G. The actin nucleating Arp2/3 complex contributes to the formation of axonal filopodia and branches through the regulation of actin patch precursors to filopodia. Developmental neurobiology. 2011;71:747–758. doi: 10.1002/dneu.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Merianda TT, Twiss JL, Gallo G. Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell reports. 2013;5:1564–1575. doi: 10.1016/j.celrep.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodori RM, Betini J, de Oliveira LS, Sobral LL, Takeda SY, de Lima Montebelo MI. Swimming exercise in the acute or late phase after sciatic nerve crush accelerates nerve regeneration. Neural plasticity. 2011;2011:783901. doi: 10.1155/2011/783901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff W, Alexander SW, Miller FD, Bisby MA. Response of facial and rubrospinal neurons to axotomy: changes in mRNA expression for cytoskeletal proteins and GAP-43. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1991;11:2528–2544. doi: 10.1523/JNEUROSCI.11-08-02528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Sandrow-Feinberg HR, Miller K, Santi L, Connors T, Lemay MA, Houlé JD. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:14881–14890. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DE, van Niekerk EA, Sasaki Y, Mesngon M, Merianda TT, Williams GG, Kendall M, Smith DS, Bassell GJ, Twiss JL. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. The Journal of cell biology. 2007;178:965–980. doi: 10.1083/jcb.200703209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Aebischer P, Bunge MB. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Experimental neurology. 1995;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- Yao J, Sasaki Y, Wen Z, Bassell GJ, Zheng JQ. An essential role for beta-actin mRNA localization and translation in Ca2+-dependent growth cone guidance. Nature neuroscience. 2006;9:1265–1273. doi: 10.1038/nn1773. [DOI] [PubMed] [Google Scholar]
- Young W. Spinal cord regeneration. Cell transplantation. 2014;23:573–611. doi: 10.3727/096368914X678427. [DOI] [PubMed] [Google Scholar]
- Zhou S, Zhou J. Neuritin, a neurotrophic factor in nervous system physiology. Current medicinal chemistry. 2014;21:1212–1219. doi: 10.2174/0929867321666131218093327. [DOI] [PubMed] [Google Scholar]