Abstract
Purpose
The EGFR tyrosine kinase inhibitors (TKIs), erlotinib and afatinib, have transformed the treatment of advanced EGFR mutant lung adenocarcinoma. However, almost all patients who respond develop acquired resistance on average ~1 year after starting therapy. Resistance is commonly due to a secondary mutation in EGFR (EGFRT790M). We previously found that the combination of the EGFR TKI afatinib and the EGFR antibody cetuximab could overcome EGFRT790M-mediated resistance in preclinical models. This combination has shown a 29% response rate in a clinical trial in patients with acquired resistance to first-generation TKIs. An outstanding question is whether this regimen is beneficial when used as front-line therapy.
Experimental Design
Using mouse models of EGFR mutant lung cancer, we tested whether the combination of afatinib plus cetuximab delivered upfront to mice with TKI-naïve EGFRL858R-induced lung adenocarcinomas delayed tumor relapse and drug-resistance compared to single agent TKI.
Results
Afatinib plus cetuximab markedly delayed the time to relapse and incidence of drug-resistant tumors, which occurred in only 63% of the mice, in contrast to erlotinib or afatinib treatment where 100% of mice developed resistance. Mechanisms of tumor escape observed in afatinib plus cetuximab resistant tumors include the EGFRT790M mutation and Kras mutations. Experiments in cell lines and xenografts confirmed that the afatinib plus cetuximab combination does not suppress the emergence of EGFRT790M.
Conclusions
These results highlight the potential of afatinib plus cetuximab as an effective treatment strategy for patients with TKI-naïve EGFR mutant lung cancer and indicate that clinical trial development in this area is warranted.
Keywords: lung adenocarcinoma, EGFR mutation, targeted therapy, drug resistance, mouse models
INTRODUCTION
Tyrosine kinase inhibitors (TKI), such as erlotinib and afatinib, are approved for front-line treatment of lung adenocarcinomas with somatic mutations in exons encoding the tyrosine kinase (TK) domain of the Epidermal Growth Factor Receptor (EGFR). The most common lung adenocarcinoma-associated EGFR mutations are either in-frame deletions in exon 19 that eliminate an LREA motif in the protein (EGFREx19del) or a point mutation in exon 21 that results in the substitution of a leucine for an arginine at position 858 (EGFRL858R). These mutations cause conformational changes in the EGFR kinase domain leading to unregulated activation of the receptor tyrosine kinase. Erlotinib is a reversible EGFR-TKI that blocks activation of the receptor by competing with adenosine triphosphate (ATP) for binding to the ATP-binding pocket of the receptor. Afatinib, another ATP-inhibitor, binds EGFR covalently, to irreversibly block the activity of EGFR. The clinical success of these reversible and irreversible TKIs for the treatment of EGFR mutant lung adenocarcinomas lies in their higher binding affinity for mutant EGFR (EGFRex19del and EGFRL858R) than wild type EGFR (1, 2).
While most patients with EGFR mutant lung adenocarcinoma experience significant clinical benefit and radiographic response to treatment with EGFR TKIs, median progression-free survival is approximately twelve months (3, 4). In the majority of drug-resistant tumors, the mutant EGFR allele has acquired a secondary point mutation in exon 20, which leads to substitution of methionine for threonine at position 790 (T790M) in the kinase domain (5). The EGFRT790M mutation restores the receptor affinity for ATP to wild-type levels, thus reducing the effect of the ATP-competitive TKIs (6). New generation TKIs, such as AZD9291 and CO-1686, which bind covalently to the mutant EGFR, are showing clinical activity especially in the setting of T790M-positive disease (7–10).
A previous study conducted in transgenic mice with EGFRL858R+T790M-induced lung adenocarcinomas demonstrated that the combination of a second generation TKI afatinib with the anti-EGFR antibody cetuximab can overcome T790M-mediated resistance, while neither drug alone is effective (11). Based on these data a Phase IB/II clinical trial of this drug combination was conducted in patients that developed progressive disease after erlotinib or gefitinib. A 29% radiographic was observed with a median duration of response of 5.7 months (12).
Given the promising results in the resistance setting and data presented in this manuscript, a randomized phase II/III trial of afatinib plus cetuximab versus afatinib alone in treatment-naïve patients with advanced EGFR mutant lung cancer is ongoing by the South West Oncology Group (SWOG).
We hypothesized that patients may derive an even greater benefit from upfront treatment with the combination of afatinib plus cetuximab, with the goal of delaying the development of resistance. Here we investigated the therapeutic effect of first line afatinib plus cetuximab combination therapy vs. erlotinib or afatinib alone in a mouse model of lung cancer driven by EGFRL858R which we previously developed (13).
MATERIALS AND METHODS
Transgenic mice
TetO-EGFRL858R mice and CCSP-rtTA mice were previously described (13). Doxycycline was administered by feeding mice with doxycycline-impregnated food pellets (625 ppm; Harlan-Tekland). Erlotinib and afatinib (obtained from the Organic Synthesis Core Facility at MSKCC) were suspended in 0.5% (w/v) methylcellulose. Erlotinib was administered intraperitoneally (i.p., 25 mg/kg, 5 days a week) while afatinib was administered orally (p.o., 25 mg/kg, 5 days a week). Cetuximab (Erbitux; Bristol-Myers Squibb and Eli Lilly Pharmaceuticals) was administered intraperitoneally (i.p., 1 mg twice a week). Our initial intent was to compare afatinib plus cetuximab to erlotinib, therefore mice were randomized to treatment with these agents. Afatinib treatment was included later as we began to develop the concept of the front-line trial of afatinib vs. afatinib plus cetuximab. At the end of the study, mice were euthanized by CO2 asphyxiation. All animals were kept in pathogen-free housing under guidelines approved by the Yale University Institutional Animal Care & Use Committee (IACUC).
Magnetic resonance imaging (MRI)
All procedures were performed in accordance with protocols approved by the Yale University IACUC and in agreement with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Respiratory gated, gradient-echo MR images of mice were collected with a 4T (31 cm bore) small-animal Bruker horizontal-bore spectrometer (Bruker AVANCE, Billerica, MA). All data were collected using T2* weighted contrast using a custom-built 4 cm diameter 1H Bollinger coil. Prior to the imaging experiments, mice were anesthetized with isoflurane and were maintained on isoflurane/O2 (2–2.5% v/v) throughout data collection. Animal core-body temperature was maintained at 37 ± 1°C by circulation of warm air through the bore of the magnet. Although during the MR imaging, the respiration rates for all mice were regular, MR data collection was synchronized with animal respiration using an MR compatible small animal monitoring and gating system (SA instruments, Inc, Stony Brook, NY), which allowed gated MRI acquisition in the same phase of the breathing cycle. All the MR images were collected during post-expiratory periods with the following MR parameters: field of view = 2.56 × 2.56 × 1.80 cm3, image matrix = 256 × 128 × 24, repetition time = 100 ms, Echo time = 4.5 ms, Flip angle= 30 degree, 2 averages. These scan parameters were chosen to maximize the contrast between healthy lung tissue and tumor. Following a treatment period, mice in different treatment groups were scanned repeatedly over weeks off drug to evaluate the presence of recurrent tumors. Tumor volume was quantified by calculating the area of visible lung opacities present in each image sequence per mouse using BioImage Suite 3.01 (14).
Sequencing
Freshly harvested tumors and adjacent normal lung tissue were pulverized in liquid nitrogen and RNA was extracted using the RNeasy platform (Qiagen, #74104). RNA was then treated with DNase I (RNase-Free DNA Set, Qiagen #79254). cDNA was synthesized using the Superscript III First-Strand cDNA Synthesis Kit (Invitrogen, #18080-051). The cDNA was used as template to amplify the EGFR transgene and Kras cDNA. PCR products were sequenced by Sanger sequencing and sequence tracings were manually reviewed in the forward and reverse directions. The presence of the EGFRL858R mutation was confirmed with the following primers: EGFR-2445F: 5′-caactggtgtgtgcagatcg-3′, EGFR-3616R: 5′-cactgcttggtggcgcgac-3′. The presence of the EGFRT790M mutation was evaluated using the following primers: EGFR-2074F: 5′-cttacacccagtggagaagc-3′, EGFR-2502R: 5′-caccaagcgacggtcctcca-3′. The presence of Kras mutations were evaluated using the following primers: Kras-Fw: 5′-agagaggcctgctgaaaatg-3′, Kras-432Rv: 5′-ccctccccagttctcatgta-3′. Mutations in mouse Pi3k catalytic subunit alpha (Pik3ca) and beta (Pik3cb) were investigated using the following oligos: Pik3ca-F1: 5′-ggcctggggaaacataaact-3′, Pik3ca-R1: 5′-ttctaagcaccgaacagca-3′, Pik3ca-F3: 5′-tggctcaaggacaagaacaa-3′, Pik3ca-R3: 5′-ctgcttgatggtgtggaagt-3′, Pik3cb-F2: 5′-tgagctggaagaaatgctga-3′, Pik3cb-R2: 5′-gagggcacaatcgagaaaag-3′.
Cell lines and in vitro EGFRT790M selection
Human lung adenocarcinoma cell lines, PC-9 and PC-9/BRc1 were cultured in RPMI + L-glutamine (Corning), supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals) and 1% penicillin/streptomycin (Corning). PC-9 cells were obtained from Varmus laboratory and maintained in Pao laboratory since 2004. The isogenic afatinib-resistant cell line PC-9/BRc1 was derived from parental PC-9 cells as previously described (15). Both cell lines were routinely tested for mycoplasma contamination and authentication was performed by confirming the presence of the predicted EGFR kinase domain mutations and using STR profiling (GenePrint 10 System) at the Yale University DNA Analysis Facility in July 2015.
Aliquots of cell mixtures containing 75% PC-9 parental cells (T790M-negative) and 25% PC-9/BRc1 cells (T790M-positive) were prepared and plated in separate dishes. One aliquot was saved as the pre-treatment sample to empirically determine the starting T790M allele frequency. Cell mixtures were harvested following 7 days of treatment with EGFR inhibitors. Drugs were refreshed every 72 hours. Genomic DNA was extracted using the DNeasy kit (Qiagen #69504) and was subjected to SNaPshot sequencing for T790M (16). T790M allele frequency was determined by measuring the relative heights of T790M mutant versus wild-type EGFR peaks [(mutant peak height) / (mutant + wild-type peak height)].
Xenografts and in vivo EGFRT790M selection
Aliquots of cell mixtures containing 75% PC-9 parental cells (T790M-negative) and 25% PC-9/BRc1 cells (T790M-positive) were prepared. Eight-week old athymic nude mice were injected s.c. with 10 million cells from the mixture. When tumors reached approximately 250 mm3, animals were randomized for immediate tumor harvesting or to receive either vehicle or the combination of afatinib (p.o., 25 mg/kg, 5 days a week) and cetuximab (i.p., 50 mg/kg, twice a week) for 10 days. Tumor samples were collected after 10 days of treatment and flash-frozen in liquid nitrogen. Genomic DNA was extracted by phenol-chloroform extraction and subjected to SNaPshot sequencing for T790M (16). T790M allele frequency was determined by measuring the relative heights of mutant versus wild-type EGFR peaks at the 2369 EGFR residue [(mutant peak height)/(mutant + wild-type peak height)].
Quantitative PCR
Genomic DNA from pulverized tumors and adjacent normal lung was extracted using the Wizard genomic purification kit (Promega, #A1120). Quantitative PCR was performed with TaqMan copy number assays (Applied Biosystems) using a ViiA7 Real Time PCR System (Applied Biosystems). 10 ng of genomic DNA were used in the reaction. Amplification was carried out for 40 cycles (10 minutes at 95°C, 15 seconds at 95°C, 1 minute at 60°C). Quadruplicate CT values were averaged and normalized to genomic DNA from the tail of a C57BL/6J mouse. TaqMan copy number reference assay mouse Tfrc (Applied Biosystems) was used for all the reactions. Met copy number was evaluated using the following primers: Mm00193012_cn and Mm00192999_cn; Erbb2 copy number was evaluated with the primers: Mm00341635_cn and Mm00342296_cn. Egfr copy number was evaluated with the following primers: Mm00341576_cn and Mm00340936_cn.
Real-time PCR
Real-time PCR was used to determine the levels of expression of the Egfr transgene in the tumors and normal adjacent lungs using the ViiA7 Real Time PCR System (Applied Biosystems) and the pre-designed TaqMan primer Hs01076078_m1. RNA was extracted using the RNeasy kit according to the manufacturer’s protocol (Qiagen, #74104). cDNA was synthesized from DNase I-treated RNA using the Superscript III First-Strand cDNA Synthesis Kit (Invitrogen). 15 ng of cDNA were used in the reaction (amplification 40 cycles, 2 minutes at 50°C, 10 minutes at 95°C, 15 seconds at 95°C, 1 minute at 60°C). Quadruplicate CT values were averaged and normalized to mouse actin-beta (TaqMan, Actb 4352933E).
Histology and immunohistochemistry
After sacrifice, normal and tumor lung were macrodissected and fixed in 4% paraformaldehyde (PFA) overnight at room temperature, placed in 70% ethanol and sent for paraffin embedding and sectioning (Histology@Yale). 4 μm sections were used for hematoxylin and eosin (H&E) and phospho-histone H3 (1:200, CST #9701) staining.
Immunoblotting
Crushed tumors were lysed in ice-cold RIPA lysis buffer [50 mM Tris, pH 8.0, 150 mM NaCl, 5 mM MgCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, protease and phosphatase inhibitor cocktail (Thermo Scientific)]. Equal amounts of total protein were separated by SDS-PAGE and probed as indicated. Signals were detected using either SuperSignal West Pico or Femto chemiluminescent substrates (Pierce Biotechnology). Antibodies for immunoblotting against EGFRL858R (#3197), phospho-EGFR-Y1068 (#2234), phospho-Erbb2-Y1248 (#2247), AKT (#2938), phospho-AKT (#4060), ERK1/2 (#9102), phospho-ERK (#4376), S6 (#2217), phospho-S6 (#5364), GAPDH (#2118) and the secondary anti-rabbit HRP antibody (#7074) were from Cell Signaling Technology (CST). Additional antibodies include: Erbb2 (Millipore, 06-562), and SPC (Abcam, #ab90716). All the antibodies were used at the dilutions suggested by manufacturer.
Statistics
Statistical analysis was performed with GraphPad Prism 6.0 software with the appropriate tests as indicated in the text and figure legends.
RESULTS
Afatinib plus cetuximab combination therapy delays relapse compared to erlotinib or afatinib alone
As a first step in testing whether the afatinib plus cetuximab combination was more effective than single agent TKI in the initial treatment of EGFR mutant lung cancer, we measured tumor relapse following treatment with the different regimens. CCSP-rtTA; TetO-EGFRL858R tumor-bearing mice were treated with afatinib plus cetuximab, afatinib, or erlotinib for 4 weeks after which treatment was interrupted (Fig. 1). Doxycycline treatment, to induce transgene expression was initiated at weaning and maintained throughout the life of the mice. To evaluate response to each treatment, tumor volume was quantified by MRI at the beginning and at the end of the 4 weeks of treatment. MR images taken at the end of the treatment period showed dramatic responses in all three treatment groups: the median tumor volume change on erlotinib, afatinib and afatinib+cetuximab was −100% (Supplementary Fig. S1A, S1B and Table S1).
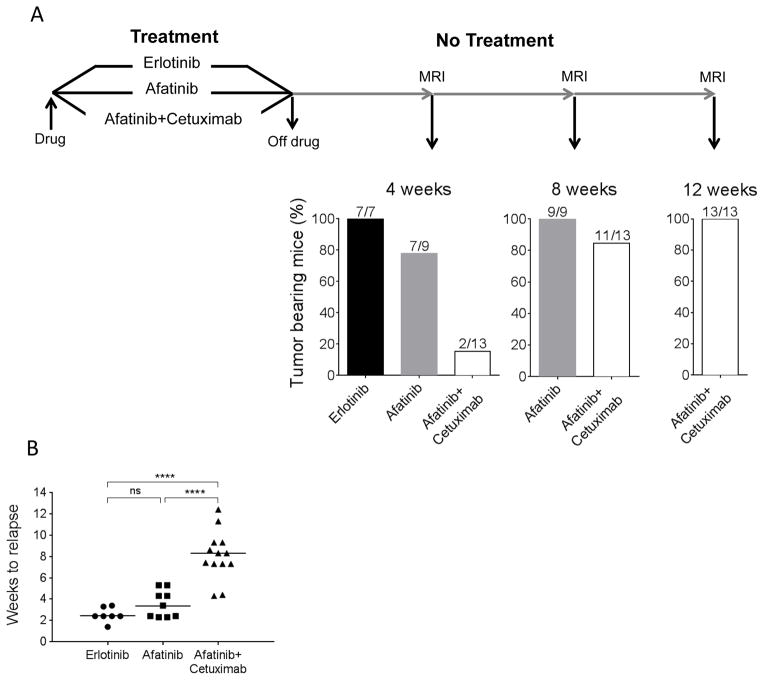
Figure 1. Tumor relapse in erlotinib-, afatinib- or afatinib plus cetuximab-treated tumors.
A. Schematic representation of the protocol used to evaluate tumor relapse after erlotinib, afatinib or afatinib plus cetuximab treatment. CCSP-rtTA; TetO-EGFRL858R mice with lung adenocarcinomas were treated for 4 weeks with erlotinib, afatinib or afatinib plus cetuximab and monitored by MRI for tumor recurrence. Histograms show the percentage of tumor-bearing mice 4, 8 and 12 weeks after drug withdrawal. B. Scatter plot showing the time to tumor relapse in the three treatment groups. The median time is 2 weeks for erlotinib, 3 weeks for afatinib and 8 weeks for afatinib plus cetuximab. Statistical significance was assessed using the one-way ANOVA Bonferroni multiple comparison test (ns= non significant, **** p<0.0001).
After 4 weeks treatment was halted, mice were monitored by MRI 4, 8 and 12 weeks off drug to evaluate the presence of recurrent tumors (Fig. 1). In some cases imaging was performed before the 4-week interval was complete as the mice were showing signs of respiratory distress. After 4 weeks off drug, only 15.4% of the afatinib plus cetuximab-treated mice showed measurable recurrent tumors (defined as tumor volume ≥100mm3), in contrast to 77.8% of afatinib-treated and 100% of erlotinib-treated mice. This percentage increased to 84.6% after 8 weeks off drug in the afatinib plus cetuximab group and reached 100% only after 12 weeks off-drug (Fig. 1). By 8 weeks off drug tumors in all mice treated with afatinib as a single agent had recurred. The median tumor burden pre-treatment in the afatinib plus cetuximab group was similar to that in the erlotinib and afatinib (erlotinib 225.7 mm3, afatinib 320.7 mm3, afatinib plus cetuximab 206.7 mm3) groups, indicating that the longer time to relapse was not a function of the initial tumor volume (Supplementary Fig. S1 and Table S1). These data show that afatinib plus cetuximab delays tumor re-emergence by two-fold in our mouse model of EGFR mutant lung cancer when compared to erlotinib and afatinib, indicating that the drug combination is more potent than the single agents in eradicating tumor cells.
Generation of afatinib plus cetuximab resistant lung adenocarcinomas
To investigate whether long-term afatinib plus cetuximab treatment of EGFRL858R mutant lung adenocarcinomas in mice leads to the emergence of drug-resistant tumors, we subjected mice with EGFRL858R-induced lung adenocarcinomas to an intermittent drug-dosing schedule, previously used to successfully generate erlotinib-resistant tumors (17). Mice were treated with erlotinib, afatinib, or afatinib plus cetuximab for 4 weeks (see Materials and Methods for details), after which treatment was discontinued until the detection of recurrent tumors by MRI (defined as tumors that grew in the presence of drugs). Upon recurrence, treatment was reinitiated for another 4 weeks. This on/off drug-treatment cycle was repeated until the emergence of resistance or for a maximum of 5 times (Fig. 2A and Supplementary Table S2 and Table S3). All seven mice intermittently treated with erlotinib developed resistance within 4 drug cycles, confirming previously published results (17) (Fig. 2B). The emergence of drug resistance was also observed in all 5 mice treated with afatinib (Fig. 2B). Consistent with these data, our results indicate that afatinib induces resistance and does not delay the onset of resistance compared to erlotinib (Fig. 2B and 2C).
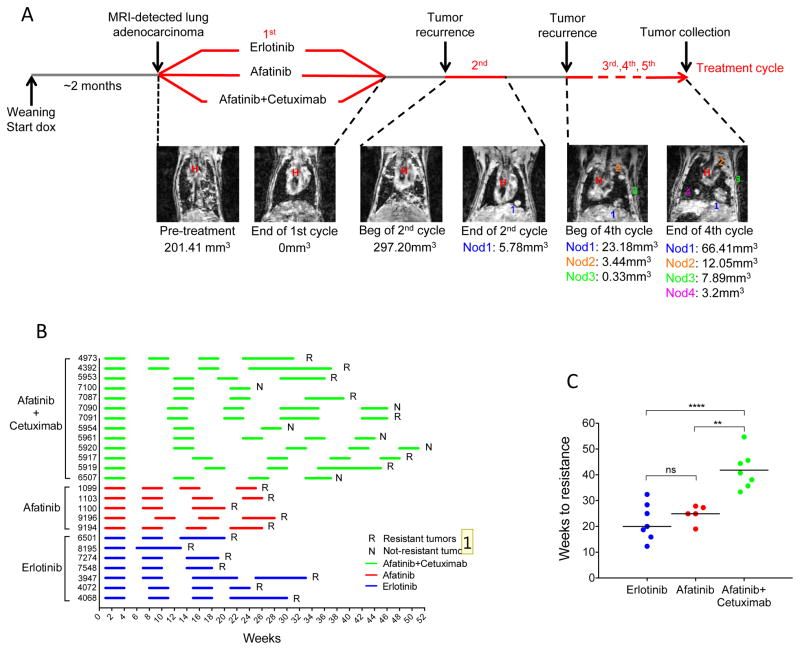
Figure 2. Generation of erlotinib, afatinib or afatinib plus cetuximab resistant tumors.
A. Schematic representation of the intermittent dosing protocol used to generate acquired resistance to erlotinib, afatinib and afatinib plus cetuximab in CCSP-rtTA; TetO-EGFRL858R mice. Doxycycline administration was initiated at weaning and subsequently kept constant throughout the life of the animal. Tumor response was evaluated by MRI at the beginning and at the end of every drug treatment cycle (see Material and Methods for details). Intermittent drug dosing was repeated until the emergence of resistance. Coronal MR images of a CCSP-rtTA; TetO-EGFRL858R mouse subjected to intermittent afatinib plus cetuximab treatment are shown. Tumor volume measurements are at the bottom of each image (H= heart, nod= nodule). B. Line chart showing the treatment schedule of individual mice. Mice were treated 5 days a week for 4 weeks, as highlighted by horizontal lines (in blue, erlotinib treatment; in red, afatinib treatment; in green, afatinib plus cetuximab treatment), and then stopped. Treatment was resumed at disease recurrence as assessed by MRI and clinical presentation. R= mice that developed resistance to treatment, N= mice that were sacrificed because they did not develop resistance after 4 to 5 cycles of treatment or were still showing a complete response after the third cycle. C. Scatter plot showing the time to acquired resistance to erlotinib, afatinib and afatinib plus cetuximab. The median time is 20 weeks for erlotinib, 25 weeks for afatinib and 41 weeks for afatinib plus cetuximab-treated tumors. Significance was assessed using a One-way ANOVA Bonferroni multiple comparison test between erlotinib and afatinib plus cetuximab (** p<0.01) and afatinib and afatinib plus cetuximab (**** p<0.0001). ns= non significant.
The emergence of resistance to afatinib plus cetuximab was evaluated in 13 CCSP-rtTA; TetO-EGFRL858R mice. The afatinib plus cetuximab drug combination was well-tolerated by mice treated with the intermittent dosing protocol as evidenced by their weight patterns compared to TKI treatment alone (Supplementary Fig. S2). Six out of thirteen mice that went through at least 3 cycles of afatinib plus cetuximab did not develop resistant disease (Fig. 2B and Supplementary Table S3). Four out of 11 mice that went through at least 4 cycles of afatinib plus cetuximab, did not develop resistant disease (Fig. 2B and Supplementary Table S3). Therefore, in contrast to erlotinib and afatinib, resistance to afatinib plus cetuximab occurred only in 63% (seven out of eleven) mice that went through the same number of drug cycles. Since afatinib plus cetuximab delayed tumor relapse, each off-drug cycle was extended in this cohort of mice. Therefore, in the afatinib plus cetuximab-treated mice, the overall time for resistant tumors to emerge was longer (>2-fold) compared to those treated with single agent (Fig. 2C). In summary, our data reveal that drug resistance is delayed and occurs with decreased incidence in mice treated with afatinib plus cetuximab compared to single-agent TKI.
EGFRT790M and Kras mutations are found in afatinib plus cetuximab resistant lung adenocarcinomas
To investigate the mechanisms of resistance in tumors that grew out following an initial response to EGFR inhibitor treatment, drug-resistant tumors generated using either continuous or intermittent dosing of the drugs were collected for molecular analysis and histopathological evaluation. In some cases multiple resistant-tumors per mouse were observed by MRI and at the time of necropsy. However, in most cases only one nodule was large enough for molecular studies. We first evaluated whether EGFRT790M could account for drug resistance in all three groups of treatment. For this purpose, we extracted RNA from the resistant tumors and the adjacent normal lung and generated cDNA that was used to sequence the TK domain of EGFR. All the erlotinib- (n=4) and the afatinib- (n=6) resistant tumors analyzed contained the cytosine to thymine point mutation at position 2369, leading to the T790M amino acid substitution. Interestingly, the same mutation was detected in only seven out of thirteen afatinib plus cetuximab-resistant tumors studied (53.8%) (Table 1 and Supplementary Fig. S3A). All matched adjacent normal lungs from the same animals were negative for EGFRT790M (Table 1 and Supplementary Fig. S3A). Expression of the EGFRL858R mutant was confirmed in all the resistant tumors and adjacent lungs as expected (Supplementary Fig. S3B).
Table 1.
Summary of resistance mechanisms in murine drug-resistant tumors.
| Mouse | Drug treatment | Sample | Primary EGFR mutation | Secondary EGFR mutation | Kras mutation |
|---|---|---|---|---|---|
| 6501 | E | Normal | L858R | Neg | Neg |
| Nodule 1 | L858R | T790M | Neg | ||
| 8814* | E | Normal | L858R | Neg | Neg |
| Nodule 1 | L858R | T790M | Neg | ||
| 8817* | E | Normal | L858R | Neg | Neg |
| Nodule 1 | L858R | T790M | Neg | ||
| 8818* | E | Normal | L858R | Neg | Neg |
| Nodule 1 | L858R | T790M | Neg | ||
| 9194 | A | Normal | L858R | Neg | Neg |
| Nodule 1 | L858R | T790M | Neg | ||
| Nodule 2 | L858R | T790M | Neg | ||
| 9196 | A | Normal | L858R | Neg | Neg |
| Nodule 1 | L858R | T790M | Neg | ||
| Nodule 2 | L858R | T790M | Neg | ||
| 1100 | A | Normal | L858R | Neg | Neg |
| Nodule 1 | L858R | T790M | Neg | ||
| Nodule 2 | L858R | T790M | Neg | ||
| 4392 | A+C | Normal | L858R | Neg | Neg |
| Nodule 1 | L858R | Neg | G12R | ||
| Nodule 2 | L858R | T790M | Neg | ||
| 4973 | A+C | Normal | L858R | Neg | Neg |
| Nodule 2 | L858R | T790M | Neg | ||
| Nodule 3 | L858R | T790M | Neg | ||
| Nodule 4 | L858R | T790M | Neg | ||
| 5917 | A+C | Nodule | L858R | Neg | G12V |
| 5919 | A+C | Nodule | L858R | T790M | Neg |
| 5953 | A+C | Normal | L858R | Neg | Neg |
| Nodule | L858R | Neg | G12D | ||
| 7087 | A+C | Normal | L858R | Neg | Neg |
| Nodule1 | L858R | Neg | G12D | ||
| Nodule2 | L858R | Neg | Neg | ||
| 7091 | A+C | Nodule | L858R | Neg | G12D |
| 9072* | A+C | Normal | L858R | Neg | Neg |
| Nodule 1 | L858R | T790M | Neg | ||
| Nodule 2 | L858R | T790M | Neg |
E, erlotinib; A, afatinib; A+C, afatinib+cetuximab
Mice underwent continuous and not intermittent drug treatment.
These data suggest that afatinib plus cetuximab treatment does not suppress the emergence of EGFRT790M even though it delays its emergence. To further explore this possibility, we performed mixing experiments in cell culture, in which PC-9 cells (T790M negative) and TKI-resistant PC-9/BRc1 cells (T790M positive) were mixed in a 3:1 ratio. The cells were then treated with afatinib, cetuximab or afatinib plus cetuximab for 1 week after which the T790M allele frequency was determined. Consistent with data from the transgenic mice, we found that afatinib and afatinib plus cetuximab treatment both selected for EGFRT790M to a similar extent (Fig. 3A). We further examined EGFRT790M selection in a xenograft model by injecting a 3:1 ratio of PC-9 cells and PC-9/BRc1 cells into the flanks of immunodeficient mice and treating animals with afatinib plus cetuximab for 10 days. By comparing T790M allele frequency pre- and post- treatment, we again found that afatinib plus cetuximab selected for T790M in vivo (Fig. 3B). Interestingly, in these mixing experiments the abundance of T790M in the afatinib plus cetuximab treatments did not differ from the single agent studies, in contrast to results in the transgenic mice. This can potentially be explained by this experimental design in which cells with the T790M mutation are mixed with cells without it facilitating the emergence of T790M compared to transgenic mice where the mutation must arise spontaneously. In summary, our data are consistent with the notion that an increase in T790M allele frequency may mediate resistance to treatment with afatinib plus cetuximab in a subset of cases.
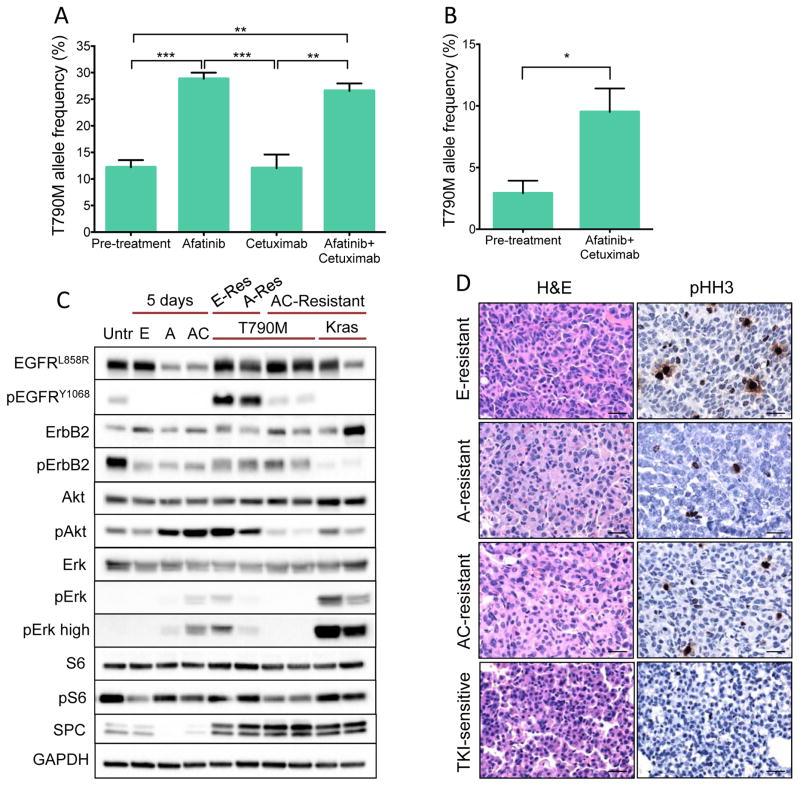
Figure 3. Mechanisms of resistance to afatinib plus cetuximab.
A. Addition of cetuximab does not affect selection of the T790M allele in the presence of afatinib. T790M allele frequency following treatment with afatinib (100 nM), cetuximab (10 μg/mL), or afatinib plus cetuximab for 7 days. T790M allele frequency following treatment is compared to T790M allele frequency in the initial cell mixture of 75% PC-9 cells (T790M-negative) plus 25% PC-9/BRc1 cells (T790M-positive). Data are expressed as mean ± SE. Statistical significance was assessed using the one-way ANOVA Bonferroni multiple comparison test (** p<0.01, *** p<0.001). The difference in the T790M frequency between pre-treatment and cetuximab-treated groups is not statistically significant. B. Afatinib plus cetuximab selects for the T790M allele in vivo. T790M allele frequency following treatment with afatinib plus cetuximab for 10 days in vivo. Cell mixtures of 75% PC-9 cells (T790M-negative) plus 25% PC-9/BRc1 cells (T790M-positive) were injected s.c. in immunodeficient mice and allowed to grow to ~250 mm3. Tumors were then either extracted (Pre-treatment; n=5) or treated for 10 days with the combination of afatinib plus cetuximab (Afatinib + Cetuximb; n=6). T790M allele frequency following treatment is compared to T790M allele frequency in the pre-treatment tumor. Data are expressed as mean ± SE of measurements from 5–6 mice (unpaired t-test * p<0.01). C. Signaling pathway activation in erlotinib-, afatinib- and afatinib plus cetuximab-sensitive and resistant tumors. Immunoblotting analyses of tumor lysates from lung adenocarcinomas derived from untreated (Untr), erlotinib- (E), afatinib- (A) and afatinib+cetuximab (AC)-sensitive (5 days treated) and resistant CCSP-rtTA; TetO-EGFRL858R mice. Lysates were probed with the indicated antibodies. (p= phospho). D. H&E and immunohistochemical staining for phospho-histone H3 performed on paraffin sections of erlotinib, afatinib and afatinib plus cetuximab-resistant tumors compared to a TKI-sensitive tumor (tumor treated for 5 days with erlotinib is shown). 20x magnification is shown. Bars= 50 μm.
We further explored mechanisms of resistance to afatinib plus cetuximab in the remainder of T790M-negative drug-resistant tumors. Since resistance to cetuximab treatment has been reported to occur via KRAS mutations in metastatic colorectal cancer (18), we checked whether mutations in this gene could be associated with resistance to afatinib plus cetuximab, especially in those tumors without EGFRT790M mutations. For this purpose, we sequenced endogenous Kras in all the resistant tumors and normal adjacent lung. Interestingly, 5 resistant tumors that were negative for the T790M mutation had acquired a point mutation that changed the glycine amino acid at position 12. We detected a guanine-to-cytosine transversion at position 34, leading to G12R, a guanine-to-thymine transversion at position 35, leading to G12V, and three guanine-to-adenosine transition at position 35, leading to G12D (Table 1 and Supplementary Fig. S3C). Importantly, these tumors with Kras mutations retained production of the EGFR L858R protein (Fig. 3C).
Since MET, ERBB2 or EGFR amplification are also associated with resistance to EGFR TKIs (19–21), we looked at alterations in copy number of these genes in the resistant tumors and normal adjacent lung. None of resistant tumors showed amplification of Met, ErbB2 or Egfr (Supplementary Fig. S3D). However, variations in the levels of expression of EGFR were found in one afatinib-resistant tumor that displayed a >3fold-increased expression level, and two afatinib plus cetuximab-resistant tumors that had ~3-fold increased level of expression of the human transgene (Supplementary Fig. S3E). Increased EGFR copy number has also been described in human EGFR mutant TKI-resistant lung adenocarcinomas (21, 22).
Signaling pathway activation in resistant tumors
Blockade of EGFR by targeted therapy results in the inhibition of the MAPK/ERK as well as the PIK3/AKT pathways in tumor cells. By gaining the somatic T790M mutation, such cells maintain activation of these downstream pathways. To confirm this scenario in our samples, we performed immunoblotting on lysates from untreated, drug-sensitive and resistant tumors (Fig. 3C). Indeed, phosphorylated EGFR levels were restored to untreated levels in EGFRT790M positive tumors but not in those harboring a Kras mutation. Interestingly, the EGFRT790M tumors also showed increased Erbb2 phosphorylation that was not detected in the Kras mutant tumors. In the Kras mutant tumors, instead, elevated phospho-Erk levels were found (Fig. 3C). Staining for the mitotic marker phospho-histone H3 on tumor sections revealed active proliferation in EGFRT790M positive and Kras mutant afatinib plus cetuximab-resistant tumors, indicating that regardless of the mechanism these tumors have escaped drug treatment allowing them to proliferate (Fig. 3D).
Lung adenocarcinomas with somatic mutations in EGFR are characterized by activation of PI3K-AKT-mTOR pathway (23) that can be further engaged in settings of resistance to TKIs and thus attenuate apoptosis and enhance proliferation (24). To investigate the contribution of the mTOR pathway to resistance to afatinib plus cetuximab, we performed immunoblotting for pS6, a widely used marker of mTOR activation. After 5 days of treatment with erlotinib or afatinib alone and afatinib plus cetuximab decreased levels of pS6 were observed. We did not observe an increase in the pS6 levels in resistant tumors, consistent with the mutationally-driven mechanisms of resistance found (Fig. 3C).
DISCUSSION
The emergence of acquired resistance to single agent EGFR TKI inhibitor treatment is the barrier to achieving long-term benefit from these targeted therapies in patients with EGFR mutant lung cancer. Therefore, there is an urgent need for therapeutic regimens that can delay or prevent the emergence of drug resistance. Here we show that dual targeting of mutant EGFR with the irreversible TKI afatinib and the EGFR antibody cetuximab in the first-line setting reduces the incidence and delays drug resistance in mice with EGFRL858R-induced lung adenocarcinomas. Moreover, we investigated mechanisms of resistance in these afatinib plus cetuximab-resistant tumors. Our data highlight the potential of this drug combination for the front-line treatment of patients with EGFR mutant lung cancer.
Several new strategies and drugs have been developed in recent years aimed at overcoming resistance to first and second-generation TKIs. The afatinib plus cetuximab combination was originally found to lead to tumor regression in transgenic mice harboring EGFRL858R+T790M-induced lung adenocarcinomas (11). In a clinical trial of this combination, 29% of patients with TKI-refractory lung adenocarcinomas (both with and without the T790M mutation) responded to these agents (12). One of the disadvantages of this combination, however, is the increased toxicity observed due to inhibition of wild-type EGFR. More recently, third-generation EGFR TKIs, such as AZD9291 and CO1686, which specifically target mutant EGFR, including the EGFRT790M mutation, have been developed and are showing promise in clinical trials in patients with TKI-resistant EGFR mutant tumors (7, 8, 25, 26). While it is at present unknown how to best sequence these different therapies, emerging preclinical studies suggest that appropriate sequencing of the agents will be important (27). Indeed, resistance to erlotinib, afatinib and afatinib plus cetuximab can be overcome using the third-generation TKI AZD9291, but the reverse does not occur (27). Together with this information, our data indicate that afatinib plus cetuximab is superior to afatinib or erlotinib alone when used as front-line therapy, suggesting a potential treatment scenario in which afatinib plus cetuximab are given upfront followed by third-generation TKI treatment if and when resistance emerges. A Cooperative group Phase II/III trial of afatinib plus cetuximab vs. afatinib alone in patients with TKI-naïve EGFR mutant lung cancer is underway. Patients with Exon 19 deletion mutant tumors exhibit improved survival upon upfront afatinib treatment (28) and whether they benefit differently from combined afatinib plus cetuximab treatment compared to patients with L858R-induced tumors remains to be determined. Importantly, our study compares afatinib vs. afatinib plus cetuximab in mice harboring the L858R point mutation and not EGFR Exon 19 deletion mutations.
Afatinib plus cetuximab can effectively lead to the regression of tumors harboring the EGFRT790M mutation (11). Prior to this work, however, it was unclear whether this mutation could emerge as a mechanism of resistance to afatinib plus cetuximab upon treatment of TKI naïve tumors. We show that in mice with EGFRL858R-induced tumors, long-term treatment with afatinib plus cetuximab can lead to the emergence of EGFRT790M. Further supporting this result, when we mixed cells with and without EGFRT790M and treated them with afatinib plus cetuximab, cells harboring EGFRT790M outgrew the EGFRT790M negative cells. Our data support the possibility that one of the mechanisms of resistance to this drug combination is the EGFRT790M mutation, a finding that will be confirmed in the clinical trial of this drug combination. These results, also suggest that additional mediators of sensitivity and resistance to afatinib plus cetuximab are likely to exist since we know that tumors harboring EGFRT790M mutations can be responsive to this drug combination in mice and humans (11, 12).
Previously, we had found mTOR pathway activation as a mechanism of resistance to afatinib plus cetuximab in tumors already harboring an EGFRT790M mutation (24). Interestingly, in the afatinib plus cetuximab-resistant tumors examined here, we did not observe activation of this pathway above baseline levels (Fig. 3C). This is likely due to the fact that all of the resistant tumors examined in this study either had acquired the EGFRT790M mutation or a Kras mutation and suggests that the mechanisms of resistance to afatinib plus cetuximab in TKI-naïve and resistant tumors may be different. Importantly, our study points to specific potential resistance mechanisms that should and will be examined in the Phase II/III cooperative group trial of afatinib vs. afatinib plus cetuximab.
One of the surprising findings from our study was that ~50% of afatinib plus cetuximab resistant tumors in the EGFRL858R mouse model harbored mutations in Kras. Mutations in Kras are a well-established mechanism of primary resistance to EGFR TKIs (29), but have not been found to emerge following successful TKI treatment (e.g. acquired resistance) in patients (30). Previously, we found that Kras mutations could emerge in transgenic models of EGFR mutant lung cancer following erlotinib treatment (17). The discrepancy between humans and mice could be due to the fact that Kras mutations can arise spontaneously in aged mouse lungs (31); thus, it is possible that the Kras mutant tumors arise independently of EGFR mutations and/or drug treatment. Alternatively, we cannot exclude that the afatinib plus cetuximab drug regimen may contribute to the emergence of Kras mutations. Indeed, Kras mutations have been identified in colorectal cancers that have acquired resistance to cetuximab (18). EGFR and KRAS mutations are mutually exclusive in human lung cancer, possibly reflecting the lethality of expressing mutations in both genes (32). In the resistant tumors, however, suppression of EGFR activity may be permissive for the survival of Kras mutant cells. Analysis of samples from the clinical trials of these agents will further shed light on this issue. Although the EGFRT790M mutation and Kras mutations were the only mechanisms of resistance identified in our study, we expect that a broader array of mechanisms will be found in the context of the planned Phase II/III clinical trial. Indeed, plans for the molecular analysis of repeat biopsy specimens at the time of acquired resistance to afatinib or afatinib plus cetuximab in this trial include whole exome sequencing, analysis of receptor tyrosine kinase amplification and protein levels and will provide a comprehensive picture of the mechanistic basis for resistance to these agents in patients with EGFR mutant lung cancer.
In conclusion, further investigation of the afatinib plus cetuximab combination therapy for patients with untreated EGFR mutant lung cancer warrants investigation, despite the potential for added toxicity, and may represent an alternative to single agent TKI treatment that could delay the emergence of drug resistance.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
The first-generation tyrosine kinase inhibitors (TKI), erlotinib and gefitinib, have improved progression-free survival in patients with lung adenocarcinomas harboring activating mutations in the Epidermal Growth Factor Receptor (EGFR) gene. Despite the effectiveness of these compounds, patients inevitably develop progressive disease after a median of approximately one year of starting treatment. Effective strategies to delay the emergence of this resistance are needed. Here, we report on a preclinical study that demonstrates how the combination of the EGFR tyrosine kinase inhibitor (TKI) afatinib and the EGFR antibody cetuximab decreases the incidence and delays drug resistance in transgenic mouse models of EGFR mutant lung cancer. This work lays the foundation for a clinical trial of the combination of afatinib and cetuximab in patients with TKI-naïve EGFR mutant lung cancer.
Acknowledgments
Financial Support: This work was funded by NIH/NCI grants R01CA120247 (KP), R01CA121210 (WP and KP), R01CA140102 (FH), P30NS052519 (FH), P30CA68485 (WP), P30CA008748 (EdS), the American Italian Cancer Foundation (VP), Uniting Against Lung Cancer (KP) and Yale University. C.B. Meador was supported by Public Health Service Award T32 GM07347 from the National Institute of General Medical Studies for the Vanderbilt Medical-Scientist Training Program and the VICC Melly Family Scholarship. Sarah Goldberg is funded by the Department of Defense, the Hope Foundation, and AstraZeneca. Deborah Ayeni was supported by an NSF Pre-doctoral fellowship (DGE-1122492).
We thank Mary Ann Melnick for expert technical assistance and critical reading of the manuscript.
Footnotes
Conflicts of Interest: Rights to a patent application for EGFRT790M testing were licensed on behalf of KP and WP by Memorial Sloan-Kettering Cancer Center to Molecular MD. KP has served as a consultant for Takeda and her lab receives research support from AstraZeneca and Kolltan.
References
- 1.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science (New York, NY. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Rosell R, Viteri S, Molina MA, Benlloch S, Taron M. Epidermal growth factor receptor tyrosine kinase inhibitors as first-line treatment in advanced nonsmall-cell lung cancer. Curr Opin Oncol. 2010;22:112–20. doi: 10.1097/CCO.0b013e32833500d2. [DOI] [PubMed] [Google Scholar]
- 4.Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 5.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS medicine. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, Meyerson M, et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer cell. 2007;11:217–27. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walter AO, Sjin RT, Haringsma HJ, Ohashi K, Sun J, Lee K, et al. Discovery of a Mutant-Selective Covalent Inhibitor of EGFR that Overcomes T790M-Mediated Resistance in NSCLC. Cancer discovery. 2013;3:1404–15. doi: 10.1158/2159-8290.CD-13-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an Irreversible EGFR TKI, Overcomes T790M-Mediated Resistance to EGFR Inhibitors in Lung Cancer. Cancer discovery. 2014;4:1046–61. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sequist LV, Soria JC, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. The New England journal of medicine. 2015;372:1700–9. doi: 10.1056/NEJMoa1413654. [DOI] [PubMed] [Google Scholar]
- 10.Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. The New England journal of medicine. 2015;372:1689–99. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 11.Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. The Journal of clinical investigation. 2009;119:3000–10. doi: 10.1172/JCI38746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janjigian YY, Smit EF, Groen HJ, Horn L, Gettinger S, Camidge DR, et al. Dual Inhibition of EGFR with Afatinib and Cetuximab in Kinase Inhibitor-Resistant EGFR-Mutant Lung Cancer with and without T790M Mutations. Cancer discovery. 2014;4:1036–45. doi: 10.1158/2159-8290.CD-14-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Politi K, Zakowski MF, Fan PD, Schonfeld EA, Pao W, Varmus HE. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes & development. 2006;20:1496–510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papademetris X, Jackowski MP, Rajeevan N, DiStasio M, Okuda H, Constable RT, et al. BioImage Suite: An integrated medical image analysis suite: An update. The insight journal. 2006;2006:209. [PMC free article] [PubMed] [Google Scholar]
- 15.Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, et al. Optimization of Dosing for EGFR-Mutant Non-Small Cell Lung Cancer with Evolutionary Cancer Modeling. Science translational medicine. 2011;3:90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su Z, Dias-Santagata D, Duke M, Hutchinson K, Lin YL, Borger DR, et al. A platform for rapid detection of multiple oncogenic mutations with relevance to targeted therapy in non-small-cell lung cancer. J Mol Diagn. 2011;13:74–84. doi: 10.1016/j.jmoldx.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Politi K, Fan PD, Shen R, Zakowski M, Varmus H. Erlotinib resistance in mouse models of epidermal growth factor receptor-induced lung adenocarcinoma. Dis Model Mech. 2010;3:111–9. doi: 10.1242/dmm.003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–6. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science (New York, NY. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 20.Takezawa K, Pirazzoli V, Arcila ME, Nebhan CA, Song X, de Stanchina E, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer discovery. 2012;2:922–33. doi: 10.1158/2159-8290.CD-12-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Science translational medicine. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 23.Li D, Shimamura T, Ji H, Chen L, Haringsma HJ, McNamara K, et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer cell. 2007;12:81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Pirazzoli V, Nebhan C, Song X, Wurtz A, Walther Z, Cai G, et al. Acquired Resistance of EGFR-Mutant Lung Adenocarcinomas to Afatinib plus Cetuximab Is Associated with Activation of mTORC1. Cell reports. 2014;7:999–1008. doi: 10.1016/j.celrep.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sequist LV, Soria JC, Gadgeel SM, Wakelee HA, Camidge DR, Varga A, et al. First-in-human evaluation of CO-1686, an irreversible, highly selective tyrosine kinase inhibitor of mutations of EGFR (activating and T790M) J Clin Oncol. 2014;32:5s. Abstr 8010. [Google Scholar]
- 26.Janne P, Ramalingam SS, Yang JC-H, Ahn MJ, Kim DW, Kim SW, et al. Clinical activity of the mutant-selective EGFR inhibitor AZD9291 in patients (pts) with EGFR inhibitor–resistant non-small cell lung cancer (NSCLC) J Clin Oncol. 2014;32:5s. doi: 10.3978/j.issn.2218-6751.2014.08.02. abstr 8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meador CB, Jin H, de Stanchina E, Nebhan CA, Pirazzoli V, Wang L, et al. Optimizing the sequence of anti-EGFR targeted therapy in EGFR-mutant lung cancer. Molecular cancer therapeutics. 2014;14:542–52. doi: 10.1158/1535-7163.MCT-14-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. The lancet oncology. 2015;16:141–51. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 29.Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS medicine. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohashi K, Sequist LV, Arcila ME, Moran T, Chmielecki J, Lin YL, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2127–33. doi: 10.1073/pnas.1203530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dragani TA, Manenti G, Pierotti MA. Genetics of murine lung tumors. Adv Cancer Res. 1995;67:83–112. doi: 10.1016/s0065-230x(08)60711-3. [DOI] [PubMed] [Google Scholar]
- 32.Unni AM, Lockwood WW, Zejnullahu K, Lee-Lin SQ, Varmus H. Evidence that synthetic lethality underlies the mutual exclusivity of oncogenic KRAS and EGFR mutations in lung adenocarcinoma. eLife. 2015:4. doi: 10.7554/eLife.06907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.