Abstract
Purpose
The aims of this study were to determine if pre-treatment plasma levels of cytokines and immune activation-associated molecules changed following treatment for AIDS-NHL with rituximab plus infusional EPOCH, and to determine if pre-treatment levels of these molecules were associated with response to treatment and/or survival.
Experimental design
We quantified plasma levels of B cell activation-associated molecules (sCD27, sCD30, sCD23) and cytokines (IL-6, IL-10, CXCL13) prior to and after the initiation of treatment in persons with AIDS-NHL (n=69) in the AIDS Malignancies Consortium (AMC) 034 study, which evaluated treatment of AIDS-NHL with EPOCH chemotherapy and rituximab.
Results
Treatment resulted in decreased plasma levels of some of these molecules (CXCL13, sCD27, sCD30), with decreased levels persisting for one year following the completion of treatment. Lower levels of CXCL13 before treatment were associated with complete responses following lymphoma therapy. Elevated levels of IL-6 pre-treatment were associated with decreased overall survival, while higher IL-10 levels were associated with shorter progression-free survival, in multivariate analyses. Furthermore, patients with CXCL13 or IL-6 levels higher than the median levels for the NHL group, as well as those who had detectable IL-10, had lower overall survival and PFS, in Kaplan Meier analyses.
Conclusions
These results indicate that CXCL13, IL-6 and IL-10 have significant potential as prognostic biomarkers for AIDS-NHL.
INTRODUCTION
The risk for developing B cell non-Hodgkin’s lymphoma (NHL) is significantly and markedly increased in persons living with HIV infection (1–5). The introduction of combination antiretroviral therapy (HAART) has had a significant impact on overall survival of persons living with HIV infection (6–10). The incidence of AIDS-NHL has decreased in the HAART era, but not to the same extent as that of Kaposi’s sarcoma or other AIDS-defining conditions. Additionally, the widespread availability of HAART appears to have had a differential effect on the incidence of AIDS-NHL subtypes, with a marked decrease in the incidence of primary central nervous system lymphoma (PCNSL), while that of other forms of AIDS-NHL, such as Burkitt’s lymphoma (BL) or diffuse large B cell lymphoma (DLBCL), either has not decreased or has remained unchanged (7, 11). Therefore, lymphoma remains a significant clinical problem in the HAART era. In fact, NHL appears to be the most common AIDS-related cancer in populations with ready access to HAART, and remains a significant cause of morbidity and mortality in HIV+ persons in the post-HAART era (12–13).
B cell hyperactivation, as well as loss of regulation of Epstein-Barr virus (EBV) infected B cells, are believed to play important roles in the development of AIDS-NHL (14–17). We and others have shown elevated serum/plasma levels of several B cell-stimulatory cytokines, including IL-6, IL-10 and CXCL13, are present over a period of several years prior to the diagnosis of AIDS-NHL (18–29). Elevated levels of circulating IL-6 or IL-10 also are seen after AIDS-NHL diagnosis (30–31). AIDS-NHL cell lines also are known to produce cytokines, including IL-6 and IL-10 (32). Additionally, we saw elevated levels of the expression of activation-induced cytidine deaminase (AICDA), a DNA mutating enzyme, in circulating mononuclear cells, preceding the diagnosis of AIDS-NHL (33). AICDA expression has also been reported to be elevated in B cells and lymphoma cells infected with HCV (34). HIV can directly induce B cell AICDA expression, as well as their secretion of several cytokines (IL-6, IL-10) and surface molecules (CD23), in vitro (17, 35).
In the present study, we evaluated plasma levels of several B cell activation-associated molecules (sCD23, sCD27, sCD30, IgE) and B cell-stimulatory cytokines (IL-6, IL-10, CXCL13), in persons who had an AIDS-NHL diagnosis, prior to and after the initiation of treatment, with the aim of better defining post-diagnosis, pre-treatment levels of these molecules in AIDS-NHL, and to determine how levels of these immune system stimulatory molecules are affected by treatment for AIDS-NHL. We found that AIDS-NHL patients had high pre-treatment plasma levels of several B cell activation-associated molecules (IL-6, IL-10, CXCL13, sCD27, sCD30), when compared to HIV+ and HIV-negative reference groups who did not have NHL. Additionally, treatment of NHL resulted in a rapid decrease in plasma levels of most of these molecules, with decreased levels persisting for one year following the completion of treatment. Importantly, pre-treatment levels of some of these molecules were associated with response to lymphoma therapy, as well as overall survival.
MATERIALS AND METHODS
Study population
Of the 106 AIDS NHL patients enrolled in an AIDS Malignancy Consortium (AMC) trial, AMC protocol #034 (AMC-034), which compared infusional combination chemotherapy (EPOCH: etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) with concurrent or sequential rituximab (36), plasma specimens were available from 69 patients with intermediate- or high-grade HIV-associated B-cell NHL (50 patients had DLBCL, 17 BL, and 2 were classified only as lymphoma). The median age of lymphoma patients was 42.6 ± 8.8 years. Lymphoma patients had a median HIV plasma level of 9908 (inter-quartile rage [IQR] = 492.5 – 45,660), and a median CD4 number of 187 cells/mm3 (IQR = 82 – 333). Plasma was collected prior to the initiation of therapy, at the end of the first cycle (within a week or less of treatment), and at 6 months and one year following the completion of treatment. Clinical responses were defined as described in the report detailing the AMC-034 trial results (36).
Rituximab, EPOCH, supportive care, and clinical evaluation
Details regarding the treatment protocol can be found in Sparano et al (36). Clinical responses were defined by the International Response Criteria for Non-Hodgkin Lymphoma (which uses anatomic but not functional imaging). Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (Version 2.0). Response was evaluated after every 2 cycles of EPOCH therapy (with computerized tomography of the chest, abdomen, and pelvis) and continued for 2 cycles beyond achieving a CR (for a minimum of 4 and maximum of 6 cycles), including after completion of R-EPOCH in the concurrent arm, and after completion of EPOCH alone and by rituximab alone in the sequential arm. No patients received rituximab when not approved as part of the study protocol. All patients were required to have bone marrow biopsy and lumbar puncture for cerebrospinal fluid cytologic examination at baseline. A repeat bone marrow biopsy was required if the original study demonstrated lymphomatous marrow involvement, and if the physical examination and imaging studies were consistent with a complete response (36).
Determination of cytokines and soluble receptor molecules and IgE in plasma samples
Plasma levels of B cell stimulatory cytokines and molecules associated with immune system activation were assessed by enzyme-linked immunosorbent assay (ELISA). IL-6 was measured using an ultrasensitive assay (Biosource/Invitrogen, Carlsbad, Calilfornia), with color development time extended to 40 minutes to ensure consistent low-level detection (detection limit = 0.2 pg/ml). IL-10 was measured using a human IL-10-specific assay (Biosource/Invitrogen) that does not cross react with EBV viral IL-10 (21), modified to increase sensitivity by extending the standard curve (detection limit = 2 pg/ml), increasing sample incubation time to 3 hours, and performing all incubations on a microtiter plate rotator (500 rpm). CXCL13/BCA-1 was measured using the R&D Systems ELISA kit according to the manufacturer’s protocol, with a 1:2 dilution (detection limit = 7.8 pg/ml). sCD27 was determined using the PeliKine-compact ELISA kit and Toolset according to the manufacturer’s protocol (CLB/Sanquin, Netherlands), with 1:20 dilutions on all HIV+ samples (detection limit = 32 units/ml, taking dilution into account). Assays for sCD23 (detection limit = 13 units/ml) and sCD30 (detection limit = 6 units/ml) were performed according to the manufacturer’s protocols (Bender MedSystems USA, San Bruno, California). Total plasma IgE was determined utilizing the CIA-7.12 and CIA-4.15 monoclonal antibodies (37) as previously described (38), with the following modifications: IgE ELISA plates were blocked with 10% fetal bovine plasma in PBS-Tween buffer, and all plasma samples were diluted 1:10 using PBS-Tween buffer prior to addition to the ELISA plate (19). Diluted sera and all subsequent reagents were added at 50µl per well, and all incubations were performed on a microtiter plate rotator (500 rpm). The IgE standard was pooled normal plasma (generously provided by Drs. Andrew Saxon and Ke Zhang); when referenced to the WHO IgE standard NIBSC 75/502 (which is also pooled human sera), the mean conversion factor was 0.67 ng per IU. The concentration of the lowest IgE standard was 0.8 ng/ml; taking the dilution into account, the lower limit of detection in plasma samples was 8 ng/ml. Plasma samples used for the measurement of IL-6, IL-10, sCD27, sCD30 and IgE, were frozen and thawed once. Samples used for the measurement of CXCL13 and sCD23 were frozen and thawed twice.
Statistics
The Wilcoxon rank sum test was used to compare biomarker levels of complete responders with patients who did not achieve a complete response with treatment for lymphoma. To assess their association with outcome measures (complete response, overall survival and progression-free survival), levels for CXCL13, CD23, CD27, CD30, IL-6 and LDH were dichotomized at their median value. Fisher’s exact test was used to compare those who achieved complete response and those who did not with respect to international prognostic index (IPI) score (age-adjusted) and each biomarker. Those biomarkers that were associated with complete response at the 0.05 significance level were incorporated into a stepwise logistic regression model.
Proportional hazards models were used to evaluate the association of each individual biomarker with overall survival and progression-free survival. Those factors associated with overall and progression-free survival at the 0.05 level were incorporated into a stepwise proportional hazards model.
RESULTS
Plasma levels of B cell-stimulatory cytokines and immune activation molecules were detected in persons with AIDS-NHL
Plasma levels of B cell-stimulatory cytokines (IL-6, IL-10, CXCL13), and of molecules associated with B cell activation (sCD23, sCD27, sCD30, IgE), were measured by ELISA in specimens collected, pre- and post-treatment, from persons enrolled in an AIDS Malignancy Consortium trial comparing infusional combination chemotherapy (EPOCH: etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) with concurrent or sequential rituximab (AMC protocol #034). There was a negative correlation between CD4 counts and CXCL13 levels (p=0.042); correlations of CD4 with the other biomarkers were not significant.
Plasma levels of CXCL13 decreased following NHL treatment
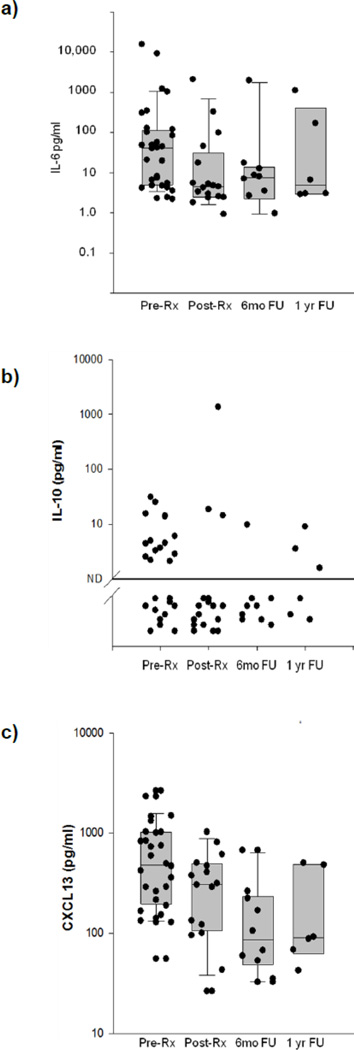
A central aim of this study was to determine if there is a change in the levels of these cytokines and immune activation molecules following treatment for AIDS-NHL. In order to study this, we measured plasma levels of these molecules before (Pre-RX), during lymphoma treatment (Post-Rx), as well as 6 months (6mo FU) and one year (1 year FU) after lymphoma treatment. Treatment was seen to result in a marked decrease in plasma CXCL13 levels following the initiation of treatment (p=0.005, Wilcoxon signed rank test) (Figure 1); this decrease was a consequence of treatment and not of survival. This decrease was maintained over time, with decreased plasma CXCL13 levels seen at 6 months and 1 year after the completion of treatment (p=0.016 and 0.031, respectively, Wilcoxon signed rank test).
Figure 1. Levels of B cell-stimulatory cytokines pre- and post-NHL treatment.
Plasma IL-6 (a), IL-10 (b) and CXCL13 (c) levels were measured in samples form subjects that had been diagnosed with NHL, in samples from the same subjects collected after lymphoma treatment (at the end of the first cycle of tretment, within a week after treatment initiation), 6 months after treatment and 1 year after treatment. Each filled circle represents a sample from a single subject.
In contrast, plasma levels of IL-6 or IL-10 were not seen to decrease significantly following treatment (p=0.421 and 0.492, respectively), nor at 6 months (p=0.688 and 0.625, respectively) or 12 months post-treatment (p=1.00 and not evaluable, respectively) (Figure 1).
Plasma levels of some immune stimulatory molecules decreased following NHL treatment
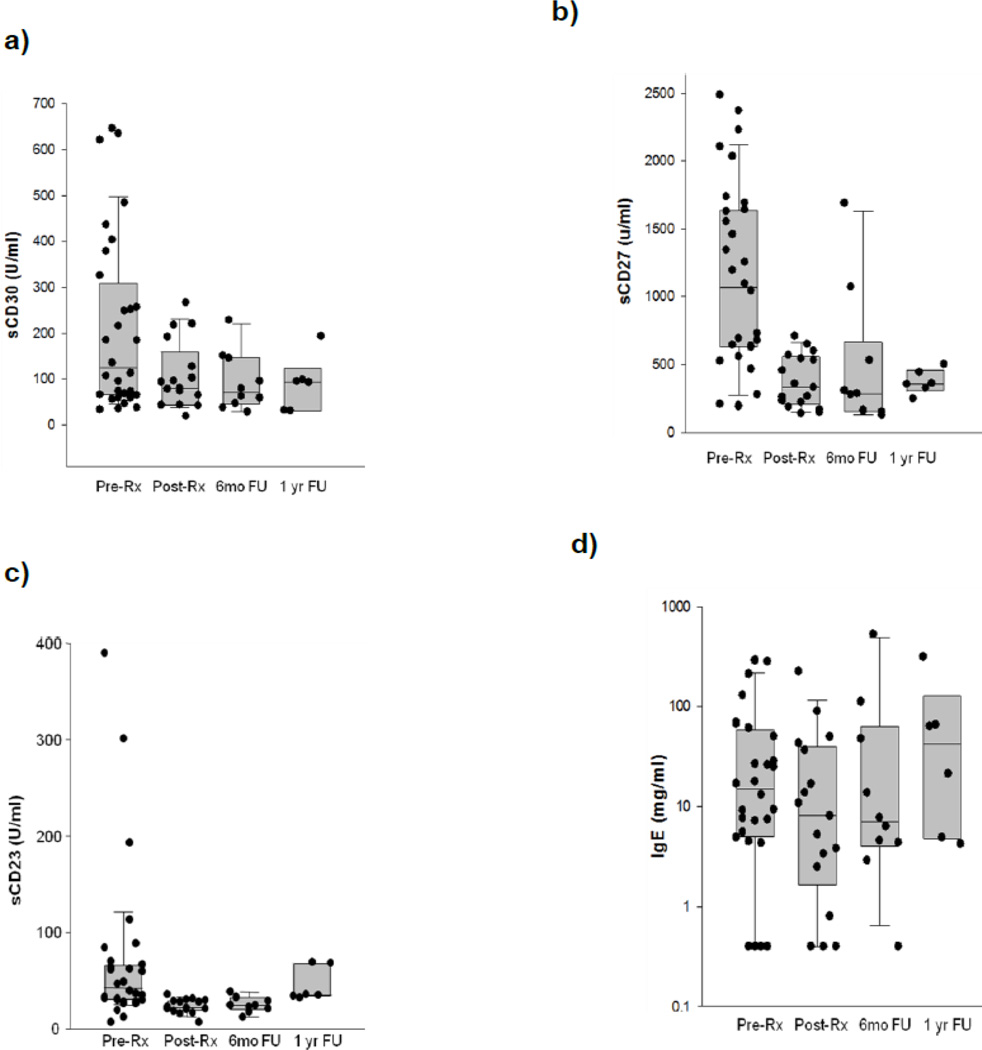
Compared with pre-treatment, sCD30 plasma levels were seen to decrease significantly (p<0.004, Wilcoxon signed rank test) (Figure 2), and appeared to remain at lower levels at six months and one year after treatment completion, although this was not statistically significant (p=0.297 and 0.063, respectively).
Figure 2. Levels of immune activation biomarkers pre- and post-NHL treatment.
Plasma sCD30 (a), sCD27 (b), sCD23 (c) and IgE (d) levels were measured in samples form subjects that had been diagnosed with NHL, in samples from the same subjects collected after lymphoma treatment (at the end of the first cycle of tretment, within a week after treatment initiation), 6 months after treatment and 1 year after treatment. Each filled circle represents a sample from a single subject.
Similarly, plasma sCD27 levels were significantly decreased following the initiation of treatment (p<0.001) (Figure 2), and remained lower at 6 months (p=0.016) and 1 year after the completion of lymphoma treatment (p=0.063), although this decrease was not statistically significant at one year post-treatment.
Plasma levels of sCD23 were seen to decrease significantly (p<0.001, Wilcoxon signed rank test) after the initiation of lymphoma treatment and at 6 months post-treatment (p=0.16), but not at 1 year after the completion of treatment (p=0.219) (Figure 2).
Plasma levels of IgE appeared unchanged after lymphoma treatment (Figure 2).
Association of biomarker levels with type of treatment
In this study, patients were treated with EPOCH with concurrent rituximab, or administration of rituximab after EPOCH treatment was complete. There were no significant differences seen between patients receiving concurrent or sequential rituximab, in terms of plasma levels of these biomarkers (not shown).
Association of biomarker levels with clinical response to lymphoma therapy, and survival
Lower levels of CXCL13, IL-10, IL-6, and LDH, as well as IPI scores, were significantly associated with complete response to therapy, in univariate analyses (Table 1). Similarly, when we assessed the association of these biomarkers with any clinical response (complete and partial responders vs non-responders), CXCL13, LDH and IPI remained significant predictors of a clinical response and detectable IL-10 was associated with non-responders (not significant) (Table 2). Additionally, when a stepwise logistic regression was used to assess the relative contribution of these factors to complete response, only CXCL13 was seen to be associated with complete response (p=0.003; OR=5.5 [1.81–16.68]).
Table 1.
Mean (IQR) of cytokines and stimulatory molecules and p values, when comparing complete responders vs non- or partial-responders before treatment.
| Complete Responders [median(IQR)] n = 37 |
Non-Responders or Partial Responders [median(IQR)] n=30–32 |
p-value1 | |
|---|---|---|---|
| IL6 (pg/mL) | 6.3 (4.0 – 32.2) | 44.4 (8.2 – 96.1) | 0.043 |
| IL10 (% detectable) | 41% | 72% | 0.0148 |
| sCD23 (U/mL) | 69.5 (29.8 – 94.0) | 39.4 (26.0 – 68.7) | 0.228 |
| sCD30 (U/mL) | 102 (76.1 – 227) | 157 (62.4 – 376) | 0.586 |
| CXCL13 (pg/mL) | 238 (155 – 395) | 617 (252 – 1030) | 0.001 |
| IgE (ng/mL) | 21.8 (8.2 – 29.2) | 40.2 (7.7 – 146) | 0.190 |
| sCD27 (U/mL) | 800 (559 – 1250) | 758 (526 – 1480) | 0.966 |
| LDH | 305 (164 – 619) | 555 (240 – 1370) | 0.0442 |
| IPI (% who had IPI score of 0–1) | 92% | 66% | 0.0198 |
Table 2.
Median (IQR) of cytokines and stimulatory molecules and p values, when comparing all responders (complete plus partial) vs non-responders before treatment.
| Complete and Partial Responders [median (IQR)] n = 44–52 |
Non-Responders [median (IQR)] n=16–18 |
p-value1 | |
|---|---|---|---|
| IL6 (pg/mL) | 8.1 (4.2 – 44.4) | 45.7 (4.2 – 84.1) | 0.469 |
| IL10 (% detectable) | 50% | 71% | 0.168 |
| sCD23 (U/mL) | 47.1 (23.1 – 79.8) | 43.4 (30.9 – 84.2) | 0.485 |
| sCD30 (U/mL) | 96.6 (68.0 – 227) | 171 (70.4 – 362) | 0.273 |
| CXCL13 (pg/mL) | 247 (166 – 481) | 663 (389 – 1490) | 0.003 |
| IgE (ng/mL) | 26.4 (9.4 – 84.5) | 14.6 (7.1 – 88.6) | 0.466 |
| sCD27 (U/mL) | 794 (523 – 1250) | 729 (525 – 1270) | 0.903 |
| LDH | 320 (190 – 691) | 980 (260 – 1660) | 0.044 |
| IPI (% who had IPI score of 0–1) | 44% | 12% | 0.020 |
We also looked at the association of these biomarkers with overall survival or progression free survival (PFS). In univariate analyses, the following factors were significantly associated with overall survival: IPI, CXCL13, IL-6, IL-10, and LDH (Table 3). However, when they were incorporated into a stepwise proportional hazard model for overall survival, only IL-6 (P=0.010, OR=0.30 [0.12, 0.75]) was associated with overall survival.
Table 3.
Relationship between biomarkers and outcome measures
| Factor | N | Complete response rate (%) |
1-year OS (%) (95% CI) |
1-year PFS (%) (95% CI) |
|
|---|---|---|---|---|---|
| IPI score | |||||
| 0–1 | 25 | 72 | 95.8 (73.9, 99.4) | 95.8 (73.9, 99.4) | |
| 2–4 | 44 | 43 | 62.8 (46.6, 75.3) | 54.0 (38.1, 67.4) | |
| OR/HR3 | 3.38 (1.06. 11.47) | 0.40 (0.15, 1.09) | 0.39 (0.16, 0.97) | ||
| P-value | 0.0261 | 0.0282 | 0.0152 | ||
| CXCL13 | |||||
| < median | 35 | 74 | 88.0 (71.2, 95.3) | 85.0 (67.6, 93.5) | |
| > median | 34 | 32 | 61.3 (42.8, 75.4) | 52.9 (35.1, 67.9) | |
| OR/HR3 | 6.04 (1.90, 19.68) | 0.31 (0.13, 0.74) | 0.41 (0.19, 0.89) | ||
| P-value | <0.0011 | 0.0082 | 0.0242 | ||
| sCD27 | |||||
| < median | 34 | 53 | 70.1 (51.5, 82.6) | 61.1 (42.5, 75.3) | |
| > median | 34 | 56 | 81.9 (64.1, 91.4) | 79.1 (61.1, 89.5) | |
| OR/HR3 | 0.89 (0.31, 2.56) | 1.78 (0.78, 4.08) | 0.77 (0.83, 3.78) | ||
| P-value | 1.0001 | 0.1712 | 0.1412 | ||
| sCD23 | |||||
| < median | 34 | 47 | 82.1 (64.5, 91.6) | 73.1 (54.7, 85.0) | |
| > median | 35 | 60 | 68.0 (49.6, 80.8) | 65.2 (46.9, 78.5) | |
| OR/HR3 | 0.59 (0.20, 1.71) | 0.43 (0.18, 1.01) | 0.64 (0.31, 1.35) | ||
| P-value | 0.3381 | 0.0512 | 0.2452 | ||
| sCD30 | |||||
| < median | 35 | 60 | 69.5 (50.7, 82.3) | 58.0 (39.6, 72.7) | |
| > median | 34 | 47 | 79.8 (62.2, 89.8) | 79.8 (62.2, 89.8) | |
| OR/HR3 | 1.69 (0.59, 4.88) | 1.59 (0.71, 3.56) | 1.62 (0.77, 3.41) | ||
| P-value | 0.3381 | 0.2612 | 0.2072 | ||
| IL-6 | |||||
| < median | 34 | 68 | 90.8 (74.1, 96.9) | 85.0 (67.7, 93.5) | |
| > median | 34 | 41 | 61.2 (42.6, 75.3) | 55.1 (36.9, 70.1) | |
| OR/HR3 | 2.99 (1.00, 9.08) | 0.26 (0.10, 0.64) | 0.34 (0.15, 0.76) | ||
| P-value | 0.0511 | 0.0042 | 0.0082 | ||
| IL-10 | |||||
| Undetectable | 31 | 71 | 93.3 (75.8, 98.3) | 90.1 (72.3, 96.7) | |
| Detectable | 38 | 39 | 60.0 (42.6, 73.6) | 52.6 (35.8, 67.0) | |
| OR/HR3 | 3.75 (1.23, 11.77) | 0.25 (0.09–0.67)) | 0.31 (0.13, 0.74) | ||
| P-value | 0.0151 | 0.0062 | 0.0082 | ||
| LDH | |||||
| < median | 30 | 60 | 85.9 (66.7, 94.5) | 79.6 (60.1, 90.3) | |
| > median | 30 | 40 | 60.0 (40.5, 75.0) | 53.3 (34.3, 69.1) | |
| OR/HR3 | 2.25 (0.71, 7.18) | 0.41, 0.17, 0.98) | 0.45 (0.20, 0.98) | ||
| P-value | 0.1961 | 0.0442 | 0.0462 | ||
Fisher’s exact test
log rank test
Odds ratio (OR) and 95% confidence interval for complete responses; hazard ratio (HR) and 95% confidence interval for OS and PFS (unadjusted)
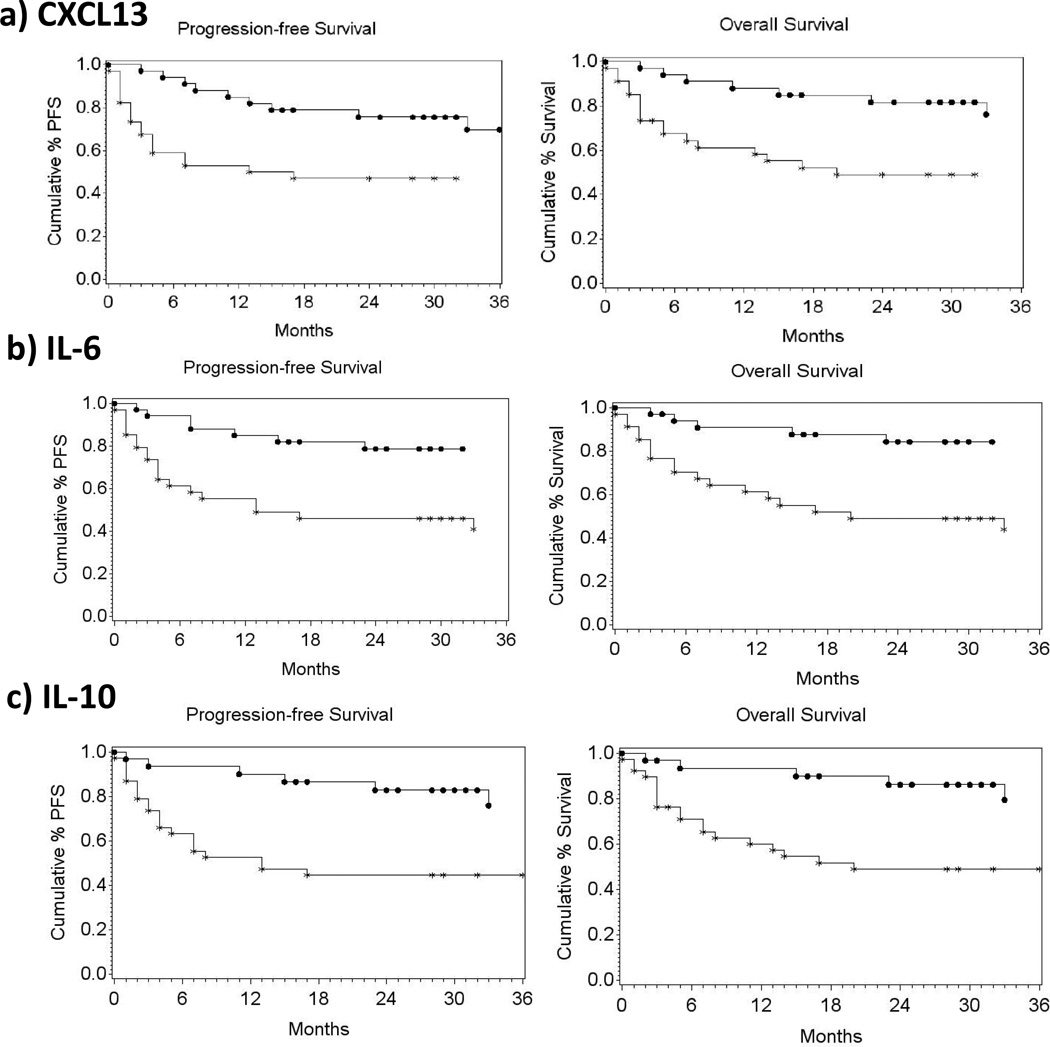
The following factors were significantly associated with PFS in univariate analyses: IPI, CXCL13, IL-6, IL-10 and LDH (Table 3). However, when these markers were incorporated into a stepwise proportional hazard model, only IL-10 (P=0.024; OR=2.86 [1.15, 7.15]) was significantly associated with PFS. Furthermore, patients with CXCL13, IL-6, or IL-10 levels higher than the median levels for the NHL group as a whole had lower overall survival and PFS, in Kaplan Meier analyses (Figure 3).
Figure 3. Association of CXCL13, IL-6 or IL-10 plasma levels and progression free survival and overall survival.
Progression free survival and overall survival of subjects with a) pre-treatment CXCL13 values higher than median (353 pg/ml), b) pre-treatment IL-6 values higher than median (18.03 pg/ml), or c) detectable pre-treatment IL-10 values. AIDS-NHL cases with CXCL13 or IL-6 levels lower than median, or with no detectable plasma IL-10, are represented by filled circles); cases with CXCL13 or IL-6 levels higher than median, or with detectable IL-10, are represented by asterisks.
We also assessed the association of cytokine/activation markers with response to treatment by NHL pathologic types (DLBCL, BL). With respect to OS and PFS, the direction of the hazard ratios was the same for BL and DBLCL, but frequently achieved statistical significance only when all patients are included (not shown). Therefore, while there was insufficient statistical power to definitively determine if there was a difference in the results observed for these two NHL subgroups, it does not appear that there were marked differences seen between DLBCL and BL.
DISCUSSION
In this study we found that plasma levels of several molecules that are associated with immune activation and inflammation are detectable in those who have untreated AIDS-NHL, and that plasma levels of some of these molecules (sCD23, sCD27, sCD30, CXCL13) showed marked reductions after EPOCH and Rituximab treatment. We did not see any differences in the levels of these biomarkers between the concurrent or sequential EPOCH and Rituximab treatment groups.
Most notably, we saw that pre-treatment levels of CXCL13, IL-6, IL-10, and LDH, as well as IPI, were significantly lower in those patients who went on to have complete responses to treatment. After conducting a stepwise logistic regression analysis, CXCL13 was the only factor that significantly correlated with subsequent treatment response. Similarly, multivariate analyses showed that only pre-treatment IL-6 levels were associated with overall survival, and IL-10 levels with PFS.
We also assessed the association of cytokine/activation markers with response to treatment by the major NHL pathologic types (DLBCL, BL) included in the AMC-034 study. While there was insufficient statistical power to definitively determine if there was a difference in the results observed for these two NHL subgroups, it did not appear that there were marked differences seen between DLBCL and BL.
Overall, these results suggest that plasma levels of CXCL13, IL-6 and IL-10 have significant potential as prognostic biomarkers for AIDS-NHL, and may add additional information over LDH or IPI, which are commonly used to assess prognosis (39). Certainly, the prognostic value of these cytokines needs to be confirmed in larger studies. Additionally, it is important to determine if the levels of these cytokines reflect tumor burden, or alternatively, if they are markers for the inability of patients to respond to therapy for other reasons relating to their poor health. Also, further studies are needed to define the prognostic value of measuring these cytokines when measured after the initiation of treatment for AIDS-NHL. However, these molecules show promise as new tools for the assessment of prognosis, and potentially, for the selection of treatment regimens for AIDS-NHL.
CXCL13 is a chemokine that directs the normal trafficking of B cells (40). It is expressed by T follicular helper cells, dendritic cells, and stromal cells in secondary lymphoid tissue (41). Plasma levels of the chemokine, CXCL13, are elevated during HIV infection (42), and decrease with anti-retroviral drug treatment (43). Other reports indicate that there are abnormalities in the CXCR5/CXCL13 system during HIV infection, including loss of expression of CXCR5 on mature B cells (44), and expression of CXCL13 by recirculating B cells (45). Together, these observations raise the possibility that the CXCR5/CXCL13 system may contribute to the abnormalities that are seen in the B cell compartment during HIV infection, and thus could be involved in the genesis of AIDS-NHL. CXCL13 has been shown to be associated with Sjögren’s disease, in which CXCL13 contributes to the organization of ectopic reactive lymphoid tissue (46). Additionally, elevated serum levels of CXCL13 have been seen prior to the diagnosis of non-AIDS NHL (47, 48), and CXCL13 and/or CXCR5 have been shown to be associated with several subtypes of non-AIDS-related B cell lymphomas (49–50).
IL-6 and IL-10 are inflammation-associated cytokines that are secreted by monocytes, lymphocytes and other cell types, and can enhance B cell proliferation, survival and antibody production. IL-6 and IL-10 are known to be elevated prior to lymphoma diagnosis, and their elevated levels are associated with risk for the development of NHL in HIV+ persons (19–29). It is unclear how IL-6 and IL-10 are contributing to the development of lymphoma. They may be directly promoting the growth and/or viability of cancer cells and/or they may affect other immune cells, presumably creating a favorable environment for the development or growth of lymphoma cells. Additionally, they may be secreted by the tumor cells. Thus, the reduction of these cytokines seen following treatment may be due to loss of tumor cells, as well as to the loss of tumor-reactive cells. Alternatively, the loss of cytokines maybe due to survival time bias, as partial responders and non-responders are more likely not to survive.
In prior work, we reported that serum/plasma levels of sCD23 were significantly elevated some years prior to the development of AIDS-NHL, but sCD23 levels did not differ between AIDS-NHL cases and controls when measurements were made closer (<1 year) to the time of lymphoma diagnosis (19). Therefore, this molecule seems to be elevated several years prior to lymphoma diagnosis, but then drops as NHL diagnosis is approached. In this sense, the observed levels of sCD23 seen in NHL patients in this study are consistent with a progressive decrease in plasma sCD23, going from elevated several years prior to NHL diagnosis to decreased post-diagnosis. It is possible that sCD23 plays an etiologic role in early events in lymphomagenesis, but not in supporting the progressive growth of these cancers. These results are consistent with the known role of sCD23 in promoting IgH class switch recombination (CSR), a molecular event thought to contribute to the genesis of lymphomagenic chromosomal translocations that lead to BL (17).
As mentioned above, we and others have reported that these cytokines and molecules are elevated preceding AIDS-NHL (19–29). The results presented here extend, and are generally in agreement with, those prior studies, and support the notion that a B cell stimulatory environment is associated with the development and progression of AIDS-NHL. In addition to this, some of these AIDS-NHL-associated plasma molecules, especially CXCL13, IL-6 and IL-10, appear to have potential value as indicators of subsequent response to NHL treatment and survival.
STATEMENT OF TRANSLATIONAL RELEVANCE.
HIV infection greatly increases the risk for non-Hodgkin lymphoma (NHL), an AIDS-defining cancer. In fact, NHL is now the most common AIDS-related cancer in populations that have access to treatment with effective combination anti-retroviral drug treatment regimens (HAART). While elevated serum/plasma levels of several cytokines and immune activation-associated molecules have been seen to precede AIDS-NHL diagnosis, little is known about the their prognostic value. Defining new prognostic biomarkers is of importance, as common techniques for assessing NHL prognosis (i.e., positron emission tomography [PET]) have significant limitations when used in HIV-infected patients. The results presented here indicate that CXCL13, IL-6 and IL-10, B cell-stimulatory cytokines, have the potential to serve as prognostic biomarkers in AIDS-NHL.
Acknowledgments
GRANT SUPPORT
This work was supported by grants from the National Institutes of Health (U01-CA-121947, R01-CA-121195, and R01-CA-168482). This work was carried out in the facilities of the UCLA AIDS Institute, which are supported, in part, by funds from NIH grant AI-028697, UCLA Center for AIDS Research (CFAR).
Footnotes
DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The authors declare no competing financial interests.
AUTHOR’ CONTRIBUTIONS
Conception and design of this study: R.F. Ambinder, M. Epeldegui, and O. MartÌnez-Maza
AMC-034 clinical trial administration and oversight: R. Mitsuyasu, R.F. Ambinder, J.A. Sparano
Laboratory studies and acquisition of data: A.C. MartÌnez, M. Epeldegui, L.I. Magpantay
Statistical analysis and interpretation: J.Y. Lee and D. Regidor
Interpretation of results, manuscript preparation, review and revision: M. Epeldegui, J.Y. Lee, A.C. MartÌnez, D.P. Widney, L.I. Magpantay, D. Regidor, R. Mitsuyasu, J.A. Sparano, R.F. Ambinder and O. MartÌnez-Maza
REFERENCES
- 1.Beral V, Peterman T, Berkelman R, Jaffe H. AIDS-associated non-Hodgkin lymphoma. Lancet. 1991;337:805–809. doi: 10.1016/0140-6736(91)92513-2. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler JL, Bragg K, Abrams D, Beckstead J, Cogan M, Volberding P, et al. High-grade non-Hodgkin's lymphoma in patients with AIDS. Ann N Y Acad Sci. 1984;437:412–419. doi: 10.1111/j.1749-6632.1984.tb37161.x. [DOI] [PubMed] [Google Scholar]
- 3.Armenian HK, Hoover DR, Rubb S, Metz S, Martinez-Maza O, Chmiel J, et al. Risk factors for non-Hodgkin's lymphomas in acquired immunodeficiency syndrome (AIDS) Am J Epidemiol. 1996;143:374–379. doi: 10.1093/oxfordjournals.aje.a008751. [DOI] [PubMed] [Google Scholar]
- 4.Biggar RJ, Rosenberg PS, Cote T. Kaposi's sarcoma and non-Hodgkin's lymphoma following the diagnosis of AIDS. Multistate AIDS/Cancer Match Study Group. Int J Cancer. 1996;68:754–758. doi: 10.1002/(SICI)1097-0215(19961211)68:6<754::AID-IJC11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Goedert JJ, Cote TR, Virgo P, Scoppa SM, Kingma DW, Gail MH, et al. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;351:1833–1839. doi: 10.1016/s0140-6736(97)09028-4. [DOI] [PubMed] [Google Scholar]
- 6.Hessol NA, Seaberg EC, Preston-Martin S, Massad LS, Sacks HS, Silver S, et al. Cancer risk among participants in the women's interagency HIV study. Jaids-Journal of Acquired Immune Deficiency Syndromes. 2004;36:978–985. doi: 10.1097/00126334-200408010-00013. [DOI] [PubMed] [Google Scholar]
- 7.Biggar RJ. AIDS-related cancers in the era of highly active antiretroviral therapy. Oncology (Huntingt) 2001;15:439–448. discussion 48–9. [PubMed] [Google Scholar]
- 8.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, Biggar RJ. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 9.Seaberg EC, Hessol N, Jacobson L, MartÌnez-Maza O, Sutcliffe C, Levine A. Cancer incidence before and during the era of HAART. 9th International Workshop on HIV Observational Databases Budapest; Hungary. 2005. [Google Scholar]
- 10.Seaberg EC, Wiley D, Martinez-Maza O, Chmiel JS, Kingsley L, Tang Y, et al. Cancer incidence in the multicenter aids cohort study before and during the HAART era: 1984 to 2007. Cancer. 2010;116:5507. doi: 10.1002/cncr.25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:3786–3791. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnet F, Balestre E, Thiebaut R, Morlat P, Pellegrin JL, Neau D, et al. Factors associated with the occurrence of AIDS-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy: Aquitaine Cohort, France. Clin Infect Dis. 2006;42:411–417. doi: 10.1086/499054. [DOI] [PubMed] [Google Scholar]
- 13.Bonnet F, Lewden C, May T, Heripret L, Jougla E, Bevilacqua S, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101:317–324. doi: 10.1002/cncr.20354. [DOI] [PubMed] [Google Scholar]
- 14.van Baarle D, Hovenkamp E, Callan MF, Wolthers KC, Kostense S, Tan LC, et al. Dysfunctional Epstein-Barr virus (EBV)-specific CD8(+) T lymphocytes and increased EBV load in HIV-1 infected individuals progressing to AIDS-related non-Hodgkin lymphoma. Blood. 2001;98:146–155. doi: 10.1182/blood.v98.1.146. [DOI] [PubMed] [Google Scholar]
- 15.Epeldegui M, Widney DP, Martinez-Maza O. Pathogenesis of AIDS lymphoma: role of oncogenic viruses and B cell activation-associated molecular lesions. Curr Opin Oncol. 2006;18:444–448. doi: 10.1097/01.cco.0000239882.23839.e5. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Maza O, Breen EC. B-cell activation and lymphoma in patients with HIV. Curr Opin Oncol. 2002;14:528–532. doi: 10.1097/00001622-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Epeldegui M, Vendrame E, Martinez-Maza O. HIV-associated immune dysfunction and viral infection - role in the pathogenesis of AIDS-related lymphoma. Immunologic Research. 2010 doi: 10.1007/s12026-010-8168-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambinder RF, Bhatia K, Martinez-Maza O, Mitsuyasu R. Cancer biomarkers in HIV patients. Curr Opin HIV AIDS. 2010;5:531–537. doi: 10.1097/COH.0b013e32833f327e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breen EC, Hussain SK, Magpantay L, Jacobson LP, Detels R, Rabkin CS, et al. B-Cell Stimulatory Cytokines and Markers of Immune Activation Are Elevated Several Years Prior to the Diagnosis of Systemic AIDS-Associated Non-Hodgkin B-Cell Lymphoma. Cancer Epidemiol Biomarkers Prev. 2011;20:1303–1314. doi: 10.1158/1055-9965.EPI-11-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pluda JM, Venzon DJ, Tosato G, Lietzau J, Wyvill K, Nelson DL, et al. Parameters affecting the development of non-Hodgkin's lymphoma in patients with severe human immunodeficiency virus infection receiving antiretroviral therapy. J Clin Oncol. 1993;11:1099–1107. doi: 10.1200/JCO.1993.11.6.1099. [DOI] [PubMed] [Google Scholar]
- 21.Breen EC, Boscardin WJ, Detels R, Jacobson LP, Smith MW, Chmiel JS, et al. Non-Hodgkin's B cell lymphoma in persons with acquired immunodeficiency syndrome is associated with increased serum levels of IL10, or the IL10 promoter-592 C/C genotype. Clin Immunol. 2003;109:119–129. doi: 10.1016/s1521-6616(03)00214-6. [DOI] [PubMed] [Google Scholar]
- 22.Breen EC, van der Meijden M, Cumberland W, Kishimoto T, Detels R, Martinez-Maza O. The development of AIDS-associated Burkitt's/small noncleaved cell lymphoma is preceded by elevated serum levels of interleukin 6. Clin Immunol. 1999;92:293–299. doi: 10.1006/clim.1999.4760. [DOI] [PubMed] [Google Scholar]
- 23.Ouedraogo DE, Makinson A, Kuster N, Nagot N, Rubbo PA, Bollore K, et al. Increased T-cell activation and Th1 cytokine concentrations prior to the diagnosis of B-cell lymphoma in HIV infected patients. J Clin Immunol. 2013;33:22–29. doi: 10.1007/s10875-012-9766-0. [DOI] [PubMed] [Google Scholar]
- 24.Widney DP, Gui D, Popoviciu LM, Said JW, Breen EC, Boscardin WJ, et al. Expression and function of the chemokine, CXCL13, and its receptor, CXCR5, in AIDS-assocaited non-Hogkni's lymphoma. AIDS Research and Treatment. 2010;2010:164586. doi: 10.1155/2010/164586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussain SK, Zhu W, Chang SC, Breen EC, Vendrame E, Magpantay L, et al. Serum levels of the chemokine CXCL13, genetic variation in CXCL13 and its receptor CXCR5, and HIV-associated non-hodgkin B-cell lymphoma risk. Cancer Epidemiol Biomarkers Prev. 2013;22:295–307. doi: 10.1158/1055-9965.EPI-12-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hussain SK, Hessol NA, Levine AM, Crabb Breen E, Anastos K, Cohen M, D’Souza A, Gustafson D, Silver S, Martίnez-Maza O. Serum biomarkers of immune activation and subsequent risk of non-Hodgkin B-cell lymphoma among HIV-infected women. Cancer Epidemiol Biomarkers Prev. 2013;22:2084–2093. doi: 10.1158/1055-9965.EPI-13-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vendrame E, Hussain SK, Crabb Breen E, Magpantay LI, Widney DP, Jacobson LP, et al. Serum levels of cytokines, and biomarkers for inflammation and immune activation, and HIV-associated non-Hodgkin B cell lymphoma risk. Cancer Epidemiol Biomarkers Prev. 2014;23:343–349. doi: 10.1158/1055-9965.EPI-13-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yawetz S, Cumberland WG, van der Meyden M, Martinez-Maza O. Elevated serum levels of soluble CD23 (sCD23) precede the appearance ofacquired immunodeficiency syndrome--associated non-Hodgkin's lymphoma. Blood. 1995;85:1843–1849. [PubMed] [Google Scholar]
- 29.Widney D, Gundapp G, Said JW, van der Meijden M, Bonavida B, Demidem A, et al. Aberrant expression of CD27 and soluble CD27 (sCD27) in HIV infection and in AIDS-associated lymphoma. Clin Immunol. 1999;93:114–123. doi: 10.1006/clim.1999.4782. [DOI] [PubMed] [Google Scholar]
- 30.Levine AM, Scadden DT, Zaia JA, Krishnan A. Hematologic Aspects of HIV/AIDS. Hematology (Am Soc Hematol Educ Program) 2001:463–478. doi: 10.1182/asheducation-2001.1.463. [DOI] [PubMed] [Google Scholar]
- 31.Edelman L, Deveau C, Raphael M, Monchatre E, Gabarre J, Deville-Chabrol A, et al. Serum interleukin-10 in acquired immunodeficiency syndrome lymphoma patients. Seroco-Hemoco Study Group. Eur Cytokine Netw. 1996;7:785–791. [PubMed] [Google Scholar]
- 32.Pastore C, Gaidano G, Ghia P, Fassone L, Cilia AM, Gloghini A, et al. Patterns of cytokine expression in AIDS-related non-Hodgkin's lymphoma. Br J Haematol. 1998;103:143–149. doi: 10.1046/j.1365-2141.1998.00927.x. [DOI] [PubMed] [Google Scholar]
- 33.Epeldegui M, Breen EC, Hung YP, Boscardin WJ, Detels R, Martinez-Maza O. Elevated expression of activation induced cytidine deaminase in peripheral blood mononuclear cells precedes AIDS-NHL diagnosis. AIDS. 2007;21:2265–2270. doi: 10.1097/QAD.0b013e3282ef9f59. [DOI] [PubMed] [Google Scholar]
- 34.Machida K, Cheng KT, Sung VM, Shimodaira S, Lindsay KL, Levine AM, et al. Hepatitis C virus induces a mutator phenotype: enhanced mutations of immunoglobulin and protooncogenes. Proc Natl Acad Sci U S A. 2004;101:4262–4267. doi: 10.1073/pnas.0303971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imbeault M, Ouellet M, Giguere K, Bertin J, BÈlanger D, Martin G, Tremblay MJ. Acquisition of host-derived CD40L by HIV-1 in vivo and its functional consequences in the B-cell compartment. J Virol. 2011;85:2189–2200. doi: 10.1128/JVI.01993-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sparano JA, Lee JY, Kaplan LD, Levine AM, Ramos JC, Ambinder RF, et al. Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood. 2010;115:3008–3016. doi: 10.1182/blood-2009-08-231613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanowith-Klein S, Saxon A. Fc epsilon receptors on human cell lines and peripheral blood lymphocytes detected by binding of IgE immune complexes. J Clin Immunol. 1985;5:38–45. doi: 10.1007/BF00915167. [DOI] [PubMed] [Google Scholar]
- 38.Zhang K, Clark EA, Saxon A. CD40 stimulation provides an IFN-gamma-independent and IL-4-dependent differentiation signal directly to human B cells for IgE production. J Immunol. 1991;146:1836–1842. [PubMed] [Google Scholar]
- 39.Barta SK, Lee JY, Kaplan LD, Noy A, Sparano JA. Pooled analysis of AIDS malignancy consortium trials evaluating rituximab plus CHOP or infusional EPOCH chemotherapy in HIV-associated non-Hodgkin lymphoma. Cancer. 2011 doi: 10.1002/cncr.26723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller G, Hopken UE, Stein H, Lipp M. Systemic immunoregulatory and pathogenic functions of homeostatic chemokine receptors. J Leukoc Biol. 2002;72:1–8. [PubMed] [Google Scholar]
- 41.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Widney DP, Breen EC, Boscardin WJ, Kitchen SG, Alcantar JM, Smith JB, et al. Serum levels of the homeostatic B cell chemokine, CXCL13, are elevated during HIV infection. J Interferon Cytokine Res. 2005;25:702–706. doi: 10.1089/jir.2005.25.702. [DOI] [PubMed] [Google Scholar]
- 43.Regidor DL, Detels R, Breen EC, Widney DP, Jacobson L, Palella F, et al. Effect of highly active antiretroviral therapy on biomarkers of B-lymphocyte activation and inflammation. AIDS. 2011;25:303–314. doi: 10.1097/QAD.0b013e32834273ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Förster R, Schweigard G, Johann S, Emrich T, Kremmer E, Nerl C, Lipp M. Abnormal expression of the B-cell homing chemokine receptor BLR1 during the progression of acquired immunodeficiency syndrome. Blood. 1997;90:520–525. [PubMed] [Google Scholar]
- 45.Cagigi A, Mowafi F, Phuong Dang LV, Tenner-Racz K, Atlas A, Grutzmeier S, et al. Altered expression of the receptor-ligand pair CXCR5/CXCL13 in B cells during chronic HIV-1 infection. Blood. 2008;112:4401–4410. doi: 10.1182/blood-2008-02-140426. [DOI] [PubMed] [Google Scholar]
- 46.Barone F, Bombardieri M, Rosado MM, Morgan PR, Challacombe SJ, De Vita S, et al. CXCL13, CCL21, and CXCL12 expression in salivary glands of patients with Sjogren's syndrome and MALT lymphoma: association with reactive and malignant areas of lymphoid organization. J Immunol. 2008;180:5130–5140. doi: 10.4049/jimmunol.180.7.5130. [DOI] [PubMed] [Google Scholar]
- 47.De Roos AJ, Mirick D, Edlefsen K, LaCroix AZ, Kopecky K, Madeleine M, et al. Markers of B-cell activation in relation to risk of non-Hodgkin lymphoma. Cancer Research. 2012;72:4733–4743. doi: 10.1158/0008-5472.CAN-12-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Purdue MP, Hofmann JN, Kemp TJ, Chaturvedi AK, Lan Q, Park JH, et al. A prospective study of 67 serum immune and inflammation markers and risk of non-Hodgkin lymphoma. Blood. 2013;122:951–957. doi: 10.1182/blood-2013-01-481077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Husson H, Freedman AS, Cardoso AA, Schultze J, Munoz O, Strola G, et al. CXCL13 (BCA-1) is produced by follicular lymphoma cells: role in the accumulation of malignant B cells. Br J Haematol. 2002;119:492–495. doi: 10.1046/j.1365-2141.2002.03832.x. [DOI] [PubMed] [Google Scholar]
- 50.Trentin L, Cabrelle A, Facco M, Carollo D, Miorin M, Tosoni A, et al. Homeostatic chemokines drive migration of malignant B cells in patients with non-Hodgkin lymphomas. Blood. 2004;104:502–508. doi: 10.1182/blood-2003-09-3103. [DOI] [PubMed] [Google Scholar]