Abstract
Purpose
Tumor infiltrating lymphocytes (TILs) become hypofunctional, although the mechanisms are not clear. Our goal was to generate a model of human tumor-induced TIL hypofunction to study mechanisms and to test anti-human therapeutics.
Experimental Design
We transduced human T cells with a published, optimized T-cell receptor (TCR) that is directed to a peptide within the cancer testis antigen NY-ESO-1. After demonstrating antigen-specific in-vitro activity, these cells were used to target a human lung cancer line that expressed NY-ESO-1 in the appropriate HLA context growing in immunodeficient mice. The ability of anti-PD1 antibody to augment efficacy was tested.
Results
Injection of transgenic T cells had some antitumor activity, but did not eliminate the tumors. The injected T cells became profoundly hypofunctional accompanied by upregulation of PD1, Tim3, and Lag3 with co-expression of multiple inhibitory receptors in a high percentage of cells. This model allowed us to test reagents targeted specifically to human T cells. We found that injections of an anti-PD1 antibody in combination with T cells led to decreased TIL hypofunction and augmented the efficacy of the adoptively transferred T cells.
Conclusion
This model offers a platform for preclinical testing of adjuvant immunotherapeutics targeted to human T cells prior to transition to the bedside. Because the model employs engineering of human T cells with a TCR clone instead of a CAR, it allows for study of the biology of tumor-reactive TILs that signal through an endogenous TCR. The lessons learned from TCR-engineered TILs can thus be applied to tumor-reactive TILs.
Introduction
The field of adoptive T cell transfer (ATC) has made impressive progress over the last decade. Expanding from early experiences using ex-vivo-expanded tumor-infiltrating lymphocytes in metastatic melanoma(1), the field is now exploring the use of autologous peripheral blood T cells that are genetically modified to express chimeric antigen receptors (CARs) or modified T cell receptors (TCRs) to redirect them toward tumor-associated antigens (TAA)(2). The most impressive successes have involved the use of CARs directed against the B-cell antigen, CD19, where a high percentage of complete clinical responses have been observed in both adults and children with chronic and acute leukemias(3).
In contrast, the treatment of solid tumors with ATC (or any other form of immunotherapy) has so far proven to be more challenging, though not without some success. There have been some promising results with ATC using TCR-engineered T cells derived from peripheral blood T cells. These T cells are genetically altered to express optimized TCRs that operate under restriction of the TCR-peptide-MHC complex, but offer the benefits of the full spectrum of activation signals that are exhibited by the wild-type receptor(4, 5), show more physiologically-relevant levels of affinity for their cognate antigen(6), and can also be directed against antigen-expressing stromal cells and intracellular TAAs(7). Antigens that have been targeted by TCR engineering, to date, have included relatively immunogenic antigens derived from spontaneously occurring tumor-specific T cells in patients, such as the melanocyte differentiation antigens MART-1(8), glycoprotein 100 (gp100)(9), the melanoma-associated antigen (MAGE)(10), and New York esophageal squamous cell carcinoma antigen (NYESO1)(11). Using these targets, anti-tumor responses have been reported in a subset of patients enrolled in early phase clinical trials of TCR-engineered T cells targeting melanoma(1, 9), colon cancer(12), and synovial cell sarcoma(13).
It is likely that the lack of consistent success of ATC for solid tumors seen so far is due to the same set of obstacles encountered by cancer vaccines and other forms of immunotherapy in general, that include: 1) inadequate T cell trafficking(14), 2) intra-tumoral metabolic and hypoxic challenges(15), and 3) an immune-inhibitory tumor microenvironment (TME) milieu that includes stroma, suppressive immune cells, and soluble factors(16). In addition, there is expression of a set of surface inhibitory receptors (IRs) (i.e. CTLA4, PD1, Tim3, and Lag3) and intracellular checkpoints (i.e. SHP-1, diacylglycerol kinase, and the transcription factor Ikaros) that are naturally upregulated after TCR engagement in order to block continued activation and thus prevent autoimmunity (17–19). Although elucidating these factors in mouse models of immunotherapy has been the basis of much of our understanding of tumor immunology, it is clear that there are many important differences between mouse and human T cell and tumor biology. It would therefore be of great value to be able to study human T cells targeted to human tumors in experimentally manipulatable animal models.
Recently, our lab published the details of such an animal model of human solid tumor where injection of “second generation” CAR-engineered human T cells exerted some antitumor effects, but became reversibly hypofunctional after infiltration into tumors due to a variety of mechanisms (20). This hypofunction phenomenon was similar to that described in TILs isolated from human tumors (21–23). The goal of this study was to generate a similar model, however, using human T cells expressing a specific TCR, rather than a CAR. We reasoned that this model would not only provide a tool to study ATC using transgenic T cells, but, since all signaling occurs through a defined and “authentic” TCR, we could more feasibly extrapolate our findings to TILs observed in the majority of human cancers.
To accomplish this goal, we transduced human T cells with a previously published, optimized TCR (called Ly95) that is directed to a peptide within the cancer testis antigen (CTA) NY-ESO-1(24). These cells were then used to target a human lung cancer line that expresses NY-ESO-1 in the appropriate HLA context growing in immunodeficient mice. Injection of these transgenic T cells had some anti-tumor activity, but did not eliminate the tumors. We observed that the injected T cells become hypofunctional and noted upregulation of PD1 and other IRs. These findings allowed us to take advantage of a major strength of this model – the ability to test reagents targeted specifically to human T cells. We found that PD1 blockade using an anti-human PD1 antibody led to decreased T cell hypofunction and augmented the efficacy of the adoptively transferred T cells.
Materials and Methods
Cell culture conditions
(See Supplemental Methods)
Lentivirus preparation
The NY-ESO1-reactive Ly95 TCR construct is an affinity-enhanced variant of the wild-type IG4 TCR identified from T cells recognizing the HLA-A2 restricted NY-ESO-1:157–165 peptide antigen. In the mutant form, the threonine at residue position 95 is substituted by leucine and the serine at residue position 96 is substituted by tyrosine. It was constructed using an overlapping PCR method(25) based on the description and sequences published previously(24) and incorporated into the lentiviral expression vector pELNS bearing the EF1α promoter (provided by Dr. Carl June at the University of Pennsylvania). Packaging of each plasmid into lentivirus has been previously described(26). Titering of lentiviral concentration was performed by transduction of Sup-T1 cells (ATCC CRL-1942) at different virus dilutions and measurement of transgenic TCR expression by flow cytometric analysis using an anti-human Vβ13.1 TCR chain antibody (Beckman Coulter, CA);
Isolation, bead activation, transduction, and expansion of primary human T lymphocytes
(See Supplemental Methods)
Generation of the target lung cancer cell line
(See Supplemental Methods)
FACS Analysis
(See Supplemental Methods)
Flow Cytometric T cell Activation Assay
(See Supplemental Methods)
In vitro testing of tumor cell killing by Ly95 TCR T cells
Control tumor cells and A549-A2-ESO cells were plated in a flat-bottom 96-well plate at 5000 cells per well in triplicates. After overnight incubation, Ly95 T cells were co-cultured at different effector:target (E:T) ratios. After 18hrs of incubation at 37°C and 5%CO2, supernatant from the wells were aspirated for cytokine analysis by ELISA, wells were washed, the remaining tumor cells were lysed, and luminescence was read in a Modulus II Microplate Multimode Plate Reader after addition of 100ul of luciferin reagent (Promega E1501, Madison, WI). The same assay was used to examine the tumor killing ability of tumor-infiltrating lymphocytes obtained from our in vivo experiments (see below).
Measurement of Ly95 T cell IFNγ secretion by ELISA
(See Supplemental Methods)
In vivo xenograft experiments
A total of 5x106 A549-A2-ESO tumor cells were injected in the flanks of NSG mice in a solution of X-Vivo media (Lonza, NJ) and Matrigel (BD Biosciences, CA). After tumors were established (100–200 mm3), the mice were randomly assigned to one of three intravenous (tail-vein) treatment groups: (i) saline, ii) 10x106 mock-transduced and expanded (mock) T cells, and iii) 10x106 Ly95 expressing T cells. In the experiments combining anti-PD-1 antibody with T cells, two additional groups were included: (iv) every 5-day intraperitoneal (IP) injection of 10mg/kg anti-PD1 antibody (Ultra-LEAF™, Biolegend, CA), and (v) 10x106 Ly95 T cells IV plus every 5-day IP injection of 10mg/kg anti-PD1 antibody. Tumors were measured using calipers and tumor volumes were calculated using the formula (π/6) (length) x (width)2. When predefined protocol endpoints were reached, tumors were harvested, micro-dissected, and digested in a solution of 1:2 DNase:collagenase in a shaker incubator at 37°C for 2 hours. Digested tumors were then filtered through 70-μm nylon mesh cell strainers, and red blood cells were lysed if needed (BD Pharm Lyse; BD Biosciences, CA). Spleens harvested from the same mice were also filtered through 70-μm nylon mesh cells trainers with red blood cell lysis. 1x106 cells from single-cell suspensions were placed in standard FACS tubes and were stained with anti-human CD45, CD8, CD4, and TCRVβ13.1 antibodies to assess degree of infiltration of adoptively transferred T cells. Additionally, we also stained cells with anti-PD1, anti-Tim3, and anti-Lag3 antibodies to measure expression of IRs on TILs. The in vivo experiments were repeated three times in an independent fashion. Groups contained 5–10 mice each.
Ex vivo TIL analysis
After digestion of harvested tumors, necrotic debris was first removed by processing the single cell suspension using a Dead Cell Removal Kit (Miltenyi Biotech, CA). TILs were subsequently isolated using an anti-human CD45-PE antibody (BD Biosciences, CA) with the EasySEP PE Selection Kit (STEMCELL Technologies, Vancouver, Canada). Once isolated, functional analyses for TILs were performed in two different ways: (i) luciferase-based killing assays, and (ii) measurement of antigen-induced T cell IFNγ secretion by ELISA (see above). Pooling of samples was required in order to isolate sufficient numbers of viable TILs after processing (e.g. harvest, digestion, single cell preparation via multiple filter and wash steps, dead cell removal, CD45 magnetic separation) to perform in vitro coculture killing experiments.
Statistical Analysis
(See Supplemental Methods)
Animals
(See Supplemental Methods)
Results
An engineered TCR can be efficiently expressed on the surface of human T cells
Transduction of human CD4 and CD8 T cells undergoing anti-CD3/CD28 bead activation with high-titer lentivirus that encodes the Ly95 TCR recognizing NY-ESO-1 resulted in ~50% expression as measured by FACS analysis of T cells stained with an anti-human TCRVβ13.1 antibody (Ab). At the time of analysis, approximately 70% of the T cells were CD8+ and 29% were CD4+ (Fig. 1A).
Figure 1. Transduction and function of human T cells transduced with the Ly95 TCR.
A) Human T cells were activated using anti-CD3/CD28 microbeads and transduced with high-titer lentivirus encoding Ly95 TCR. After expansion in vitro, FACS analysis was performed using anti-CD4 and anti-TCR (TCRVβ13.1) antibodies. Results show greater than 50% of cultured T cells expressing the engineered TCR with the majority of the lymphocyte population being CD8 T cells.
B) Ly95 T cells alone or Ly95 T cells co-cultured with A549-A2 or A549-A2-ESO cells at an E:T ratio of 10:1 for 24 hours, were evaluated for upregulation of activation markers CD25, IFNγ, Granzyme B, and CD107a. Only A549-A2-ESO cells stimulated the T cells.
C) Ly95 T cells were co-cultured with different human tumor cell lines including the target A549-A2-ESO cells for 18 hours at E:T ratios of 1:1, 5:1, and 10:1 for 18hrs. Ly95 T cells demonstrated (A) efficient killing of and (B) high secretion of IFNγ in response to A549-A2-ESO tumor cells in a dose dependent manner. (* p<0.05, ** p<0.01).
Ly95 engineered human T cells demonstrate reactivity to A549-A2-ESO tumor cells in an antigen-specific fashion
After the Ly95 T cells were “rested down”, they were co-cultured with A549-A2-ESO (A549 expressing NY-ESO-1 in the context of HLA-A2) or A549-A2 tumor cells (A549 expressing HLA-A2 but not NY-ESO-1) for 24hrs, and stained with different markers of activation and for intracellular cytokine secretion. Compared to T cells that were not co-cultured with tumor cells, or T cells co-cultured with control cells not expressing NY-ESO-1, T cells co-cultured with A549-A2-ESO cells demonstrated increases in the percent of cells expressing: 1) intracellular IFNγ (~2% to 15%), 2) granzyme B (~2% to 25%), 3) CD107a (~3% to 22%), and 4) CD25 (~10% to 47%) (Fig. 1B).
We next measured specific lysis of tumor cells by Ly95 T cells via co-culture killing assays (Fig. 1C). Ly95 T cells demonstrated high efficiency killing of A549-A2-ESO tumor cells in a dose-dependent fashion, but did not kill a variety of control cells that did not express HLA-2, NY-ESO-1, or both (Fig. 1C, upper panel). We observed the same pattern of high level of dose-dependent IFNγ secretion in response to A549-A2-ESO tumor cells with no IFNγ secretion after exposure to the control cell lines (Figure 1C, lower panel).
Ly95 T cells are able to slow progression but not induce regression of A549-A2-ESO flank tumors in NSG mice
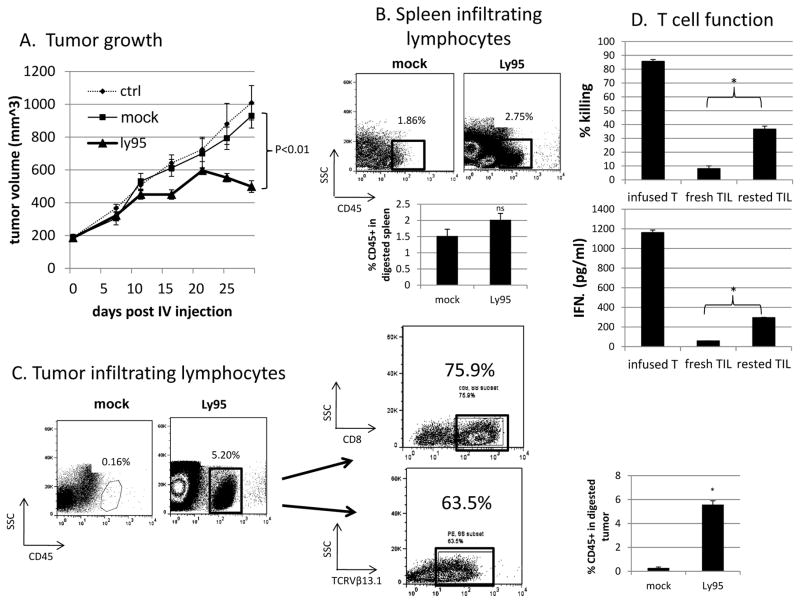
To test the in vivo activity of Ly95 T cells, 10 million Ly95-expressing T cells were injected once intravenously in NSG mice bearing large established A549-A2-ESO flank tumors (approximately 200 mm3 in size). We observed slowing of tumor growth after 11 days post-adoptive transfer. At Day 30, the tumors in the Ly95 T cell-treated group were approximately 50% smaller than those of the untreated group (p<0.01). Administration of mock-transduced T cells (to control for allogenic responses) had no significant effect on flank tumor growth (Figure 2A).
Figure 2. In vivo Activity of Ly95 T cells in a mouse tumor model.
A) NSG mice (n=10 per group) were injected subcutaneously in the flank with A549-A2-ESO tumor cells. After tumors were established and grew to about 200mm3, either saline, 1x107 mock transduced T cells, or 1x107 Ly95 T cells were injected intravenously once and tumor volume was monitored. Ly95 T cells were able to significantly (p<0.01) control tumor growth as far as 30 days post T cell injection compared to mock transduced T cells.
B and C) At 30 days, spleens and A549-A2-ESO flank tumors were harvested, digested, processed into single-cell suspensions. Anti-hCD45 FACS analysis confirmed infiltration into spleens (B) and tumors (C) of injected mice. Dot plots demonstrate representative analyses of spleens and tumors. Bar graphs demonstrate average frequencies of CD45+ events ± S.E. from individual mice. There was no difference in infiltration into spleens (2.0% vs. 1.5%, p>0.05) whereas infiltration into tumors was significantly greater in Ly95 treated mice vs. mock treated mice (5.6% vs. 0.3%, p<0.01)
D) hCD45 positive cells were isolated using magnetic beads. Isolated TILs were cocultured with A549-A2-ESO tumor cells in 20:1 E:T ratio (numbers calculated based on percentage positive TCRVβ13.1 staining) for 18 hours. A portion of the isolated TILs were rested overnight at 37°C, 5% CO2. Killing ability of these fresh and rested TILs were also compared with Ly95 T cells that had been cryopreserved and not injected. Freshly harvested Ly95 TILs demonstrated marked hypofunction in both killing ability (upper panel) and ability to secrete IFNγ (lower panel) in response to tumor reactivity. However, when isolated TILs were rested overnight, they recovered a significant portion of their function both in terms of killing and cytokine secretion ability. (* p<0.01) Bar graphs represent average % killing ± S.E. from triplicates.
Ly95 T cells successfully infiltrate A549-A2-ESO flank tumors after intravenous administration
At 30 days post-adoptive transfer, flank tumors and spleens were harvested, digested into single-cell suspension, and analyzed by flow cytometry. There was some infiltration detected in the spleens of adoptively transferred mice (2.8% of total cells in Ly95 treated tumors, 1.5% of total cells in mock-transduced T cell treated tumors) (Fig. 2B), however this was increased in the tumors, where we observed a distinct population of human CD45+ T cells comprising 5.8% of the single-cell tumor suspension in the “Ly95 tumors” vs. 0.3% of that in “mock tumors” (p<0.05) (Figure 2C). The majority of TILs were CD8+ (76%) and Ly95+ (64%) (Figure 2C).
Ly95 TILs are reversibly hypofunctional
Although a significant number of live Ly95 TILs with confirmed surface expression of the engineered TCR were detected within A549-A2-ESO flank tumors, their ability to kill freshly cultured A549-A2-ESO cells (Fig. 2D- upper panel) and secrete IFNγ (Fig. 2D- lower panel) was dampened after isolation from tumors (“fresh TIL”) at a 20:1 E:T ratio compared to the infusion product- that is cryopreserved Ly95 T cells (“infused T”). However, when the isolated Ly95 “fresh TILs” were cultured in cell culture medium (“rested”) overnight in 37°C and 5%CO2 (without IL-2), and then co-cultured with tumor cells the following day, they exhibited significant improvements in effector function and IFNγ secretion (Fig. 2D, right columns).
Hypofunctional Ly95 TILs have increased expression of inhibitory receptors compared to cryopreserved Ly95 T cells
We sought to compare the expression of three IRs on freshly isolated Ly95 TILs versus that on infused T cells (the latter serving as baseline) to determine if the hypofunctionality of freshly isolated Ly95 TILs could at least be partially attributed to increased IR expression. In the infused T cells, CD8 and CD4 cells showed similar expression of PD1 (both 3%), Tim3 (2% and 3%, respectively), and Lag3 (1% and 3%, respectively) (Fig. 3A, upper panel). In comparison to infused T cells, both CD4 and CD8 freshly isolated Ly95 TILs exhibited increased expression of all three IRs (Fig. 3A, lower panel and Fig. 3B). When repeated independently two additional times, upregulation of all three IRs was consistently seen on both CD8 and CD4 TILs compared to CD8 and CD4 infused T cells. (Supplemental Figure 1).
Figure 3. Expression of Inhibitory Receptors (IRs) on human T cells.
TILs were isolated as described in Figure 2 and subjected to FACS using anti-human CD45, CD4, TCRVβ13.1, PD1, Tim3, Lag3 antibodies.
A) Expression of IRs was examined on the CD4 positive and negative (CD8) cells. The number in the box represents the percentage of IR+ T cells in the CD4 or CD8 subset.
B) Upregulation of IR expression was similar between CD8 and CD4 TILs.
C) Expression of IRs was examined on the TCR+ positive (TCRVβ13.1+) and negative cells. The number in the box represents the percentage of IR+ T cells in the TCRVB13.1+ or TCRVB13.1− subset.
D) Compared to TCR− TILs, TCR+ TILs demonstrated significantly greater upregulation of PD1 (74% vs. 36%, p<0.01) and Tim3 (49% vs. 41%, p<0.05).
Dot-plots display representative analyses from individual single mouse. Bar graph displays average frequency of IR+ events in TCR+ and TCR− gates ± S.E. from individual mice.
We were also interested in comparing IR expression on the Ly95-expressing versus non-Ly95-expressing T cells from the same tumors. We first examined the phenotype of the infused T cells. As shown in Figure 3C (upper panels- small boxes), the percent of cells expressing PD1, Tim3 and Lag 3 was low, but very similar between the TCR− and the TCR+ (Vβ13.1 positive) cells. When we examined the isolated TILs (Fig 3C, lower panels and Fig 3D), we noted increased expression of all of the IRs. However, there were differenced in the expression levels of T cells with the transgenic TCRs compared to the T cells not expressing the TCR. Specifically, TCR+ TILs, as compared to TCR− TILs, showed greater upregulation of levels of PD1 (74% vs. 36%, p<0.01) and Tim3 (49% vs 41%, p<0.05), but no increases in Lag3. (Fig. 3B, bottom panel and bar graph)
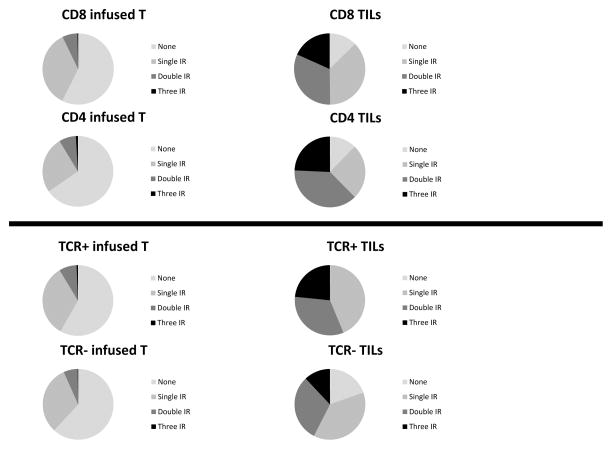
Additionally, we analyzed the frequency of cells that expressed more than one IR (Figure 4 and Supplemental Fig. 2). A clear increase in cells expressing multiple IRs was seen in the TILs versus the infused T cells (Fig. 4 upper panels). Interestingly, the percentage of cells expressing two or three IR positive cells was consistently higher on TCR+ TILs compared to TCR− TILs (Fig. 4, lower panels); comparing TCR+ TILs and TCR− TILs, 23% and 12% expressed all three IR, whereas of the infused TCR+ and TCR− cryopreserved T cells, only 0.6% and 0.2% expressed all three IR, respectively.
Figure 4. Expression of Multiple IRS on human T cells.
CD8, CD4, TCR+, and TCR− subsets of Ly95 TILs were analyzed to see the proportional makeup of TILs expressing single, double, triple, or no IRs compared to cryopreserved controls.
A) Expression profiles of injected CD8 and CD4 cells (cryo T cells) are compared to TILs. Both CD4 and CD8 TILs expressed more IRs than the cryo T cells.
B) Expression profiles of injected TCR+ and TCR negative cells (cryo T cells) are compared to TILs. TCR+ TILs had higher proportions of single IR, double IR, and triple IR TILs than TCR− TILs. Also, all TCR+ TILs has upregulation of at least one IR.
Repeated intraperitoneal injections of anti-human PD1 antibody augments the efficiency of Ly95 T cells in controlling the growth of A549-A2-ESO tumors, and preserves Ly95 TIL function
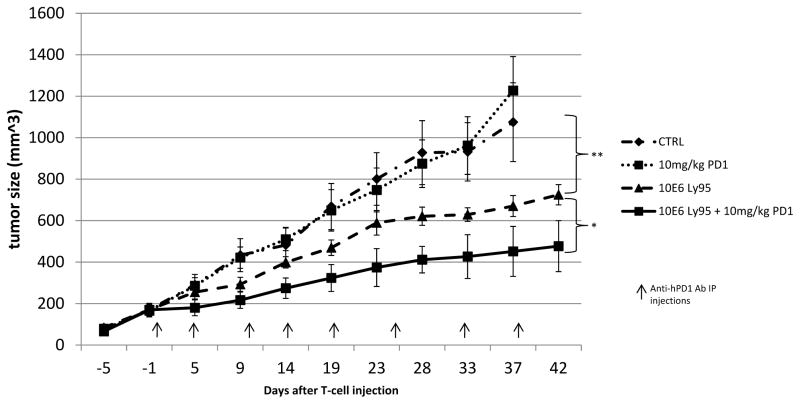
Given the increased levels of PD1 that we observed in hypofunctional TILs, we hypothesized that this upregulation had functional significance, and that blocking PD1 with an anti-human PD1 antibody might augment T cell function and anti-tumor efficacy. Accordingly, NSG with established A549-A2-ESO flank tumors were treated with both Ly95 T cells and repeated doses of anti-PD1 antibody. As shown in Figure 5, the anti-PD1 antibody alone had no effect on tumor growth compared to control, while Ly95 T cells significantly reduced tumor growth, as above. However, tumor-bearing mice that received repeated IP injections of PD1 Ab in addition to a single IV injection of 10 million Ly95 T cells, demonstrated the slowest rate of tumor growth for six weeks from the start of treatment. At the end of the experiment, the Ly95 T cell + PD1 Ab-treated group had tumors that were, on average, 35% smaller than those in the Ly95 T cell-treated group (p<0.05). This experiment was repeated with similar results.
Figure 5. Anti-PD1 antibody Augments the Efficacy of Ly95 T cells in vivo.
NSG mice (n= 10 per group) were injected in the flank with A549-A2-ESO tumor cells. After tumors were established and grew to about 200mm3, mice were injected with either saline, anti-PD1 at a dose of 10mg/kg every 5 days intraperitoneally, 1x107 Ly95 T-cells injected intravenously, or both anti-PD1 antibody and Ly95 T cells. Tumor volume was monitored. Anti-PD1 antibody had no effect by itself. Ly95 T cells were able to slow tumor growth compared to tumors in the control group (725mm3 Ly95 vs. 1074mm3 control, ** p<0.01). Anti-PD1 antibody was able to enhance tumor control when combined with Ly95 T cells (477mm3 vs. 725mm3, * p<0.05).
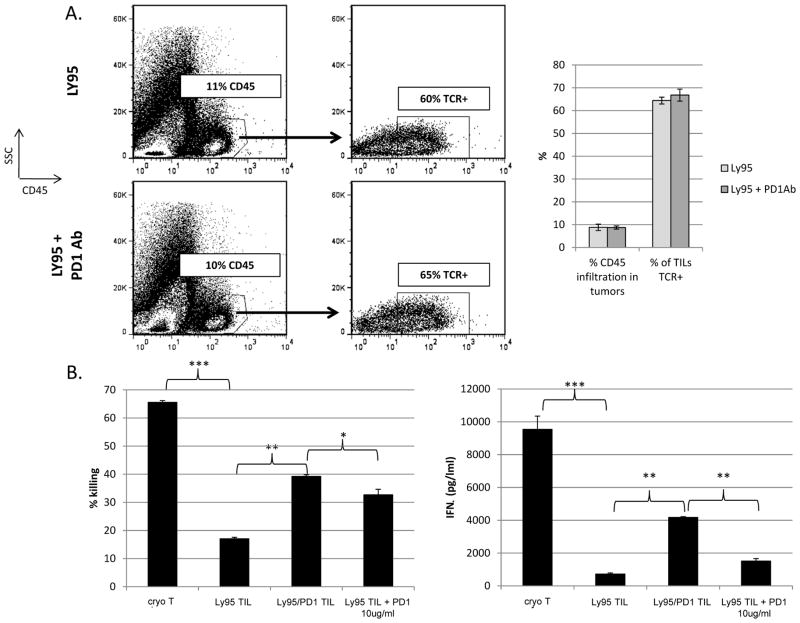
At this time point, the tumors were harvested/digested and processed into single-cell suspensions. After dead cell removal, FACS analysis revealed that the degree of T cell infiltration into the tumors and enrichment of TCR-engineered T cells was similar between Ly95 T cell and Ly95 T cell + PD1 Ab-treated mice (Fig. 6A). However, when Ly95 TILs were isolated and co-cultured with A549-A2-ESO tumor cells at 20:1 E:T ratio for 18hrs, TILs from Ly95 T cell + PD1 Ab-treated mice exhibited less hypofunction than those treated with Ly95 T cells in terms of improved killing (39% vs. 17%) (Fig 6B, left panel) and increased IFNγ secretion (4180 pg/ml vs. 720 pg/ml) (p<0.01) (Fig. 6B- right panel). TILs from Ly95 T cell-treated mice were also co-cultured ex vivo with A549-A2-ESO in the presence of 10ug/ml of PD1 Ab. The addition of PD1 Ab during the ex vivo killing assay led to partial recovery of Ly95 TIL function as measured by killing (33% vs. 17%, p<0.01) and IFNγ secretion (1510 pg/ml vs. 720 pg/ml, p<0.05) (Fig. 6B).
Figure 6. Analysis of TIL from the anti-PD1 animal study.
At Day 42 in the study shown in Figure 5, flank tumors from Ly95 and Ly95 + PD1 Ab treated mice were harvested, digested and processed into single-cell suspension.
A) FACS analysis using anti-hCD45 (upper panels) revealed equal degree of TIL infiltration. FACs analysis using the TCRVβ13.1 antibody, showed an equal percentage of Ly95 TCR+ cells in both groups. Dot-plots display representative analyses from experiment with pooled samples. Bar graph displays averaged results ± S.E. from independently experiment repeats analyzing pooled samples.
B) Purified TILs (see Figure 2 Legend) from each group were cocultured with A549-A2-ESO tumors at 20:1 E:T ratio for 18hrs. TILs from the Ly95 group demonstrated profound hypofunctional killing and cytokine secretion ability. TILs from the Ly95 + anti-PD1 antibody group demonstrated significantly better killing and cytokine secretion ability. When 10ug/ml of anti-human PD1 Ab was added to the coculture of Ly95 TIL, killing ability was partially restored (p<0.05). However, TIL function even when exposed to PD1 Ab was not has high as that of TILs isolated from Ly95 + PD1 Ab treated group (* p<0.05, ** p<0.01, *** p<0.001). Bar graphs display average values ± S.E. from triplicates.
Interestingly, the A549-A2-ESO tumor cells which upregulate PDL1 in response to IFNγ in-vitro (Supplemental Figure 3A) also increased PDL1 expression in-vivo when Ly95 T cells were injected with PD1 Ab. (Supplemental Figure 3B).
Discussion
Dysfunction of antigen-specific T cells infiltrating murine tumors is now well established (27). A number of investigators have documented that although TILs are often found in high numbers within tumors, they are usually hypofunctional upon isolation, with an inability to kill tumor cells or release cytokines. Key features of this hypofunction phenomenon in most models include a proximal TCR signaling defect and reversible dysfunction when they removed from the tumor microenvironment (27). Thus, it appears that tumor-induced hypofunction is distinct from what has been described in anergy, tolerance, senescence, or exhaustion where dysfunction is usually not reversible (28, 29).
Much less is known about human tumor-induced T cell dysfunction. A limited number of studies have functionally analyzed human TIL (30) and, in general, the findings of reversible TIL hypofunction seem similar (see Supplemental Table 1 for a summary). Our data are consistent with these findings. However, it should be noted that the mechanisms may be somewhat variable and perhaps tumor-specific. For example, in an examination of renal cell cancers, TILs were reversibly hypofunctional, but had normal proximal TCR signaling and faulty distal signaling with high DGK levels and decreased MAPK activation(31), whereas in an examination of melanoma tumors, TILs were irreversibly hypofunctional and demonstrated abnormal proximal TCR signaling, specifically downregulation of CD3ζ and Lck(32). In addition, PD1 signaling has been shown to be a significant contributing factor to TIL hypofunction in patients (including those with lung cancer and human TILs with NY-ESO-1 reactivity), similar to our model (33, 34).
Despite extensive investigation, the mechanisms responsible for tumor-induced T cell dysfunction have yet to be fully elucidated, although many factors have been implicated in murine models (see introduction). Given the complexity of this process and the importance of the tumor microenvironment, TIL hypofunction must be studied using intact tumor models, rather than cell culture or in vitro studies. Although experiments using murine tumor models have been important, it is clear that human and mouse T cells and tumors are very different, requiring tractable models of human TIL. To date, analysis of human TIL have been largely restricted to correlation analyses (35–38) or very short term ex vivo studies using digested tumor extracts (21, 31), thus significantly limiting possible experimental manipulations.
In this manuscript, we describe an experimental system that allows the long term study and manipulation of antigen-specific human T cells reacting with human tumor cells in an intact (although murine – see below) tumor microenvironment. To do so, we transduced activated human T cells with a modified transgenic TCR (already being used in clinical trials (39)) that targets the tumor antigen NY-ESO-1 in the context of HLA-A2 restriction on tumor cells. These effector T cells were highly active in vitro. After injection into immunosuppressed mice bearing large human tumors, the T cells infiltrated the tumors, persisted, and proliferated. They were able to significantly slow tumor growth, however, they did not induce actual tumor regression. Importantly, after the transgenic TILs were isolated and interrogated for functional analyses ex vivo, we found them to be very hypofunctional. That is, when compared to infusion product (cryopreserved) T cells, the TILs were significantly inhibited in their ability to kill target tumor cells and to secrete IFNγ. Importantly, this hypofunction was reversible by isolating the TILs away from tumor and “resting” them overnight. This phenotype is very similar to that of actual human TIL as described above.
We believe this model offers unique opportunities to study human TIL biology. First, it provides the ability to isolate large numbers of TILs in a controlled, reproducible laboratory setting. Many clinical studies have encountered difficulties in isolating sufficient TILs for analysis due to difficulty in obtaining sufficient quantities of tumor freshly harvested from the operating room. In addition, there is obviously a large amount of patient heterogeneity. Due to these issues, many previous patient studies have been limited to analyses of tumor-reactive T cells isolated from peripheral blood instead of from actual tumors(40, 41), an obviously suboptimal approach given the importance of the tumor microenvironment.
Second, since we are using T cells with a defined transgenic TCR, we can conduct ex vivo studies examining the reaction of the T cells to their known tumor antigen and look at their actual tumor cell killing ability, as well as stimulation of the T cells (i.e. cytokine release and CD107a upregulation). This is in contrast to the vast majority of patient-derived TIL studies which rely on non-physiologic stimulation of TIL using agents such as CD3/CD28 antibodies or phorbol ester/ionomycin and can only examine surrogate markers of T cell killing such as IFNγ secretion or CD107a upreguation by TILs.
Third, we can isolate tumor-reactive versus non-tumor reactive T cells from within the same tumor microenvironment. Since patient TILs are polyclonal with varying degrees of reactivity to the tumor, further analysis of the specific tumor-reactive TIL population is very difficult. In our model, we have just two distinct populations—tumor reactive T cells assessed by TCRVβ13.1 positivity (the majority of the TILs due to enrichment in the tumor) or tumor non-reactive T cells (TCRVβ13.1 negative cells). Using flow cytometry, within one tumor sample, we can thus compare the phenotype of TCR-engineered TILs and compare them to TILs which do not bear the TCR transgene by flow cytometry. Isolation and genetic analysis of these populations is also possible.
Finally, and perhaps most importantly, a major opportunity of this model is the ability to study and manipulate the adoptively transferred human T cells within the tumor microenvironment over time, using anti-human directed reagents. Unlike observational human TIL studies, it is possible to alter the T cells themselves via genetic manipulation at the time of transduction or to administer various agents that affect the tumor microenvironment and/or the T cells themselves. As an example, we describe studies using an anti-human antibody directed to the immune inhibitory receptor PD1 (see below). However, ongoing studies in our lab using shRNA, dominant negative constructs, and other antibodies are examining the effects of other types of IR’s, chemokine receptors, phosphatases (such as SHP-1), T cell enzymes (DGK and protein kinase A), and transcription factors (Ikaros). Manipulations of the tumor-microenvironment can also be done, either prior to T cell transfer, during T cell transfer, or after T cell infiltration to tumor. For example, we are studying the effects of cyclooxygenase inhibition or targeting tumor-associated fibroblasts to breakdown tumor stroma (42) prior to transfer of tumor reactive T cells.
In this manuscript, we utilized our model to study cell surface T cell inhibitory receptors. IRs operate at multiple levels to ensure appropriate T cell homeostasis, activation, and differentiation(43). Many studies have demonstrated significant upregulation of IRs on human TILs that negatively impact their anti-tumor function (44, 45). More recently, studies have shown that Lag3 and PD1 can be co-expressed on tolerized TILs, suggesting that they may contribute to tumor-induced immune suppression(33, 46). Consistent with these data from human TIL, when we compared the phenotype of the hypofunctional Ly95 CD4 and CD8 TILs with the infused, cryopreserved Ly95 T cells, there was significant upregulation of surface expression of PD1, Tim3, and Lag3 (Fig. 3). In addition, a significant percentage of TILs, particularly TCR+ TILs, had upregulation of more than one IR, with 25% of TCR+ TILs and 12% of TCR− TILs expressing PD1, Tim3, and Lag3 (Fig. 4). Of direct relevance to our model, Matsuzaki et al. showed that NY-ESO-1 reactive TILs from patients with ovarian cancer had high expression of both Lag3 and PD1 and were blunted in their ability to secrete cytokines(33).
Our model also allowed us to extend these observations and pursue a number of more novel and interesting questions. For example, we could study if the increases in PD1 and Lag3 expression were due to chronic TCR activation or external microenvironmental influences. We were able to address this question by using flow cytometry to compare the IR expression on the TILs expressing the TCR transgene versus those that did not in the same tumor. As shown in Figure 3, all of the IRs increased in both the TCR-expressing (TCRVβ13.1+) TILs and the TCR− T cells, especially for Lag3 and Tim3. This suggests that the overall tumor microenvironment plays an important role. In contrast, the increase in PD1 was more prominent in the TCR-expressing (TCRVβ13.1+) TILs, indicating a more important role for chronic antigen stimulation for this IR. These data thus suggest that IR upregulation is a complex process that involves intrinsic factors such as stimulation of the TCR on the T cell, as well as microenvironmental factors (i.e. cytokines).
A second question that we were able to address in our model was the functional importance of TIL PD1 expression. As shown in Figure 6, the addition of an anti-PD1 antibody to our ex vivo hypofunctional TILs was able to partially restore function. More importantly, we were able to test the effects of repeated injections of an anti-human PD1 antibody in animals in combination with the Ly95 T cells. As we hypothesized, injections of anti-PD1 antibody significantly augmented tumor control by the single injection of 10 million Ly95 T cells by over 30%. This finding is similar to what was published recently by John et al. in a model of murine ATC(47), however, to our knowledge, this is the first description of TCR-engineered human T cells being augmented by IR antibody blockade against a human cancer model. Mechanistically, although we did not note increased numbers of TILs within the treated tumors, we did observe that when isolated, the TILs from the anti-PD1 antibody-treated mice had enhanced ex vivo killing and cytokine function (Fig. 6).
These findings have some interesting translational implications for human IR therapy. Most importantly, our data support clinical trials using IR antibodies in human T cell transfer approaches. This could apply to T cells expressing the same TCR used in our study, as NY-ESO-1-targeted transgenic T cells have shown promise in trials of sarcomas (13) and NY-ESO-1 is also expressed in up to 30% of lung cancer specimens (48). IR therapy could also be of value in other transgenic TCR approaches, for TIL therapy, and for chimeric antigen receptor-based therapies. However, the fact that anti-PD1 antibody augmented the slowing of tumor growth, but did not induce tumor regression, supports the idea that the TME-induced immunosuppression is multifactorial, and blockade of other inhibitory pathways or other inhibitory factors in a combinatorial fashion will need to be tested. Finally, our data suggest that analysis of the TILs, rather than just the tumor cells, may be useful in determining the choice of adjuvant IR blocking therapy and predicting response to therapy. In this animal model, we would predict that blockade of Lag3 and Tim3 might show added benefits. We have preliminary data that support this approach. This is consistent with other studies that have demonstrated that hypofunctional TILs express more than one IR(33, 44) and blockade of multiple IRs is needed to achieve optimal anti-tumor effects(45, 49)
Limitations of the model must also be acknowledged. The NSG mice lack a complete immune system, thus limiting the ability to study the effects of important tumor immune cells such at T-regulatory cells, NK cells, dendritic cells, B cells, and some myeloid cells. The innate immune cells present in NSG mice are not isologous with the human T cells and tumor cells. This could result in some species non-compatibility issues (i.e. some ligands in the murine microenvironment might not react with human T cell receptors). For example, murine inteferons are not able to effect human tumor or T cells. The adoptive transfer of other human cell types (i.e. T-regs) or the use of mice with more fully “humanized” immune systems may be advantageous. Additionally, non-tumor-derived signals (even if murine) could potentially influence adoptively transferred human T cells as supported by descriptions of xenoreactive/homeostatic signals that can affect adoptively transferred cells.(50, 51) Although possible, there is data to support the observation that the upregulation of IR expression on the TILs are due to the tumor microenvironment and not just to non-tumor xenoreactive/homeostatic signals. One piece of data is that the the TCR-positive TILs had much higher proportions of double and triple IR positive cells than the TCR-negative TILs (Figure 4.) Perhaps even stronger data is that the infiltrating human lymphocytes isolated from the mouse spleens (which were subject to the same xenoreactive and homeostatic expansion as the tumor TILs) show much less IR upregulation than do the TILs. (Supplemental Figure 4.)
An area that have not yet explored is the issue of tumor heterogeneity. Response to adoptively transferred T cell therapy appears to vary among tumor types, even when they express the same targeted tumor associated antigen. (13, 39) Response to checkpoint blockade also varies among different tumors. (52) It will thus be of interest to compare T cell efficacy, hypofunction, and IR upregulation among different tumors. To address this, we are currently transfecting other human tumor cell lines with HLA-A2 and NY-ESO-1. We will then compare the degree of TIL hypofunction, expression of various inhibitory receptors, and responses to anti-PD1 and other antibodies.
In summary, despite these potential limitations, the human Ly95 TILs isolated from the human tumors do seem to strongly resemble human TIL suggesting that many of the key factors that induce T cell hypofunction are present, without these other cell types. We thus believe the model will be useful in studying human adoptive T cell transfer and further understanding the biology of tumor-reactive TILs that signal through an endogenous TCR. The model should also serve as an efficient and straightforward platform for preclinical testing of new human specific immunotherapeutics prior to transition to the bedside.
Supplementary Material
Statement of Translational Significance.
Adoptive T cell transfer (ATC) holds promise for cancer therapeutics, however solid tumors pose significant hurdles for ATC, including the upregulation of inhibitory receptors (IRs) on T cells after successful infiltration into tumor. Robust preclinical models are needed to study the IR expression pattern on tumor infiltrating lymphocytes (TILs) and test the effects of tailored blockade of IRs on anti-tumor activity of tumor reactive T cells. We present such a model, where tumor-reactive (via TCR engineering) human T cells targeting human solid tumor successfully infiltrate into established tumors, but demonstrate hypofunction due, at least in part, to the upregulation of IRs (e.g. PD1, Tim3, Lag3), with PD1 being significantly upregulated on TCR engineered TILs, especially those engineered with enhanced tumor reactivity. We further describe the ability to augment the activity of transferred engineered T cells by combining with injected anti-PD1 antibody.
Acknowledgments
The authors gratefully acknowledge Drs. Linda Snyder and Raluca Verona of Janssen R&D for providing the anti-human PD1 antibody used for in vivo experiments.
Grant Support:
The research was supported by funding from a K08 (K08 CA163941-04) from the National Cancer Institute (NIH)
Footnotes
The authors have declared that no conflict of interest exists.
References
- 1.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.June CH, Maus MV, Plesa G, Johnson LA, Zhao Y, Levine BL, et al. Engineered T cells for cancer therapy. Cancer Immunol Immunother. 2014;63:969–75. doi: 10.1007/s00262-014-1568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123:2625–35. doi: 10.1182/blood-2013-11-492231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brocker T. Chimeric Fv-zeta or Fv-epsilon receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood. 2000;96:1999–2001. [PubMed] [Google Scholar]
- 5.Geiger TL, Leitenberg D, Flavell RA. The TCR zeta-chain immunoreceptor tyrosine-based activation motifs are sufficient for the activation and differentiation of primary T lymphocytes. J Immunol. 1999;162:5931–9. [PubMed] [Google Scholar]
- 6.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–6. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 7.Schumacher TN. T-cell-receptor gene therapy. Nat Rev Immunol. 2002 Jul;2(7):512–9. doi: 10.1038/nri841. [DOI] [PubMed] [Google Scholar]
- 8.Chodon T, Comin-Anduix B, Chmielowski B, Koya RC, Wu Z, Auerbach M, et al. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin Cancer Res. 2014;20:2457–65. doi: 10.1158/1078-0432.CCR-13-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang G, Wang L, Cui H, Wang X, Ma J, Han H, et al. Anti-melanoma activity of T cells redirected with a TCR-like chimeric antigen receptor. Sci Rep. 2014;4:3571. doi: 10.1038/srep03571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinnasamy N, Wargo JA, Yu Z, Rao M, Frankel TL, Riley JP, et al. A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J Immunol. 2011;186:685–96. doi: 10.4049/jimmunol.1001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol. 2005;174:4415–23. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620–6. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–24. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher DT, Chen Q, Appenheimer MM, Skitzki J, Wang WC, Odunsi K, et al. Hurdles to lymphocyte trafficking in the tumor microenvironment: implications for effective immunotherapy. Immunol Invest. 2006;35:251–77. doi: 10.1080/08820130600745430. [DOI] [PubMed] [Google Scholar]
- 15.Chappert P, Schwartz RH. Induction of T cell anergy: integration of environmental cues and infectious tolerance. Curr Opin Immunol. 2010;22:552–559. doi: 10.1016/j.coi.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 17.Baitsch L, Legat A, Barba L, Fuertes Marraco SA, Rivals JP, Baumgaertner P, et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS One. 2012;7:e30852. doi: 10.1371/journal.pone.0030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jotereau F, Gervois N, Labarriere N. Adoptive transfer with high-affinity TCR to treat human solid tumors: how to improve the feasibility? Target Oncol. 2012;7:3–14. doi: 10.1007/s11523-012-0207-z. [DOI] [PubMed] [Google Scholar]
- 19.Kunert A, Straetemans T, Govers C, Lamers C, Mathijssen R, Sleijfer S, et al. TCR-Engineered T Cells Meet New Challenges to Treat Solid Tumors: Choice of Antigen, T Cell Fitness, and Sensitization of Tumor Milieu. Front Immunol. 2013;4:363. doi: 10.3389/fimmu.2013.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J, et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res. 2014;20:4262–73. doi: 10.1158/1078-0432.CCR-13-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reichert TE, Strauss L, Wagner EM, Gooding W, Whiteside TL. Signaling abnormalities, apoptosis, and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin Cancer Res. 2002;8:3137–45. [PubMed] [Google Scholar]
- 22.Zhang Y, Huang S, Gong D, Qin Y, Shen Q. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol. 2010;7:389–95. doi: 10.1038/cmi.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–85. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 24.Robbins PF, Li YF, El-Gamil M, Zhao Y, Wargo JA, Zheng Z, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180:6116–31. doi: 10.4049/jimmunol.180.9.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–9. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 26.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–5. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vazquez-Cintron EJ, Monu NR, Frey AB. Tumor-induced disruption of proximal TCR-mediated signal transduction in tumor-infiltrating CD8+ lymphocytes inactivates antitumor effector phase. J Immunol. 2010;185:7133–40. doi: 10.4049/jimmunol.1001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25:214–21. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiou SH, Sheu BC, Chang WC, Huang SC, Hong-Nerng H. Current concepts of tumor-infiltrating lymphocytes in human malignancies. J Reprod Immunol. 2005;67:35–50. doi: 10.1016/j.jri.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Prinz PU, Mendler AN, Masouris I, Durner L, Oberneder R, Noessner E. High DGK-alpha and disabled MAPK pathways cause dysfunction of human tumor-infiltrating CD8+ T cells that is reversible by pharmacologic intervention. J Immunol. 2010;188:5990–6000. doi: 10.4049/jimmunol.1103028. [DOI] [PubMed] [Google Scholar]
- 32.Rabinowich H, Banks M, Reichert TE, Logan TF, Kirkwood JM, Whiteside TL. Expression and activity of signaling molecules in T lymphocytes obtained from patients with metastatic melanoma before and after interleukin 2 therapy. Clin Cancer Res. 1996;2:1263–74. [PubMed] [Google Scholar]
- 33.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–80. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang SF, Fouquet S, Chapon M, Salmon H, Regnier F, Labroquere K, et al. Early T cell signalling is reversibly altered in PD-1+ T lymphocytes infiltrating human tumors. PLoS One. 2011;6:e17621. doi: 10.1371/journal.pone.0017621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–8. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma C, Zhang Q, Ye J, Wang F, Zhang Y, Wevers E, et al. Tumor-infiltrating gammadelta T lymphocytes predict clinical outcome in human breast cancer. J Immunol. 2012;189:5029–36. doi: 10.4049/jimmunol.1201892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuda M, Petersson M, Lenkei R, Taupin JL, Magnusson I, Mellstedt H, et al. Alterations in the signal-transducing molecules of T cells and NK cells in colorectal tumor-infiltrating, gut mucosal and peripheral lymphocytes: correlation with the stage of the disease. Int J Cancer. 1995;61:765–72. doi: 10.1002/ijc.2910610605. [DOI] [PubMed] [Google Scholar]
- 38.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robbins PF, Kassim SH, Tran TL, Crystal JS, Morgan RA, Feldman SA, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T cell receptor: Long term follow up and correlates with response. Clin Cancer Res. 2015;21:1019–27. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osanto S, Schiphorst PP, Weijl NI, Dijkstra N, Van Wees A, Brouwenstein N, et al. Vaccination of melanoma patients with an allogeneic, genetically modified interleukin 2-producing melanoma cell line. Hum Gene Ther. 2000;11:739–50. doi: 10.1089/10430340050015635. [DOI] [PubMed] [Google Scholar]
- 41.Germeau C, Ma W, Schiavetti F, Lurquin C, Henry E, Vigneron N, et al. High frequency of antitumor T cells in the blood of melanoma patients before and after vaccination with tumor antigens. J Exp Med. 2005;201:241–8. doi: 10.1084/jem.20041379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang LC, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res. 2014;2:154–66. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grosso JF, Goldberg MV, Getnet D, Bruno TC, Yen HR, Pyle KJ, et al. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J Immunol. 2009;182:6659–69. doi: 10.4049/jimmunol.0804211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19:5636–46. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 48.Jungbluth AA, Chen YT, Stockert E, Busam KJ, Kolb D, Iversen K, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–60. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 49.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alcantar-Orozco EM, Gornall H, Baldan V, Hawkins RE, Gilham DE. Potential limitations of the NSG humanized mouse as a model system to optimize engineered human T cell therapy for cancer. Hum Gene Ther Methods. 2013;24:310–20. doi: 10.1089/hgtb.2013.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volk A, Hartmann S, Muik A, Geiss Y, Konigs C, Dietrich U, et al. Comparison of three humanized mouse models for adoptive T cell transfer. J Gene Med. 2012;14:540–8. doi: 10.1002/jgm.2652. [DOI] [PubMed] [Google Scholar]
- 52.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.