Abstract
Toxoplasma gondii is a protozoan that infects 30% of humans as intermediate hosts. T Sexual reproduction can occur only within the intestinal tract of felines, however, infection in other mammals and birds is associated with asexual replication and interconversion between the tachyzoite and bradyzoite stages. Bradyzoites are slow growing forms found in tissue cysts in latent infection. Recently, our group described the biological behavior of the EGS strain that forms thick walled cysts spontaneously in tissue culture, constituting a useful tool for examining the developmental biology of T. gondii. To further improve the usefulness of this model, we constructed genetically modified EGS parasites that express fluorescent tags under the control of stage specific promoters. The promoter regions for SAG-1 (tachyzoite specific), BAG-1 and LDH-2 (bradyzoite specific) were amplified by PCR and plasmids were constructed with mCherry (redT) and sfGFP (greenB) sequences, respectively. Strains of parasites were selected using FACS to arrive at single fluorescent and dual fluorescent strains of EGS expressing tags in a stage specific manner. In cell cultures, vacuoles labeled by immunofluorescence assay using anti-CST-1 a marker for T. gondii cyst wall contained parasites that were positive for BAG1-GFP and negative for SAG1-mCherry. Tachyzoites and bradyzoites harvested from the mice expressed stage specific mCherry and GFP proteins, respectively. These new dual fluorescent transgenic EGS strains are a promising tool to elucidate the mechanisms of Toxoplasma gondii differentiation both in vitro and in vivo.
Keywords: Toxoplasma gondii, bradyzoite, cyst, conversion, atypical strain
1. Introduction
Toxoplasma gondii, the causative agent of toxoplasmosis, is an obligate intracellular protozoan parasite found on all continents [1]. One of the main features of this protozoan is its ability to infect different classes of animals, from birds to mammals, including humans. The three infective stages of T. gondii: bradyzoites, tachyzoites and sporozoites can rapidly penetrate into nucleated cells, replicate within these cells and can undergo stage specific differentiation [2]. For example, the rapidly dividing tachyzoites, associated with acute toxoplasmosis, can convert into the long term resistant form, the bradyzoites, which divide slowly inside a modified parasitophorous vacuole (PV) with a cyst wall [3], under pressure of the host immune response. Doubtlessly, the conversion of tachyzoites into bradyzoites plays a fundamental role in the maintenance of T. gondii in nature, especially by transmitting the parasite between intermediate hosts through carnivorism [4].
Bradyzoites are found within intracellular cysts, protected from the immune system and are the main form seen during the chronic phase of toxoplasmosis [5]. Under natural conditions, Toxoplasma cysts are preferentially found in neurons and muscle cells [6]. This stage can persist for the whole life of the host [7]. There is, however, a risk of reactivation of the acute phase of the infection, especially in situations in which the immune system is compromised, as occurs in HIV infected patients and during treatment with radiation, corticosteroids, immune modulating antibodies, or anti neoplastic drugs [8–10].
Initial studies of T. gondii cysts relied primarily on material isolated from mouse brains [11]; however, insights into the factors leading to cyst formation and the role of the parasite and the host cell were significantly facilitated by reagents that allowed studies of this process of differentiation in vitro [12–17]. Several authors have demonstrated that different strains of T. gondii can form cysts in cell cultures from different origins [15–17]. It is possible to obtain bradyzoites by spontaneous conversion of tachyzoites isolated from cystogenic strains and to enhance this process by various treatments in culture such as alkalization from 7.2 to 8.0 [18], the use of IFN-γ and NO inductors [19,20], increase in the temperature from 37 °C to 42 °C, and chemical stress with sodium arsenite [21]. Under such stress conditions bradyzoite differentiation rates of at least 70% have been attained, whereas, in a non stressed environment, only about 10% of vacuoles contain bradyzoites [21].
Previous work has shown the ability to form cysts is correlated with virulence in mice [22]. A classification system based on genetic studies of circulating strains of T. gondii in Europe and North America has confirmed the presence of the three major clonal lineages I, II and III in humans and domestic animals, as well as the type 12 (haplotype X and A) lineage in wildlife. Type I is considered the most virulent and lethal, while types II and III are considered avirulent and are associated with chronic infection [22]. However, strains isolated in Asia and South America display a different genetic pattern, with many recombinant genotypes that display variations in virulence and cystogenic competence in mice [23].
Ferreira et al. [24] demonstrated that 20 isolates of T. gondii from Brazil were recombinant genotypes, including the EGS strain. This strain was isolated from the human amniotic fluid of a patient that acquired toxoplasmosis during pregnancy from Minas Gerais state in 1998 and the baby was subsequently diagnosed with congenital toxoplasmosis [25,26]. The EGS strain is a recombinant type I/III strain and is both virulent and cystogenic in mice [27,28].
We have found that the EGS strain, in many different cell types under physiological pH [29] conditions, has a high rate of spontaneous differentiation, with about 30% of infected cells containing cysts/bradyzoites. Furthermore, the wall of cysts formed in vitro is thick and resembles the wall of mature cysts isolated from mice. EGS, therefore, represents a useful strain for investigations of differentiation in T. gondii.
There are many aspects of cyst formation that are still unknown, and tools that would help follow this conversion process in vitro and in vivo would be extremely useful. To this end, molecular and immunological tools have been recently developed to allow a dynamic characterization of the conversion in vitro, using the expression of stage specific proteins combined with expression of fluorescent proteins, the employment of antibodies against stage specific proteins [30] and the genetic manipulation of genes to study the functional role of this on the cyst process [31]. Artificial inductors of encystment and genetically modified parasites have been developed that express fluorescent proteins. Dzierszinski and colleagues [32] demonstrated reorganization of organelles in bradyzoites and the movement of bradyzoties among neighbor cells by fluorescence and video microscopy. Di Cristina [33] modified parasites of a T, gondii strain expressing luciferase to localize cysts in vivo. However, a strain with dual expression of fluorescent tags was still unavailable. With this purpose, Unno et al. [34] and Zhang et al [35] developed PLK strains expressing both BAG1 and SAG1 with fluorescent proteins, therefore establishing a type II strain that could be used for studies of differentiation. However, PLK has lost its ability to form oocysts in cats. In this paper, we describe the development of genetically modified parasites of EGS strain that expresses stage specific fluorescent tags which enables one to follow stage conversion in vitro or in vivo at a high frequency without any need for exogenous stress inducers such as pH shifts.
2. Materials and methods
2.1. Host cells
LLC-MK2 cells (ATCC©) (epithelial kidney cells from Macaca mullata), Human foreskin fibroblasts (HFF - human foreskin fibroblast; ATCC© # SCRC-1041™) were maintained in medium (RPMI INVITROGEN - for LLC-MK2 and DEMEM High Glucose (INVITROGEN - for HFF) supplemented with 10% FBS and 2 mg/ml gentamicin. Serial passages were conducted by trypsinization when the cell density approached confluence in a monolayer and maintained at 37 °C in 5% CO2.
2.2. Parasites
Tachyzoites of the EGS strain were obtained from Swiss mice intraperitoneally infected with 100 to 200 cysts harvested from brains. After 5 to 7 days of infection, parasites were collected from peritoneal fluid and allowed to infect confluent monolayer of LLC-MK2 cells at a ratio of 10:1 parasites per cell. The maintenance of the cultures presenting cysts was performed by trypsinization of the monolayers and expansion to new flasks [29]. Infected cultures were kept for a maximum of 2 months in vitro, as after 8 to 10 passages spontaneous cyst formation slows down, and the capacity is recovered after animal passage. The experimental protocols to maintain the EGS strain in vivo were established by Ferreira and colleagues [27]. The EGS strain was maintained in Swiss mice by subsequent passages of 50 cysts obtained from mice previously infected orally for at least 4 weeks.
2.3. Construction of transfer vector
Type I/III EGS strain was used as the background strain for the development of cystogenesis sensor strains. The promoter region of the bradyzoite-specific antigen 1 (BAG-1), lactate dehydrogenase 2 (LDH-2) and tachyzoite specific surface antigen (SAG-1) genes were PCR-amplified from genomic DNA of EGS strain tachyzoites isolated by Wizard® Genomic DNA Purification Kit (Promega), following the manufactures instructions. The primers and probe sequences used in this study are listed in Table 1.
Table 1.
Forward and reverse primers used in the development of transfer vectors.
| Genes | Forward | Reverse |
|---|---|---|
| superfoldedGFP | ATGGTGAGCAAGGGCGAGGA | GGCATGGACGAGCTGTACAAGTAATTAATTAAGACTACG |
| mCherry | ATGGTGAGCAAGGGCGAGGA | CGGCATGGACGAGCTGTACAAGTAGTTAATTAAGACTACG |
| SAG-1 TGME49_233460 | ACGGTATCGATAAGCTTATTTAAAT | CATTGTCGTGTAAACACACGGTTGTATGGTGAGCAAGGGC |
| BAG-1 TGME49_059020 | TAAGCTTATTTAAATTGCAGAGGGGTGCCCGCGT | CCCAGGTCCCGTATGATATTCAAAAAAGATGGTGAGCAAGGGC |
| LDH-2 TGME49_291040 | TAAGCTTATTTAAATGGTTCCATCGTACCCTGAATGGAAG | TTCGCCTTTACGCATGGTGGAAGTGAAGTACGAATGCCG |
| Sc Vector | ACGGTATCGATAAGCTTATTTAAAT | TTAATTAAGACTACGACGAAAGTGA |
The DNA sequence sfGFP and mCherry DNA were amplified by PCR from plasmids containing sfGFP (plasmid cSFpEGFP-N1 containing human codon optimized superfolder GFP gift of Dr. Erik Lee Snapp Albert Einstein College of Medicine) and mCherry (plasmid gift of Dr. William Jacobs, Albert Einstein College of Medicine). The fragments of stage specific promoters and fluorescent proteins were concatenated into puc19 shuttle vector (Clontech) using an InFusion® kit (Clonthec) following the company instructions briefly, this enzymatic DNA recombination is based on the overlap of 15 bp between the sequences that would be concatenated. For that, all primers were designed with this overlap based on the plasmid map designed using the Geneoius software. Three plasmids for transfection were constructed: (1) pBAG-1/sfGFP, (2) pLDH-2/sfGFP and (3) pSAG-1/mcherry. After the recombination, the plasmids were cloned into Stellar competent cells (Clonthec). The recombination was checked by PCR and the plasmids was isolated using QIAprep Spin Miniprep Kit (QIAGEN) following the manufacturer’s protocol. Plasmids were sequenced in the AECOM Genomic Core Facility (ABI 3730) and the results were checked by alignment using Geneious software. The plasmids containing the correct inserts were subcloned using DH5α™ (Invitrogen) competent cells following the manufactures protocol and ampicilin resistant colonies were selected and grown in LB containing 100 μg/ml media for 12 h at 37 °C, for posterior isolation using NucleoBond® PC 500 (MACHEREY-NAGEL).
2.4. Transfection and parasite selection
The plasmids were linearized using BAM HI (NEB) for 1 h at 37°C and SWAI (NEB) for 1 h at 25°C, and the linearization checked by DNA electrophoresis. Following this, 50 μg of DNA was isolated using standard ethanol precipitation techniques (1/10 vol/vol 3 M sodium acetate and 2 times the total volume of 100% ethanol for 1 h at −70 °C followed by centrifugation and the DNA pellet washed with 75% ethanol and dried at room temperature). Following purification, the DNA was resuspended in 100 μl of cytomix buffer [36]. Transfection was performed in a BTX electroporator using standard conditions for T. gondii [37], at 2000V, 25 Ω and 50 μ F. For each plasmid, 50 μg of DNA was used in a 107 EGS tachyzoites isolated and purified from 30% lysed infected HFF culture.
When necessary, a fourth plasmid was used: TUB-CAT plasmid with a chloramphenicol acetyltransferase (CAT) selectable marker driven by the α-tubulin promoter permitted selection with 20 μM chloramphenicol as previously described [37], 25 μg this plasmid was used in the co-transfection [38]. After the electroporation the parasites were cultivated in HFF cells. The transfection strategies are described in the table 2.
Table 2.
Transfection strategies of EGS parasites used to develop the sensor strains for cyst formation.
| Plasmids | Transfection | Selection | Population |
|---|---|---|---|
| SAG-mcherry | Unique | FACS+subcloning | Tred |
| LDH-2 sfGFP | Unique | FACS+subcloning | Bgreen |
| SAG-mcherry + BAG-1 sfGFP | Sequenced | FACS+subcloning (2 times) | Double 1 |
| SAG-mcherry + LDH-2 sfGFP | Sequenced | FACS+subcloning (2 times) | Double 2 |
| SAG-mcherry + LDH-2 sfGFP + TUB CAT | Co-transfection | Drug selection + FACS+subcloning | Double Cat |
2.5. Selection of parasites
Transient transfected parasites were selected after 1 intracellular cycle in HFF cultures using Cell Sorting. Selection for parasites expressing the fluorescent labels were done using flow cytometer cell sorting employing a FACS ARIA II (BD- at AECOM Flow Cytometer Core Facility). A population of 108 parasites were sorted under sterile conditions the positive parasites were then cultivated in HFF cells. In order to enrich the positive parasites population, when these parasites lysed the host cell cultures, FACS was repeated. After 3 rounds of FACS, the enrichment of the positive population reached about 30% and the parasites were sub cloned into 96 well plates. Fluorescent positive colonies were selected and expanded. For the DoubleCat transfected group just the colonies that were double positive were isolated and subcloned.
2.6. Host cell infection assays
EGS parasites collected from the supernatant of a 10 day-old infected culture were allowed to interact for 45 min with fresh LLC-MK2 monolayers in ratios of 10:1 parasites per host cell. The monolayers were washed twice with RPMI in order to remove remaining extracellular parasites and incubated for 96 h at 37 °C in 5% CO2. for cyst formation. Afterwards, infected cells were processed for, immunofluorescence (IFA) or bright field microscopy, as described below.
2.7. Immunofluorescence microscopy
Immunofluorescence assays (IFA) were performed using a specific monoclonal antibody against bradyzoite antigen 1 (mAb 7E5, anti-BAG-1/hsp30) and polyclonal rabbit antibody against tachyzoite surface antigen 1 (anti-SAG-1). The cultures were fixed for 20 min at room temperature in 4% freshly prepared formaldehyde diluted in phosphate buffered saline (PBS) at 7.2 pH. The samples were permeabilized with 0.5% Triton X-100 for 20 min and incubated in a solution containing 1.5% bovine serum albumin (BSA), 0.025% fish gelatin (Sigma Chemical Co.) in PBS at 7.2 pH, for 1 h at room temperature, followed by incubation for 1 h with anti-BAG-1 (1:200) and anti-SAG-1 (1:300), consecutively. Afterwards, the coverslips were incubated with goat anti-mouse antibody conjugated with Alexa 546 or with goat anti-rabbit antibody conjugated with Alexa 488 (Molecular Probes) at a 1:400 dilution. Controls were performed omitting the primary antibodies. After that, the coverslips were incubated with 0.5 μg/ml DAPI (4,6-diamidino-2-phenylindole; Sigma Chemical Co.) for 5 minutes. To label the cyst wall, the cover slips were incubated for 1h with anti CST-1 SalmonE [31] 1:100. The coverslips were then mounted on slides with Prolong Gold Antifade (Invitrogen). The samples were examined under a Nikon Epifluorescence microscope (Albert Einstein College of Medicine) and Leica DMI 6000 Epifluorescence (Federal University of Rio de Janeiro).
2.8. In vivo stage conversion of GMO
To observe parasitic fluorescence during the acute phase in vivo, 4-week-old female BALB/c DM1-mice (n=3) were infected intraperitoneally (i.p.) with 106 SAG1mcherry parasites or Double SAG1mCherry/BAG1sfGFP parasites, 48 hours post infection the ascitic fluid was collected and checked under UV light. In order to check the expression of fluorescent proteins during chronic stage, the mice were infected for 35 days with 103 LDH2sfGFP positive parasites or double SAG1mCherry/BAG1sfGFP parasites followed by 10 days under sulfadiazine treatment. The brains were isolated and homogenized by syringe serial passages. Presence of cysts was determined in Nikon epifluorescence microscope.
3. Results
3.1. Transformation of EGS parasites
Stable lines of EGS strain expressing stage specific fluorescent proteins were constructed as follows: (1) pSAG1-mCherry to generate a red expressing tachyzoite specific strain (Tred); (2) pLDH2 sfGFP to generate a green expressing bradyzoite specific strain (greenB); and (3) pSAG1mCherry, pBAG1-sfGFP and ptubCAT to generate a parasite line having red tachyzoites and green bradyzoites (DoubleCat) and (4) pSAG1mCherry, pBAG1-sfGFP for dual expression of stage specific promoters with no selectable marker (Double). The efficiency of transfection was evaluated using a control plasmid pGRA1-GFP that expresses GFP in both tachyzoites and bradyzoites. Using this plasmid 13% of infected EGS T. gondii expressed GFP while 6.6% of EGS infected parasites expressed mCherry when transfected with pSAG1mCherry after 24 h of transfection (Data not shown). The 3 constructs were successfully integrated in transient transfection of EGS strain as demonstrated in figures 1 A, B and C for pSAG1, pBAG1 and pLDH2, respectively.
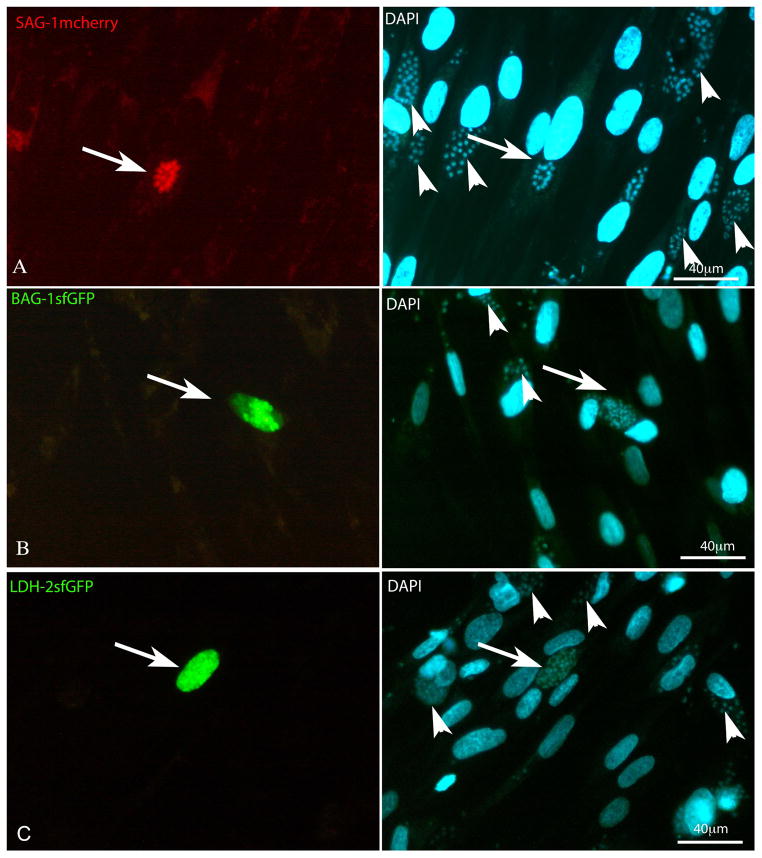
Figure 1. Transient Transfection of stage specific plasmids.
HFF cells infected with parasites immediately after the transfection. A) Tachyzoites transiently expressing SAG1-mCherry (arrow - red) after 30 h of infection: in the even panel the cells were stained with DAPI and it is possible to localize non-fluorescent parasites (arrowheads) B) Bradyzoites transiently expressing BAG1-sfGFP (arrow-green) and in the even panel the cells were stained with DAPI and it is possible localize the non-fluorescent parasites (arrowheads) after 96 h of infection. C) LDH2-sfGFP (arrow-green) after 72 h post infection: in the even panel the cells were stained with DAPI and it is possible to localize the non-fluorescent parasites (arrowheads).
3.2. Selection and cultivation of the genetically modified EGS parasites
The constructs used in this study to allow expression of stage specific reporter proteins do not posses a selectable marker and in order to select the fluorescent parasites flow cytometry was employed. After transfection, parasites were allowed to infect HFF and then parasites which egressed from the monolayer were selected using FACS. The efficiency of recovery of fluorescent parasites was 1% from the total population screened on the first FACS. In order to enrich the positive population two additional FACS selections were performed, in the second FACS, 14% fluorescent parasites were recovered and in the third FACS 30%. Clonal populations of Tred and Bgreen were obtained by subcloning using serial dilution. The best clonal populations of Bgreen and Tred were then selected by fluorescence microscopy.
Dual expression strains were produced using two strategies: (1) triple transfection of pSAG/mCherry, pBAG/sfGFP and pTUB/CAT constructions and (2) a sequenced transfection plus selection of Double positive clones. The DoubleCat lineage was obtained by selection in the presence of 20 μM Chloramphenicol, after the triple transfection, this was followed by FACS selection for the double positive parasites (14% of the parasite population) and subsequently cloning by serial dilution. The best clonal populations of DoubleCat were then chosen by fluorescence microscopy. The development of the Double strain, that is dual expression with no selectable marker, was done in a sequenced mode of transformation. The population Tred was transfected with pBAG1/sfGFP, selected by FACS, as described before, and subcloned.
These four clonal lineages: redT, greenB DoubleCat and Double parasites were easily cultivated in HFF cells and the in vitro intracellular cycle was 5 days similar to that of the wild type EGS (parental) strain demonstrating that the growth rate for the genetic modified parasite was not altered during the integration of fluorescent proteins. When the GMO (genetic modified organism) EGS lineages were passed in vitro 8 ~10 times, after that time the number of cyst spontaneously formed dramatically decreased and the number of tachyzoites increased compared to low passage EGS strains. The phenotype of a high rate of spontaneous cyst formation was recovered by passage in mice. The lineages were cryopreserved and after recovery from mice the parasites maintained their fluorescent protein expression capacity. The maintenance of low passage strains in mice was, therefore, essential for the high in vitro conversion capacity.
3.3. The genetically modified EGS parasites are able to form cysts in vitro and in vivo
The spontaneous conversion phenotype of the genetically modified EGS strain was tested in cell culture detecting the cyst wall by IFA. The maintenance of stage specific fluorescent tag expression was also tested in vivo. For this, parasites were cultivated for 96h in HFF cells and IFA was performed to detect the cyst wall formation. In the Tred infection, vacuoles positively stained by CST-1 were negative for mCherry (Figure 2 A) while in the Bgreen infection, cyst wall staining was positive in vacuoles containing GFP positive parasites (Figure 2B). Cell cultures infected with the DoubleCat population were also analyzed, and mCherry positive vacuoles and GFP positive cyst-like vacuoles were clearly distinguished (Figure 2C). The expression of fluorescent tags was also tested by induction using low CO2 concentrations and high ph shift [20], and the expression of the promoters was similar to that seen under physiological non stress conditions (Data not shown).
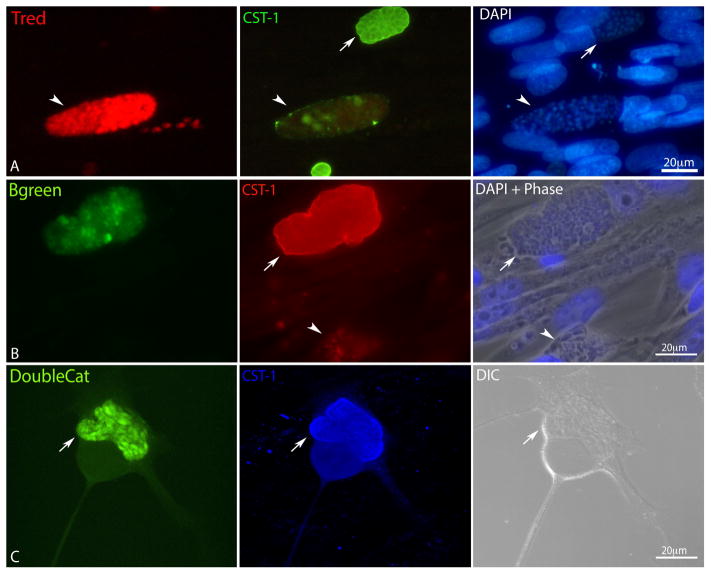
Figure 2. Conversion of the stable lineages Tred, Bgreen and DoubleCat after 96 h post infection.
A) HFF infected with redT and immunolabeled with anti CST-1. The arrowhead points to a SAG1-mCherry positive vacuole, which is negative for CST-1 (middle panel) arrow points to a CST-1 positive cyst, which is negative for SAG1 mCherry. B) HFF infected with greenB line and immunolabeled with anti CST-1. The arrow points to LDH2-GFP positive cyst, which is positive for CST-1 (middle panel). The arrowhead points to LDH2 and CST-1 negative vacuole. C) HFF infected with DoubleCat. The arrow points to BAG1-GFP positive cyst, which is positive for CST-1 (middle panel).
Infections in animals were performed to verify the maintenance of expression of stage specific fluorescent proteins in vivo. After 6 days, Tred parasites were collected from ascitic fluid of infected mice and efficiently infected HFF cells, showing that the population keeps the mCherry protein expression after animal passage (Figure 3A). The Bgreen population was used to chronically infect mice and after 35 days, the brains were dissected and processed to check the presence of cysts. All cysts investigated were GFP positive (Figure 3B). The same procedure was made for the DoubleCat strain, the parasites recovered from peritoneal ascitic fluid kept the mCherry expression (data not shown); and cysts recovered from infected brains kept the GFP expression (Figure 3 C).
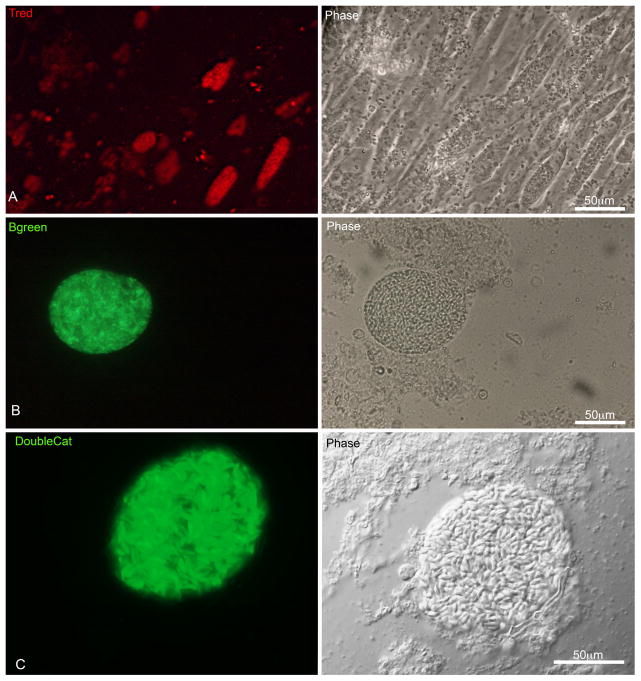
Figure 3. In vivo Infection of HFF cells with the stable lineages redT and greenB and DoubleCat.
A) HFF cells infected for 48 h with the clonal lineage redT harvested from ascitic fluid of an infected mouse, showing all parasites mCherry positive. B) A LDH2-GFP positive cyst harvested from a mouse brain after 35 days of infection. C) A BAG1-GFP positive cyst harvested from a brain infected for 35 days.
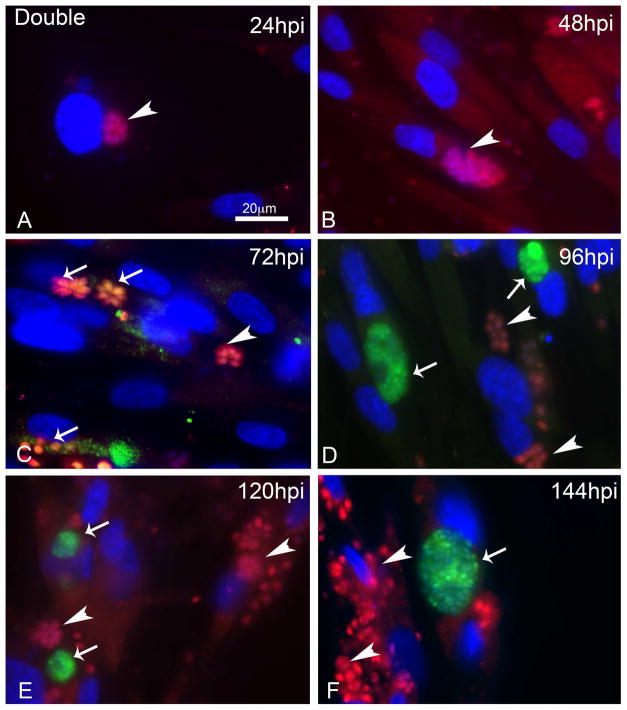
The dual expression strain was followed in a time course way to monitor the conversion during 144 h. At 24 hpi and 48 hpi, mCherry vacuoles were seen (Figure 4A and B respectively), at 72 hpi intermediated profiles were observed (Figure 4C), and at 96 hpi GFP positive cysts and mCherry positive vacuoles were observed (Figure 4D). The profiles at 120 and 144 hpi (Figure 4 E and F) were similar to that seen at 96 hpi.
Figure 4. Time course of infection of HFF cells with the stable lineage DoubleCat BAG1-sfGFP and SAG1-mCherry during 144h.
A) Infected cells at 24hpi with vacuoles SAG1-mCherry positives (arrowhead). B) Infected cells at 48hpi with vacuoles SAG1-mCherry positives (arrowhead). C) Infected cells at 72hpi with vacuoles SAG1-mCherry positives (arrowhead) and intermediate profiles (arrows). D) Infected cells at 96hpi with vacuoles SAG1-mCherry positives (arrowhead) and cysts BAG1-GFP positives (arrows). E) Infected cells at 120 hpi with vacuoles SAG1-mCherry positives (arrowhead) and cysts BAG1-GFP positives (arrows). F) Infected cells at 96hpi with vacuoles SAG1-mCherry positives (arrowhead) and cysts BAG1-GFP positives (arrows).
4.4. Discussion
The development of new tools for the study of cyst formation in T. gondii is useful for the analysis of differentiation in vitro and in vivo. To this end, several groups have developed reagents that permit dynamic characterization of stage conversion in vitro. Dzierszinski and colleagues [32] showed the reorganization of organelles in bradyzoites and Di Cristina [33] modified parasites of a Toxoplasma strain expressing luciferase to localize cysts in vivo. A strain with dual expression of fluorescent tags was developed by Zhang et al [35] (PLK expressing both BAG and SAG with fluorescent proteins). However, the rate of spontaneous cyst formation in this PLK strain was low in vitro, and this strain needed significant stress to see induction. Furthermore, PLK can no longer form oocysts in cats. In this work we described the development of genetically modified parasites of EGS strain that expresses stage specific fluorescent tags. This tool is useful to follow stage conversion in vitro or in vivo at a high frequency without any need for exogenous stress inducers such as pH shift.
The use of these stage specific reporters in the EGS strain of T. gondii will be able to facilitate studies on the mechanisms of differentiation as well as studies to identify drug targets that are active against bradyzoites or for screening agents that prevent differentiation [29]. As the visualization of stage specific fluorescent proteins is the easiest and fastest way to track the dynamics of the conversion process, the strains developed in this work will be useful to determine new triggers or essential genes of cyst formation by chemical or genetic interference. The EGS strain was found to be as easy to manipulate as the frequently used laboratory strains as RH and PRU [39,40], in contrast to the difficulties reported with the recently isolated strain GT-1 [41]. The possibility of using genetic disruption tools together with stage specific fluorescent protein expression has the potential to be a very effective strategy to determine unexplored essential aspects of differentiation and should permit the use of new DNA editing technologies such as CRIPR/Cas9 system [42]. As the current EGS reporters have no selectable markers, except for DoubleCat, this allows the use of all of the traditional markers in these EGS strains for the manipulation of other genes.
The molecular and cellular host cell mechanisms that contribute to T. gondii interconversion process are not yet fully elucidated, although the role of host cell type and metabolism is important in differentiation [43]. Stress induction affects the host cell as well as the parasite by introducing another variable in studies of differentiation. The high rate of spontaneous conversion in EGS (30% bradyzoites in epithelial cells after 96 hours) suggests it will be a useful model to study induction in the absence of exogenous stress [29], [44],
Furthermore, the use of an atypical Brazilian strain could also help elucidate new aspects of host parasite interaction and virulence aspects as showed for other atypical strains [45]. Thus, EGS Tred, Bgreen, DoubleCat and Double strains adds another powerful tool to study differentiation and pathogenesis of T. gondii caused by atypical strains, which have been reported to result in severe disease even in immune competent patients and are an ongoing health problem in Brazil [46]. So far, all evidence gathered shows that the EGS strains generated and presented here constitute reliable tools to study mechanisms of cyst formation both in vitro and in vivo.
Acknowledgments
The authors are thankful to Albert Einstein College of Medicine Flow Cytometer Core Facility staff and Brunno Verçoza for technical assistance at Leica Epifluorescence DMI 6000 at Numpex Bio-UFRJ, and Fernando Almeida for technical assistance at Leica SP3 confocal microscope at CENABIO-UFRJ. This work was supported by CAPES, CNPq, FAPERJ, Forgaty/NIH D43 TW007129-10, and NIH/NIAID AI93220 (LMW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–58. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Speer CA, Dubey JP, McAllister MM, Blixt JA. Comparative ultrastructure of tachyzoites, bradyzoites, and tissue cysts of Neospora caninum and Toxoplasma gondii. Int J Parasitol. 1999;29:1509–19. doi: 10.1016/s0020-7519(99)00132-0. [DOI] [PubMed] [Google Scholar]
- 3.Lainson R. Observations on the development and nature of pseudocysts and cysts of Toxoplasma gondii. Trans R Soc Trop Med Hyg. 1958;52:396–407. doi: 10.1016/0035-9203(58)90123-8. [DOI] [PubMed] [Google Scholar]
- 4.Work K. Toxoplasmosis. With special reference to transmission and life cycle of Toxoplasma gondii. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;221:1–51. [PubMed] [Google Scholar]
- 5.Weiss LM, Kim K. The development and biology of bradyzoites of Toxoplasma gondii. Front Biosci. 2000;5:D391–405. doi: 10.2741/weiss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manger ID, Hehl A, Parmley S, Sibley LD, Marra M, Hillier L, et al. Expressed Sequence Tag Analysis of the Bradyzoite Stage of Toxoplasma gondii: Identification of Developmentally Regulated Genes. Infect Immun. 1998;66:1632–7. doi: 10.1128/iai.66.4.1632-1637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson DJ, Hutchison WM. An ultrastructural study of the early development and tissue cyst formation of Toxoplasma gondii in the brains of mice. Parasitol Res. 1987;73:483–91. doi: 10.1007/BF00535321. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–8. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 9.Innes EA. Toxoplasmosis: comparative species susceptibility and host immune response. Comp Immunol Microbiol Infect Dis. 1997;20:131–8. doi: 10.1016/s0147-9571(96)00038-0. [DOI] [PubMed] [Google Scholar]
- 10.Gross U, Kempf MC, Seeber F, Lüder CG, Lugert R, Bohne W. Reactivation of chronic toxoplasmosis: is there a link to strain-specific differences in the parasite? Behring Inst Mitt. 1997:97–106. [PubMed] [Google Scholar]
- 11.Wanko T, Jacobs L, Gavin MA. Electron microscope study of Toxoplasma cysts in mouse brain. J Protozool. 1962;9:235–42. doi: 10.1111/j.1550-7408.1962.tb02611.x. [DOI] [PubMed] [Google Scholar]
- 12.Matsubayashi H, Akao S. Morphological studies on the development of the Toxoplasma cyst. Am J Trop Med Hyg. 1963;12:321–33. doi: 10.4269/ajtmh.1963.12.321. [DOI] [PubMed] [Google Scholar]
- 13.Shimada K, O’Connor GR, Yoneda C. Cyst formation by Toxoplasma gondii (RH strain) in vitro. The role of immunologic mechanisms. Arch Ophthalmol. 1974;92:496–500. doi: 10.1001/archopht.1974.01010010510010. [DOI] [PubMed] [Google Scholar]
- 14.Hoff RL, Dubey JP, Behbehani AM, Frenkel JK. Toxoplasma gondii cysts in cell culture: new biologic evidence. J Parasitol. 1977;63:1121–4. [PubMed] [Google Scholar]
- 15.Lindsay DS, Mitschler RR, Toivio-Kinnucan MA, Upton SJ, Dubey JP, Blagburn BL. Association of host cell mitochondria with developing Toxoplasma gondii tissue cysts. Am J Vet Res. 1993;54:1663–7. [PubMed] [Google Scholar]
- 16.Guimarães EV, Carvalho L, Santos Barbosa H. Primary Culture of Skeletal Muscle Cells as a Model for Studies of Toxoplasma gondii Cystogenesis. J Parasitol. 2008;94:72–83. doi: 10.1645/GE-1273.1. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira-da-silva F, Taka AC, Barbosa HS, Gross U, Lu CGK. Primary skeletal muscle cells trigger spontaneous Toxoplasma gondii tachyzoite-to-bradyzoite conversion at higher rates than fibroblasts. 2009;299:381–8. doi: 10.1016/j.ijmm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Soete M, Fortier B, Camus D, Dubremetz JF. Toxoplasma gondii: kinetics of bradyzoite-tachyzoite interconversion in vitro. Exp Parasitol. 1993;76:259–64. doi: 10.1006/expr.1993.1031. [DOI] [PubMed] [Google Scholar]
- 19.Bohne W, Heesemann J, Gross U. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun. 1994;62:1761–7. doi: 10.1128/iai.62.5.1761-1767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss LM, Laplace D, Takvorian PM, Tanowitz HB, Cali A, Wittner M. A cell culture system for study of the development of Toxoplasma gondii bradyzoites. J Eukaryot Microbiol. 1995;42:150–7. doi: 10.1111/j.1550-7408.1995.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 21.Soête M, Camus D, Dubremetz JF. Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro. Exp Parasitol. 1994;78:361–70. doi: 10.1006/expr.1994.1039. [DOI] [PubMed] [Google Scholar]
- 22.Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–6. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 23.Sibley LD, Khan A, Ajioka JW, Rosenthal BM. Genetic diversity of Toxoplasma gondii in animals and humans. 2009:2749–61. doi: 10.1098/rstb.2009.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira ADM, Vitor RWA, Gazzinelli RT, Melo MN. Genetic analysis of natural recombinant Brazilian Toxoplasma gondii strains by multilocus PCR-RFLP. Infect Genet Evol. 2006;6:22–31. doi: 10.1016/j.meegid.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Castro FC, Jorge M, Viegas B, Carlos A, Cabral V, Ferreira AM, et al. Prenatal toxoplasmosis diagnosis from amniotic fluid by PCR Diagnóstico pré-natal da toxoplasmose no líquido amniótico através da técnica de PCR. Infect Genet Evol. 2001;35:1–6. [Google Scholar]
- 26.Vieira P, Vidigal T, Vítor D, Santos V, Castro FC, César J. Prenatal toxoplasmosis diagnosis from amniotic fluid by PCR. Rev Soc Bras Med Trop. 2002;35:1–6. doi: 10.1590/s0037-86822002000100001. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira AM, Vitor RWA, Carneiro ACAV, Brandão GP, Melo MN. Genetic variability of Brazilian Toxoplasma gondii strains detected by random amplified polymorphic DNA-polymerase chain reaction (RAPD-PCR) and simple sequence repeat anchored-PCR (SSR-PCR) Infect Genet Evol. 2004;4:131–42. doi: 10.1016/j.meegid.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira AM, Martins MS, Vitor RW. Virulence for BALB/c mice and antigenic diversity of eight Toxoplasma gondii strains isolated from animals and humans in Brazil. Parasite. 2001;8:99–105. doi: 10.1051/parasite/2001082099. [DOI] [PubMed] [Google Scholar]
- 29.Paredes-Santos TC, Martins-Duarte ES, Vitor RWA, de Souza W, Attias M, Vommaro RC. Spontaneous cystogenesis in vitro of a Brazilian strain of Toxoplasma gondii. Parasitol Int. 2013;62:181–8. doi: 10.1016/j.parint.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson DJP. Use of molecular and ultrastructural markers to evaluate stage conversion of Toxoplasma gondii in both the intermediate and definitive host. Int J Parasitol. 2004;34:347–60. doi: 10.1016/j.ijpara.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 31.Tomita T, Bzik DJ, Ma YF, Fox BA, Markillie LM, Taylor RC, et al. The Toxoplasma gondii cyst wall protein CST1 is critical for cyst wall integrity and promotes bradyzoite persistence. PLoS Pathog. 2013;9:e1003823. doi: 10.1371/journal.ppat.1003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dzierszinski F, Nishi M, Ouko L, Roos DS. Dynamics of Toxoplasma gondii differentiation. Eukaryot Cell. 2004;3:992–1003. doi: 10.1128/EC.3.4.992-1003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Cristina M, Marocco D, Galizi R, Proietti C, Spaccapelo R, Crisanti A. Temporal and spatial distribution of Toxoplasma gondii differentiation into bradyzoites and tissue cyst formation in vivo. Infect Immun. 2008;76:3491–501. doi: 10.1128/IAI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unno A, Suzuki K, Batanova T, Cha S-Y, Jang H-K, Kitoh K, et al. Visualization of Toxoplasma gondii stage conversion by expression of stage-specific dual fluorescent proteins. Parasitology. 2009;136:579–88. doi: 10.1017/S0031182009005836. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Zhang Y, Cao J, Zhou Y, Wang N, Zhou J. Determination of stage interconversion in vitro and in vivo by construction of transgenic Toxoplasma gondii that stably express stage-specific fluorescent proteins. Exp Parasitol. 2013;134:275–80. doi: 10.1016/j.exppara.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 36.van den Hoff MJ, Moorman AF, Lamers WH. Electroporation in “intracellular” buffer increases cell survival. Nucleic Acids Res. 1992;20:2902. doi: 10.1093/nar/20.11.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim K, Soldati D, Boothroyd JC. Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science. 1993;262:911–4. doi: 10.1126/science.8235614. [DOI] [PubMed] [Google Scholar]
- 38.Soldati D, Boothroyd JC. Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science. 1993;260:349–52. doi: 10.1126/science.8469986. [DOI] [PubMed] [Google Scholar]
- 39.Huynh MH, Carruthers VB. Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot Cell. 2009;8:530–9. doi: 10.1128/EC.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox BA, Falla A, Rommereim LM, Tomita T, Gigley JP, Mercier C, et al. Type II Toxoplasma gondii KU80 knockout strains enable functional analysis of genes required for Cyst development and latent infection. Eukaryot Cell. 2011;10:1193–206. doi: 10.1128/EC.00297-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, et al. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314:1776–80. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- 42.Shen B, Brown KM, Lee TD, Sibley LD. Efficient Gene Disruption in Diverse Strains of Toxoplasma gondii Using CRISPR/CAS9. MBio. 2014;5:e01114–14. doi: 10.1128/mBio.01114-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weilhammer DR, Iavarone AT, Villegas EN, Brooks GA, Sinai AP, Sha WC. Host metabolism regulates growth and differentiation of Toxoplasma gondii. Int J Parasitol. 2012;42:947–59. doi: 10.1016/j.ijpara.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferreira-da-Silva MDF, Rodrigues RM, De Andrade EF, De Carvalho L, Gross U, Lüder CGK, et al. Spontaneous stage differentiation of mouse-virulent Toxoplasma gondii RH parasites in skeletal muscle cells: an ultrastructural evaluation. Mem Inst Oswaldo Cruz. 2009;104:196–200. doi: 10.1590/s0074-02762009000200012. [DOI] [PubMed] [Google Scholar]
- 45.Melo MB, Nguyen QP, Cordeiro C, Hassan MA, Yang N, McKell R, et al. Transcriptional Analysis of Murine Macrophages Infected with Different Toxoplasma Strains Identifies Novel Regulation of Host Signaling Pathways. PLoS Pathog. 2013;9:1–17. doi: 10.1371/journal.ppat.1003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubey JP, Lago EG, Genari SM, Su C, Jones JL. Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology. Parasitology. 2012;139:1375–424. doi: 10.1017/S0031182012000765. [DOI] [PubMed] [Google Scholar]