Abstract
Treatment of iron deficiency anemia in malaria endemic areas is complicated as iron supplementation increases malaria risk while malaria decreases iron absorption. Here we measured the influence of hepcidin expression and nonheme iron during iron supplementation on hepatic Plasmodium berghei numbers in anemic and nonanemic mice. Despite elevated hepatic nonheme iron on the high iron diet, elevated hepcidin expression is associated with less parasite bioavailable iron and lower hepatic parasite loads in anemic, iron deficient mice after both two and six weeks of supplementation. A marginal trend to lower parasite hepatic numbers was seen in nonanemic, iron replete mice. In a transgenic model of severe anemia, mice with a deletion in Sec15l1, which reportedly have normal liver iron and normal hepcidin expression, there were no changes in liver parasite numbers or bloodstage numbers or outcome in the lethal P. yoelii model. In summary during iron supplementation the lower hepatic malaria numbers are regulated more by hepcidin than the absolute level of nonheme hepatic iron.
Keywords: Anemia, Iron-Deficiency, malaria, hepcidin
1. Introduction
Each year approximately 200 million cases of human malaria occur, with most of the morbidity and mortality in children under five in SubSaharan Africa [1, 2]. Malaria infection often coincides geographically with iron deficiency anemia. Malaria contributes to anemia by decreasing intestinal iron absorption, restricting erythropoiesis and sequestering bioavailable iron in ferritin stores and hemozoin [3]. Prevalence of anemia can be as high as 95% in malaria hyperendemic areas [4]. Studies examining malaria, anemia and iron supplementation have resulted in contradictory findings. Some older studies associated injectable iron or oral refeeding in famine stricken refugees with a high percentage of symptomatic malaria [5, 6]. However, other studies including a meta-analysis did not show exacerbation of human malaria with oral iron replacement [4, 7-10]. Policy on iron supplementation of anemic individuals has been intensely debated after the study in the Island of Pemba, hyperendemic for malaria in the 1990's and early 2000's, showed that both iron and folate supplementation together increased the risk for hospitalization and death presumed to be from malaria in iron replete rather than iron deficient children regardless of anemia status [11]. More specifically the supplementation had an apparent significant risk of adverse outcomes and deaths in children iron replete (Zinc protoporphyrin IX (ZnPPIX) as a metric) with or without anemia (hemoglobin as metric), a protective effect in children who were both iron deficient and anemic and no effect in children iron deficient and with no anemia.
Hepatic malaria stages are required for maturation and replication of parasites before generating the erythrocytic forms that cause the symptoms [12]. Iron supplementation followed by sporozoite infection within 48 hours increases murine hepatic parasite number and iron chelators decrease the development of these exoerythrocytic forms [13-15]. The level of hepcidin, an important iron regulator, is increased in blood stage malaria to high levels which limit liver stage malaria [13]. Hepcidin induces ferroportin degradation which limits iron absorption from intestinal cells and prevents release from macrophages [16-18]. Hepcidin is also induced by inflammation [19, 20] and is down-regulated by hypoxia or iron deficiency [21]. Iron supplementation increases hepcidin over the span of hours to days, preventing further absorption of iron supplements [18].
We hypothesized that hepcidin expression and hepatic iron stores regulate iron available to the malaria parasite. We further hypothesized that this bioavailable iron influences hepatic parasite number during iron replenishment and changes malaria outcome. In the present study, hepatic stage malaria parasite numbers were compared in nonphlebotomized, nonanemic, iron-replete wild type mice and in phlebotomized, anemic, iron-deficient wild type mice fed on either high or low iron diets at two time durations. Additionally, an anemic mouse with a deletion in a gene for transferrin recycling was tested.
2. Materials And Methods
2.1. Iron deficiency from phlebotomy and iron diets
For the first biologic replicate, mice raised on the standard iron diet were purchased at 5 months of age. To induce both anemia and iron deficiency, phlebotomy was performed with removal of 500 μl of blood 3 times in the course of one week followed by a low iron diet (Fig. 1). Three weeks after phlebotomy, the murine anemia was measured by hemoglobin and iron status was measured by the ZnPPIX/heme ratio. Two days post anemia confirmation, four groups were created with nonphlebotomized, nonanemic, iron-replete mice or phlebotomized, anemic, iron-deficient mice placed on either low or high iron diets. Food and ultrapure water (Quality Biological cat #351-029-131) were provided ad libitum. The high iron diet contained the same protein, carbohydrate, lipid base with 20 g iron/kg [22] (Harlan, Madison, WI-TD.08714). Both diets contained 2.8 mg/kg of folic acid.
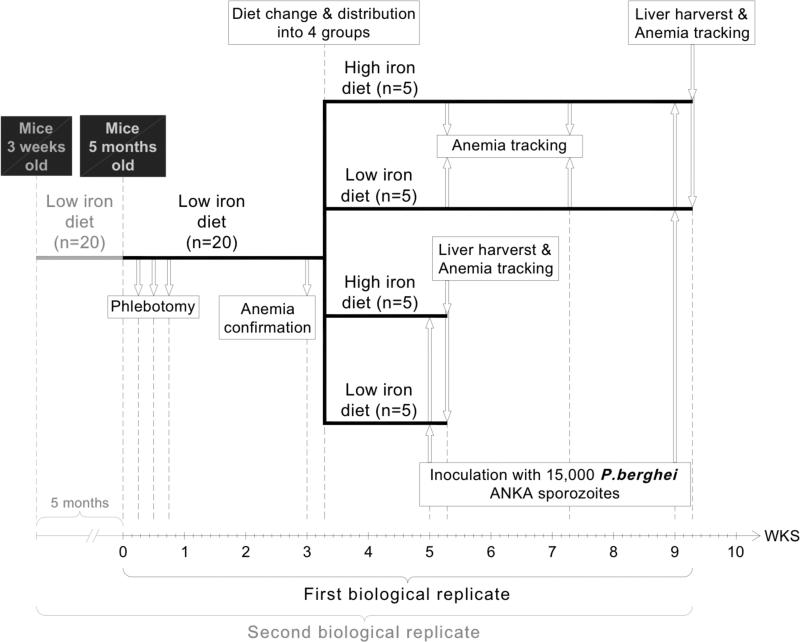
Fig 1. Schedule for the biologic replicates, diet, sporozoite infection and liver harvest.
In replicate 1 mice were purchased at 5 months of age and split into two groups based on phlebotomy (nonphlebotomized groups are not shown on the shcme; they followed the same pattern of diet change after anemia confirmation on phlebotomized mice) with generation of four groups based on two different diets which were then sampled after 2 and 6 weeks with a change in diet. For the second replicate 3 week old mice were maintained on a low iron diet which did not change hemoglobin or ZnPPIX and after 5 months the mice on the low iron diet were phlebotomized and split into groups as in replicate 1.
Anemia status was checked again every 2 weeks until day of sacrifice with a removal of 100 μl of blood. For the second biological replicate, three- week old female Balb/c mice were purchased from Jackson Laboratories (Maine, USA). Half the mice were maintained on a low iron diet of 2-6 mg iron/kg [23-25] (Harlan, Madison, WI-TD.99397) for 5 months, which did not induce anemia. Half the mice were phlebotomized as above.
2.2 Animal ethics
All experiments were performed according to protocol “Iron and Murine Malaria” (Approval ID-MO09H403) approved by The Johns Hopkins Animal Care and Use Committee in accordance with institutional standards.
2.3. Iron assays
For survival experiments, blood was collected by retro-orbital bleeding from Avertin anesthetized mice. Perimortem, blood was collected by cardiac puncture. All whole blood was collected into EDTA microtainer tubes (BD, Franklin Lakes, NJ). Mouse hemoglobin levels were determined based on the absorbance of samples at 540 nm using the Drabkin's reagent (Sigma, St Louis, MO) following the instructions provided by the manufacturer. An aliquot of blood was placed on a cover glass (Aviv Biomedical Inc., Lakewood, NJ) to quantify the ZnPPIX/heme ratio on the ZPP hematofluorometer (Aviv Biomedical Inc., Lakewood, NJ). Normal mouse ratios of ZnPPIX/heme in the literature range from 80 to 120 μM ZnPPI/M heme [26-28]. Livers and spleens from P. berghei ANKA infected female Balb/c mice were collected 40 hours post infection and stored at −80°C. Tissue nonheme iron was quantified as described by Cook with minor modifications [29, 30]. Briefly, tissues were accurately weighted and digested in an acid solution containing HCl 3M and 0.61M trichloroacetic acid for 50 h at 65°C. Fifty microliters of the tissue extract were added to 1 ml of working chromogen solution (saturated sodium acetate, and 0.01% bathophenanthroline sulfonate and 0.1% thioglycolic acid). Samples were incubated for 10 minutes at room temperature. Absorbance was determined at 535 nm. An iron standard curve was performed with an iron standard diluted in acid solution with concentrations ranging from 200 μg/dl to 1000 μg/dl. Measurements were expressed as micrograms of iron per gram of tissue.
2.4. qReal-Time PCR quantification of liver infection and murine hepcidin level
Mouse livers were collected and homogenized in 12 ml TRizol (Invitrogen, Carlsbad, CA) and stored at −80°C. Total RNA was isolated by adding chloroform and isopropanol and resuspending the RNA pellet in 75% ethanol. Pellets were finally dissolved in DEPC treated water and stored at −80°C. Reverse transcription was performed with: random hexamers (50 μM), PCR buffer (1X), MgCl2 (5mM), dNTPs (4mM), RNAse inhibitor (1U/μl) and MuLV reverse transcriptase (2.5U/μl) from Applied Biosystems, Branchburg, NJ. The conditions for cDNA synthesis were: 25°C for 10 min, 42°C for 20 min and 95°C for 5 min.
Liver infection and murine hepcidin level were quantified by using P. berghei ANKA (PbA) 18s rRNA [31] and mouse hepcidin specific primers respectively. Multiplex qRT-PCR reactions included: 1X IQ Multiplex Power Mix (BioRad, Hercules, CA), 0.2 μM 18s and hepcidin primers, 0.5 μM Hypoxanthine Guanine Phosphoriboayltransferase (hprt) primers (Gagliardi et al., 2011), 0.2 μM of each TaqMan probe and 3 μl (around 540 ng/μl) cDNA for a total volume of 10 μl. Cycling conditions were followed according to the BioRad CFX96 protocol: 95°C for 3 min and 39 cycles of 95°C for 10 sec and 55°C for 30 sec. The gene copy number was normalized against the mouse hprt housekeeping gene. The specific sequences of primers (Sigma Aldrich, Saint Louis, MO) were: 5’- GGAGATTGGTTTTGACGTTTATGCG-3’ and 5’- AAGCATTAAATAAAGCGAATACATCCTTA-3’ for 18s; 5’-TGCAGAAGAGAAGGAAGAGAGACA-3’ and 5’- CACACTGGGAATTGTTACAGCATT-3’ for hepcidin and 5’- TCCCAGCGTCGTGATTAGC-3’ and 5’-CGGCATAATGATTAGGTATACAAAACA-3’ for hprt. The specific sequences for the probes (Integrated DNA Technologies, Coralville, IO) were: 5’ 6-FAM/ZENCAATTGGTTTACCTTTTGCTCTTT-3’IBFC for 18s; 5’ TETCAACTTCCCCATCTGCATCTTCTGCTGT- 3’IBFQ for hepcidin and 5’ Cy5-TGATGAACCAGGTTATGACC-3’BHQ-2 for hprt. Standardization curves were made with cDNA plasmids cloned with the TOPO TA cloning kit (Invitrogen, Carlsbad, CA).
2.5. Sporozoite infection
Mice were infected with 15,000 P. berghei ANKA sporozoites by tail vein injection on four separate occasions at both two weeks and six weeks post diet change for each of the two biologic replicates. Forty hours post infection; mice were sacrificed. Livers were collected for qRT-PCR quantification of P. berghei ANKA load and mouse hepcidin expression; whole blood was collected by cardiac puncture for determination of anemia and iron deficiency status.
2.6 In vivo murine transgenic anemic mice
Hbd mice have an exon deletion in gene Sec15l1 (a component of the exocyst complex involved in the cycling of transferrin) making the uptake of transferrin iron defective in the erythroid precursor. Sec15l1 is homologous to a gene encoding a member of the exocyst pathway in yeast. These mice have hypochromic anemia, with a decrease in hemoglobin and normal liver iron stores [32]. Six month female C57BL/6 mice were used as controls.
2.7 In vivo lethal P. yoelii challenge
The P. yoelii 17X lethal strain was the gift of James Burns (Drexel University). 10 million P. yoelii infected erythrocytes from donor mouse were given intraperitoneally to hbd mice and C57Bl/6 mice in groups of 4 [33].
2.7. Statistical analysis
For analysis of blood P. yoelii levels in hbd mice, hepatic P. berghei ANKA levels in hbd, phlebotomized and in nonphlebotomized mice versus iron diets, hepcidin levels in phlebotomized and in nonphlebotomized livers versus iron diets and nonheme iron quantification in the liver and spleen of phlebotomized and in nonphlebotomized mice versus diet we performed Student t-tests. All statistics were evaluated at an alpha level of 0.05 [34].
3. Results
3.1 Persistence of phlebotomy induced anemia and iron deficiency in mice
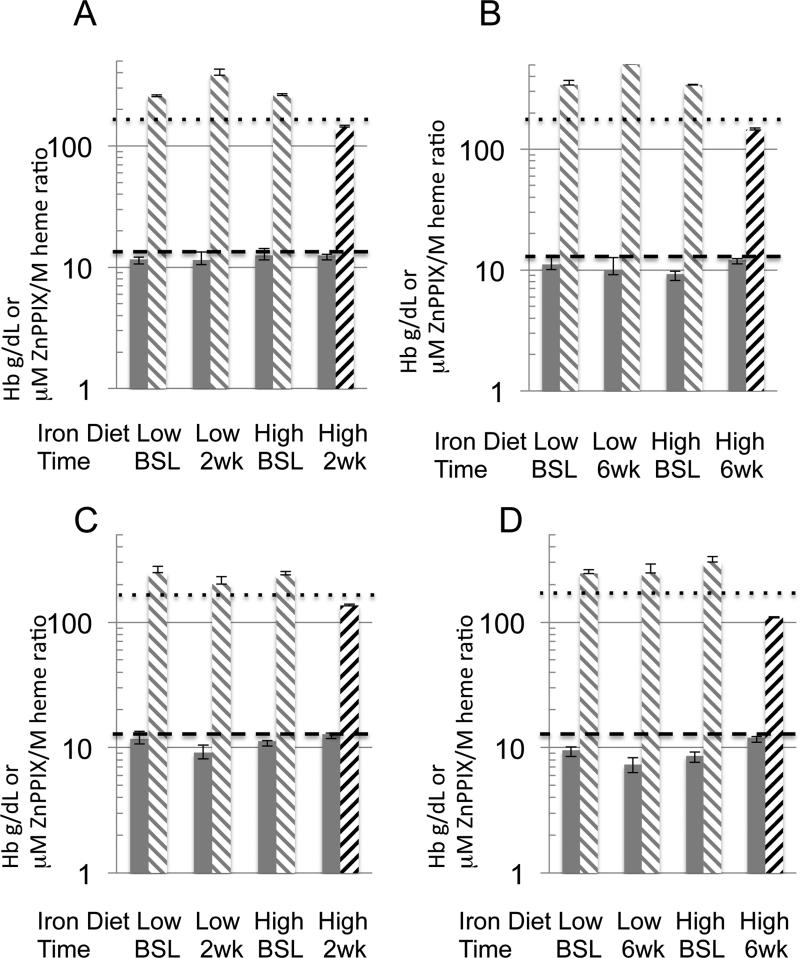
Phlebotomies induced anemia measured by hemoglobin and iron deficiency measured by ZnPPIX/heme ratios (FIG. 2). Mice with hemoglobin lower than 13.0 g/dL were considered anemic [35, 36]. Mice with a ratio higher than 170 μM ZnPPIX /M heme were considered iron deficient. In the setting of iron deficiency erythroprogenitor cells insert zinc instead of iron via ferrochelatase elevating this ratio as a marker of chronic iron deficiency [37, 38]. Phlebotomized mice remained anemic after 2 and 6 weeks on a low or high iron diet. The high iron diet alone normalized iron deficiency measured by the ZnPPIX ratios at two and six weeks, while the phlebotomized mice on the low iron diet at two and six weeks did not.
Fig. 2. Murine anemia and iron deficiency status after phlebotomy from baseline to liver harvest.
First biological experiment (A, B) for mice on a low or high iron diet at two weeks (A) and six weeks (B) show all mice anemic with hemoglobin less than 13 g/dL (gray bars). In general while all the ZnPPIX/heme ratios were elevated above 170 μM ZnPPIX/M heme (gray stripes downward left to right) at baseline, only the phlebotomized mice on the high iron diet normalized (black stripes upward left to right) ZnPPIX/heme ratio at both two and six weeks. The second biological repetition (C, D) mirrored changes from baseline to two (C) or six (D) weeks. Standard error of the mean with five mice in a group is shown. The dotted line is the 170 μM ZnPPIX/M heme mark and the dashed line is the 13 g/dL mark.
At the time of liver harvest comparing anemia and iron deficiency status amongst those who had phlebotomy and those that were not phlebotomized, all phlebotomized mice remained anemic on both high or low iron diets as above. However for nonphlebotomized mice the hemoglobin levels were normal on both diets at two weeks (for the first biologic replicate) or six weeks (for the second biologic replicate), while more variable for the other time points attributed to a narrower range of hemoglobin levels in this group. In contrast the ZnPPIX/heme ratios were more robust in segregating diet and phlebotomy outcomes. The nonphlebotmized mice as expected did not alter the iron deficiency status measured by ZnPPIX/heme ratio after two weeks and had a small increase to 199 μM ZnPPIX /M heme after six weeks of a low iron diet in the first group only compared to values of 250 to 500 μM ZnPPIX /M heme in the phlebotomized mice on the low iron diet. Based on our hemoglobin cut off, anemia was a consistent feature of phlebotomized mice, even 6 weeks after supplementation with a high iron diet. However, iron deficiency (as determined by elevated ZnPPIX /M heme ratio) was only a feature of phlebotomized mice fed a low iron diet.
3.2 Nonheme iron quantification in the liver and spleen after change in diets
The hemoglobin and μM ZnPPIX /M heme ratios are noninvasive indirect measures of bioavailable body iron status. We measured direct liver and spleen nonheme tissue iron at time of liver harvest for the parasite and hepcidin indicators below. Levels of nonheme iron were highest in the livers in both anemic and nonanemic mice after two weeks of the high iron diet at about 800-1000 μg iron/g tissue compared to the low iron diet of less than 55 μg iron/g tissue (Table 1). This high level of liver nonheme iron mostly redistributed to the spleen by 6 weeks time with an inversion of liver and spleen iron levels. By six weeks, both phlebotomized and nonphlebotomized mice had similar levels of nonheme iron when on the same diet.
TABLE 1.
Nonheme iron from spleens and livers from nonphlebotomized and phlebotomized mice after low and high iron diet from the first biologic replicate. Measurements are expressed as micrograms of iron per gram of tissue. P values by the Student t test compare levels between phlebotomized and nonphlebotomized mice. Five mice in a group.
| Liver |
Spleen |
|||||
|---|---|---|---|---|---|---|
| Weeks | Iron diet | Mice | Total nonheme iron mean (μg/g) (SEM) | P value | Total nonheme iron mean (μg/g) (SEM) | P value |
| 2 | Low | Nonphlebotomized | 50.2 (7.6) | 0.024 | 130.5 (6.4) | <0.001 |
| Phlebotomized | 18.6 (1.2) | 30.3 (5.5) | ||||
| High | Nonphlebotomized | 798.3 (53.9) | 0.336 | 485.4 (30.8) | 0.193 | |
| Phlebotomized | 1040.3 (96.5) | 393.9 (48.0) | ||||
| 6 | Low | Nonphlebotomized | 51.0 (9.0) | 0.110 | 127.2 (35.6) | 0.053 |
| Phlebotomized | 38.9 (7.0) | 16.7 (2.9) | ||||
| High | Nonphlebotomized | 285.4 (34.2) | 0.654 | 1085.3 (150.0) | 0.105 | |
| Phlebotomized | 312.1 (45.0) | 745.6 (44.9) | ||||
3.3 Hepatic Plasmodium parasite loads after high or low iron diets
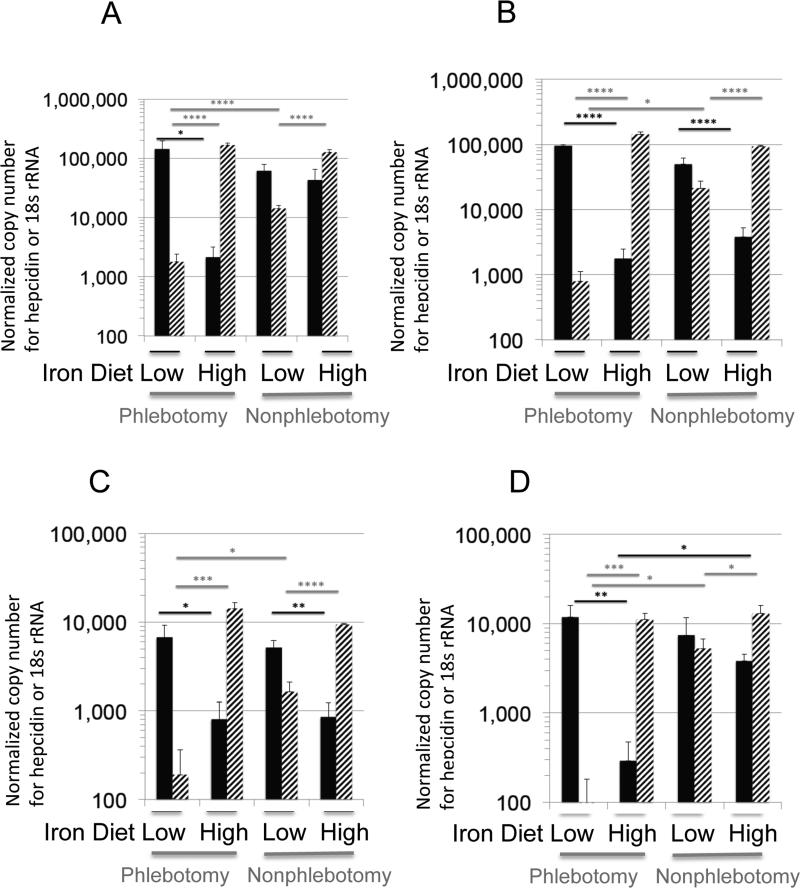
Both nonphlebotomized (nonanemic and iron replete) and phlebotomized (anemic and iron deficient) Balb/c mice fed on a low or a high iron diet were infected with approximately 15,000 P. berghei ANKA sporozoites at two and six weeks in both biologic replicates. In phlebotomized anemic mice after both two and six weeks (Fig. 3), liver parasite numbers on a low iron diet versus the high iron diet were 50 to 80-fold higher. The high iron diet decreases hepatic parasite loads in phlebotomized and nonphlebotomized mice at 2 and 6 weeks. In the high iron diet group at 6 weeks there were more liver stage parasites in the nonphlebotomized mice compared to phlebotomized with a trend to higher liver loads in nonphlebotomized groups compared to phlebotomized on this high iron diet with p values close to 0.1 in the first replicate group (Fig. 3 A and B). Hepatic parasite reduction in nonphlebotomized mice was variable, but a trend for reduced parasite reduction with increased hepcidin was somewhat apparent.
Fig. 3. Hepatic levels of P. berghei ANKA 18S rRNA and murine hepcidin from phlebotomized and nonphlebotomized mice fed with low and high iron diets.
Nonphlebotomized nonanemic, iron replete and postphlebotomy, anemic, iron deficient mice, (n=5) were fed with low or high iron diets ad libitum for 2 (A, C) weeks or 6 (B, D) weeks before blood inoculum in four separate biologic replicates with 15,000 P. berghei ANKA sporozoites. The hepatic parasite 18s rRNA level (solid black bars) and murine hepcidin level (striped bars) was quantified by qRT-PCR and normalized to mouse hprt expression. Results are graphed as mean and S.E.M. Only significant values determined by a one tailed Student t-test are shown with *p<0.05, **p<0.005, *** p<0.0005 and **** p<.00005. Comparisons were not made between different diets and different phlebotomy status.
3.4 Hepcidin levels after high or low iron diets
Hepcidin expression was consistently increased with high iron diets in both phlebotomized and nonphlebotomized mice at both two and six weeks (Fig. 3). All nonphlebotomized mice on a low iron diet have a higher expression of liver hepcidin than phlebotomized mice on a low iron diet (p< 0.5), suggesting suppression of hepcidin in response to erythropoietic demand. In contrast, hepcidin expression level was similar in mice on the high iron diet whether anemic or not. The lowest parasite loads were observed in phlebotomized mice transferred to the high iron diet.
3.5 Anemic hbd mice have normal liver parasite numbers
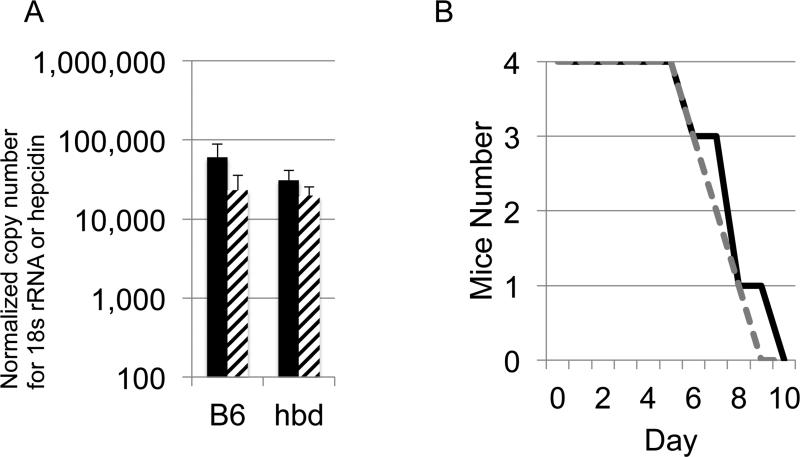
The hbd mice have normal liver iron stores and considerable anemia from an erythrocyte defect in transferrin-mediated iron uptake [32]. In our group of mice the mean ZnPPIX/ heme ratio was very high at 1038 (SEM 43) while matched wild C57Bl/6 had ZnPPIX/heme at 119 (SEM 20). Hemoglobin was 9.9 g/dL (SEM 1.2) with hbd mice, while hemoglobin was 12.6 g/dL (SEM 0.8) with these matched C57BL6 mice. While we did not determine nonheme iron on these hbd mice, in a separate published report, the iron load of hbd mice was 67 ± 19 per g wet weight with matched controls at 52 ± 5 per g wet weight [32]. hbd mice and nonanemic wild type mice (C57BL/6) were infected with 15,000 P. berghei ANKA sporozoites by tail vein injection. The qReal-time PCR performed on livers harvested 40 hours post infection revealed similar parasite loads and hepcidin levels compared to wild C57BL/6 (Fig. 4A). The hbd mice showed an insignificant 14% reduction with p value greater than 0.1.
Fig. 4. Hepatic levels of P. berghei ANKA 18S rRNA and hepcidin in hemoglobin deficientmice.
(A) hemoglobin deficient mice (hbd) (n=4) were infected in separate biologic replicates with 15,000 P. berghei ANKA sporozoites and the hepatic parasite load (18s) and hepcidin was quantified by qRT-PCR, then normalized to mouse hprt expression. Wild type mice C57BL6 (B6) were used as controls. The parasite load in hbd was similar compared to control mice (p-value = 0.21). Hepcidin normalized levels were also not different (p-value = 0.41). Mean and S.E.M. are shown. (B) Survival curves for lethal blood stage P. yoelii infection in hbd mice (black line and wild type mice gray dotted line (n=4).
The greatly elevated ZnPPIX/heme ratio raised the question if this by itself might protect from lethal murine bloodstage malaria by the ability of ZnPPIX to inhibit heme crystallization similar to the quinolones [39]. For analysis of this bloodstage response in contrast to the liver stage response studied as above, we injected 10 million lethal P. yoelii XL in both groups and by day 4 both hbd and wild type mice had similar 30% parasitemias. Time to death was also the same (FIG. 4B). From these data, we conclude elevated erythrocyte ZnPP does not protect from blood stage infection.
4. Discussion
This study focused on the liver stage numbers of Plasmodium rather than blood stage numbers during iron supplementation. Mice on the high iron diets showed an increase in liver nonheme iron (Fig. 5 and Table 1). Based on the hypothesis that iron supplementation enhances Plasmodium parasite burden, we would expect increased liver Plasmodium numbers as seen in Portugal et al [13]. However our hepatic parasite numbers were decreased in our mice supplemented with a high iron diet. Across this panel of experiments, mice on the low iron diet generally had the highest liver Plasmodium numbers and the lowest hepcidin expression. Our prior collaborative work published by Portugal et al, showed that hepcidin reduced liver stage Plasmodium infection [13]. Likewise, Wang and colleagues observed a protective effect of hepcidin on the host after blood stage P. berghei infection in the murine model [40]. Wang and colleagues also demonstrated that neutralization of hepcidin was detrimental to the murine host following P. berghei infection [40]. We therefore hypothesize that the strong induction of hepcidin, which occurred when anemic, iron-deficient mice were fed a high iron diet and had an increase in liver and spleen iron (Fig. 5), provided a protective effect similar to induction of hepcidin in the context of malaria superinfection [13]. While the high iron diet did not result in such a strong induction of hepcidin in mice that were not previously phlebotomized, a similar protective effect of hepcidin induction was a consistent finding. Of note is that hepcidin induction in response to multiple different stimuli (inflammation in the case of bloodstage malaria versus iron supplementation) similarly results in protection against hepatic infection [13, 40, 41].
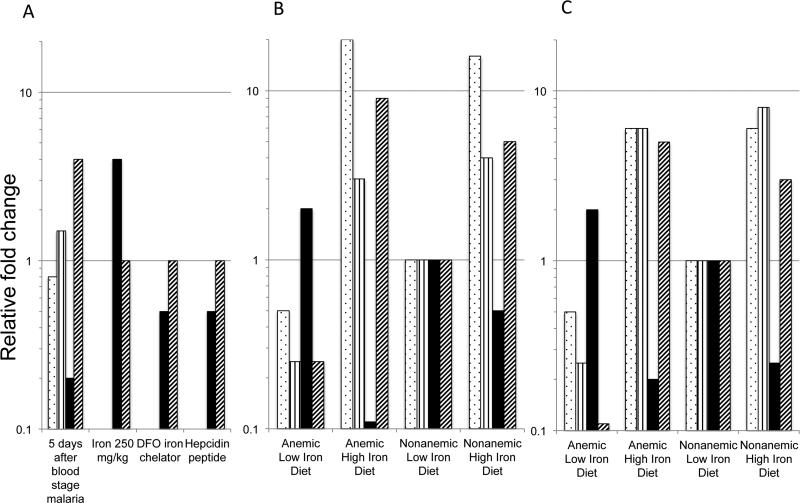
Fig. 5. Model of temporal pattern of iron and hepcidin regulation of Plasmodium liver loads.
A) Relative fold change of measured values compared to untreated control mice for liver nonheme iron (dotted bars), spleen iron (vertical stripe bars), Plasmodium liver load (black bar) and hepcidin levels (diagonal stripe bars) are shown 5 days after a bloodstage murine malaria infection, after iron citrate at 250 mg/kg for 24-48 hours, after desferoximine (DFO) an iron chelator at 250 mg/kg and after hepcidin peptide from reference [13]. B) Two weeks or C) six weeks after low or high iron diets on either anemic or nonanemic mice, the relative liver and spleen nonheme iron, Plasmodium liver loads and hepcidin levels are shown relative to nonanemic mice on a low iron diet as the 1x control.
Prior studies have demonstrated that infection of mice with higher liver iron loads, result in higher liver parasite loads and shorter times to patency[13] [15]. We expect that this occurs without substantial changes in hepcidin expression. The sum of these data suggests the Plasmodium may be sensitive to a poorly defined hepatic iron or heme pool. In contrast with prior studies done within a few days of iron supplementation, our infections were performed two and six weeks after iron supplementation with higher levels of hepcidin transcripts and higher liver and spleen nonheme iron than mice fed a low iron diet (Fig. 5).
By six weeks, we saw the nonphlebotomized (nonanemic and iron replete) mice on high iron supplementation had modestly higher liver parasite numbers than the phlebotomized (anemic, iron deficient) mice also on high iron supplementation although both were lower than parasite numbers on a low iron diet. One possible interpretation of the data obtained in mice on high iron supplementation is that higher hepcidin induced by iron supplementation results in lower liver stage parasite numbers in anemic mice compared to nonanemic mice. In this way the lower cumulative dose of liver stage parasites progressing to bloodstage parasites in anemic, iron deficient individuals compared to higher cumulative numbers in nonanemic, iron replete individuals results in more malaria morbidity and mortality in the nonanemic, iron replete individuals. This interpretation brings up the discussion of the dose and time dependence of liver inoculums having an impact on blood stage mortality. In semimmune individuals, the sporozoite dose effect leading to more severe malaria has been demonstrated in large cohorts of Kenyan children treated once to clear infections and followed over many weeks for malaria morbidity [42]. This epidemiologic study observed a strong association of mean infective bite exposure or cumulative dose of infective mosquitoes delivering more liver stage parasites over time with more severe malaria indices. In hyperendemic areas with at least an infective mosquito bite each week, iron supplementation in nonanemic, iron replete children would be expected to result in higher cumulative exposure to more parasites released from the liver resulting in higher malaria morbidity and mortality. This line of reasoning is also used to support that the RTS.S vaccine targeting liver stages in malaria endemic communities which is able to have an effect on reducing severe blood stage malaria also.
Anemia with normal liver stores in the hbd mice did not change liver parasite number. Likewise the blood stage parasitemias were unaltered using the lethal P. yoelii mouse model of malaria. The extremely high ZnPPIX/heme ratios did not influence bloodstage outcome.
The hepcidin values that prevent any hepatic infection during blood stage Plasmodium infection are greatly elevated in comparison to our values seen in these iron supplementation studies. Human studies also support the greatly elevated hepcidin levels associated with malaria versus those seen with iron supplementation. In Gambian children hepcidin levels were generally at 25 ng/ml after malaria compared to 12-16 ng/ml after iron supplementation [18].
A recent report of growth of bloodstage P. falciparum in iron replete and iron deficient erythrocytes from noninfected American patients showed that iron deficient erythrocytes exhibited less invasion of P. falciparum merozoites in the iron deficient erythrocytes. The erythrocytes harvested after iron supplementation from the same individuals supported more P. falciparum growth from an associated increase in reticulocytosis. Blood from iron replete individuals did have an erythrocyte growth advantage over the iron deficient blood [43]. We did not investigate time to bloodstage patency in the iron supplementation experiments because of the analysis of two independent liver and bloodstage variables a) the lower liver stage parasite numbers that lengthen time to patency with normal erythrocyte iron and also b) the blood stage development that may be influenced by alterations in erythrocyte iron. An earlier report noted 25% and 7% mortalities in iron deficient mice given P. chaubaudi compared to 100% in iron replete mice. The protected iron deficient mice when supplemented with iron had 100% recrudescent malaria while those maintained on the iron deficient diet never demonstrated positive blood films [44].
These studies indicate a dynamic over time for iron regulation of liver stage malaria. In the first few days, iron supplementation increases hepatic parasite numbers which later the hepatic numbers are decreased in the setting of a recent increase in hepcidin [45]. Hepcidin dominates in control of liver stage Plasmodium numbers over absolute iron levels. Nonanemic mice and anemic mice on iron supplemented diet for 6 weeks had similar levels of hepcidin. However, nonanemic mice treated with iron tended to have higher parasite numbers than anemic mice treated with iron. Iron supplementation was shown to increase human malaria blood stage risk from 6 to 12 weeks [43]. This work adds to the evidence that consideration for malaria chemoprophylaxis along with iron supplementation may optimize iron absorption while decreasing malaria risk.
Acknowledgements
DJS and CNR were supported by the National Institute of Child Health and Human Development under award number U01HD061241. We thank Jorge A. Ferrer for assistance with scheme figure design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–7. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . World malaria report 2014. World Health Organization; Geneva: 2014. p. 242. [Google Scholar]
- 3.Spivak JL. Iron and the anemia of chronic disease. Oncology. 2002;16:25–33. [PubMed] [Google Scholar]
- 4.Mebrahtu T, Stoltzfus RJ, Chwaya HM, Jape JK, Savioli L, Montresor A, Albonico M, Tielsch JM. Low-dose daily iron supplementation for 12 months does not increase the prevalence of malarial infection or density of parasites in young Zanzibari children. J Nutr. 2004;134:3037–41. doi: 10.1093/jn/134.11.3037. [DOI] [PubMed] [Google Scholar]
- 5.Murray MJ, Murray AB, Murray MB, Murray CJ. The adverse effect of iron repletion on the course of certain infections. Br Med J. 1978;2:1113–5. doi: 10.1136/bmj.2.6145.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray MJ, Murray NJ, Murray AB, Murray MB. Refeeding-malaria and hyperferraemia. Lancet. 1975;1:653–4. doi: 10.1016/s0140-6736(75)91758-4. [DOI] [PubMed] [Google Scholar]
- 7.Menendez C, Schellenberg D, Quinto L, Kahigwa E, Alvarez L, Aponte JJ, Alonso PL. The effects of short-term iron supplementation on iron status in infants in malaria-endemic areas. Am J Trop Med Hyg. 2004;71:434–40. [PubMed] [Google Scholar]
- 8.Menendez C, Kahigwa E, Hirt R, Vounatsou P, Aponte JJ, Font F, Acosta CJ, Schellenberg DM, Galindo CM, Kimario J, Urassa H, Brabin B, Smith TA, Kitua AY, Tanner M, Alonso PL. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet. 1997;350:844–50. doi: 10.1016/S0140-6736(97)04229-3. [DOI] [PubMed] [Google Scholar]
- 9.Berger J, Dyck JL, Galan P, Aplogan A, Schneider D, Traissac P, Hercberg S. Effect of daily iron supplementation on iron status, cell-mediated immunity, and incidence of infections in 6-36 month old Togolese children. Eur J Clin Nutr. 2000;54:29–35. doi: 10.1038/sj.ejcn.1600888. [DOI] [PubMed] [Google Scholar]
- 10.Gera T, Sachdev HP. Effect of iron supplementation on incidence of infectious illness in children: systematic review. BMJ. 2002;325:1142. doi: 10.1136/bmj.325.7373.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, Kabole FM. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–43. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 12.Prudencio M, Rodriguez A, Mota MM. The silent path to thousands of merozoites: the Plasmodium liver stage. Nat Rev Microbiol. 2006;4:849–56. doi: 10.1038/nrmicro1529. [DOI] [PubMed] [Google Scholar]
- 13.Portugal S, Carret C, Recker M, Armitage AE, Goncalves LA, Epiphanio S, Sullivan D, Roy C, Newbold CI, Drakesmith H, Mota MM. Host-mediated regulation of superinfection in malaria. Nat Med. 2011;17:732–7. doi: 10.1038/nm.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stahel E, Mazier D, Guillouzo A, Miltgen F, Landau I, Mellouk S, Beaudoin RL, Langlois P, Gentilini M. Iron chelators: in vitro inhibitory effect on the liver stage of rodent and human malaria. Am J Trop Med Hyg. 1988;39:236–40. doi: 10.4269/ajtmh.1988.39.236. [DOI] [PubMed] [Google Scholar]
- 15.Goma J, Renia L, Miltgen F, Mazier D. Iron overload increases hepatic development of Plasmodium yoelii in mice. Parasitology. 1996;112(Pt 2):165–8. doi: 10.1017/s0031182000084729. [DOI] [PubMed] [Google Scholar]
- 16.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 17.Cercamondi CI, Egli IM, Ahouandjinou E, Dossa R, Zeder C, Salami L, Tjalsma H, Wiegerinck E, Tanno T, Hurrell RF, Hounhouigan J, Zimmermann MB. Afebrile Plasmodium falciparum parasitemia decreases absorption of fortification iron but does not affect systemic iron utilization: a double stable-isotope study in young Beninese women. Am J Clin Nutr. 2010;92:1385–92. doi: 10.3945/ajcn.2010.30051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prentice AM, Doherty CP, Abrams SA, Cox SE, Atkinson SH, Verhoef H, Armitage AE, Drakesmith H. Hepcidin is the major predictor of erythrocyte iron incorporation in anemic African children. Blood. 2012;119:1922–8. doi: 10.1182/blood-2011-11-391219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, Ho LP, Townsend AR, Drakesmith H. Hepcidin regulation by innate immune and infectious stimuli. Blood. 2011;118:4129–39. doi: 10.1182/blood-2011-04-351957. [DOI] [PubMed] [Google Scholar]
- 20.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piperno A, Galimberti S, Mariani R, Pelucchi S, Ravasi G, Lombardi C, Bilo G, Revera M, Giuliano A, Faini A, Mainini V, Westerman M, Ganz T, Valsecchi MG, Mancia G, Parati G. Modulation of hepcidin production during hypoxia-induced erythropoiesis in humans in vivo: data from the HIGHCARE project. Blood. 2011;117:2953–9. doi: 10.1182/blood-2010-08-299859. [DOI] [PubMed] [Google Scholar]
- 22.Chua AC, Klopcic BR, Ho DS, Fu SK, Forrest CH, Croft KD, Olynyk JK, Lawrance IC, Trinder D. Dietary iron enhances colonic inflammation and IL-6/IL-11-Stat3 signaling promoting colonic tumor development in mice. PLoS One. 2013;8:e78850. doi: 10.1371/journal.pone.0078850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson RJ. Dietary iron levels and hypoxia independently affect iron absorption in mice. J Nutr. 1996;126:1858–64. doi: 10.1093/jn/126.7.1858. [DOI] [PubMed] [Google Scholar]
- 24.Krijt J, Frydlova J, Kukackova L, Fujikura Y, Prikryl P, Vokurka M, Necas E. Effect of iron overload and iron deficiency on liver hemojuvelin protein. PLoS One. 2012;7:e37391. doi: 10.1371/journal.pone.0037391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dowling P, Klinker F, Amaya F, Paulus W, Liebetanz D. Iron-deficiency sensitizes mice to acute pain stimuli and formalin-induced nociception. J Nutr. 2009;139:2087–92. doi: 10.3945/jn.109.112557. [DOI] [PubMed] [Google Scholar]
- 26.Ohgami RS, Campagna DR, Antiochos B, Wood EB, Sharp JJ, Barker JE, Fleming MD. nm1054: a spontaneous, recessive, hypochromic, microcytic anemia mutation in the mouse. Blood. 2005;106:3625–31. doi: 10.1182/blood-2005-01-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walden TL, Draganac PS, Farkas WR. The elevation of blood levels of zinc protoporphyrin in mice following whole body irradiation. Blood. 1984;63:1159–67. [PubMed] [Google Scholar]
- 28.Friedman JS, Lopez MF, Fleming MD, Rivera A, Martin FM, Welsh ML, Boyd A, Doctrow SR, Burakoff SJ. SOD2-deficiency anemia: protein oxidation and altered protein expression reveal targets of damage, stress response, and antioxidant responsiveness. Blood. 2004;104:2565–73. doi: 10.1182/blood-2003-11-3858. [DOI] [PubMed] [Google Scholar]
- 29.Torrance J, Bothwell T. Tissue Iron Stores. Churchill Livingstone; New York, NY: 1980. [Google Scholar]
- 30.Cook JD. Iron. Churchill Livingstone; New York: 1980. [Google Scholar]
- 31.Bruna-Romero O, Hafalla JC, Gonzalez-Aseguinolaza G, Sano G, Tsuji M, Zavala F. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasit. 2001;31:1499–502. doi: 10.1016/s0020-7519(01)00265-x. [DOI] [PubMed] [Google Scholar]
- 32.Lim JE, Jin O, Bennett C, Morgan K, Wang F, Trenor CC, 3rd, Fleming MD, Andrews NC. A mutation in Sec15l1 causes anemia in hemoglobin deficit (hbd) mice. Nat Genet. 2005;37:1270–3. doi: 10.1038/ng1659. [DOI] [PubMed] [Google Scholar]
- 33.Ferrer P, Tripathi AK, Clark MA, Hand CC, Rienhoff HY, Jr., Sullivan DJ., Jr. Antimalarial iron chelator, FBS0701, shows asexual and gametocyte Plasmodium falciparum activity and single oral dose cure in a murine malaria model. PLoS One. 2012;7:e37171. doi: 10.1371/journal.pone.0037171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.R.R. Team . A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna Austria: 2008. [Google Scholar]
- 35.Raabe BM, Artwohl JE, Purcell JE, Lovaglio J, Fortman JD. Effects of weekly blood collection in C57BL/6 mice. J Am Assoc Lab Anim Sci. 2011;50:680–5. [PMC free article] [PubMed] [Google Scholar]
- 36.Moreau R, Tshikudi Malu D, Dumais M, Dalko E, Gaudreault V, Romero H, Martineau C, Kevorkova O, Dardon JS, Dodd EL, Bohle DS, Scorza T. Alterations in bone and erythropoiesis in hemolytic anemia: comparative study in bled, phenylhydrazine-treated and Plasmodium-infected mice. PLoS One. 2012;7:e46101. doi: 10.1371/journal.pone.0046101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labbe RF, Vreman HJ, Stevenson DK. Zinc protoporphyrin: A metabolite with a mission. Clin Chem. 1999;45:2060–72. [PubMed] [Google Scholar]
- 38.Cook JD. Diagnosis and management of iron-deficiency anaemia. Best Pract Res Clin Haematol. 2005;18:319–32. doi: 10.1016/j.beha.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Iyer JK, Shi L, Shankar AH, Sullivan DJ., Jr. Zinc protoporphyrin IX binds heme crystals to inhibit the process of crystallization in Plasmodium falciparum. Mol Med. 2003;9:175–82. doi: 10.2119/2003-00010.sullivan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang HZ, He YX, Yang CJ, Zhou W, Zou CG. Hepcidin is regulated during blood-stage malaria and plays a protective role in malaria infection. J Immunol. 2011;187:6410–6. doi: 10.4049/jimmunol.1101436. [DOI] [PubMed] [Google Scholar]
- 41.Spottiswoode N, Fried M, Drakesmith H, Duffy PE. Implications of malaria on iron deficiency control strategies. Adv Nutr. 2012;3:570–8. doi: 10.3945/an.111.001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McElroy PD, Beier JC, Oster CN, Onyango FK, Oloo AJ, Lin X, Beadle C, Hoffman SL. Dose- and time-dependent relations between infective Anopheles inoculation and outcomes of Plasmodium falciparum parasitemia among children in western Kenya. Am J Epidemiol. 1997;145:945–56. doi: 10.1093/oxfordjournals.aje.a009054. [DOI] [PubMed] [Google Scholar]
- 43.Clark MA, Goheen MM, Fulford A, Prentice AM, Elnagheeb MA, Patel J, Fisher N, Taylor SM, Kasthuri RS, Cerami C. Host iron status and iron supplementation mediate susceptibility to erythrocytic stage Plasmodium falciparum. Nat Commun. 2014;5:4446. doi: 10.1038/ncomms5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harvey PW, Bell RG, Nesheim MC. Iron deficiency protects inbred mice against infection with Plasmodium chabaudi. Infect Immun. 1985;50:932–4. doi: 10.1128/iai.50.3.932-934.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spottiswoode N, Duffy PE, Drakesmith H. Iron, anemia and hepcidin in malaria. Front Pharmacol. 2014;5:125. doi: 10.3389/fphar.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]