Abstract
Problem
Human gamma delta (GD) T cells play a well-documented role in epithelial barrier surveillance and protection. Two subsets of GD T cells, defined by the use of either the Vdelta2 (GD2) or Vdelta1 (GD1) TCR, predominate. We hypothesized that endocervical GD T cells play important role in lower genital tract anti-HIV immune responses.
Method of Study
HIV infected (n=18) and HIV uninfected (n=19) pre-menopausal women participating in the WIHS cohort were recruited. Frequency and phenotype of GD T cells were determined in endocervical cytobrush samples and peripheral blood by multicolor flow cytometry.
Results
We found depletion of GD2 cells in the blood of HIV infected women as well as significant decrease in the frequency of endocervical GD1 cells compared to uninfected women.
Conclusions
We report for the first time, the GD1 cells are a predominant endocervical T cell subset that is significantly decreased in HIV infected women.
Keywords: gamma delta T cells, HIV, female reproductive tract, biomarker
Introduction
In the United States, women represent 20% of new HIV infections and the majority of these infections occur via vaginal intercourse (http://www.cdc.gov/hiv/risk/gender/women/facts/index.html#refc).
The female reproductive tract (FRT) is the initial site of HIV replication and better knowledge of the genital mucosa is essential for understanding pathogenicity of HIV infection in women and for development of efficient HIV prevention strategies. Within the FRT, the mucosal immune system serves as the first line of defense1-3, uniquely balancing effective level of protection against pathogens with reproductive demands. Mucosal tissues sequester the largest proportion of T lymphocytes in the body 4 . While T cell populations in the gastrointestinal tract have been well characterized, characterization of immune cell populations in the lower genital tract is largely unknown and remains an essential step in understanding the pathogenesis of HIV in the FRT.
Gamma delta (GD) T cells are unconventional T cells recognizing antigens via their gamma delta T-cell receptor (TCR) in a way that is fundamentally different from conventional alpha beta T cells 5. GD T cells are usually divided into subsets according to the type of V gamma (G) and/or V delta (D) chain they express in their TCR. There is considerable heterogeneity of the GD T cell populations across body compartments in terms of cellular phenotype, nature of antigen recognized and effector functions employed 6. In the peripheral blood, GD T cells represent only minor subset of T lymphocytes (less than 10%). About 50-90% of peripheral blood GD T cells express Vdelta2 chains (combined with Vgamma9) (GD2) 7. In contrast, GD T cells expressing the Vdelta1 chain, (paired with a variety of V gamma chains) (GD1), are enriched in tissues, such as skin, respiratory, urogenital tract, and intestinal epithelia (up to 60% of small intestinal intraepithelial lymphocytes, IEL, are GD T cells) 8. Intraepithelial GD T cells contribute to the earliest stages of immune responses against infection through the epithelial surfaces. It has been well documented that GD deletion by genetic knock out or treatment by specific antibodies renders mice more susceptible to infection with a variety of microbes9, 10. Also, a large number of studies in human and murine system have confirm the presence of GD T cells in uterine lymphocytes during pregnancy but GD T cells have not been evaluated in the female lower FRT 11-14.
While phenotypic changes in GD T subset of peripheral blood and rectal mucosa 15-17 have been described in HIV, GD T subsets have not previously been examined in the endocervical mucosa, the primary site of HIV infection in women. Since mucosal GD T cells may play an important role in trans mucosal HIV infection and control of HIV replication, it is important that effect of HIV on these cells be examined. In an attempt to elucidate the biology and functional properties of human endocervical GD T cells, in this study we evaluate endocervical GD T cells in women participating in the Women's Interagency HIV Infection Study (WIHS), the largest longitudinal cohort of women with HIV infection and at risk for HIV infection in the United States.
Methods
Ethics statement
Institutional Review Board (University of Miami Miller School of Medicine) approval was obtained prior to recruitment and any assessment or study related procedures. Participants were provided with information about the study and assured of confidentiality of information and study records. Voluntary signed informed consent was obtained from all participants prior to participating in the study.
Study procedures
Study activities took place at University of Miami HIV Research Unit in collaboration with the Miami Women's HIV Interagency Study (WIHS) and the Miami Center for AIDS Research (CFAR). Participants were women participating in the WIHS study in Miami, aged 18 to 45 years of age, sexually active, not pregnant and not on contraceptive medications or with an intrauterine device. Participants completed a web based questionnaire assessing demographic, sexual risk factors and medical history. Participants underwent a 10 milliliter blood draw and vaginal examination with collection of endocervical cytobrush samples.
Women without documentation of HIV status underwent HIV testing. HIV testing was performed by using rapid HIV test (OraQuick ADVANCE Rapid HIV-1/2 Antibody, OraSure Technologies Inc. Bethlehem, PA, USA). Women with history of HIV infection presented documentation of HIV infection (HIV western blood results, medical records, or any laboratory result with a detectable HIV viral load). Chlamydia and gonorrhea testing was performed in endocervical swabs using ProbeTec Chlamydia and Gonorrhea Displacement Assay, Becton Dickinson, Sparks, MD, USA).
Demographics and sexual risk factors
The demographic and sexual risk factors questionnaire included age, race, number of partners, and history of exchanging sex for money, use of condoms, alcohol and drugs.
Medical History
Medical history included history of genital infections including Sexually Transmitted Infections and use of antiretroviral drugs. HIV+ women on antiretroviral therapy (ART) were receiving at least 3 drugs with a combination of 2 NRTI (nucleoside reverse transcriptase inhibitor) and boosted protease inhibitor or integrase inhibitor or non-nucleoside reverse transcriptase inhibitor.
In women with HIV infection plasma CD4 T cells/milliliter and HIV RNA viral load was performed as part of the study.
Endocervical brush sample collection and processing
Participants underwent a vaginal examination. Cervical os was visualized and cleaned from mucous. Endocervical samples were collected by inserting Cytobrush Plus GT (CareFusion, San Diego, CA, USA) cell collector into the endocervix until the bottom-most bristles were exposed. The brush was then rotated 360 degrees clockwise. The brush was removed and inserted in a 15 mL tube containing 5 mL of IMDM. Any visible blood contamination was noted. The tube containing cytobrush was place on ice immediately after collection. The cytobrush was processed sequentially and the extracted cells were combined for staining and flow cytometric analysis
First, tube containing cytobrush was vortex for 1 min and then centrifuged for 10 min at 250 g. Second, cytobrush was taken out and additional wash was performed by placing cytobrush on the 100mm cell strainer. Pellet was combined with the additional cytobrush wash (20 ml IMDM) and centrifuged for additional 10 min at 250g. Supernatant was decanted and pelleted cells resuspended in 1 ml IMDM and counting was performed using a Beckman Coulter automated cell counter (The Vi-CELL Series Cell Viability Analyzer) with the trypan blue dye exclusion method (total viable cell concentration were reported).
Peripheral blood processing
Blood was collected in heparin anti-coagulated vacutainer tubes (BD Biosciences, San Jose, CA, USA). Plasma HIV viremia was assessed by measuring plasma HIV-1 RNA viral load respectively using real time COBAS amplicor HIV PCR testing (Roche Molecular Systems Inc, Branchburg, NJ,USA). Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation using Ficoll-Histopaque (Sigma- Aldrich, St. Louis, MO, USA).
Flow cytometry
Cells were re-suspended in FACS buffer (PBS containing 1% bovine serum albumin (Sigma-Aldrich) and stained with the LIVE/DEAD Fixable Yellow Dead Cell Stain kit (Life Technologies, Grand Island, NY,USA) and with the antibody panel for phenotyping (Table S1). After 30 min incubation on +4°C, cells were washed with FACS buffer and re-suspended in 300 mL 1% paraformaldehyde (Sigma-Aldrich). Samples were acquired on Fortessa flow cytometer (BD, San Jose, CA, USA), equipped with 405 nm, 488 nm, and 635 nm lasers.
Antibody panel optimization and titrations were performed in PBMCs, followed by confirmation using cells isolated from cytobrushes. Compensation controls were prepared simultaneously with sample processing, using UltraComp eBeads, (eBioscience, San Diego, CA, USA) for antibodies and ArC Amine Reactive Compensation Beads (Life Technologies, Grand Island, NY, USA) for the viability stain.
Numbers of GD1+ /GD2+ cells enumerated from endocervical cytobrush samples. Number on the Y-axis represents the cell numbers (×106) calculated using flow cytometry (total of 10.000 events/cells collected in CD3+live cells+ gate in each analyzed sample). Absolute counts were determined by multiplying the frequency of each subset relative to lymphocytes (determined by scatter gating) by the absolute lymphocyte count obtained from a complete cell count analyzed by a Coulter automated cell counter (The Vi-CELL Series Cell Viability Analyzer).
Data analysis
FACS data files were analyzed using FlowJo 9.6 for PC (Tree Star, Ashland, OR, USA) and Prism 5.01 for Windows
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5 (San Diego, California, USA). Shapiro–Wilks test for normality was applied to determine the distribution of the grouped samples. Mann–Whitney U test was applied for nonparametric independent sample comparisons and Wilcoxon signed rank tests were applied to matched samples for nonparametric comparison. Kruskal–Wallis ANOVA tests were used for non-parametric assessments of variation between groups, with Dunn's post-test applied to test for the effect of multiple comparisons. For comparison of frequencies, the Chi-square test was used to compare groups. All tests were two-tailed and p-values of 0.05 were considered significant.
Data analysis
FACS data files were analyzed using FlowJo 9.6 for PC (Tree Star, Ashland, OR, USA) and Prism 5.01 for Windows
Results
Characteristics of women
Thirty seven pre-menopausal women participated in the study: 18 HIV infected (HIV+) and 19 HIV uninfected (HIV-). Median age was 34.4 (SD = 6.3) and was similar in both HIV+ and HIV- women (p=0.60). The majority was Black/African American (24, 64.8%, p=0.68) and engaged in high risk behaviors: 30 (81.0%) had more than one partner in the prior month and 14 (37.8%) reported a history of exchanging sex for money or drugs and was similar in both HIV+ and HIV- women (p=0.29). Reported condom use was low as only 12 (37.5%) reported using condoms in all sexual encounters in the prior month and was not different by HIV status (HIV- 41.7% and HIV+58.3%; p=1.10).
Twenty two (59%) of participants (reported having been diagnosed with a genital infection in their lifetime (Chlamydia, gonorrhea, syphilis, trichomoniasis or bacterial vaginosis) and was not different by HIV status (p=0.61).
Genital examination was unremarkable in the majority of participants: 5 participants had increased vaginal discharged of normal characteristics, one participant had white discharge and 3 had friability of the cervix. One participant tested positive for chlamydia and one for gonorrhea. Median pH was 5.2 (4 – 7) and was not different by HIV status.
The median time from the first day of the last menstrual cycle was 18.5 days (SD=7.4) and was not statistically different in HIV- vs HIV+ women (day 16 of the cycle for HIV- and day 19 for HIV+ women). The Shapiro-Wilk test of normality indicated that the gamma-delta T cell data was not normally distributed (p=0.001). Spearman's rho was calculated for the relationship between menstrual day and gamma delta frequency. No significant relationship was found, regardless of HIV infection (p=0.302). When the calculations were repeated for HIV positive and HIV negative women in the cohort no significant relationships were found (p=0.135 and p=0.929, respectively).
Among those participants with HIV infection, most were on antiretroviral medications (n=14, 74%) and had CD4 counts over 500 cells per milliliter (14, 74%). The median CD4 count was 1,030 cells per milliliter (SD = 606). Plasma viral load was available for 15 participants and 10 (66.6%) had undetectable plasma viremia as defined as less than 400 copies per milliliter (median viral load = VL= 1.7 log10, SD=2.1).
The number of collected total cervical cells were similar in samples from HIV- and HIV+ women (median number of 700,000, SD=1.22). The percentage of CD45+ cells was 2.78 % (SD = 4.91) in HIV- and 2.10 % (SD = 3.39) in HIV+ women (p=0.70). Endocervical cells were isolated from a single cytobrush yielding a median number of viable CD45+ leukocytes of 25,000 (4,000 - 90,000) in HIV- and 10,000 (2,000 - 170,000) in HIV+ women (p= 0.63). The viability of CD45+ leukocytes were similar between two groups of samples 76.6% in HIV- and 66.9% in HIV+).
Although, cytobrush samples yielded similar numbers of cervical CD3+ T cells: 12,000 (SD = 6,29) in HIV- and 9,100 (SD = 2,69) in HIV+ (p= 0.56), HIV+ women had a significantly skewed percent of CD4+ T cells within endocervical CD3+ cells compared to HIV- women (3.38% (SD=0.94) in HIV- and 0.36% (SD=0.14) in HIV+ women; p=0.0001).
Flow cytometric analysis of human gamma delta T cells in the blood and endocervical brush samples
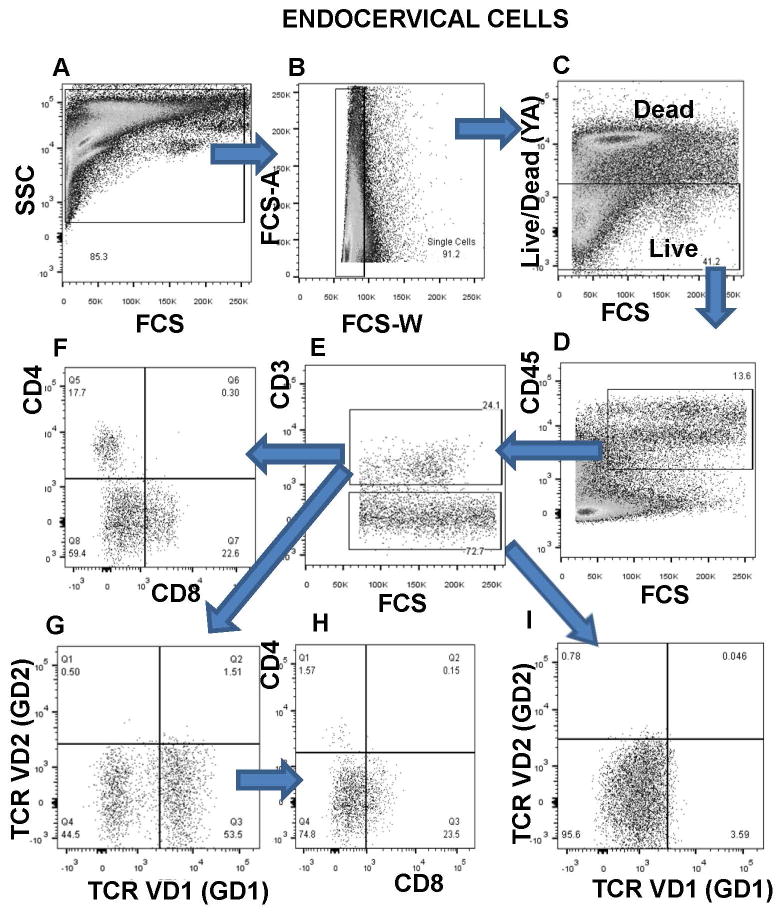
Diverse methods have been developed to study the immune populations and environment of the FRT (cervical biopsy, cervical cytobrushes and cervicovaginal lavages)18-22. It is important to note that these methods sample distinct portions of the FRT (cytobrush, sample a region of a single layer of columnar epithelium, in contrast to cervicovaginal lavage and biopsy, that sample a region of squamous stratified epithelium). Also, nature of leukocyte populations differs significantly between these sample regions21, 22. We hypothesized that intraepithelial gamma delta T cells will be observed among the endocervical mononuclear cell population from uninfected and HIV infected women. Using multiparametar flow cytometry-based immunophenotyping, we describe for the first time a unique population of endocervical gamma delta V1 (GD1) T cells (Figure 1). The isolation of endocervical brush samples (described in Materials and Methods) was performed within 1 h of sample collection. Furthermore, for most samples time from collection to processing was less than 30 min. Analysis was always compared to autologous PBMC samples. A representative gating strategy for HIV uninfected endocervical and PBMC sample is shown in Figure 1 and Supplementary Figure 1. Large gate is set on Forward/Side scatter of acquired endocervical cytobrush cells (Figure 1 A). The FCS-A versus FSC-W plot allows for the exclusion of cell doublets (Figure 1B). Viability is defined by exclusion of dead cells using LIVE/DEAD Fixable Yellow Amine Dead Cell Stain (Figure 1C). Live cells are defined as leukocytes by CD45 expression (Figure 1D) and within the live CD45+ cells, T cells are defined by CD3 expression (Figure 1E). Subpopulations of CD3+ cells that express GD TCR (TCR gamma delta V1 or TCR gamma delta V2) are defined as gamma delta 1 (GD1) or gamma delta 2 (GD2) cells and percentage of GD1 or GD2 cells within CD3+ T cells was reported (Figure 1G). In Figure 1 I we confirmed that only CD3+ T cells express TCR gamma delta since gated CD3- T cells do not express TCR gamma delta 1 or delta V2. Also, we analyzed the frequency of cervical CD4+ and CD8+ T cells as well as double negative CD4-CD8- T cell subpopulation (Figure 1F). Phenotypic analysis of gated CD3+GD1+ cells revealed (Figure 1H), that majority of GD1 cells (∼74%) are CD4-CD8-.
Figure 1. Representative flow cytometry gating strategy for enumeration of gamma delta (GD) T cell subpopulation in endocervical mucosa.
Identification of endocervical T cell populations by following gating strategy: A) Large gate is set on endocervical cytobrush cells B) Exclusion of cell doublets C) Live cells are viability dye-negative population D) CD45 expression on live cells E) CD3 expression on CD45+ cells F) CD4 and CD8 expression on CD3+ cells G) TCRVD1 (GD1) and TCRVD2 (GD2) expression on CD3+ cells H) CD4 and CD8 expression on GD1+ cells I) TCRVD1 expression on gated CD3 negative cells.
Increased frequency of GD1 T cell subset in the blood of HIV infected women
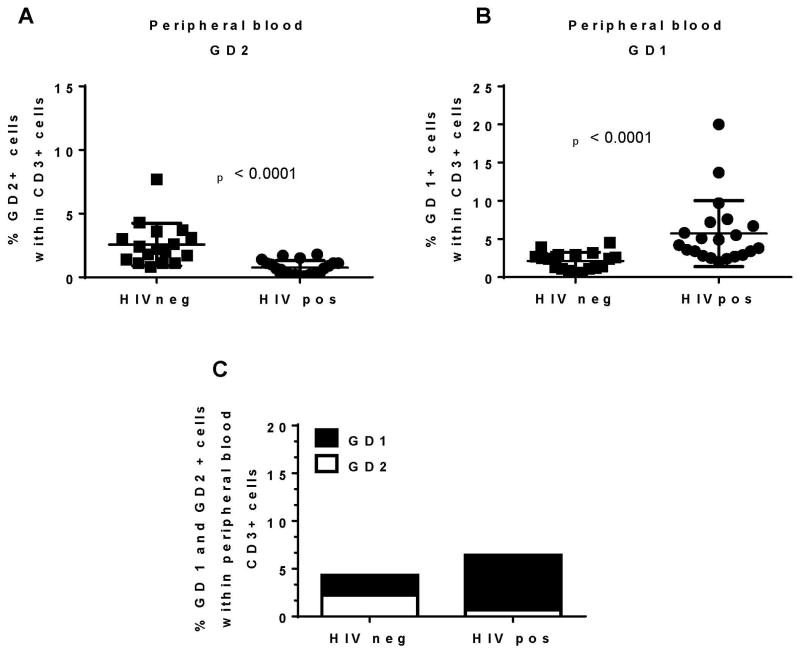
Alterations of GD T cell subsets in blood occur during progressive SIV and HIV infection23, 24. We evaluated the frequency of two gamma delta T cell subsets (GD1 and GD2) in peripheral blood of HIV- and HIV+ women recruited in our study (Figure 2A and B). We found statistically significant decrease in the frequency of GD2 subset in the peripheral blood of HIV + women compared to HIV - women (2.2%, SD = 1.6 in HIV- vs 0.7%, SD = 0.55 in HIV+; p<0.0001) (Figure 2A). We also found increase in the frequency of GD1 subset in the blood of HIV+ women (4.2, SD = 4.3 in HIV+ vs 2.4, SD = 1.1 in HIV-; p<0.0001) (Figure 2B). We reported an increased GD1 / GD2 ratio in the blood of HIV infected women compared to HIV uninfected women (Figure 2C). In summary, consistent with previous reports 25, 26, we found that the GD2 subset is depleted in HIV disease while GD1 T cells represent the major GD T cell subset in peripheral blood of HIV infected women (Figure 2). Brenchley's group26 reported that GD1 T cells expansion in SIV infected monkeys was related to pathological changes in the intestinal epithelium that increased bacterial translocation causing higher levels of bacterial products in circulation that would stimulate GD1 T cells.
Figure 2. Increased GD1 / GD2 T cells ratio in the blood of HIV infected women.
PBMC samples from HIV infected and uninfected women were analyzed by flow cytometry A) frequency of GD2+ cells and GD1+ (B) within endocervical CD3+ cells C) combined data from A and B
GD1 cells are major endocervical GD T cell subpopulation in both HIV infected and uninfected women
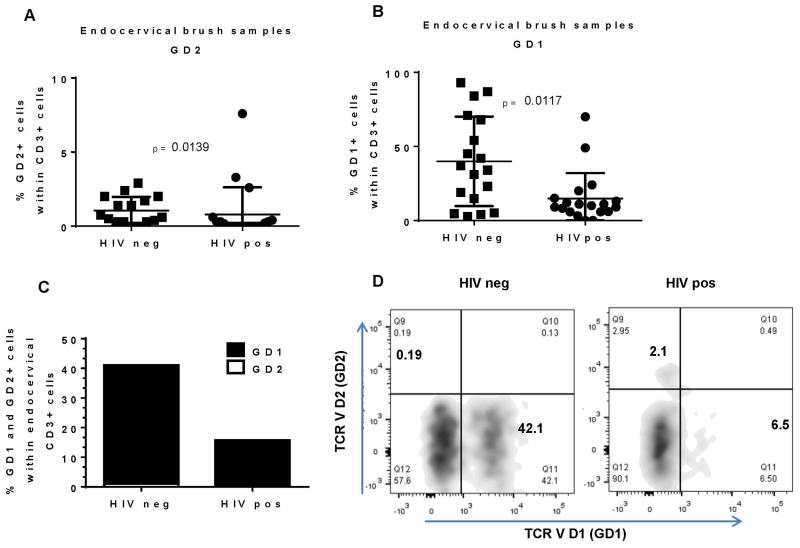
Gamma delta T cells, located at peripheral interfaces (including FRT and decidua) 13, are associated with responsiveness to viral, bacterial and protozoal antigens 27. Because of their well described role in epithelial barrier protection, we propose that these cells play a major role in the first line of defense against HIV in endocervical mucosa. Similarly to the blood, we observed decrease in the frequency of GD2 cells in HIV+ compared to HIV- women (0.67%, SD = 0.91 for HIV- vs 0.08%, SD = 1.83 for HIV+; p=0.0139) (Figure 3A).
Figure 3. Endocervical GD1 are predominant GD T cell subset in HIV infected and uninfected women.
Endocervical brush samples form HIV infected and uninfected women were analyzed by flow cytometry A) frequency of GD2+ cells and GD1+ (B) within endocervical CD3+ cells C) combined data from A and B D) representative flow cytometry data for HIV negative and HIV positive endocervical CD3+ cells showing expression of TCR VD2 (GD2) and TCR VD1 (GD1).
As shown on Figure 3B and C, frequency of GD1 cells among all CD3+ cells was significantly higher in HIV- than in HIV+ women (35.50%, SD = 30.27 in HIV- vs 10.00%, SD = 17.17 in HIV+; p=0.0117) (Figure 3B and C and representative dot plot D).
In summary, we report for the first time that GD1 cells represent the major intraepithelial GD T cell subset in the endocervix of uninfected women. In HIV infection there is a decrease in both, frequency and the absolute number (Supplementary Figure 2) of intraepithelial GD1 T cells. High abundance of GD1 T cells in the uninfected endocervical mucosa supports immunoregulatory role and GD1 involvement in the epithelial barrier homeostasis. Therefore, the loss of intraepithelial GD1 T cells and establishment of proinflammatory environment leads to higher mucosal vulnerability and pathogen (HIV) susceptibility.
Endocervical GD T cells express CD4 and CCR5
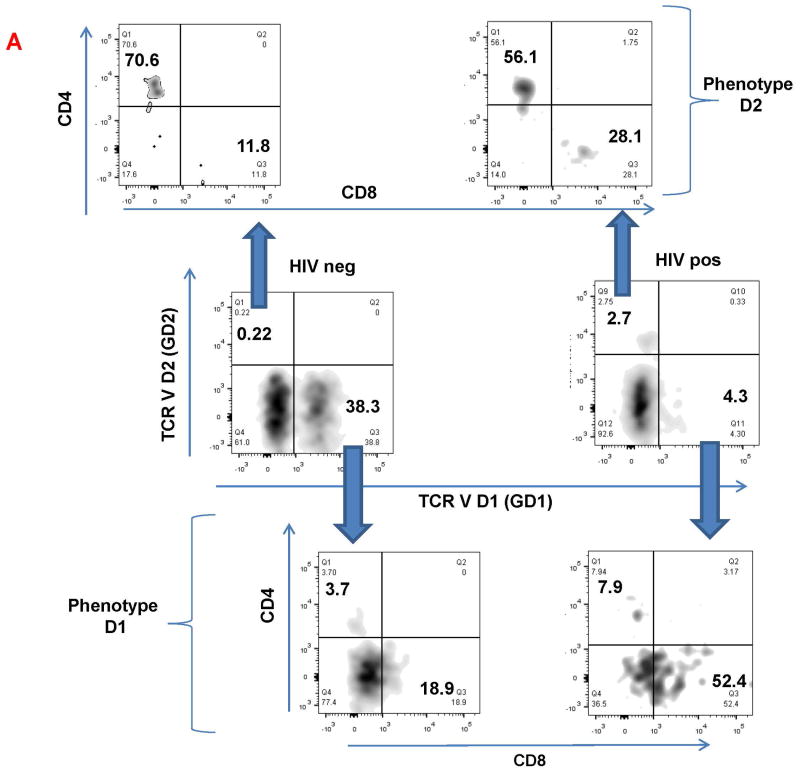
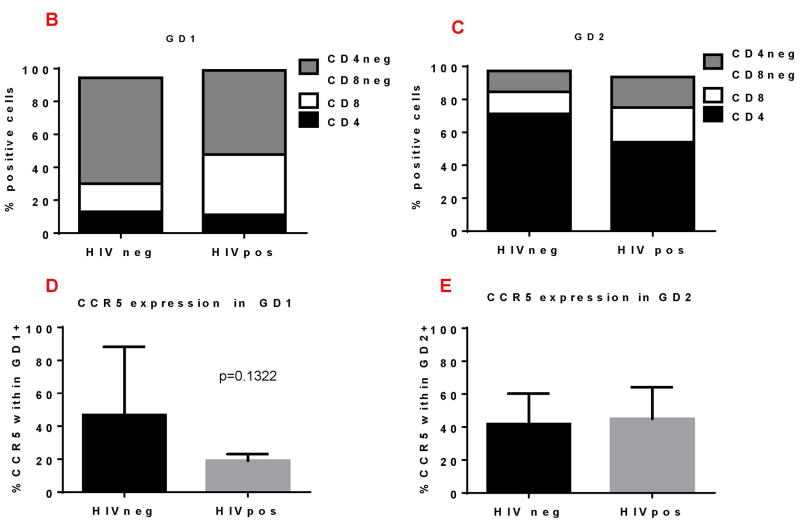
We compared the phenotype of endocervical GD1 and GD2 T cells (Figure 4A-C) in HIV negative and HIV positive women. We observed that endocervical GD2 cells, in both HIV negative and positive women, predominantly express CD4 (Figure 4A, upper two panels and Figure 4C). In contrast, endocervical GD1 T cells from HIV negative and positive women express very few CD4 molecules on their surface (Figure 4A, lower two panels and Figure 4B). Majority of GD1 T cells were double negative cells (CD4-CD8-). Interestingly, HIV+ women had higher frequency of endocervical GD1 and GD2 T cells that express CD8 molecule (Figure 4A, lower right panel and Figure 4B-C).
Figure 4. Phenotypic characteristics of endocervical GD1+ and GD2+ T cells in HIV positive (+) and negative (-) women.
Endocervical GD1+ and GD2+ cells were analyzed for expression of surface molecules: CD4, CD8 and CCR5. A) Representative flow cytometry data of gated endocervical CD3+ cells expressing TCR VD2 (GD2+) and TCR VD1 (GD1+) (middle panels). Expression of CD4 and CD8 on GD2+cells (upper panels) and GD1+cells (lower panels). Numbers in the quadrants are showing percent of positive cells. B) Percent of CD4, CD8, double negative (CD4-CD8-) and CCR5+ cells within the GD1 (B and D) and GD2 cells (C and E) in HIV+ and HIV- women.
The CCR5 is the major co-receptor by which HIV infects cells and previous reports have demonstrated expression of this molecule on FRT T cells28-30. We were interested to confirm its expression on endocervical GD T cells (Figure 4C). We found that both endocervical GD1 and GD2 T cells from all women (HIV neg and HIV pos) express CCR5 on their surface. In summary, our data demonstrate predominance of double negative (CD4-CD8-) endocervical GD1 cells and high CD4 and CCR5 expression on endocervical GD2 T cells supporting our hypothesis that two major intraepithelial GD subset could have a different role in HIV pathogenesis and HIV transmission. Striking difference in the expression of major HIV co-receptor CD4 on GD1 and GD2 T cells, indicate that GD2 T cells could represent the major HIV target cell.
Discussion
HIV acquisition and sexual transmission are dependent on the hormonal and immune environment of the FRT31-33. Mucosal CD4 T lymphocytes in the vagina, cervix and uterus are thought to be the primary targets for sexual acquisition of HIV in women30, 34-36. HIV infects discrete subset of CD4 T cells, which express phenotypic receptors and co-receptors that are necessary for HIV to gain intracellular access 30, 37, 38. As the major component in the mucosal immune system, unconventional T cells, GD T cells may have a decisive role in early phases of HIV infection. Effect of the HIV infection on peripheral blood GD T cells has been studied previously15, 25, however nothing is known of the phenotype or functionality of mucosal FRT GD T cells. The endocervix is lined by a single layer of columnar epithelium and intraepithelial lymphocytes represent a unique T cell subset that can rapidly initiate immune response when encounter pathogenic microbes. Understanding the immunological events in the endocervical intraepithelial compartment therefore is important for the design of effective strategies to prevent HIV infection.
In the present study, we demonstrate for the first time the feasibility of utilizing endocervical brush samples to explore the role of intraepithelial GD T cells in the FRT. We also demonstrate that GD T cells are present in the cervix and HIV infection is associated with alterations in endocervical GD T cells. Furthermore, we found that endocervical brush samples from both, HIV infected and uninfected women predominantly harbor GD1 T cell subset. Our study is the first to confirm the presence of GD1 T cells in endocervical intraepithelial compartment and to show the phenotypic differences between two GD T cell subsets (GD1 and GD2).
One major difference between the circulating and resident GD T cells is that they are using different variable (V) delta chains – the resident GD T cells are Vdelta1+ while the circulating counterpart is Vdelta2+39. Studies of the phenotype of two major GD T subsets in humans reveal that the Vdelta2+ cells are similar in surface markers to the αβT cells while Vdelta1 T cells have a phenotype more like mucosal lymphocytes and IELs.39 It has been reported that only few of either the GD1 or the GD2 cells in the peripheral blood expressed CD840. In contrast, majority of the small intestine intraepithelial GD T lymphocytes express homodimer CD8αα41. Expression of CD8 or CD4 molecules on the surface of GD cells has been associated with cytotoxic (CD8αα+ small intestine intraepithelial GD T cells highly express effector molecules like granzyme and perforin 41, 42) as well as immunoregulatory functions (peripheral blood CD4+GD1+ T cells are precursors of naïve αβ T cells 43). There were no reports regarding the expression of CD8 and CD4 on reproductive tract GD T cells, so we wanted to determine the intraepithelial endocervical phenotype in more details. Peripheral blood GD T cells express CD4, CCR5 and CXCR423, 44, 45 and are susceptible to infection with HIV46. Since, the primary target cells for the human immunodeficiency virus (HIV) infection in the genital tract are CD4 T cells that express CCR5, we hypothesized that alterations in endocervical GD T cells that express CD4 and CCR5 could impact HIV acquisition risk. We found that majority of endocervical GD2 T cells in both, uninfected and infected women express CD4, while predominant population of GD1 T cells are mainly double negative cells, CD4-CD8- (Figure 4 A-C). It is known that natural intraepithelial lymphocytes, are double negative cells that migrate to the intestine and express molecules associated with immune regulation (LAG3, TGF-beta, FGL2, prothymosin beta4) and several killer cell immunoglobulin-like receptors (KIRs) that can function as activating receptors and trigger cytotoxic responses. The concomitant expression of effector and regulatory molecules implies that these “activated yet resting” natural IELs may gain a full activation status to serve self-antigen-directed protective and/or regulatory functions8, 41. Well described regulatory phenotype of human decidual GD T cells (secretion of IL-10 and TGF beta) clearly supports GD T cell potential to contribute toward maintenance of non-inflammatory micro-environment11, 12, 47. Involvement of other cell subsets, like B cells, in supporting GD T cells regulatory phenotype should be considered in the future studies, since the role of B cells in the establishment tolerance at the fetal-maternal interface is well recognized 48, 49. At the same time, higher expression of CD8 on the surface of GD1 T cells in HIV infection implies the effector/cytotoxic potential of these cells.
Our data show that by eliminating cervical GD1, HIV cripples an important antiviral effector subset and removes a normal control over inflammation resulting in HIV persistence and progressing disease. The loss of intraepithelial GD1 T cells will lead to a lack of immunoregulatory environment with subsequent activation of pro-inflammatory cytotoxic pathways.
In this study, we found that in GD1 and GD2 from uninfected and HIV infected women, express CCR5 (Figure 4D-E). GD2 cells that highly express CD4 and CCR5 represent the major HIV target cells. Since, low levels of CD4, undetectable by flow, may be sufficient for productive HIV infection23, 50, endocervical GD1 T cells can also be potential HIV targets. Importantly, predominance of intraepithelial GD1 cells over GD2 in intraepithelial compartment supports the hypothesis that GD1 cell, in a steady state, are true surveillance cells, while GD2 cells can be recruited in this compartment under microbe-initiated activation. The possibility of increased mucosal recruitment of GD2 T cells is supported by Poles et al 16 finding of increased expression of CCR9 and CD103 on peripheral blood GD T cells during HIV infection. Furthermore, the ability of GD2 T cells to respond to bacterial metabolites and produce key mediators of intestinal immunity, including IFN-g, TNF-a, and IL-17A 51, 52, indicates considerable potential for this population to further augment conventional pro-inflammatory T cell responses. Alterations in the normal microbiota, influenced by many different factors, can result in pro-inflammatory milieu and robust immunity that can increase susceptibility to HIV infection. Future experiments will address the association of endocervical GD2 cells with FRT microbiota.
Understanding the immunological milieu of endocervical intraepithelial compartment is important for the design of effective strategies to prevent HIV infections and other sexually transmitted infections and for assessing the safety of future microbicide and vaccine candidates.
Development of markers of mucosal vulnerability in women has a very high impact for all clinical studies addressing the risk of HIV acquisition and transmission. In summary, we propose that GD1 cells in endocervical mucosa are antiviral surveillant cells with the primary aim to defend host without inducing pro-inflammatory responses. In addition, endocervical GD2 cells that express high levels of CD4 and CCR5, may play a role in HIV persistence and transmission. Further investigation of how GD1 and GD2 activation and cell trafficking in the human endocervical mucosa can be modified by environmental factors may lead to the development of novel prevention strategies.
Supplementary Material
Acknowledgments
We thank the Miami WIHS study participants for their willingness to participate in this work. Data in this manuscript were collected by the Miami Women's Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). This work was supported by National Institute of Allergy and Infectious Diseases and Women's Interagency HIV Infection Study (WIHS) [grant number U01 AI103397], National Institute of Allergy and Infectious Diseases, National Institutes of Health [ grant number P30AI073961], National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities, National Institute of Health [grant number UL1TR000460 and 1KL2TR000461], and National Institute of Child Health and Human Development, National Institute of Health [ grant number K23HD074489].
Footnotes
Disclosure: Authors do not have commercial or other association that might pose a conflict of interest (e.g., pharmaceutical stock ownership, consultancy, advisory board membership, relevant patents, or research funding).
References
- 1.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. Aids. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wira CR, Fahey JV, Rodriguez-Garcia M, Shen Z, Patel MV. Regulation of mucosal immunity in the female reproductive tract: the role of sex hormones in immune protection against sexually transmitted pathogens. American journal of reproductive immunology. 2014;72:236–258. doi: 10.1111/aji.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Garcia M, Patel MV, Wira CR. Innate and adaptive anti-HIV immune responses in the female reproductive tract. Journal of reproductive immunology. 2013;97:74–84. doi: 10.1016/j.jri.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayday A, Gibbons D. Brokering the peace: the origin of intestinal T cells. Mucosal immunology. 2008;1:172–174. doi: 10.1038/mi.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nature reviews Immunology. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 6.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nature reviews Immunology. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 7.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nature reviews Immunology. 2011;11:445–456. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annual review of immunology. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 10.Nakasone C, Yamamoto N, Nakamatsu M, Kinjo T, Miyagi K, Uezu K, Nakamura K, Higa F, Ishikawa H, O'Brien RL, Ikuta K, Kaku M, Fujita J, Kawakami K. Accumulation of gamma/delta T cells in the lungs and their roles in neutrophil-mediated host defense against pneumococcal infection. Microbes and infection / Institut Pasteur. 2007;9:251–258. doi: 10.1016/j.micinf.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Mincheva-Nilsson L, Hammarstrom S, Hammarstrom ML. Human decidual leukocytes from early pregnancy contain high numbers of gamma delta+ cells and show selective down-regulation of alloreactivity. Journal of immunology. 1992;149:2203–2211. [PubMed] [Google Scholar]
- 12.Nagaeva O, Jonsson L, Mincheva-Nilsson L. Dominant IL-10 and TGF-beta mRNA expression in gammadeltaT cells of human early pregnancy decidua suggests immunoregulatory potential. American journal of reproductive immunology. 2002;48:9–17. doi: 10.1034/j.1600-0897.2002.01131.x. [DOI] [PubMed] [Google Scholar]
- 13.Szekeres-Bartho J, Barakonyi A, Miko E, Polgar B, Palkovics T. The role of gamma/delta T cells in the feto-maternal relationship. Seminars in immunology. 2001;13:229–233. doi: 10.1006/smim.2000.0318. [DOI] [PubMed] [Google Scholar]
- 14.Exley MA, Boyson JE. Protective role of regulatory decidual gammadelta T cells in pregnancy. Clinical immunology. 2011;141:236–239. doi: 10.1016/j.clim.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Autran B, Triebel F, Katlama C, Rozenbaum W, Hercend T, Debre P. T cell receptor gamma/delta+ lymphocyte subsets during HIV infection. Clinical and experimental immunology. 1989;75:206–210. [PMC free article] [PubMed] [Google Scholar]
- 16.Poles MA, Barsoum S, Yu W, Yu J, Sun P, Daly J, He T, Mehandru S, Talal A, Markowitz M, Hurley A, Ho D, Zhang L. Human immunodeficiency virus type 1 induces persistent changes in mucosal and blood gammadelta T cells despite suppressive therapy. Journal of virology. 2003;77:10456–10467. doi: 10.1128/JVI.77.19.10456-10467.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pauza CD, Cairo C. Evolution and function of the TCR Vgamma9 chain repertoire: It's good to be public. Cellular immunology. 2015 doi: 10.1016/j.cellimm.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liebenberg LJ, Gamieldien H, Mkhize NN, Jaumdally SZ, Gumbi PP, Denny L, Passmore JA. Stability and transport of cervical cytobrushes for isolation of mononuclear cells from the female genital tract. Journal of immunological methods. 2011;367:47–55. doi: 10.1016/j.jim.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirbod T, Kaldensjo T, Broliden K. In situ distribution of HIV-binding CCR5 and C-type lectin receptors in the human endocervical mucosa. PloS one. 2011;6:e25551. doi: 10.1371/journal.pone.0025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasselrot K, Cheruiyot J, Kimani J, Ball TB, Kaul R, Hirbod T. Feasibility and safety of cervical biopsy sampling for mucosal immune studies in female sex workers from Nairobi, Kenya. PloS one. 2012;7:e47570. doi: 10.1371/journal.pone.0047570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKinnon LR, Hughes SM, De Rosa SC, Martinson JA, Plants J, Brady KE, Gumbi PP, Adams DJ, Vojtech L, Galloway CG, Fialkow M, Lentz G, Gao D, Shu Z, Nyanga B, Izulla P, Kimani J, Kimwaki S, Bere A, Moodie Z, Landay AL, Passmore JA, Kaul R, Novak RM, McElrath MJ, Hladik F. Optimizing viable leukocyte sampling from the female genital tract for clinical trials: an international multi-site study. PloS one. 2014;9:e85675. doi: 10.1371/journal.pone.0085675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juno JA, Boily-Larouche G, Lajoie J, Fowke KR. Collection, isolation, and flow cytometric analysis of human endocervical samples. Journal of visualized experiments : JoVE. 2014 doi: 10.3791/51906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodara VL, Parodi LM, Chavez D, Smith LM, Lanford R, Giavedoni LD. Characterization of gammadeltaT cells in naive and HIV-infected chimpanzees and their responses to T-cell activators in vitro. Journal of medical primatology. 2014;43:258–271. doi: 10.1111/jmp.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosub DA, Lehrman G, Milush JM, Zhou D, Chacko E, Leone A, Gordon S, Silvestri G, Else JG, Keiser P, Jain MK, Sodora DL. Gamma/Delta T-cell functional responses differ after pathogenic human immunodeficiency virus and nonpathogenic simian immunodeficiency virus infections. Journal of virology. 2008;82:1155–1165. doi: 10.1128/JVI.01275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pauza CD, Poonia B, Li H, Cairo C, Chaudhry S. gammadelta T Cells in HIV Disease: Past, Present, and Future. Frontiers in immunology. 2014;5:687. doi: 10.3389/fimmu.2014.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris LD, Klatt NR, Vinton C, Briant JA, Tabb B, Ladell K, Lifson J, Estes JD, Price DA, Hirsch VM, Brenchley JM. Mechanisms underlying gammadelta T-cell subset perturbations in SIV-infected Asian rhesus macaques. Blood. 2010;116:4148–4157. doi: 10.1182/blood-2010-05-283549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayday AC, Spencer J. Barrier immunity. Seminars in immunology. 2009;21:99–100. doi: 10.1016/j.smim.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Patterson BK, Landay A, Andersson J, Brown C, Behbahani H, Jiyamapa D, Burki Z, Stanislawski D, Czerniewski MA, Garcia P. Repertoire of chemokine receptor expression in the female genital tract: implications for human immunodeficiency virus transmission. The American journal of pathology. 1998;153:481–490. doi: 10.1016/S0002-9440(10)65591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meditz AL, Moreau KL, MaWhinney S, Gozansky WS, Melander K, Kohrt WM, Wierman ME, Connick E. CCR5 expression is elevated on endocervical CD4+ T cells in healthy postmenopausal women. Journal of acquired immune deficiency syndromes. 2012;59:221–228. doi: 10.1097/QAI.0b013e31823fd215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shanmugasundaram U, Critchfield JW, Pannell J, Perry J, Giudice LC, Smith-McCune K, Greenblatt RM, Shacklett BL. Phenotype and functionality of CD4+ and CD8+ T cells in the upper reproductive tract of healthy premenopausal women. American journal of reproductive immunology. 2014;71:95–108. doi: 10.1111/aji.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wira CR, Veronese F. Hormone regulation of the mucosal environment in the reproductive tract and the prevention of HIV infection. American journal of reproductive immunology. 2014;71:487–489. doi: 10.1111/aji.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaul R, Pettengell C, Sheth PM, Sunderji S, Biringer A, MacDonald K, Walmsley S, Rebbapragada A. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. Journal of reproductive immunology. 2008;77:32–40. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 34.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saba E, Grivel JC, Vanpouille C, Brichacek B, Fitzgerald W, Margolis L, Lisco A. HIV-1 sexual transmission: early events of HIV-1 infection of human cervico-vaginal tissue in an optimized ex vivo model. Mucosal immunology. 2010;3:280–290. doi: 10.1038/mi.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Garcia M, Barr FD, Crist SG, Fahey JV, Wira CR. Phenotype and susceptibility to HIV infection of CD4+ Th17 cells in the human female reproductive tract. Mucosal immunology. 2014;7:1375–1385. doi: 10.1038/mi.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, Grossman Z, Dybul M, Oxenius A, Price DA, Connors M, Koup RA. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 38.Kader M, Wang X, Piatak M, Lifson J, Roederer M, Veazey R, Mattapallil JJ. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal immunology. 2009;2:439–449. doi: 10.1038/mi.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen ZW. Comparative biology of gamma delta T cells. Science progress. 2002;85:347–358. doi: 10.3184/003685002783238762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Rosa SC, Mitra DK, Watanabe N, Herzenberg LA, Herzenberg LA, Roederer M. Vdelta1 and Vdelta2 gammadelta T cells express distinct surface markers and might be developmentally distinct lineages. Journal of leukocyte biology. 2001;70:518–526. [PubMed] [Google Scholar]
- 41.Shires J, Theodoridis E, Hayday AC. Biological insights into TCRgammadelta+ and TCRalphabeta+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE) Immunity. 2001;15:419–434. doi: 10.1016/s1074-7613(01)00192-3. [DOI] [PubMed] [Google Scholar]
- 42.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nature immunology. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 43.Ziegler H, Welker C, Sterk M, Haarer J, Rammensee HG, Handgretinger R, Schilbach K. Human Peripheral CD4(+) Vdelta1(+) gammadeltaT Cells Can Develop into alphabetaT Cells. Frontiers in immunology. 2014;5:645. doi: 10.3389/fimmu.2014.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kabelitz D, Wesch D. Features and functions of gamma delta T lymphocytes: focus on chemokines and their receptors. Critical reviews in immunology. 2003;23:339–370. doi: 10.1615/critrevimmunol.v23.i56.10. [DOI] [PubMed] [Google Scholar]
- 45.Zheng NN, McElrath MJ, Sow PS, Mesher A, Hawes SE, Stern J, Gottlieb GS, De Rosa SC, Kiviat NB. Association between peripheral gammadelta T-cell profile and disease progression in individuals infected with HIV-1 or HIV-2 in West Africa. Journal of acquired immune deficiency syndromes. 2011;57:92–100. doi: 10.1097/QAI.0b013e318215a877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace M, Scharko AM, Pauza CD, Fisch P, Imaoka K, Kawabata S, Fujihashi K, Kiyono H, Tanaka Y, Bloom BR, Malkovsky M. Functional gamma delta T-lymphocyte defect associated with human immunodeficiency virus infections. Molecular medicine. 1997;3:60–71. [PMC free article] [PubMed] [Google Scholar]
- 47.Mincheva-Nilsson L. Pregnancy and gamma/delta T cells: taking on the hard questions. Reproductive biology and endocrinology : RB&E. 2003;1:120. doi: 10.1186/1477-7827-1-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashour HM, Seif TM. The role of B cells in the induction of peripheral T cell tolerance. Journal of leukocyte biology. 2007;82:1033–1039. doi: 10.1189/jlb.0507310. [DOI] [PubMed] [Google Scholar]
- 49.Fettke F, Schumacher A, Costa SD, Zenclussen AC. B cells: the old new players in reproductive immunology. Frontiers in immunology. 2014;5:285. doi: 10.3389/fimmu.2014.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imlach S, Leen C, Bell JE, Simmonds P. Phenotypic analysis of peripheral blood gammadelta T lymphocytes and their targeting by human immunodeficiency virus type 1 in vivo. Virology. 2003;305:415–427. doi: 10.1006/viro.2002.1759. [DOI] [PubMed] [Google Scholar]
- 51.Poccia F, Agrati C, Martini F, Capobianchi MR, Wallace M, Malkovsky M. Antiviral reactivities of gammadelta T cells. Microbes and infection / Institut Pasteur. 2005;7:518–528. doi: 10.1016/j.micinf.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poccia F, Agrati C, Martini F, Mejia G, Wallace M, Malkovsky M. Vgamma9Vdelta2 T cell-mediated non-cytolytic antiviral mechanisms and their potential for cell-based therapy. Immunology letters. 2005;100:14–20. doi: 10.1016/j.imlet.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.