Abstract
Objective
Assess nevirapine (NVP) resistance in infants who became infected in the three arms of the Breastfeeding, Antiretrovirals, and Nutrition (BAN) study: daily infant NVP prophylaxis, triple maternal antiretrovirals (ARV), or no extra intervention for 28 weeks of breastfeeding.
Design
Prospective cohort study.
Methods
The latest available plasma or dried blood spot specimen was tested from infants who became HIV-positive between 3 and 48 weeks of age. Population sequencing was used to detect mutations associated with reverse transcriptase inhibitor resistance. Sequences were obtained from 22/25 transmissions in the infant-NVP arm, 23/30 transmissions in the maternal-ARV arm, and 33/38 transmissions in the control arm.
Results
HIV-infected infants in the infant-NVP arm were significantly more likely to have NVP resistance than infected infants in the other two arms of the trial, especially during breastfeeding through 28 weeks of age (56% in infant-NVP arm vs. 6% in maternal-ARV arm and 11% in control arm, p=0.004). There was a nonsignificant trend suggesting infants with NVP resistance tended to be infected earlier and exposed to NVP while infected for a greater duration than infants without resistance.
Conclusions
Infants on NVP prophylaxis during breastfeeding are at reduced risk of acquiring HIV, but are at increased risk of NVP resistance if they do become infected. These findings point to the need for frequent HIV testing of infants while on NVP prophylaxis, and for availability of antiretroviral regimens excluding NVP for treating infants who become infected while on such a prophylactic regimen.
Keywords: antiretroviral, breastfeeding, HIV transmission, nevirapine resistance, PMTCT
Introduction
There have been several clinical trials aimed at reducing transmission of HIV from infected mothers to their infants via breastfeeding using antiretroviral drugs (ARV) as treatment or prophylaxis. These trials have included increasing duration of infant nevirapine (NVP) prophylaxis from 6 weeks up to 6 months [1–3], or ARV for the mother for up to 6 months [4–6]. Longer infant prophylaxis or maternal ARV resulted in reduced HIV transmission in these trials [7]. Resistance to ARV, either in mothers or in infants who become infected, may limit the effectiveness of prophylaxis and/or limit effective treatment regimens in mothers and infants who do become infected with HIV.
The Breastfeeding, Antiretrovirals, and Nutrition (BAN) study was a randomized trial occurring between 2004 and 2010 in Lilongwe, Malawi, comparing three strategies during 28 weeks of breastfeeding: (1) ARV given to breastfeeding mothers with CD4 lymphocyte counts >200/mm3, (2) daily NVP prophylaxis for the infant, and (3) no intervention (control, which was standard-of-care at the time). Mothers were advised to wean between 24 and 28 weeks, interventions were stopped at 28 weeks, and both mothers and infants were scheduled to be followed for an additional 20 weeks. Both interventions were effective in reducing the risk of HIV transmission during breastfeeding (1.7% infected in infant-NVP arm and 2.9% in maternal-ARV arm, vs. 5.7% in the control arm) [8]. During the follow-up period, an additional 28 infants became infected [9].
ARV-resistant HIV in the mother is a possible risk factor for NVPR virus in the HIV-infected infants in all three BAN arms. SdNVP was given to all of the mothers in the BAN study to prevent transmission during delivery, and NVPR commonly occurs after a single dose [10], although mothers in the BAN study were also given a week of AZT/3TC treatment after delivery to reduce the development of maternal NVPR [11]. Mothers in the maternal-ARV arm could also develop resistant HIV with poor adherence to their regimen. If the mothers had resistant virus prior to transmission via breastfeeding, the infants should have the same resistance mutations in their HIV as found in their mothers. The objectives of this study were to determine whether the infants in the intervention arms had more frequent detection of NVPR or other RT mutations over the control arm and compare resistance mutations between infants and mothers.
Methods
Study participants
Mother-infant pairs were enrolled in the BAN study with informed consent. The trial was approved by the Malawi National Health Science Research Committee and by the institutional review boards at the University of North Carolina at Chapel Hill and the US Centers for Disease Control and Prevention (CDC). At the time of delivery, all mothers and infants were given single-dose NVP (sdNVP) and one week of zidovudine (AZT)/lamivudine (3TC) to reduce NVP resistance (NVPR) [11]. After delivery, mother-infant pairs were randomized to one of three arms: the maternal-ARV arm in which the mother received ARV therapy (AZT/3TC/NVP, AZT/3TC/nelfinivir, or AZT/3TC/ritonavir-boosted lopinavir) for 28 weeks, the infant-NVP arm in which the infant received daily NVP prophylaxis for 28 weeks, or the control arm in which neither the mother nor the child received additional ARV [12]. Only the first few mothers randomized to the maternal-ARV arm received NVP in their ARV regimen before NVP was replaced with other drugs. Infants were tested for HIV infection at birth and at 2, 12, 28, and 48 weeks of age (Roche Amplicor DNA Assay, v1.5, on whole blood pellets). Positive tests were confirmed with a Roche DNA test on a follow-up blood sample. Mother-infant pairs in which the infant tested HIV-positive at birth or 2 weeks were removed from the cohort. Retrospective testing was performed on dried blood spots (DBS) (collected at 4, 8 10, 16, 20, 24, and 36 weeks) from the HIV-positive infants to narrow the window of infection [8,9]. The days from birth to HIV infection were estimated as the midpoint between the date of the last specimen that tested negative and the date of the first specimen that tested positive. The days on NVP prophylaxis were calculated for each infant from date of birth to the either the documented cessation of NVP or the first visit date after their first HIV-positive screen date, when the infant would have had its positive test confirmed and the mother would have been told to stop NVP prophylaxis. The days on NVP while HIV-positive were estimated as the difference between the days on NVP and the days from birth to HIV infection.
Specimens
The latest available infant plasma or DBS after the infant became HIV-positive was used for sequencing (median interval between first positive test and sequencing of 85 days; range 0–378 days). Infant plasma was unavailable at many visits due to missed visits [13] or insufficient blood volume drawn. Some of the DBS could not be amplified. Therefore, there were large gaps between the first positive visit and the visit that was sequenced for most of the infants. Breastmilk was collected from mothers starting at delivery and continuing every 2–6 weeks until weaning by 28 weeks; maternal plasma was collected at screening during pregnancy, at delivery, and every 2–6 weeks until 48 weeks post-delivery. Maternal plasma from the closest time point to transmission was used for sequencing when available (19 from same visit as the infant tested positive, eight from 2–6 weeks before, two from 4 weeks after, and one each from 12 weeks after, 12 weeks before, and 18 weeks before; samples were not available from all mothers). In one case, only breastmilk from the time of transmission was available (same visit) and was used for sequencing.
Sequencing
HIV-1 RNA was isolated either from plasma using a Qiagen Viral RNA Mini Kit, or from DBS or whole breastmilk using the Abbott RNA Sample Prep on the m2000sp. HIV-1 RNA was reverse transcribed, amplified, and sequenced as described previously [11]. Amplification was unsuccessful for some specimens; alternate samples were sought in these cases, including DBS. The complete RT region of interest (approximately the first two-thirds of the gene where the RT inhibitor mutations are found) [11] could not be amplified from DBS-derived RNA, so alternate primers were designed to amplify two smaller amplicons that encompassed the NVP resistance mutations. The first set was for a 419 bp amplicon (first round primers KCRTUP1 (5′-TGGGCCTGAAAATCC-3′) and KCRTDN1 (5′-GCTCTATGTTGCCCTATTTCTAAGTC-3′); second round primers KCRTUP2 (5′-CCATATAACACTCCAGTATTTGC-3′) and KCRTDN2 (5′-TCTAAGTCAGATCCTACATACAAGTC-3′)). The second set was for a 312 bp amplicon (first round primers KCRTUP3 (5′-GAACTCAAGACTTTTGGGAAGTTC-3′) and KCRTDN1; second round primers KCRTUP4 (5′-TCAATTAGGAATACCACACCC-3′) and KCRTDN2). These alternate primers were used on the DBS RNA to get the longest amplicon possible. Sequences were aligned and phylogenetic trees were made with CLC Sequence Viewer (CLC bio A/S) to ensure linkage between maternal and infant sequences in a mother-infant pair with no cross-contamination between any samples. Sequences have been submitted to GenBank under accession numbers KP981253-KP981362.
Statistical Analyses
Associations between the presence of NVP resistance in infants and each predictor were assessed using Fisher’s exact test for binary variables and the Wilcoxon rank-sum test for continuous variables. P-values less than 0.05 were considered statistically significant; no adjustments were made for multiple testing.
Results
Of the 93 infants infected with HIV in the BAN study between 3 and 48 weeks of age, unique RT sequences were generated from 78. Infants in the infant-NVP arm were significantly more likely to have NVPR mutations (41%) than infants in the other two trial arms (4% in the maternal-ARV arm and 9% in the control arm, p=0.001; Table 1). For infants infected between 3 and 28 weeks, the frequency of NVPR was 56% among 9 sequenced HIV-infected infants in the infant-NVP arm compared to significantly smaller percentages of NVPR in the HIV-infected infants in the other arms (6% of 16 sequenced infected infants in the maternal-ARV arm and 11% of 28 sequenced infected infants in the control arm, p=0.004; Table 1). After the ARV interventions stopped at 28 weeks, infants who became HIV-infected in the infant-NVP arm still had a high frequency of NVPR (31%), with no resistance in infected infants from the other two arms (Table 1).
Table 1.
Detection of NVP resistance mutations in HIV-positive infants by arm in the BAN study.
| Time period | Arm | Infant HIV infections | Infant specimens available | NVPR* | P-value‡ |
|---|---|---|---|---|---|
| Entire study 3–48 weeks | Infant-NVP | 25 | 22 | 9 (41%) | 0.001 |
| Maternal-ARV | 30 | 23 | 1 (4%) | ||
| Control | 38 | 33 | 3 (9%) | ||
| Intervention 3–28 weeks | Infant-NVP | 12 | 9 | 5 (56%) | 0.004 |
| Maternal-ARV | 21 | 16 | 1 (6%) | ||
| Control | 32 | 28 | 3 (11%) | ||
| Follow-up 29–48 weeks | Infant-NVP | 13 | 13 | 4 (31%) | 0.10 |
| Maternal-ARV | 9 | 7 | 0 (0%) | ||
| Control | 6 | 5 | 0 (0%) |
NVPR = nevirapine resistant
Fisher’s exact test comparing infant arm vs. maternal/control arms combined.
Retrospective testing of the infants who first tested positive for HIV after 2 weeks of age revealed that among 86 infants with no loss to follow-up, 24 (28%) infants had their first HIV-positive specimen when they were diagnosed (no delay). The other infants had delays between their first HIV-positive specimen from retrospective testing and actual diagnosis, and thus had continued exposure to prophylactic/sub-therapeutic levels of NVP until diagnosis was confirmed: 26 (30%) infants had up to a 6 week delay, 30 (35%) had up to a 12 week delay, 5 (6%) had a 15–17 week delay, and 1 (1%) had a 27 week delay (an extended time period between the post-weaning visit at 28 weeks and the final visit at 48 weeks). Among infants in the infant-NVP arm who were infected from 3–28 weeks, infants with NVPR HIV tended to test HIV-positive earlier (median 108.5 days of age with NVPR vs. 166.0 days with NVP-sensitive (NVPS), and to thus have longer exposure to prophylactic NVP after becoming infected (median 76.5 days) than infants with NVPS HIV (median 25.0 days), although neither of these differences were statistically significant (Table 2). Other potential predictors of NVPR in infected infants were examined by univariate analyses. Gender, birth weight, pre-delivery maternal CD4 count, and pre-delivery maternal viral load were not statistically significantly associated with NVPR in the infected infants, either in the infant arm alone or among all of the infected infants sequenced (data not shown).
Table 2.
Timing of HIV infection and length of NVP prophylaxis for infants infected from 3–28 weeks of age, infant-NVP arm of the BAN study.
| NVPR* (n=5) |
NVPS* (n=4) |
P-value‡ | |
|---|---|---|---|
| Median days from birth to HIV infection (IQR†) |
108.5 (35.0–129.0) |
166.0 (108.5–187.5) |
0.18 |
| Median days on NVP prophylaxis (IQR) |
127.0 (55.0–227.0) |
187.5 (143.0–217.5) |
0.54 |
| Median days on NVP prophylaxis while HIV+ (IQR) |
76.5 (23.0–92.0) |
25.0 (6.0–64.5) |
0.46 |
NVPR = nevirapine resistant, NVPS = nevirapine sensitive
Wilcoxon rank-sum test
IQR = interquartile range
To determine whether the infants with NVPR acquired resistant HIV from their mothers, we sequenced the RT genes from maternal plasma (and in one case breastmilk) as close to the time of transmission as samples were available (Fig. 1). For the 9 infants with NVPR in the infant-NVP arm, 4 of the mothers had no NVPR mutations, 3 mothers had the same NVPR mutation as their infants, and 2 mothers had different NVPR mutations from their infants (Table 3). Limited resistance was seen in mothers in the other two arms of the trial whose infants had NVPR or other RT resistance mutations, although we didn’t sequence many of the mothers because there were few cases of resistance in the infants. Of note, none of the few mothers in the maternal arm who were taking a NVP-containing regimen transmitted HIV to their infants. The transmitting mothers in the maternal-ARV arm had detectable virus in plasma and/or breast milk at most time points tested, so non-adherence was likely in these mothers (Davis et al., in preparation). The mother of the only infant with NVPR in the maternal-ARV arm did not have any resistance mutations; this infant had 2 NVPR mutations and one 3TC resistance mutation. Of the three mothers in the control arm whose infants had NVPR, one mother had no NVPR mutations, one mother had the same NVPR mutation as her infant mixed with the wild type, and one mother had the complicated situation of having one NVPR mutation shared with her infant, one unique NVPR mutation, and missing the 3TC resistance mutation that her infant had (mother-infant pairs 46–48, respectively; Table 3).
Figure 1.
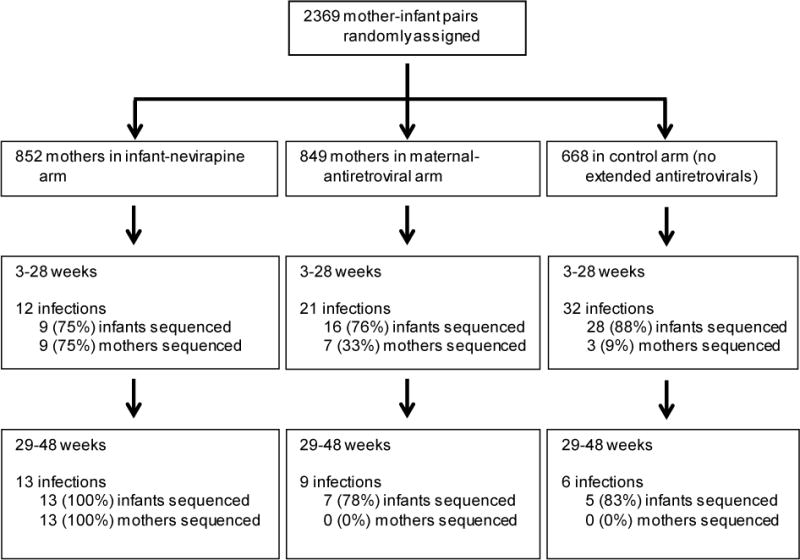
Details on infected infants and mothers in the BAN study whose HIV reverse transcriptase gene was sequenced. Samples were unavailable or sequencing was unsuccessful for 15 of the infected infants; there was no statistically significant difference between infected infants with and without sequence in terms of study arm or number of days until first HIV-positive test.
Table 3.
Results of HIV reverse transcriptase gene sequencing for NVP (AZT/3TC) resistance mutations, BAN study
| Mother-infant paira | Age infant tested positive in weeks | Infant NVP (AZT/3TC) resistance mutations | Maternal NVP resistance mutations |
|---|---|---|---|
| Infant-NVP arm | |||
| 1 | 6 | Y181C | None |
| 2 | 8 | K103Ne | None (breastmilk) |
| 3 | 19 | Y181C | K103N |
| 4 | 24 | K103N | K103N |
| 5 | 24 | K103Nd | None |
| 6 | 32 | K103N | K103KN |
| 7 | 37 | K103N | K103KT |
| 8 | 37 | Y181C | None |
| 9 | 46 (24)b | K103N | K103N |
| 10 | 12 | None | None |
| 11 | 24 | None | None |
| 12 | 28 | None | None |
| 13 | 29 | None | V106AMTV |
| 14 | 32 | None (K65R) | None |
| 15 | 35 | Nonee | None |
| 16 | 36 | Noned | None |
| 17 | 36 | Noned | None |
| 18 | 36 | None | None |
| 19 | 36 | None | None |
| 20 | 37 | None | None |
| 21 | 43 (24)b | None | None |
| 22 | 43 (12)b | None | None |
| Maternal-ARV arm | |||
| 23 | 4 | K103N, Y181C (K70E) | None |
| 24 | 6 | (M184V) | Not tested |
| 25–30c | 4–29 | None | None |
| 31 | 4 | Noned | Not tested |
| 32 | 4 | Nonee | Not tested |
| 33 | 11 | Nonee | Not tested |
| 34 | 28 | Nonee | Not tested |
| 35 | 36 | Noned | Not tested |
| 36–45c | 4–48 | None | Not tested |
| Control arm | |||
| 46 | 4 | K103N | K103KN |
| 47 | 6 | K103N (M184V) | None |
| 48 | 6 | K103N (M184MI) | A98AG, K103KN |
| 49–78c | 4–42 | None | Not tested |
Pairs are listed if infant was infected after 2 weeks of age and an RT sequence was obtained from the infant (n=22 for infant-NVP arm, n=23 for maternal-ARV arm, and n=33 for control arm.
Time of first positive HIV test using more sensitive testing shown in parentheses [17].
Multiple infants with the same resistance result are grouped together in the same row.
DBS was used for sequencing, 419 bp amplicon was obtained.
DBS was used for sequencing, 312 bp amplicon was obtained.
Maternal plasma was also sequenced for the rest of the HIV-infected infants in the infant-NVP arm (all 13 mothers had plasma available) and five of the HIV-infected infants in the maternal-ARV arm to determine whether mothers of infants with NVPS HIV had any resistance mutations that were not transmitted (Table 3). Among these mothers, one (7.7% in the infant-NVP arm) carried a NVPR mutation that her infant did not have (mother-infant pair 13, Table 3). The maternal sample was from 20 weeks, while her infant first tested HIV-positive at 29 weeks.
In the control arm of the BAN study, 38 of 668 (5.7%) infants were observed to become infected between 3 and 48 weeks of age, whereas in the infant-NVP arm, 25 of 852 (2.9%) infants were observed to become infected at 3–48 weeks of age. Therefore, we can estimate that approximately 24 more HIV infections would have been observed had, contrary to fact, these infants not been assigned NVP prophylaxis. Of the infants in the infant-NVP arm who had their specimens sequenced in this study, 40.9% had NVP resistance mutations. In contrast, only 9.1% of the infants in the control arm that were sequenced had NVP resistance mutations. Assuming infected infants in the infant- NVP arm would still have been infected if they had not received NVP prophylaxis, we estimate that approximately 8 of the 25 infected infants (32%) in the infant-NVP arm had resistance attributable to NVP prophylaxis. The rest of observed NVPR would presumably be due to NVP exposure at birth, the same as in the control and maternal-ARV arms.
Discussion
Mother-to-child transmission of HIV via breastfeeding is preventable by giving the infant NVP prophylactically and/or treating the mother with ARV therapy to suppress viral replication. In the BAN study, in which these two interventions were compared with a control arm that received neither intervention, NVP prophylaxis during breastfeeding decreased the risk of HIV transmission with approximately 24 infections averted among 852 infants in the infant-NVP arm [8]. However, 56% of the infants who became infected while receiving NVP prophylaxis had NVPR, which was significantly more than observed in infants in the other arms of the BAN study. Other studies of infant NVP prophylaxis during breastfeeding have found even higher rates of NVPR among infants infected while taking prophylaxis (92% of infants who became positive between 1 and 6 weeks of age in the SWEN study (which used NVP prophylactically for 6 weeks), 83% in the PEPI-Malawi study (NVP prophylaxis given for 14 weeks), and 75% in the HPTN046 Study (NVP prophylaxis given for 6 months))[14–16]. The lower rate in BAN may be due to fewer mothers developing NVPR after single dose peripartum NVP [11], since all of the mothers (and infants) in BAN received a week of AZT/3TC specifically to reduce NVPR. Alternatively, the lower rate observed in BAN may be due to chance; the small numbers of infections in the infant NVP arm preclude precise inference about the rate of NVPR in BAN. Only 3 of 9 infants with NVPR in the infant-NVP arm had the same resistance patterns as their mothers, suggesting that 1 in 3 infants with NVPR received a resistant strain from their mothers and the rest were initially infected with NVPS virus that subsequently became resistant in the infant. This is in agreement with data from the SWEN study [14].
We found a nonsignificant trend suggesting that infants with NVPR tended to have longer exposure to NVP while infected than did infants without NVPR. Testing the infants more often would prevent this problem, but that would require additional costs for visits and testing, adding to the strain of very busy clinics and laboratories in resource-limited settings. Our results also emphasize the importance of having treatment regimens available for these infants that exclude the drug used for prophylaxis.
We observed an increased proportion of NVPR in the infant-NVP arm, compared with the maternal-ARV and control arms, among infants infected between 29 and 48 weeks, after the prophylactic interventions had ended. The observed prevalence of NVPR in the infant-NVP arm (31%) in this timeframe was higher than the 15% NVPR among infants who became infected after the 6 weeks of extended NVP in the SWEN study [14]. Mothers in the SWEN study continued breastfeeding after the 6 weeks of prophylaxis for their infants, contrary to the BAN study where mothers were advised to stop at 28 weeks postpartum. While there was a fraction of BAN mothers who did not wean by 28 weeks [9], more extensive testing of some of the late infections in BAN with very sensitive assays indicated that at least some of these “late” infections occurred prior to 28 weeks, but were not detected by the in-country test until after 28 weeks [17] (Table 3). The rates of resistance in the BAN and SWEN studies may be more similar if many of the late infection infants with NVPR were actually infected while they were taking NVP prophylaxis, but we lack the specimens to more accurately date the time of infection for all of the “late” infections. Nevertheless, the HIV transmissions after 28 weeks point to the importance of continuing prophylaxis until breastfeeding has completely stopped.
Differences in resistance mutations between mothers and infants in the majority of cases of infant NVP resistance indicate that many infants acquired a resistance mutation after transmission of NVPS virus from the mothers due to sub-therapeutic NVP dosing. In addition, we saw potential evidence that the presence of maternal NVPR does not mean that NVP prophylaxis will fail: the infants born to three NVPR mothers did not test HIV positive until well after the prophylaxis was stopped (mother-infant pairs 6, 7, and 9, Table 3), implying the infants were protected while they took the NVP. However, more sensitive testing [17] found that one of these three infants (9) actually became HIV-positive before stopping NVP prophylaxis, so it may be that the other two mothers transmitted earlier, but we do not have the specimens for more sensitive testing.
Only two of the 23 infected infants with sequence data in the maternal-ARV arm had resistance to any RT inhibitors (one to NVP and AZT, the other to 3TC; Table 3) despite the mothers being treated with 2 or 3 RT inhibitors. In the Kisumu Breastfeeding Study where mothers were given ARV during 6 months of breastfeeding, 67% of infected infants had any resistance at 6 months [16], while in the current study we found that only 2 of 16 infected infants by 28 weeks (12.5%) had any resistance to NVP or AZT/3TC. Even though we only sequenced virus from the mother of the one infant in the maternal-ARV arm with NVPR plus 6 randomly selected mothers of infected infants in that arm, the lack of resistance in the infants implies that infants became infected for reasons other than resistance in the mothers. Among mother-infant pairs in the control arm, we did find instances of resistance to NVP and other RT inhibitors (2/3 mothers sequenced and 3/33 infants sequenced). Of note, all of the infants with NVPR in the maternal-ARV and control arms had tested HIV-positive by 6 weeks of age, making it likely that the sdNVP and week of AZT/3TC given after delivery to mothers and infants led to the resistance in these infants. A previous study of BAN and non-BAN mothers showed that addition of a week of AZT/3TC to the sdNVP at delivery prevented most, but not all, NVPR in the mothers [11], so the few cases we observed could be attributed to the low level of NVPR in spite of AZT/3TC use.
Limitations of this trial included the small number of infections and missing samples for some of the infants and mothers, both of which decrease our effective sample size; and the temporal gap between collection of maternal and infant samples and actual time of transmission. The temporal gap may affect our calculations of time on NVP while HIV-positive and how well the sequences we found in the mother represent the viruses that may have been transmitted by the mother. The analysis examining the association between NVPR and duration of NVP exposure after infection was based only on 10 infants and therefore had low statistical power (Table 2). Another limitation was the use of population sequencing to look for resistance in the mothers and infants. Comparison of mother and infant sequences confirmed transmission and ruled out contamination of the samples, but transmission of a resistant variant when both mother and infant have the same mutation cannot be confirmed by these methods. Mixtures of NVPS and NVPR virus were seen in three mothers whose infants did not have the same resistance mutations (mother-infant pairs 7, 13, and 48, Table 3) and in two mothers whose infants had the same resistance mutation (pairs 6 and 46, Table 3).
Our data indicate that infants given NVP prophylaxis are largely protected from HIV infection during breastfeeding, but among those infants who become infected, they are at increased risk of developing NVPR, especially if HIV testing is infrequent. These infected infants will need access to ARV regimens that exclude NVP. Our results highlight the importance of early infant diagnosis of HIV for infants exposed via breastfeeding, with repeated testing necessary to identify and treat infected infants.
Acknowledgments
We are grateful to the BAN Study Team at University of North Carolina Chapel Hill, Centers for Disease Control and Prevention, Atlanta, and UNC Project team in Lilongwe including: Linda Adair, Yusuf Ahmed, Mounir Ait-Khaled, Sandra Albrecht, Shrikant Bangdiwala, Ronald Bayer, Margaret Bentley, Brian Bramson, Emily Bobrow, Nicola Boyle, Sal Butera, Charles Chasela, Charity Chavula, Joseph Chimerang’ambe, Maggie Chigwenembe, Maria Chikasema, Norah Chikhungu, David Chilongozi, Grace Chiudzu, Lenesi Chome, Anne Cole, Amanda Corbett, Amy Corneli, Anna Dow, Ann Duerr, Henry Eliya, Sascha Ellington, Joseph Eron, Sherry Farr, Yvonne Owens Ferguson, Susan Fiscus, Valerie Flax, Ali Fokar, Shannon Galvin, Laura Guay, Chad Heilig, Irving Hoffman, Elizabeth Hooten, Mina Hosseinipour, Michael Hudgens, Stacy Hurst, Lisa Hyde, Denise Jamieson, George Joaki (deceased), David Jones, Elizabeth Jordan-Bell, Zebrone Kacheche, Esmie Kamanga, Gift Kamanga, Coxcilly Kampani, Portia Kamthunzi, Deborah Kamwendo, Cecilia Kanyama, Angela Kashuba, Damson Kathyola, Dumbani Kayira, Peter Kazembe, Caroline C. King, Rodney Knight, Athena P. Kourtis, Robert Krysiak, Jacob Kumwenda, Hana Lee, Edde Loeliger, Dustin Long, Misheck Luhanga, Victor Madhlopa, Maganizo Majawa, Alice Maida, Cheryl Marcus, Francis Martinson, Navdeep Thoofer, Chrissie Matiki (deceased), Douglas Mayers, Isabel Mayuni, Marita McDonough, Joyce Meme, Ceppie Merry, Khama Mita, Chimwemwe Mkomawanthu, Gertrude Mndala, Ibrahim Mndala, Agnes Moses, Albans Msika, Wezi Msungama, Beatrice Mtimuni, Jane Muita, Noel Mumba, Bonface Musis, Charles Mwansambo, Gerald Mwapasa, Jacqueline Nkhoma, Megan Parker, Richard Pendame, Ellen Piwoz, Byron Raines, Zane Ramdas, John Rublein, Mairin Ryan, Ian Sanne, Christopher Sellers, Diane Shugars, Dorothy Sichali, Wendy Snowden, Alice Soko, Allison Spensley, Jean-Marc Steens, Gerald Tegha, Martin Tembo, Roshan Thomas, Hsiao-Chuan Tien, Beth Tohill, Charles van der Horst, Esther Waalberg, Elizabeth Widen, Jeffrey Wiener, Cathy Wilfert, Patricia Wiyo, Innocent Zgambo, Chifundo Zimba. We also wish to thank, most especially, all the women and infants that agreed to participate in the study.
Source of Funding: This project was supported by a grant from the Prevention Research Centers Special Interest Project of the Centers for Disease Control and Prevention (SIP13-01 U48-CCU409660-09), the IMPAACT Network (U01-AI068632) through the UNC IMPAACT Specialty Laboratory, the NIH Fogarty AIDS International Training and Research Program (DHHS/NIH/FIC 2-D43 TW01039-06), the Fogarty International Clinical Research Scholars Program (R24 TW007988), and the UNC Center for AIDS Research (P30-AI50410). The antiretrovirals used in the BAN study were donated by Abbott Laboratories, GlaxoSmithKline, Boehringer Ingelheim, Roche Pharmaceuticals, and Bristol-Myers Squibb. The Call to Action PMTCT program was supported by the Elizabeth Glaser Pediatric AIDS Foundation, the United Nations Children’s Fund, the World Food Program, the Malawi Ministry of Health and Population, Johnson & Johnson, and the U.S. Agency for International Development.
Footnotes
Conflicts of Interest and Source of Funding: No conflicts of interest were declared by any of the authors.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Note: This work was presented in part at the Conference on Retroviral and Opportunistic Infections, February 2015, Seattle, Washington, USA. Abstract 909.
References
- 1.Omer SB, Six Week Extended Dose Nevirapine (SWEN) Study Team Twelve-month follow-up of Six Week Extended Dose Nevirapine randomized controlled trials: differential impact of extended-dose nevirapine on mother-to-child transmission and infant death by maternal CD4 cell count. AIDS Lond Engl. 2011;25:767–776. doi: 10.1097/QAD.0b013e328344c12a. [DOI] [PubMed] [Google Scholar]
- 2.Taha TE, Li Q, Hoover DR, Mipando L, Nkanaunena K, Thigpen MC, et al. Postexposure prophylaxis of breastfeeding HIV-exposed infants with antiretroviral drugs to age 14 weeks: updated efficacy results of the PEPI-Malawi trial. J Acquir Immune Defic Syndr 1999. 2011;57:319–325. doi: 10.1097/QAI.0b013e318217877a. [DOI] [PubMed] [Google Scholar]
- 3.Coovadia HM, Brown ER, Fowler MG, Chipato T, Moodley D, Manji K, et al. Efficacy and safety of an extended nevirapine regimen in infant children of breastfeeding mothers with HIV-1 infection for prevention of postnatal HIV-1 transmission (HPTN 046): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;379:221–228. doi: 10.1016/S0140-6736(11)61653-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kesho Bora Study Group. de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11:171–180. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 5.Thomas TK, Masaba R, Borkowf CB, Ndivo R, Zeh C, Misore A, et al. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding–the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med. 2011;8:e1001015. doi: 10.1371/journal.pmed.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro RL, Kitch D, Ogwu A, Hughes MD, Lockman S, Powis K, et al. HIV transmission and 24-month survival in a randomized trial of HAART to prevent MTCT during pregnancy and breastfeeding in Botswana. AIDS Lond Engl. 2013;27:1911–1920. doi: 10.1097/qad.0b013e32836158b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudgens MG, Taha TE, Omer SB, Jamieson DJ, Lee H, Mofenson LM, et al. Pooled individual data analysis of 5 randomized trials of infant nevirapine prophylaxis to prevent breast-milk HIV-1 transmission. Clin Infect Dis Off Publ Infect Dis Soc Am. 2013;56:131–139. doi: 10.1093/cid/cis808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamieson DJ, Chasela CS, Hudgens MG, King CC, Kourtis AP, Kayira D, et al. Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48-week follow-up of the BAN randomised controlled trial. Lancet. 2012;379:2449–2458. doi: 10.1016/S0140-6736(12)60321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Permar SR, Salazar MG, Gao F, Cai F, Learn GH, Kalilani L, et al. Clonal amplification and maternal-infant transmission of nevirapine-resistant HIV-1 variants in breast milk following single-dose nevirapine prophylaxis. Retrovirology. 2013;10:88. doi: 10.1186/1742-4690-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farr SL, Nelson JAE, Ng’ombe TJ, Kourtis AP, Chasela C, Johnson JA, et al. Addition of 7 days of zidovudine plus lamivudine to peripartum single-dose nevirapine effectively reduces nevirapine resistance postpartum in HIV-infected mothers in Malawi. J Acquir Immune Defic Syndr 1999. 2010;54:515–523. doi: 10.1097/qai.0b013e3181e3a70e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van der Horst C, Chasela C, Ahmed Y, Hoffman I, Hosseinipour M, Knight R, et al. Modifications of a large HIV prevention clinical trial to fit changing realities: a case study of the Breastfeeding, Antiretroviral, and Nutrition (BAN) protocol in Lilongwe, Malawi. Contemp Clin Trials. 2009;30:24–33. doi: 10.1016/j.cct.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sellers CJ, Lee H, Chasela C, Kayira D, Soko A, Mofolo I, et al. Reducing lost to follow-up in a large clinical trial of prevention of mother-to-child transmission of HIV: the Breastfeeding, Antiretrovirals and Nutrition study experience. Clin Trials Lond Engl. 2015;12:156–165. doi: 10.1177/1740774514562031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moorthy A, Gupta A, Bhosale R, Tripathy S, Sastry J, Kulkarni S, et al. Nevirapine resistance and breast-milk HIV transmission: effects of single and extended-dose nevirapine prophylaxis in subtype C HIV-infected infants. PLoS ONE. 2009;4:e4096. doi: 10.1371/journal.pone.0004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogel J, Hoover DR, Sun J, Mofenson LM, Fowler MG, Taylor AW, et al. Analysis of nevirapine resistance in HIV-infected infants who received extended nevirapine or nevirapine/zidovudine prophylaxis. AIDS Lond Engl. 2011;25:911–917. doi: 10.1097/QAD.0b013e328344fedc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fogel JM, Mwatha A, Richardson P, Brown ER, Chipato T, Alexandre M, et al. Impact of maternal and infant antiretroviral drug regimens on drug resistance in HIV-infected breastfeeding infants. Pediatr Infect Dis J. 2013;32:e164–169. doi: 10.1097/INF.0b013e31827f44ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King CC, Kourtis AP, Ziemniak C, Hudgens MG, Nelson JAE, Tegha G, et al. Delayed HIV detection in infants exposed to postnatal ARV prophylaxis during breastfeeding. AIDS Lond Engl. doi: 10.1097/QAD.0000000000000794. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


