Abstract
Background
The blood brain barrier (BBB) is critical for maintaining central nervous system (CNS) homeostasis by restricting entry of potentially toxic substances. However, the BBB is a major obstacle in the treatment of neurotoxicity and neurological disorders due to the restrictive nature of the barrier to many medications. Intranasal delivery of active enzymes to the brain has therapeutic potential for the treatment of numerous CNS enzyme deficiency disorders and CNS toxicity caused by chemical threat agents.
New method
The aim of this work is to provide a sensitive model system for analyzing the rapid delivery of active enzymes into various regions of the brain with therapeutic bioavailability.
Results
We tested intranasal delivery of chloramphenicol acetyltransferase (CAT), a relatively large (75 kD) enzyme, in its active form into different regions of the brain. CAT was delivered intranasally to anaesthetized rats and enzyme activity was measured in different regions using a highly specific High Performance Thin Layer Chromatography (HP-TLC)-radiometry coupled assay. Active enzyme reached all examined areas of the brain within 15 min (the earliest time point tested). In addition, the yield of enzyme activity in the brain was almost doubled in the brains of rats pre-treated with matrix metalloproteinase-9 (MMP-9).
Comparison with existing method (s)
Intranasal administration of active enzymes in conjunction with MMP-9 to the CNS is both rapid and effective.
Conclusion
The present results suggest that intranasal enzyme therapy is a promising method for counteracting CNS chemical threat poisoning, as well as for treating CNS enzyme deficiency disorders.
Keywords: glymphatic system, catalytic bioscavenger, bioavailability, organophosphate chemical threat agents, enzyme deficiency diseases
1. Introduction
A major obstacle in the treatment of neurotoxicity caused by chemical threat agents (CTA) and neurological disorders resulting from enzyme deficiencies is the inability of active bio-scavengers or catalytically active enzymes to transverse the BBB. Researchers with promising concepts have been forced to abandon research into drugs with high therapeutic potential due to their inability to cross the BBB in therapeutic concentrations (Djupesland, Messina et al., 2014). The intranasal route of administration is associated with a number of advantages over systemic administration including rapid CNS bioavailability, early onset of effects and a growing record of success with approved formulations. Intranasal delivery has been the most successful non-invasive, alternative route of entry into the brain for substances that cannot traverse the BBB (Lochhead and Thorne, 2012). An additional advantage of intranasal administration of drugs and proteins to the brain includes the avoidance of first-pass hepatic metabolism. Research into whether the intranasal route might deliver potentially therapeutic amounts of larger biologics such as proteins to the CNS was first described two decade ago (Thorne, Emory et al., 1995). Thorne and coworkers proposed that intranasal delivery of solutes to the brain occurs through pathways associated with the olfactory and trigeminal nerves. Solutes applied to the nasal epithelium would be transported to the olfactory bulb and brainstem, respectively, before the distribution to other CNS areas (Thorne, Hanson et al., 2008; Thorne, Pronk et al., 2004). This distribution throughout the brain is thought to be accomplished through paravascular spaces between vascular endothelial cells and the surrounding astrocyte sheath which allows bulk flow of fluid and solutes. This system has been termed the glymphatic system because of the involvement of astrocytes and the functional similarities to the lymphatic system (Iliff, Wang et al., 2012). Recently, Lochhead et al., (Lochhead, Wolak et al., 2015) proposed that bulk flow within these paravascular compartments is responsible for the entry of therapeutics to olfactory bulbs and brainstem, and the subsequent distribution to the rest of the brain.
A number of reports have demonstrated protein uptake into brain parenchyma after intranasal administration. Small proteins such as insulin (~5.8 kD) (Frey, 2013), insulin-like growth factor 1 (~7.6 kD) (Liu, Fawcett et al., 2001), and nerve growth factor (~26.5 kD) (Zhu, Cheng et al., 2011) have been successfully delivered to the brain by the intranasal route, but larger proteins do not gain access to brain parenchyma as readily. Studies comparing the movement of fluorescent tracers with molecular masses of 3 kD and 10 kD showed that compounds within this size range can gain access to brain parenchyma after intranasal administration, and that the use of the extracellular matrix degrading enzyme MMP-9 increased uptake of both compounds into the brain (Lochhead, Wolak et al., 2015). MMP-9 is a zinc-dependent endopeptidase responsible for both physiological and pathophysiological tissue remodeling and plays a major role in the degradation of the extracellular matrix. Reports for successful intranasal delivery of active enzymes to brain parenchyma are scanty. One study reported that intranasal delivery of an active enzyme to the brain was accomplished when administering α-L-iduronidase, an 85 kD lysosomal enzyme, to mice deficient in the enzyme (Wolf, Hanson et al., 2012). Enzyme activity was detectable in the brains of the enzyme deficient mice. Whether the enzyme reached all areas of the brain remained unclear.
Our objective was to provide a simple model system for studying intranasal delivery of enzymes to the brain in their active form. We selected the well-characterized bacterial enzyme chloramphenicol acetyltransferase (CAT), which functions as a trimer with a molecular weight of 75 kD. The mammalian brain does not express significant CAT-like activity (McMahon, Novak et al., 1984), making this enzyme a good candidate for intranasal administration and bioavailability studies. We also tested the effect of intranasal pretreatment with MMP-9 to augment epithelial permeability.
An emerging use for catalytic bio-scavenging enzymes is in the detoxification of organophosphate CTAs as a countermeasure to protect the CNS from excess cholinergic activation after the CTA poisoning (Worek, Seeger et al., 2014). However, intravenous administration of active organophosphate degrading enzymes will not deliver them to brain parenchyma, thus limiting their efficacy in protecting the CNS. We developed the current method of intranasal delivery of active enzyme to the CNS in order to improve our understanding of enzyme bioavailability via this route with the future goal applying this strategy as an adjunct therapy for CTA poisoning.
2. Materials and methods
2.1. Reagents
CAT (Cat No-C2900-25KU) and acetyl-CoA were purchased from Sigma Aldrich (St. Louis, MO). MMP-9 was purchased from Sino Biologicals (Beijing, China). [14C]-labelled chloramphenicol (Cat No- ARC 0401) was obtained from American Radiolabeled Chemicals (St. Louis, MO). High performance thin layer chromatography (HP-TLC) plates were obtained from Merck and phosphor imaging plates were purchased from GE Healthcare Life Sciences (Pittsburgh, PA). Other chemicals were obtained from Sigma Aldrich. The aerosol propelled precision olfactory delivery device used for intranasal administration was provided by Impel NeuroPharma Inc. (Seattle, WA).
2.2. Animals
Male Sprague-Dawley rats were obtained from Taconic Biosciences (Hudson, NY). The animals were allowed free access to food and water, and all procedures were performed in accordance with guidelines of the National Institutes of Health and the animal protocol was approved by Institutional Animal Care and Use Committee of the Uniformed Services University of the Health Sciences, Bethesda, MD.
2.3. Treatment
Weight matched (250 ± 50 g) male rats were assigned to five groups: control rats, CAT-15 min, CAT+MMP-9–15 min, CAT-30 min and CAT+MMP-9–30 min with 3 animals per group. Animals were placed in an anesthetic chamber for 5 min at 5% isoflurane and they were then placed in supine position and were administered lyophilized CAT diluted in 100 mM Tris-HCl (pH = 7.0) and MMP-9 diluted in saline using the olfactory delivery device. The tip of the device was inserted 8–10 mm into the rat’s nostril, angled toward the olfactory epithelium and propellant in the device was discharged for one second expelling the solution onto the olfactory epithelium. The time of administration was recorded as the time at which the animals received the dose to both nostrils. The animals in the CAT-15 min and CAT-30 min groups received 10µl diluted enzyme in each nostril (total 20µl/animal, the total applied enzyme activity per animal was 2941 IU). Control animals were treated intranasally with saline. The animals in the CATMMP-9–15 min and CAT-MMP-9–30 min groups also received same dose of enzyme 30 min after intranasal treatment with total 20 µl of MMP-9 (2 nmol/animal).
2.4. Tissue collection
After the respective time periods animals were anesthetized with 5% isoflurane for 5 min and euthanized by decapitation. Brains were rapidly dissected on ice into specific regions including olfactory bulb (OB), frontal cortex (FC: including frontal cortex and striatum), brainstem (BS; including medulla and pons), cerebellum (CB), midbrain (MB; including mesencephalon and thalamus), hippocampus (HP), and cortex, (CT; including parietal, occipital and external temporal cortex), and the samples were rapidly frozen on dry ice and stored at −80 °C until analyzed.
2.5. Radiometric assay of CAT activity
CAT enzyme activity in different brain regions was assayed by an HP-TLC procedure, utilizing C−14 labelled chloramphenicol as the substrate. The CAT assay was adapted from that of Gorman et al. (Gorman, Moffat et al., 1982), with modifications from Crabb and Dixon (Crabb and Dixon, 1987). Samples from the dissected brain areas (OB, FC, BS, CB, MB, HP and CT) were sonicated separately in 100 mM Tris-HCl (pH-7) ice cold buffer to make a 20% homogenate. Homogenates were centrifuged at 5000 rpm for 5 min and the supernatants were collected. The assay mixture contained 50µl of supernatant in 100µl of Tris HCl buffer with 10 µl of 1 µCi [14C]-chloramphenicol and incubated at 60°C for 30 min to eliminate any endogenous CAT-like activity and thus reduce the background. Bacterial CAT is resistant to heat inactivation at this temperature. Then 30 µl of 1 mM acetyl-CoA was added and incubations were continued at 37 °C for 1 hr. Standards contained 2 µl of CAT (294.1 IU) in place of tissue homogenates. After incubation the reactions were stopped and acetylated chloramphenicol was analyzed by adding 50 µl ethyl acetate to the reaction mixtures, and 40 µl of the ethyl acetate containing fractions were spotted on HP-TLC plates and allowed to separate with chloroform and methanol (9:1) as the mobile phase. After separation, the HP-TLC plates were exposed to radiation-sensitive phosphor imaging plates and the intensity of the acetylated chloramphenicol bands were measured using a Fujifilm FLA-5100 Phosphorimeter (Fujifilm Medical System, Stamford, CT). Enzyme activity was calculated in different brain areas by measuring the intensity of the mono-acetylated derivative of chloramphenicol with corresponding intensity of the standard enzyme reaction mixture. One di-acetylated and two mono-acetylated (C1 and C3 positions) products were detected under the assay conditions. We focused on the major monoacetylated form (C-3’ position) for our analysis (McMahon, Novak et al., 1984). We also determined background activities of endogenous acetyltransferases in brain homogenates by using samples from control rats who were administered saline intranasally instead of the active enzyme.
2.6. Statistical analysis
The results were analyzed using SPSS/PC+, Version 22 (SPSS Inc.). Values were expressed as means + SD. Student’s t-tests (2-tailed, unequal variance) were used to determine significance, and p ≤ 0.05 was considered to be significant.
3. Results
We have analyzed the levels of CAT enzymatic activity, delivered by intranasal administration, in seven regions of the rat brain by HP-TLC coupled with radiometric assays. We observed four major bands on the HP-TLC plates representing non-acetylated chloramphenicol, two bands of mono-acetylated chloramphenicol due to the acetylation of the 1’ and 3’ carbon positions, as well as di-acetylated chloramphenicol. These findings demonstrate that the active enzyme was transported to the brain where it remained active throughout the tissue processing and assay procedures.
3.1. CAT activity at 15 minutes
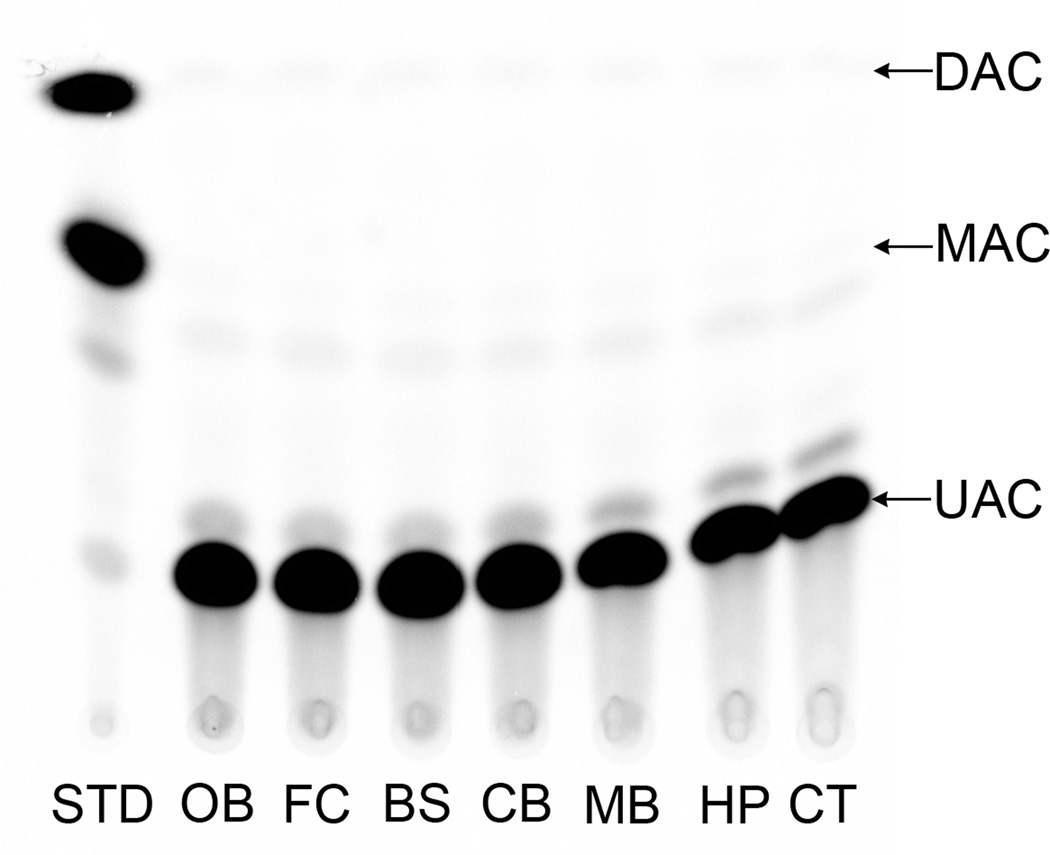
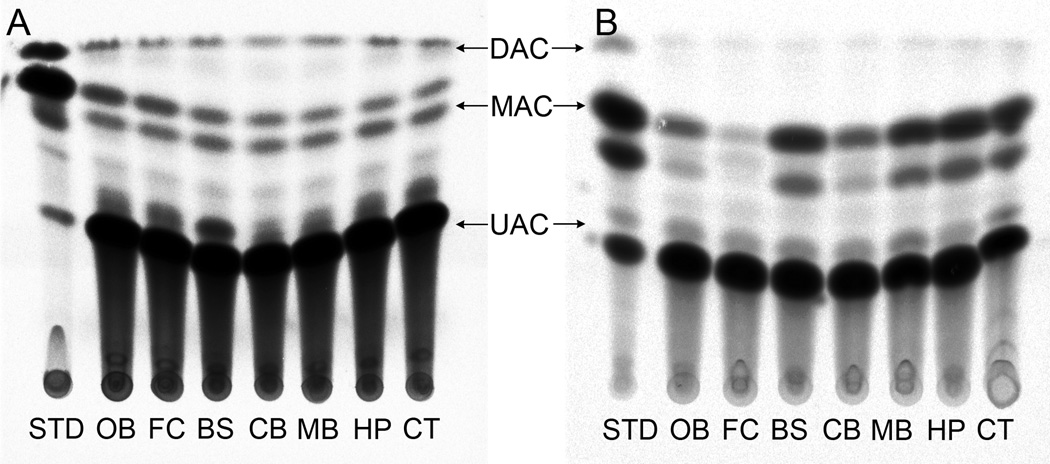
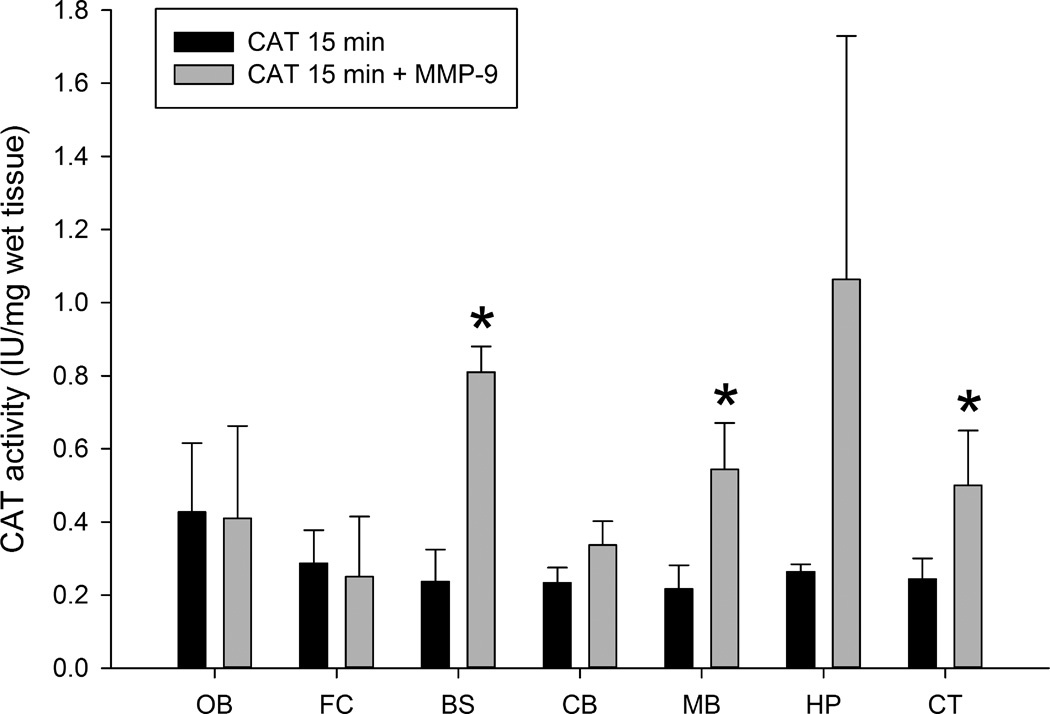
We did not observe endogenous CAT-like activity in control animals under the described assay conditions. No significant CAT activity was detected in any of the brain regions tested from the control group (Fig. 1). However, we observed radiolabeled product (mono and di-acetylated chloramphenicol) resulting from CAT enzymatic activity in all brain regions in animals treated intranasally with both CAT-15 min and CAT+MMP-9–15 min groups (Figs. 2 & 3) and higher activity was found in combination with MMP-9 than CAT alone. The increase in detectable enzyme activity with MMP-9 at the 15 min time point was significant in the brainstem, midbrain, and cortex. The yield of CAT activity was 16 ± 1.98% and 32 ± 7% with respect to total administered enzyme activity per animal in CAT-15 min and CAT+MMP-9–15 min groups respectively. In short, enzyme activity was detected in all brain regions tested, and the total yield of enzyme activity was almost doubled in CAT-MMP-9–15 min group. We note that this yield may be an overestimate since the yield was calculated based on both high and low enzyme activities measured under a less than optimized assay conditions.
Figure 1.
Image of a representative HP-TLC plate showing minimal endogenous CAT activity in control samples. The UAC band represents un-acetylated chloramphenicol, MAC represents mono-acetylated chloramphenicol and DAC is di-acetylated chloramphenicol. Abbreviations: OB = olfactory bulb, FC = frontal cortex, BS = brainstem, CB = cerebellum, MB = midbrain, HP = hippocampus and CT = cortex.
Figure 2.
Activity of CAT in different brain areas at the 15 min time point. Fig. 2A shows schematic representation of a HP-TLC plate from the CAT-15 min group and Fig. 2B shows a plate from the CAT+MMP-9–15 min group. The UAC band represents un-acetylated chloramphenicol, the MAC band represents mono-acetylated chloramphenicol and DAC is diacetylated chloramphenicol. Abbreviations: OB = olfactory bulb, FC = frontal cortex, BS = brainstem, CB = cerebellum, MB = midbrain, HP = hippocampus and CT = cortex.
Figure 3.
Comparative levels of CAT activity in different brain regions in samples from the CAT 15 min and CAT 15 min+MMP-9 groups expressed in IU/mg wet tissue. Abbreviations: OB = olfactory bulb, FC = frontal cortex, BS = brainstem, CB = cerebellum, MB = midbrain, HP = hippocampus and CT = cortex. All values are expressed as means ± SD with n = 3 rats per group (*significant difference between CAT-15 min and CAT+MMP-9–15 min groups; p ≤ 0.05).
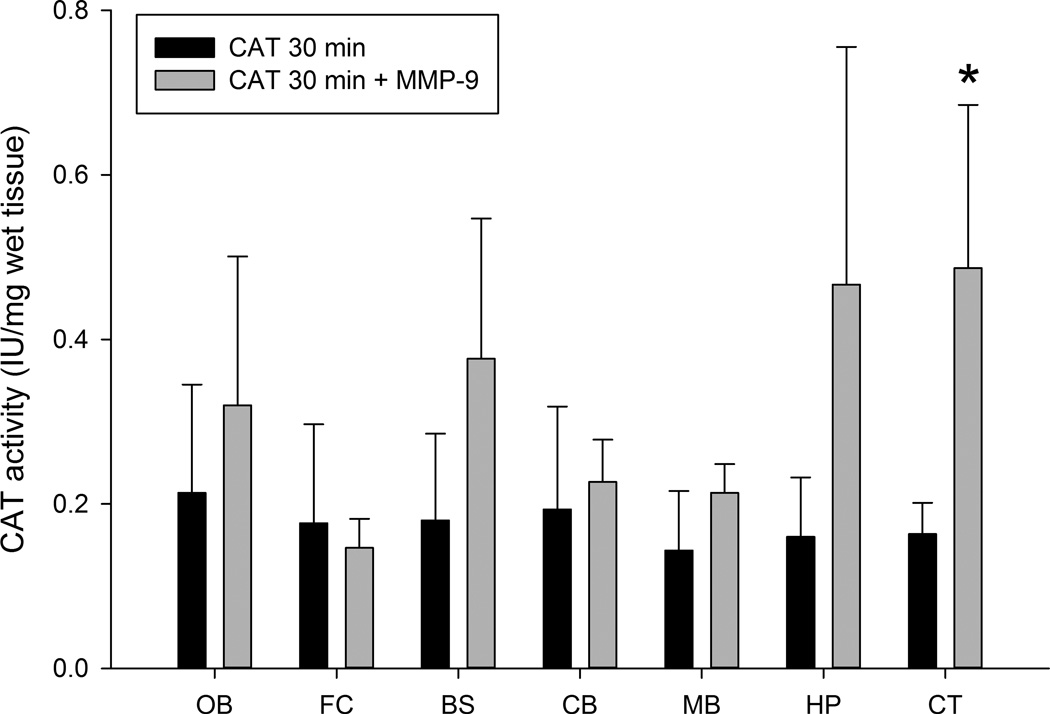
3.2. CAT activity at 30 minutes
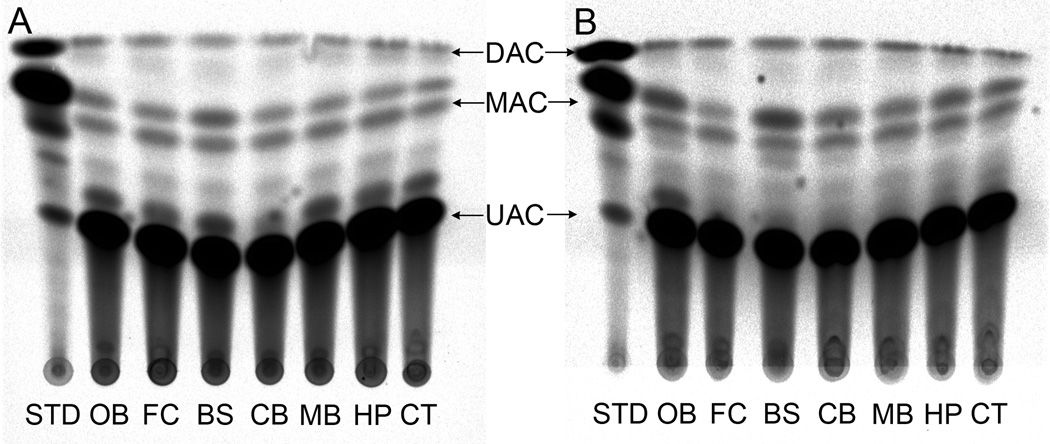
We found that significant activity of CAT in all brain areas tested in the CAT-30 min group, with an increase of activity in several regions from the MMP-9 treated groups. However, the increase only reached significance in the cortex (Figs 4 and 5). In comparison with the 15 min time point there was a decrease in the activity of CAT in both 30 min groups. The yield of enzyme activity in whole brain of CAT-30 min and CAT+MMP-9–30 min groups were approx. 11.42 ± 2.78% and 23 ± 5.3% respectively with respect to the total enzyme delivered per animal.
Figure 4.
Activity of CAT in different brain areas at the 30 min time point. Fig. 4A shows schematic representation of a HP-TLC plate from the CAT-30 min group and Fig. 4B shows a plate from the CAT+MMP-9–30 min group. The UAC band represents un-acetylated chloramphenicol, the MAC band represents mono-acetylated chloramphenicol and DAC is diacetylated chloramphenicol. Abbreviations: OB = olfactory bulb, FC = frontal cortex, BS = brainstem, CB = cerebellum, MB = midbrain, HP = hippocampus and CT = cortex.
Figure 5.
CAT activity in different regions of the brain of CAT-30 min and CAT+MMP-9–30 min groups and expressed in IU/mg wet tissue. Abbreviations: OB = olfactory bulb, FC = frontal cortex, BS = brainstem, CB = cerebellum, MB = midbrain, HP = hippocampus and CT = cortex. All values are expressed as means ± SD with n = 3 rats per group (*significant difference between CAT-30 min and CAT+MMP-9–30 min groups; p < 0.05).
Overall results showed that intranasally delivered, enzymatically active CAT was delivered to all areas of brain tested in both groups. The activity and yield of CAT at the 15 min time point was significantly increased in brain in the presence of MMP-9, in brainstem, midbrain, and cortex. The yield was approximately doubled in both MMP-9 pre-treated 15 and 30 min groups and achieved about 32 ± 7.1% and 23 ± 5.3% respectively. CAT activity was significantly decreased at the 30 min time point, suggesting rapid degradation or clearance of the foreign protein once internalized. It remains to be determined if mammalian-derived enzymes would also be subject to the same rapid clearance.
4. Discussion
The aim of this study was to develop a model system for studying the intranasal delivery of active enzymes to the brain with good yield. We chose a bacterial enzyme with resistance to heat inactivation in order to avoid endogenous acetylation activity against chloramphenicol. The nasal mucosa is an advantageous administration route for rapid drug absorption into the CNS. Intranasal brain delivery provides a practical, non-invasive delivery route for therapeutic agents, including higher molecular weight biologics, with the advantages of bypassing the BBB and avoiding first pass hepatic metabolism (Frey, 2013; Lochhead, Wolak et al., 2015;Thorne, Hanson et al., 2008; Thorne, Pronk et al., 2004). Limitations with the intranasal route include poor contact of the formulations with the nasal mucosa and permeability of drugs (Arora, Sharma et al., 2002).
We chose the 92 kD type IV collagenase MMP-9 to increase the permeability of nasal epithelium because it has previously been shown to increase the uptake of a 10 kD dextran tracer across the olfactory epithelia (Lochhead, Wolak et al., 2015). Additionally, type IV collagen is the one of the more abundant ECM proteins in nasal epithelium (Benali, Tournier et al., 1993), suggesting that MMP-9 would be an effective enzyme for increasing permeability there. Our results showed that the 75 kDa CAT was transported into all the regions of brain within 15 min. Pretreatment with MMP-9, 30 min before intranasal administration of CAT resulted in an increase in the CAT activity and yield in several brain regions including the brainstem. Intranasally administered therapeutics reach the CNS via the olfactory and trigeminal neural pathways. Both the olfactory and trigeminal nerves innervate the nasal cavity, providing a direct connection with the CNS (Chapman, Frey et al., 2013). One notable distinction between our results and those of Lochhead and colleagues (Lochhead, Wolak et al., 2015) was that we observed significant uptake and transport of active CAT enzyme into the brain after intranasal application, even in the absence of pre-treatment with MMP-9. Lochhead et al. found very little uptake of a fluorescent labeled 10 kD dextran tracer after intranasal administration without MMP-9 pretreatment. This may indicate that the methods used in the current study are somewhat more sensitive to lower levels of transport than the use of fluorescent tracers.
It remains to be determined if intranasal MMP-9 treatment is safe for prolonged use. MMP-9 has other biological functions in addition to degradation of the extracellular matrix, and increased expression in the brain is associated with pathological conditions (reviewed in Vafadari, Salamian et al., 2015). There are potential mechanisms in the nasal mucosa that may attenuate the action of intranasally administered MMP-9 over time. For example, mucus is transported at a rate of 5 mm/min and its transit time in human nasal cavity is reported to be 15–20 min. Nasal muco-ciliary clearance is a potential physiological barrier that reduces the residential time of intranasally administered therapeutics in contact with the nasal mucosa (Illum, 2003). Further, previous studies have shown the strong expression of tissue inhibitors of metalloproteinase (TIMP-1 and TIMP-2) in the nasal mucosa (Shaida, Kenyon et al., 2001). Due to the ciliary action and presence of high expression of TIMPs in nasal mucosa, MMP-9 action may be limited in duration thus preventing damage to epithelial tissue.
Transport of the enzyme from the olfactory mucosa and epithelia to the brain involves bulk flow of fluid through perivascular spaces surrounding penetrating blood vessels entering the brain (Thorne and Frey, 2001). This fluid transport efficiently moves solutes from the nasal mucosa and epithelia to the CNS. In the rats tested 30 min after intranasal administration of enzyme, the activity of CAT was found in all the regions of the brain and the pattern was similar to that of 15 min groups. However, in comparison with 15 min groups, the activity of CAT in the 30 min groups was significantly lower. It is likely that the enzyme is inactivated by normal processing, or that the foreign protein is cleared quickly from the brain diminishing enzyme activity by the 30 min time point.
5. Conclusion
Using a sensitive radiometric assay we detected radiolabeled product resulting from CAT enzyme activity in the brain at 15 minutes and 30 minutes post intranasal administration. We detected enzyme activity throughout the brain, with significantly higher levels of activity observed in some brain areas after pre-treatment with MMP-9. This method can be used to examine the delivery of active enzyme to the brain for a variety of purposes including the possibility of using catalytic bioscavengers to detoxify CNS poisons including organophosphate CTAs, or testing the efficacy of intranasal delivery of enzymes in CNS diseases involving enzyme deficiency.
Highlights.
-
➢
The BBB is a major obstacle in the treatment of CNS disorders and toxicity
-
➢
Enzymes given intranasally can treat toxicity and enzyme deficiency disorders
-
➢
Intranasally administered active enzyme reached all brain areas within 15 min.
-
➢
Therapeutic bioavailability was doubled by pretreatment with MMP-9
-
➢
Model for catalytic bioscavengers to detoxify CNS poisons and enzyme deficiencies
Acknowledgements
We acknowledge Impel NeuroPharma, Inc. (Seattle, WA) for making their intranasal delivery device available for our use. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as reflecting the views of the Department of Defense. This work was supported by the NIH grant NS076448. Graphical abstract adapted from (Swanson, 2004) (http://larrywswanson.com/?page_id=164).
Abbreviations
- BBB
blood brain barrier
- CAT
chloramphenicol acetyltransferase
- CNS
central nervous system
- CTA
chemical threat agent
- HP-TLC
High Performance Thin Layer Chromatography
- MMP-9
matrix metalloproteinase-9
- TIMP
tissue inhibitors of metalloproteinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflicts of interest.
Reference List
- Arora P, Sharma S, Garg S. Permeability issues in nasal drug delivery. Drug Discov. Today. 2002;7:967–975. doi: 10.1016/s1359-6446(02)02452-2. [DOI] [PubMed] [Google Scholar]
- Benali R, Tournier JM, Chevillard M, Zahm JM, Klossek JM, Hinnrasky J, Gaillard D, Maquart FX, Puchelle E. Tubule formation by human surface respiratory epithelial cells cultured in a three-dimensional collagen lattice. Am. J. Physiol. 1993;264:L183–L192. doi: 10.1152/ajplung.1993.264.2.L183. [DOI] [PubMed] [Google Scholar]
- Chapman CD, Frey WH, Craft S, Danielyan L, Hallschmid M, Schioth HB, Benedict C. Intranasal treatment of central nervous system dysfunction in humans. Pharm. Res. 2013;30:2475–2484. doi: 10.1007/s11095-012-0915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb DW, Dixon JE. A method for increasing the sensitivity of chloramphenicol acetyltransferase assays in extracts of transfected cultured cells. Anal. Biochem. 1987;163:88–92. doi: 10.1016/0003-2697(87)90096-0. [DOI] [PubMed] [Google Scholar]
- Djupesland PG, Messina JC, Mahmoud RA. The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview. Ther. Deliv. 2014;5:709–733. doi: 10.4155/tde.14.41. [DOI] [PubMed] [Google Scholar]
- Frey WH. Intranasal insulin to treat and protect against posttraumatic stress disorder. J. Nerv. Ment. Dis. 2013;201:638–639. doi: 10.1097/NMD.0b013e318298302e. [DOI] [PubMed] [Google Scholar]
- Gorman CM, Moffat LF, Howard BH. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol. Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illum L. Nasal drug delivery--possibilities, problems and solutions. J. Control. Release. 2003;87:187–198. doi: 10.1016/s0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- Liu XF, Fawcett JR, Thorne RG, DeFor TA, Frey WH. Intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage. J. Neurol. Sci. 2001;187:91–97. doi: 10.1016/s0022-510x(01)00532-9. [DOI] [PubMed] [Google Scholar]
- Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012;64:614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Lochhead JJ, Wolak DJ, Pizzo ME, Thorne RG. Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J. Cereb. Blood. Flow. Metab. 2015;35:371–381. doi: 10.1038/jcbfm.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon AP, Novak TJ, Britten RJ, Davidson EH. Inducible expression of a cloned heat shock fusion gene in sea urchin embryos. Proc. Natl. Acad. Sci. U. S. A. 1984;81:7490–7494. doi: 10.1073/pnas.81.23.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaida A, Kenyon G, Devalia J, Davies RJ, MacDonald TT, Pender SL. Matrix metalloproteinases and their inhibitors in the nasal mucosa of patients with perennial allergic rhinitis. J. Allergy. Clin. Immunol. 2001;108:791–796. doi: 10.1067/mai.2001.119024. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain: Third Edition. Amsterdam: Elsevier; 2004. [Google Scholar]
- Thorne RG, Emory CR, Ala TA, Frey WH. Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995;692:278–282. doi: 10.1016/0006-8993(95)00637-6. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Frey WH. Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin. Pharmacokinet. 2001;40:907–946. doi: 10.2165/00003088-200140120-00003. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Hanson LR, Ross TM, Tung D, Frey WH. Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neurosci. 2008;152:785–797. doi: 10.1016/j.neuroscience.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Pronk GJ, Padmanabhan V, Frey WH. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neurosci. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Vafadari B, Salamian A, Kaczmarek L. MMP-9 in Translation: From Molecule to Brain Physiology, Pathology and Therapy. J. Neurochem. 2015 doi: 10.1111/jnc.13415. [DOI] [PubMed] [Google Scholar]
- Wolf DA, Hanson LR, Aronovich EL, Nan Z, Low WC, Frey WH, McIvor RS. Lysosomal enzyme can bypass the blood-brain barrier and reach the CNS following intranasal administration. Mol. Genet. Metab. 2012;106:131–134. doi: 10.1016/j.ymgme.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Worek F, Seeger T, Goldsmith M, Ashani Y, Leader H, Sussman JS, Tawfik D, Thiermann H, Wille T. Efficacy of the rePON1 mutant IIG1 to prevent cyclosarin toxicity in vivo and to detoxify structurally different nerve agents in vitro. Arch. Toxicol. 2014;88:1257–1266. doi: 10.1007/s00204-014-1204-z. [DOI] [PubMed] [Google Scholar]
- Zhu W, Cheng S, Xu G, Ma M, Zhou Z, Liu D, Liu X. Intranasal nerve growth factor enhances striatal neurogenesis in adult rats with focal cerebral ischemia. Drug Deliv. 2011;18:338–343. doi: 10.3109/10717544.2011.557785. [DOI] [PubMed] [Google Scholar]