Abstract
Obesity and type 2 diabetes mellitus (T2DM) convey an increased risk for developing dementia. The microtubule associated protein tau is implicated in neurodegenerative disease by undergoing hyperphosphorylation and aggregation, leading to cytotoxicity and neurodegeneration. Enzymes involved in the regulation of tau phosphorylation, such as GSK3β, are tightly associated with pathways found to be dysregulated in T2DM. We have shown previously that leptin resistant mice, which develop obesity and a diabetic phenotype, display elevated levels of tau phosphorylation. Here we show cells cultured with leptin, an adipokine shown to have neuroprotective effects, reduces tau phosphorylation. To explore how this mechanism works in vivo we transduced an existing diabetic mouse line (Leprdb/db) with a tau mutant (tauP301L) via adeno-associated virus. The resulting phenotype included a striking increase in tau phosphorylation and the number of neurofibrillary tangles found within the hippocampus. We conclude that leptin resistance-induced obesity and diabetes accelerates the development of tau pathology. This model of metabolic dysfunction and tauopathy provides a new system in which to explore the mechanisms underlying the ways in which leptin resistance and diabetes influence development of tau pathology, and may ultimately be related to the development of neurofibrillary tangles.
Keywords: Tau, Leptin, Obesity, Diabetes, Alzheimer disease
Introduction
Type 2 diabetes mellitus (T2DM) affects over 25 million individuals in the U.S. (Adeghate et al., 2006, Prevention, 2011). T2DM is a metabolic disorder associated with insulin resistance, dysregulated intracellular signaling (elevated glucagon release, failure of glucose receptor recruitment, and reduced lipolysis to name a few), and pancreatic β-cell degeneration in late stages of the disease, which ultimately result in systemic glucose mishandling (Baker et al., 2011, Accardi et al., 2012, Bergman, 2013). Obesity is a common driving factor for the development of T2DM and the two diseases are highly associated, with 85.2% of T2DM patients being classified as overweight or obese (Ford et al., 1997, Resnick et al., 2000, Mokdad et al., 2003, Prevention, 2011). Chronic obesity and T2DM often result in a series of secondary pathologies, including cardiovascular disease, renal dysfunction, and dementias (Rewers et al., 2004, Xu et al., 2009, Seshasai et al., 2011, Association, 2013).
Due to improved therapeutics, individuals with T2DM are living longer and are, therefore, living into the ages where neurodegenerative diseases develop. However, these patients are at a greater risk of developing neurodegenerative disease, such as Alzheimer’s disease (AD), mild cognitive impairment, and vascular dementia, than healthy, similarly aged counterparts (Stewart and Liolitsa, 1999, Yaffe et al., 2004, Craft, 2005, Messier, 2005, Profenno et al., 2010, Chen et al., 2012). Though this link is well-established, the underlying mechanisms involved in the development of pathology remain unclear. Previous studies have focused on amyloid or tau accumulation, inflammation, and cerebrovascular disease driven by diabetes and/or obesity (Anguiano et al., 2002, Desai et al., 2014, Ferreira et al., 2014).
Tau binds to microtubules which not only support cellular structure, but also provide a physical pathway for important axonal transporters, such as dynein and kinesin (Weingarten et al., 1975). Tau is regulated by many different kinases and phosphatases which modify its phosphorylation state and microtubule-binding capabilities (Morishima-Kawashima et al., 1995, Augustinack et al., 2002, Cavallini et al., 2013). When tau becomes hyperphosphorylated, it can no longer bind to, and stabilize, microtubules. In addition, hyperphosphorylated tau has a tendency to aggregate, resulting in the formation of higher order structures, such as oligomers, paired helical filaments (PHFs), and neurofibrillary tangles (NFTs) (Augustinack et al., 2002, Iqbal et al., 2005). NFTs are a classic hallmark of tauopathy observed in many neurodegenerative diseases, such as AD, frontotemporal dementia (FTD), and may be observed in other neurodegenerative diseases such as Parkinson’s disease (Kosik et al., 1986, Wood et al., 1986, Williams, 2006). Though it is unclear whether NFTs directly induce neurodegeneration, their presence is associated with neuronal death (Kril et al., 2002). On the other hand, it has been suggested that NFTs may form as a protective mechanism to counter the effects of oxidative stress or to sequester the cytotoxic oligomeric species of tau (Sayre et al., 2000, Maeda et al., 2006, Lasagna-Reeves et al., 2012).
Multiple studies have demonstrated that tau pathology can be modulated by diabetes or obesity. Streptozotocin-induced diabetes results in tau hyperphosphorylation in mice (Planel et al., 2007). Alterations in tau splice patterns and increases in tau phosphorylation have been observed in rodent models of T2DM (Kim et al., 2009, Jung et al., 2011). Additionally, hyperinsulinemic rats display increases in tau hyperphosphorylation (Freude et al., 2005). These changes to tau regulation are likely due to dysfunction within the myriad of pathways impacted by obesity and diabetes (Virkamaki et al., 1999, Schmelzle et al., 2006, Rains and Jain, 2011). For instance, insulin signaling transiently modulates tau phosphorylation in primary cortical neurons when stimulated with Insulin-like growth factor 1 (Lesort and Johnson, 2000). Collectively, these data suggest that diabetes associated metabolic dysfunction influences tau phosphorylation, and may promote pathogenesis (Freude et al., 2005).
Insulin signaling is only one of the signaling pathways disrupted in the chronically obese or diabetic state. Leptin is a hormone secreted from adipose tissue that regulates satiety and energy expenditure via the hypothalamus. The leptin receptor is expressed throughout the brain, suggesting it plays additional roles outside hypothalamic regulation (Couce et al., 1997, Shioda et al., 1998, Burguera et al., 2000). Leptin signaling has also been shown to reduce tau phosphorylation (Greco et al., 2008, Marwarha et al., 2010). Individuals who are obese and diabetic often develop resistance to leptin signaling (Campfield et al., 1995, Halaas et al., 1995, Considine et al., 1996, Ostlund et al., 1996). Leptin signaling classically regulates transcription via Janus Kinase and Signal Transducer and Activator of Transcription (JAK-STAT) pathways to modulate energy metabolism and satiety (Hakansson and Meister, 1998, Elias et al., 1999, Munzberg et al., 2003). However, leptin signaling also involves a variety of other signaling cascades including the PI3K/AKT, MAPK, and mTOR pathways (Banks et al., 2000, Bjorbaek et al., 2001, Niswender et al., 2001, Zhao et al., 2002, Rahmouni et al., 2003, Cota et al., 2006, Cota et al., 2008). Plasma leptin levels have shown to be inversely correlated with dementia risk, and leptin treatment has been shown to increase cognition and reduce amyloid pathology in transgenic mice (Harvey, 2007, Lieb et al., 2009, Greco et al., 2010). In addition, our lab has demonstrated that leptin reduces β-amyloid production, likely as a result of lower γ-secretase expression (Niedowicz et al., 2013).
Though other studies have examined the effect of diabetes on tau phosphorylation, to our knowledge, no studies have looked directly at the effects of obesity and diabetes on the development of tau pathology, the neurodegenerative driving hallmark. In this study, we transduced leptin resistant mice (Leprdb/db), which develop obesity and diabetes with AAV1 tauP301L. We show that obesity and diabetes promote tau hyperphosphorylation and the accumulation of tau pathology.
EXPERIMENTAL PROCEDURES
Mice
All animal work was approved by the University of Kentucky Institutional Animal Care and Use Committee (IACUC) (Protocol Number: 2010-0673), and was performed in accordance with PHS guidelines. All procedures were performed under conditions designed to minimize pain and distress. The University of Kentucky is an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) approved institution, and follows the current version of the Guide for the Care and Use of Laboratory Animals (8th Edition), as adopted by the Office of Laboratory Animal Welfare (OLAW). The work with recombinant virus was approved by the University of Kentucky Institutional Biosafety Committee (B11-1629).
Leptin receptor deficient mice were purchased from the Jackson Laboratory (B6.BKS(D)-Leprdb/J, stock # 000697) and were housed two to three mice per cage with ab libitum access to food and water and maintained under a 12 hour light/dark cycle. All husbandry and treatment procedures were conducted with prior approval of the University of Kentucky’s Institutional Animal Care and Use Committee, in accordance with PHS guidelines. Heterozygous males and females were used for breeding (as mice homozygous for the db mutation are infertile). The offspring were genotyped by Leprdb single-nucleotide polymorphism-specific qPCR using a Taqman® genotyping kit (Applied Biosystems by Life Technologies; Grand Island, NY) with Quanta Accustart Genotyping Toughmix® (Quanta Biosciences; Gaithersburg, MD). Previous studies using AAV1 vectors to transduce the tauP301L mutation indicated that extensive tangle pathology develops by 6 months of age, therefore we chose 6 months to be our endpoint for these mice (Klein et al., 2004b). Mice were subjected to glucose tolerance tests (GTTs) at 22 weeks and Morris water maze (MWM) at 23 weeks of age. Mice were euthanized at 6 months by administration of a lethal dose of Beuthanasia-D (Henry Schein Animal Health; Dublin, OH). Upon euthanasia, brains were collected and divided along the sagittal plane; one half of the brain was drop-fixed in 10% PBS-buffered formalin, and then transferred (after a minimum of 24 hours) to phosphate buffered saline (PBS) with 0.05% NaN3 for storage. Whole blood was collected in tubes containing EDTA (Starstedt; Newton, NC), centrifuged at 1,500 × g for 10 minutes, and the plasma collected and frozen for future analysis
Adeno-Associated Virus (AAV)
For this study we utilized an AAV1 construct as this serotype is known to produce robust expression in the brain and maintain expression for up to nine months (Zincarelli et al., 2008). The tauP301 expression constructs containing the chicken β-actin promoter and the 3′ enhancer woodchuck hepatitis virus post-transcriptional regulatory element (WPRE), was a kind gift from Dr. Ronald Klein (Louisiana State University in Shreveport, Shreveport, LA). The tauP301L mutation was discovered as familial mutation which promotes the development of pathology and induces neurodegeneration and dementia in patients with frontotemporal dementia and parkinsonism linked to chromosome-17 (FTDP-17), and is commonly used in studying tauopathies (Poorkaj et al., 1998, Spillantini and Goedert, 2000, Morris et al., 2001, Lin et al., 2003). The tauP301L plasmid (pcDNA3), pAdΔF6 adeno-helper plasmid (pZac2.1), and rep/cap (AAV2/1) plasmids (pZac2.1) were co-transfected into HEK293A cells (ATCC; Manassas, VA) (Klein et al., 2004b, a). After 72 hours, the cells were harvested and the resulting virus purified by iodixonal gradient (15–54%), and reconstituted in PBS. The AAV vector was then titered for copies of vector genomes by WPRE inclusion by quantitative real-time PCR using WPRE-specific primers (Forward: GGCTGTTGGGCACTGACAAT, Reverse: CCGAAGGGACGTAGCAGAAG) and RT2 SYBR Green/ROX qPCR Master Mix SABiosciences; Frederick, MA). The AAV copy numbers were between 1 × 1012 and 1×1013 genomes/mL. These vectors were created both in lab and by the UK Viral Core following the same protocol. A control non-expressing AAV1 (CAG.Flex.eGFP.WPRE.bGH) was purchased from the University of Pennsylvania Vector Core.
Viral Transduction in vivo
Viral transduction in mouse brain was conducted as previously described (Levites et al., 2006, Liu et al., 2009, Liu et al., 2010, Furman et al., 2012). Briefly, newborn mice, postnatal day 0–2 (P0–P2), were gently placed in an aluminum foil envelope and cryoanesthetized on wet ice for approximately 5 minutes. AAVs (2 μL/injection) were injected bilaterally into the intracerebroventricular space using a 5 μL syringe (Hamilton; Reno, NV) with a 26 gauge needle, freehand. Immediately after injection, the pups were warmed under a heat lamp until fully recovered before returning them to their home cage. The pups were monitored for three days following injection, then weekly for the remainder of the study. A small number of mice were left uninjected to use as controls (62 AAV1 tauP301L injected, 28 control AAV1 injected, and 58 uninjected controls).
Glucose Tolerance Test (GTT)
GTTs were performed two weeks before the end of the study (22 weeks). Mice were fasted for 6 hours and fasting blood glucose was taken via tail prick using a hand held glucometer (Breeze2, Bayer HealthCare, LLC; Tarrytown, NY). Immediately after the baseline glucose measure was taken, the mice were administered an intraperitoneal injection of glucose (Hospira; Lake Forest, IL) at a dose of 2g/kg body weight. Blood glucose levels were then measured at 15, 30, 60 and 120 minutes after the injection. Any reading of ‘HI’ (above the detectable range) was recorded as 700 mg/dL for data analysis.
Behavioral Testing
One week before the end of the study (23 weeks), mice were tested in the Morris water maze (MWM) to gauge cognitive function. Mice were trained over five days to locate a submerged platform (11 cm) using external cues. The platform was in the northeast quadrant of a 134.5 cm diameter pool filled with opaque water (25°C) containing nontoxic white tempura paint. Mice were given 60 seconds to find the platform, and if they did not find it within this time period, they were placed on the platform for 15 seconds to acquaint them with its location. Mice were trained over four 60-second trials each day (starting from a different quadrant on each trial), with a minimum five-minute interval between trials. At the end of training on the fifth day the platform was removed and a probe trial was performed. Finally, a single visual acuity test was performed during which the platform was moved from its training location to the opposite side of the pool and cued with a small white flag. All trials were recorded using a video camera and analyzed with EthoVision XT software (Noldus Information Technology; Leesburg, VA) to measure swim distance, escape latency, and velocity.
Cell Culture
HEK293 cells stably expressing the tauP301L mutation (a kind gift from Dr. Chad Dickey, University of South Florida) were grown in DMEM (Invitrogen) with 10% fetal bovine serum (HyClone; Logan, UT), 1% penicillin-streptomycin (Cellgro; Manassas, VA) and 120 ng/mL G418 (HyClone) for selective pressure. We have observed functional leptin receptor expression in multiple cell lines, including in HEK cells (not shown)(Niedowicz et al., 2013). Cells were treated with various concentrations (0, 10, 50, and 100 ng/mL) of recombinant human leptin (Sigma-Aldrich; St. Louis, MO) for 48 hours and lysed in RIPA buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50mM Tris, and 150 mM sodium chloride, pH = 8.0) supplemented with protease inhibitor cocktail (Amresco; Solon, OH) and phosphatase inhibitor (EMD Millipore; Billerica, MA).
Western Blotting and ELISAs
Frozen brain tissue was homogenized with an AHS200 PowerMax polytron (VWR; Radnor, PA) in Tris-buffered saline (50mM Tris, 274mM NaCl, and 5mM KCl, pH = 7.4) with protease (Amresco) and phosphatase inhibitors (EMD Millipore) at 1mL per 100 mg tissue. The homogenate was centrifuged at 13,000 × g for 15 minutes at 4°C and the supernatant separated and stored at −80°C; samples were resuspended in standard 2% SDS Laemmli loading buffer. The protein concentrations of the supernatants were determined by bicinchoninic acid assay (Pierce; Rockford, IL). Extracts (25 μg) were separated by SDS-PAGE on 4–12% Bis-Tris Criteron gels in MOPS running buffer (Bio-Rad; Hercules, CA) and transferred to a 0.2 μm nitrocellulose membrane (Bio-Rad). Alternatively, some samples were examined by slot blot (Niedowicz et al., 2013, Niedowicz et al., 2014). Following transfer, the membranes were blocked overnight in PBS with 1% bovine serum albumin and 2% BlockAce (AbD Serotec, Raleigh, NC). Blots were then probed with AT8 (Greco et al., 2010, Koike et al., 2010, Martinez-Coria et al., 2010a, van Eersel et al., 2010) for pSer202/pThr205 tau (Pierce; 1:500), AT180 (Koike et al., 2010, Martinez-Coria et al., 2010a) for pThr231 tau (Pierce, 1:100), PHF-1 (Martinez-Coria et al., 2010a) for pSer396/pS404 (courtesy of Dr. Peter Davies, Albert Einstein College of Medicine, Bronx, NY 1:1000), HT7 (Koike et al., 2010, Martinez-Coria et al., 2010a, van Eersel et al., 2010) for total tau (Pierce, 1:1000) which has been shown to detect both human and rodent total tau (Oddo et al., 2007, Resende et al., 2008, Ke et al., 2009, Martinez-Coria et al., 2010b), and anti-GAPDH (ab9385, Abcam; Cambridge, MA, 1:5000) antibodies then probed with HRP-conjugated rabbit anti-mouse secondary antibody (Rockland Immunochemicals; Gilbertsville, PA, 1:10,000). Membranes were then incubated with SuperSignal West Dura chemiluminescent substrate (Pierce), and exposed to film. Films were scanned, and densitometric analysis was performed using Image J software (NIH; www.imagej.net). Total tau (LifeTechnologies), pSer199 tau (LifeTechnologies), and pThr181 tau (Fujirebio; Malvern, PA) were measured by sandwich ELISA using commercially available kits according to manufacturer’s instructions. Absorbance was measured at 450 nm using a multiwell plate reader (BioTek, Winooski, VT).
Immunohistochemistry (IHC)
Formalin-fixed hemibrains were serially cryoprotected in sucrose (10%, 20%, and 30% sucrose for 24 hours each). Brains were sectioned at 25 μm on a freezing, sliding microtome and stored in PBS with 0.05% NaN3. Immunohistochemistry was performed using PHF-1 (courtesy of Dr. Peter Davies, 1:100) to stain for neurofibrillary tangles/tau pathology, and a mouse on mouse (M.O.M) peroxidase kit (Vector Laboratories; Burlingame, CA) with 3,3′diaminobenzidine (DAB; Vector Laboratories) for development. Fluorescent double-labeling was also performed for PHF-1 to stain for neurofibrillary tangles/tau pathology with Alexa Fluor® 568 secondary antibody (Life Technologies, 1:200), and astrocytes were labeled with Rabbit anti-glial fibrillary acidic protein (GFAP) antibody (#PA1-10019, Pierce, 1:1000) and Alexa Fluor® 488 secondary antibody (Life Technologies,1:200). Slides were cover slipped with VECTASHIELD® Mounting Media with DAPI (Vector Laboratories). Images were taken using an Olympus BX51 microscope (Olympus; Melville, NY) and an Olympus Q-Color5 digital camera (Olympus). Images were processed using Image J software (NIH) to perform densitometric analysis.
Statistical Analysis
Analyses were performed using SPSS software version 22 (IBM; Armonk, NY) or Prism version 5 (GraphPad Software, La Jolla, CA). Group comparison data were analyzed by general linear model ANOVA and post-hoc comparisons made using Tukey-HSD or Dunnett’s tests. Repeated measures were analyzed using linear mixed models with time as a categorical predictor.
RESULTS
Leptin Regulates Tau Phosphorylation
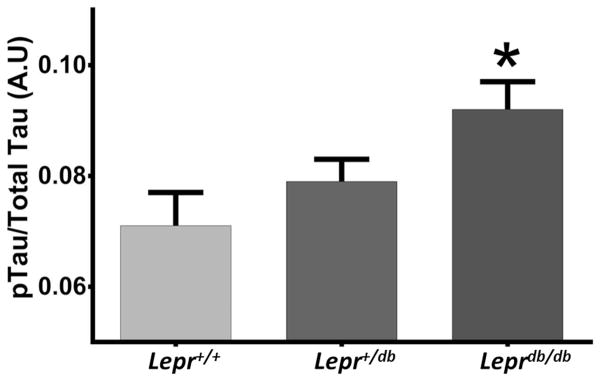
Recently we crossed a mouse model of diabetes (Leprdb/db) and amyloid pathology (APP ΔNL/PS1P264L) knock-in line to study the effects of diabetes and obesity on amyloid-driven dementia (Niedowicz et al., 2014). While no increase in amyloid deposition occurred, these mice (db/AD) developed severe aneurysms, ischemic strokes, and cognitive impairment. In these mice we observed that mice homozygous for the db mutation displayed significantly increased levels of tau phosphorylation (p = 0.03, n = 22 Lepr+/+, 50 Lepr+/db, 24 Leprdb/db) (Fig. 1).
Fig. 1. Tau Phosphorylation Is Increased in db Homozygous Mice.
Homozygous db mice showed an increase in tau phosphorylation, as determined by 96-well slot blot (shown: phospo-tau [AT8]; total tau [HT7] and β-actin [AC15]; p < 0.02, n = 22 Lepr+/+, 50 Lepr+/db, 24 Leprdb/db). The increase was also significant when not standardized to total tau (p < 0.03, not shown). No difference between sexes was observed (p = 0.182, n = 45 female, 51 male).
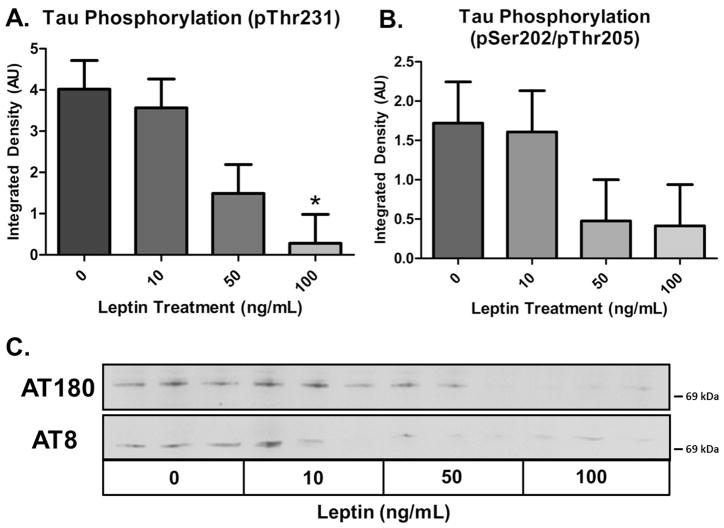
The key characteristic that drives obesity and diabetes in Leprdb/db mice is the abolishment of leptin signaling due to a point mutation in the leptin receptor, abolishing leptin signaling and simulating leptin resistance (Leprdb/db mice are a model of leptin resistance). To determine if the observed increase in tau phosphorylation in Leprdb/db mice is directly related to loss of the leptin signaling axis, we evaluated the impact of leptin signaling on tau phosphorylation in vitro. HEK293 cells stably expressing tauP301L were treated with recombinant human leptin at concentrations consistent with the observed plasma levels in mice. Leptin treatment induced a dose-dependent reduction in tau phosphorylation at the pSer202/Thr205 (AT8) and pThr231 (AT180) epitopes as shown via Western blot (Fig. 2). Although HEK cells are not an ideal model for determining the mechanism underlying this effect, these results indicate that leptin is capable of modulating the phosphorylation state of tau, and may thus play a neuroprotective role. It stands to reason that a reduction in leptin signaling would promote tau phosphorylation, similar to what we observed in the Leprdb/db mice.
Fig. 2. Leptin Treatment Reduces Tau Phosphorylation in HEK293 TauP301L Cells.
HEK293 cells stably transfected with tauP301L display a dose dependent reduction in tau phosphorylation when treated with recombinant human leptin (n = 3 for all treatments; AT180: p ≤ 0.05; AT8 p = 0.108).
Characteristics of the Leprdb/db Mouse Model
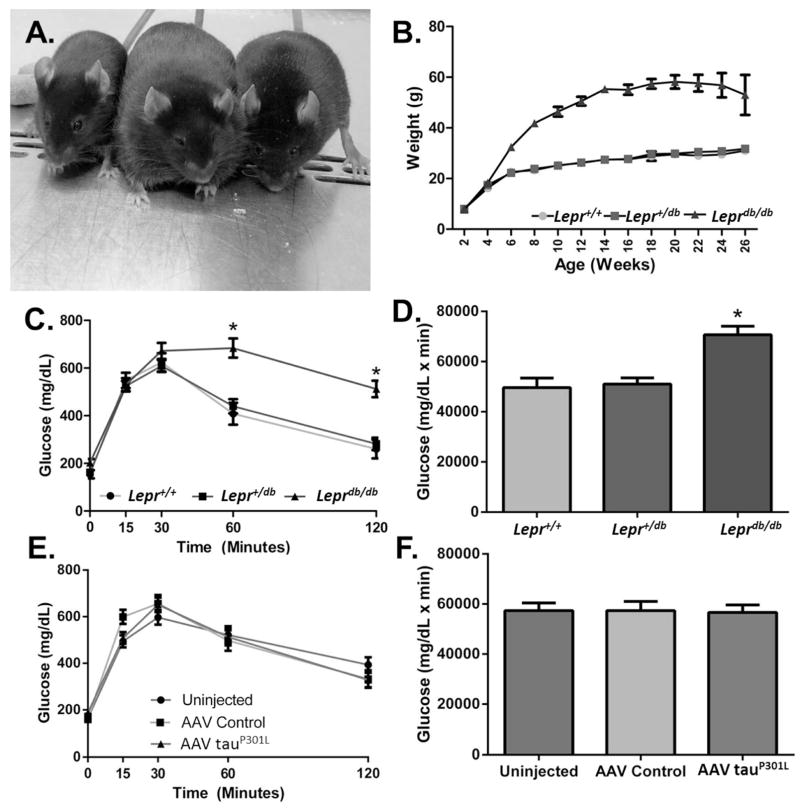
Rodent tau does not naturally form PHFs or NFTs. It is, thus, not possible to study the effect of diabetes on tangle pathology in the db/AD model. We, therefore, turned to AAV technology, which facilitates rapid production of a large number of dual transgenic animals (Platt et al., 2013). We only used Leprdb/db mice in this study as we wanted to determine the effects of leptin resistance-induced obesity and diabetes independent of amyloid pathology. We transduced the brains of Leprdb/db mice with a tau mutant, tauP301L using AAV. Though the tauopathy observed in AD does not occur due to a genetic defect, tau mutations are found in other tauopathies. The tauP301L mutation is commonly used to produce tangle pathology in rodent models (Lewis et al., 2000, Gotz et al., 2001). Characterization of these mice revealed that mice homozygous for the db mutation (Leprdb/db) weighed significantly more than wild-type (Lepr+/+) or heterozygous (Lepr+/db) littermates (p = 0.001, n = 18 Lepr+/+, 42 Lepr+/db, 30 Leprdb/db), and displayed accelerated weight gain from weaning (Fig. 3A–B). No weight differences were observed between genders until five and six months where males of all genotypes weighed significantly more than their female counterparts (data not shown; 5 months: p < 0.001, 6 months: p < 0.001; n = 7F/11M, Lepr+/+, 18F/24M Lepr+/db, 14F/16M Leprdb/db). Additionally, GTT analysis showed that mice homozygous for the db mutation displayed impaired glucose handling compared to wild-type and heterozygous littermates (Fig. 3C–D: p < 0.001, n = 18 Lepr+/+, 45 Lepr+/db, 25 Leprdb/db). Males displayed an even greater impairment in glucose handling compared to females (data not shown; p < 0.01, n = 8F/10M, Lepr +/+, 18F/27M Lepr+/db, 14F/13M Leprdb/db). AAV injection with either the control or tauP301L constructs had no effect on glucose handling (Fig. 3E–F: p = 0.001, n = 26 uninjected, 27 control AAV1, 35 AAV1 tauP301L). Similarly, AAV injection had no overall effect on weight (AAV Control: 38.8 ± 1.5 g; AAV tauP301L: 38.17 ± 1.9 g; p = 0.766), and there was no interaction between AAV injection and body weight by genotype (p = 0.714). These data indicate that mice homozygous for the db mutation have metabolic dysfunction, consistent with previous reports (Chua et al., 1996, Koch et al., 2010), and that expression of mutant tau had no impact on this phenotype.
Fig. 3. Characteristics of Leprdb/db Mice Injected with AAV TauP301L.
Mice homozygous for the db genotype were obese, whereas wild-type and heterozygous mice maintained a normal body weight (A–B) (4 month old littermates, right: Lepr+/db; middle: Leprdb/db; left: Lepr+/+. ANOVA, p < 0.001, n =18 Lepr+/+, 45 Lepr+/db 25 Leprdb/db). At five and six months males of all genotypes weighed more than female counterparts (data not shown; 5 months: p < 0.001, 6 months: p < 0.001; n = 7F/11M, Lepr+/+, 18F/24M Lepr+/db, 14F/16M Leprdb/db). Mice homozygous for the db genotype had impaired glucose handling as determined by glucose tolerance test (C–D) (Repeated measures ANOVA, * = p < 0.001, n = 18 Lepr+/+, 45 Lepr+/db, 25 Leprdb/db). Males displayed a greater impairment in glucose tolerance (data not shown; p < 0.01, n = 8F/10M, Lepr+/+, 18F/27M Lepr+/db, 14F/13M Leprdb/db). AAV injection had no effect on glucose handling (E–F) (ANOVA, p < 0.879, n = 26 uninjected, 27 control AAV1 injected, 35 tauP301L injected).
Mice Transduced with TauP301L Express Higher Levels of the Tau Protein
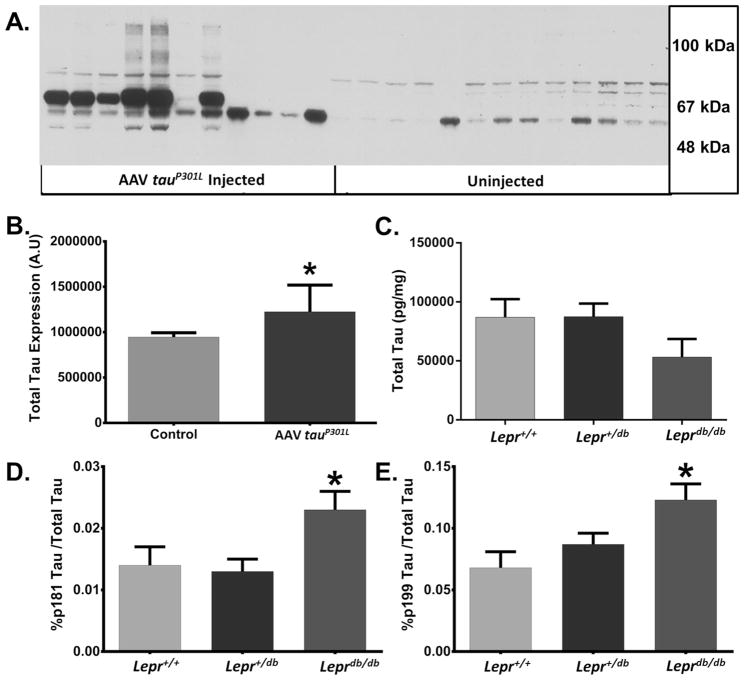
At six months of age, mice were euthanized and their tissues harvested. Mice transduced with AAV1 tauP301L expressed significantly higher levels of tau than control animals (Fig. 4A–B: p < 0.005, n = 13 uninjected [5 Lepr+/+, 3 Lepr+/db, 5 Leprdb/db]/11 tauP301L [5 Lepr+/+, 3 Lepr+/db, 3 Leprdb/db]). It is also interesting to note that some AAV1 tauP301L samples display large protein smears on Western blot (Fig. 4A) indicating that tau aggregates are likely present in the homogenate, which may be indicative of tangle pathology. However, as this is an analysis of soluble tau isolated from the TBS fraction, there may be a substantial proportion of less soluble material still present in the pellet. Total tau levels were not significantly different between genotypes (Fig. 4C: p = 0.172, n = 10 Lepr+/+, 23 Lepr+/db, 10 Leprdb/db; the slight reduction of total tau in homozygous db mice was not significant, p = 0.172). Similarly, the absolute amounts of pThr 181 (p = 0.874, values in pg/mg: Lepr+/+, 1031 ± 115; Lepr+/db, 982 ± 80; Leprdb/db, 944 ± 121) and pSer 199 (p = 0.473, values in pg/mg: Lepr+/+, 6156 ± 1439; Lepr+/db, 7421 ± 995; Leprdb/db, 5295 ± 1509) phospho-tau were unchanged across genotypes. However, tau phosphorylation was significantly higher in homozygous db mice as a proportion of total tau, as compared to the other genotypes (Fig. 4D–E: pSer 199 tau p < 0.05, pThr 181 tau p < 0.05). As only the AAV tauP301L injected mice display increased tau expression uninjected and non-expressing AAV injected mice will collectively be referred to as the ‘control’ group henceforth. These data support the hypothesis that leptin resistance, obesity, and diabetes increase tau phosphorylation in vivo.
Fig. 4. AAV1 TauP301L Injected Mice Display Increased Levels of Total Tau.
(A–B) A cohort of mice were injected with AAV1 tauP301L at P0–P2 and expression levels of total tau (HT7) were evaluated by Western blot after six months (p < 0.05; n = 13 uninjected [5 Lepr+/+, 3 Lepr+/db, 5 Leprdb/db]/11 tauP301L [5 Lepr+/+, 3 Lepr+/db, 3 Leprdb/db]). (C) Leprdb/db mice did not displayed significantly different levels of total tau (TBS fraction), as measured by sandwich ELISA, though they did appear to have slightly less total tau (p = 0.172, n = 10 Lepr+/+, 23 Lepr+/db, 10 Leprdb/db) (D–E) Phospho-tau Ser199 and Thr181 levels were also measured by ELISA. Both epitopes showed a significant increase in the percentage of tau phosphorylation, standardized to total tau, in db homozygotes compared to other genotypes (ANOVA, * = p < 0.05; n = 10 Lepr+/+, 23 Lepr+/db, 10 Leprdb/db).
To determine if tau pathology was exacerbated by diabetes, we examined brain sections from injected mice by IHC. AAV1 tauP301L-injected mice developed tau pathology in the neocortex and hippocampus (Fig. 5A), typically present in large pyramidal neurons (Fig. 5B). Direct comparison of AAV1 tauP301L-injected mice (Fig. 5C & E) to control mice (Fig. 5D & F), illustrated the dramatic extent to which tau pathology developed in mice expressing the tauP301L mutation, indicating that this is a viable model of tauopathy. To determine the interaction between genotype and the burden of tau accumulation, five sections from 15 mice, five of each genotype, were labeled with PHF-1 (Fig. 6A–C). Consistent with the observed increase in tau phosphorylation, db homozygotes displayed more extensive tau pathology than their wild-type or heterozygous littermates (Fig. 6D). Interestingly, other cells resembling astrocytes were also observed throughout the brain as harboring tau pathology (Fig. 7A). Fluorescent co-labeling with PHF1 and anti-GFAP antibodies indicated that tau accumulation was indeed present in a small subset of astrocytes (Fig. 7B).
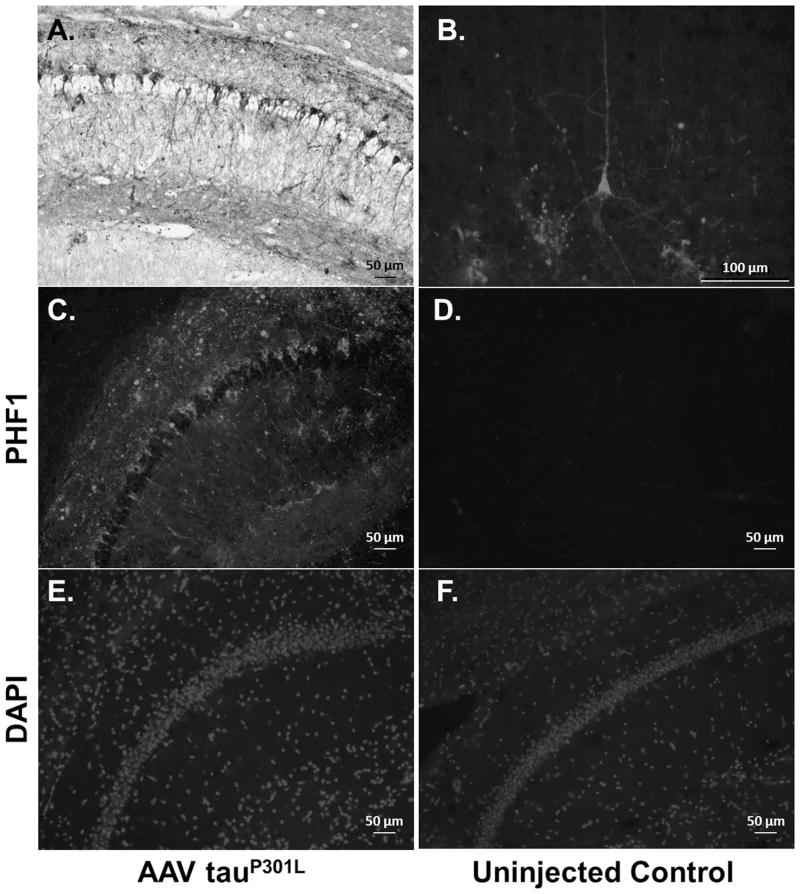
Fig. 5. Tau Expression in AAV1 TauP301L Injected Mice.
(A) AAV1 tauP301L injected brain section of a db homozygous mouse stained with PHF-1 primary antibody. Extensive tau pathology developed in the hippocampus of these mice at 6 months of age. (B) Extensive tau pathology could also be observed in the cortex. (C – F) TauP301L injected mice displayed extensive PHF1 immunoreactivity compared to uninjected controls (Top: PHF-1, Bottom: Nuclei stained with DAPI for anatomical reference). Note that no PHF1 immunoreactivity is observed in mice not injected with AAV1 tauP301L (D).
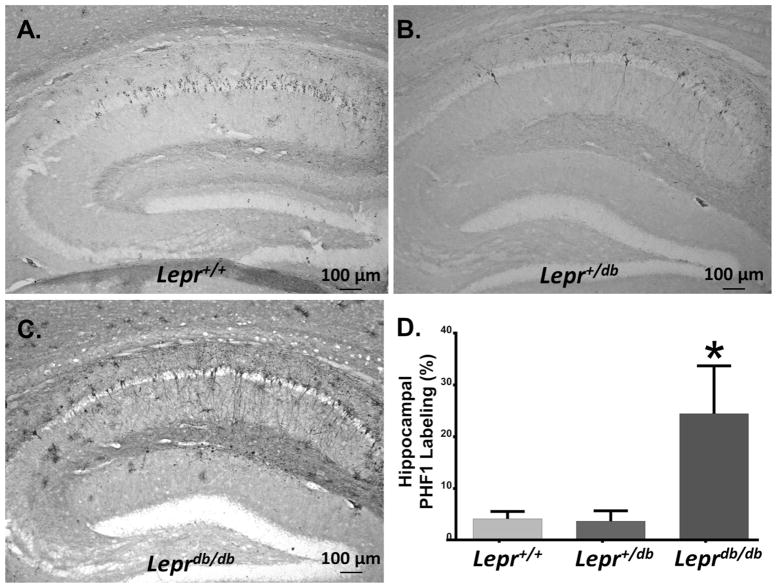
Fig. 6. db Homozygotes Display Tau Pathology Within the Hippocampus.
(A–C) AAV1 tauP301L injected hemibrains were sectioned and stained for PHF-1 to determine the extent of tau pathology in the hippocampus (A: Lepr+/+ B: Lepr+/db, C: Leprdb/db). (D) Densitometric analysis of these sections indicated that db homozygotes displayed increased PHF1 immunoreactivity than the wild-types or heterozygotes (p < 0.05, n = 5 Lepr+/+, 5 Lepr+/db, 5 Leprdb/db).
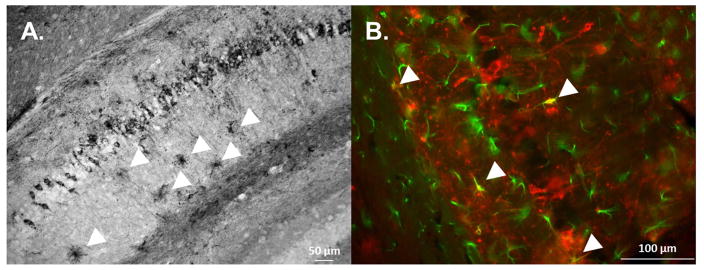
Fig. 7. Astrocytic Tau Pathology Is Present in AAV1 TauP301LInjected Mice.
(A) In addition to tau accumulation in pyramidal neurons, tau pathology was also present in astrocytes (arrows). (B) Colabeling with PHF1 and anti-GFAP antibodies revealed colocalization of tau accumulation within a subset of astrocytes (arrows).
Cognitive Function
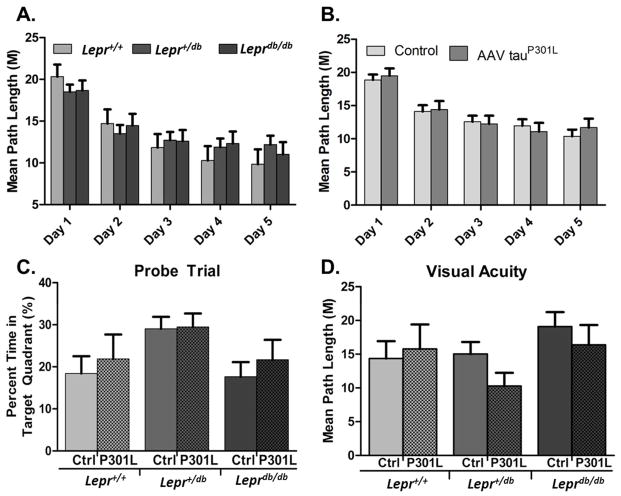
Finally, in order to determine if the observed tau pathology induced cognitive deficits we performed a basic Morris Water Maze test. For all training days, swim distance was not significantly different between the three genotypes (Fig. 8A: p = 0.959, n = 18 Lepr+/+, 45 Lepr+/db, 26 Leprdb/db). Escape latency for db homozygous animals was significantly higher, a finding likely explained by the slight reduction in observed swim speed (data not shown; escape latency: p < 0.001; swim speed: p < 0.001). There was no difference in swim distance between AAV control and AAV1 tauP301L injected animals (Fig. 8B: p = 0.862, n = 54 control, 35 AAV1 tauP301L), and no interaction between genotype and AAV tauP301L injection was observed (data not shown; p = 0.312). After the fifth day of acquisition trials, a probe trial was performed to test spatial memory. Interestingly, db heterozygotes spent significantly more time in the target quadrant than the other genotypes (p = 0.044 compared to Lepr+/+), whereas AAV1 tauP301L injection had no effect (p = 0.992; Fig. 8C: genotype: n = 18 Lepr+/+, 45 Lepr+/db, 26 Leprdb/db; treatment: p = 0.473, n = 35 AAV1 tauP301L, 54 control).
Fig. 8. Mice Have No Deficits in Memory or Learning Based on Morris Water Maze Analysis.
(A – B) There were no significant differences by genotype or treatment (genotype: p = 0.837, n = 18 Lepr+/+, 45 Lepr+/db, 26 Leprdb/db, treatment: p = 0.633, n = 54 control, 35 AAV1 tauP301L injected). (C) On the last day of training after the final trial, the platform was removed and mice underwent a probe trial. Heterozygotes performed significantly better at this trial than their counterparts, and treatment had no effect (genotype: p < 0.05, n = 18 Lepr+/+, 45 Lepr+/db, 26 Leprdb/db; treatment: p = 0.473, n = 54 control, 35 AAV1 tauP301L injected) (D) After the probe trial the platform was placed in the tank within the southwest quadrant and flagged to make the platform visible. Neither genotype nor treatment had any significant effect (genotype: p = 0.118, n = 18 Lepr+/+, 45 Lepr+/db, 26 Leprdb/db, treatment: p = 0.59, n = 54 control, 35 AAV1 tauP301L injected).
Diabetic retinopathy is a common secondary complication of diabetes and has previously been reported in Leprdb/db mice as early as 22 weeks of age (Midena et al., 1989, Clements et al., 1998, Cheung et al., 2005). Therefore, in order to determine if there was a visual impairment in db homozygotes that could potentially affect MWM performance, we performed a visual acuity test. In this test all mice displayed similar swim distances to the flagged platform (Fig. 8D: genotype: p = 0.118, n = 18 Lepr+/+, 45 Lepr+/db, 26 Leprdb/db; treatment: p = 0.59, n = 35 AAV1 tauP301L, 54 control) regardless of genotype or treatment, indicating that there were no significant visual deficits.
DISCUSSION
The influence of leptin resistance, obesity, and diabetes on tau pathology in neurodegeneration remains unclear, largely due to the complex metabolic state of these conditions. Leptin has been shown to be protective against stroke, excitotoxicity, and neuropathy (Dicou et al., 2001, Zhang et al., 2007, Guo et al., 2008, Lieb et al., 2009, Perez-Gonzalez et al., 2011). Reports have also indicated that leptin may improve cognition and modulate hippocampal synaptic plasticity (Shanley et al., 2001, Wayner et al., 2004, Farr et al., 2006, Oomura et al., 2006, Paz-Filho et al., 2008). Similarly, several studies have demonstrated that deficient leptin signaling, as a result of mutation or resistance, can impair cognition (Li et al., 2002, Harvey and Ashford, 2003, Winocur et al., 2005). In this study, we demonstrate that leptin resistance-induced obesity and diabetes modulate tau phosphorylation pathways, resulting in increased tau phosphorylation, and that this in turn leads to accelerated development of tau pathology.
Leptin functions through JAK/STAT signaling pathways, activating downstream regulatory kinases such as MAPK, AKT, and mTOR, all of which play key roles in regulating tau’s phosphorylation state (Augustinack et al., 2002, Cavallini et al., 2013). It is also interesting to note that these three regulatory pathways inhibit glycogen synthase kinase-3β (GSK3β), thought to be the primary kinase involved in pathologic tau hyperphosphorylation (Frame and Cohen, 2001, Lee, 2011). Leptin-mediated inhibition of GSK3β activity has been reported, and it is logical to deduce that GSK3β activity would be increased in the absence of leptin signaling, leading to increased tau phosphorylation (Valerio et al., 2006).
Despite increases in tau pathology, these mice displayed no impairments in learning or memory, as tested by the MWM. Other traditional transgenic models, which utilize the tauP301L mutation, display cognitive deficits at around 16 months of age (Harris et al., 2012). Our model has more diffuse expression than the tau models used in those studies and may display significant cognitive deficits at ages beyond six months. It is quite possible that neurodegeneration is minimal at this age though pathology is developing in the brains of these mice. One barrier that we encountered when using the Leprdb/db model is that these mice have a substantially decreased lifespan (approximately 15 to 16 months) and can develop severe complications associated with obesity and metabolic dysfunction. It is occasionally observed that db/db mice have deficits in the Morris water maze (Chen et al., 2014), these studies are mostly confined to younger animals (~3 months of age) than those used in the current study (~6 months). Although the precise reason for this discrepancy is beyond the scope of the current study, these deficits are typically quite small, and it is possible that they are transitory and can be compensated for as the mice mature. While the Leprdb/db line has proven to be an excellent model of obesity and diabetes, any future studies with these mice will need to balance the possible age requirements needed induce neurodegeneration with the innate morbidity and mortality of these mice.
In addition to future studies in aged mice, altering the AAV vector itself could provide some benefit. This study utilized an AAV serotype 1 vector. While injected mice displayed extensive tau pathology, utilizing another serotype could provide slightly better results. Other AAV serotypes, such as 8 and 9, have the highest transduction efficiencies within the CNS, are able to provide robust expression within the hippocampus, and cross the blood-brain barrier more easily than other serotypes (Cearley and Wolfe, 2006, Harding et al., 2006, Klein et al., 2008, Duque et al., 2009). Using a different AAV serotype may allow for higher transduction efficiencies and more advanced pathology, though further experiments would be required to determine if they would yield better results than our current AAV serotype. Though the CBA promoter of the virus should transduce all cell types, it is possible other promoters could enhance transgene expression. Studies indicate that the human synapsin promoter, which selectively transduces neurons, can induce more potent transgene expression than other promoters, including the CBA promoter (Shevtsova et al., 2005).
In conclusion, we have demonstrated that a model of dysfunctional leptin signaling, obesity, and T2DM has increased tau phosphorylation and tau pathology. This study establishes a connection between metabolic dysfunction and tauopathies. This study provides a solid foundation for future work exploring mechanisms linking metabolic dysfunction and tauopathy. Such studies could initially be pursued in model cell lines, such as primary neuronal cultures from transgenic mice or stem cell-derived cultures from AD patients, that better model both the human disease state, and leptin signaling pathways in the brain. Additionally, studies into the activity states of important tau kinases warrant investigation, and the development of potential therapeutics could be developed from these findings.
Highlights.
Obesity, diabetes, and leptin resistance promote tau phosphorylation in vivo.
Leptin resistance promotes tau phosphorylation in vitro
Obesity, diabetes, and leptin resistance enhance NFT pathology in tauP301L expressing mice
Acknowledgments
We would like to thank Dr. Ronald Klein for the use of his AAV constructs, Dr. Peter Davies for his donation of the PHF1 antibody to the Sanders-Brown Neuropathology Core, Dr. Chad Dickey for his donation of the HEK tauP301L cell line, and Dr. Michael Mendenhall for assistance in AAV generation. This work was funded by the NIH (NS058382, NS083692, AG045809, GM103486, DK020579), the Coins for Alzheimer’s Research Trust (CART), the American Heart Association (13IRG14330016) and the Alzheimer’s Association (IIRG-10-172905).
Abbreviations
- AAV1
adeno-associated virus serotype 1
- AD
Alzheimer’s disease
- AMPK
AMP-activated protein kinase
- APP
amyloid precursor protein
- AU
arbitrary unit
- FTD
frontotemporal dementia
- JAK
janus kinase
- MAPK
mitogen-activated protein kinase
- mTOR
mammalian target of rapamycin
- NFT
neurofibrillary tangle
- PI3K
phosphatidylinositol-4,5-bisphosphate 3-kinase
- PS1
presenilin-1
- STAT
signal Transducer and Activator of Transcription
- T2DM
type-2 diabetes mellitus
Footnotes
The authors declare no competing financial interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accardi G, Caruso C, Colonna-Romano G, Camarda C, Monastero R, Candore G. Can Alzheimer disease be a form of type 3 diabetes? Rejuvenation Res. 2012;15:217–221. doi: 10.1089/rej.2011.1289. [DOI] [PubMed] [Google Scholar]
- Adeghate E, Schattner P, Dunn E. An update on the etiology and epidemiology of diabetes mellitus. Ann N Y Acad Sci. 2006;1084:1–29. doi: 10.1196/annals.1372.029. [DOI] [PubMed] [Google Scholar]
- Anguiano M, Nowak RJ, Lansbury PT., Jr Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry. 2002;41:11338–11343. doi: 10.1021/bi020314u. [DOI] [PubMed] [Google Scholar]
- Association AD. Prevention and Management of Diabetes Complications. Alexandria, Va, USA: American Diabetes Association; 2013. [Google Scholar]
- Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68:51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- Bergman M. Pathophysiology of prediabetes and treatment implications for the prevention of type 2 diabetes mellitus. Endocrine. 2013;43:504–513. doi: 10.1007/s12020-012-9830-9. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG, Jr, Flier JS. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- Burguera B, Couce ME, Long J, Lamsam J, Laakso K, Jensen MD, Parisi JE, Lloyd RV. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain. Neuroendocrinology. 2000;71:187–195. doi: 10.1159/000054536. [DOI] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Cavallini A, Brewerton S, Bell A, Sargent S, Glover S, Hardy C, Moore R, Calley J, Ramachandran D, Poidinger M, Karran E, Davies P, Hutton M, Szekeres P, Bose S. An unbiased approach to identifying tau kinases that phosphorylate tau at sites associated with Alzheimer disease. J Biol Chem. 2013;288:23331–23347. doi: 10.1074/jbc.M113.463984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther. 2006;13:528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Chen J, Liang L, Zhan L, Zhou Y, Zheng L, Sun X, Gong J, Sui H, Jiang R, Zhang F, Zhang L. ZiBuPiYin recipe protects db/db mice from diabetes-associated cognitive decline through improving multiple pathological changes. PLoS One. 2014;9:e91680. doi: 10.1371/journal.pone.0091680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Jiang XZ, Zhao XH, Qin YL, Gu Z, Gu PL, Zhou B, Zhu ZH, Xu LY, Zou YF. Risk factors of mild cognitive impairment in middle aged patients with type 2 diabetes: a cross-section study. Ann Endocrinol (Paris) 2012;73:208–212. doi: 10.1016/j.ando.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Cheung AK, Fung MK, Lo AC, Lam TT, So KF, Chung SS, Chung SK. Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes. 2005;54:3119–3125. doi: 10.2337/diabetes.54.11.3119. [DOI] [PubMed] [Google Scholar]
- Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- Clements RS, Jr, Robison WG, Jr, Cohen MP. Anti-glycated albumin therapy ameliorates early retinal microvascular pathology in db/db mice. J Diabetes Complications. 1998;12:28–33. doi: 10.1016/s1056-8727(97)00051-2. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Cota D, Matter EK, Woods SC, Seeley RJ. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci. 2008;28:7202–7208. doi: 10.1523/JNEUROSCI.1389-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Couce ME, Burguera B, Parisi JE, Jensen MD, Lloyd RV. Localization of leptin receptor in the human brain. Neuroendocrinology. 1997;66:145–150. doi: 10.1159/000127232. [DOI] [PubMed] [Google Scholar]
- Craft S. Insulin resistance syndrome and Alzheimer’s disease: age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiol Aging. 2005;26(Suppl 1):65–69. doi: 10.1016/j.neurobiolaging.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Desai G, Zheng C, Geetha T, Mathews ST, White BD, Huggins KW, Zizza CA, Broderick TL, Babu JR. The Pancreas-Brain Axis: Insight into Disrupted Mechanisms Associating Type 2 Diabetes and Alzheimer’s Disease. Journal of Alzheimer’s disease: JAD. 2014 doi: 10.3233/JAD-140018. [DOI] [PubMed] [Google Scholar]
- Dicou E, Attoub S, Gressens P. Neuroprotective effects of leptin in vivo and in vitro. Neuroreport. 2001;12:3947–3951. doi: 10.1097/00001756-200112210-00019. [DOI] [PubMed] [Google Scholar]
- Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, Douar AM, Fyfe J, Moullier P, Colle MA, Barkats M. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther. 2009;17:1187–1196. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–1425. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Ferreira ST, Clarke JR, Bomfim TR, De Felice FG. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2014;10:S76–83. doi: 10.1016/j.jalz.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol. 1997;146:214–222. doi: 10.1093/oxfordjournals.aje.a009256. [DOI] [PubMed] [Google Scholar]
- Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freude S, Plum L, Schnitker J, Leeser U, Udelhoven M, Krone W, Bruning JC, Schubert M. Peripheral hyperinsulinemia promotes tau phosphorylation in vivo. Diabetes. 2005;54:3343–3348. doi: 10.2337/diabetes.54.12.3343. [DOI] [PubMed] [Google Scholar]
- Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD, Van Eldik LJ, Norris CM. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J Neurosci. 2012;32:16129–16140. doi: 10.1523/JNEUROSCI.2323-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, Chen F, Barmettler R, Nitsch RM. Tau filament formation in transgenic mice expressing P301L tau. J Biol Chem. 2001;276:529–534. doi: 10.1074/jbc.M006531200. [DOI] [PubMed] [Google Scholar]
- Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N, Casadesus G. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2010;19:1155–1167. doi: 10.3233/JAD-2010-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco SJ, Sarkar S, Johnston JM, Zhu X, Su B, Casadesus G, Ashford JW, Smith MA, Tezapsidis N. Leptin reduces Alzheimer’s disease-related tau phosphorylation in neuronal cells. Biochem Biophys Res Commun. 2008;376:536–541. doi: 10.1016/j.bbrc.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Jiang H, Xu X, Duan W, Mattson MP. Leptin-mediated cell survival signaling in hippocampal neurons mediated by JAK STAT3 and mitochondrial stabilization. J Biol Chem. 2008;283:1754–1763. doi: 10.1074/jbc.M703753200. [DOI] [PubMed] [Google Scholar]
- Hakansson ML, Meister B. Transcription factor STAT3 in leptin target neurons of the rat hypothalamus. Neuroendocrinology. 1998;68:420–427. doi: 10.1159/000054392. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Harding TC, Dickinson PJ, Roberts BN, Yendluri S, Gonzalez-Edick M, Lecouteur RA, Jooss KU. Enhanced gene transfer efficiency in the murine striatum and an orthotopic glioblastoma tumor model, using AAV-7- and AAV-8-pseudotyped vectors. Hum Gene Ther. 2006;17:807–820. doi: 10.1089/hum.2006.17.807. [DOI] [PubMed] [Google Scholar]
- Harris JA, Koyama A, Maeda S, Ho K, Devidze N, Dubal DB, Yu GQ, Masliah E, Mucke L. Human P301L-mutant tau expression in mouse entorhinal-hippocampal network causes tau aggregation and presynaptic pathology but no cognitive deficits. PLoS One. 2012;7:e45881. doi: 10.1371/journal.pone.0045881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol. 2007;7:643–647. doi: 10.1016/j.coph.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, Ashford ML. Leptin in the CNS: much more than a satiety signal. Neuropharmacology. 2003;44:845–854. doi: 10.1016/s0028-3908(03)00076-5. [DOI] [PubMed] [Google Scholar]
- Iqbal K, del Alonso AC, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Jung HJ, Park SS, Mok JO, Lee TK, Park CS, Park SA. Increased expression of three-repeat isoforms of tau contributes to tau pathology in a rat model of chronic type 2 diabetes. Exp Neurol. 2011;228:232–241. doi: 10.1016/j.expneurol.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Ke YD, Delerue F, Gladbach A, Gotz J, Ittner LM. Experimental diabetes mellitus exacerbates tau pathology in a transgenic mouse model of Alzheimer’s disease. PloS one. 2009;4:e7917. doi: 10.1371/journal.pone.0007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Backus C, Oh S, Hayes JM, Feldman EL. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology. 2009;150:5294–5301. doi: 10.1210/en.2009-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Tatom JB, Henderson KM, Henning PP. AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol Ther. 2008;16:89–96. doi: 10.1038/sj.mt.6300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Lin WL, Dickson DW, Lewis J, Hutton M, Duff K, Meyer EM, King MA. Rapid neurofibrillary tangle formation after localized gene transfer of mutated tau. The American journal of pathology. 2004a;164:347–353. doi: 10.1016/S0002-9440(10)63124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Lin WL, Dickson DW, Lewis J, Hutton M, Duff K, Meyer EM, King MA. Rapid neurofibrillary tangle formation after localized gene transfer of mutated tau. Am J Pathol. 2004b;164:347–353. doi: 10.1016/S0002-9440(10)63124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C, Augustine RA, Steger J, Ganjam GK, Benzler J, Pracht C, Lowe C, Schwartz MW, Shepherd PR, Anderson GM, Grattan DR, Tups A. Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity. J Neurosci. 2010;30:16180–16187. doi: 10.1523/JNEUROSCI.3202-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike MA, Green KN, Blurton-Jones M, Laferla FM. Oligemic hypoperfusion differentially affects tau and amyloid-{beta} Am J Pathol. 2010;177:300–310. doi: 10.2353/ajpath.2010.090750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kril JJ, Patel S, Harding AJ, Halliday GM. Neuron loss from the hippocampus of Alzheimer’s disease exceeds extracellular neurofibrillary tangle formation. Acta Neuropathol. 2002;103:370–376. doi: 10.1007/s00401-001-0477-5. [DOI] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, Kiritoshi T, Neugebauer V, Jackson GR, Kayed R. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci Rep. 2012;2:700. doi: 10.1038/srep00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB. Obesity, leptin, and Alzheimer’s disease. Ann N Y Acad Sci. 2011;1243:15–29. doi: 10.1111/j.1749-6632.2011.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesort M, Johnson GV. Insulin-like growth factor-1 and insulin mediate transient site-selective increases in tau phosphorylation in primary cortical neurons. Neuroscience. 2000;99:305–316. doi: 10.1016/s0306-4522(00)00200-1. [DOI] [PubMed] [Google Scholar]
- Levites Y, Jansen K, Smithson LA, Dakin R, Holloway VM, Das P, Golde TE. Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid beta, amyloid beta40, and amyloid beta42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice. J Neurosci. 2006;26:11923–11928. doi: 10.1523/JNEUROSCI.2795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, Roubenoff R, Auerbach S, DeCarli C, Wolf PA, Seshadri S. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. Jama. 2009;302:2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW. Filamentous tau in oligodendrocytes and astrocytes of transgenic mice expressing the human tau isoform with the P301L mutation. Am J Pathol. 2003;162:213–218. doi: 10.1016/S0002-9440(10)63812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Studzinski C, Beckett T, Guan H, Hersh MA, Murphy MP, Klein R, Hersh LB. Expression of neprilysin in skeletal muscle reduces amyloid burden in a transgenic mouse model of Alzheimer disease. Mol Ther. 2009;17:1381–1386. doi: 10.1038/mt.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Studzinski C, Beckett T, Murphy MP, Klein RL, Hersh LB. Circulating neprilysin clears brain amyloid. Molecular and cellular neurosciences. 2010;45:101–107. doi: 10.1016/j.mcn.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Sahara N, Saito Y, Murayama S, Ikai A, Takashima A. Increased levels of granular tau oligomers: an early sign of brain aging and Alzheimer’s disease. Neurosci Res. 2006;54:197–201. doi: 10.1016/j.neures.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Martinez-Coria H, Green KN, Billings LM, Kitazawa M, Albrecht M, Rammes G, Parsons CG, Gupta S, Banerjee P, LaFerla FM. Memantine improves cognition and reduces Alzheimer’s-like neuropathology in transgenic mice. Am J Pathol. 2010a;176:870–880. doi: 10.2353/ajpath.2010.090452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Coria H, Green KN, Billings LM, Kitazawa M, Albrecht M, Rammes G, Parsons CG, Gupta S, Banerjee P, LaFerla FM. Memantine improves cognition and reduces Alzheimer’s-like neuropathology in transgenic mice. The American journal of pathology. 2010b;176:870–880. doi: 10.2353/ajpath.2010.090452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwarha G, Dasari B, Prasanthi JR, Schommer J, Ghribi O. Leptin reduces the accumulation of Abeta and phosphorylated tau induced by 27-hydroxycholesterol in rabbit organotypic slices. J Alzheimers Dis. 2010;19:1007–1019. doi: 10.3233/JAD-2010-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier C. Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobiol Aging. 2005;26(Suppl 1):26–30. doi: 10.1016/j.neurobiolaging.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Midena E, Segato T, Radin S, di Giorgio G, Meneghini F, Piermarocchi S, Belloni AS. Studies on the retina of the diabetic db/db mouse. I. Endothelial cell-pericyte ratio. Ophthalmic Res. 1989;21:106–111. doi: 10.1159/000266787. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Jama. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Titani K, Ihara Y. Proline-directed and non-proline-directed phosphorylation of PHF-tau. J Biol Chem. 1995;270:823–829. doi: 10.1074/jbc.270.2.823. [DOI] [PubMed] [Google Scholar]
- Morris HR, Khan MN, Janssen JC, Brown JM, Perez-Tur J, Baker M, Ozansoy M, Hardy J, Hutton M, Wood NW, Lees AJ, Revesz T, Lantos P, Rossor MN. The genetic and pathological classification of familial frontotemporal dementia. Arch Neurol. 2001;58:1813–1816. doi: 10.1001/archneur.58.11.1813. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–2131. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- Niedowicz DM, Reeves VL, Platt TL, Kohler K, Beckett TL, Powell DK, Lee TL, Sexton TR, Song ES, Brewer LD, Latimer CS, Kraner SD, Larson KL, Ozcan S, Norris CM, Hersh LB, Porter NM, Wilcock DM, Murphy MP. Obesity and diabetes cause cognitive dysfunction in the absence of accelerated beta-amyloid deposition in a novel murine model of mixed or vascular dementia. Acta Neuropathol Commun. 2014;2:64. doi: 10.1186/2051-5960-2-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedowicz DM, Studzinski CM, Weidner AM, Platt TL, Kingry KN, Beckett TL, Bruce-Keller AJ, Keller JN, Murphy MP. Leptin regulates amyloid beta production via the gamma-secretase complex. Biochim Biophys Acta. 2013;1832:439–444. doi: 10.1016/j.bbadis.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Cheng D, Jouleh B, Torp R, LaFerla FM. Genetically augmenting tau levels does not modulate the onset or progression of Abeta pathology in transgenic mice. J Neurochem. 2007;102:1053–1063. doi: 10.1111/j.1471-4159.2007.04607.x. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Matsumiya T, Ishibashi M, Aou S, Li XL, Kohno D, Uramura K, Sougawa H, Yada T, Wayner MJ, Sasaki K. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27:2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Ostlund RE, Jr, Yang JW, Klein S, Gingerich R. Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab. 1996;81:3909–3913. doi: 10.1210/jcem.81.11.8923837. [DOI] [PubMed] [Google Scholar]
- Paz-Filho GJ, Babikian T, Asarnow R, Delibasi T, Esposito K, Erol HK, Wong ML, Licinio J. Leptin replacement improves cognitive development. PLoS One. 2008;3:e3098. doi: 10.1371/journal.pone.0003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Gonzalez R, Antequera D, Vargas T, Spuch C, Bolos M, Carro E. Leptin induces proliferation of neuronal progenitors and neuroprotection in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2011;24(Suppl 2):17–25. doi: 10.3233/JAD-2011-102070. [DOI] [PubMed] [Google Scholar]
- Planel E, Tatebayashi Y, Miyasaka T, Liu L, Wang L, Herman M, Yu WH, Luchsinger JA, Wadzinski B, Duff KE, Takashima A. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J Neurosci. 2007;27:13635–13648. doi: 10.1523/JNEUROSCI.3949-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt TL, Reeves VL, Murphy MP. Transgenic models of Alzheimer’s disease: better utilization of existing models through viral transgenesis. Biochim Biophys Acta. 2013;1832:1437–1448. doi: 10.1016/j.bbadis.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- Prevention CoDCa. Diabetes Fact Sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services; 2011. [Google Scholar]
- Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Haynes WG, Morgan DA, Mark AL. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension. 2003;41:763–767. doi: 10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende R, Ferreiro E, Pereira C, Oliveira CR. ER stress is involved in Abeta-induced GSK-3beta activation and tau phosphorylation. Journal of neuroscience research. 2008;86:2091–2099. doi: 10.1002/jnr.21648. [DOI] [PubMed] [Google Scholar]
- Resnick HE, Valsania P, Halter JB, Lin X. Relation of weight gain and weight loss on subsequent diabetes risk in overweight adults. J Epidemiol Community Health. 2000;54:596–602. doi: 10.1136/jech.54.8.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewers M, Zaccaro D, D’Agostino R, Haffner S, Saad MF, Selby JV, Bergman R, Savage P. Insulin sensitivity, insulinemia, and coronary artery disease: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2004;27:781–787. doi: 10.2337/diacare.27.3.781. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Perry G, Harris PL, Liu Y, Schubert KA, Smith MA. In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer’s disease: a central role for bound transition metals. J Neurochem. 2000;74:270–279. doi: 10.1046/j.1471-4159.2000.0740270.x. [DOI] [PubMed] [Google Scholar]
- Schmelzle K, Kane S, Gridley S, Lienhard GE, White FM. Temporal dynamics of tyrosine phosphorylation in insulin signaling. Diabetes. 2006;55:2171–2179. doi: 10.2337/db06-0148. [DOI] [PubMed] [Google Scholar]
- Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njolstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21:RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevtsova Z, Malik JM, Michel U, Bahr M, Kugler S. Promoters and serotypes: targeting of adeno-associated virus vectors for gene transfer in the rat central nervous system in vitro and in vivo. Experimental physiology. 2005;90:53–59. doi: 10.1113/expphysiol.2004.028159. [DOI] [PubMed] [Google Scholar]
- Shioda S, Funahashi H, Nakajo S, Yada T, Maruta O, Nakai Y. Immunohistochemical localization of leptin receptor in the rat brain. Neurosci Lett. 1998;243:41–44. doi: 10.1016/s0304-3940(98)00082-2. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Goedert M. Tau mutations in familial frontotemporal dementia. Brain. 2000;123( Pt 5):857–859. doi: 10.1093/brain/123.5.857. [DOI] [PubMed] [Google Scholar]
- Stewart R, Liolitsa D. Type 2 diabetes mellitus, cognitive impairment and dementia. Diabet Med. 1999;16:93–112. doi: 10.1046/j.1464-5491.1999.00027.x. [DOI] [PubMed] [Google Scholar]
- Valerio A, Ghisi V, Dossena M, Tonello C, Giordano A, Frontini A, Ferrario M, Pizzi M, Spano P, Carruba MO, Nisoli E. Leptin increases axonal growth cone size in developing mouse cortical neurons by convergent signals inactivating glycogen synthase kinase-3beta. J Biol Chem. 2006;281:12950–12958. doi: 10.1074/jbc.M508691200. [DOI] [PubMed] [Google Scholar]
- van Eersel J, Ke YD, Liu X, Delerue F, Kril JJ, Gotz J, Ittner LM. Sodium selenate mitigates tau pathology, neurodegeneration, and functional deficits in Alzheimer’s disease models. Proc Natl Acad Sci U S A. 2010;107:13888–13893. doi: 10.1073/pnas.1009038107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virkamaki A, Ueki K, Kahn CR. Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Invest. 1999;103:931–943. doi: 10.1172/JCI6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner MJ, Armstrong DL, Phelix CF, Oomura Y. Orexin-A (Hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides. 2004;25:991–996. doi: 10.1016/j.peptides.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR. Tauopathies: classification and clinical update on neurodegenerative diseases associated with microtubule-associated protein tau. Intern Med J. 2006;36:652–660. doi: 10.1111/j.1445-5994.2006.01153.x. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, McEwen BS. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005;119:1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- Wood JG, Mirra SS, Pollock NJ, Binder LI. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986;83:4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes. 2009;58:71–77. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke. 2007;38:2329–2336. doi: 10.1161/STROKEAHA.107.482786. [DOI] [PubMed] [Google Scholar]
- Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci. 2002;5:727–728. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]
- Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Molecular therapy: the journal of the American Society of Gene Therapy. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]