Abstract
Chronic Lymphocytic Leukemia (CLL) is a malignancy of mature B lymphocytes which are highly dependent on interactions with the tissue microenvironment for their survival and proliferation. Critical components of the microenvironment are monocyte-derived nurselike cells (NLCs), mesenchymal stromal cells, T cells and NK cells, which communicate with CLL cells through a complex network of adhesion molecules, chemokine receptors, tumor necrosis factor (TNF) family members, and soluble factors. (Auto-) antigens and/or autonomous mechanisms activate the B-cell receptor (BCR) and its downstream signaling cascade in secondary lymphatic tissues, playing a central pathogenetic role in CLL. Novel small molecule inhibitors, including the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib and the phosphoinositide-3-kinase delta (PI3Kδ) inhibitor idelalisib, target BCR signaling and have become the most successful new therapeutics in this disease. We here review the cellular and molecular characteristics of CLL cells, and discuss the cellular components and key pathways involved in the cross-talk with their microenvironment. We also highlight the relevant novel treatment strategies, focusing on immunomodulatory agents and BCR signaling inhibitors and how these treatments disrupt CLL-microenvironment interactions.
Keywords: CLL, nurselike cells, stromal cells, BCR, BCR signaling, BCR signaling inhibitors
1. Background
Chronic Lymphocytic Leukemia (CLL) is the most frequent leukemia in the Western World, with an estimated incidence of about 4.5 new cases per 100.000 individuals annually and a median age at diagnosis of 72 years. CLL is characterized by the clonal expansion and accumulation of mature CD19+CD5+ B lymphocytes in the peripheral blood, bone marrow and secondary lymphoid organs. CLL cells are phenotypically similar to antigen-experienced B cells, and express high levels of surface molecules (such as CD23, CD25, CD69 and CD71) that are up-regulated after antigen encounter, and low levels of markers down-regulated following cellular activation, such as CD22, Fc gamma receptor IIb and CD79b [1]. In addition, they express the memory B-cell marker CD27 [2] and show gene expression profiles similar to memory B cells [3]. The cellular origin of CLL is still debated, although transcriptome analyses of CLL and normal B-cell subsets from human blood and spleen revealed that CLL cells carrying unmutated immunoglobulin heavy chain variable region (IGHV) genes (U-CLL) derive from unmutated mature CD5+ B cells and CLL cells carrying mutated IGHV genes (M-CLL) derive from a distinct, previously unrecognized CD5+CD27+ post-germinal center B-cell subset [4].
2. Biological and genetic features of CLL cells
CLL has a very heterogeneous clinical course; some patients experience very stable disease without requirement for therapy, while others show more aggressive disease and require early treatment. Clinical and biological prognostic factors have been identified that help to define the risk for disease progression in individual patients and to develop personalized treatment strategies. The most important prognostic factors are the clinical staging systems developed by Rai [5] and Binet [6], serum markers including β2 microglobulin levels [7], thymidine kinase levels [8], and soluble CD23 levels [9], cellular markers including CD38 [10] and ζ chain associated protein kinase 70 (ZAP70) [11, 12], and genetic parameters including the mutational status of IGHV genes [10, 13], and cytogenetic aberrations [14].
CD38 is a transmembrane protein that supports B-cell interaction and differentiation through the binding of CD31 [15], a cell-adhesion molecule expressed by cells of the CLL microenvironment, including nurselike cells (NLCs) [16] and T lymphocytes [17]. Patients with high CD38 expression have a faster progression and a shorter life expectancy [10]. ZAP70 is a key signaling molecule in T and NK cells, and is structurally homologous to spleen tyrosine kinase (SYK). ZAP70 enhances BCR signaling [18] and patients whose cells express high levels of ZAP70 protein have a more aggressive disease course [11, 12]. The mutational status of IGHV genes has a very strong prognostic significance. U-CLL cases carry BCRs with ≥98% homology with the corresponding germline sequence and show a more aggressive disease and a shorter median survival time compared to M-CLL (<98% homology) [10, 13]. Additional categorization of CLL into “subsets” based on common IGHV gene expression and shared BCR structure has been described (reviewed in [19]). There is a significant correlation between selected cytogenetic abnormalities and CLL patients’ survival. In previously untreated CLL patients, frequently found aberrations are 13q deletions (55%), chromosome 12 trisomy (15%), 11q deletions (12%) and 17p deletions (8%) [14, 20]. Patients carrying 13q deletions generally have low-risk disease and a favourable outcome [14]. The deleted region comprises two miRNAs, miR-15-a and mir-16-1, that are highly expressed in normal CD5+ B cells, where they may act as negative regulators of the anti-apoptotic molecule B-cell lymphoma 2 (BCL2) [14]. A mouse model with a targeted deletion of miR-15-a and mir-16-1 locus has been generated and recapitulates many features of CLL [21]. 17p and 11q deletions, comprising the p53 and the ataxia telangiectasia mutated (ATM) genes, respectively, are predictors of poor clinical outcome [14]. Whole genome/exome sequencing analyses of CLL samples identified additional recurrent mutations (> 5% cases at diagnosis) (reviewed in [22]) affecting NOTCH1 [23, 24], splicing factor 3B subunit 1 (SF3B1) [25, 26], baculoviral IAP repeat containing 3 (BIRC3) [27] and myeloid differentiation primary response (MYD88) genes [23]. These mutations are generally enriched in CLL patients that have transformed to Richter’s syndrome [24], or in progressive/refractory CLL cases [25, 26]. Recent studies [28, 29] have provided insight into the accumulation of subclonal variants in the CLL clone overtime, including ATM [28], TP53 [28, 29], SF3B1 [29] and NOTCH1 mutations [29], which depends both on the ability of each mutation to provide survival advantage to the cells in terms of proliferation and/or protection from apoptosis, as well as on the accumulation of selected high-risk mutations after treatment.
3. The CLL microenvironment
CLL cell interactions with the supportive tissue microenvironment play a critical role in disease pathogenesis [30]. CLL cells recirculate between peripheral blood and secondary lymphoid organs, where they proliferate in distinct tissue areas, termed “pseudofollicles”, at a daily birth rate of approximately 1–2% of the entire clone, as determined by deuterated water labeling [31]. Homing to tissues is dependent on a tightly regulated interaction between chemokines that are secreted by stromal cells within the tissues, which attract and retain CLL cells to tissues sites via corresponding chemokine receptors, in cooperation with adhesion molecules on the leukemia cells and respective tissue ligands. Over the years, several cellular components of the CLL microenvironment have been described, along with the signaling pathways involved in CLL homing, survival and proliferation, which now provides a rationale for targeting the CLL microenvironment.
3.1 Nurselike cells and mesenchymal stromal cells
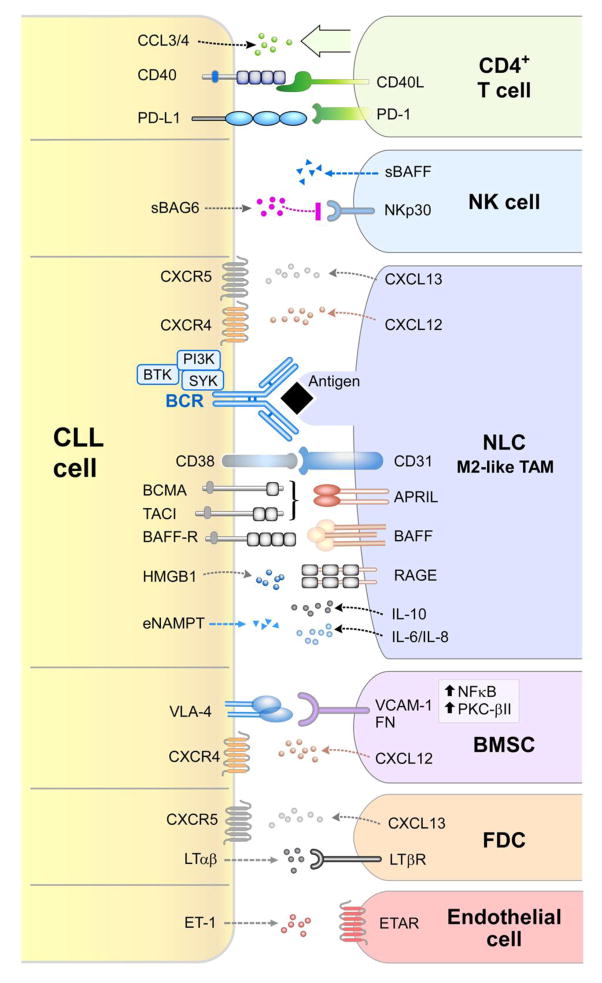
NLCs represent a critical component of the CLL microenvironment (Figure 1 and Table 1). NLCs are cells of monocytic origin, which spontaneously differentiate in vitro from monocytes in high-density cultures of CLL peripheral blood mononuclear cells [32] and which can be found in situ in lymphoid organs from CLL patients [33, 34]. Gene expression profile analyses of CLL cells after CLL-NLC co-culture showed that NLCs activate the BCR and nuclear factor kappa B (NF-κB) signaling pathways in CLL cells [35]; similar gene signatures were identified in CLL cells isolated from lymph nodes of patients [36], demonstrating that NLCs are a valid model for studying the CLL microenvironment. NLCs induce chemotaxis and promote survival of CLL cells through secretion of chemokines C-X-C motif ligand 12 (CXCL12) [32] and CXCL13 [34], and expression of TNF family members B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) [37], and they promote CLL disease progression in vivo in mouse models of CLL [38, 39]. NLCs express antigens that can activate the BCR on CLL cells, including vimentin and calreticulin [40]. They also express CD31, the ligand for CD38, which is expressed on CLL cells [16]. The mechanism through which NLCs differentiate in vitro remains incompletely defined; a recent study demonstrated that high mobility group box 1 (HMGB1) released by CLL cells can stimulate NLC differentiation through activation of the receptor for advanced glycation end-product (RAGE)- toll like receptor 9 (TLR9) pathway [41]. Gene expression profile analyses have shown that NLCs exhibit an M2-like phenotype of tumor associated macrophages (TAMs) [42, 43] and it was recently demonstrated that the M2-phenotype skewing is further promoted by nicotinamide phosphorybosiltransferase (NAMPT), the enzyme responsible for nicotinamide adenine dinucleotide (NAD) biosynthesis, which is produced at high levels by CLL cells [44]. Additional studies have shown that monocytes, protect CLL cells from in vitro apoptosis by secreting soluble CD14, which activates NF-κB in CLL cells [45], and induce gene expression profile changes in CLL including inflammatory cytokine production [46].
Figure 1. Cellular and molecular components of the CLL microenvironment.
Contact between CLL cells and nurselike cells (NLCs) is established and maintained by chemokine receptors and adhesion molecules expressed on CLL cells and corresponding ligands on NLCs, which show phenotypic features similar to M2-like TAMs [42, 43]. BCR signaling is activated in CLL cells after co-culture with NLCs [35], possibly by direct recognition of CLL-BCR ligands expressed by NLCs [40]. Pro-survival pathways activated by the NLC-CLL interaction include the CD38-CD31 axis [15, 16] and the TNF family members APRIL and BAFF, which interact with corresponding receptors BCMA, TACI and BAFF-R [37]. Extracellular release of eNAMPT by CLL cells further promotes M2-skewing of TAMs, with associated release of tumor promoting (i.e. IL-6, IL-8) and immunosuppressive (i.e. IL-10) cytokines [44]. Differentiation of NLCs is promoted by HMGB1-RAGE) interactions [41]. NLCs attract CLL cells by secreting CXCL12 [32, 83, 87, 89] and CXCL13 chemokines [34, 66], which interact with their cognate receptors CXCR4 and CXCR5, which are expressed at high levels on CLL cells. BCR stimulation induces CCL3 and CCL4 chemokine secretion [35], which recruit T cells and monocytes to tissue microenvironments. The CD40/CD40L axis favors survival and proliferation of CLL cells [68, 101, 102], and interaction of PD-L1 ligand with PD-1, which is expressed at high levels on the surface of T cells from CLL patients, favors immune evasion of CLL cells from T-cell cytotoxicity [70, 72, 76]. Several factors contribute to reduced NK-cell cytotoxicity, including low expression of NK-cell activating receptors, such as NKp30 [79, 80], soluble BAFF release by NK cells [82], and soluble BAG6 release by CLL cells [77]. Adhesion to bone marrow stromal cells (BMSCs) is mediated by VCAM-1 or FN interaction with VLA-4 integrins [96], and chemotaxis towards BMSCs involves the CXCR4-CXCL12 axis [85]. Cross-talk between CLL cells and follicular dendritic cells (FDCs) through the CXCR5-CXCL13 and LTαβ-LTβR axis is essential for CLL positioning within lymphoid follicles and leukemia progression in vivo [66]. CLL cells additionally secrete ET-1, which interacts with ETAR receptor on endothelial cells and promotes survival and drug resistance [63].
Table 1.
Signaling axes in the CLL microenvironment: cellular interactions and functions.
| Receptor | Ligand | CLL interaction partner | Function | References |
|---|---|---|---|---|
| BCR | Antigen | NLC | BCR signaling activation, chemotaxis, CCL3 and CCL4 chemokine secretion, survival | [35, 40] |
| CXCR4 | CXCL12 | NLC BMSC |
Chemotaxis | [32, 83, 87, 89] |
| CXCR5 | CXCL13 | NLC FDC |
Chemotaxis, CLL positioning within lymphoid follicles | [34, 66] |
| BAFFR | BAFF | NLC | Survival | [37] |
| BCMA/TACI | APRIL | NLC | Survival | [37] |
| CD31 | CD38 | NLC | Adhesion, survival, proliferation | [15, 16] |
| RAGE | HMGB1 | NLC | NLC differentiation | [41] |
| LTβR | LTαβ | FDC | CXCL13 release by FDC | [66] |
| VCAM-1 | VLA-4 | BMSC | Adhesion | [96] |
| PD-1 | PD-L1 | CD4+ T CD8+ T |
T-cell dysfunction, impaired immune synapse formation | [70, 72, 76] |
| CD40 | CD40L | CD4+ T | Survival, CCL17 and CCL22 chemokine secretion | [68, 101, 102] |
| ETAR | ET-1 | Endothelium | Survival, drug resistance | [63] |
Mesenchymal stromal cells, such as bone marrow stromal cells (BMSCs), are “feeder” layers for normal hematopoietic progenitor cells and contribute to normal bone marrow architecture. Mesenchymal stromal cells are also commonly found in secondary lymphatic tissues of CLL patients [47], where they provide survival and migration signals to CLL cells (Figure 1 and Table 1). CLL cells are protected from spontaneous and drug-induced apoptosis by direct contact with BMSCs [48, 49], and they are able to co-opt and disrupt normal bone marrow architecture [50]. Stromal cells constitutively secrete chemokines, which organize CLL-cell trafficking and tissue homing [51], and provide additional signals that support CLL survival and promote drug resistance. BMSCs induce up-regulation of aggressive disease markers in CLL cells, including ZAP70 and CD38, as well as down-regulation of C-X-C motif receptor 4 (CXCR4) [52]. BMSCs have also been recently shown to down-modulate CD20 expression from the surface of CLL cells [53], with implications for resistance to anti-CD20 antibody treatment. In addition, stromal cells promote glutathione synthesis in CLL cells [54], and induce glycolysis through NOTCH-mediated c-MYC activation, thus promoting cell survival and drug resistance [55]. Not only CLL cells benefit from bone marrow stroma contact, the stromal cells in turn also become activated by CLL cells, with induction of protein kinase C beta II (PKCβII) expression and NF-κB pathway activation [56]. CLL cells are also able to release microvesicles, which are enriched in activated signaling proteins [57] and can activate the AKT pathway in BMSCs [58], supporting the relevance of a bidirectional cross-talk between CLL cells and stromal cells.
3.2 Endothelial cells and follicular dendritic cells
Additional cellular elements in the CLL microenvironment include endothelial cells and follicular dendritic cells (FDCs) (Figure 1 and Table 1), which are essential for tissue homing and CLL retention to tissues. Adhesion to microvascular endothelial cells promotes CLL survival, activation and drug resistance [59–63]. CLL cells bind to β1 and β2 integrins [62] and to BAFF and APRIL on the surface of microvascular endothelial cells [60]. In addition, endothelin 1 (ET-1) engagement on CLL cells by endothelin subtype A receptor (ETAR) on endothelial cells promotes CLL survival and drug resistance, which can be blocked by ETAR inhibition [63]. In vitro culture with FDCs rescues CLL cells from spontaneous apoptosis by direct cell contact, dependent on ligation of CD44 on CLL cells and subsequent up-regulation of myeloid cell leukemia 1 (MCL1), a member of the BCL2 family of anti-apoptotic proteins [64]. The CD100/plexinB1 cross-talk also appears to be involved in this context [65]. Reciprocal cross-talk between CLL cells and FDCs via the CXCR5-CXCL13 and the lymphotoxin beta receptor (LTβR)/lymphotoxin alpha beta (LTαβ) signaling pathways is essential for CLL positioning within lymphoid follicles and for leukemia progression in vivo in the EμTCL1 mouse model of CLL [66].
3.3 T and NK cells
Interactions between CD40-expressing B cells and CD40 ligand (CD40L) on activated CD4+ T cells are critical in the context of antigen presentation and induction of normal B-cell responses [67]. Similarly, activation of malignant B cells by CD40 ligation promotes survival of CLL cells [68] (Figure 1 and Table 1). In CLL, the overall number of circulating T cells, oligoclonal in both the CD4+ and the CD8+ compartment, is increased, though their functionality appears to be compromised [69]. Increased numbers of effector memory CD4+ cells and terminally differentiated CD8+ lymphocytes associate with a more advanced disease stage [70]. CD4+ and CD8+ cells fail to form functional immune synapses [71, 72], show reduced Rho GTPase mediated T-cell motility [73] and display higher expression of exhaustion markers including programmed cell death protein 1 (PD-1) [70, 74, 75]; accordingly, CLL cells express high levels of PD-1 ligand (PD-L1) [70, 72]. Interference with the PD-1/PD-L1 axis by PD-L1 blocking antibodies prevents CLL development and restores immune effector functions, including those of T cells and macrophages, in the EμTCL1 adoptive transfer model of CLL [76]. T cells from CLL patients additionally show increased expression of the inhibitory receptor cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) [77] and increased proliferation when CTLA-4 is blocked with anti-CTLA-4 antibodies [77]. Over the years, several studies have also reported defective NK-cell function. Human leukocyte antigen G (HLA-G) molecule overexpression in the plasma of CLL patients [78] induces NK-cell apoptosis and impairs NK-cell mediated cytotoxicity. Reduced NK-cell cytotoxicity has been associated to low expression levels of the activating receptors natural killer cell p30-related protein (NKp30) [79, 80] and natural killer group 2 member D (NKGD2) [81]; NK cells can also produce soluble BAFF, which interferes with NK-cell mediated CLL cell lysis after rituximab administration [82], and show reduced responses to the activating soluble BCL2-associated athanogene 6 (BAG6) ligand produced by CLL cells [79]. Taken together, these findings indicate that both the T and NK-cell compartments have overall reduced effector activities, which can explain the evasion of CLL cells from immune-mediated destruction.
3.4 Chemokines and adhesion molecules
CLL cell trafficking and homing to tissue microenvironments is tightly regulated, involving activation of chemokine receptors and adhesion molecules on the CLL cells. CLL chemotaxis towards stromal cells is promoted by the chemokine CXCL12 (previously called stromal cell derived factor 1 or SDF-1), which is secreted both by BMSCs [83] and by NLCs [32] (Figure 1 and Table 1). Additionally, NLCs secrete the chemokine CXCL13 [34], which attracts CLL cells through interaction with its receptor, CXCR5 [34]. High levels of CXCR4+ cells have been associated with higher risk of lymphoid organ infiltration and poorer disease outcome [84], as well as higher responsiveness to BCR stimulation [85]. CXCR4 surface expression is stabilized through hyper-phosphorylation mediated by proviral integration site for moloney murine leukemia virus (PIM) kinase [86] and regulated by receptor endocytosis after CXCL12 binding [87]; consequently, CXCR4 surface levels are low in lymph nodes and bone marrow of CLL patients, where CXCL12 levels are high [88]. CXCR4 is in close proximity to CD38 on the surface of CLL cells, and CD38 synergizes with CXCR4 signaling to promote homing and chemotaxis to CXCL12 [89]. CLL cells expressing high levels of ZAP70 [90], CD38 [15] and very late antigen-4 (VLA-4) integrins [91] show higher chemotaxis towards CXCL12, and a higher degree of extravasation in an in vitro model of CLL migration [92]. CXCR4 stimulation is associated to prolonged CLL cell survival in vitro [32, 37] and activation of extracellular signal-regulated kinase (ERK) kinase and signal transducer and activator of transcription 3 (STAT-3) signaling [93, 94]. The integrin VLA-4 interacts with CD38 molecule [95], is involved in CLL-cell adhesion to stroma [96] and its expression is associated with inferior clinical outcome of patients [97]. Another layer of complexity is added by chemokines secreted by the CLL cells themselves. Activated CLL cells secrete high levels of the chemokines C-C motif ligand 3 (CCL3) and CCL4 following BCR stimulation or after co-culture with NLCs [35] and higher plasma levels of CCL3 and CCL4 in CLL patients are associated with an inferior clinical outcome [98]. CCL3 and CCL4 presumably recruit T cells and monocyte/macrophages to tissue sites for interactions with CLL cells [99, 100]. In addition, CLL cells activated via CD40 secrete CCL17 and CCL22 [101, 102], which also can recruit T cells [101].
3.5 Angiogenic factors
In the normal bone marrow, balanced expression of pro- and anti-angiogenic factors, in concert with chemokines and cytokines, supports stable tissue maintenance and tissue homeostasis; imbalances between pro- and anti-angiogenic factors result in pathological angiogenesis. There is now more than circumstantial evidence supporting a role for angiogenesis in CLL pathogenesis. Microvessel density is increased in bone marrow biopsies of CLL patients [103] and angiogenic factors are expressed by CLL cells, including vascular endothelial growth factor (VEGF) [104], which is also a negative prognostic indicator [105]. CLL cells express VEGF receptors [106], and high levels of neuropilin-1 (NRP1), a VEGF coreceptor [107]. The levels of two other important angiogenic factors, basic fibroblast growth factor (bFGF) [108] and platelet-derived growth factor (PDGF) [109] are increased in CLL patients and correlate with disease stage and chemotherapy resistance. Interaction of mesenchymal stromal cells with CLL cells increases the production of VEGF and PDGF [110]; in turn, PDGF binding activates the AKT pathway in stromal cells with subsequent secretion of additional VEGF [109]. High levels of angiogenic factors may decrease the stability of the endothelial cell layer, thus allowing neo-angiogenesis and transendothelial migration of CLL cells. Neo-angiogenesis can be targeted with immunomodulatory agents including lenalidomide, which reduces both VEGF and bFGF levels and increases the stability of the endothelium [111].
4. B-cell receptor signaling in CLL
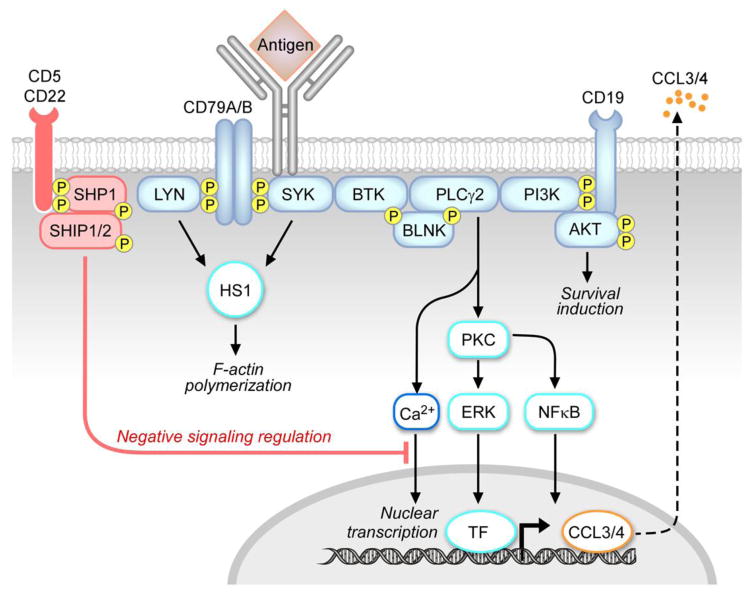
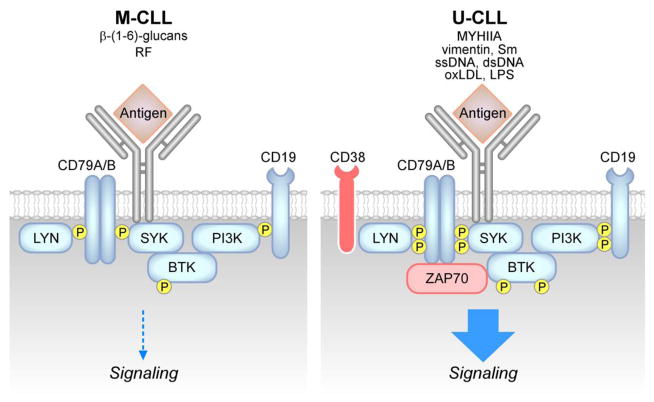
The B-cell receptor is a multimeric complex composed by the antigen-specific surface immunoglobulin (sIg) and the Ig-α/Ig-β hetero-dimers (CD79A, CD79B) (Figure 2). Antigen binding to the sIg induces activation of upstream kinases, including SYK and the Src kinase LYN, which phosphorylate immunoreceptor tyrosine-based activation motifs (ITAMs) in the cytoplasmatic tails of CD79A and CD79B. This, in turn, activates the cytoskeleton, including hematopoietic cell-specific LYN substrate-1 (HS1) protein [112, 113] and the related F-actin polymerization, as well as other upstream kinases, including BTK and PI3Ks and downstream pathways, including phospholipase C gamma 2 (PLC-γ2), calcium signaling, PKC, NF-κB signaling, mitogen-activated protein kinases (MAPKs) and nuclear transcription. Activation of phosphatases, including Src homology 2 (SH2) domain containing protein tyrosine phosphatase-1 (SHP1) and SH2 domain containing inositol 5-phosphatases 1/2 (SHIP1/2), and of negative co-receptors (e.g. CD22, CD5) contributes to negative regulation of the BCR signaling response. There is increasing evidence that BCR signaling plays a relevant role in CLL pathogenesis [114, 115]. CLL-BCRs show differential degrees of somatic mutations, which correlate with the clinical prognosis of patients [10,13] and one third of CLL patients express quasi-identical (“stereotyped”) BCRs [116], suggesting that common antigens may be relevant to disease pathogenesis across patients subsets. CLL cells show features of mature B cells [3, 4], and most of them express surface immunoglobulins of both IgM and IgD isotypes [117, 118]. Cells expressing high levels of CD38 [10, 117], ZAP70 [119] and carrying unmutated IGHV genes [10, 120] are generally more responsive to IgM stimulation. On the other hand, M-CLL cells usually show constitutive phosphorylation of signaling proteins, including ERK kinase [121, 122] and reduced levels of responsiveness to BCR stimulation, generally referred to as “anergy”. Despite one single study, which identified mutations in the CD79B gene [123], there is general consensus on the absence of somatic mutation on both CD79A and CD79B, which may lead to constitutive activation of BCR signaling, a phenomenon observed in the ABC subtype of diffuse large B-cell lymphoma [124]. The role of IgD signaling in CLL is less defined [118, 125, 126]. IgD stimulation can cause CLL-cell apoptosis [125] or survival and plasma cell differentiation [126] and differential responsiveness to IgD stimulation has been linked to clinical outcome [127]. Although the nature of the antigens stimulating CLL-BCRs in patients is still poorly defined, some reports have shown that U-CLL BCRs are polyreactive and mostly recognize autoantigens and other environmental antigens [40, 128–137] including cytoskeletal non-muscle myosin heavy chain IIA (MYHIIA), vimentin, cofilin-1, Fc tail of IgG, single-stranded DNA (ssDNA) or double-stranded DNA (dsDNA), lipopolysaccharide (LPS), apoptotic cells, oxidized low-density lipoprotein (ox-LDL), lupus-associated ribonuclear protein Smith (Sm), human immunodeficiency virus 1 and hepatitis C viral antigens and bacterial antigens (Figure 3). In contrast, affinity-matured BCRs from M-CLL cases bind to a restricted set of more specific antigens, including β-(1,6)-glucans from yeast and fungi [138] and the Fc tails of rheumatoid factors [131–133, 139] (Figure 3). Binder et al., also demonstrated that recombinant CLL-BCRs from U-CLL patients are able to recognize vimentin and calreticulin proteins exposed on the surface of NLCs and these interactions are responsible for stroma-mediated anti-apoptotic effects [40]. Interestingly, when M-CLL BCR sequences are reverted to their germline equivalent, the CLL-BCRs regain polyreactivity [130]. In addition to antigen-dependent signaling responses, autonomous signaling capacity of CLL-BCRs due to self-recognition of epitopes within the BCR third complementarity-determining region of the heavy chain (HCDR3) has been described [140, 141] and has been recently reported to be involved, together with BCR responses to low-affinity autoantigens, in leukemia development in vivo in the EμTCL1 mouse model of CLL [115]. Therefore, both antigen-dependent and antigen-independent/autonomous signaling responses appear to be involved in CLL pathogenesis.
Figure 2. The BCR signaling pathway.
BCR triggering by an antigen induces activation of early kinases, including LYN and SYK [199], which then transduce the signal to cytoskeletal activators, including HS1 protein [112, 113], and to other early effectors of the signaling response, including BTK kinase [161]. Through the BLNK adaptor, BTK activates PLCγ2, and subsequent downstream responses, including calcium signaling (Ca2+), PKC, NFκB and ERK kinase [121, 122], and nuclear transcription factors (TF). The positive co-receptor CD19 contributes to the activation of the PI3K-AKT pathway and to survival induction [182]. The signaling response ultimately promotes activation of nuclear transcription, including CCL3 and CCL4 chemokine genes, which are then produced and secreted [35]. The signaling response is tightly modulated by negative coreceptors (e.g. CD22, CD5) and phosphatases, including SHP1 and SHIP1/2.
Figure 3. Differences between M-CLL and U-CLL signaling pathways.
M-CLL cells show constitutive phosphorylation of signaling proteins and reduced activation of the signaling response after BCR triggering by external antigens [121, 122], including β-(1,6)-glucans [138] and rheumatoid factors (RF) [131–133, 139]. U-CLL cells express BCRs specific for autoantigens, including non-muscle myosin heavy chain IIA (MYHIIA), vimentin, lupus associated ribonuclear protein Smith (Sm), single-stranded DNA (ssDNA), double-stranded DNA (dsDNA), oxidized low-density lipoprotein (oxLDL) as well as microbial antigens, including lipo-polysaccaride (LPS) [40, 128–137]. U-CLL are generally highly responsive to antigenic stimulation [10, 120], as well as those expressing high levels of CD38 [10, 117] and ZAP70 [119].
4.1 Targeting the microenvironment: lenalidomide and CXCR4/CXCL12 inhibitors
Lenalidomide is an immunomodulatory agent, which interferes with multiple components of the CLL microenvironment. Lenalidomide only induces mild apoptosis of leukemic cells, but reduces CLL proliferation through a cereblon/p21 dependent mechanism [142] and interferes with NLC-mediated [143] and endothelium-mediated [111] survival support. Lenalidomide has pleiotropic effects on the CLL microenvironment: it increases CD4+ T-mediated antigen presentation, proliferation and activity [144, 145], and enhances NK and CD4+ T-cell mediated anti-tumor immune responses [146, 147]. Lenalidomide restores functional immune synapse formation between T and CLL cells, down-regulates the immunosuppressive axis PD-1/PD-L1, and enhances T-cell motility [71, 73]; it also causes B-cell activation [148], and interferes with the activity and proliferation of T-regulatory cells [149]. Lenalidomide is active alone, in CLL relapsed/refractory patients [150, 151], or as initial treatment for elderly patients [152, 153] or in combination with rituximab [150, 154] and is currently tested in combination with ibrutinib [155].
The CXCR4/CXCL12 signaling axis represents another important therapeutic target in CLL. CXCR4 antagonists have been developed, including peptide CXCR4 antagonists (BKT140), small molecule CXCR4 antagonists (AMD3100, now called plerixafor), and antibodies to CXCR4 (MDX-1338/BMS 93656) [156]. Plerixafor inhibits CXCL12-mediated signaling activation on CLL cells, along with chemotaxis and F-actin polymerization and interferes with CLL-NLC and CLL-BMSC interactions [157]. Mobilization of CLL cells to the peripheral blood was observed in a phase I clinical trial of plerixafor used in combination with rituximab in relapsed CLL patients [158]. CXCL12 targeting has been achieved through the use of RNA oligonucleotides, such as NOX-A12, which inhibit CLL-cell migration in vitro and sensitize CLL cells towards cytotoxic agents [159]. NOX-A12 is currently tested in a phase II trial in combination with bendamustine and rituximab in relapsed CLL patients [160].
4.2 Targeting BCR-associated kinases BTK, PI3K, and SYK: ibrutinib, idelalisib, fostamatinib and novel small molecule inhibitors
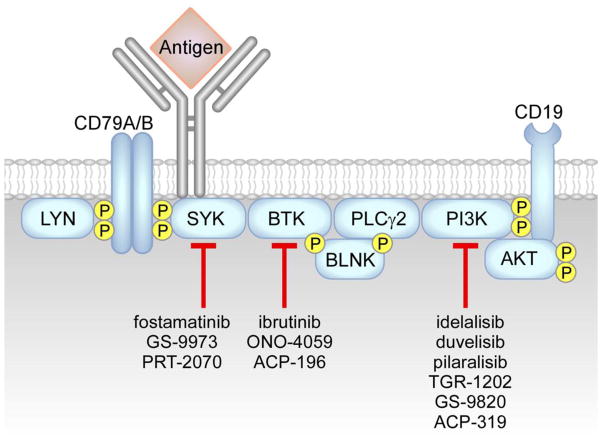
Several small molecule BCR signaling inhibitors, mainly targeting BTK, PI3K and SYK kinases, have been generated and have shown excellent clinical activity (Figure 4 and Table 2). BTK is a non-receptor tyrosine kinase of the Tec family, and is rapidly phosphorylated by both LYN and SYK kinases upon BCR engagement, resulting in the activation of PLCγ2, AKT and ERK kinases, and NF-κB signaling [161]. BTK mutations in humans are associated to X-linked agammaglobulinemia, a primary immunodeficiency characterized by the absence of peripheral blood B cells and decreased levels of serum immunoglobulins [161]. In addition, BTK is also involved in the regulation of migration and adhesion via CXCR4/CXCR5 and integrin signaling [162]. Ibrutinib is an orally bioavailable inhibitor which was approved in 2014 for the treatment of mantle cell lymphoma and CLL; it blocks BTK kinase activity by forming a covalent bond with cysteine-481 residue [163, 164]. Ibrutinib is capable of overcoming pro-survival signals derived from the CLL microenvironment in vitro, including those from NLC- contact, CD40 ligation, BAFF, fibronectin (FN), IL-6, IL-4, TNFα [165] and BCR stimulation [166]. Ibrutinib inhibits CLL-cell proliferation [166], chemotaxis towards CXCL12 and CXCL13 [166, 167], integrin-mediated adhesion [167], CLL-cell release of exosomes [168] and of CCL3 and CCL4 chemokines, in vitro and in CLL patients receiving ibrutinib therapy [166]. Ibrutinib has been tested either alone [169, 170], or in combination with chemo-immunotherapy, including rituximab [171] or bendamustine and rituximab, or fludarabine cyclophosphamide and rituximab [172]. High rates of durable remissions in patients with relapsed refractory disease, including patients with high-risk genetic lesions (e.g. del17p and del11q) [169, 171, 173, 174], as well as in previously untreated older patients (>65 years) have been reported [173, 175]. Early lymphocytosis and organomegaly reduction followed by lymphocyte count normalization are typical effects of ibrutinib treatment [176, 177, 178], linked to CLL-cell inhibition of proliferation and induction of cell death in vivo. Mutations in BTK and PLCγ2 were identified through whole-exome sequencing of peripheral blood samples from patients experiencing relapse after ibrutinib treatment, including a cysteine-to-serine mutation at position 481 in BTK (C481S) leading to a protein product with reduced kinase activity, that is only partially inhibited by ibrutinib [179, 180], and three putative gain-of-function mutations in PLCγ2, including arginine-to-tryptophan at position 665 (R665W), leucine to phenylalanine at position 845 (L845F) and serine to tyrosine at position 707 (S707Y) [179]. In addition to ibrutinib, novel small molecules inhibitors of BTK kinase have been tested and are currently under early clinical development, including ACP-196 and ONO-4059 [181].
Figure 4. BCR signaling inhibitors.
Interference with the BCR signaling axis can be obtained with inhibitors of SYK kinase, including fostamatinib [200], GS-9973 [202], and PRT-2070, of BTK kinase, including ibrutinib [163–180], ACP-196 and ONO-4059 [181], and of PI3K kinases, including idelalisib (δ inhibitor) [165, 183], duvelisib (also called IPI-145, γ,δ inhibitor) [192], pilaralisib (also called SAR245408, pan-PI3K inhibitor) [196], GS-9820 (β,δ inhibitor), TGR-1202 (δ inhibitor) [197], and ACP-319 (δ inhibitor).
Table 2.
Current therapeutic strategies using BCR signaling inhibitors
| Inhibitor | Target | Phase of study | Treatment | Patients | Reference/clinicaltrial.gov identifier |
|---|---|---|---|---|---|
| Ibrutinib | BTK | III | Monotherapy vs. Ofatumumab (anti-CD20) | R/R | [170] |
| II | Monotherapy | R/R and untreated >65yrs | [173] | ||
| II | Monotherapy | R/R | [174] | ||
| II | + R | R/R and untreated > 65 yrs | [171] | ||
| Ib-II | Monotherapy | R/R | [169] | ||
| Ib-II | Monotherapy | untreated >65yrs | [175] | ||
| Ib | + BR or FCR | R/R | [203] | ||
| I | + Lenalidomide | R/R | [155] | ||
| ACP-196 | BTK | II | Monotherapy | R/R or untreated | NCT02337829 |
| Ib-II | +Pembrolizumab (anti-PD1) | untreated | NCT02362035 | ||
| I | +Obinutuzumab (anti-CD20) | R/R or untreated >65yrs | NCT02296918 | ||
| ONO-4059 | BTK | I | Monotherapy | R/R | [181] |
| Idelalisib | PI3Kδ | III | +R vs. placebo + R | R/R | [191] |
| II | + R | untreated >65yrs | [190] | ||
| II | Monotherapy | untreated >65yrs | [187] | ||
| I | Monotherapy | R/R | [186] | ||
| I | + B, BR, F, Chl or ChlR | R/R | [189] | ||
| Duvelisib (IPI-145) | PI3Kγ,δ | III | Monotherapy vs. Ofatumumab | R/R | NCT02004522 |
| I | Monotherapy | R/R | [194] | ||
| I | Monotherapy | R/R previously treated with ibrutinib | [195] | ||
| I | +Obinutuzumab | R/R to prior BTK inhibitor therapy | NCT02292225 | ||
| Pilaralisib (SAR245408) | pan-PI3K | I | Monotherapy | R/R | [196] |
| GS-9820 | PI3Kβ,δ | I | Monotherapy | R/R | NCT01705847 |
| TGR-1202 | PI3Kδ | I | Monotherapy | R/R | [198] |
| I | + Obinutuzumab and Chl | untreated | NCT02100852 | ||
| I | + Ublituximab (anti-CD20) and/or ibrutinib | R/R | [197] | ||
| I-Ib | + ibrutinib | R/R | NCT02268851 | ||
| ACP-319 | PI3Kδ | I | + ACP-196 | R/R | NCT02157324 |
| Fostamatinib | SYK | II | Monotherapy | R/R | [200] |
| GS-9973 | SYK | II | Monotherapy | R/R | [202] |
| II | + idelalisib | R/R | NCT01796470 | ||
| PRT-2070 | SYK | Pending phase I | n.a. | n.a. | n.a. |
R/R: relapsed/refractory; F: Fludarabine; C: Cyclophosphamide; R: Rituximab; B: Bendamustine; Chl: Chlorambucil; n.a.: not applicable.
PI3Ks are divided into 3 classes (I through III) and class I is further composed by four different isoforms (α,β,γ,δ). PI3Ks are responsible for the activation of AKT kinase along the BCR signaling pathway, as well as they exert effects on cell metabolism, migration, proliferation, and survival [182]. The predominant form expressed by hematopoietic cells is PI3Kδ, which plays a critical role in B-cell homeostasis and function. Idelalisib is a PI3Kδ inhibitor, which was approved by the FDA in 2014 for the treatment of previously treated CLL patients when used in combination with rituximab. Idelalisib is a highly selective PI3Kδ inhibitor [183], which antagonizes CLL-survival signals coming from the microenvironment, such as NLC-contact [184], CD40 ligation, TNFα, fibronectin and BCR stimulation [165, 183]. Idelalisib reduces CLL-cell chemotaxis towards CXCL12 and CXCL13 [184], CCL3 and CCL4 release by CLL cells in vitro and in patients receiving idelalisib therapy [184], and synergizes with ibrutinib in reducing CLL adhesion to vascular cell-adhesion molecule-1 (VCAM-1) and fibronectin [185]. Idelalisib has been tested as single agent [186, 187], or in combination strategies [188–191]. Similar to patients receiving ibrutinib, idelalisib induces early lymphocytosis followed by lymphocyte count normalization. Additional PI3K inhibitors are currently under development, including duvelisib, also called IPI-145, a potent PI3K γ–δ inhibitor, which antagonizes BCR and microenvironment interactions in vitro [192], even in cells carrying the BTK C481S mutation [193], and has been tested either alone [194, 195] or in combination with anti-CD20 antibodies. Additional small molecule inhibitors in early clinical development include the pan-PI3K inhibitor pilaralisib, also called SAR245408 [196], the PI3K β,δ inhibitor GS-9820, and the PI3Kδ inhibitors ACP-319 and TGR-1202 [197, 198].
SYK kinase belongs to the SYK/ZAP70 family of non-receptor kinases, and activates signaling pathways downstream of the BCR, chemokine and integrin receptors, suggesting that SYK participates in tissue homing and retention of activated B cells [199]. Fostamatinib (FosD, R788) is an orally available inhibitor of SYK, which induced partial responses in relapsed CLL patients in phase I/II study [200], but further development of this drug focused on rheumatoid arthritis [201]. Additional SYK-specific inhibitors are under development, including GS-9973, which has been tested alone [202] or in combination with idelalisib, and PRT-2070.
5. Conclusions and perspective
A plethora of cellular and molecular components shape the CLL microenvironment, and the mechanisms involved in CLL proliferation and survival have been progressively unraveled. The CLL microenvironment has gained extensive attention during the last few years, thanks to the introduction of small molecule inhibitors, which target the CLL-microenvironment cross-talk. The BCR signaling pathway is central to CLL activation and likely to be triggered by antigens expressed in the tissue microenvironment. Inhibitors targeting BCR-associated kinases, including ibrutinib and idelalisib, have changed the landscape of treatment for CLL patients, inducing durable remissions in relapsed/refractory patients, including those carrying unfavorable genetic alterations (e.g. del17p, del11q). Recently, point mutations in one of the drug targets, BTK kinase, and activating mutations in closely related BCR pathway molecules (i.e. PLCγ2) have been linked to resistance [179]. Randomized trials comparing new drugs and/or their combinations with standard chemo-immunotherapy regimens are ongoing and will allow a better definition of optimal treatment strategies. The complexity of the cross-talk between CLL cells and their microenvironment, as well as the mechanisms of drug resistance and treatment failure still need to be better defined and are currently investigated.
Highlights.
CLL cells are dependent on interactions with their microenvironment for survival
Nurselike cells, T and stromal cells are key components of the CLL microenvironment
B-cell receptor signaling has a central pathogenetic role in CLL
BCR signaling inhibitors are the most successful new therapeutics for CLL
Acknowledgments
The work was supported by a Leukemia & Lymphoma Society Scholar Award in Clinical Research (J.A.B.), and MD Anderson’s Moon Shot Program in CLL. This research is also supported in part by the MD Anderson Cancer Center Support Grant CA016672.
Abbreviations
- APRIL
a proliferation-inducing ligand
- ATM
ataxia telangiectasia mutated
- BAFF
B-cell activating factor
- BAFFR
BAFF receptor
- BAG6
BCL2-associated athanogene 6
- BCMA
B-cell maturation antigen
- BCL2
B-cell lymphoma 2
- BCR
B cell receptor
- bFGF
basic fibroblast growth factor
- BIRC3
baculoviral IAP repeat containing 3
- BMSC
bone marrow stromal cell
- BTK
Bruton’s tyrosine kinase
- CCL
C-C motif ligand
- CD40L
CD40 ligand
- CLL
Chronic Lymphocytic Leukemia
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- CXCL
C-X-C motif ligand
- CXCR
C-X-C motif receptor
- dsDNA
double-stranded DNA
- ERK
extracellular signal-regulated kinase
- ET-1
endothelin 1
- ETAR
endothelin subtype A receptor
- FDC
follicular dendritic cell
- FN
fibronectin
- HCDR3
third complementarity-determining region of the heavy chain
- HLA-G
human leukocyte antigen G
- HMGB1
high mobility group box 1
- HS1
hematopoietic cell-specific LYN substrate-1
- IGHV
immunoglobulin heavy chain variable region
- IL
interleukin
- ITAM
immunoreceptor tyrosine-based activation motif
- LPS
lipopolysaccharide
- LTαβ
lymphotoxin alpha beta
- LTβR
lymphotoxin beta receptor
- MAPK
mitogen-activated protein kinase
- MCL1
myeloid cell leukemia 1
- M-CLL
mutated IGHV-gene carrying CLL
- MYD88
myeloid differentiation primary response
- MYHIIA
non-muscle myosin heavy chain IIA
- NAD
nicotinamide adenine dinucleotide
- NAMPT
nicotinamide phosphorybosiltransferase
- NF-κB
nuclear factor kappa B
- NKGD2
natural killer group 2 member D
- NKp30
natural killer cell p30-related protein
- NLC
nurselike cells
- NRP1
neuropilin-1
- ox-LDL
oxidized low-density lipoprotein
- PD-1
programmed cell death protein 1
- PDGF
platelet-derived growth factor
- PD-L1
PD-1 ligand
- PI3K
phosphoinositide-3-kinase
- PIM
proviral integration site for moloney murine leukemia virus
- PKC
protein kinase C
- PLC-γ2
phospholipase C gamma 2
- RAGE
receptor for advanced glycation end-product
- SDF-1
stromal cell derived factor 1
- SF3B1
splicing factor 3B subunit 1
- SH2
Src homology 2
- SHP1
SH2 domain containing protein tyrosine phosphatase-1
- SHIP1/2
SH2 domain containing inositol 5-phosphatases 1/2
- sIg
surface immunoglobulin
- Sm
lupus-associated ribonuclear protein Smith
- ssDNA
single-stranded DNA
- STAT-3
signal transducer and activator of transcription 3
- SYK
spleen tyrosine kinase
- TACI
transmembrane activator and calcium modulator and cyclophilin ligand interactor
- TAM
tumor associated macrophage
- TLR
toll like receptor
- TNF
tumor necrosis factor
- U-CLL
unmutated IGHV-gene carrying CLL
- VCAM-1
vascular cell-adhesion molecule-1
- VEGF
vascular endothelial growth factor
- VLA-4
very late antigen-4
- ZAP70
ζ chain associated protein kinase 70
Footnotes
Conflict of interest disclosure
J. A. Burger has received commercial research grants from Pharmacyclics, Gilead, and Portola Pharmaceuticals, and is a consultant/advisory board member for Noxxon, Boehringer Ingelheim Pharma, Janssen, and Pharmacyclics. E. ten Hacken declares no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. The New England journal of medicine. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Damle RN, Ghiotto F, Valetto A, Albesiano E, Fais F, Yan XJ, Sison CP, Allen SL, Kolitz J, Schulman P, Vinciguerra VP, Budde P, Frey J, Rai KR, Ferrarini M, Chiorazzi N. B-cell chronic lymphocytic leukemia cells express a surface membrane phenotype of activated, antigen-experienced B lymphocytes. Blood. 2002;99:4087–4093. doi: 10.1182/blood.v99.11.4087. [DOI] [PubMed] [Google Scholar]
- 3.Klein U, Tu Y, Stolovitzky GA, Mattioli M, Cattoretti G, Husson H, Freedman A, Inghirami G, Cro L, Baldini L, Neri A, Califano A, Dalla-Favera R. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. The Journal of experimental medicine. 2001;194:1625–1638. doi: 10.1084/jem.194.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seifert M, Sellmann L, Bloehdorn J, Wein F, Stilgenbauer S, Durig J, Kuppers R. Cellular origin and pathophysiology of chronic lymphocytic leukemia. The Journal of experimental medicine. 2012;209:2183–2198. doi: 10.1084/jem.20120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 6.Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, Vaugier G, Potron G, Colona P, Oberling F, Thomas M, Tchernia G, Jacquillat C, Boivin P, Lesty C, Duault MT, Monconduit M, Belabbes S, Gremy F. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198–206. doi: 10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.Keating MJ. Chronic lymphocytic leukemia. Semin Oncol. 1999;26:107–114. [PubMed] [Google Scholar]
- 8.Hallek M, Langenmayer I, Nerl C, Knauf W, Dietzfelbinger H, Adorf D, Ostwald M, Busch R, Kuhn-Hallek I, Thiel E, Emmerich B. Elevated serum thymidine kinase levels identify a subgroup at high risk of disease progression in early, nonsmoldering chronic lymphocytic leukemia. Blood. 1999;93:1732–1737. [PubMed] [Google Scholar]
- 9.Sarfati M, Chevret S, Chastang C, Biron G, Stryckmans P, Delespesse G, Binet JL, Merle-Beral H, Bron D. Prognostic importance of serum soluble CD23 level in chronic lymphocytic leukemia [see comments] Blood. 1996;88:4259–4264. [PubMed] [Google Scholar]
- 10.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, Buchbinder A, Budman D, Dittmar K, Kolitz J, Lichtman SM, Schulman P, Vinciguerra VP, Rai KR, Ferrarini M, Chiorazzi N. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. [PubMed] [Google Scholar]
- 11.Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M, Marce S, Lopez-Guillermo A, Campo E, Montserrat E. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. The New England journal of medicine. 2003;348:1764–1775. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 12.Wiestner A, Rosenwald A, Barry TS, Wright G, Davis RE, Henrickson SE, Zhao H, Ibbotson RE, Orchard JA, Davis Z, Stetler-Stevenson M, Raffeld M, Arthur DC, Marti GE, Wilson WH, Hamblin TJ, Oscier DG, Staudt LM. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood. 2003;101:4944–4951. doi: 10.1182/blood-2002-10-3306. [DOI] [PubMed] [Google Scholar]
- 13.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 14.Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, Dohner K, Bentz M, Lichter P. Genomic aberrations and survival in chronic lymphocytic leukemia. The New England journal of medicine. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 15.Deaglio S, Vaisitti T, Aydin S, Bergui L, D’Arena G, Bonello L, Omede P, Scatolini M, Jaksic O, Chiorino G, Efremov D, Malavasi F. CD38 and ZAP-70 are functionally linked and mark CLL cells with high migratory potential. Blood. 2007;110:4012–4021. doi: 10.1182/blood-2007-06-094029. [DOI] [PubMed] [Google Scholar]
- 16.Deaglio S, Vaisitti T, Bergui L, Bonello L, Horenstein AL, Tamagnone L, Boumsell L, Malavasi F. CD38 and CD100 lead a network of surface receptors relaying positive signals for B-CLL growth and survival. Blood. 2005;105:3042–3050. doi: 10.1182/blood-2004-10-3873. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka Y, Albelda SM, Horgan KJ, van Seventer GA, Shimizu Y, Newman W, Hallam J, Newman PJ, Buck CA, Shaw S. CD31 expressed on distinctive T cell subsets is a preferential amplifier of beta 1 integrin-mediated adhesion. The Journal of experimental medicine. 1992;176:245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Huynh L, Apgar J, Tang L, Rassenti L, Weiss A, Kipps TJ. ZAP-70 enhances IgM signaling independent of its kinase activity in chronic lymphocytic leukemia. Blood. 2008;111:2685–2692. doi: 10.1182/blood-2006-12-062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agathangelidis A, Vardi A, Baliakas P, Stamatopoulos K. Stereotyped B-cell receptors in chronic lymphocytic leukemia. Leukemia & lymphoma. 2014:2252–2261. doi: 10.3109/10428194.2013.879715. [DOI] [PubMed] [Google Scholar]
- 20.Stilgenbauer S, Lichter P, Dohner H. Genetic features of B-cell chronic lymphocytic leukemia. Rev Clin Exp Hematol. 2000;4:48–72. doi: 10.1046/j.1468-0734.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 21.Klein U, Lia M, Crespo M, Siegel R, Shen Q, Mo T, Ambesi-Impiombato A, Califano A, Migliazza A, Bhagat G, Dalla-Favera R. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 2010;17:28–40. doi: 10.1016/j.ccr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Rossi D, Ciardullo C, Spina V, Gaidano G. Molecular bases of chronic lymphocytic leukemia in light of new treatments. Immunol Lett. 2013;155:51–55. doi: 10.1016/j.imlet.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Puente XS, Pinyol M, Quesada V, Conde L, Ordonez GR, Villamor N, Escaramis G, Jares P, Bea S, Gonzalez-Diaz M, Bassaganyas L, Baumann T, Juan M, Lopez-Guerra M, Colomer D, Tubio JM, Lopez C, Navarro A, Tornador C, Aymerich M, Rozman M, Hernandez JM, Puente DA, Freije JM, Velasco G, Gutierrez-Fernandez A, Costa D, Carrio A, Guijarro S, Enjuanes A, Hernandez L, Yague J, Nicolas P, Romeo-Casabona CM, Himmelbauer H, Castillo E, Dohm JC, de Sanjose S, Piris MA, de Alava E, San Miguel J, Royo R, Gelpi JL, Torrents D, Orozco M, Pisano DG, Valencia A, Guigo R, Bayes M, Heath S, Gut M, Klatt P, Marshall J, Raine K, Stebbings LA, Futreal PA, Stratton MR, Campbell PJ, Gut I, Lopez-Guillermo A, Estivill X, Montserrat E, Lopez-Otin C, Campo E. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475:101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossi D, Rasi S, Fabbri G, Spina V, Fangazio M, Forconi F, Marasca R, Laurenti L, Bruscaggin A, Cerri M, Monti S, Cresta S, Fama R, De Paoli L, Bulian P, Gattei V, Guarini A, Deaglio S, Capello D, Rabadan R, Pasqualucci L, Dalla-Favera R, Foa R, Gaidano G. Mutations of NOTCH1 are an independent predictor of survival in chronic lymphocytic leukemia. Blood. 2012;119:521–529. doi: 10.1182/blood-2011-09-379966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quesada V, Conde L, Villamor N, Ordonez GR, Jares P, Bassaganyas L, Ramsay AJ, Bea S, Pinyol M, Martinez-Trillos A, Lopez-Guerra M, Colomer D, Navarro A, Baumann T, Aymerich M, Rozman M, Delgado J, Gine E, Hernandez JM, Gonzalez-Diaz M, Puente DA, Velasco G, Freije JM, Tubio JM, Royo R, Gelpi JL, Orozco M, Pisano DG, Zamora J, Vazquez M, Valencia A, Himmelbauer H, Bayes M, Heath S, Gut M, Gut I, Estivill X, Lopez-Guillermo A, Puente XS, Campo E, Lopez-Otin C. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat Genet. 2012;44:47–52. doi: 10.1038/ng.1032. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Lawrence MS, Wan Y, Stojanov P, Sougnez C, Stevenson K, Werner L, Sivachenko A, DeLuca DS, Zhang L, Zhang W, Vartanov AR, Fernandes SM, Goldstein NR, Folco EG, Cibulskis K, Tesar B, Sievers QL, Shefler E, Gabriel S, Hacohen N, Reed R, Meyerson M, Golub TR, Lander ES, Neuberg D, Brown JR, Getz G, Wu CJ. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. The New England journal of medicine. 2011;365:2497–2506. doi: 10.1056/NEJMoa1109016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi D, Fangazio M, Rasi S, Vaisitti T, Monti S, Cresta S, Chiaretti S, Del Giudice I, Fabbri G, Bruscaggin A, Spina V, Deambrogi C, Marinelli M, Fama R, Greco M, Daniele G, Forconi F, Gattei V, Bertoni F, Deaglio S, Pasqualucci L, Guarini A, Dalla-Favera R, Foa R, Gaidano G. Disruption of BIRC3 associates with fludarabine chemorefractoriness in TP53 wild-type chronic lymphocytic leukemia. Blood. 2012;119:2854–2862. doi: 10.1182/blood-2011-12-395673. [DOI] [PubMed] [Google Scholar]
- 28.Landau DA, Carter SL, Stojanov P, McKenna A, Stevenson K, Lawrence MS, Sougnez C, Stewart C, Sivachenko A, Wang L, Wan Y, Zhang W, Shukla SA, Vartanov A, Fernandes SM, Saksena G, Cibulskis K, Tesar B, Gabriel S, Hacohen N, Meyerson M, Lander ES, Neuberg D, Brown JR, Getz G, Wu CJ. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152:714–726. doi: 10.1016/j.cell.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baliakas P, Hadzidimitriou A, Sutton LA, Rossi D, Minga E, Villamor N, Larrayoz M, Kminkova J, Agathangelidis A, Davis Z, Tausch E, Stalika E, Kantorova B, Mansouri L, Scarfo L, Cortese D, Navrkalova V, Rose-Zerilli MJ, Smedby KE, Juliusson G, Anagnostopoulos A, Makris AM, Navarro A, Delgado J, Oscier D, Belessi C, Stilgenbauer S, Ghia P, Pospisilova S, Gaidano G, Campo E, Strefford JC, Stamatopoulos K, Rosenquist RCLL. European Research Initiative on, Recurrent mutations refine prognosis in chronic lymphocytic leukemia. Leukemia. 2015;29:329–336. doi: 10.1038/leu.2014.196. [DOI] [PubMed] [Google Scholar]
- 30.Caligaris-Cappio F, Bertilaccio MT, Scielzo C. How the microenvironment wires the natural history of chronic lymphocytic leukemia. Seminars in cancer biology. 2014;24:43–48. doi: 10.1016/j.semcancer.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Messmer BT, Messmer D, Allen SL, Kolitz JE, Kudalkar P, Cesar D, Murphy EJ, Koduru P, Ferrarini M, Zupo S, Cutrona G, Damle RN, Wasil T, Rai KR, Hellerstein MK, Chiorazzi N. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. The Journal of clinical investigation. 2005;115:755–764. doi: 10.1172/JCI23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–2663. [PubMed] [Google Scholar]
- 33.Tsukada N, Burger JA, Zvaifler NJ, Kipps TJ. Distinctive features of “nurselike” cells that differentiate in the context of chronic lymphocytic leukemia. Blood. 2002;99:1030–1037. doi: 10.1182/blood.v99.3.1030. [DOI] [PubMed] [Google Scholar]
- 34.Burkle A, Niedermeier M, Schmitt-Graff A, Wierda WG, Keating MJ, Burger JA. Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B-cell chronic lymphocytic leukemia. Blood. 2007;110:3316–3325. doi: 10.1182/blood-2007-05-089409. [DOI] [PubMed] [Google Scholar]
- 35.Burger JA, Quiroga MP, Hartmann E, Burkle A, Wierda WG, Keating MJ, Rosenwald A. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood. 2009;113:3050–3058. doi: 10.1182/blood-2008-07-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herishanu Y, Perez-Galan P, Liu D, Biancotto A, Pittaluga S, Vire B, Gibellini F, Njuguna N, Lee E, Stennett L, Raghavachari N, Liu P, McCoy JP, Raffeld M, Stetler-Stevenson M, Yuan C, Sherry R, Arthur DC, Maric I, White T, Marti GE, Munson P, Wilson WH, Wiestner A. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishio M, Endo T, Tsukada N, Ohata J, Kitada S, Reed JC, Zvaifler NJ, Kipps TJ. Nurselike cells express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of SDF-1alpha. Blood. 2005;106:1012–1020. doi: 10.1182/blood-2004-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Troeger A, Johnson AJ, Wood J, Blum WG, Andritsos LA, Byrd JC, Williams DA. RhoH is critical for cell-microenvironment interactions in chronic lymphocytic leukemia in mice and humans. Blood. 2012;119:4708–4718. doi: 10.1182/blood-2011-12-395939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinart N, Nguyen PH, Boucas J, Rosen N, Kvasnicka HM, Heukamp L, Rudolph C, Ristovska V, Velmans T, Mueller C, Reiners KS, Pogge von Strandmann E, Krause G, Montesinos-Rongen M, Schlegelberger B, Herling M, Hallek M, Fingerle-Rowson G. Delayed development of chronic lymphocytic leukemia in the absence of macrophage migration inhibitory factor. Blood. 2013;121:812–821. doi: 10.1182/blood-2012-05-431452. [DOI] [PubMed] [Google Scholar]
- 40.Binder M, Lechenne B, Ummanni R, Scharf C, Balabanov S, Trusch M, Schluter H, Braren I, Spillner E, Trepel M. Stereotypical chronic lymphocytic leukemia B-cell receptors recognize survival promoting antigens on stromal cells. PloS one. 2010;5:e15992. doi: 10.1371/journal.pone.0015992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia L, Clear A, Liu FT, Matthews J, Uddin N, McCarthy A, Hoxha E, Durance C, Iqbal S, Gribben JG. Extracellular HMGB1 promotes differentiation of nurse-like cells in chronic lymphocytic leukemia. Blood. 2014;123:1709–1719. doi: 10.1182/blood-2013-10-529610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ysebaert L, Fournie JJ. Genomic and phenotypic characterization of nurse-like cells that promote drug resistance in chronic lymphocytic leukemia. Leukemia & lymphoma. 2011;52:1404–1406. doi: 10.3109/10428194.2011.568078. [DOI] [PubMed] [Google Scholar]
- 43.Filip AA, Cisel B, Koczkodaj D, Wasik-Szczepanek E, Piersiak T, Dmoszynska A. Circulating microenvironment of CLL: are nurse-like cells related to tumor-associated macrophages? Blood cells, molecules & diseases. 2013;50:263–270. doi: 10.1016/j.bcmd.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Audrito V, Serra S, Brusa D, Mazzola F, Arruga F, Vaisitti T, Coscia M, Maffei R, Rossi D, Wang T, Inghirami G, Rizzi M, Gaidano G, Garcia JG, Wolberger C, Raffaelli N, Deaglio S. Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood. 2015;125:111–123. doi: 10.1182/blood-2014-07-589069. [DOI] [PubMed] [Google Scholar]
- 45.Seiffert M, Schulz A, Ohl S, Dohner H, Stilgenbauer S, Lichter P. Soluble CD14 is a novel monocyte-derived survival factor for chronic lymphocytic leukemia cells, which is induced by CLL cells in vitro and present at abnormally high levels in vivo. Blood. 2010;116:4223–4230. doi: 10.1182/blood-2010-05-284505. [DOI] [PubMed] [Google Scholar]
- 46.Schulz A, Toedt G, Zenz T, Stilgenbauer S, Lichter P, Seiffert M. Inflammatory cytokines and signaling pathways are associated with survival of primary chronic lymphocytic leukemia cells in vitro: a dominant role of CCL2. Haematologica. 2011;96:408–416. doi: 10.3324/haematol.2010.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruan J, Hyjek E, Kermani P, Christos PJ, Hooper AT, Coleman M, Hempstead B, Leonard JP, Chadburn A, Rafii S. Magnitude of stromal hemangiogenesis correlates with histologic subtype of non-Hodgkin’s lymphoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:5622–5631. doi: 10.1158/1078-0432.CCR-06-1204. [DOI] [PubMed] [Google Scholar]
- 48.Lagneaux L, Delforge A, Bron D, De Bruyn C, Stryckmans P. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood. 1998;91:2387–2396. [PubMed] [Google Scholar]
- 49.Kurtova AV, Balakrishnan K, Chen R, Ding W, Schnabl S, Quiroga MP, Sivina M, Wierda WG, Estrov Z, Keating MJ, Shehata M, Jager U, Gandhi V, Kay NE, Plunkett W, Burger JA. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood. 2009;114:4441–4450. doi: 10.1182/blood-2009-07-233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janel A, Dubois-Galopin F, Bourgne C, Berger J, Tarte K, Boiret-Dupre N, Boisgard S, Verrelle P, Dechelotte P, Tournilhac O, Berger MG. The chronic lymphocytic leukemia clone disrupts the bone marrow microenvironment. Stem cells and development. 2014;23:2972–2982. doi: 10.1089/scd.2014.0229. [DOI] [PubMed] [Google Scholar]
- 51.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114:3367–3375. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Purroy N, Abrisqueta P, Carabia J, Carpio C, Palacio C, Bosch F, Crespo M. Co-culture of primary CLL cells with bone marrow mesenchymal cells, CD40 ligand and CpG ODN promotes proliferation of chemoresistant CLL cells phenotypically comparable to those proliferating in vivo. Oncotarget. 2015;6:7632–7643. doi: 10.18632/oncotarget.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marquez ME, Hernandez-Uzcategui O, Cornejo A, Vargas P, Da Costa O. Bone marrow stromal mesenchymal cells induce down regulation of CD20 expression on B-CLL: implications for rituximab resistance in CLL. British journal of haematology. 2015;169:211–218. doi: 10.1111/bjh.13286. [DOI] [PubMed] [Google Scholar]
- 54.Zhang W, Trachootham D, Liu J, Chen G, Pelicano H, Garcia-Prieto C, Lu W, Burger JA, Croce CM, Plunkett W, Keating MJ, Huang P. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nature cell biology. 2012;14:276–286. doi: 10.1038/ncb2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jitschin R, Braun M, Qorraj M, Saul D, Le Blanc K, Zenz T, Mougiakakos D. Stromal cell-mediated glycolytic switch in CLL-cells involves Notch-c-Myc signaling. Blood. 2015 doi: 10.1182/blood-2014-10-607036. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 56.Lutzny G, Kocher T, Schmidt-Supprian M, Rudelius M, Klein-Hitpass L, Finch AJ, Durig J, Wagner M, Haferlach C, Kohlmann A, Schnittger S, Seifert M, Wanninger S, Zaborsky N, Oostendorp R, Ruland J, Leitges M, Kuhnt T, Schafer Y, Lampl B, Peschel C, Egle A, Ringshausen I. Protein kinase c-beta-dependent activation of NF-kappaB in stromal cells is indispensable for the survival of chronic lymphocytic leukemia B cells in vivo. Cancer Cell. 2013;23:77–92. doi: 10.1016/j.ccr.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghosh AK, Secreto C, Boysen J, Sassoon T, Shanafelt TD, Mukhopadhyay D, Kay NE. The novel receptor tyrosine kinase Axl is constitutively active in B-cell chronic lymphocytic leukemia and acts as a docking site of nonreceptor kinases: implications for therapy. Blood. 2011;117:1928–1937. doi: 10.1182/blood-2010-09-305649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghosh AK, Secreto CR, Knox TR, Ding W, Mukhopadhyay D, Kay NE. Circulating microvesicles in B-cell chronic lymphocytic leukemia can stimulate marrow stromal cells: implications for disease progression. Blood. 2010;115:1755–1764. doi: 10.1182/blood-2009-09-242719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Badoux X, Bueso-Ramos C, Harris D, Li P, Liu Z, Burger J, O’Brien S, Ferrajoli A, Keating MJ, Estrov Z. Cross-talk between chronic lymphocytic leukemia cells and bone marrow endothelial cells: role of signal transducer and activator of transcription 3. Human pathology. 2011;42:1989–2000. doi: 10.1016/j.humpath.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cols M, Barra CM, He B, Puga I, Xu W, Chiu A, Tam W, Knowles DM, Dillon SR, Leonard JP, Furman RR, Chen K, Cerutti A. Stromal endothelial cells establish a bidirectional crosstalk with chronic lymphocytic leukemia cells through the TNF-related factors BAFF, APRIL, and CD40L. Journal of immunology. 2012;188:6071–6083. doi: 10.4049/jimmunol.1102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamilton E, Pearce L, Morgan L, Robinson S, Ware V, Brennan P, Thomas NS, Yallop D, Devereux S, Fegan C, Buggins AG, Pepper C. Mimicking the tumour microenvironment: three different co-culture systems induce a similar phenotype but distinct proliferative signals in primary chronic lymphocytic leukaemia cells. British journal of haematology. 2012;158:589–599. doi: 10.1111/j.1365-2141.2012.09191.x. [DOI] [PubMed] [Google Scholar]
- 62.Maffei R, Fiorcari S, Bulgarelli J, Martinelli S, Castelli I, Deaglio S, Debbia G, Fontana M, Coluccio V, Bonacorsi G, Zucchini P, Narni F, Torelli G, Luppi M, Marasca R. Physical contact with endothelial cells through beta1- and beta2- integrins rescues chronic lymphocytic leukemia cells from spontaneous and drug-induced apoptosis and induces a peculiar gene expression profile in leukemic cells. Haematologica. 2012;97:952–960. doi: 10.3324/haematol.2011.054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maffei R, Bulgarelli J, Fiorcari S, Martinelli S, Castelli I, Valenti V, Rossi D, Bonacorsi G, Zucchini P, Potenza L, Vallisa D, Gattei V, Poeta GD, Forconi F, Gaidano G, Narni F, Luppi M, Marasca R. Endothelin-1 Promotes Survival and Chemoresistance in Chronic Lymphocytic Leukemia B Cells through ETA Receptor. PloS one. 2014;9:e98818. doi: 10.1371/journal.pone.0098818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pedersen IM, Kitada S, Leoni LM, Zapata JM, Karras JG, Tsukada N, Kipps TJ, Choi YS, Bennett F, Reed JC. Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood. 2002;100:1795–1801. [PubMed] [Google Scholar]
- 65.Granziero L, Circosta P, Scielzo C, Frisaldi E, Stella S, Geuna M, Giordano S, Ghia P, Caligaris-Cappio F. CD100/Plexin-B1 interactions sustain proliferation and survival of normal and leukemic CD5+ B lymphocytes. Blood. 2003;101:1962–1969. doi: 10.1182/blood-2002-05-1339. [DOI] [PubMed] [Google Scholar]
- 66.Heinig K, Gatjen M, Grau M, Stache V, Anagnostopoulos I, Gerlach K, Niesner RA, Cseresnyes Z, Hauser AE, Lenz P, Hehlgans T, Brink R, Westermann J, Dorken B, Lipp M, Lenz G, Rehm A, Hopken UE. Access to follicular dendritic cells is a pivotal step in murine chronic lymphocytic leukemia B-cell activation and proliferation. Cancer discovery. 2014;4:1448–1465. doi: 10.1158/2159-8290.CD-14-0096. [DOI] [PubMed] [Google Scholar]
- 67.van Kooten C, Banchereau J. CD40-CD40 ligand. Journal of leukocyte biology. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 68.Kitada S, Zapata JM, Andreeff M, Reed JC. Bryostatin and CD40-ligand enhance apoptosis resistance and induce expression of cell survival genes in B-cell chronic lymphocytic leukaemia. British journal of haematology. 1999;106:995–1004. doi: 10.1046/j.1365-2141.1999.01642.x. [DOI] [PubMed] [Google Scholar]
- 69.Riches JC, Gribben JG. Understanding the immunodeficiency in chronic lymphocytic leukemia: potential clinical implications. Hematol Oncol Clin North Am. 2013;27:207–235. doi: 10.1016/j.hoc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 70.Brusa D, Serra S, Coscia M, Rossi D, D’Arena G, Laurenti L, Jaksic O, Fedele G, Inghirami G, Gaidano G, Malavasi F, Deaglio S. The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica. 2013;98:953–963. doi: 10.3324/haematol.2012.077537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramsay AG, Johnson AJ, Lee AM, Gorgun G, Le Dieu R, Blum W, Byrd JC, Gribben JG. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. The Journal of clinical investigation. 2008;118:2427–2437. doi: 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood. 2012;120:1412–1421. doi: 10.1182/blood-2012-02-411678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramsay AG, Evans R, Kiaii S, Svensson L, Hogg N, Gribben JG. Chronic lymphocytic leukemia cells induce defective LFA-1-directed T-cell motility by altering Rho GTPase signaling that is reversible with lenalidomide. Blood. 2013;121:2704–2714. doi: 10.1182/blood-2012-08-448332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Riches JC, Davies JK, McClanahan F, Fatah R, Iqbal S, Agrawal S, Ramsay AG, Gribben JG. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121:1612–1621. doi: 10.1182/blood-2012-09-457531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.te Raa GD, Pascutti MF, Garcia-Vallejo JJ, Reinen E, Remmerswaal EB, ten Berge IJ, van Lier RA, Eldering E, van Oers MH, Tonino SH, Kater AP. CMV-specific CD8+ T-cell function is not impaired in chronic lymphocytic leukemia. Blood. 2014;123:717–724. doi: 10.1182/blood-2013-08-518183. [DOI] [PubMed] [Google Scholar]
- 76.McClanahan F, Hanna B, Miller S, Clear AJ, Lichter P, Gribben JG, Seiffert M. PD-L1 Checkpoint Blockade Prevents Immune Dysfunction and Leukemia Development in a Mouse Model of Chronic Lymphocytic Leukemia. Blood. 2015 doi: 10.1182/blood-2015-01-622936. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Motta M, Rassenti L, Shelvin BJ, Lerner S, Kipps TJ, Keating MJ, Wierda WG. Increased expression of CD152 (CTLA-4) by normal T lymphocytes in untreated patients with B-cell chronic lymphocytic leukemia. Leukemia. 2005;19:1788–1793. doi: 10.1038/sj.leu.2403907. [DOI] [PubMed] [Google Scholar]
- 78.Rizzo R, Audrito V, Vacca P, Rossi D, Brusa D, Stignani M, Bortolotti D, D’Arena G, Coscia M, Laurenti L, Forconi F, Gaidano G, Mingari MC, Moretta L, Malavasi F, Deaglio S. HLA-G is a component of the chronic lymphocytic leukemia escape repertoire to generate immune suppression: impact of the HLA-G 14 base pair (rs66554220) polymorphism. Haematologica. 2014;99:888–896. doi: 10.3324/haematol.2013.095281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reiners KS, Topolar D, Henke A, Simhadri VR, Kessler J, Sauer M, Bessler M, Hansen HP, Tawadros S, Herling M, Kronke M, Hallek M, Pogge von Strandmann E. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood. 2013;121:3658–3665. doi: 10.1182/blood-2013-01-476606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Veuillen C, Aurran-Schleinitz T, Castellano R, Rey J, Mallet F, Orlanducci F, Pouyet L, Just-Landi S, Coso D, Ivanov V, Carcopino X, Bouabdallah R, Collette Y, Fauriat C, Olive D. Primary B-CLL resistance to NK cell cytotoxicity can be overcome in vitro and in vivo by priming NK cells and monoclonal antibody therapy. J Clin Immunol. 2012;32:632–646. doi: 10.1007/s10875-011-9624-5. [DOI] [PubMed] [Google Scholar]
- 81.Huergo-Zapico L, Acebes-Huerta A, Gonzalez-Rodriguez AP, Contesti J, Gonzalez-Garcia E, Payer AR, Villa-Alvarez M, Fernandez-Guizan A, Lopez-Soto A, Gonzalez S. Expansion of NK cells and reduction of NKG2D expression in chronic lymphocytic leukemia. Correlation with progressive disease. PloS one. 2014;9:e108326. doi: 10.1371/journal.pone.0108326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wild J, Schmiedel BJ, Maurer A, Raab S, Prokop L, Stevanovic S, Dorfel D, Schneider P, Salih HR. Neutralization of (NK-cell-derived) B-cell activating factor by Belimumab restores sensitivity of chronic lymphoid leukemia cells to direct and Rituximab-induced NK lysis. Leukemia. 2015 doi: 10.1038/leu.2015.50. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 83.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) The Journal of experimental medicine. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Calissano C, Damle RN, Hayes G, Murphy EJ, Hellerstein MK, Moreno C, Sison C, Kaufman MS, Kolitz JE, Allen SL, Rai KR, Chiorazzi N. In vivo intraclonal and interclonal kinetic heterogeneity in B-cell chronic lymphocytic leukemia. Blood. 2009;114:4832–4842. doi: 10.1182/blood-2009-05-219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coelho V, Krysov S, Steele A, Sanchez Hidalgo M, Johnson PW, Chana PS, Packham G, Stevenson FK, Forconi F. Identification in CLL of circulating intraclonal subgroups with varying B-cell receptor expression and function. Blood. 2013;122:2664–2672. doi: 10.1182/blood-2013-02-485425. [DOI] [PubMed] [Google Scholar]
- 86.Decker S, Finter J, Forde AJ, Kissel S, Schwaller J, Mack TS, Kuhn A, Gray N, Follo M, Jumaa H, Burger M, Zirlik K, Pfeifer D, Miduturu CV, Eibel H, Veelken H, Dierks C. PIM Kinases Are Essential for Chronic Lymphocytic Leukemia Cell Survival (PIM2/3) and CXCR4-Mediated Microenvironmental Interactions (PIM1) Mol Cancer Ther. 2014;13:1231–1245. doi: 10.1158/1535-7163.MCT-13-0575-T. [DOI] [PubMed] [Google Scholar]
- 87.Burger JA, Burger M, Kipps TJ. Chronic Lymphocytic Leukemia B Cells Express Functional CXCR4 Chemokine Receptors That Mediate Spontaneous Migration Beneath Bone Marrow Stromal Cells. Blood. 1999;94:3658–3667. [PubMed] [Google Scholar]
- 88.Mittal AK, Chaturvedi NK, Rai KJ, Gilling-Cutucache CE, Nordgren TM, Moragues M, Lu R, Opavsky R, Bociek GR, Weisenburger DD, Iqbal J, Joshi SS. Chronic lymphocytic leukemia cells in a lymph node microenvironment depict molecular signature associated with an aggressive disease. Molecular medicine. 2014;20:290–301. doi: 10.2119/molmed.2012.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vaisitti T, Aydin S, Rossi D, Cottino F, Bergui L, D’Arena G, Bonello L, Horenstein AL, Brennan P, Pepper C, Gaidano G, Malavasi F, Deaglio S. CD38 increases CXCL12-mediated signals and homing of chronic lymphocytic leukemia cells. Leukemia. 2010;24:958–969. doi: 10.1038/leu.2010.36. [DOI] [PubMed] [Google Scholar]
- 90.Richardson SJ, Matthews C, Catherwood MA, Alexander HD, Carey BS, Farrugia J, Gardiner A, Mould S, Oscier D, Copplestone JA, Prentice AG. ZAP-70 expression is associated with enhanced ability to respond to migratory and survival signals in B-cell chronic lymphocytic leukemia (B-CLL) Blood. 2006;107:3584–3592. doi: 10.1182/blood-2005-04-1718. [DOI] [PubMed] [Google Scholar]
- 91.Brachtl G, Sahakyan K, Denk U, Girbl T, Alinger B, Hofbauer SW, Neureiter D, Hofbauer JP, Egle A, Greil R, Hartmann TN. Differential bone marrow homing capacity of VLA-4 and CD38 high expressing chronic lymphocytic leukemia cells. PloS one. 2011;6:e23758. doi: 10.1371/journal.pone.0023758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walsby E, Buggins A, Devereux S, Jones C, Pratt G, Brennan P, Fegan C, Pepper C. Development and characterization of a physiologically relevant model of lymphocyte migration in chronic lymphocytic leukemia. Blood. 2014;123:3607–3617. doi: 10.1182/blood-2013-12-544569. [DOI] [PubMed] [Google Scholar]
- 93.Burger M, Hartmann T, Krome M, Rawluk J, Tamamura H, Fujii N, Kipps TJ, Burger JA. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106:1824–1830. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 94.O’Hayre M, Salanga CL, Kipps TJ, Messmer D, Dorrestein PC, Handel TM. Elucidating the CXCL12/CXCR4 signaling network in chronic lymphocytic leukemia through phosphoproteomics analysis. PloS one. 2010;5:e11716. doi: 10.1371/journal.pone.0011716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zucchetto A, Vaisitti T, Benedetti D, Tissino E, Bertagnolo V, Rossi D, Bomben R, Dal Bo M, Del Principe MI, Gorgone A, Pozzato G, Gaidano G, Del Poeta G, Malavasi F, Deaglio S, Gattei V. The CD49d/CD29 complex is physically and functionally associated with CD38 in B-cell chronic lymphocytic leukemia cells. Leukemia. 2012;26:1301–1312. doi: 10.1038/leu.2011.369. [DOI] [PubMed] [Google Scholar]
- 96.Burger JA, Zvaifler NJ, Tsukada N, Firestein GS, Kipps TJ. Fibroblast-like synoviocytes support B-cell pseudoemperipolesis via a stromal cell-derived factor-1- and CD106 (VCAM-1)-dependent mechanism. The Journal of clinical investigation. 2001;107:305–315. doi: 10.1172/JCI11092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gattei V, Bulian P, Del Principe MI, Zucchetto A, Maurillo L, Buccisano F, Bomben R, Dal-Bo M, Luciano F, Rossi FM, Degan M, Amadori S, Del Poeta G. Relevance of CD49d protein expression as overall survival and progressive disease prognosticator in chronic lymphocytic leukemia. Blood. 2008;111:865–873. doi: 10.1182/blood-2007-05-092486. [DOI] [PubMed] [Google Scholar]
- 98.Sivina M, Hartmann E, Kipps TJ, Rassenti L, Krupnik D, Lerner S, Lapushin R, Xiao L, Huang X, Werner L, Neuberg D, Kantarjian H, O’Brien S, Wierda WG, Keating MJ, Rosenwald A, Burger JA. CCL3 (MIP-1{alpha}) plasma levels and the risk for disease progression in chronic lymphocytic leukemia (CLL) Blood. 2011;117:1662–1669. doi: 10.1182/blood-2010-09-307249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krzysiek R, Lefevre EA, Zou W, Foussat A, Bernard J, Portier A, Galanaud P, Richard Y. Antigen receptor engagement selectively induces macrophage inflammatory protein-1 alpha (MIP-1 alpha) and MIP-1 beta chemokine production in human B cells. Journal of immunology. 1999;162:4455–4463. [PubMed] [Google Scholar]
- 100.Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nature immunology. 2001;2:1126–1132. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 101.Ghia P, Strola G, Granziero L, Geuna M, Guida G, Sallusto F, Ruffing N, Montagna L, Piccoli P, Chilosi M, Caligaris-Cappio F. Chronic lymphocytic leukemia B cells are endowed with the capacity to attract CD4+, CD40L+ T cells by producing CCL22. Eur J Immunol. 2002;32:1403–1413. doi: 10.1002/1521-4141(200205)32:5<1403::AID-IMMU1403>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 102.Scielzo C, Apollonio B, Scarfo L, Janus A, Muzio M, Ten Hacken E, Ghia P, Caligaris-Cappio F. The functional in vitro response to CD40 ligation reflects a different clinical outcome in patients with chronic lymphocytic leukemia. Leukemia. 2011;25:1760–1767. doi: 10.1038/leu.2011.149. [DOI] [PubMed] [Google Scholar]
- 103.Kini AR, Kay NE, Peterson LC. Increased bone marrow angiogenesis in B cell chronic lymphocytic leukemia. Leukemia. 2000;14:1414–1418. doi: 10.1038/sj.leu.2401825. [DOI] [PubMed] [Google Scholar]
- 104.Baban D, Murray J, Earl H, Kerr D, Seymour L. Quantitative analysis of vascular endothelial growth factor expression in chronic lymphocytic leukaemia. International journal of oncology. 1996;8:29–34. [PubMed] [Google Scholar]
- 105.Molica S, Vitelli G, Levato D, Gandolfo GM, Liso V. Increased serum levels of vascular endothelial growth factor predict risk of progression in early B-cell chronic lymphocytic leukaemia. British journal of haematology. 1999;107:605–610. doi: 10.1046/j.1365-2141.1999.01752.x. [DOI] [PubMed] [Google Scholar]
- 106.Bairey O, Boycov O, Kaganovsky E, Zimra Y, Shaklai M, Rabizadeh E. All three receptors for vascular endothelial growth factor (VEGF) are expressed on B-chronic lymphocytic leukemia (CLL) cells. Leukemia research. 2004;28:243–248. doi: 10.1016/s0145-2126(03)00256-x. [DOI] [PubMed] [Google Scholar]
- 107.Piechnik A, Dmoszynska A, Omiotek M, Mlak R, Kowal M, Stilgenbauer S, Bullinger L, Giannopoulos K. The VEGF receptor, neuropilin-1, represents a promising novel target for chronic lymphocytic leukemia patients. International journal of cancer Journal international du cancer. 2013;133:1489–1496. doi: 10.1002/ijc.28135. [DOI] [PubMed] [Google Scholar]
- 108.Menzel T, Rahman Z, Calleja E, White K, Wilson EL, Wieder R, Gabrilove J. Elevated intracellular level of basic fibroblast growth factor correlates with stage of chronic lymphocytic leukemia and is associated with resistance to fludarabine. Blood. 1996;87:1056–1063. [PubMed] [Google Scholar]
- 109.Ding W, Knox TR, Tschumper RC, Wu W, Schwager SM, Boysen JC, Jelinek DF, Kay NE. Platelet-derived growth factor (PDGF)-PDGF receptor interaction activates bone marrow-derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood. 2010;116:2984–2993. doi: 10.1182/blood-2010-02-269894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ding W, Nowakowski GS, Knox TR, Boysen JC, Maas ML, Schwager SM, Wu W, Wellik LE, Dietz AB, Ghosh AK, Secreto CR, Medina KL, Shanafelt TD, Zent CS, Call TG, Kay NE. Bi-directional activation between mesenchymal stem cells and CLL B-cells: implication for CLL disease progression. British journal of haematology. 2009;147:471–483. doi: 10.1111/j.1365-2141.2009.07868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maffei R, Fiorcari S, Bulgarelli J, Rizzotto L, Martinelli S, Rigolin GM, Debbia G, Castelli I, Bonacorsi G, Santachiara R, Forconi F, Rossi D, Laurenti L, Palumbo GA, Vallisa D, Cuneo A, Gaidano G, Luppi M, Marasca R. Endothelium-mediated survival of leukemic cells and angiogenesis-related factors are affected by lenalidomide treatment in chronic lymphocytic leukemia. Experimental hematology. 2014;42:126–136 e121. doi: 10.1016/j.exphem.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 112.Scielzo C, Ghia P, Conti A, Bachi A, Guida G, Geuna M, Alessio M, Caligaris-Cappio F. HS1 protein is differentially expressed in chronic lymphocytic leukemia patient subsets with good or poor prognoses. The Journal of clinical investigation. 2005;115:1644–1650. doi: 10.1172/JCI24276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.ten Hacken E, Scielzo C, Bertilaccio MT, Scarfo L, Apollonio B, Barbaglio F, Stamatopoulos K, Ponzoni M, Ghia P, Caligaris-Cappio F. Targeting the LYN/HS1 signaling axis in chronic lymphocytic leukemia. Blood. 2013;121:2264–2273. doi: 10.1182/blood-2012-09-457119. [DOI] [PubMed] [Google Scholar]
- 114.Burger JA, Chiorazzi N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013;34:592–601. doi: 10.1016/j.it.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iacovelli S, Hug E, Bennardo S, Duehren-von Minden M, Gobessi S, Rinaldi A, Suljagic M, Bilbao D, Bolasco G, Eckl-Dorna J, Niederberger V, Autore F, Sica S, Laurenti L, Wang H, Cornall RJ, Clarke SH, Croce CM, Bertoni F, Jumaa H, Efremov DG. Two types of BCR interactions are positively selected during leukemia development in the Emu-TCL1 transgenic mouse model of CLL. Blood. 2015;125:1578–1588. doi: 10.1182/blood-2014-07-587790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Agathangelidis A, Darzentas N, Hadzidimitriou A, Brochet X, Murray F, Yan XJ, Davis Z, van Gastel-Mol EJ, Tresoldi C, Chu CC, Cahill N, Giudicelli V, Tichy B, Pedersen LB, Foroni L, Bonello L, Janus A, Smedby K, Anagnostopoulos A, Merle-Beral H, Laoutaris N, Juliusson G, di Celle PF, Pospisilova S, Jurlander J, Geisler C, Tsaftaris A, Lefranc MP, Langerak AW, Oscier DG, Chiorazzi N, Belessi C, Davi F, Rosenquist R, Ghia P, Stamatopoulos K. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood. 2012;119:4467–4475. doi: 10.1182/blood-2011-11-393694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lanham S, Hamblin T, Oscier D, Ibbotson R, Stevenson F, Packham G. Differential signaling via surface IgM is associated with VH gene mutational status and CD38 expression in chronic lymphocytic leukemia. Blood. 2003;101:1087–1093. doi: 10.1182/blood-2002-06-1822. [DOI] [PubMed] [Google Scholar]
- 118.Krysov S, Dias S, Paterson A, Mockridge CI, Potter KN, Smith KA, Ashton-Key M, Stevenson FK, Packham G. Surface IgM stimulation induces MEK1/2-dependent MYC expression in chronic lymphocytic leukemia cells. Blood. 2012;119:170–179. doi: 10.1182/blood-2011-07-370403. [DOI] [PubMed] [Google Scholar]
- 119.Chen L, Widhopf G, Huynh L, Rassenti L, Rai KR, Weiss A, Kipps TJ. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2002;100:4609–4614. doi: 10.1182/blood-2002-06-1683. [DOI] [PubMed] [Google Scholar]
- 120.Allsup DJ, Kamiguti AS, Lin K, Sherrington PD, Matrai Z, Slupsky JR, Cawley JC, Zuzel M. B-cell receptor translocation to lipid rafts and associated signaling differ between prognostically important subgroups of chronic lymphocytic leukemia. Cancer research. 2005;65:7328–7337. doi: 10.1158/0008-5472.CAN-03-1563. [DOI] [PubMed] [Google Scholar]
- 121.Muzio M, Apollonio B, Scielzo C, Frenquelli M, Vandoni I, Boussiotis V, Caligaris-Cappio F, Ghia P. Constitutive activation of distinct BCR-signaling pathways in a subset of CLL patients: a molecular signature of anergy. Blood. 2008;112:188–195. doi: 10.1182/blood-2007-09-111344. [DOI] [PubMed] [Google Scholar]
- 122.Apollonio B, Scielzo C, Bertilaccio MT, Ten Hacken E, Scarfo L, Ranghetti P, Stevenson F, Packham G, Ghia P, Muzio M, Caligaris-Cappio F. Targeting B-cell anergy in chronic lymphocytic leukemia. Blood. 2013;121:3879–3888. S3871–3878. doi: 10.1182/blood-2012-12-474718. [DOI] [PubMed] [Google Scholar]
- 123.Thompson AA, Talley JA, Do HN, Kagan HL, Kunkel L, Berenson J, Cooper MD, Saxon A, Wall R. Aberrations of the B-cell receptor B29 (CD79b) gene in chronic lymphocytic leukemia. Blood. 1997;90:1387–1394. [PubMed] [Google Scholar]
- 124.Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nature reviews Drug discovery. 2013;12:229–243. doi: 10.1038/nrd3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tavolaro S, Peragine N, Chiaretti S, Ricciardi MR, Raponi S, Messina M, Santangelo S, Marinelli M, Di Maio V, Mauro FR, Del Giudice I, Foa R, Guarini A. IgD cross-linking induces gene expression profiling changes and enhances apoptosis in chronic lymphocytic leukemia cells. Leukemia research. 2013;37:455–462. doi: 10.1016/j.leukres.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 126.Zupo S, Massara R, Dono M, Rossi E, Malavasi F, Cosulich ME, Ferrarini M. Apoptosis or plasma cell differentiation of CD38-positive B-chronic lymphocytic leukemia cells induced by cross-linking of surface IgM or IgD. Blood. 2000;95:1199–1206. [PubMed] [Google Scholar]
- 127.Morabito F, Cutrona G, Gentile M, Fabbi M, Matis S, Colombo M, Reverberi D, Megna M, Spriano M, Callea V, Vigna E, Rossi E, Lucia E, Festini G, Zupo S, Molica S, Neri A, Ferrarini M. Prognostic relevance of in vitro response to cell stimulation via surface IgD in binet stage a CLL. British journal of haematology. 2010;149:160–163. doi: 10.1111/j.1365-2141.2009.08032.x. [DOI] [PubMed] [Google Scholar]
- 128.Chu CC, Catera R, Hatzi K, Yan XJ, Zhang L, Wang XB, Fales HM, Allen SL, Kolitz JE, Rai KR, Chiorazzi N. Chronic lymphocytic leukemia antibodies with a common stereotypic rearrangement recognize nonmuscle myosin heavy chain IIA. Blood. 2008;112:5122–5129. doi: 10.1182/blood-2008-06-162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chu CC, Catera R, Zhang L, Didier S, Agagnina BM, Damle RN, Kaufman MS, Kolitz JE, Allen SL, Rai KR, Chiorazzi N. Many chronic lymphocytic leukemia antibodies recognize apoptotic cells with exposed nonmuscle myosin heavy chain IIA: implications for patient outcome and cell of origin. Blood. 2010;115:3907–3915. doi: 10.1182/blood-2009-09-244251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Herve M, Xu K, Ng YS, Wardemann H, Albesiano E, Messmer BT, Chiorazzi N, Meffre E. Unmutated and mutated chronic lymphocytic leukemias derive from self-reactive B cell precursors despite expressing different antibody reactivity. The Journal of clinical investigation. 2005;115:1636–1643. doi: 10.1172/JCI24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Broker BM, Klajman A, Youinou P, Jouquan J, Worman CP, Murphy J, Mackenzie L, Quartey-Papafio R, Blaschek M, Collins P, et al. Chronic lymphocytic leukemic (CLL) cells secrete multispecific autoantibodies. J Autoimmun. 1988;1:469–481. doi: 10.1016/0896-8411(88)90068-6. [DOI] [PubMed] [Google Scholar]
- 132.Sthoeger ZM, Wakai M, Tse DB, Vinciguerra VP, Allen SL, Budman DR, Lichtman SM, Schulman P, Weiselberg LR, Chiorazzi N. Production of autoantibodies by CD5-expressing B lymphocytes from patients with chronic lymphocytic leukemia. The Journal of experimental medicine. 1989;169:255–268. doi: 10.1084/jem.169.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kostareli E, Gounari M, Janus A, Murray F, Brochet X, Giudicelli V, Pospisilova S, Oscier D, Foroni L, di Celle PF, Tichy B, Pedersen LB, Jurlander J, Ponzoni M, Kouvatsi A, Anagnostopoulos A, Thompson K, Darzentas N, Lefranc MP, Belessi C, Rosenquist R, Davi F, Ghia P, Stamatopoulos K. Antigen receptor stereotypy across B-cell lymphoproliferations: the case of IGHV4-59/IGKV3-20 receptors with rheumatoid factor activity. Leukemia. 2012;26:1127–1131. doi: 10.1038/leu.2011.311. [DOI] [PubMed] [Google Scholar]
- 134.Borche L, Lim A, Binet JL, Dighiero G. Evidence that chronic lymphocytic leukemia B lymphocytes are frequently committed to production of natural autoantibodies. Blood. 1990;76:562–569. [PubMed] [Google Scholar]
- 135.Lanemo Myhrinder A, Hellqvist E, Sidorova E, Soderberg A, Baxendale H, Dahle C, Willander K, Tobin G, Backman E, Soderberg O, Rosenquist R, Horkko S, Rosen A. A new perspective: molecular motifs on oxidized LDL, apoptotic cells, and bacteria are targets for chronic lymphocytic leukemia antibodies. Blood. 2008;111:3838–3848. doi: 10.1182/blood-2007-11-125450. [DOI] [PubMed] [Google Scholar]
- 136.Krysov S, Potter KN, Mockridge CI, Coelho V, Wheatley I, Packham G, Stevenson FK. Surface IgM of CLL cells displays unusual glycans indicative of engagement of antigen in vivo. Blood. 2010;115:4198–4205. doi: 10.1182/blood-2009-12-254847. [DOI] [PubMed] [Google Scholar]
- 137.Gounari M, Ntoufa S, Apollonio B, Papakonstantinou N, Ponzoni M, Chu CC, Rossi D, Gaidano G, Chiorazzi N, Stamatopoulos K, Ghia P. Excessive antigen reactivity may underlie the clinical aggressiveness of chronic lymphocytic leukemia stereotyped subset 8. Blood. 2015 doi: 10.1182/blood-2014-09-603217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hoogeboom R, van Kessel KP, Hochstenbach F, Wormhoudt TA, Reinten RJ, Wagner K, Kater AP, Guikema JE, Bende RJ, van Noesel CJ. A mutated B cell chronic lymphocytic leukemia subset that recognizes and responds to fungi. The Journal of experimental medicine. 2013;210:59–70. doi: 10.1084/jem.20121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoogeboom R, Wormhoudt TA, Schipperus MR, Langerak AW, Dunn-Walters DK, Guikema JE, Bende RJ, van Noesel CJ. A novel chronic lymphocytic leukemia subset expressing mutated IGHV3-7-encoded rheumatoid factor B-cell receptors that are functionally proficient. Leukemia. 2013;27:738–740. doi: 10.1038/leu.2012.238. [DOI] [PubMed] [Google Scholar]
- 140.Duhren-von Minden M, Ubelhart R, Schneider D, Wossning T, Bach MP, Buchner M, Hofmann D, Surova E, Follo M, Kohler F, Wardemann H, Zirlik K, Veelken H, Jumaa H. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 2012;489:309–312. doi: 10.1038/nature11309. [DOI] [PubMed] [Google Scholar]
- 141.Binder M, Muller F, Frick M, Wehr C, Simon F, Leistler B, Veelken H, Mertelsmann R, Trepel M. CLL B-cell receptors can recognize themselves: alternative epitopes and structural clues for autostimulatory mechanisms in CLL. Blood. 2013;121:239–241. doi: 10.1182/blood-2012-09-454439. [DOI] [PubMed] [Google Scholar]
- 142.Fecteau JF, Corral LG, Ghia EM, Gaidarova S, Futalan D, Bharati IS, Cathers B, Schwaederle M, Cui B, Lopez-Girona A, Messmer D, Kipps TJ. Lenalidomide inhibits the proliferation of CLL cells via a cereblon/p21(WAF1/Cip1)-dependent mechanism independent of functional p53. Blood. 2014;124:1637–1644. doi: 10.1182/blood-2014-03-559591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schulz A, Durr C, Zenz T, Dohner H, Stilgenbauer S, Lichter P, Seiffert M. Lenalidomide reduces survival of chronic lymphocytic leukemia cells in primary cocultures by altering the myeloid microenvironment. Blood. 2013;121:2503–2511. doi: 10.1182/blood-2012-08-447664. [DOI] [PubMed] [Google Scholar]
- 144.Aue G, Njuguna N, Tian X, Soto S, Hughes T, Vire B, Keyvanfar K, Gibellini F, Valdez J, Boss C, Samsel L, McCoy JP, Jr, Wilson WH, Pittaluga S, Wiestner A. Lenalidomide-induced upregulation of CD80 on tumor cells correlates with T-cell activation, the rapid onset of a cytokine release syndrome and leukemic cell clearance in chronic lymphocytic leukemia. Haematologica. 2009;94:1266–1273. doi: 10.3324/haematol.2009.005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee BN, Gao H, Cohen EN, Badoux X, Wierda WG, Estrov Z, Faderl SH, Keating MJ, Ferrajoli A, Reuben JM. Treatment with lenalidomide modulates T-cell immunophenotype and cytokine production in patients with chronic lymphocytic leukemia. Cancer. 2011;117:3999–4008. doi: 10.1002/cncr.25983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Acebes-Huerta A, Huergo-Zapico L, Gonzalez-Rodriguez AP, Fernandez-Guizan A, Payer AR, Lopez-Soto A, Gonzalez S. Lenalidomide induces immunomodulation in chronic lymphocytic leukemia and enhances antitumor immune responses mediated by NK and CD4 T cells. BioMed research international. 2014;2014:265840. doi: 10.1155/2014/265840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, Schafer P, Bartlett JB. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:4650–4657. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 148.Lapalombella R, Andritsos L, Liu Q, May SE, Browning R, Pham LV, Blum KA, Blum W, Ramanunni A, Raymond CA, Smith LL, Lehman A, Mo X, Jarjoura D, Chen CS, Ford R, Jr, Rader C, Muthusamy N, Johnson AJ, Byrd JC. Lenalidomide treatment promotes CD154 expression on CLL cells and enhances production of antibodies by normal B cells through a PI3-kinase-dependent pathway. Blood. 2010;115:2619–2629. doi: 10.1182/blood-2009-09-242438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, Todryk S, Chen R, Muller G, Stirling D, Schafer P, Bartlett JB, Dalgleish AG. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58:1033–1045. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ferrajoli A, Lee BN, Schlette EJ, O’Brien SM, Gao H, Wen S, Wierda WG, Estrov Z, Faderl S, Cohen EN, Li C, Reuben JM, Keating MJ. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chanan-Khan A, Miller KC, Lawrence D, Padmanabhan S, Miller A, Hernandez-Illatazurri F, Czuczman MS, Wallace PK, Zeldis JB, Lee K. Tumor flare reaction associated with lenalidomide treatment in patients with chronic lymphocytic leukemia predicts clinical response. Cancer. 2011;117:2127–2135. doi: 10.1002/cncr.25748. [DOI] [PubMed] [Google Scholar]
- 152.Badoux XC, Keating MJ, Wen S, Lee BN, Sivina M, Reuben J, Wierda WG, O’Brien SM, Faderl S, Kornblau SM, Burger JA, Ferrajoli A. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood. 2011;118:3489–3498. doi: 10.1182/blood-2011-03-339077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen CI, Bergsagel PL, Paul H, Xu W, Lau A, Dave N, Kukreti V, Wei E, Leung-Hagesteijn C, Li ZH, Brandwein J, Pantoja M, Johnston J, Gibson S, Hernandez T, Spaner D, Trudel S. Single-agent lenalidomide in the treatment of previously untreated chronic lymphocytic leukemia. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:1175–1181. doi: 10.1200/JCO.2010.29.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.James DF, Werner L, Brown JR, Wierda WG, Barrientos JC, Castro JE, Greaves A, Johnson AJ, Rassenti LZ, Rai KR, Neuberg D, Kipps TJ. Lenalidomide and rituximab for the initial treatment of patients with chronic lymphocytic leukemia: a multicenter clinical-translational study from the chronic lymphocytic leukemia research consortium. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32:2067–2073. doi: 10.1200/JCO.2013.51.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pollyea DA, Coutre S, Gore L, Adler N, Harris P, Phelps MA, Johnson AJ, Ling Y, Li H, Gutman JA, Byrd JC. A Dose Escalation Study of Ibrutinib with Lenalidomide for Relapsed and Refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Blood (ASH annual meeting abstracts) 2014;124:1987. [Google Scholar]
- 156.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 157.Stamatopoulos B, Meuleman N, De Bruyn C, Pieters K, Mineur P, Le Roy C, Saint-Georges S, Varin-Blank N, Cymbalista F, Bron D, Lagneaux L. AMD3100 disrupts the cross-talk between chronic lymphocytic leukemia cells and a mesenchymal stromal or nurse-like cell-based microenvironment: pre-clinical evidence for its association with chronic lymphocytic leukemia treatments. Haematologica. 2012;97:608–615. doi: 10.3324/haematol.2011.052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Andritsos L, Byrd JC, Jones JA, Hewes B, Kipps TJ, Hsu FJ, Burger JA. Preliminary Results From A Phase I Dose Escalation Study to Determine the Maximum Tolerated Dose of Plerixafor In Combination with Rituximab In Patients with Relapsed Chronic Lymphocytic Leukemia. Blood. 2010;116:1017a. [Google Scholar]
- 159.Hoellenriegel J, Zboralski D, Maasch C, Rosin NY, Wierda WG, Keating MJ, Kruschinski A, Burger JA. The Spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood. 2014;123:1032–1039. doi: 10.1182/blood-2013-03-493924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Steurer M, Montillo M, Scarfo L, Romana Mauro F, Andel J, Wildner S, Trentin L, Janssens A, Burgstaller D, Kruschinski A, Dummler T, Riecke K, Ghia P, Caligaris-Cappio F, Gobbi M. Results from a Phase IIa Study of the Anti-CXCL12 Spiegelmer Olaptesed Pegol (NOX-A12) in Combination with Bendamustine/Rituximab in Patients with Chronic Lymphocytic Leukemia. Blood (ASH annual meeting abstracts) 2014;124:1996. [Google Scholar]
- 161.Takata M, Kurosaki T. A role for Bruton’s tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-gamma 2. The Journal of experimental medicine. 1996;184:31–40. doi: 10.1084/jem.184.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.de Gorter DJ, Beuling EA, Kersseboom R, Middendorp S, van Gils JM, Hendriks RW, Pals ST, Spaargaren M. Bruton’s tyrosine kinase and phospholipase Cgamma2 mediate chemokine-controlled B cell migration and homing. Immunity. 2007;26:93–104. doi: 10.1016/j.immuni.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 163.Rushworth SA, Bowles KM, Barrera LN, Murray MY, Zaitseva L, MacEwan DJ. BTK inhibitor ibrutinib is cytotoxic to myeloma and potently enhances bortezomib and lenalidomide activities through NF-kappaB. Cellular signalling. 2013;25:106–112. doi: 10.1016/j.cellsig.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 164.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, Buggy JJ. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, Flynn J, Jones J, Blum KA, Buggy JJ, Hamdy A, Johnson AJ, Byrd JC. Bruton’s tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, Keating MJ, O’Brien S, Chiorazzi N, Burger JA. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, Pals ST, Spaargaren M. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 168.Yeh YY, Ozer HG, Lehman AM, Maddocks K, Yu L, Johnson AJ, Byrd JC. Characterization of CLL exosomes reveals a distinct microRNA signature and enhanced secretion by activation of B-cell receptor signaling. Blood. 2015 doi: 10.1182/blood-2014-12-618470. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Sukbuntherng J, Chang BY, Clow F, Hedrick E, Buggy JJ, James DF, O’Brien S. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, Coutre S, Tam CS, Mulligan SP, Jaeger U, Devereux S, Barr PM, Furman RR, Kipps TJ, Cymbalista F, Pocock C, Thornton P, Caligaris-Cappio F, Robak T, Delgado J, Schuster SJ, Montillo M, Schuh A, de Vos S, Gill D, Bloor A, Dearden C, Moreno C, Jones JJ, Chu AD, Fardis M, McGreivy J, Clow F, James DF, Hillmen P, Investigators R. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. The New England journal of medicine. 2014;371:213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Burger JA, Keating MJ, Wierda WG, Hartmann E, Hoellenriegel J, Rosin NY, de Weerdt I, Jeyakumar G, Ferrajoli A, Cardenas-Turanzas M, Lerner S, Jorgensen JL, Nogueras-Gonzalez GM, Zacharian G, Huang X, Kantarjian H, Garg N, Rosenwald A, O’Brien S. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. The Lancet Oncology. 2014;15:1090–1099. doi: 10.1016/S1470-2045(14)70335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Brown JR, Barrientos JC, Barr PM, Flinn IW, Burger JA, Tran A, Clow F, James DF, Graef T, Friedberg JW, Rai K, O’Brien S. The Bruton tyrosine kinase inhibitor ibrutinib with chemoimmunotherapy in patients with chronic lymphocytic leukemia. Blood. 2015;125:2915–2922. doi: 10.1182/blood-2014-09-585869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Farooqui MZ, Valdez J, Martyr S, Aue G, Saba N, Niemann CU, Herman SE, Tian X, Marti G, Soto S, Hughes TE, Jones J, Lipsky A, Pittaluga S, Stetler-Stevenson M, Yuan C, Lee YS, Pedersen LB, Geisler CH, Calvo KR, Arthur DC, Maric I, Childs R, Young NS, Wiestner A. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. The Lancet Oncology. 2015;16:169–176. doi: 10.1016/S1470-2045(14)71182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.O’Brien S, Jones JA, Coutre S, Mato AR, Hillmen P, Tam C, Osterborg A, Siddiqi T, Thirman MJ, Furman RR, Ilhan O, Keating MJ, Call TG, Brown JR, Stevens-Brogan M, Li Y, Fardis M, Clow F, James DF, Chu AD, Hallek M, Stilgenbauer S. Efficacy and Safety of Ibrutinib in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia or Small Lymphocytic Leukemia with 17p Deletion: Results from the Phase II RESONATE -17 Trial. Blood (ASH annual meeting abstracts) 2014;124:327. [Google Scholar]
- 175.O’Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, Grant B, Richards DA, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Izumi R, Hamdy A, Chang BY, Graef T, Clow F, Buggy JJ, James DF, Byrd JC. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. The Lancet Oncology. 2014;15:48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Smith DD, Goldstein L, Cheng M, James DF, Kunkel LA, Fardis M, Hamdy A, Izumi R, Buggy JJ, Clow F. Modeling absolute lymphocyte counts after treatment of chronic lymphocytic leukemia with ibrutinib. Annals of hematology. 2015;94:249–256. doi: 10.1007/s00277-014-2187-9. [DOI] [PubMed] [Google Scholar]
- 177.Wodarz D, Garg N, Komarova NL, Benjamini O, Keating MJ, Wierda WG, Kantarjian H, James D, O’Brien S, Burger JA. Kinetics of CLL cells in tissues and blood during therapy with the BTK inhibitor ibrutinib. Blood. 2014;123:4132–4135. doi: 10.1182/blood-2014-02-554220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Burger J, Kelvin L, Keating MJ, Sivina M, Ferrajoli A, Jalayer A, Huang X, Kantarjian H, Wierda WG, O’Brien S, Hellerstein M, Turner S, Emson C, Chen S, Yan X, Chiorazzi N. Functional Evidence from Deuterated Water Labeling That the Bruton Tyrosine Kinase Inhibitor Ibrutinib Blocks Leukemia Cell Proliferation and Trafficking and Promotes Leukemia Cell Death in Patients with Chronic Lymphocytic Leukemia and small Lymphocytic Lymphoma. Blood (ASH annual meeting abstracts) 2014;124:326. [Google Scholar]
- 179.Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS, Xue L, Li DH, Steggerda SM, Versele M, Dave SS, Zhang J, Yilmaz AS, Jaglowski SM, Blum KA, Lozanski A, Lozanski G, James DF, Barrientos JC, Lichter P, Stilgenbauer S, Buggy JJ, Chang BY, Johnson AJ, Byrd JC. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. The New England journal of medicine. 2014;370:2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fegan C, Bagshawe J, Salles G, Karlin L, Rule S, Shah N, Morschhauser F, Terriou L, Dyer MJ, Walter H, Hutchinson C, Cartron G, Sharpe J, Duffy K, Nishimura A, Abe S, Honda H, Yasuhiro T, Yoshizawa T, Birkett J, Courtenay-Luck N. The Bruton’s Tyrosine Kinase (BTK) Inhibitor ONO-4059: Promising Single Agent Activity and Well Tolerated in Patients with High Risk Chronic Lymphocytic Leukaemia (CLL) Blood (ASH annual meeting abstracts) 2014;124:3328. [Google Scholar]
- 181.Salles GA, Karlin L, Rule S, Shah N, Morschhauser F, Terriou L, Dyer MJ, Hutchinson C, Fegan C, Cartron G, Knurowski T, Saunders AJ, Wright JG, Honda H, Mazur A, Yoshizawa T, Kawabata K, Birkett JT. A phase I study of the oral BTK inhibitor ONO-4059 in patients with relapsed/refractory and high risk chronic lymphocytic leukaemia (CLL) Blood (ASH annual meeting abstracts) 2013;122:676. [Google Scholar]
- 182.Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3:317–330. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- 183.Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, Byrd JC, Tyner JW, Loriaux MM, Deininger M, Druker BJ, Puri KD, Ulrich RG, Giese NA. CAL-101, a p110{delta} selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, Giese N, O’Brien S, Yu A, Miller LL, Lannutti BJ, Burger JA. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.de Rooij MF, Kuil A, Kater AP, Kersten MJ, Pals ST, Spaargaren M. Ibrutinib and idelalisib synergistically target BCR-controlled adhesion in MCL and CLL: a rationale for combination therapy. Blood. 2015;125:2306–2309. doi: 10.1182/blood-2014-12-619163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, Spurgeon SE, Kahl BS, Bello C, Webb HK, Johnson DM, Peterman S, Li D, Jahn TM, Lannutti BJ, Ulrich RG, Yu AS, Miller LL, Furman RR. Idelalisib, an inhibitor of phosphatidylinositol 3 kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:3390–3397. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zelenetz AD, Lamanna N, Kipps TJ, Coutre S, O’Brien S, Aiello M, Cho Y, Dubowy RL, Flinn IW. A Phase 2 Study of Idelalisib Monotherapy in Previously Untreated Patients ≥65 Years with Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL) Blood (ASH annual meeting abstracts) 2014;124:1986. [Google Scholar]
- 188.Sharman JP, Coutre S, Furman RR, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, Zelenetz AD, Kipps TJ, Flinn I, Ghia P, Hallek M, Coiffier B, O’Brien S, Tausch E, Kreuzer K, Jiang W, Lazarov M, Li D, Jahn TM, Stilgenbauer S. Second Interim Analysis of a Phase 3 Study of Idelalisib (ZYDELIG®) Plus Rituximab (R) for Relapsed Chronic Lymphocytic Leukemia (CLL): Efficacy Analysis in Patient Subpopulations with Del(17p) and Other Adverse Prognostic Factors. Blood (ASH annual meeting abstracts) 2014;124:330. [Google Scholar]
- 189.Barrientos JC, Coutre S, de Vos S, Wagner-Johnston MT, Boyd TE, Rai KR, Leonard JP, Kim Y, Viggiano A, Jahn TM, Furman RR. Long-Term Follow-up of a Phase 1 Trial of Idelalisib (ZYDELIG®) in Combination with Bendamustine (B), Bendamustine/Rituximab (BR), Fludarabine (F), Chlorambucil (Chl), or Chlorambucil/Rituximab (ChlR) in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL) Blood (ASH annual meeting abstracts) 2014;124:4696. [Google Scholar]
- 190.O’Brien S, Lamanna N, Kipps TJ, Flinn IW, Zelenetz AD, Burger JA, Holes LM, Cho Y, Dubowy RL, Coutre SE. Update on a Phase 2 Study of Idelalisib in Combination with Rituximab in Treatment-Naïve Patients ≥65 Years with Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL) Blood (ASH annual meeting abstracts) 2014;124:1994. [Google Scholar]
- 191.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, Zelenetz AD, Kipps TJ, Flinn I, Ghia P, Eradat H, Ervin T, Lamanna N, Coiffier B, Pettitt AR, Ma S, Stilgenbauer S, Cramer P, Aiello M, Johnson DM, Miller LL, Li D, Jahn TM, Dansey RD, Hallek M, O’Brien SM. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Balakrishnan K, Peluso M, Fu M, Rosin NY, Burger JA, Wierda WG, Keating MJ, Faia K, O’Brien S, Kutok JL, Gandhi V. The Phosphoinositide-3-Kinase (PI3K)-Delta and Gamma Inhibitor, IPI-145, Overcomes Signals from the PI3K/AKT/S6 Pathway and Promotes Apoptosis in CLL. Leukemia. 2015 doi: 10.1038/leu.2015.105. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dong S, Guinn D, Dubovsky JA, Zhong Y, Lehman A, Kutok J, Woyach JA, Byrd JC, Johnson AJ. IPI-145 antagonizes intrinsic and extrinsic survival signals in chronic lymphocytic leukemia cells. Blood. 2014;124:3583–3586. doi: 10.1182/blood-2014-07-587279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.O’Brien S, Patel M, Kahl BS, Horwitz SM, Foss FM, Porcu P, Sweeney J, Allen K, Faia K, Stern HM, Kelly P, Flinn I. Duvelisib (IPI-145), a PI3K-δ,γ Inhibitor, Is Clinically Active in Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia. Blood (ASH annual meeting abstracts) 2014;124:3334. [Google Scholar]
- 195.Porcu P, Flinn I, Kahl BS, Horwitz SM, Oki Y, Byrd JC, Sweeney J, Allen K, Faia K, Ni M, Stern HM, Kelly KR, O’Brien S. Clinical Activity of Duvelisib (IPI-145), a Phosphoinositide-3-Kinase-δ,γ Inhibitor, in Patients Previously Treated with Ibrutinib. Blood (ASH annual meeting abstracts) 2014;124:3335. [Google Scholar]
- 196.Brown JR, Davids MS, Rodon J, Abrisqueta P, Kasar SN, Lager J, Jiang J, Egile C, Awan FT. Phase I trial of the pan-PI3K inhibitor pilaralisib (SAR245408/XL147) in patients with chronic lymphocytic leukemia (CLL) or relapsed/refractory lymphoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015 doi: 10.1158/1078-0432.CCR-14-3262. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 197.Lunning MA, Vose JM, Schreeder MT, Fowler N, Nastoupil LJ, Siddiqi T, Blumel S, Pauli EK, Cutter K, Tse W, Miskin HP, Sportelli P, Weiss MS, Vakkalanka S, Viswanadha S, O’Brien S. Ublituximab, a Novel Glycoengineered Anti-CD20 Monoclonal Antibody (mAb), in Combination with TGR-1202, a Next Generation Once Daily PI3kδ Inhibitor, Demonstrates Activity in Heavily Pre-Treated and High-Risk Chronic Lymphocytic Leukemia (CLL) and B-Cell Lymphoma. Blood (ASH annual meeting abstracts) 2014;124:801. [Google Scholar]
- 198.Burris HA, Patel MR, Brander DM, O’Connor OA, Deng C, Fenske TS, Gutierrez M, Jones S, Kuhn J, Miskin HP, Sportelli P, Vakkalanka S, Flinn I. TGR-1202, a Novel Once Daily PI3Kδ Inhibitor, Demonstrates Clinical Activity with a Favorable Safety Profile, Lacking Hepatotoxicity, in Patients with Chronic Lymphocytic Leukemia and B-Cell Lymphoma. Blood (ASH annual meeting abstracts) 2014;124:1984. [Google Scholar]
- 199.Kurosaki T, Hikida M. Tyrosine kinases and their substrates in B lymphocytes. Immunological reviews. 2009;228:132–148. doi: 10.1111/j.1600-065X.2008.00748.x. [DOI] [PubMed] [Google Scholar]
- 200.Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, Schaefer-Cutillo J, De Vos S, Sinha R, Leonard JP, Cripe LD, Gregory SA, Sterba MP, Lowe AM, Levy R, Shipp MA. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Weinblatt ME, Kavanaugh A, Genovese MC, Musser TK, Grossbard EB, Magilavy DB. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. The New England journal of medicine. 2010;363:1303–1312. doi: 10.1056/NEJMoa1000500. [DOI] [PubMed] [Google Scholar]
- 202.Sharman J, Hawkins M, Kolibaba K, Boxer M, Klein L, Wu M, Hu J, Abella S, Yasenchak C. An open-label phase 2 trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in chronic lymphocytic leukemia. Blood. 2015;125:2336–2343. doi: 10.1182/blood-2014-08-595934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Brown JR, Barrientos JC, Barr PM, Flinn I, Burger J, Salman Z, Clow F, James DF, Graef T, Friedberg JW, Rai KR, O’Brien S. Ibrutinib in combination with bendamustine and rituximab is active and tolerable in patients with relapsed/refractory CLL/SLL: final results of a phase 1b study. Blood (ASH Annual Meeting Abstracts) 2013;122:525. [Google Scholar]